Abstract
Background
The clinical significance of premature ventricular complexes (PVCs) in heart failure (HF) remains unclear. We aimed to clarify the associations of PVC burden with re‐hospitalization and cardiac death in HF patients.
Methods
We studied 435 HF patients (271 men, mean age 65 years). All patients were hospitalized for worsening HF. After optimal medications, echocardiography, 24 hours Holter monitoring and cardiopulmonary exercise testing were performed before discharge. The clinical characteristics and outcomes of the HF patients were investigated.
Results
During a median follow‐up period of 2.3 years, there were 125 (28.7%) cardiac events (re‐hospitalization due to worsening HF, fatal arrhythmias, or cardiac death). The patients with cardiac events had higher PVC burden compared to those without (median 0.374%/d [interquartile range 0.013‐1.510] vs median 0.026%/d [interquartile range 0.000‐0.534], P < .001). We examined cutoff value of PVC burden for predicting cardiac events. Receiver‐operating characteristic analysis showed PVC burden (>0.145%/d) to be a predictive factor of cardiac events (area under the curve: 0.64). Kaplan‐Meier analysis demonstrated that cardiac events were more frequent in patients with high‐PVC burden (>0.145%/d, n = 194) compared to those with low‐PVC burden (≤0.145%/d, n = 241). Furthermore, the high‐PVC burden patients had left ventricular (LV) and atrial dilatation, reduced LV ejection fraction, and impaired exercise capacity, compared to the low‐PVC burden patients. In Cox proportional hazards analysis, high‐PVC burden was significantly associated with cardiac events with a hazard ratio of 2.028 (95% confidence interval: 1.418‐2.901, P < .001).
Conclusion
These results suggest that PVC burden is an important predictor of cardiac events in HF patients.
Keywords: cardiac death, heart failure, Holter monitoring, premature ventricular complex, readmission
The frequency of premature ventricular complex after optimal medication was significantly associated appropriate implantable cardioverter defibrillator therapy, readmission due to worsening heart failure or cardiac death in hospitalized patients with heart failure, even if the frequency was low (>0.145%/d).
1. INTRODUCTION
A premature ventricular complex (PVC) is defined as early electric depolarizations originating in the ventricular myocardium. PVCs are often seen on electrocardiography irrespective of cardiac diseases, and are commonly considered to be benign.1, 2 However, it has been demonstrated that frequent PVCs not only cause various types of disease symptoms such as palpitations, atypical chest discomfort, and syncope but also contribute to cardiac remodeling and subsequent heart failure (HF).1, 3
Recent therapeutic advances improve clinical outcome of HF patients. However, the high rate of re‐hospitalization and high cardiac mortality are still serious issues.4 The frequency of PVCs is considered to be higher in HF patients compared with general populations.5 However, the relationship between PVC burden and the progression of HF has not been fully elucidated. It was previously reported that an increasing PVC burden of >24%/d on 24 hours Holter monitoring was significantly related with left ventricular (LV) remodeling in the patients with idiopathic PVCs undergoing radiofrequency catheter ablation (RFCA).6 On the other hand, in HF patients, even a low‐PVC frequency may have a significant impact on LV dysfunction and poor prognosis. Thus, we aimed to validate the clinical significance of PVC burden on cardiac function, exercise capacity, and outcome in hospitalized patients with HF.
2. METHODS
2.1. Subjects and study protocol
This study consisted of 435 patients hospitalized with decompensated HF at Fukushima Medical University Hospital between 2010 and 2015. The diagnosis of decompensated HF was determined by several physicians using the HF guidelines.7, 8 All subjects received optimal medication for HF and underwent echocardiography, 24 hours Holter monitoring and cardiopulmonary exercise testing at stable condition before discharge. Before study enrollment, 23 patients had undergone implantable cardioverter defibrillator (ICD, n = 10), cardiac resynchronization therapy (CRT)‐pacemaker (CRT‐P, n = 3), or CRT‐defibrillator (CRT‐D, n = 10) in accordance with established criteria.9, 10 The exclusion criteria in our study were acute coronary syndrome and receiving hemodialysis. The methodology of the present study has been validated in our previous work.11 All subjects gave written informed consent. The study protocol was approved by the ethics committee of Fukushima Medical University (approval number: 1656).
2.2. Determination of risk factors
The definition of target comorbidities (ie, hypertension, diabetes, dyslipidemia, chronic kidney disease, and anemia) was mentioned in a previous work.11, 12 The definition of hypertension was the recent use of antihypertensive medications, a systolic blood pressure of ≥140 mm Hg, and/or a diastolic blood pressure of >90 mm Hg. The definition of diabetes was the recent use of antidiabetic medications (insulin or antidiabetic drugs), a fasting blood glucose value of >126 mg/dL, and/or a hemoglobin A1c value of >6.5%. The definition of dyslipidemia was the recent use of cholesterol‐lowering medications, a triglyceride value of >150 mg/dL, a low‐density lipoprotein cholesterol value of >140 mg/dL, and/or a high‐density lipoprotein cholesterol value of <40 mg/dL. The measurement of estimated glomerular filtration rate (GFR) was performed based on the modification of diet in renal disease formula.13 The definition of chronic kidney disease was an estimated GFR of <60 mL/min/1.73 m.13, 14 The definition of anemia was hemoglobin of <12.0 g/dL in women and <13.0 g/dL in men.14
2.3. Identification of cardiac events during the follow‐up
The follow‐up of cardiac events continued until March 2017. The definition of cardiac events was appropriate ICD therapy (antitachycardia pacing or shock therapy), re‐hospitalization due to worsening HF, or cardiac death, which were the clinical endpoints of our study. ICD programing was performed at the physician's discretion according to patient's background. The diagnosis of worsening HF was determined using HF guidelines.7, 8 Cardiac death was confirmed by independent attending physicians and included death due to worsened HF, ventricular fibrillation documented by electrocardiogram, or sudden death. Status and dates of death were investigated in detail based on the patients’ medical records or their referring cardiologists. Survival time was calculated from the date of Holter monitoring until the date of ICD therapy, re‐hospitalization, cardiac death, or last follow‐up. The study subjects were divided into two groups: cardiac event group (n = 125) and noncardiac event group (n = 310). Clinical features and parameters obtained from Holter monitoring and echocardiography were compared between patients with and without cardiac events.
2.4. Echocardiography
Echocardiography was performed using standard techniques by experienced echocardiographers using an ultrasound system (ACUSON Sequoia, Siemens Medical Solutions USA, Inc) at our hospital.15 Interventricular septum thickness, posterior wall thickness, and LV end‐diastolic diameter were measured in parasternal long axis view at end diastole. LV end‐systolic diameter and left atrial (LA) dimension were measured in parasternal long axis view at end systole. LV ejection fraction (LVEF) was assessed using the modified Simpson's method.
2.5. Holter monitoring
All patients underwent Holter monitoring during the 24 hours recording period at stable status before hospital discharge. In the present study, PVC burden (the percentage of total beats that were PVCs), multifocal PVCs (PVCs of at least two morphologies), non‐sustained ventricular tachycardia (NSVT), and atrial fibrillation (AF) were estimated. NSVT was defined as three or more consecutive beats arising below the atrioventricular node, with a mean RR interval of ≤600 ms and lasting less than 30 seconds. AF included paroxysmal (lasting more than 30 seconds)16 and persistent AF during Holter monitoring. QRS morphology was automatically evaluated by a digitized Holter analyzer (SCM‐6600, Fukuda Denshi) and confirmed by experienced medical technologist who were blinded to other clinical data in the present study. Initial and mean axes of the QRS complex and the T‐wave between two PVCs were manually assessed for discrimination of multifocal PVCs.
2.6. Cardiopulmonary exercise testing
The patients underwent incremental symptom‐limited exercise testing using an upright cycle ergometer with a ramp protocol (Strength Ergo 8; Fukuda Denshi). Breath‐by‐breath oxygen consumption (VO2), carbon dioxide production (VCO2), and minute ventilation (VE) were assessed during exercise using an AE‐300S respiratory monitor (Minato Medical Science).17 Peak VO2 was measured as an average of the last 30 seconds of exercise. Ventilatory response to exercise (expressed as a VE/VCO2 slope) was calculated as the regression slope relating VE to CO2 from the start of exercise until the respiratory compensation point (the time at which ventilation is stimulated by CO2 output and end‐tidal CO2 tension begins to decrease).18
2.7. Statistical analysis
Parametric variables were presented as mean ± SD, nonparametric variables were presented as a median and interquartile range, and categorical variables were expressed as numbers and percentages. Parametric variables were compared using Student's t test, nonparametric variables were compared using the Mann‐Whitney U test, and the chi‐square test was used for comparisons of categorical variables. The correlation relationships were investigated by Spearman's rank correlation coefficient. The Cox proportional hazard regression models determined which variables were associated with cardiac events. In addition to the electrocardiographic parameters, to prepare for potential confounding, we considered the following clinical factors, which are generally known to affect prognosis in patients with HF: age, sex, comorbidities, and treatment. The variables selected for testing in the multivariable analysis were those with a P < .05 in the univariable models. Sensitivity, specificity, areas under the receiver‐operating characteristic (ROC) curve, and the optimal cutoff value for identification of cardiac events at the 1‐year follow‐up were calculated using ROC analysis. In the follow‐up period, Kaplan‐Meier survival curves were used to analyze event‐free survival data in patients with or without cardiac events. Because several confounding factors have multicollinearity (eg, chronic kidney disease and anemia, PVC burden and, LVEF, β blockers, and PVCs and NSVT), we performed subgroup analyses to evaluate potential heterogeneity of the relationship between PVC burden and cardiac events. Interactions between PVC burden and clinically relevant variables that are known to affect the risk of cardiac events in patients with HF (prevalence of older age [>65 years], male gender, ischemic etiology, New York Heart Association (NYHA) functional class III or IV, reduced LVEF [<50%], AF, hypertension, diabetes, dyslipidemia, chronic kidney disease, anemia, receiving device therapy and use of β blockers, angiotensin‐converting enzyme inhibitors [ACE inhibitors]/angiotensin II receptor blockers [ARBs], amiodarone and digitalis) were estimated by a Cox proportional hazard regression model. A value of P < .05 was considered statistically significant. Statistical analyses were performed with SPSS statistical software (version 22.0, SPSS Institute).
3. RESULTS
3.1. Baseline characteristics
The baseline characteristics of the present study's subjects are summarized in Table 1. There were 125 (28.7%) cardiac events including appropriate ICD therapy (n = 5), re‐hospitalization due to worsening HF (n = 71), or cardiac death (n = 49) due to HF (n = 37), ventricular fibrillation (n = 4), and sudden death (n = 8) during a median follow‐up period of 2.3 years. Compared to the noncardiac event group, the cardiac event group had a higher prevalence of NYHA class III/IV, hypertension, diabetes, chronic kidney disease, anemia, receiving device therapy (ICD, CRT‐P, or CRT‐D), as well as intake of ACE inhibitors/ARBs and amiodarone. In addition, age and PVC burden were significantly higher, and LVEF was significantly lower in the cardiac event group than in the noncardiac event group. However, gender, prevalence of ischemic etiology, dyslipidemia, and other medications were not different between the two groups.
Table 1.
Comparison of clinical characteristics between cardiac and noncardiac events
|
Total (n = 435) |
Noncardiac events (n = 310) |
Cardiac events (n = 125) |
P value | |
|---|---|---|---|---|
| Age | 65.1 ± 13.9 | 63.8 ± 14.0 | 68.3 ± 13.3 | .002 |
| Male (n, %) | 271 (62.2) | 194 (62.5) | 77 (61.6) | .849 |
| Ischemic etiology (n, %) | 75 (17.2) | 52 (16.7) | 23 (18.4) | .685 |
| NYHA class III/IV (n, %) | 9 (2.0) | 2 (0.6) | 7 (5.6) | .003 |
| LVEF (%) | 46.0 ± 16.6 | 47.8 ± 16.6 | 41.6 ± 15.7 | .001 |
| Holter monitoring | ||||
| Atrial fibrillation (n, %) | 127 (29.1) | 82 (26.4) | 45 (36.0) | .047 |
| PVC burden (%/d)a | 0.071 (0.000‐0.960) | 0.026 (0.000‐0.534) | 0.374 (0.013‐1.510) | <.001 |
| NSVT (n, %) | 139 (31.9) | 83 (26.7) | 56 (44.8) | <.001 |
| Comorbidity | ||||
| Hypertension (n, %) | 335 (77.0) | 228 (73.5) | 107 (85.6) | .007 |
| Diabetes (n, %) | 154 (35.4) | 95 (30.6) | 59 (47.2) | .001 |
| Dyslipidemia (n, %) | 334 (76.7) | 236 (76.1) | 98 (78.4) | .612 |
| Chronic kidney disease (n, %) | 230 (52.8) | 142 (45.8) | 88 (70.4) | <.001 |
| Anemia (n, %) | 217 (49.8) | 134 (43.2) | 83 (66.4) | <.001 |
| Device therapy | ||||
| Pace maker (n, %) | 10 (2.2) | 6 (1.9) | 4 (3.2) | .483 |
| ICD/CRT‐P/CRT‐D (n, %) | 23 (5.2) | 9 (2.9) | 14 (11.2) | .001 |
| ICD (n, %) | 10 (2.2) | 6 (1.9) | 4 (3.2) | |
| CRT‐P (n, %) | 3 (0.6) | 1 (0.3) | 2 (1.6) | |
| CRT‐D (n, %) | 10(2.2) | 2 (0.6) | 8 (6.4) | |
| Medication | ||||
| β‐blockers (n, %) | 372 (85.5) | 259 (83.5) | 113 (90.4) | .066 |
| ACE inhibitors/ARBs (n, %) | 353 (81.1) | 244 (78.7) | 109 (87.5) | .040 |
| Amiodarone (n, %) | 88 (20.2) | 42 (13.5) | 46 (36.8) | <.001 |
| Digitalis (n, %) | 72 (16.5) | 47 (15.1) | 25 (20.0) | .219 |
Abbreviations: ACE inhibitors, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; CRT‐D, cardiac resynchronization therapy‐defibrillator; CRT‐P, cardiac resynchronization therapy‐pacemaker; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NSVT, non‐sustained ventricular tachycardia; NYHA, New York Heart Association; PVC, premature ventricular complex.
Median (interquartile range).
3.2. Predictive impact of PVC burden on cardiac events
The ability of variables to predict cardiac events was examined by the univariable and multivariable Cox proportional hazard analyses, and the data are shown in Table 2. The univariable analysis revealed significant associations of PVC burden (%/d) with cardiac events. In addition, age, NYHA class III/IV, LVEF, Holter‐based AF, diabetes, chronic kidney disease, anemia, receiving device therapy (ICD, CRT‐P, or CRT‐D), and the use of amiodarone were also associated with cardiac events. To determine the predictive accuracy of PVC burden (%/d), we next performed multivariable Cox proportional hazard analysis. The results of the analysis showed that PVC burden (%/d) was significantly associated with an increased risk of cardiac events with a hazard ratio (HR) of 1.036 (95% confidence interval [CI]: 1.005‐1.068, P = .021) after adjusting for multiple confounders (ie, age, NYHA class III/IV, LVEF, Holter‐based AF, diabetes, chronic kidney disease, anemia, receiving devise therapy, and the use of amiodarone).
Table 2.
Univariable and multivariable Cox proportional hazard models for predicting cardiac events
|
Univariable HR (95% CI) |
P value |
Multivariable HR (95% CI) |
P value | |
|---|---|---|---|---|
| Age (per 1 y increase)a | 1.022 (1.007‐1.037) | .003 | 1.019 (1.002‐1.035) | .026 |
| Male | 0.938 (0.654‐1.346) | .729 | ||
| Ischemic etiology | 1.078 (0.685‐1.694) | .746 | ||
| NYHA class III/IV | 5.444 (2.521‐11.755) | <.001 | 5.950 (2.516‐14.068) | <.001 |
| LVEF (per 1% increase)a | 0.981 (0.970‐0.992) | .001 | 0.989 (0.977‐1.002) | .095 |
| Holter monitoring | ||||
| Average heart rate (per 1 beat increase)a | 0.997 (0.985‐1.009) | .590 | ||
| Atrial fibrillation | 1.519 (1.054‐2.191) | .025 | 1.622 (1.083‐2.429) | .019 |
| PVC burden (per 1% increase)a | 1.046 (1.021‐1.071) | <.001 | 1.036 (1.005‐1.068) | .021 |
| Hypertension | 1.466 (0.888‐2.420) | .134 | ||
| Diabetes | 1.609 (1.132‐2.286) | .008 | 1.454 (0.993‐2.127) | .054 |
| Dyslipidemia | 1.060 (0.692‐1.624) | .788 | ||
| Chronic kidney disease | 2.435 (1.657‐3.578) | <.001 | 1.632 (1.051‐2.535) | .029 |
| Anemia | 2.230 (1.538‐3.233) | <.001 | 1.881 (1.243‐2.847) | .003 |
| Device therapy | ||||
| Pace maker | 1.346 (0.497‐3.647) | .559 | ||
| ICD/CRT‐P/CRT‐D | 3.007 (1.722‐5.248) | <.001 | 1.408 (0.749‐2.650) | .288 |
| Medication | ||||
| β‐blockers | 1.700 (0.938‐3.083) | .080 | ||
| ACE inhibitors/ARBs | 1.537 (0.909‐2.598) | .109 | ||
| Amiodarone | 2.844 (1.976‐4.094) | <.001 | 3.064 (2.004‐4.683) | <.001 |
| Digitalis | 1.416 (0.913‐2.196) | .121 | ||
Abbreviations: CI, confidence interval; HR, hazard ratio. The other abbreviations are as in Table 1.
Continuous variables.
In ROC analysis, the optimal cutoff value of PVC burden for predicting cardiac events at the 1‐year follow‐up was 0.145%/d (95% CI: 0.570‐0.714, P < .001, sensitivity of 64.2%, specificity of 59.0%, and areas under the curve of 0.64). The study subjects (n = 435) were then divided into two groups: high‐PVC burden (>0.145%/d, n = 194) and low‐PVC burden (n = 241). The patients with high‐PVC burden had higher prevalence of NSVT and multifocal PVCs, LV and LA dilatation, lower LVEF, and impaired exercise capacity, compared to the patients with low‐PVC burden, as shown in Table 3. Furthermore, PVC burden was negatively correlated with LVEF and peak VO2 (R = −.247, P = .005 and R = −.139, P = .035, respectively) and positively correlated with LV end‐diastolic diameter and LA dimension (R = .328, P < .001 and R = .156, P = .002, respectively). During a median follow‐up period of 2.3 years, cardiac event‐free survival was significantly lower in the high‐PVC burden group than in the low‐PVC burden group (P < .001), as shown in Figure 1. We conducted subgroup analyses and examined interaction terms to assess potential heterogeneity of impact of high‐PVC burden (>0.145%/d: vs low‐PVC burden) on cardiac events (Table 4). The univariable analysis showed that high‐PVC burden was significantly associated with cardiac events with a HR of 2.028 (95% CI: 1.418‐2.901, P < .001). There were no interactions except for a significant interaction with presence of hypertension (P = .015) or anemia (P = .049), and prescribed ACE inhibitors/ARBs use (P = .028) in associations of high‐PVC burden and cardiac events between subgroups. Although the cardiac event group had a higher intake of amiodarone compared with the noncardiac event group in Table 1, there was no significant interaction with an intake of amiodarone (P = .245) in association of high‐PVC burden and cardiac events.
Table 3.
Comparison of data of echocardiography and cardiopulmonary exercise testing between high‐PVC burden and low‐PVC burden
|
Low‐PVC burden (≤0.145%/d) (N = 241) |
High‐PVC burden (>0.145%/d) (N = 194) |
P value | |
|---|---|---|---|
| Holter monitoring | |||
| NSVT | 30 (12.4%) | 109 (56.1%) | <.001 |
| Multifocal PVCs | 188 (78.0%) | 187 (96.3%) | <.001 |
| Echocardiography | |||
| IVST (mm) | 11.4 ± 3.2 | 11.1 ± 2.6 | .340 |
| LVEDD (mm) | 50.6 ± 10.2 | 57.5 ± 11.3 | <.001 |
| LVESD (mm) | 36.7 ± 11.9 | 45.4 ± 12.9 | <.001 |
| PWT (mm) | 11.1 ± 2.5 | 11.2 ± 3.3 | .965 |
| LVEF (%) | 49.9 ± 16.6 | 41.2 ± 15.3 | <.001 |
| LAD (mm) | 42.2 ± 8.2 | 45.4 ± 9.9 | .001 |
| Cardiopulmonary exercise testing | |||
| Peak VO2 (ml·kg−1·min−1) | 16.4 ± 5.0 | 15.0 ± 3.9 | .021 |
| VE/ VCO2 slope | 32.9 ± 7.7 | 34.3 ± 6.9 | .173 |
Abbreviations: IVST, interventricular septum thickness; LAD, left atrial diameter; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; NSVT, non‐sustained ventricular tachycardia; peak VO2, peak oxygen uptake, and VE/ VCO2 slope, rate of increase in ventilation per unit increase in carbon dioxide; PVC, premature ventricular complex; PWT, posterior wall thickness.
Figure 1.
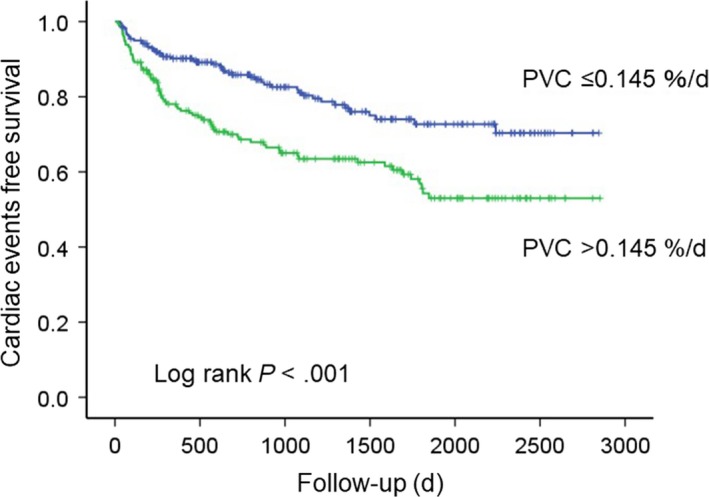
Cardiac events in heart failure patients. Kaplan‐Meier analysis of PVC burden for cardiac events. PVC, Premature ventricular complex
Table 4.
Subgroup analyses for cardiac events: the impact of PVC burden high vs low
| Factor | Subgroup | N | HR (95% CI) | P value |
Interaction P value |
|---|---|---|---|---|---|
| Total | — | 435 | 2.028 (1.418‐2.901) | <.001 | — |
| Age | ≥65 y | 253 | 1.681 (1.091‐2.590) | .019 | 0.231 |
| <65 y | 182 | 2.634 (1.388‐4.999) | .003 | ||
| Sex | Male | 271 | 2.086 (1.300‐3.348) | .002 | 0.938 |
| Female | 164 | 2.163 (1.225‐3.821) | .008 | ||
| Ischemic etiology | Yes | 75 | 1.156 (0.503‐2.656) | .732 | 0.185 |
| No | 360 | 2.265 (1.521‐3.372) | <.001 | ||
| NYHA functional class at discharge | I or II | 426 | 2.044 (1.413‐2.955) | <.001 | 0.771 |
| III or IV | 9 | 1.237 (0.272‐5.629) | .783 | ||
| LVEF | ≥50% | 233 | 1.575 (0.841‐2.951) | .156 | 0.637 |
| <50% | 202 | 1.924 (1.219‐3.039) | .005 | ||
| AF | Yes | 127 | 1.444 (0.781‐2.672) | .241 | 0.254 |
| No | 308 | 2.223 (1.427‐3.465) | <.001 | ||
| NSVT | Yes | 139 | 1.982 (0.939‐4.183) | .073 | 0.471 |
| No | 296 | 1.454 (0.886‐2.387) | .139 | ||
| Multifocal PVCs | Yes | 375 | 2.009 (1.368‐2.949) | <.001 | 0.833 |
| No | 60 | 0.042 (0.000‐292.3) | .482 | ||
| Hypertension | Yes | 335 | 1.648 (1.125‐2.415) | .010 | 0.015 |
| No | 100 | 8.366 (2.417‐28.954) | <.001 | ||
| Diabetes | Yes | 154 | 1.556 (0.925‐2.617) | .095 | 0.264 |
| No | 281 | 2.374 (1.448‐3.890) | .001 | ||
| Dyslipidemia | Yes | 334 | 1.776 (1.191‐2.647) | .005 | 0.153 |
| No | 101 | 3.428 (1.448‐8.113) | .005 | ||
| Chronic kidney disease | Yes | 230 | 1.544 (1.006‐2.369) | .047 | 0.163 |
| No | 205 | 2.642 (1.370‐5.095) | .004 | ||
| Anemia | Yes | 217 | 1.462 (0.946‐2.258) | .087 | 0.049 |
| No | 218 | 3.209 (1.689‐6.099) | <.001 | ||
| Pace maker | Yes | 10 | 29.958 (0.000‐3335438.6) | .481 | 0.943 |
| No | 425 | 1.997 (1.390‐2.868) | <.001 | ||
| ICD/CRT‐P/CRT‐D | Yes | 23 | 2.119 (0.632‐7.105) | .224 | 0.842 |
| No | 412 | 1.944 (1.332‐2.836) | .001 | ||
| Usage of β blockers at discharge | Yes | 372 | 1.868 (1.283‐2.720) | .001 | 0.295 |
| No | 63 | 2.993 (0.897‐9.979) | .074 | ||
| Usage of ACE inhibitors/ARBs at discharge | Yes | 353 | 2.398 (1.624‐3.541) | <.001 | 0.028 |
| No | 82 | 0.711 (0.258‐1.961) | .510 | ||
| Usage of amiodarone at discharge | Yes | 88 | 1.280 (0.690‐2.375) | .433 | 0.245 |
| No | 347 | 1.990 (1.276‐3.104) | .002 | ||
| Usage of digitalis at discharge | Yes | 72 | 2.667 (1.064‐6.685) | .036 | 0.533 |
| No | 363 | 1.872 (1.261‐2.780) | .002 |
4. DISCUSSION
In the present study, there were several important findings. High‐PVC burden (>0.145%/d) was significantly associated with an increased risk of cardiac events in hospitalized patients after optimal medication for HF. The patients with high‐PVC burden had LV and LA dilatation, reduced LVEF, and impaired exercise capacity when compared to the patients with low‐PVC burden (≤0.145%/d). In subgroup analyses, the impact of high‐PVC burden on cardiac events was found to be more distinct in HF patients without hypertension or anemia (HR 8.366, P < .001 and HR 3.209, P < .001, respectively).
4.1. Impact of PVC on HF patients
It has been considered that suppression or elimination of PVCs using β‐blocker therapy and RFCA is an effective strategy to reverse cardiac remodeling with frequent PVCs.19, 20 Therefore, PVC may have a significant impact on the progression of LV dysfunction, resulting in poor prognosis. Indeed, in the current study, our Cox hazard analysis revealed that high‐PVC burden is significantly associated with an increased risk of cardiac events in hospitalized patients with HF. In HF patients, the frequency of PVCs is increased by the impaired oscillation of sympathetic21 or vagal activity.5, 22 In addition, it has been demonstrated that cardiac autonomic dysfunction, such as impaired heart rate turbulence in stable compensated HF, is associated with readmission due to worsening HF and high cardiac mortality.11 Thus, the patients with frequent PVCs after optimal medication for HF may be associated with high risk of cardiac events.
In the present study, the multivariable analysis indicated that LVEF was not an independent predictor of cardiac events. One possible reason for this result was that LVEF of our study was not severely reduced (46.0 ± 16.6%). Although LVEF is a powerful predictor of cardiovascular events, a previous study demonstrated that cardiovascular death declined with increasing LVEF up to 45%.23 In addition, it has been reported that the majority of deaths in HF with preserved ejection fraction are cardiovascular deaths.24
4.2. Relation of PVC burden to the progression of HF
It was previously demonstrated that an increased PVC burden was related with LVEF reduction and LV dilatation assessed by echocardiography in patients with idiopathic PVCs undergoing RFCA.25 In another study, non‐HF patients with high‐PVC burden (0.123%‐17.7%/d) had a 48% increased risk of incident HF and a 31% increased risk of mortality over a median follow‐up >13 years compared with those with low‐PVC burden (0%‐0.002%/d).26 In the present study, the HF patients with a PVC burden of >0.145%/d had reduced LVEF, dilated LV and an increased incidence of appropriate ICD therapy, readmission, and cardiac mortality during a median follow‐up period of 2.3 years compared with those with a PVC burden of ≤0.145%/d. Therefore, in the HF patients, even a low‐PVC frequency might be associated with adverse events in a short time compared with non‐HF patients. In addition, the HF patients with high‐PVC burden also had a higher prevalence of NSVT and multifocal PVCs, LA dilatation, and impaired exercise capacity compared with those with low‐PVC burden. The prevalence of NSVT and multifocal PVCs is associated with the development of cardiac death attributed to HF.27, 28 LA overload due to LV dysfunction leads to LA enlargement, and LA remodeling is an independent predictor of mortality.29, 30 Although the exercise capacity of HF patients with frequent PVCs has not been fully investigated, exercise capacity is generally considered to be a powerful predictor of mortality.31 Therefore, although the frequency was low (>0.145%/d), HF patients with high‐PVC burden after administration of optimal medication had higher risk of cardiac events during follow‐up period. In subgroup analyses, significant associations between high‐PVC burden and cardiac events were found in HF patients without hypertension or anemia. We could not fully explain the reason of these interaction. However, these associations may be explained by the fact that hypertension and anemia are important predictive factors of worsening HF.32, 33 Also, the usage of ACE inhibitors/ARBs might be associated with cardiac events because there was a significant interaction with hypertension in associations of high‐PVC burden and cardiac events.
One possible mechanism responsible for HF progression due to PVCs is LV dyssynchrony. LV mechanical alterations due to the irregular beats of frequent PVCs can lead to increases in the LV filling pressure and the LA overload, resulting in the progression of HF in the general population.34 However, it has been considered that LV dilatation due to idiopathic PVCs appears to be a reversible and functional abnormality.19, 20 In this study, we found that PV burden after optimal medication was an important predictor of cardiac events in hospitalized patients with HF. In addition, PVC burden was negatively correlated with LVEF reduction and exercise intolerance, whereas it was positively correlated with LV and LA dilatation. It remains unclear whether adjustment of medical therapy and RFCA for PVCs is effective on LV remodeling or improvement of outcome in HF patients. Thus, further investigation into the exact effect of PVCs on the development of HF and cardiac death in HF patients is necessary.
4.3. Limitations
There are some limitations in this study. First, the present study was performed in a single institution with a relatively small number of subjects. Second, since only the baseline data at hospital discharge were used for the analyses, any changes after discharge in any parameters including Holter monitoring were not considered. Finally, we have performed both subgroup and multivariable Cox proportional hazard analyses and statistically adjusted differences in clinical backgrounds to validate a clinical impact of PVCs. However, these differences between the two groups might not be completely adjusted. Therefore, it remains unclear whether small amount of PVCs contribute to the pathophysiology of progressive HF or it functions merely as a marker of the progression of HF. We would consider performing a study on these issues in the future.
5. CONCLUSIONS
The assessment of PVC burden after optimal medication is useful for predicting appropriate ICD therapy, readmission due to worsening HF, or cardiac death in hospitalized patients with HF. Although the frequency is low (>0.145%/d), PVC burden is an important predictive factor of cardiac events in patients with HF.
CONFLICT OF INTEREST
Authors declare no conflict of interests for this article.
Yamada S, Yoshihisa A, Sato T, et al. Prognostic significance of premature ventricular complex burden on hospitalized patients with heart failure. J Arrhythmia. 2020;36:134–142. 10.1002/joa3.12259
REFERENCES
- 1. Simpson RJ Jr, Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2002;143:535–40. [DOI] [PubMed] [Google Scholar]
- 2. Messineo FC. Ventricular ectopic activity: prevalence and risk. Am J Cardiol. 1989;64:53J–56J. [DOI] [PubMed] [Google Scholar]
- 3. Lee GK, Klarich KW, Grogan M, Cha YM. Premature ventricular contraction‐induced cardiomyopathy: a treatable condition. Circ Arrhythm Electrophysiol. 2012;5:229–36. [DOI] [PubMed] [Google Scholar]
- 4. Jhund PS, MacIntyre K, Simpson CR, Lewsey JD, Stewart S, Redpath A, et al. Long‐term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation. 2009;119:515–23. [DOI] [PubMed] [Google Scholar]
- 5. La Rovere MT, Specchia G, Mortara A, Schwartz PJ. Baroreflex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction: a prospective study. Circulation. 1988;78:816–24. [DOI] [PubMed] [Google Scholar]
- 6. Baman TS, Lange DC, Ilg KJ, Gupta SK, Liu T‐Y, Alguire C, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–9. [DOI] [PubMed] [Google Scholar]
- 7. Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–15. [DOI] [PubMed] [Google Scholar]
- 8. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 9. Vardas PE, Auricchio A, Blanc JJ, Daubert JC, Drexler H, Ector H, et al. European Society of Cardiology; European Heart Rhythm Association. Guidelines for cardiac pacing and cardiac resynchronization therapy: the Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Eur Heart J. 2007;28:2256–95. [DOI] [PubMed] [Google Scholar]
- 10. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 Guidelines for Device‐Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–408. [DOI] [PubMed] [Google Scholar]
- 11. Yamada S, Yoshihisa A, Sato Y, Sato T, Kamioka M, Kaneshiro T, et al. Utility of heart rate turbulence and T‐wave alternans to assess risk for readmission and cardiac death in hospitalized heart failure patients. J Cardiovasc Electrophysiol. 2018;29:1257–64. [DOI] [PubMed] [Google Scholar]
- 12. Yoshihisa A, Suzuki S, Sato Y, Kanno Y, Abe S, Miyata M, et al. Relation of testosterone levels to mortality in men with heart failure. Am J Cardiol. 2018;121:1321–7. [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. [DOI] [PubMed] [Google Scholar]
- 14. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–69. [DOI] [PubMed] [Google Scholar]
- 15. Yoshihisa A, Kimishima Y, Kiko T, Sato YU, Watanabe S, Kanno Y, et al. Liver fibrosis marker, 7S domain of collagen type IV, in patients with pre‐capillary pulmonary hypertension. Int J Cardiol. 2018;258:269–74. [DOI] [PubMed] [Google Scholar]
- 16. Pappone C, Augello G, Sala S, Gugliotta F, Vicedomini G, Gulletta S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol. 2006;48:2340–7. [DOI] [PubMed] [Google Scholar]
- 17. Kanno Y, Yoshihisa A, Watanabe S, Takiguchi M, Yokokawa T, Sato A, et al. Prognostic significance of insomnia in heart failure. Circ J. 2016;80:1571–7. [DOI] [PubMed] [Google Scholar]
- 18. Ponikowski P, Francis DP, Piepoli MF, Davies LC, Chua TP, Davos CH, et al. Enhanced ventilatory response to exercise in patients with chronic heart failure and preserved exercise tolerance: marker of abnormal cardiorespiratory reflex control and predictor of poor prognosis. Circulation. 2001;103:967–72. [DOI] [PubMed] [Google Scholar]
- 19. Krittayaphong R, Bhuripanyo K, Punlee K, Kangkagate C, Chaithiraphan S. Effect of atenolol on symptomatic ventricular arrhythmia without structural heart disease: a randomized placebo‐controlled study. Am Heart J. 2002;144:e10. [DOI] [PubMed] [Google Scholar]
- 20. Yamada S, Chung F‐P, Lin Y‐J, Chang S‐L, Lo L‐W, Hu Y‐F, et al. Electrocardiographic characteristics for predicting idiopathic right ventricular outflow tract premature ventricular complex‐induced cardiomyopathy. J Interv Card Electrophysiol. 2018;53:175–85. [DOI] [PubMed] [Google Scholar]
- 21. Pogwizd SM, Corr PB. Electrophysiologic mechanisms underlying arrhythmias due to reperfusion of ischemic myocardium. Circulation. 1987;76:404–26. [DOI] [PubMed] [Google Scholar]
- 22. Yamada S, Suzuki H, Kamioka M, Suzuki S, Kamiyama Y, Yoshihisa A, et al. Sleep‐disordered breathing increases risk for fatal ventricular arrhythmias in patients with chronic heart failure. Circ J. 2013;77:1466–73. [DOI] [PubMed] [Google Scholar]
- 23. Solomon SD, Anavekar N, Skali H, McMurray JJV, Swedberg K, Yusuf S, et al. Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–44. [DOI] [PubMed] [Google Scholar]
- 24. Chan MM, Lam CS. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail. 2013;15:604–13. [DOI] [PubMed] [Google Scholar]
- 25. Del Carpio Munoz F, Syed FF, Noheria A, Cha Y‐M, Friedman PA, Hammill SC, et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J Cardiovasc Electrophysiol. 2011;22:791–8. [DOI] [PubMed] [Google Scholar]
- 26. Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, et al. Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol. 2015;66:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin C‐Y, Chang S‐L, Chung F‐P, Chen Y‐Y, Lin Y‐J, Lo L‐W, et al. Long‐term outcome of non‐sustained ventricular tachycardia in structurally normal hearts. PLoS ONE. 2016;11:e0160181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin C‐Y, Chang S‐L, Lin Y‐J, Lo L‐W, Chung F‐P, Chen Y‐Y, et al. Long‐term outcome of multiform premature ventricular complexes in structurally normal heart. Int J Cardiol. 2015;180:80–5. [DOI] [PubMed] [Google Scholar]
- 29. Yamaguchi H, Yoshida J, Yamamoto K, Sakata Y, Mano T, Akehi N, et al. Elevation of plasma brain natriuretic peptide is a hallmark of diastolic heart failure independent of ventricular hypertrophy. J Am Coll Cardiol. 2004;43:55–60. [DOI] [PubMed] [Google Scholar]
- 30. Ristow B, Ali S, Whooley MA, Schiller NB. Usefulness of left atrial volume index to predict heart failure hospitalization and mortality in ambulatory patients with coronary heart disease and comparison to left ventricular ejection fraction (from the Heart and Soul Study). Am J Cardiol. 2008;102:70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. [DOI] [PubMed] [Google Scholar]
- 32. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–62. [PubMed] [Google Scholar]
- 33. Kajimoto K, Sato N, Takano T.; Investigators of the Acute Decompensated Heart Failure Syndromes (ATTEND) registry . Association between anemia, clinical features and outcome in patients hospitalized for acute heart failure syndromes. Eur Heart J Acute Cardiovasc Care. 2015;4:568–76. [DOI] [PubMed] [Google Scholar]
- 34. Wang J, Kurrelmeyer KM, Torre‐Amione G, Nagueh SF. Systolic and diastolic dyssynchrony in patients with diastolic heart failure and the effect of medical therapy. J Am Coll Cardiol. 2007;49:88–96. [DOI] [PubMed] [Google Scholar]


