Abstract
Context: Boswellia dalzielii Hutch. (Burseraceae) is an aromatic plant. The leaves are used for beverage flavouring.
Objective: This study investigates the chemical composition and biological activities of various extracts.
Materials and methods: The essential oil was prepared via hydrodistillation. Identification and quantification were realized via GC-MS and GC-FID. Consecutive extractions (cyclohexane, dichloromethane, ethyl acetate and methanol) were carried out and various chemical groups (phenolics, flavonoids, tannins, antocyanins and sugar) were quantified. The volatile compounds of organic extracts were identified before and after derivatization. Antioxidant, antihyperuricemia, anti-Alzheimer, anti-inflammatory and anticancer activities were evaluated.
Results: In the essential oil, 50 compounds were identified, including 3-carene (27.72%) and α-pinene (15.18%). 2,5-Dihydroxy acetophenone and β-d-xylopyranose were identified in the methanol extract. Higher phenolic (315.97 g GAE/kg dry mass) and flavonoid (37.19 g QE/kg dry mass) contents were observed in the methanol extract. The methanol extract has presented remarkable IC50 = 6.10 mg/L for antiDPPH, 35.10 mg/L for antixanthine oxidase and 28.01 mg/L for anti-5-lipoxygenase. For acetylcholinesterase inhibition, the best IC50 (76.20 and 67.10 mg/L) were observed, respectively, with an ethyl acetate extract and the essential oil. At 50 mg/L, the dichloromethane extract inhibited OVCAR-3 cell lines by 65.10%, while cyclohexane extract inhibited IGROV-1 cell lines by 92.60%.
Discussion and conclusion: Biological activities were fully correlated with the chemical groups of the extracts. The ethyl acetate and methanol extracts could be considered as potential alternatives for use in dietary supplements for the prevention or treatment of diseases because of these extracts natural antioxidant, antihyperuricemic and anti-inflammatory activities.
Keywords: GC-MS, derivatization, antioxidant, anti-Alzheimer, anti-inflammatory, anticancer
Introduction
Natural substances known for their medicinal properties are an inexhaustible source of chemical molecules and therefore a valuable source of molecules with pharmacological activities. Human beings have always sought these plants, not only for food but also for therapeutic applications. These herbs are used to create products with high added value (essential oils, extracts, resins, etc.) that are presented as complex mixtures. They are among the great source of bioactive molecules (Dias et al. 2012); thus, it is important to investigate unstudied plants for all such activities (Michiels 2014).
Benin has rich flora that are used in various fields (medicine, pharmacy, perfumery, cosmetics and food) for their therapeutic and organoleptic properties, though some of them have not been thoroughly studied. Boswellia dalzielii Hutch. (Burseraceae) is an aromatic plant found in the Sudano-Sahelian savannah, on rocky and dry, shallow soils (Ouédraogo et al. 2006). In Benin, B. dalzielii or the frankincense tree, is present at Segbana, in the north, and is also called ‘Tree Man’ by Gourmantches in eastern Burkina Faso. It is a tree up to 13 m tall with a rounded and clear crown. It has yellow-grey bark that peels into strips, and it exudes a white fragrant sap (Ouédraogo et al. 2006). Traditionally, a decoction of the bark is drunk as a protection against dysentery, haemorrhage, and angina. The dried and crushed bark is used in combination with other herbs to treat malaria, yellow fever, stomach ailments, and many childhood diseases (Ouédraogo et al. 2006). The bark is also used to treat rheumatism, gastrointestinal disorders, wounds, asthma, pleurisy, appendicitis, dizziness, palpitations, leprosy, diarrhoea and bloating in cattle (Arbonnier, 2000). It has antiseptic, healing, and antifungal potential and is used externally to treat sores, ulcers, and dental caries (Ouédraogo 1996). The leaves are used for beverage flavouring, while the resin associated with the bark is used as incense and fumigation to perfume and disinfect houses. In Beninese Pharmacopeia, the roots are used as a protection against syphilis. The mixture of the roots and bark is a real antidote to poisons and snakebites (Ouédraogo 1996).
Regarding B. dalzielii, no compounds have been isolated from the leaves. A few studies conducted on the stem bark reported incensole, gallic acid, protocatechuic acid, 4′-methoxy-(E)-resveratrol-3-orutinoside and β-sitosterol (Alemika et al. 2004, 2006). Gokaraju et al. (2010) proved the presence of sesquiterpenes, diterpenes and triterpenes in the gum resin of B. dalzielii. An analysis carried on samples roots and bark samples indicated the presence of sterols, terpenes, carbohydrates, saponins, tannins, flavonoids and glycosides (Hassan et al. 2009).
In such a context, the objectives of this work are (i) the study of the chemical composition of the essential oil and various extracts obtained from B. dalzielii leaves, and (ii) the evaluation of their antioxidant, antihyperuricemia, anti-Alzheimer, anticancer and anti-inflammatory activities.
Materials and methods
Chemical and plant material
All chemicals used were of analytical reagent grade. All reagents were purchased from Sigma-Aldrich-Fluka (Saint-Quentin, France). The fresh leaves of B. dalzielii were collected in the morning in December 2012 from Ségbana region (North of Benin). Identification was made by Dr Agbani Pierre, responsible of botanic garden in the Abomey-Calavi’s University (Benin) and voucher specimens (reference 2140) was deposited at the Herbarium of the National herb of the same institution.
Preparation of extracts and essential oil
The collected leaves of B. dalzielii were dried in shade at room temperature and transformed to powder. The average particle size obtained after sieving of grinding (using laboratory knife grinder) was 0.8 mm. The plant extracts were obtained successively by maceration of four solvents with croissant polarity (cyclohexane, dichloromethane, ethyl acetate and methanol). So, 200 g of powder was successively extracted with 2 L for each solvent during 4 h. The filtrates were obtained using Whatman filter paper (Fisher, France). Extracts were obtained by rotary evaporator under vacuum at 35 °C and then stored in freezer at 4 °C until further analyses.
For essential oil, 1.2 kg of dry leaves was used for extraction by hydrodistillation using a Clevenger-type apparatus during 4 h in the Laboratory of physical organic chemistry and synthesis in Benin. Essential oil is yellow (visually).
Gas chromatography
Essential oil analysis
The chemical identification and quantification of the essential oil is quoted in the work of Afoulous et al. (2011). Gas chromatography-flame ionization detection (GC-FID) analyses was carried on a Varian Star 3400 C × chromatograph (Les Ulis, France) fitted with a fused silica capillary DB-5MS column (5% phenylmethylpolysyloxane, 30 × 0.25 mm, film thickness 0.25 μm). Chromatographic conditions were 60–260 °C temperature rise with a gradient of 5 °C/min and 15 min isotherm at 260 °C. A second gradient was applied to 340 °C at 40 °C/min. For analysis purposes, the essential oil was dissolved in petroleum ether. One microliter was injected in the split mode ratio of 1:10. Helium was used as carrier gas at 1 mL/min. The injector was operated at 200 °C. The gas chromatography-mass spectrometry (GC-MS) system (Varian Saturn 2000 ion trap GC/MS with CP-3800 GC) was used with the same chromatographic conditions for GC-FID. Mass spectrometer was adjusted for an emission current of 10 μA and electron multiplier voltage between 1400 and 1500 V. Trap temperature was 250 °C and that of the transfer line was 270 °C. Mass scanning was from 40 to 650 amu.
Compounds were identified by (i) comparison of their retention index (RI) relative to C5-C24 n-alkanes obtained on a nonpolar DB-5MS column, with those provided in the literature and (ii) by comparison of their mass spectra with those recorded in NIST 08 (National Institute of Standards and Technology), or reported in published articles or by co-injection of available reference compounds. The percentage composition of the essential oil was computed by the normalization method from the GC peak areas, assuming identical mass response factor for all compounds.
Volatile compounds of extracts
The identification of volatile compounds from the organic extracts, before or after derivatization, was carried with the same equipment (GC-MS) (Les Ulis, France) but we applied the following gradient: 5 min at 60 °C, 60–270 °C at 15 °C/min, 6 min at 270 °C, 270–300 °C at 50 °C/min and finally 4.5 min at 300 °C. The entire chromatographic program lasted 30 min. The derivatization method used (Tzing & Ding 2010) consisted of dissolving 5 mg of each extract in 1 mL of acetonitrile and adding 150 μL of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) reagent and 1.5 μL of chlorotrimethylsilane (TMCS). After stirring, the nitrogen was bubbled for 20 s and then homogenized via ultrasound followed by incubation for 15 min in a heated bath at 40 °C.
Quantification of total phenolic content
The total phenolics of each extract were determined via the Folin–Ciocalteu method (Bekir et al. 2013). The diluted solution of each extract (0.5 mL) was mixed with the Folin–Ciocalteu reagent (0.2 N, 2.5 mL) and rested at room temperature for 5 min, then sodium carbonate solution (75 g/L in water, 2 mL) was added. After 1 h of incubation, the absorbance was measured at 765 nm against a blank. A standard calibration curve was plotted using gallic acid (0–300 mg/L). The results were expressed as g of gallic acid equivalents (GAE) per kg of dry mass (DM).
Quantification of total flavonoids content
The total flavonoid content was estimated according to Bekir et al. (2013) method. In a 96-well plate, 100 μL of each extract was mixed with a solution (100 μL) of aluminium trichloride in methanol (2%). The absorbance of the mixture was measured at 510 nm against a reagent blank of methanol (100 μL) and a plant extract (100 μL) without AlCl3. Quercetin was used for calibration, and results were expressed as g of quercetin equivalent (QE) per kg of DM.
Quantification of total condensed tannin content
The condensed tannin content of the extracts was determined via the vanillin method (Bekir et al. 2013): 50 μL of extract solution was mixed with 150 μL of vanillin (1% in 7 M H2SO4) in an ice bath and then incubated at 25 °C. After 15 min, the absorbance of the solution was read at 500 nm. The results were expressed as g of catechin equivalents (CE) per kg of DM.
Quantification of total anthocyanin content
Total anthocyanin content was determined by a pH differential method (Bekir et al. 2013) using two buffers: hydrochloric acid-potassium chloride (pH 1.0, 0.2 M) and acetic acid–sodium acetate (pH 4.5, 1 M). The extract (20 μL) was mixed with 180 μL of corresponding buffers and the absorbance was measured at 510 and 700 nm after 15 min of incubation. Absorbance (A) was calculated using A = [(A510 – A700) pH1.0 – (A510 – A700) pH4.5] using a molar extinction coefficient of 29,600. The final results were expressed as mg cyanidin-3-glucoside equivalent (C3GE) per kg of DM.
Sugars quantification
For sugar quantification, 100 μL of each extract at 5 mg/mL were mixed with 150 μL of 3,5-dinitrosalicylic acid (DNS) solution (0.05 M). After stirring, followed by incubation for 5 min in a bath-husband at 100 °C, 750 μL of water was added. After a second stirring, the absorbance of the mixture was measured at 530 nm against a blank consisting of the same sample in which the DNS was replaced by 5% dimethyl sulfoxide (DMSO) and against a negative control wherein the extract was replaced by 5% DMSO. Each experiment was performed three times. The amount of sugar was determined in mg of glucose equivalent per gram of dry mass (DM).
Antioxidant activity
Antioxidant scavenging activity was studied using 1,1-diphenyl-2-picrylhydrazyl free radical (DPPH) as described by Bekir et al. (2013): 20 μL of various dilutions of extracts were mixed with 180 μL of a 0.2 mM methanol DPPH solution. After an incubation period of 30 min at 25 °C, the absorbance at 520 nm was recorded as A(sample). A blank experiment was also carried applying the same procedure to a solution without extract and the absorbance was recorded as A(blank). The free radical-scavenging activity of each solution was calculated as percentage of inhibition according to the following equation: % inhibition =100 (A(blank) – A(sample))/A(blank)
Antioxidant activity of the extract was expressed as IC50, defined as the concentration of the test material required to cause a 50% decrease in initial DPPH concentration. Vitamin C was used as a standard. All measurements were performed in triplicate.
Anti-inflammatory activity
The anti-inflammatory activity of the extracts was determined using soybean 5-lipoxygenase, as described by Bekir et al. (2013) with some modifications. Twenty milliliters extracts of various concentrations were mixed individually with sodium phosphate buffer (pH 7.4) containing 5-lipoxygenase and 60 μL of linoleic acid (3.5 mM), yielding a final volume of 1 mL. However, the blank did not contain the substrate, but did include 30 μL of buffer solution. All extracts were re-suspended in the DMSO followed by dilution in the buffer so that the DMSO would not exceed 1%. The mixture was incubated at 25 °C for 10 min, and the absorbance was determined at 234 nm. Nordihydroguaiaretic acid was used as a positive control. The enzyme activity percentage was plotted against the concentration of the leaf extract. The IC50 value is the concentration of the flower extract that caused 50% enzyme inhibition.
Antiacetylcholinesterase activity
Acetylcholinesterase (AChE) inhibitory activity was measured using Ellman’s method, as previously reported (Bekir et al. 2013). 50 μL of 0.1 M sodium phosphate buffer (pH 8.0), 25 μL of AChE, 25 μL of extract and 125 μL of 5,5′-dithiobis-(2-nitrobenzoic acid) were added to a 96-well microplate and incubated for 15 min at 25 °C. All extracts were re-suspended in the DMSO followed by dilution in the buffer so that the DMSO did not exceed 1%. The reaction was then initiated with the addition of 25 μL of acetylthiocholine iodide and measured at a wavelength of 412 nm. The concentration of the extracts that caused 50% inhibition of the AChE activity (IC50) was calculated via nonlinear regression analysis. The percentage of inhibition was calculated from (1−S/E) × 100, where E and S were the enzyme activities without and with the test sample, respectively. Galanthamine was used as a positive control.
Cytotoxicity evaluation
Cytotoxicity of samples were estimated on human ovarian cell lines (IGROV-1 and OVCAR-3) as described by Bekir et al. (2013) with modification. Cells were distributed in 96-well plates at 3 × 104 cells/well in 100 μL, and then 100 μL of culture medium containing sample at various concentrations were added. Cell growth was estimated by the 3-(4, 5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. MTT is a yellow water-soluble tetrazolium salt. Optical density was measured at 540 nm. All extracts were re-suspended in the DMSO followed by dilution in the buffer so that the DMSO does not exceed 1%. Tamoxifen was used as a positive control.
Xanthine oxidase (XOD) inhibition assay
The antihyperuricemia test consisted of inhibiting xanthine oxidase activity, and hence reducing the formation of uric acid. This xanthine oxidase inhibition can be explained by the formation of uric acid. The method of Lin et al. (2000) was used, with some modifications. The assay mixture consisted of 50 μL of extract solution (100 mg/L), 60 μL of 70 mM phosphate buffer (pH =7.5) and 30 μL of enzyme solution (0.1 units/mL in the same buffer). After the first incubation for 15 min at 25 °C, the reaction were then initiated via the addition of 60 μL of substrate solution (150 μM xanthine in the same buffer), all in a microplate of 96 wells, and the final volume of each well was 200 μL. The absorbance of the mixture was then measured at 295 nm after the second incubation, for 5 min, against a blank consisting of the same sample without the enzyme solution. Allopurinol was used as a positive control. Each experiment was performed three times. The inhibition percentage was calculated in the following way:
where Asample is the real absorbance of the extract containing the reaction and Acontrol is the real absorbance of the reaction control.
Superoxide dismutase (SOD) inhibition assay
SOD is an antioxidant that inhibits the autoxidation of pyrogallol. Thus, when it is effectively inhibited by the extract, the absorbance of the product formed after the autoxidation of pyrogallol is high. The principle of the assay (Li 2012) performed is based on the kinetics study of the competition between the inhibitory activity of SOD against pyrogallol and the kinetics of the inhibition of SOD by extracts (indirect analysis). In fact, the following mixtures were prepared: the sample was consisted of 50 μL of extract (in DMSO 5%) and 120 μL of SOD (0.1 unit/mL), whereas the blank sample consisted of 50 μL of DMSO 5% and 120 μL of SOD (0.1 unit/mL). Similarly, a control solution was prepared by mixing 50 μL of extract (in DMSO 5%) and 120 μL of buffer trizma/diethylenetriaminepentaacetic acid (DTPA) (50 mM) while the blank control solution consisted of 50 μL of DMSO 5% and 120 μL of buffer trizma/DTPA (50 mM). After stirring for 5 s at 25 °C followed by incubation for 4 min, 30 μL of pyrogallol solution (30 mM) was added to each mixture. After stirring for 5 s, another incubation was performed for 7 min at 25 °C. After this incubation, the first absorbance was immediately read at 37 °C. Four other absorbances were read every minute for 4 min at 37 °C. The absorbance difference (ΔDO325nm/min) of the blank control must be equal to 0.04 ± 0.01 and that of blank sample must be equal to 0.004 ± 0.001 before proceeding to the calculation of the percentage inhibition from the average ΔDO325 nm/min obtained for each sample:
Statistical analysis
All data were expressed as means ± standard deviations of triplicate measurements. The confidence limits were set at p < .05. Correlations and regressions were carried using Excel program. Data analysis procedure (ANOVA) was performed in to assess the data.
Results
Extraction yields
The yields of various extracts are shown in Table 1. The highest yield was recorded with the methanol extract (16.08%), followed by the hexane extract (3.37%), the dichloromethane extract (1.57%) and finally the ethyl acetate extract (0.03%). For the essential oil, the yield was 1.12% of the mass of dry leaves.
Table 1.
Extraction yields (%) and chemical composition of B. dalzielii extracts.
| Samples | Yields (%) | Phenolics (GAE)a | Flavonoids (QE)a | Tannins (CE)a | Anthocyanins (C3GE)b | Sugar (GE) |
|---|---|---|---|---|---|---|
| Cyclohexane | 3.37b | 3.58 ± 0.09d | 4.09 ± 0.26d | ndc | 0.82 ± 0.01a | nd |
| Dichloromethane | 1.57c | 48.23 ± 0.22c | 31.95 ± 0.39c | 10.81 ± 0.72b | ndc | nd |
| Ethyl acetate | 0.03e | 148.81 ± 1.44b | 35.26 ± 0.50b | 35.18 ± 0.46a | 0.23 ± 0.00b | nd |
| Methanol | 16.08a | 315.97 ± 4.12a | 37.19 ± 0.42a | ndc | ndc | nd |
| Essential oil | 1.12d |
nd: not detected. Standard deviations (SD) did not exceed 5%.
ag/kg dry mass; bmg/kg dry mass.
Chemical composition of essential oil
Table 2 showed 50 compounds identified, representing to 98.87% of all compounds, with 11 monoterpene hydrocarbons (68.58%), 10 monoterpenes oxygenated (5.14%), 20 sesquiterpene hydrocarbons (19.07%), 6 oxygenated sesquiterpenes (5.21%) and 3 other compounds (0.90%). Among all the compounds identified, the compounds 3-carene (27.72%), α-pinene (15.18%), p-cymene (9.54%), β-phellandrene (8.48%), isolongifolene (6.15%) and myrcene (5.72%) were the predominant compounds and Z-β-farnesene (4.53%), β-selinene (2.13%), torreyol (1.93%), 1,6-humulanedien-3-ol (1.61%) and terpinen-4-ol (1.41%) were the least predominant compounds.
Table 2.
Chemical composition of B. dalzielii leaves essential oil.
| No. | RI | Noum | %Area |
|---|---|---|---|
| 1 | 939 | α-Pinene | 15.18 |
| 2 | 953 | Camphene | 0.26 |
| 3 | 983 | E-isolimonene | 0.37 |
| 4 | 993 | Myrcene | 5.72 |
| 5 | 1011 | 3-Carene | 27.72 |
| 6 | 1019 | α-Terpinene | 0.45 |
| 7 | 1027 | p-Cymene | 9.54 |
| 8 | 1032 | β-Phellandrene | 8.48 |
| 9 | 1045 | Z-β-ocimene | 0.28 |
| 10 | 1057 | γ-Terpinene | 0.20 |
| 11 | 1084 | Isoterpinolene | 0.40 |
| 12 | 1093 | E-sabinene hydrate | 0.67 |
| 13 | 1116 | 1S-β-Fenchol | 0.19 |
| 14 | 1132 | 3-Terpinenol | 0.17 |
| 15 | 1158 | E-pinocamphone | 0.69 |
| 16 | 1162 | Z-chrysanthemol | 0.64 |
| 17 | 1167 | Lavandulol | 0.42 |
| 18 | 1179 | Terpinen-4-ol | 1.41 |
| 19 | 1189 | Methyl salicylate | 0.52 |
| 20 | 1221 | β-cyclocitral | 0.38 |
| 21 | 1230 | Z-carveol | 0.33 |
| 22 | 1255 | Carvenone | 0.24 |
| 23 | 1292 | Undecan-2-one | 0.27 |
| 24 | 1345 | α-Cubebene | 0.70 |
| 25 | 1354 | α-Longipinene | 0.23 |
| 26 | 1379 | β-Patchoulene | 0.25 |
| 27 | 1387 | Isolongifolene | 6.15 |
| 28 | 1394 | Cyperene | 0.20 |
| 29 | 1396 | Italicene | 0.17 |
| 30 | 1418 | β-Caryophyllene | 0.75 |
| 31 | 1436 | E-α-bergamotene | 0.20 |
| 32 | 1439 | Aromadendrene | 0.79 |
| 33 | 1445 | Z-β-farnesene | 4.53 |
| 34 | 1451 | Z-4,5-muuroladiene | 0.24 |
| 35 | 1456 | α-Patchoulene | 0.47 |
| 36 | 1462 | β-Santalene | 0.91 |
| 37 | 1468 | 9-Epicaryophyllene | 0.23 |
| 38 | 1476 | β-Chamigrene | 0.43 |
| 39 | 1484 | β-selinene | 2.13 |
| 40 | 1487 | Z-β-guaiene | 0.22 |
| 41 | 1493 | Viridiflorene | 0.16 |
| 42 | 1498 | β-Himachalene | 0.20 |
| 43 | 1521 | Lilial | 0.11 |
| 44 | 1548 | E-α-bisabolene | 0.12 |
| 45 | 1559 | Caryophyllene alcohol | 0.35 |
| 46 | 1590 | Viridiflorol | 0.41 |
| 47 | 1598 | Widdrol | 0.38 |
| 48 | 1621 | 1,6-Humulanedien-3-ol | 1.61 |
| 49 | 1630 | γ-Eudesmol | 0.53 |
| 50 | 1645 | Torreyol-α-cadinol | 1.93 |
| Monoterpene hydrocarbons | 68.58 | ||
| Monoterpenes oxygenated | 5.14 | ||
| Sesquiterpeneshydrocarbons | 19.07 | ||
| Sesquiterpenes oxygenated | 5.21 | ||
| Others | 0.90 | ||
| Total | 98.87 |
RI: retention index.
Chemical group contents
The largest amount of phenolics was obtained via methanol (315.97 ± 1.44 g GAE/kg DM) (Table 1), followed by the ethyl acetate (148.81 ± 1.14 g GAE/kg DM), the dichloromethane (48.23 ± 0.22 g GAE/kg DM) and cyclohexane (3.58 ± 0.09 g GAE/kg DM).
The largest amount of flavonoids was found in the methanol extract (37.19 ± 0.42 g QE/kg DM), followed by the ethyl acetate extract (35.26 ± 0.5 g QE/kg DM), the dichloromethane extract (31.95 ± 0.39 g QE/kg DM) and finally the cyclohexane extract (4.09 ± 0.26 g QE/kg DM). The amount of tannins was unevenly distributed across the various extracts of B. dalzielii. The largest quantity (35.18 ± 0.46 g CE/kg DM) was observed in the ethyl acetate extract, followed by the dichloromethane extract (10.81 ± 0.72 g CE/kg DM). No amount of tannin was determined in the methanol or cyclohexane extracts. The presence of anthocyanins was only observed in the ethyl acetate and cyclohexane extracts, and even there, they were seen in only very small amount (0.23 ± 00 and 0.82 ± 01 mg C3GE/kg DM, respectively). No reducing sugar content was quantified in the extracts.
Compounds identified from extracts via GC-MS before and after derivatization
Next, the chemical compounds were identified via GC-MS. The amount of a given compound is variable and symbolized by ‘+’ (Table 3). O-Cymene and α-cadinol were present in the cyclohexane and ethyl acetate extracts; (5E)-2,4,4,7-tetramethyl-5,7-octadien-3-ol was present in the cyclohexane, dichloromethane and ethyl acetate extracts; caryophyllene oxide was found in the cyclohexane and dichloromethane extracts; 6-isopropenyl-4,8a-dimethyl-3,5,6,7,8,8a-hexahydro-2 (H)-naphthalenone was identified in the cyclohexane, ethyl acetate and methanol extracts and longipinocarvone was presented in the cyclohexane and methanol extracts. The specific presence of methyl 8,11,14-heptadecatrienoate was identified in the cyclohexane extract. Similarly, 2-furanmethanol tetrahydro-acetate, (−)-β-copaene, 2-propenoic acid, 3-[2-(aminocarbonyl) phenyl]-,(E) and (2E,6Z)-3,7,11-trimethyl-2,6,10-dodecatrienyl hexofuranoside were identified only in the dichloromethane extract; Z-linalool oxide, thymol, phytol acetate, urs-12-en-24-oic acid, 3-oxo-,methyl ester and 1,2,3,5,6,7,8,8a-octahydronaphthalen-2-yl ester, 3-hydroxy-6-isopropenyl-4,8a-dimethyl, acetic acid were also found only in the ethyl acetate extract. The methanol extract contained 6-isopropenyl-4,8a-dimethyl-3,5,6,7,8,8a-hexahydro-2(H)-naphtalenone.
Table 3.
Compounds identified for B. dalzielii extracts before and after derivatization.
| No. | Tr (min) | Compounds | Structure | Cyclohexane | Dichloromethane | Ethyl acetate | Methanol |
|---|---|---|---|---|---|---|---|
| Before derivatization | |||||||
| 1 | 6.35 | Tetrahydrofurfuryl acetate | 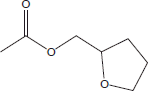 |
+++ | |||
| 2 | 7.10 | α-Pinene | 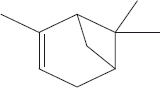 |
+++ | |||
| 3 | 8.56 | p-Cymene | 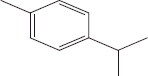 |
+++ | ++ | ||
| 4 | 9.46 | Z-linalooloxide | 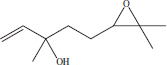 |
++ | |||
| 5 | 10.80 | Thymol | 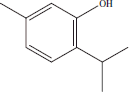 |
+ | |||
| 6 | 12.00 | (5E)-2,4,4,7-tetramethyl-5,7-octadien-3-ol | 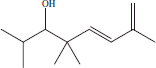 |
+++ | ++ | ++ | |
| 7 | 13.12 | β-Caryophyllene | 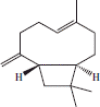 |
+++ | |||
| 8 | 13.57 | (−)-β-Copaene | 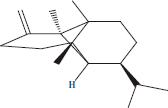 |
+++ | |||
| 9 | 13.59 | (E)-α-bergamotene | 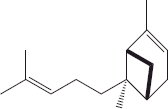 |
+++ | |||
| 10 | 14.56 | Caryophyllene alcohol | 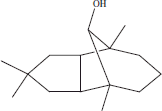 |
+++ | ++ | ||
| 11 | 15.05 | α-Cadinol | 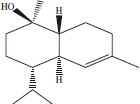 |
+++ | + | ||
| 12 | 16.26 | Phytol acetate | ++ | ||||
| 13 | 17.89 | 2-Propenoic acid, 3-[2-(amino carbonyl) phenyl]-, (E) | 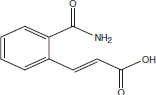 |
+++ | |||
| 14 | 18.02 | Methyl 8,11,14-heptadecatrienoate | 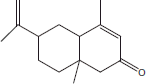 |
+++ | |||
| 15 | 21.05 | 6-Isopropenyl-4,8a-dimethyl-3,5,6,7,8,8a-Hexahydro-2(H)-naphtalenone | 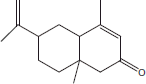 |
+++ | ++ | + | |
| 16 | 21.35 | Urs-12-en-24-oic acid, 3-oxo-,methyl ester | 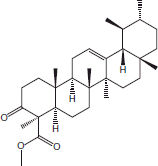 |
+++ | |||
| 17 | 22.55 | Longipinocarvone | 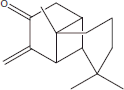 |
+++ | + | ||
| 18 | 23.05 | 1,2,3,5,6,7,8,8a-Octahydronaphthalen-2-yl ester, 3-hydroxy-6-isopropenyl-4,8a-dimethyl, acetic acid | 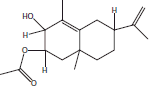 |
+++ | |||
| 19 | 24.29 | α-d-Mannofuranoside, farnesyl | 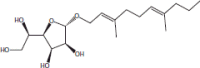 |
+++ | |||
| After derivatization | |||||||
| 1 | 16.55 | 2,5-Dihydroxy acetophenone | 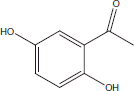 |
+++ | |||
| 2 | 16.62 | β-d-Xylopyranose | 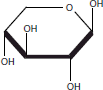 |
+++ | |||
+++: high presence; ++: average presence; +: low presence.
The same organic extracts were derivatized and GC-MS analysis revealed the presence of 2,5-dihydroxy acetophenone and β-d-xylopyranose in the methanol extract. No volatile compounds were identified in the others extracts after their derivatization.
Biologicals activities
Antioxidant activity: DPPH• assay
The essential oil provided 11.54 ± 0.20% antioxidant activity at 100 mg/L against DPPH radicals (Table 4). The antioxidant activity of methanol extract (IC50 = 6.10 ± 0.01 mg/L) was greater, followed by the ethyl acetate extract (IC50 = 15.20 ± 0.01 mg/L), dichloromethane extract (IC50 = 78.10 ± 0.10 mg/L) and cyclohexane extract (IC50 > 100 mg/L).
Table 4.
Antioxidant and antixanthine oxidase activities of B. dalzielii extracts and essential oil.
| DPPH• assay |
XOD assay |
|||
|---|---|---|---|---|
| Sample | % Inhibition (100 mg/L) | IC50 (mg/L) | % Inhibition (100 mg/L) | IC50 (mg/L) |
| Cyclohexane | 17.07 ± 0.43c | >100e | 15.58 ± 0.06d | >100e |
| Dichloromethane | 59.99 ± 0.70b | 78.10 ± 0.10d | 58.54 ± 0.32c | 88.30 ± 1.03d |
| Ethyl acetate | 95.33 ± 0.13a | 15.20 ± 0.04c | 90.08 ± 0.17a | 30.20 ± 0.04b |
| Methanol | 96.35 ± 0.10a | 6.10 ± 0.20b | 86.41 ± 0.17b | 35.10 ± 0.06c |
| Essential oil | 11.54 ± 0.20d | >100e | 10.74 ± 0.13e | >100e |
| Vitamin C | 5.3 ± 0.05a | |||
| Allopurinol | 0.95 ± 0.02a | |||
Antixanthine oxidase activity
The ethyl acetate extract (IC50 = 30.20 ± 0.01 mg/L) was the highly active in terms of antixanthine oxidase activity (Table 4), followed by the methanol extract (IC50 = 35.10 ± 0.01 mg/L), dichloromethane extract (IC50 = 88.30 ± 1.00 mg/L) and cyclohexane extract (IC50 > 100 mg/L). The inhibition percentage of the essential oil was the lowest (10.74 ± 0.13% at 100 mg/L).
AChE inhibition assay
The strongest ACHE inhibition was observed with the ethyl acetate extract (IC50 = 76.10 ± 0.02 mg/L) (Table 5), followed by the methanol extract (IC50 = 85.01 ± 0.02 mg/L), the dichloromethane extract (45.79 ± 0.01% at 100 mg/L) and the cyclohexane extract (22.79 ± 0.04% at 100 mg/L). For the essential oil, we noted a percentage inhibition equal to 85.37 ± 0.01% at 100 mg/L and an IC50 equal to 67.10 ± 0.03 mg/L.
Table 5.
Anti5-lipoxygenase, antiacetylcholinesterase and antisuperoxide dismutase activities of B. dalzielii extracts and essential oil.
| 5-lipoxygenase assay |
AChE assay |
SOD essay |
|||
|---|---|---|---|---|---|
| Sample | % inhibition (100 mg/L) | IC50 (mg/L) | % inhibition (100 mg/L) | IC50 (mg/L) | % inhibition (100 mg/L) |
| Cyclohexane | 29.39 ± 1.20e | >100f | 22.79 ± 0.04e | >100e | 13.96 ± 0.04a |
| Dichloromethane | 83.63 ± 3.30b | 58.01 ± 0.16d | 45.79 ± 0.01d | >100e | 4.14 ± 0.01c |
| Ethyl acetate | 94.12 ± 3.05a | 45.10 ± 0.01c | 59.92 ± 0.04b | 76.20 ± 0.02c | 8.27 ± 0.01b |
| Methanol | 78.87 ± 3.55c | 28.01 ± 1.07b | 53.30 ± 0.02c | 85.01 ± 0.02d | 8.03 ± 0.02b |
| Essential oil | 57.53 ± 1.73d | 70.01 ± 0.15e | 85.37 ± 0.01a | 67.10 ± 0.03b | ndd |
| Nordihydroguaiaretic acid | 1.60 ± 0.01a | ||||
| Galanthamine | 1.30 ± 0.01a | ||||
nd: not determined.
5-Lipoxygenase inhibition assay
All extracts had good inhibitory activity against 5-lipoxygenase (IC50 = 58.01 ± 0.16, 45.10 ± 0.01 and 28.01 ± 0.10 mg/L for the dichloromethane, ethyl acetate and methanol extracts, respectively) (Table 5), except the cyclohexane extract, which had the lowest level of inhibitory activity 29.39 ± 1.20% at 100 mg/L. The essential oil had good inhibitory activity at IC50 = 70.01 ± 0.15 mg/L.
SOD inhibition assay
The inhibition of SOD was tested at 100 mg/L. The inhibition percentages for the cyclohexane, dichloromethane, ethyl acetate and methanol extracts, were 13.96 ± 0.04%, 4.14 ± 0.01%, 8.27 ± 0.01% and 8.03 ± 0.02%, respectively (Table 5).
Cytotoxicity against tumour cell lines
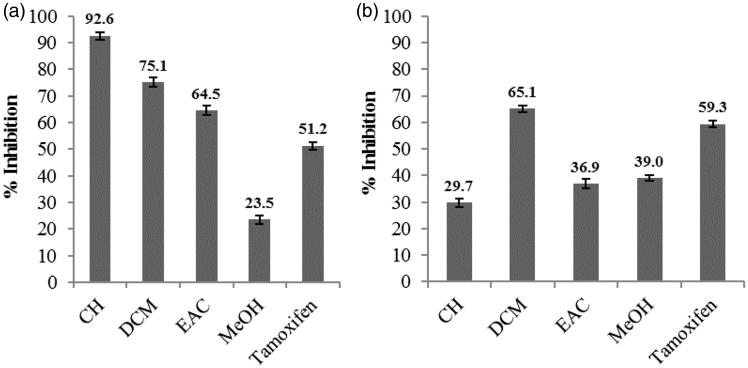
The cytotoxicity of various extracts was evaluated at 50 mg/L against two ovarian tumour cell lines, OVCAR-3 and IGROV-1 (Figure 1). Concerning the inhibition of cell line IGROV-1, the results showed that apart from the methanol extract (23.50 ± 2.50%), all other extracts, cyclohexane, dichloromethane and ethyl acetate showed good inhibition, 92.60 ± 1.40, 75.10 ± 2.80 and 64.50 ± 2.80%, respectively (Figure 1). However, regarding cancer cell line OVCAR-3, the dichloromethane extract showed the highest percentage inhibition (65.10 ± 3.20%) and all other samples were moderately anticancer at 29.70 ± 6.60, 36.90 ± 3.80 and 39.01 ± 9.10% for the cyclohexane, ethyl acetate and methanol extracts, respectively.
Figure 1.
IGROV-1 cell line inhibition (a) and OVCAR-3 cell line inhibition (b) by cyclohexane (CH), dichloromethane (DCM), ethyl acetate (EAC) and methanol (MeOH) extracts of B. dalzielii at 50 mg/L. Tamoxifen was tested at 0.2 mg/L.
Discussion
Yield and chemical composition
The B. dalzielii leaves (Jansen et al. 2010) have been extracted via dichloromethane, methanol or decoction (water). The yields were 4.7%, 24.7% and 33.4%, respectively. Jansen et al. (2010) results are not comparable to our results, because we used additional solvents successively. Their decoction was prepared under the effect of temperature, while our macerations were performed at room temperature (25 °C). For the essential oil, our yield (1.12%) was approximately equivalent to the 1.25% obtained by Kubmarawa et al. (2006), the only other work quoting a yield.
Many new compounds were identified in our work that were not seen in the essential oil prepared by Kubmarawa et al. (2006), who cited only 29 compounds. α-Pinene (45.7%) and α-terpinene (11.5%) were the predominant compounds found by Kubmarawa et al. (2006), but in our work 3-carene (27.72%), α-pinene (15.18%), p-cymene (9.54%) and β-phellandrene (8.48%) were abundant.
To our knowledge, this is the first quantification of phenolics, tannins, flavonoids, anthocyanins and sugars for the Boswellia genus. The amount of phenolics (315 g/kg) is important and very interesting in that this extract is comparable to reference extracts rich in phenolic compounds. The amount of flavonoids increased with solvent polarity. This is in agreement with the polar nature of flavonoids.
To our knowledge, our GC-MS before and after derivatization is the first such study of the extracts of a plant of the Boswellia genus. Thus, α-pinene, p-cymene, β-caryophyllene, α-E-bergamotene, caryophyllene alcohol and torreyol have been found in the essential oil confirming their existence in the plant through their presence in the cyclohexane extract before derivatization. The presence of certain compounds in two or three extracts before derivatization was due to the fact that the compounds are released from the disrupted cells of plant material with maceration solvents at an ambient temperature.
Among the identified phytochemicals, urs-12-en-24-oic acid,3-oxo-methyl ester had already been detected via GC-MS in the ethanol extract of whole-plant Kalimeris indica (Zhong et al. 2015) and 6-isopropenyl-4,8a-dimethyl-3,5,6,7,8,8a-hexahydro-2(H)-naphtalenone was detected in methanol extract of Hypochaeris radicata roots (Jamuna & Paulsamy 2013). Similarly, methyl-8,11,14-heptadecatrienoate has been identified in petroleum and ethanol extracts of leaves (Das & Himaja 2014), and phytol acetate was found in the methanol extract of Hypochaeris radicata leaves (Jamuna & Paulsamy 2013).
It is important to point out that this research has allowed us to reveal, for the first time, the presence of molecules such as α-d-mannofuranoside, farnesy l,1,2,3,5,6,7,8,8a-octahydronaphthalen-2-yl ester, 3-hydroxy-6-isopropenyl-dimethyl-4,8a, acetic acid; 2-propenoic acid, 3-[2-(amino-carbonyl) phenyl]-,(E); (5E)-2,4,4,7-tetramethyl-5,7-octadien-3-ol; 2,5-dihydroxy acetophenone and β-d-xylopyranose in the leaves of this plant. To our knowledge, none of these molecules (except β-d-xylopyranose) have ever been found in a plant. Given the therapeutic properties of each of these molecules, this study should be continued in order to develop methods for isolating these molecules from the plant.
Biological activities
In the literature, no investigation has cited of the biological activities of B. dalzielii leaves, except for their antioxidant activity. The majority of biological activities cited here have not been studied in the Boswellia genus.
The essential oil has low antioxidant activity. In terms of the extracts, the antioxidant activity increased with the polarity of the solvent. The methanol extract was a strong antioxidant comparable to vitamin C antioxidant power (5.30 ± 0.01 mg/L) and was in good agreement with the amount of phenolics. This prompted us to investigate the correlation between the antioxidant activity of these extracts and the total phenolic and flavonoid content contained in these extracts. Some good correlation coefficients (R2) were obtained: 0.68 and 0.89, respectively, with phenolics and flavonoids. To our knowledge, there is no work on successive extracts of B. dalzielii leaves. The only study of B. dalzielii (Anago et al. 2011) showed that an ethanolic extract had an inhibition percentage of 83% at 10 mg/L.
The correlation between the xanthine oxidase activity of the extracts and phenolic and flavonoid content was evaluated, with the results indicating very good correlations of 0.91 and 0.91, respectively. These results are consistent with the work of Dew et al. (2005), who evaluated the xanthine oxidase activity of extracts of cranberry juice, purple grape juice and black tea without the quantification of phenolics. The low level of activity of the essential oil can be explained by the absence of phenolics, especially flavonoids.
The anticholinesterase activity of three essential oils obtained from the oleogum resin of three species: B. socotrana, B. ameero and B. elongata have been determined (Ali et al. 2008). B. socotrana essential oil showed the higher AChE inhibitory activity at 59.3% at a concentration of 200 μg/mL in comparison to the essential oils of B. elongata and B. ameero (29.6% and 41.6% enzyme inhibition, respectively). It has been shown that at 200 mg/L, chloroform extracts of B. socotranus and B. elongata (Bakthira et al. 2011) inhibited AChE by 71.2% and 46.3%, respectively, but methanol extracts showed an inhibitory activity below 50%. Our essential oil was more active than those mentioned above (IC50 = 67.10 ± 0.03 mg/L).
It has been reported that α-pinene, linalool, β-caryophyllene and β-caryophyllene oxide have antiAChE activity (Savelev et al. 2003, 2004). Thus, the antiAChE activity of the cyclohexane extract could be attributed to the α-pinene, Z-linalool oxide, caryopyllene and caryophyllene oxide identified via GC-MS before derivatization. Good correlations were obtained between AChE inhibition and phenolics (R2 = 0.88) and between AChE inhibition and flavonoids (R2 = 0.92).
The inhibition of AChE activity induced by the essential oil was higher than those of the organic extracts. This could be explained by the action of the majority compounds 3-carene, α-pinene, p-cymene, β-phellandrene, myrcene, Z-β-farnesene and isolongifolene.
Bekir et al. (2013) showed that the inhibition of 5-lipoxygenase is more important when the extract is rich in phenolics. The amount of phenolics obtained in these extracts could explain these good inhibitory activities against 5-lipoxygenase. This was confirmed by our correlation coefficients between 5-lipoxygenase inhibition and phenolics (R2 = 0.69) and between 5-lipoxygenase inhibition flavonoids (R2 = 0.92). However, other species such as B. serrata have been recognized as anti-inflammatory (Kimmatkar et al. 2003) due to the boswellic acids contained in frankincense. Similarly, Ali and Mansour (2011) showed the important inhibitory activity of boswellic acids, principally 3-O-acetyl-11-keto-β-boswellic acid isolated from B. carterii, against 5-lipoxygenase.
The essential oil activity is due to the combined anti-inflammatory activity of compounds such as β-caryophyllene, α-pinene, p-cymene and E-sabinene hydrate. This corroborates the results of Wei and Shibamoto (2010) who demonstrated the inhibitory effect of these compounds against 5-lipoxygenase.
The extracts were not active against SOD. Based on these results, it appears that SOD, as a biological antioxidant, could not be inhibited by the compounds in the extracts.
In view of these results, it appears that the dichloromethane extract inhibits two selected cancer cell lines similarly. The cyclohexane extract is selective for the proliferation of the IGROV-1 cell line. In contrast, the methanol extract has no toxic effect on either of the two cell lines. The ethanol extract of B. ovalifoliolata leaves (Thummuri et al. 2014) was evaluated against several cancerous cell lines. This yielded IC50 (67.48 < IC50 (mg/L) < 200), which was less interesting than the results for the majority of our extracts.
Conclusion
In this study, we have evaluated the in vitro antioxidant, antixanthine oxidase, anti-Alzheimer, anti-inflammatory and anticancer activities of B. dalzielii leaf extracts, after studying the chemical compositions of all extracts and the essential oil. In view of the results, we can conclude that the ethyl acetate and methanol extracts could be considered potential alternative natural antioxidants, antihyperuricemics and anti-inflammatories for use in the food industry and in the pharmaceutical industry for the prevention or treatment of diseases. The cyclohexane extract is selective for the proliferation of the IGROV-1 cell line. Further research must be conducted on the cyclohexane, ethyl acetate and methanol extracts in order to find additional molecules responsible for these biological activities.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Afoulous S, Ferhout H, Raoelison EG, Valentin A, Moukarzel B, Couderc F, Bouajila J.. 2011. Helichrysum gymnocephalum essential oil: chemical composition and cytotoxic, antimalarial and antioxidant activities, attribution of the activity origin by correlations. Molecules. 16:8273–8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemika TE, Onawunmi GO, Olugbade TA.. 2006. Antibacterial phenolics from Boswellia dalzielii. J Nat Prod Med. 10:108–110. [Google Scholar]
- Alemika TE, Onawunmi GO, Olugbade TA.. 2004. Isolation and characterization of incensole from Boswellia dalzielii stem bark. J Pharm Biores. 1:7–11. [Google Scholar]
- Ali A, Wurster M, Arnold N, Teichert A, Schmidt J, Lindequist U, Wessjohann L.. 2008. Chemical composition and biological activities of essential oils from the Oleogum resins of three endemic Soqotraen Boswellia species. Rec Nat Prod. 2:6–12. [Google Scholar]
- Ali EN, Mansour SZ.. 2011. Boswellic acids extract attenuates pulmonary fibrosis induced by bleomycin and oxidative stress from gamma irradiation in rats. Chin Med. 6:36–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anago E, Lagnika L, Gbenou J, Loko F, Moudachirou M, Sanni A.. 2011. Antibacterial activity and phytochemical study of six medicinal plants used in Benin. Pak J Biol Sci. 14:449–455. [DOI] [PubMed] [Google Scholar]
- Arbonnier M. 2000. Arbres, arbustes et lianes des zones sèches d’Afrique de l’Ouest. Cirad/MnhnUicn, Montpellier: CIRAD, MNHN; p. 541. [Google Scholar]
- Bakthira H, Awadh Ali NA, Arnold N, Teichert A, Wessjohann L.. 2011. Anticholinesterase activity of endemic plant extracts from soqotra. Afr J Tradit Complement Altern Med. 8:296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekir J, Mars M, Souchard JP, Bouajila J.. 2013. Assessment of anti-oxidant, anti-inflammatory, anti-cholinesterase and cytotoxic activities of pomegranate (Punica granatum) leaves. Food Chem Toxicol. 55:470–475. [DOI] [PubMed] [Google Scholar]
- Das M, Himaja M.. 2014. Phytochemical screening, GC-MS analysis and biological activities of Ipomoea eriocarpa leaf extracts. Inter J Pharm Pharmaceut Sci. 6:592–594. [Google Scholar]
- Dew TP, Day AJ, Morgan MR.. 2005. Xanthine oxidase activity in vitro: effects of food extracts and components. J Agric Food Chem. 53:6510–6515. [DOI] [PubMed] [Google Scholar]
- Dias DA, Urban S, Roessner U.. 2012. A historical overview of natural products in drug discovery. Metabolites. 2:303–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokaraju GR, Gokaraju RR, Gokaraju VKRR, Golakoti T, Bhupathiraju K.. 2010. A novel Boswellia low polar gum resin extract and its synergistic compositions. Indian Pat Appl. IN 2010CH00384 A 20100319. [Google Scholar]
- Hassan HS, Musa AM, Usman MA, Abdulaziz M.. 2009. Preliminary phytochimical and antispasmodic studies of the stem bark of Boswellia dalzielii. Niger J Pharm Sci. 8:1–6. [Google Scholar]
- Jamuna S, Paulsamy S.. 2013. GC-MS analysis for bioactive compounds in the methanolic leaf and root extracts of Hypochaeris radicata L (Asteraceae). Int J Curr Res. 5:4070–4074. [Google Scholar]
- Jansen O, Angenot L, Tits M, Nicolas JP, De Mol P, Nikiéma JB, Frédérich M.. 2010. Evaluation of 13 selected medicinal plants from Burkina Faso for their antiplasmodial properties. J Ethnopharmacol. 130:143–150. [DOI] [PubMed] [Google Scholar]
- Kimmatkar N, Thawani V, Hingorani L, Khiyani R.. 2003. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee a randomized double blind placebo controlled trial. Phytomedicine. 10:3–7. [DOI] [PubMed] [Google Scholar]
- Kubmarawa D, Ogunwande IA, Okorie Domingo AO, Nureni O, Kasali A.. 2006. Constituents of the essential oils of Boswellia dalzielii Hutch. J Ess Oil Res. 18:119–120. [Google Scholar]
- Li X. 2012. Improved pyrogallol autoxidation method: a reliable and cheap superoxide-scavenging assay suitable for all anti-oxidants. J Agric Food Chem. 60:6418–6424. [DOI] [PubMed] [Google Scholar]
- Lin JK, Chen PC, Ho CT, Lin-Shiau SY.. 2000. Inhibition of xanthine oxidase and suppression of intracellular reactive oxygen species in HL-60 cells by theaflavin-3,3′-digallate, (-)-epigallocatechin-3-gallate, and propyl gallate. J Agric Food Chem. 48:2736–2743. [DOI] [PubMed] [Google Scholar]
- Michiels C. 2014. Trade for Development Center: BTC (Belgian Development Agency). Brussels, ProFound. Belgian Development Agency. p. 112. [Google Scholar]
- Ouédraogo A, Thiombiano A, Hahn-Hadjali K, Guinko S.. 2006. Régénération sexuée de Boswellia dalzielii Hutch, un arbre médicinal de grande valeur au Burkina Faso. Bois Et Forêts Des Tropics. 289:48. [Google Scholar]
- Ouédraogo NOG. 1996. Plantes médicinales et pratiques médicinales traditionnelles au Burkina Faso, cas du plateau central. Thèse, Université d’Ouagadougou (Burkina Faso). [Google Scholar]
- Savelev S, Okello E, Perry NSL, Wilkins PM, Perry EK.. 2003. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol Biochem Behav. 75:661–668. [DOI] [PubMed] [Google Scholar]
- Savelev SU, Okello EJ, Perry EK.. 2004. Butyryl- and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother Res. 18:315–324. [DOI] [PubMed] [Google Scholar]
- Thummuri D, Jeengar MK, Shrivastava S, Areti A, Yerra VG, Yamjala S, Komirishetty P, Naidu VG, Kumar A, Sistla R.. 2014. Boswellia ovalifoliolata abrogates ROS mediated NF-κB activation, causes apoptosis and chemosensitization in triple negative breast cancer cells. Environ Toxicol Pharmacol. 38:58–70. [DOI] [PubMed] [Google Scholar]
- Tzing SH, Ding WH.. 2010. Determination of melamine and cyanuric acid in powdered milk using injection-port derivatization and gas chromatography-tandem mass spectrometry with furan chemical ionization. J Chromatogr A. 1217:6267–6273. [DOI] [PubMed] [Google Scholar]
- Wei A, Shibamoto T.. 2010. Anti-oxidant/lipoxygenase inhibitory activities and chemical compositions of selected essential oils. J Agric Food Chem. 58:7218–7225. [DOI] [PubMed] [Google Scholar]
- Zhong RF, Xu GB, Wang Z, Wang AM, Guan HY, Li J, He X, Liu JH, Zhou M, Li YJ, et al. 2015. Identification of anti-inflammatory constituents from Kalimeris indica with UHPLC-ESI-Q-TOF-MS/MS and GC-MS. J Ethnopharmacol. 165:39–45. [DOI] [PubMed] [Google Scholar]