Abstract
Objective
To determine whether ascending arousal network (AAn) connectivity is reduced in patients presenting with traumatic coma.
Methods
We performed high-angular-resolution diffusion imaging in 16 patients with acute severe traumatic brain injury who were comatose on admission and in 16 matched controls. We used probabilistic tractography to measure the connectivity probability (CP) of AAn axonal pathways linking the brainstem tegmentum to the hypothalamus, thalamus, and basal forebrain. To assess the spatial specificity of CP differences between patients and controls, we also measured CP within 4 subcortical pathways outside the AAn.
Results
Compared to controls, patients showed a reduction in AAn pathways connecting the brainstem tegmentum to a region of interest encompassing the hypothalamus, thalamus, and basal forebrain. When each pathway was examined individually, brainstem-hypothalamus and brainstem-thalamus CPs, but not brainstem-forebrain CP, were significantly reduced in patients. Only 1 subcortical pathway outside the AAn showed reduced CP in patients.
Conclusions
We provide initial evidence for the reduced integrity of axonal pathways linking the brainstem tegmentum to the hypothalamus and thalamus in patients presenting with traumatic coma. Our findings support current conceptual models of coma as being caused by subcortical AAn injury. AAn connectivity mapping provides an opportunity to advance the study of human coma and consciousness.
In patients with acute severe traumatic brain injury (TBI), coma is believed to be caused by axonal injury within the ascending arousal network (AAn), a collection of subcortical pathways that connect the rostral brainstem tegmentum to the hypothalamus, thalamus, and basal forebrain.1,2 Evidence that the AAn is essential for consciousness comes from experimentally induced lesions in rodents3 and cats4 and mapping of brainstem infarctions in humans.5 In traumatic coma, however, up to 44% of patients may not have visible brainstem pathology on conventional MRI sequences.6 Furthermore, histopathologic studies of traumatic coma in humans7 and nonhuman primates2 suggest that axonal shearing in the brainstem always occurs in the context of widespread white matter injury. Evidence specifically implicating AAn disruption in the pathogenesis of traumatic coma is limited to experimental models in pigs8 and case reports of postmortem human brain specimens.9,10 Because tools to map human AAn connectivity in vivo have only recently become available,1 it is uncertain whether AAn disconnections play a role in the pathogenesis of acute traumatic coma.
In vivo mapping of the human AAn requires noninvasive, quantitative measurement of geometrically complex axonal connections.1,11 Unlike conventional MRI and diffusion tensor imaging, high-angular-resolution diffusion imaging (HARDI) meets these methodologic requirements by measuring water diffusion along axonal pathways using a multidirectional model designed to detect crossing and branching axons.12 HARDI thus provides an opportunity to model the pathophysiologic effects of TBI on the axonal architecture of the AAn and has the potential to generate novel radiologic biomarkers for patients with traumatic coma.
In this prospective observational study, we implemented HARDI on a clinical MRI scanner in the intensive care unit (ICU) to determine whether there are differences in AAn connectivity in patients with acute traumatic disorders of consciousness compared to age- and sex-matched healthy controls. We hypothesized that patients have reduced connectivity within AAn pathways linking the rostral brainstem tegmentum to the hypothalamus, thalamus, and basal forebrain but not within subcortical motor and sensory pathways outside the AAn.
Methods
Detailed information on HARDI acquisition, processing, primary analyses, and exploratory analyses is provided in the supplementary material available from Dryad (doi.org/10.5061/dryad.43m733k).
Patients
As described previously,13 we prospectively enrolled 16 consecutive patients (median age 27.5 years, interquartile range [IQR] 21.5–33 years, 12 men) who presented with acute traumatic coma to the ICU at an academic medical center. Inclusion criteria were age between 18 and 65 years, head trauma, at least 1 neurologic examination before ICU admission consistent with coma (defined as Glasgow Coma Scale score of 6 without eye opening), and no eye opening for 24 hours after injury.13 We acquired HARDI data as soon as the treating clinicians deemed patients stable for transport. Sixteen age- and sex-matched controls (median age 27 years, IQR 21–32.5 years, 12 men) underwent identical imaging.
Image acquisition
All scans were performed on a 3T Skyra MRI scanner (Siemens Medical Solutions, Malvern, PA) with a 32-channel head coil. HARDI sequence parameters were 2-mm isotropic voxels, 60 diffusion-encoded volumes (b = 2,000 s/m2), 10 b0 volumes (b = 0 s/m2), and a 220-mm field of view. Six controls and 10 patients were scanned with a repetition time of 13,700 milliseconds, echo time of 98 milliseconds, echo spacing of 0.7 milliseconds, and epi factor of 104. Twelve control and 7 patient scans were acquired with simultaneous multislice imaging with a repetition time of 6,700 milliseconds, echo time of 100 milliseconds, echo spacing 0.7 milliseconds, and epi factor of 110. For patients and controls who were scanned with both HARDI sequences, data from the simultaneous multislice imaging sequence were used for the primary analyses.
Image processing
Preprocessing was performed in FSL (FMRIB, Oxford, UK) and included brain extraction, eddy current correction, and bulk motion correction. Diffusion parameters were estimated with bedpostx with default parameter settings. Probabilistic tractography was performed with probrackx2 with 5,000 samples per voxel, a curvature threshold of 80°, maximum of 2,000 steps, step length of 0.5 mm, minimum length of 0, no anisotropy constraining, and no distance correction. Sequential linear and nonlinear transformations were generated between each patient's diffusion space (fractional anisotropy image) and Montreal Neurological Institute (MNI) 152 T1 one-millimeter space with Advanced Normalization Tools (Philadelphia, PA) (video 1 available from Dryad, doi.org/10.5061/dryad.43m733k).
Representative transformation between a patient's fractional anisotropy (FA) image and MNI 152 T1 space. The video toggles between a patient's FA image warped to MNI space and the MNI 152 T1 template brain at two axial slices. The axial slices are at the level of the mid-thalamus and the caudal midbrain.Download Supplementary Video 1 (10.2MB, mp4) via http://dx.doi.org/10.1212/008163_Video_1
Regions of interest
We created a dorsal brainstem tegmentum seed mask using the Harvard Ascending Arousal Network Atlas (martinos.org/resources/aan-atlas),1 excluding the spatially-disparate ventral tegmental area, and filling in regions between individual nuclei (data available from Dryad (figure e-1, doi.org/10.5061/dryad.43m733k). All other AAn and non-AAn regions of interest (ROIs) were generated from publicly available atlases in FSL when possible or by manual tracing the ROIs on the MNI152 T1 one-millimeter template brain guided by a human brain atlas14 (figures e-2 and e-3 available from Dryad, doi.org/10.5061/dryad.43m733k).
Probabilistic tractography
We used probabilistic tractography to map, at the single-participant level, the pathways connecting the brainstem tegmentum seed to the thalamus, hypothalamus, and basal forebrain targets. We calculated the total number of tracts leaving the brainstem seed reaching any one of the subcortical targets and normalized this value to the total number of tracts launched from the seed. Tracts crossing the corpus callosum were excluded due to anatomic implausibility. We also calculated voxel-wise tract numbers normalized by the total number of tracts leaving the seed. Each participant’s normalized tract number map was transformed into MNI space for voxel-wise group-level analyses. In seed-to-target analyses, we defined a connectivity probability (CP) between 2 ROIs as the weighted (by number of launched tracts) average of the number of tracts reaching the target from the seed and the number of tracts reaching the seed from the target.
Statistics
Group-level voxel-wise and tract-specific analyses were performed in MATLAB (MathWorks, Natick, MA) with Wilcoxon rank-sum tests. When appropriate, we adjusted p values for multiple comparisons using Holm family-wise error (FWE) adjustment, except for the voxel-wise analysis, in which we used the less-restrictive Benjamini-Hochberg false discovery rate adjustment.
Standard protocol approvals, registrations, and patient consents
This study was approved by the Massachusetts General Hospital Institutional Review Board. Written informed consent was obtained from each participant or a surrogate.
Data availability
Data processing scripts and ROI files can be found at github.com/ComaRecoveryLab/AAn-in-Acute-Traumatic-Disorders-of-Consciousness.git. The conditions of our Institutional Review Board ethics approval do not permit public archiving of anonymized study data. Readers seeking access to the data should contact the corresponding author.
Results
AAn connectivity measures
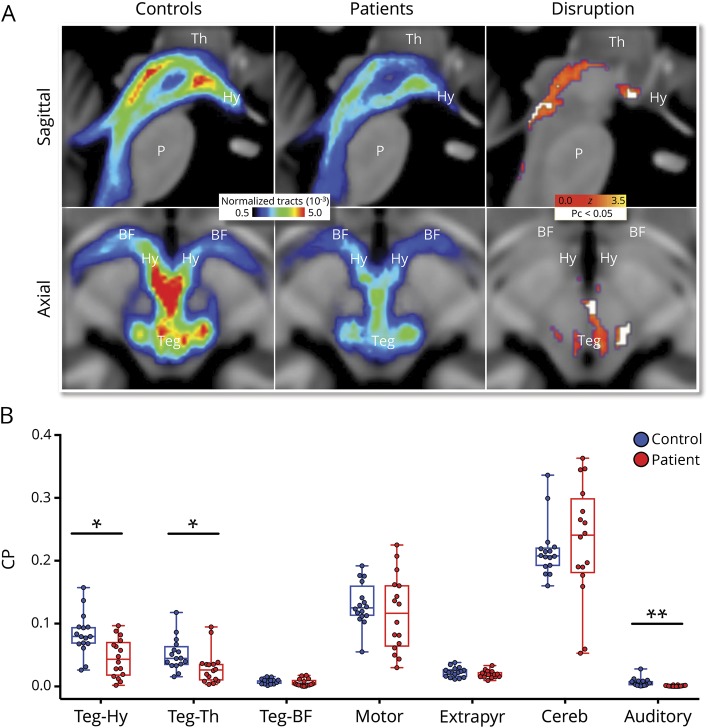
Patient clinical and radiologic characteristics are provided in table 1 and in tables e-1 through e-4 available from Dryad (doi.org/10.5061/dryad.43m733k). Compared to healthy controls, patients showed reduced normalized tract number between the brainstem tegmentum and a target encompassing the hypothalamus, thalamus, and basal forebrain (patients: median 0.08, IQR 0.04–0.1; controls: median 0.1, IQR 0.09–0.12, p = 0.006; figure 1A). Peak areas of tract reduction were seen within the left lateral midbrain tegmentum and in the rostral medial tracts connecting the tegmentum to the hypothalamus (figures 1A and 2 and figure e-4, doi.org/10.5061/dryad.43m733k and videos 2 and 3 available from Dryad). Individual seed-to-target analyses revealed significantly reduced CP for brainstem-hypothalamus (patients: 0.04, IQR 0.02–0.07; controls: 0.08, IQR 0.07–0.09, FWE pc< 0.05 and brainstem-thalamus (patients: 0.03, IQR 0.01–0.04; controls: 0.04, IQR 0.04–0.06, pc < 0.05) but not brainstem-forebrain (patients: 0.003 IQR 0.002–0.009; controls: 0.008, IQR 0.004–0.01, pc = 0.37; figure 1B). Among non-AAn pathways, we found no group-level CP differences between patients and controls in motor (ventral pons to posterior limb internal capsule) (patients: 0.12, IQR 0.07–0.16; controls: 0.12, IQR 0.11–0.15), extrapyramidal (substantia nigra to basal ganglia) (patients: 0.02, IQR 0.02–0.02; controls: 0.02, IQR 0.02–0.02), or cerebellar (superior cerebellar peduncle to red nucleus) (patients: 0.24, IQR 0.19–0.29; controls: 0.21, IQR 0.19–0.22) tracts (all pc > 0.5; figure 1B). We did, however, find a significant group-level CP reduction in auditory tracts (inferior colliculus to medial geniculate body) between patients (0.0007, IQR 0.0002–0.001) and controls (0.005, IQR 0.002–0.009, pc < 0.005; figure 1B). These results were unaffected by the exclusion of patient 16 (tables e-2 and e-3 available from Dryad, doi.org/10.5061/dryad.43m733k), the only patient who presented with an extra-axial lesion producing significant brainstem compression.
Table 1.
Patient demographics and clinical characteristics
Figure 1. AAn connectivity in patients and controls.
(A) Sagittal (top row) and axial (bottom row) images of group median normalized tract numbers for pathways connecting the brainstem tegmentum (Teg) and a combined target including the hypothalamus (Hy), thalamus (Th), and basal forebrain (BF). In the third column, white voxels indicate regions with fewer normalized tracts in patients vs controls using a corrected pc < 0.05 (after Benjamini-Hochberg false discovery rate adjustment). Red/yellow correspond to z scores of all voxels with fewer normalized tracts using an uncorrected p < 0.05. (B) Patients show reduced connectivity probability (CP) relative to controls in 2 of 3 ascending arousal network (AAn) pathways (tegmentum-hypothalamus, tegmentum-thalamus) and 1 of 4 non-AAn pathways (auditory). Points represent individual participant CP values; boxes show median and interquartile ranges; and whiskers extend from maximum to minimum values. Cereb = cerebellar pathway; Extrapyr = extrapyramidal pathway; P = pons. *Holm family-wise error adjusted pc < 0.05, **pc < 0.005.
Figure 2. Peak areas of tract disruption within the ascending arousal network.

Blue shows 3D reconstruction of median normalized tracts in controls connecting the brainstem tegmentum seed to a combined target including the hypothalamus (Hy), thalamus (Th), and basal forebrain (BF) (binarized surface, median normalized tract count > 0.0005). Red shows voxels with significantly fewer normalized tracts in patients relative to controls (Benjamini-Hochberg false discovery rate–adjusted pc < 0.05). DoC = disorders of consciousness; P = pons.
Peak regions of AAN tract disruption. In red/yellow: Ascending axial slices show median normalized tract number between the brainstem tegmentum seed and the combined hypothalamic, thalamic, and basal forebrain target in controls. Red/yellow scale: 0.0005 < Normalized Tracts < 0.005. White Overlay: Voxels with significantly fewer normalized tracts in patients as compared with controls, Benjamini-Hochberg FDR Pc < 0.05.Download Supplementary Video 2 (10.4MB, mp4) via http://dx.doi.org/10.1212/008163_Video_2
Peak regions of AAN tract disruption. In blue: Rotating 3-dimensional reconstruction of median normalized tract numbers in control subjects between the brainstem tegmentum seed and a combined subcortical target including the hypothalamus, thalamus and basal forebrain. (Binarized surface, normalized tract count > 0.0005). In red: voxels with significantly fewer normalized tracts in patients as compared with controls, Benjamini-Hochberg FDR Pc < 0.05.Download Supplementary Video 3 (14.6MB, mp4) via http://dx.doi.org/10.1212/008163_Video_3
Clinical correlation of AAn connectivity
Brainstem-hypothalamus, brainstem-thalamus, and brainstem-forebrain CPs were not correlated with days in coma or days until command following (all p ≥ 0.29 for Pearson correlation coefficients), nor were they reduced in patients with prolonged (>2 days) relative to brief (≤2 days) coma (all p ≥ 0.27).
Discussion
We provide initial in vivo evidence for reduced AAn connectivity in patients who presented to the ICU with acute traumatic coma. Within the brainstem, 2 of 3 AAn pathways but only 1 of 4 non-AAn pathways showed CP reductions compared with controls. Although severe TBI is known to cause heterogeneous subcortical axonal injury, our findings suggest a spatial specificity for AAn pathways among patients who present with coma. While these structural connectivity findings do not prove a causal relationship, they support the role of AAn disruption in the pathogenesis of acute traumatic disorders of consciousness.
The relative preservation of brainstem-forebrain CP in patients presenting in coma is surprising given evidence from animal studies that brainstem-forebrain connectivity contributes to consciousness.3 It is possible that disconnection of the brainstem from the basal forebrain is not required to produce coma,15 but preserved brainstem-forebrain connectivity is still essential for recovery of consciousness. This interpretation is supported by our observation that while all patients arrived at the hospital in coma, all surviving patients recovered consciousness by 6 months post-injury.13 Indeed, only 2 of 16 patients remained in coma and 4 of 16 had recovered to a posttraumatic confusional state at the time of MRI. The mechanism underlying recovery of consciousness after AAn injury remains unclear, but an acute reduction in CP may reflect reversible, incomplete axonal injury.16 Alternatively, if the AAn pathways with reduced CP were irreversibly injured, it is possible that structurally preserved axonal pathways within the AAn may have undergone functional reorganization to restore arousal. Preliminary evidence from AAn mapping studies in postmortem human brain specimens suggests that redundant interconnections between AAn nodes may facilitate network resilience and neuroplasticity.1
Several limitations of HARDI tractography should be considered in the interpretation of the results of this study. HARDI reconstruction of axonal pathways depends on intervoxel coherence in directional water diffusion, and focal intracellular or extracellular edema may lead to an apparent tract disruption even when the underlying axons are structurally intact. Furthermore, HARDI tractography cannot definitively distinguish between reversibly and irreversibly injured axons, which may explain the lack of correlation between HARDI-derived CP values and coma duration. These limitations of HARDI tractography highlight the need for complementary functional connectivity techniques to map the AAn in brain-injured patients.
It is also important to consider that HARDI tractography is based on water diffusion measures that are dynamic during the acute stage of traumatic axonal injury.17 Because these temporal dynamics have not been fully elucidated, it is uncertain whether HARDI-derived measures of AAn connectivity are biomarkers of injury severity, coma duration, or level of consciousness at the time of imaging. If the interval between injury and imaging varies between patients, as was the case in this study, correlations between AAn connectivity and clinical measures should be interpreted with caution.
Another limitation of this small study is its statistical power, which may have been inadequate to identify a correlation between AAn CP reduction and coma duration. It is also possible that coma duration depends on AAn functional properties that are not accounted for by AAn structural connectivity. Finally, future AAn connectivity studies may achieve more precise anatomic localization of coma-causing network disruptions by comparing comatose patients with TBI to patients with TBI with preserved consciousness rather than to healthy controls.
The structural AAn mapping methods that we developed and implemented here provide an opportunity for in vivo investigation of AAn contributions to human consciousness. HARDI-based probabilistic tractography in larger cohorts will allow specific AAn pathways to be tested for their respective roles in coma pathogenesis and for their pathophysiologic importance relative to supratentorial axonal injury. Moreover, these methods offer the potential to identify the specific combinations of AAn connections that are compatible with recovery of consciousness after traumatic coma.
Acknowledgment
The authors thank the nursing staffs of the Massachusetts General Hospital Neurosciences ICU, Multidisciplinary ICU, and Surgical ICU. They also thank Kellie Cahill, Erin Desmond, and the Massachusetts General Hospital MRI technologists for assistance with data acquisition. They are grateful to the patients and families in this study for their participation and support.
Glossary
- AAn
ascending arousal network
- CP
connectivity probability
- HARDI
high-angular-resolution diffusion imaging
- ICU
intensive care unit
- IQR
interquartile range
- MNI
Montreal Neurological Institute
- ROI
region of interest
- TBI
traumatic brain injury
Appendix. Authors

Study funding
This study was supported by the NIH National Institute of Neurological Disorders and Stroke (K23NS094538), NIH National Institute of Biomedical Imaging and Bioengineering (K01EB019474), NIH National Institute of General Medical Sciences (P01GM118269, R01GM104948), American Academy of Neurology/American Brain Foundation, James S. McDonnell Foundation, Rappaport Foundation, Tiny Blue Dot Foundation, and National Institute on Disability, Independent Living and Rehabilitation Research, Administration for Community Living (90DP0039, Spaulding-Harvard TBI Model System).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Edlow BL, Takahashi E, Wu O, et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol 2012;71:531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP. Diffuse axonal injury and traumatic coma in the primate. Ann Neurol 1982;12:564–574. [DOI] [PubMed] [Google Scholar]
- 3.Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol 2011;519:933–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindsley DB, Bowden JW, Magoun HW. Effect upon the EEG of acute injury to the brain stem activating system. Electroencephalogr Clin Neurophysiol 1949;1:475–486. [PubMed] [Google Scholar]
- 5.Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain 2003;126:1524–1536. [DOI] [PubMed] [Google Scholar]
- 6.Weiss N, Galanaud D, Carpentier A, et al. A combined clinical and MRI approach for outcome assessment of traumatic head injured comatose patients. J Neurol 2008;255:217–223. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell DE, Adams JH. Primary focal impact damage to the brainstem in blunt head injuries: does it exist? Lancet 1973;2:215–218. [DOI] [PubMed] [Google Scholar]
- 8.Smith DH, Nonaka M, Miller R, et al. Immediate coma following inertial brain injury dependent on axonal damage in the brainstem. J Neurosurg 2000;93:315–322. [DOI] [PubMed] [Google Scholar]
- 9.Edlow BL, Haynes RL, Takahashi E, et al. Disconnection of the ascending arousal system in traumatic coma. J Neuropathol Exp Neurol 2013;72:505–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenblum WI. Immediate, irreversible, posttraumatic coma: a review indicating that bilateral brainstem injury rather than widespread hemispheric damage is essential for its production. J Neuropathol Exp Neurol 2015;74:198–202. [DOI] [PubMed] [Google Scholar]
- 11.Nauta WJH, Kuypers HGJM. Some ascending pathways in the brain stem reticular formation. In: Jasper HH, Proctor LD, Knighton RS, Noshay WC, Costello RT, editors. Reticular Formation of the Brain. Boston: Little, Brown, and Company; 1958:3–30. [Google Scholar]
- 12.Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med 2002;48:577–582. [DOI] [PubMed] [Google Scholar]
- 13.Edlow BL, Chatelle C, Spencer CA, et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain 2017;140:2399–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding SL, Royall JJ, Sunkin SM, et al. Comprehensive cellular-resolution atlas of the adult human brain. J Comp Neurol 2016;524:3127–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voytko ML, Olton DS, Richardson RT, Gorman LK, Tobin JR, Price DL. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J Neurosci 1994;14:167–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma 1997;14:419–440. [DOI] [PubMed] [Google Scholar]
- 17.Edlow BL, Copen WA, Izzy S, et al. Diffusion tensor imaging in acute-to-subacute traumatic brain injury: a longitudinal analysis. BMC Neurol 2016;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative transformation between a patient's fractional anisotropy (FA) image and MNI 152 T1 space. The video toggles between a patient's FA image warped to MNI space and the MNI 152 T1 template brain at two axial slices. The axial slices are at the level of the mid-thalamus and the caudal midbrain.Download Supplementary Video 1 (10.2MB, mp4) via http://dx.doi.org/10.1212/008163_Video_1
Peak regions of AAN tract disruption. In red/yellow: Ascending axial slices show median normalized tract number between the brainstem tegmentum seed and the combined hypothalamic, thalamic, and basal forebrain target in controls. Red/yellow scale: 0.0005 < Normalized Tracts < 0.005. White Overlay: Voxels with significantly fewer normalized tracts in patients as compared with controls, Benjamini-Hochberg FDR Pc < 0.05.Download Supplementary Video 2 (10.4MB, mp4) via http://dx.doi.org/10.1212/008163_Video_2
Peak regions of AAN tract disruption. In blue: Rotating 3-dimensional reconstruction of median normalized tract numbers in control subjects between the brainstem tegmentum seed and a combined subcortical target including the hypothalamus, thalamus and basal forebrain. (Binarized surface, normalized tract count > 0.0005). In red: voxels with significantly fewer normalized tracts in patients as compared with controls, Benjamini-Hochberg FDR Pc < 0.05.Download Supplementary Video 3 (14.6MB, mp4) via http://dx.doi.org/10.1212/008163_Video_3
Data Availability Statement
Data processing scripts and ROI files can be found at github.com/ComaRecoveryLab/AAn-in-Acute-Traumatic-Disorders-of-Consciousness.git. The conditions of our Institutional Review Board ethics approval do not permit public archiving of anonymized study data. Readers seeking access to the data should contact the corresponding author.