Abstract
Background
Brugada syndrome (BrS) is an inherited arrhythmic disease associated with an increased risk of major arrhythmic events (MAE). Previous studies reported that a wide QRS complex may be useful as a predictor of MAE in BrS patients. We aimed to assess the correlation of wide QRS complex with MAE by a systematic review and meta‐analysis.
Methods
We comprehensively searched the databases of MEDLINE and EMBASE from inception to June 2019. Included studies were cohort and case control studies that reported QRS duration and the relationship between wide QRS complex (>120 milliseconds) and MAE (sudden cardiac death, sudden cardiac arrest, ventricular fibrillation, sustained ventricular tachycardia, or appropriate shock). Data from each study were combined using the random‐effects model.
Results
Twenty‐two studies from 2007 to 2018 were included in this meta‐analysis involving 4,814 BrS patients. The mean age was 46.1 ± 12.8 years. The patients were predominately men (77.6%). Wide QRS duration was an independent predictor of MAE (pooled risk ratio 1.55, 95% confidence interval: 1.04‐2.30, P = .30, I 2 = 38.4%). QRS duration was wider in BrS who had history of MAE (weight mean difference = 8.12 milliseconds, 95% confidence interval: 5.75‐10.51 milliseconds).
Conclusions
Our study demonstrated that QRS duration is wider in BrS who had history of MAE, and a wide QRS complex is associated with 1.55 times higher risk of MAE in BrS populations. Wide QRS complex can be considered for risk stratification in prediction of MAE in patients with BrS, especially when considering implantable cardioverter‐defibrillator placement in asymptomatic patients.
Keywords: Brugada syndrome, Major arrhythmic events, wide QRS
Our study demonstrated that a wide QRS complex is associated with 1.55 times higher risk of major arrhythmic events in Brugada syndrome populations. Wide QRS complex can be considered for risk stratification in prediction of MAE in patients with BrS, especially when considering implantable cardioverter‐defibrillator placement in asymptomatic patients.

Abbreviations
- BrS
Brugada syndrome
- CI
confidence interval
- ECG
electrocardiogram
- MAE
major arrhythmic events
- NOS
Newcastle‐Ottawa quality assessment scale
- RR
risk ratio
- SCA
sudden cardiac arrest
- SCD
sudden cardiac death
- VF
ventricular fibrillation
- VT
ventricular tachycardia
1. INTRODUCTION
Brugada syndrome (BrS) is an inherited arrhythmic disease associated with an increased risk of major arrhythmic events (MAE) and sudden cardiac death (SCD), and ventricular arrhythmias such as ventricular fibrillation (VF) and ventricular tachycardia (VT). It is characterized by a coved‐type ST elevation and atypical right bundle‐branch block appearances in the right precordial leads. The disease burden of BrS is difficult to determine; however, data suggest that the prevalence of asymptomatic patients with Brugada ECG pattern varies among different populations, ranging between 0% and 0.4%.1, 2, 3 With SCD being the most worrisome manifestation of BrS, identifying patients who would benefit from an implantable cardioverter‐defibrillator for primary prevention is critical; however, ventricular arrhythmia risk stratification remains challenging and controversial.
Many electrocardiogram (ECG) abnormalities have been proposed as markers for risk of MAE. Among them, conduction disturbances, such as delayed depolarization manifested as a wide QRS complex, may contribute to the development of MAE in BrS by providing or worsening a pro‐arrhythmic substrate.4 Previous studies reported that QRS duration may be useful as a predictor of MAE in BrS patients. Wide QRS complex, as defined by QRS duration >120 milliseconds measured on a standard 12‐lead ECG, has been associated with an increased risk of ventricular arrhythmia.5 Additionally, wide QRS complex was also found to be more prominent in symptomatic BrS patients.6, 7 However, data from other studies reported conflicting results, suggesting that QRS width was not useful as a risk stratification tool.8, 9 Therefore, we aimed to assess whether wide QRS complex is associated with an increased risk of MAE in BrS patients by performing a systematic review and meta‐analysis.
2. METHOD
2.1. Search strategy
Two investigators (CK and NP) independently searched for published studies indexed in MEDLINE and EMBASE databases from inception to June 2019 using a search strategy that including the terms “ECG”, “QRS”, and “Brugada” as described in File S1. Only full articles in English and studies conducted in cohorts were included. A manual search for additional pertinent studies and review articles using references from retrieved articles was also completed.
2.2. Inclusion criteria
The eligibility criteria included the following:
Cohort (prospective or retrospective), cross‐sectional, or randomized control trial studies reporting follow‐up outcome of MAE including SCD, sudden cardiac arrest (SCA), VF, sustained VT and appropriate shock in BrS patients with and without previously documented wide QRS complex as well as history of MAE as a characteristic.
QRS duration, adjusted or unadjusted risk ratio (RR), odds ratio, hazard ratio with 95% confidence interval (CI), or sufficient raw data for the calculation were provided. Patients without previously documented wide QRS complex were used as controls. Odds ratio and hazard ratio were converted to RR by previously reported principal equations.10
Study eligibility was independently determined by two investigators (RM and CT) and differences were resolved by mutual consensus. The Newcastle‐Ottawa quality assessment scale (NOS) was used to assess each study's quality in three domains, recruitment and selection of the participants, similarity and comparability between the groups, and ascertainment of the outcome of interest among cohort and case‐control studies.11
2.3. Data extraction
A standardized data collection form was used to obtain the following information from each study: title of study, name of first author, year of publication, study design, country of origin, number, gender and age of the participants, Brugada ECG pattern, available MAE outcome, follow‐up duration, leads of QRS duration measurement, and confounders that were adjusted in the multivariable analysis, if available.
Two investigators (PP and PM) independently performed this data extraction process to ensure accurate data extraction. Any data discrepancy was resolved by referring back to the original articles.
2.4. Definition
2.4.1. Wide QRS complex
Wide QRS complex was defined as QRS complex with a duration of >120 milliseconds.
2.4.2. Brugada syndrome
Brugada syndrome was diagnosed in patients with ST‐segment elevation with type 1 morphology ≥2 mm in ≥1 lead in the right precordial leads V1, V2, positioned in the 2nd, 3rd, or 4th intercostal space occurring either spontaneously or after provocative drug test with intravenous administration of class I antiarrhythmic drugs.12
2.4.3. Major arrhythmic event
Major arrhythmic events were defined by either of SCD, SCA, VF, sustained VT, or appropriate shock. VF was defined as documented VF rhythm from standard 12‐lead ECG or Holter monitoring, or as defined in each study. Sustained ventricular tachycardia was defined as a sustained ventricular rhythm, documented from standard 12‐lead ECG or Holter monitoring, faster than 100 beats per minute lasting at least 30 seconds, or requiring termination earlier because of hemodynamic instability. Only sustained VT, VF, and appropriate defibrillator intervention were included in this study. Nonsustained VT and inappropriate shock were not considered an outcome of interest.
2.4.4. Sudden cardiac death and sudden cardiac arrest
Sudden cardiac death was defined as an unexpected, nontraumatic death that occurred within 60 minutes from the onset of new or worsening symptoms or within 24 hours of last being observed alive.13 Sudden cardiac arrest was defined as a sudden cessation of cardiac activity with hemodynamic collapse for which an intervention or spontaneous reversion restores circulation.
2.5. Statistical analysis
We performed a meta‐analysis of the included studies using a random‐effects model. Studies were excluded if they did not include an outcome in each intervention group or did not have enough information required for continuous data comparison. We pooled the point estimates of RR from each study using the generic inverse‐variance method of Der Simonian and Laird.14 To examine QRS duration, we calculated the weighted mean difference and 95% CIs of QRS duration between BrS patients with and without history of MAE. The heterogeneity of effect size estimates across these studies was quantified using the I 2 statistic. The I 2 statistic ranges in value from 0% to 100% (I 2 < 25%, low heterogeneity; I 2 = 25%‐50%, moderate heterogeneity; and I 2 > 50%, substantial heterogeneity).15 Publication bias was assessed using a funnel plot and the Egger's regression test16 (P < .05 was considered significant). All data analyses were performed using the STATA SE version 14.2.
2.6. Sensitivity analysis
A sensitivity analysis was performed to assess the influence of the individual studies on the overall results by omitting one study at a time. We used a sequential exclusion strategy, as described by Patsopoulos et al, to examine whether overall estimates were influenced by the substantial heterogeneity observed 17. We sequentially and cumulatively excluded studies that accounted for the largest share of heterogeneity until I 2 was less than 50%. We then examined whether RR estimates were consistent.
3. RESULT
3.1. Search results
Our search strategy yielded 872 potentially relevant articles (498 articles from EMBASE and 374 articles from MEDLINE). After the exclusion of duplicated articles, 456 articles underwent title and abstract review. At this stage, 360 articles were excluded as they were not cohort, case‐control, or randomized controlled trials, were not conducted in BrS patients, or the titles and abstracts were not relevant. This left 96 articles for full‐length review. A further 74 studies were excluded as they did not report data regarding QRS duration, reported QRS duration as continuous data, or did not provide sufficient data to calculate HR, RR, or OR. Therefore, a total of 22 studies were included in this meta‐analysis. Figure 1 outlines the search and literature review process.
Figure 1.
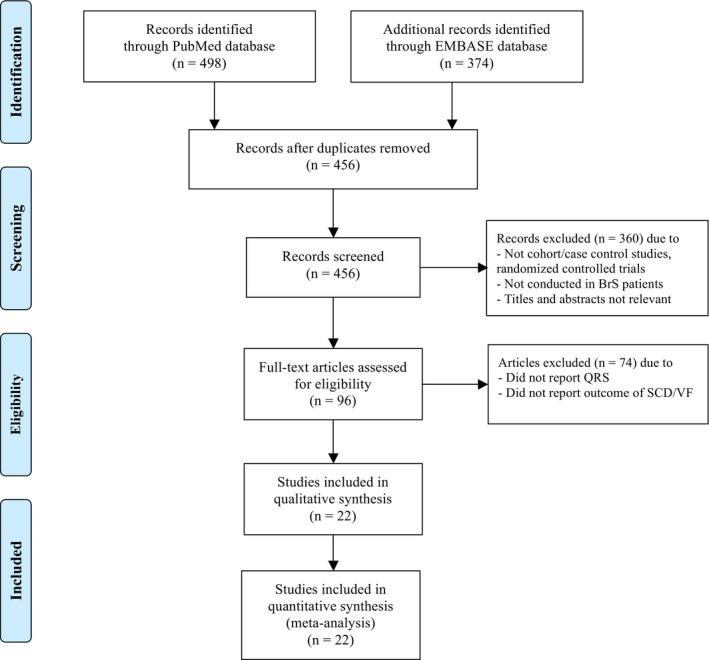
Search methodology and selection process
3.2. Description of included studies
A total of 22 studies from 2007 to 20186, 7, 9, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 were included in our meta‐analysis involving 4,814 BrS patients. The mean age was 46.1 ± 12.8 years and patients were predominately men (77.6%) and Caucasian (67.1%). A summary of study characteristics is shown in Table 1. Among 22 included studies, 16 studies6, 18, 19, 21, 22, 24, 26, 28, 29, 30, 31, 32, 33, 34, 35, 36 reported mean QRS duration compared between BrS with and without history of MAE and 7 studies7, 9, 18, 20, 23, 25, 27 reported the incidence, odds ratio, hazard ratio, or RR of MAE during follow‐up period compared between BrS with wide (>120 milliseconds) and normal QRS duration (≤120 milliseconds).
Table 1.
Summary characteristics of individual included studies of patients with a Brugada syndrome
| Study (year) | Study design | N | Country | Men (%) | Mean age (years) | Symptomatic BrS (%) | Follow‐up (months) | Factors adjusted in analyses | Leads measured | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| de Asmundis et al, (2017) | Cohort | 289 | Belgium | 70.2 | 44.8 ± 16.0 | 35.6 | 120.6 ± 55.7 | N/A | V1‐V3 | SCD or appropriate shcok |
| Benito et al (2008) | Cohort | 384 | Spain, Belgium, and Canada | 70.8 | 45.9 ± 15.3 | 21.6 | 57.9 ± 48.8 | N/A | V2 | VF or SCD |
| Calò et al (2016) | Prospective cohort | 347 | Italy | 78.4 | 45.0 ± 13.1 | 20.5 | 48.0 ± 38.6 | N/A | V2 | VF or SCD |
| Conte et al (2013) | Retrospective cohort | 503 | Belgium | 58 | 40.7 ± 12.3 | 46 | 29 ± 8 | N/A | V1‐V2 | sVT or VF |
| Furushima et al (2005) | Case‐control | 24 | Japan | 95.8 | 60.0 ± 14.4 | 62.5 | 33 ± 16 | N/A | V1‐V2 | SCA |
| Ikeda et al (2005) | Prospective cohort | 124 | Japan | 94.4 | 50.0 ± 15.0 | 33.1 | 40.0 ± 19.0 | N/A | sVT, VF, or SCD | |
| Junttila et al (2008) | Case‐control | 200 | Findland, Belgium, China, Spain, and Canada | 71.20 | 40 ± 16 | 33 | N/A | Gender, age, and SCN5A mutation | sVT, VF, or SCD | |
| Kanda et al (2012) | Retrospective cohort | 34 | Japan | 97.1 | 43.5 ± 12.4 | 100 | 38 | N/A | V5‐V6 | VF or SCA |
| Kawata et al (2013) | Prospective cohort | 49 | Japan | 94 | 46.0 ± 12.7 | 35 | 93.8 ± 45.6 | N/A | N/A | VF or SCD |
| Kawazoe et al (2016) | Case‐control | 143 | Japan | 97.9 | 46.17 ± 12.7 | 32.9 | 82.8 ± 49.0 | N/A | V6 | VF |
| Makarawate et al (2017) | Prospective cohort | 40 | Thailand | 97.5 | 43.5 ± 12.7 | 100 | 28.3 ± 11.3 | N/A | N/A | Appropriate shock |
| Morita et al (2018) | Case‐control | 62 | Japan | 100 | 50.1 ± 10.9 | 22.6 | 27‐134 | N/A | V3 | VF |
| Nakano et al (2010) | Prospective cohort | 52 | Japan | 94.2 | 42 ± 3 | 34.6 | 39 + 4 | NA | V1‐V2 | VF |
| Nishii et al (2010) | Prospective cohort | 108 | Japan | 97.2 | 46.8 ± 11.6 | 38.9 | 71.9 ± 41.3 | N/A | V5 | VF |
| Park et al (2003) | 15 | Korea | 86.7 | 44 ± 10 | 87 | 19 ± 14 | N/A | N/A | VF | |
| Probst et al (2010) | Prospective cohort | 1029 | Italy, Germany, France and The Netherlands | 72 | 45 ± 5 | 36 | 40 ± 50 | N/A | N/A | SCA |
| Sieira et al (2017) | Retrospective cohort | 400 | Belgium | 58.3 | 41.1 ± 17.8 | 32.7 | 80.7 ± 57.2 | N/A | N/A | SCD |
| Takagi et al (2007) | Retrospective cohort | 188 | Japan | 94.7 | 53 ± 14 | 47.9 | 37 ± 16 | N/A | V6 | VF |
| Take et al (2012) | Retrospective cohort | 84 | Japan | 97.6 | 47 ± 12 | 48.8 | 48 ± 48 | N/A | SAEG | VF |
| Tokioka et al (2014) | Prospective cohort | 246 | Japan | 95.9 | 47.6 ± 13.6 | N/A | 45.1 ± 44.3 | N/A | V2 | SCD, VF |
| Yamagata et al (2017) | Prospective cohort | 415 | Japan | 97 | 46 ± 14 | 45.3 | 72 | N/A | V2 | Appropriate shock, SCA or SCD |
| Zumhagen et al (2016) | Retrospective cros‐section | 78 | Germany | 73.1 | 45 + 14 | 65.4 | N/A | N/A | V1 | sVT, VF, or SCD |
Abbreviations: N/A, not applicable; SCA, sudden cardiac arrest; SCD, sudden cardiac death; sVT, sustained ventricular tachycardia; VF, ventricular fibrillation.
3.2.1. Quality assessment of included studies
The NOS of included studies are described in File S2. The NOS uses a star system (0 to 9) to evaluate included studies on 3 domains: selection, comparability, and outcomes. Higher scores represent a higher study quality.
3.3. Meta‐analysis results
3.3.1. Wide QRS complex and major arrhythmic event
Outcomes regarding MAE were available in 7 studies.7, 9, 18, 20, 23, 25, 27 There was a significant association between wide QRS complex and an increased risk of MAE (pooled RR = 1.55, 95% CI: 1.04‐2.30, P = .03, I 2 = 38.4%). QRS duration was wider in BrS who had history of MAE (weight mean difference = 8.12 milliseconds, 95% confidence interval: 5.75‐10.51 milliseconds, I 2 = 43.4%). Forest plot is demonstrated in Figures 2 and 3, respectively.
Figure 2.
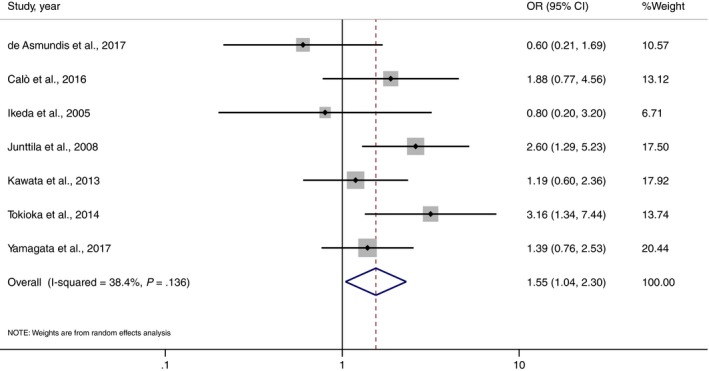
Forest plot demonstrating the association of wide QRS and MAE in patients with Brugada syndrome
Figure 3.
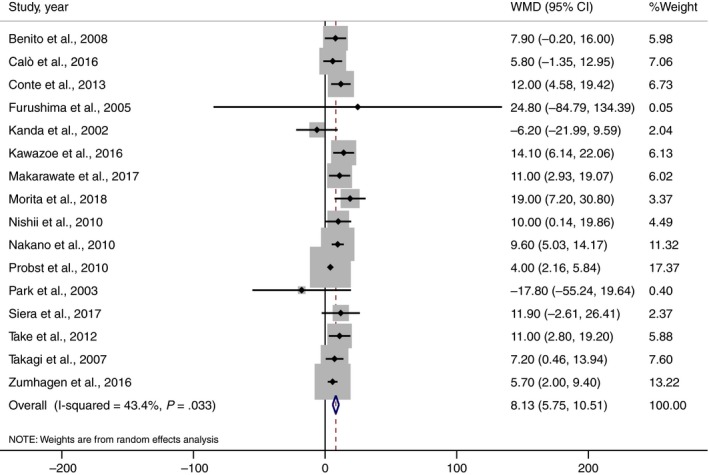
Forest plot demonstrating weight mean difference QRS duration between Brugada syndrome patient with and without history of MAE
3.3.2. Sensitivity analysis
To assess the stability of the results of the meta‐analysis, we conducted a sensitivity analysis for each outcome by excluding one study at a time. When omitting Calo et al18, Junttika et al7, Tokioka et al9, or Yamagata et al20, wide QRS still increased MAE (RR = 1.49, 1.39, 1.40, and 1.57 respectively) but the results were nonsignificant (File S3).
3.3.3. Publication bias
We aimed to investigate potential publication bias via funnel plot and Egger's test. No publication bias was observed in Egger's test (P = .051 for QRS duration weight mean difference analysis and P = .500 for wide QRS analysis) or funnel plot (Figures 4 and 5).38, 39
Figure 4.
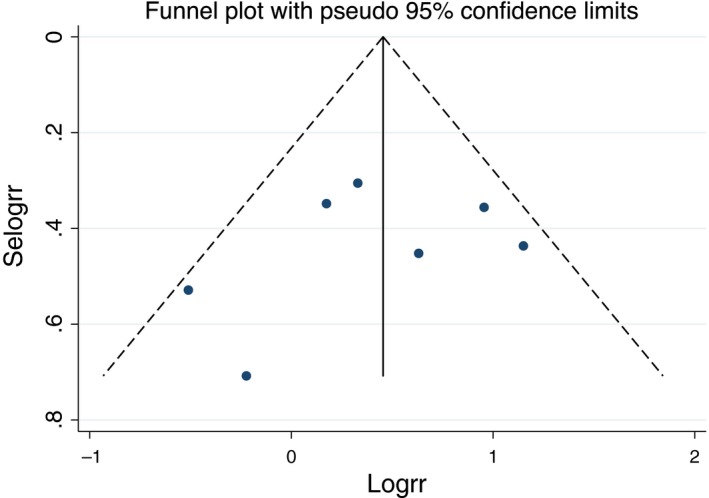
Funnel plot of the association of wide QRS and MAE in patients with Brugada syndrome
Figure 5.
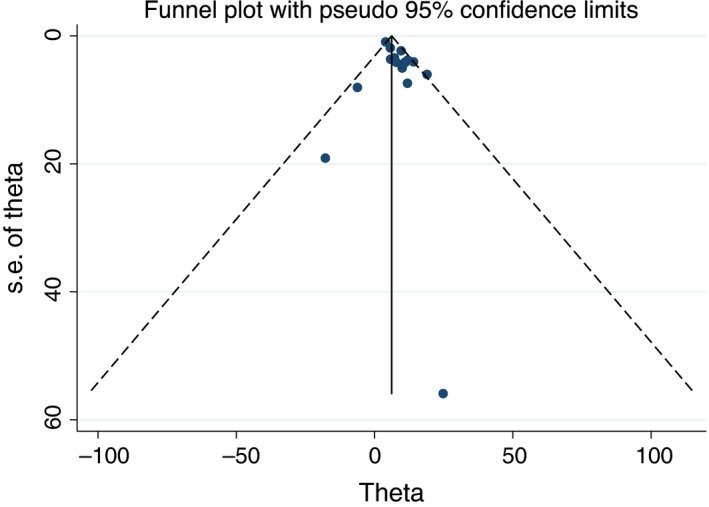
Funnel plot of weight mean difference QRS duration between Brugada syndrome patient with and without history of MAE
4. DISCUSSION
The main finding from this meta‐analysis is that the wide QRS complex on surface 12‐lead ECG is associated with an increased risk of MAE in patients with BrS.
Brugada syndrome has been reported to be responsible for up to 20% of SCD in patients with a structurally normal heart, and likely the cause in 4% of all SCD.40 Placement of ICD is a class I recommendation in BrS patients with a previous history of MAE, including SCD, SCA, VF, or sustained VT41, 42 However, a majority of newly diagnosed BrS patients are without previous history of MAE, and thus, they may not meet an indication for ICD placement.43 In addition, it remains a challenge to identify asymptomatic patients who are at risk of MAE.41
From the current evidence, the only well‐established risk factor for MAE in BrS population is spontaneous type I ECG pattern with a presence of symptoms (previous MAE or syncope presumed to be due ventricular arrhythmias).42 This pattern is more commonly found in adult male, Asians ethnicity, and patients with fever.44 Other factors that are associated with increased risk of MAE include male gender, proband status, family history of SCD, early repolarization, fragmented QRS, atrial fibrillation, prolonged atrio‐His and His‐ventricular intervals, and inducible ventricular arrhythmia during the electrophysiological study.6, 18, 21, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 To the first of the authors' knowledge, this is the first systematic review and meta‐analysis to evaluate the association between wide QRS complex and risk of MAE in BrS population.
There are three well‐known suggested mechanisms that could explain the ventricular arrhythmia in BrS,55 including the repolarization, the depolarization, microscopic fibrosis, myocyte inflammation, and the neural crest models, which are all originated from the right ventricular outflow tract abnormality found in BrS patients.56, 57, 58, 59 However, experts believe that there may be other involving factors, yet to be discovered, that also play major role in MAE in BrS patients. Mutation of the SCN5A gene, which encodes the pore‐forming region of the cardiac sodium channel, is the most common gene mutation found in BrS patients, with the impaired sodium channels predisposing patients to phase 2 reentry precipitating ventricular arrhythmias.6, 47 However, this mutation was only found in less than 30% of the patients with BrS55, 60 and only shown to be associated with MAE in Asian populations but not Caucasian populations.20, 50 Most patients with BrS do not have an identifiable mutation. This indicates that there are likely still other causal genes that are yet to be found or other factors not yet described.
A wide QRS complex is a previously described feature shown in the surface ECG of BrS pattern. This conduction abnormality is the likely result of the decreased function of the sodium channel and the abnormal sodium current influx found in an individual with SCN5A gene mutation, leading to lethal arrhythmias in BrS.4, 61 Ohkubo et al reported that QRS duration >120 milliseconds in lead V2 can be used as a predictor of ventricular arrhythmias in BrS population.5 Smits et al found that BrS patients with the SCN5A mutation showed a trend toward longer QRS duration in lead V2, with prolongation of both atrio‐His and His‐Ventricular (HV) interval, compared with the BrS patients without SCN5A mutation.62 In addition, Bordachar et al also revealed that patients with an HV interval >55 milliseconds had significantly more atrial arrhythmias than those with a normal HV interval, which subsequently predispose such patients to MAE. The etiology is unclear but could be from L‐type calcium channel mutation or microscopic fibrosis.
Nevertheless, it is important to note that BrS is a heterogeneous disease, meaning that the mechanism of MAE could differ in each patient. Wide QRS is not the only depolarization ECG marker for the increased risk of MAE, as fragmented QRS, QRS dispersion, and presence of late potentials have also been described.52, 63 Tse et al reported higher QRS dispersion in Type‐1 BrS than non‐Type‐1 BrS. However, no MAE was compared or reported.64 Our group published a comprehensive systemic review and meta‐analysis on fragmented QRS as a predictor of arrhythmic event in BrS and showed that baseline fragmented QRS increased major arrhythmic events up to 3‐fold.52 Repolarization markers, such as early repolarization and prolonged QT interval, have also been associated with MAE risk.9, 65, 66, 67 While a wide QRS complex may have a direct effect to MAE development in certain patients, in other patients this finding is just a sign of a more advanced disease.
4.1. Limitations
Our study is not without limitations. We observed moderate heterogeneity (I 2 = 38.4 and 43.4%) likely because of different patient characteristics, univariate analysis, and measurement leads. The unadjusted studies may be influenced by other confounders causing instability of the result observed in sensitivity analysis. Lastly, despite the association between wide QRS complex and MAE in BrS population, causality cannot be concluded from our study.
5. CONCLUSION
Our systematic review and meta‐analysis demonstrate that the presence of wide QRS complex on the 12‐lead ECG is associated with an increased risk of MAE in patients with BrS. This marker can be easily obtained on the surface ECG and can be used to help stratify the risk for MAE in this population.
CONFLICT OF INTEREST
The authors declare no conflict of interests for this article.
Supporting information
ACKNOWLEDGMENT
None.
Rattanawong P, Kewcharoen J, Techorueangwiwat C, et al. Wide QRS complex and the risk of major arrhythmic events in Brugada syndrome patients: A systematic review and meta‐analysis. J Arrhythmia. 2020;36:143–152. 10.1002/joa3.12290
Pattara Rattanawong and Jakrin Kewcharoen contributed equally.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
REFERENCES
- 1. Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10(12):1932–63. [DOI] [PubMed] [Google Scholar]
- 2. Vutthikraivit W, Rattanawong P, Putthapiban P, Sukhumthammarat W, Vathesatogkit P, Ngarmukos T, et al. Worldwide prevalence of Brugada syndrome: a systematic review and meta‐analysis. Acta Cardiol Sin. 2018;34(3):267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miyasaka Y, Tsuji H, Yamada K, Tokunaga S, Saito D, Imuro Y, et al. Prevalence and mortality of the Brugada‐type electrocardiogram in one city in Japan. J Am Coll Cardiol. 2001;38(3):771–4. [DOI] [PubMed] [Google Scholar]
- 4. Aiba T, Shimizu W, Hidaka I, Uemura K, Noda T, Zheng C, et al. Cellular basis for trigger and maintenance of ventricular fibrillation in the Brugada syndrome model: high‐resolution optical mapping study. J Am Coll Cardiol. 2006;47(10):2074–85. [DOI] [PubMed] [Google Scholar]
- 5. Ohkubo K, Watanabe I, Okumura Y, Ashino S, Kofune M, Nagashima K, et al. Prolonged QRS duration in lead V2 and risk of life‐threatening ventricular Arrhythmia in patients with Brugada syndrome. International Heart Journal. 2011;52(2):98–102. [DOI] [PubMed] [Google Scholar]
- 6. Takagi M, Yokoyama Y, Aonuma K, Aihara N, Hiraoka M; Japan Idiopathic Ventricular Fibrillation Study I . Clinical characteristics and risk stratification in symptomatic and asymptomatic patients with Brugada syndrome: multicenter study in Japan. J Cardiovasc Electrophysiol. 2007;18(12):1244–51. [DOI] [PubMed] [Google Scholar]
- 7. Junttila MJ, Brugada P, Hong K, Lizotte E, De zutter M, Sarkozy A, et al. Differences in 12‐lead electrocardiogram between symptomatic and asymptomatic Brugada syndrome patients. J Cardiovasc Electrophysiol. 2008;19(4):380–3. [DOI] [PubMed] [Google Scholar]
- 8. Maury P, Rollin A, Sacher F, Gourraud J‐B, Raczka F, Pasquié J‐L, et al. Prevalence and prognostic role of various conduction disturbances in patients with the Brugada syndrome. Am J Cardiol. 2013;112(9):1384–9. [DOI] [PubMed] [Google Scholar]
- 9. Tokioka K, Kusano KF, Morita H, Miura D, Nishii N, Nagase S, et al. Electrocardiographic parameters and fatal arrhythmic events in patients with Brugada syndrome: combination of depolarization and repolarization abnormalities. J Am Coll Cardiol. 2014;63(20):2131–8. [DOI] [PubMed] [Google Scholar]
- 10. Shor E, Roelfs D, Vang ZM. The "Hispanic mortality paradox" revisited: Meta‐analysis and meta‐regression of life‐course differentials in Latin American and Caribbean immigrants' mortality. Soc Sci Med. 2017;186:20–33. [DOI] [PubMed] [Google Scholar]
- 11. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 12. Sieira J, Brugada P. The definition of the Brugada syndrome. Eur Heart J. 2017;38(40):3029–34. [DOI] [PubMed] [Google Scholar]
- 13. Kuriachan VP, Sumner GL, Mitchell LB. Sudden cardiac death. Curr Probl Cardiol. 2015;40(4):133–200. [DOI] [PubMed] [Google Scholar]
- 14. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–55. [DOI] [PubMed] [Google Scholar]
- 17. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between‐study heterogeneity in meta‐analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calò L, Giustetto C, Martino A, Sciarra L, Cerrato N, Marziali M, et al. A new electrocardiographic marker of sudden death in Brugada syndrome: the S‐Wave in lead I. J Am Coll Cardiol. 2016;67(12):1427–40. [DOI] [PubMed] [Google Scholar]
- 19. Morita H, Watanabe A, Kawada S, Miyamoto M, Morimoto Y, Nakagawa K, et al. Identification of electrocardiographic risk markers for the initial and recurrent episodes of ventricular fibrillation in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2018;29(1):107–14. [DOI] [PubMed] [Google Scholar]
- 20. Yamagata K, Horie M, Aiba T, Ogawa S, Aizawa Y, Ohe T, et al. Genotype‐phenotype correlation of SCN5A mutation for the clinical and electrocardiographic characteristics of probands with Brugada syndrome: a Japanese multicenter registry. Circulation. 2017;135(23):2255–70. [DOI] [PubMed] [Google Scholar]
- 21. Benito B, Sarkozy A, Mont L, Henkens S, Berruezo A, Tamborero D, et al. Gender differences in clinical manifestations of Brugada syndrome. J Am Coll Cardiol. 2008;52(19):1567–73. [DOI] [PubMed] [Google Scholar]
- 22. Conte G, Sieira J, Sarkozy A, de Asmundis C, Di Giovanni G, Chierchia G‐B, et al. Life‐threatening ventricular arrhythmias during ajmaline challenge in patients with Brugada syndrome: incidence, clinical features, and prognosis. Heart Rhythm. 2013;10(12):1869–74. [DOI] [PubMed] [Google Scholar]
- 23. de Asmundis C, Mugnai G, Chierchia G‐B, Sieira J, Conte G, Rodriguez‐Mañero M, et al. Long‐term follow‐up of probands With Brugada syndrome. Am J Cardiol. 2017;119(9):1392–400. [DOI] [PubMed] [Google Scholar]
- 24. Furushima H, Chinushi M, Hirono T, Sugiura H, Watanabe H, Komura S, et al. Relationship between dominant prolongation of the filtered QRS duration in the right precordial leads and clinical characteristics in Brugada syndrome. J Cardiovasc Electrophysiol. 2005;16(12):1311–7. [DOI] [PubMed] [Google Scholar]
- 25. Ikeda T, Sakurada H, Sakabe K, Sakata T, Takami M, Tezuka N, et al. Assessment of noninvasive markers in identifying patients at risk in the Brugada syndrome: insight into risk stratification. J Am Coll Cardiol. 2001;37(6):1628–34. [DOI] [PubMed] [Google Scholar]
- 26. Kanda M, Shimizu W, Matsuo K, Nagaya N, Taguchi A, Suyama K, et al. Electrophysiologic characteristics and implications of induced ventricular fibrillation in symptomatic patients with Brugada syndrome. J Am Coll Cardiol. 2002;39(11):1799–805. [DOI] [PubMed] [Google Scholar]
- 27. Kawata H, Morita H, Yamada Y, Noda T, Satomi K, Aiba T, et al. Prognostic significance of early repolarization in inferolateral leads in Brugada patients with documented ventricular fibrillation: a novel risk factor for Brugada syndrome with ventricular fibrillation. Heart Rhythm. 2013;10(8):1161–8. [DOI] [PubMed] [Google Scholar]
- 28. Kawazoe H, Nakano Y, Ochi H, Takagi M, Hayashi Y, Uchimura Y, et al. Risk stratification of ventricular fibrillation in Brugada syndrome using noninvasive scoring methods. Heart Rhythm. 2016;13(10):1947–54. [DOI] [PubMed] [Google Scholar]
- 29. Makarawate P, Chaosuwannakit N, Vannaprasaht S, Sahasthas D, Koo SH, Lee EJD, et al. SCN5A genetic polymorphisms associated with increased defibrillator shocks in Brugada syndrome. J Am Heart Assoc. 2017;6:e005009 10.1161/JAHA.116.005009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakano Y, Shimizu W, Ogi H, Suenari K, Oda N, Makita Y, et al. A spontaneous Type 1 electrocardiogram pattern in lead V2 is an independent predictor of ventricular fibrillation in Brugada syndrome. Europace. 2010;12(3):410–6. [DOI] [PubMed] [Google Scholar]
- 31. Nishii N, Ogawa M, Morita H, Nakamura K, Banba K, Miura D, et al. SCN5A mutation is associated with early and frequent recurrence of ventricular fibrillation in patients with Brugada syndrome. Circ J. 2010;74(12):2572–8. [DOI] [PubMed] [Google Scholar]
- 32. Park DW, Nam GB, Rhee KS, Han GH, Choi KJ, Kim YH. Clinical characteristics of Brugada syndrome in a Korean population. Circ J. 2003;67(11):934–9. [DOI] [PubMed] [Google Scholar]
- 33. Probst V, Veltmann C, Eckardt L, Meregalli PG, Gaita F, Tan HL, et al. Long‐term prognosis of patients diagnosed with Brugada syndrome: results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121(5):635–43. [DOI] [PubMed] [Google Scholar]
- 34. Sieira J, Conte G, Ciconte G, Chierchia G‐B, Casado‐Arroyo R, Baltogiannis G, et al. A score model to predict risk of events in patients with Brugada syndrome. Eur Heart J. 2017;38(22):1756–63. [DOI] [PubMed] [Google Scholar]
- 35. Take Y, Morita H, Toh N, Nishii N, Nagase S, Nakamura K, et al. Identification of high‐risk syncope related to ventricular fibrillation in patients with Brugada syndrome. Heart Rhythm. 2012;9(5):752–9. [DOI] [PubMed] [Google Scholar]
- 36. Zumhagen S, Zeidler EM, Stallmeyer B, Ernsting M, Eckardt L, Schulze‐Bahr E. Tpeak‐Tend interval and Tpeak‐Tend/QT ratio in patients with Brugada syndrome. Europace. 2016;18(12):1866–72. [DOI] [PubMed] [Google Scholar]
- 37. Ikeda T, Takami M, Sugi K, Mizusawa Y, Sakurada H, Yoshino H. Noninvasive risk stratification of subjects with a Brugada‐type electrocardiogram and no history of cardiac arrest. Ann Noninvasive Electrocardiol. 2005;10(4):396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simmonds M. Quantifying the risk of error when interpreting funnel plots. Syst Rev. 2015;4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Debray TPA, Moons KGM, Riley RD. Detecting small‐study effects and funnel plot asymmetry in meta‐analysis of survival data: a comparison of new and existing tests. Res Synth Methods. 2018;9(1):41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111(5):659–70. [DOI] [PubMed] [Google Scholar]
- 41. Sieira J, Brugada P. Management of Brugada syndrome 2016: should all high risk patients receive an ICD? All high‐risk patients should receive an implantable cardiac defibrillator. Circ Arrhythm Electrophysiol. 2016;9:e004195 10.1161/CIRCEP.116.004195 [DOI] [PubMed] [Google Scholar]
- 42. Al‐Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72(14):e91–e220. [DOI] [PubMed] [Google Scholar]
- 43. Casado‐Arroyo R, Berne P, Rao JY, Rodriguez‐Mañero M, Levinstein M, Conte G, et al. Long‐term trends in newly diagnosed Brugada syndrome: implications for risk stratification. J Am Coll Cardiol. 2016;68(6):614–23. [DOI] [PubMed] [Google Scholar]
- 44. Shi S, Barajas‐Martinez H, Liu T, Sun Y, Yang BO, Huang C, et al. Prevalence of spontaneous Brugada ECG pattern recorded at standard intercostal leads: a meta‐analysis. Int J Cardiol. 2018;254:151–6. [DOI] [PubMed] [Google Scholar]
- 45. Sieira J, Conte G, Ciconte G, de Asmundis C, Chierchia G‐B, Baltogiannis G, et al. Prognostic value of programmed electrical stimulation in Brugada syndrome: 20 years experience. Circ Arrhythm Electrophysiol. 2015;8(4):777–84. [DOI] [PubMed] [Google Scholar]
- 46. Sieira J, Conte G, Ciconte G, de Asmundis C, Chierchia G‐B, Baltogiannis G, et al. Clinical characterisation and long‐term prognosis of women with Brugada syndrome. Heart. 2016;102(6):452–8. [DOI] [PubMed] [Google Scholar]
- 47. Sieira J, Ciconte G, Conte G, Chierchia G‐B, de Asmundis C, Baltogiannis G, et al. Asymptomatic Brugada syndrome: clinical characterization and long‐term prognosis. Circ Arrhythm Electrophysiol. 2015;8(5):1144–50. [DOI] [PubMed] [Google Scholar]
- 48. Sarkozy A, Sorgente A, Boussy T, Casado R, Paparella G, Capulzini L, et al. The value of a family history of sudden death in patients with diagnostic type I Brugada ECG pattern. Eur Heart J. 2011;32(17):2153–60. [DOI] [PubMed] [Google Scholar]
- 49. Sroubek J, Probst V, Mazzanti A, Delise P, Hevia JC, Ohkubo K, et al. Programmed ventricular stimulation for risk stratification in the Brugada syndrome: a pooled analysis. Circulation. 2016;133(7):622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rattanawong P, Chenbhanich J, Mekraksakit P, Vutthikraivit W, Chongsathidkiet P, Limpruttidham N, et al. SCN5A mutation status increases the risk of major arrhythmic events in Asian populations with Brugada syndrome: systematic review and meta‐analysis. Ann Noninvasive Electrocardiol. 2019;24(1):e12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Raharjo SB, Maulana R, Maghfirah I, Alzahra F, Putrinarita AD, Hanafy DA, et al. SCN5A gene mutations and the risk of ventricular fibrillation and syncope in Brugada syndrome patients: A meta‐analysis. J Arrhythm. 2018;34(5):473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rattanawong P, Riangwiwat T, Prasitlumkum N, Limpruttidham N, Kanjanahattakij N, Chongsathidkiet P, et al. Baseline fragmented QRS increases the risk of major arrhythmic events in Brugada syndrome: systematic review and meta‐analysis. Ann Noninvasive Electrocardiol. 2018;23(2):e12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kewcharoen J, Trongtorsak A, Kittipibul V, Prasitlumkum N, Kanitsoraphan C, Putthapiban P, et al. Fragmented QRS predicts reperfusion failure and in‐hospital mortality in ST‐Elevation myocardial infarction: a systematic review and meta‐analysis. Acta Cardiol. 2019;1–14. [DOI] [PubMed] [Google Scholar]
- 54. Rattanawong P, Vutthikraivit W, Charoensri A, Jongraksak T, Prombandankul A, Kanjanahattakij N, et al. Fever‐induced Brugada syndrome is more common than previously suspected: a cross‐sectional study from an endemic area. Ann Noninvasive Electrocardiol. 2016;21(2):136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tse G, Liu T, Li KHC, Laxton V, Chan YWF, Keung W, et al. Electrophysiological mechanisms of Brugada syndrome: insights from pre‐clinical and clinical studies. Front Physiol. 2016;7:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Antzelevitch C. The Brugada syndrome: ionic basis and arrhythmia mechanisms. J Cardiovasc Electrophysiol. 2001;12(2):268–72. [DOI] [PubMed] [Google Scholar]
- 57. Meregalli PG, Wilde AA, Tan HL. Pathophysiological mechanisms of Brugada syndrome: depolarization disorder, repolarization disorder, or more? Cardiovasc Res. 2005;67(3):367–78. [DOI] [PubMed] [Google Scholar]
- 58. Coronel R, Casini S, Koopmann TT, Wilms‐Schopman FJG, Verkerk AO, de Groot JR, et al. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome: a combined electrophysiological, genetic, histopathologic, and computational study. Circulation. 2005;112(18):2769–77. [DOI] [PubMed] [Google Scholar]
- 59. Frustaci A, Priori SG, Pieroni M, Chimenti C, Napolitano C, Rivolta I, et al. Cardiac histological substrate in patients with clinical phenotype of Brugada syndrome. Circulation. 2005;112(24):3680–7. [DOI] [PubMed] [Google Scholar]
- 60. Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, et al. An international compendium of mutations in the SCN5A‐encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7(1):33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yokokawa M, Noda T, Okamura H, Satomi K, Suyama K, Kurita T, et al. Comparison of long‐term follow‐up of electrocardiographic features in Brugada syndrome between the SCN5A‐positive probands and the SCN5A‐negative probands. Am J Cardiol. 2007;100(4):649–55. [DOI] [PubMed] [Google Scholar]
- 62. Smits JPP, Eckardt L, Probst V, Bezzina CR, Schott JJ, Remme CA, et al. Genotype‐phenotype relationship in Brugada syndrome: electrocardiographic features differentiate SCN5A‐related patients from non‐SCN5A‐related patients. J Am Coll Cardiol. 2002;40(2):350–6. [DOI] [PubMed] [Google Scholar]
- 63. Huang Z, Patel C, Li W, Xie Q, Wu R, Zhang L, et al. Role of signal‐averaged electrocardiograms in arrhythmic risk stratification of patients with Brugada syndrome: a prospective study. Heart Rhythm. 2009;6(8):1156–62. [DOI] [PubMed] [Google Scholar]
- 64. Tse G, Li KHC, Li G, Liu T, Bazoukis G, Wong WT, et al. Higher dispersion measures of conduction and repolarization in Type 1 compared to non‐type 1 Brugada syndrome patients: an electrocardiographic study from a single center. Front Cardiovasc Med. 2018;5:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Asvestas D, Tse G, Baranchuk A, Bazoukis G, Liu T, Saplaouras A, et al. High risk electrocardiographic markers in Brugada syndrome. Int J Cardiol Heart Vasc. 2018;18:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Georgopoulos S, Letsas KP, Liu T, Kalafateli M, Korantzopoulos P, Bürkle G, et al. A meta‐analysis on the prognostic significance of inferolateral early repolarization pattern in Brugada syndrome. Europace. 2018;20(1):134–9. [DOI] [PubMed] [Google Scholar]
- 67. Hiraoka M, Takagi M, Yokoyama Y, Sekiguchi Y, Aihara N, Aonuma K. Prognosis and risk stratification of young adults with Brugada syndrome. J Electrocardiol. 2013;46(4):279–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


