Abstract
Objective
To report the long-term progression in a cohort of patients with type II spinal muscular atrophy (SMA) assessed with the Hammersmith Functional Motor Scale–Expanded.
Methods
Seventy-three patients (age 2.6–25 years) were included in the study. Twenty-eight of the 73 were first assessed before the age of 5 years and had been followed up for ≈5 years or longer. We observed an overall progression that was not linear. A piecewise regression analysis showed an improvement of scores in the younger patients with a point of slope change at ≈5 years of age, a decline between 5 and 13 years of age, and stability/slower decline after that.
Results
Patients with the lowest scores at baseline had the earliest onset of scoliosis and a higher need for noninvasive ventilation compared to those with higher scores. Our results confirm that on the long-term follow-up all patients with type II SMA show a clear and progressive decline.
Conclusion
The severity of functional impairment at baseline can help to predict the magnitude of changes over time and the overall progression, including onset of scoliosis and need for noninvasive ventilation.
Spinal muscular atrophy (SMA) is an autosomal recessive disorder characterized by degeneration of alpha motor neurons in the spinal cord.1 Patients with type II SMA classically achieve the ability to sit but not to walk independently, with a disease onset generally after 6 months of age.2,3
Patients with type II SMA are often relatively stable over short intervals of time such as 6 or 12 months,4–6 but progressive loss of function is observed on long-term follow-up.7
When we assessed 12-month changes, however, the progression was not linear, with younger children showing some improvement and a drastic decline more frequently observed between the age of 5 years and puberty.5,7 Other studies have also recently reported some cross-sectional or short-term longitudinal data,8,9 but no systematic attempt has been made to establish possible patterns of progression on long-term follow-up.
The aim of this study was to report a single-center longitudinal natural history study including all patients followed up longitudinally with the use of motor functional scales. More specifically, we were interested in establishing the long-term progression in the whole cohort, assessing patterns of progression in the patients first assessed before the age of 5 years and followed up for at least 5 years and identifying whether the progression of the disease is affected by a number of possible variables such as age, baseline values, and severity of scoliosis.
Methods
This retrospective study was performed on data collected in a single center (Fondazione Policlinico Universitario Agostino Gemelli, Catholic University, Rome, Italy).
All patients had a genetically confirmed diagnosis of SMA with mutations in the SMN1 gene and a clinically confirmed diagnosis of type II SMA. Since 2006, all patients with SMA seen in our neuromuscular clinic have been routinely assessed by trained physiotherapists using structured assessments. All consecutive patients with at least 2 assessments were selected for this study. Patients enrolled in clinical trials were included until the baseline of the trial. Single assessments affected by transient pain, fractures, recent pneumonia or other infections, intercurrent surgery, or any other factor that temporarily affected performance were excluded from the analysis.
Clinical information on age, weight, and contractures was noted. Scoliosis was measured with Cobb angles. Information about need for ventilation was also noted.
Special attention was paid to the subgroup of patients who were <5 years when they were first assessed and had been followed up longitudinally for ≥5 years.
Standard protocol approvals, registrations, and patient consents
The study was approved by the Institutional Review Board (Ethics Committee). Written informed consent was obtained from all participants (or guardians of participants) in the study (consent for research).
Hammersmith Functional Motor Scale–Expanded
The scale consists of 33 items investigating the child's ability to perform various activities.10 Each activity (item) is scored on a 3-point scoring system, with a score of 2 for performs without modification, 1 for performs with modification/adaptation, and 0 for unable to perform. A total score can be achieved by summing the scores for all the individual items. The total score can range from 0, if all the activities are failed, to 66, if all the activities are achieved.
All items have to be tested without spinal jacket or orthoses. Evaluators used a procedure manual and were trained at in-person meetings that were repeated on an annual basis, including new examiners as they became available.
Data availability
Individual details of all the patients are fully shown in figures 1 and 2. Methods and the statistical plan are also fully reported in the Methods section.
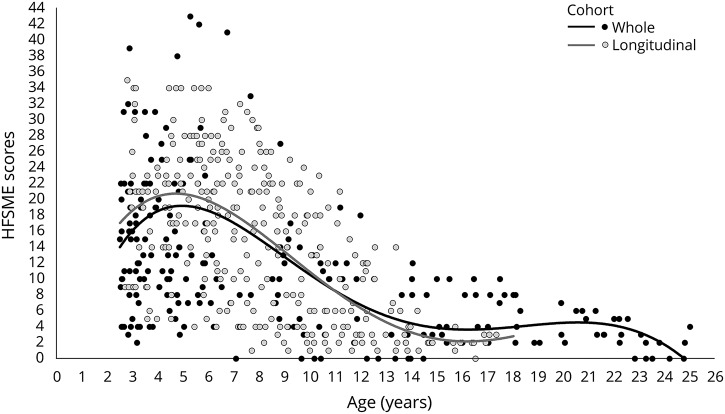
Figure 1. Details of the HFMSE distribution.
Hammersmith Functional Motor Scale–Expanded (HFMSE) score distribution of the whole cohort (black circle) and longitudinal cohort (gray circle). Interpolation line represents the HFMSE progression subdivided by whole cohort (black line) and longitudinal cohort (gray line).
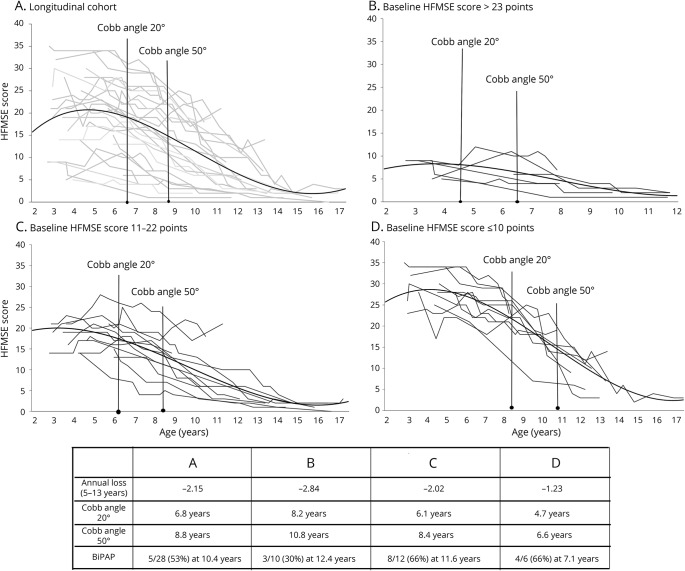
Figure 2. Details of progression.
(A–D) Individual details of the overall progression in all the 28 patients followed up longitudinally and in the subgroups divided according to their Hammersmith Functional Motor Scale–Expanded (HFMSE) scores at baseline.
Statistical analysis
The analysis of the Hammersmith Functional Motor Scale–Expanded (HFMSE) scores was performed with a linear regression analysis to define the trend of changes both in the whole cohort and in the subgroup followed up longitudinally.
To establish possible differences of progression in patients with different degree of severity, the subgroup followed up longitudinally was subdivided into 3 subgroups according to their HFMSE scores at baseline, identifying patients with scores >22, between 11 and 22, and <11. The cutoff points were arbitrarily determined on the basis of the fact that patients with scores of ≤10 (decimal ≤2.2) include those with limited mobility who generally are able to sit independently for a limited time and to raise their arm but are unable to roll over completely; patients with scores between 11 and 22 include those who are able to roll and have better trunk control in different postures; and those with scores >22 are at the higher end of the scale.
Results
Whole cohort
Data from 73 patients were included in the analysis. Their mean age at baseline was 6 years 10 months, ranging between 2 years 7 months and 25 years. Eleven of the 73 had only 2 assessments, 13 had 3, 10 had 4, 5 had 5, and 34 had >5. The mean duration of the follow-up was 4 years 8 months (SD ± 3.88), ranging between 6 months and 12 years 11 months (mean 6.64 assessments).
The mean age when patients developed a Cobb angle >20° requiring the introduction of thoracic orthosis11 was 6 years 5 months; the mean age when patients developed a Cobb angle >50°, suggesting the need for scoliosis surgery,11 was 9 years 9 months.
Ten of 73 had 2, 45 of 73 had 3, and 1 of 73 had >4 SMN2 copy numbers. In 17 patients, the copy number was not available.
Longitudinal cohort
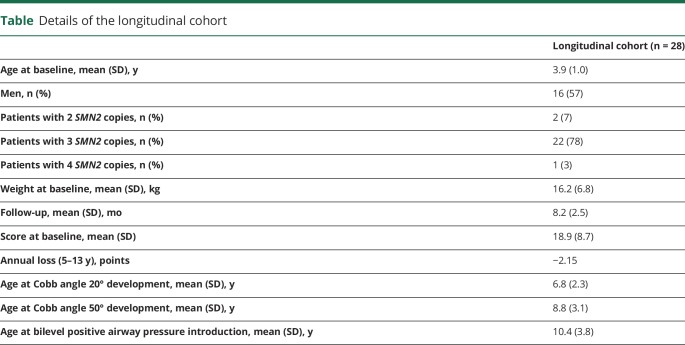
Of the 73 patients followed up in our center, 28 had more consistent longitudinal data because they were first assessed before the age of 5 years and had been followed up for ≈5 years or longer (range follow-up minimum of 4.9–maximum 12.11, average 8.2 years): 8 of 28 for ≈5 years, 7 of 28 for 7 years, 3 of 28 for 8 years, and the remaining 10 for ≥9 years (table). The SMN2 copy number was available in 25 of the 28 patients followed up longitudinally: 20 had 3 SMN2 copies, 4 had 2 copies (1 with baseline score >22, 2 with scores between 11 and 22, and 1 with a score < 10), and 1 had 4 copies. In 3 patients, the copy number was not available.
Table.
Details of the longitudinal cohort
The other 46 patients with type II SMA could not be selected because they arrived at our center after the age of 5 years or, even if first seen before the age of 5 years, had a shorter follow-up because they were still too young or because they entered clinical trials.
The mean age when patients developed a Cobb angle >20° requiring the introduction of thoracic orthosis11 was 6 years 10 months; the mean age when they developed a Cobb angle >50°, suggesting the need for scoliosis surgery,11 was 8 years 10 months; 15 had noninvasive ventilation (bilevel positive airway pressure) that was introduced at a mean age of 10 years 5 months. Twenty had 3, 4 had 2, and 1 had 4 SMN2 copy numbers.
HFMSE scores
Whole cohort
HFMSE mean total score at baseline ranged between 0 and 43 (mean 14.3, SD ± 9.8).
The results of the piecewise regression analysis showed an improvement of scores in the younger children with type II SMA. The point of slope change was ≈5 years, with a decline between the age of 5 and 13 years and a second point change associated with subsequent stability/slower decline after the age of 13 years (figure 1). The decrease in total scores was of 1.97 units per year between the ages of 5 and 13 years.
Selected longitudinal cohort
The total scores of this cohort ranged between 0 and 35.
The point of slope change was ≈5 years, with a decline after that until the age of 13 years and a second point change associated with subsequent stability/slower decline (figure 1). The decrease in total scores was 2.15 units per year between the ages of 5 and 13 years.
Figure 2 shows individual details of the 28 patients followed up longitudinally subdivided according to their HFMSE scores at baseline.
Correlation with copy number
Only 4 patients had 2 copies (1 with baseline score >22, 2 with scores between 11 and 22, and 1 with a score <10). The progression of their scores was similar to that of patients with 3 copies.
Only 1 patient had >3 copies. This patient was first seen at 3 years, had an HFMSE score of 35 and a COBB angle of 10°, and was not on ventilatory support. After 5 years 6 months of follow-up, he showed a decline of 13 point at the HFMSE and a COBB angle of 44°. The patient did not require ventilatory support for the entire duration of follow-up.
Discussion
The advent of different therapeutic options has recently changed the perspective and expectations of short- and long-term outcome in SMA.
The first open-label,12 pivotal, double-blind, randomized sham-controlled study in late-onset SMA, the Study to Assess the Efficacy and Safety of Nusinersen (ISIS 396443) in Participants With Later-Onset Spinal Muscular Atrophy (SMA) (CHERISH),13 which included mainly patients with type II SMA, showed a significant improvement over 12 months on different outcome measures. The preliminary results of the extension studies recently reported at international conferences suggest that the improvement is maintained over time. After the approval of the drug and the commercial availability of the drug in many countries, a large number of patients with type II SMA have been treated for >1 year. These include patients with a much larger range of age and functional abilities than those enrolled in the pivotal study, which had strict inclusion criteria. While the efficacy of the drug is obvious for the children achieving independent ambulation and for those showing a constant improvement of their functional scores, the interpretation of the real-world data in patients remaining stable or showing minimal changes is more challenging because long-term natural history data are scant. The interpretation becomes even more difficult in younger patients because of the possibility that they may show some physiologic improvement before the age of 5 years.5
It has therefore become important to retrieve all the available information on long-term longitudinal natural history data, especially in children followed up since the first years of life, to follow up possible positive and negative changes over time.
Recently, there has been an attempt to define more precise trajectories of progression according to age and type of SMA.5 Other studies have reported cross-sectional data8,9 or used longitudinal 12-month intervals,6 and only 1 study has reported longer follow-up.7 Our study reports for the first time the long-term outcome of young patients followed up systematically from young age, when they have a chance of improvement, until they are older, in the decline phase, using a structured functional scale specifically designed to assess motor function in SMA.
In our cohort including all patients with type II SMA followed up in our center, we confirmed that there is an overall progression and that this progression is not linear. There was an overall trend to improve until the age of 5 years, followed by a steep deterioration until puberty and a relative stabilization after that. After the age of 14 years, none of our patients had HFMSE scores >10, reflecting the loss of some abilities such as rolling that were often found in assessments performed before the age of 14 years. Some of these difficulties are probably related to the fact that by the age of 14 all our patients had a scoliosis >50° with an indication for spinal surgery that in most cases was performed before this age. These patients also had more severe contractures, especially at hips and knees (data not shown), which strongly reduces the possibility of rolling and performing many other activities on the HFMSE.
The loss of ≈2 points per year between the ages of 5 and 13 years clearly indicates a progressive loss of function. This information can be useful in the design of clinical trials or in the interpretation of different types of intervention. On the HFMSE, a loss of 2 points indicates that the patient has lost one of the functional aspects assessed in the scale. Recent surveys from patients and caregivers show that the families are aware of this progression and consider any loss of scores, even a single point, clinically meaningful.14–16
One of the limitation of our study is that a number of patients had a relatively short follow-up either because they were seen for the first time when they were already in their teens or because, over the years, many have entered clinical trials and therefore their data cannot be used to define natural history.
The selected cohort did not appear to have any obvious bias because the rate of progression and the peak of activities were similar to those found in the overall cohort of patients with type II SMA, with the slope of changes at 5.1 years and a higher risk of deterioration occurring between 5 and 14 years.
The availability of early assessments in all patients allowed subdividing them according to their baseline HFMSE scores in an attempt to identify possible differences in the progression of the disease.
Patients were subdivided into 3 subgroups using cutoff points of 10 and 22 on the HFMSE. The choice of a score of 10 was suggested by the inclusion criteria in recent clinical trials, suggesting that patients with scores <10 should not be included because they are considered too weak.13 Patients with a score <10 are classified as 2.0 or 2.1 according to the decimal classification proposed in the original article describing the Hammersmith scale.17 In contrast, a score of 22 was selected because this score is achieved in patients who are able to roll completely but are still unable to perform some activities such as crawling or standing that are generally found only in the mildest end of the spectrum.
The analysis highlighted some differences among the subgroups. First, while we confirmed that some improvements could be found in some patients before the age of 5 years (14 of 28), we observed that these were found mainly in the group with higher scores at baseline (5 of 10) and less frequently in those with intermediate scores (5 of 12) or at a later age (2 of 6).
Not surprisingly, the rate of progression measured as annual changes was apparently higher in the group with highest baseline HFMSE scores and lower in the patients with low scores at baseline because they had fewer points to lose.
The different progression among the 3 groups was also obvious when other clinical features such as onset of scoliosis were considered. The subgroup of weakest patients, with the lowest HFMSE scores at baseline, had the earliest progression of scoliosis, which was increasingly delayed in the subgroups with higher HFMSE scores at baseline. Similarly, the strongest patients, with the highest scores at baseline, had less need for introducing noninvasive ventilation (30%) compared to those with lower scores (66%).
Eighty percent of our patients had 3 copy numbers. Because only a few had 2 or ≥4 copies, no meaningful statistical analysis could be performed, but these patients did not appear to behave as outliers compared to the rest of the group with 3 copies.
Another limitation of this study is that, over the years, the method used to measure height changed from total segmental length in young children to arm span and, more recently, to ulnar length. The number of patients was also too small to allow a more accurate analysis of other factors such as scoliosis, weight, or height.
Our results confirm that, despite the variability in changes and the possibility of improvement in the first years of life, all patients with type II SMA show a clear and progressive decline on the long-term follow-up, regardless of their scores at baseline.
After the age of 5 years, stable positive changes were only occasionally observed. Similarly, after the age of 5 years, only 4 patients remained stable for ≥2 years, and they could not show any further decline because the score already indicated very low motor function (HFMSE score ≤2). We also demonstrated that the severity of functional impairment at baseline can help to predict the magnitude of changes over time and, more generally, the onset of scoliosis and need for noninvasive ventilation.
Further statistical analysis using more refined memories for assessing trajectories such as those recently used in Duchenne muscular dystrophy18,19 may help to further define the prognostic value of our findings.
Glossary
- CHERISH
A Study to Assess the Efficacy and Safety of Nusinersen (ISIS 396443) in Participants With Later-Onset Spinal Muscular Atrophy (SMA)
- HFMSE
Hammersmith Functional Motor Scale–Expanded
- SMA
spinal muscular atrophy
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study funding
Funded by GSP 13002 (Famiglie SMA/Telethon).
Disclosure
E. Mercuri received funding as a member of advisory boards from Roche, Biogen, and Avexis and as speaker in sponsored symposia. S. Lucibello, M. Pera, and S. Carnicella report no disclosures relevant to the manuscript. G. Coratti received funding from Biogen and Genesis Pharma as a speaker in sponsored symposia. R. de Sanctis received funding from Biogen as a speaker in sponsored symposia. S. Messina received funding as a member of advisory boards from Biogen and Avexis and as a speaker in sponsored symposia. E. Mazzone received funding from Biogen, Avexis, and Roche as a speaker in sponsored symposia. N. Forcina, L. Fanelli, G. Norcia, L. Antonaci, and A. Frongia report no disclosures relevant to the manuscript. M. Pane received funding as a member of advisory boards from Biogen and Avexis and as a speaker in sponsored symposia. Go to Neurology.org/N for full disclosures.
References
- 1.D'Amico A, Mercuri E, Tiziano FD, Bertini E. Spinal muscular atrophy. Orphanet J Rare Dis 2011;6:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkel R, Bertini E, Muntoni F, Mercuri E; ENMC SMA Workshop Study Group. 209th ENMC International Workshop: outcome measures and clinical trial readiness in spinal muscular atrophy 7-9 November 2014, Heemskerk, the Netherlands. Neuromuscul Disord 2015;25:593–602. [DOI] [PubMed] [Google Scholar]
- 3.Mercuri E, Bertini E, Iannaccone ST. Childhood spinal muscular atrophy: controversies and challenges. Lancet Neurol 2012;11:443–452. [DOI] [PubMed] [Google Scholar]
- 4.Tiziano FD, Bertini E, Messina S, et al. The Hammersmith functional score correlates with the SMN2 copy number: a multicentric study. Neuromuscul Disord 2007;17:400–403. [DOI] [PubMed] [Google Scholar]
- 5.Mercuri E, Finkel R, Montes J, et al. Patterns of disease progression in type 2 and 3 SMA: implications for clinical trials. Neuromuscul Disord 2016;26:126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufmann P, McDermott MP, Darras BT, et al. Observational study of spinal muscular atrophy type 2 and 3: functional outcomes over 1 year. Arch Neurol 2011;68:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufmann P, McDermott MP, Darras BT, et al. Prospective cohort study of spinal muscular atrophy types 2 and 3. Neurology 2012;79:1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chabanon A, Seferian AM, Daron A, et al. Prospective and longitudinal natural history study of patients with type 2 and 3 spinal muscular atrophy: baseline data NatHis-SMA study. PLoS One 2018;13:e0201004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadman RI, Wijngaarde CA, Stam M, et al. Muscle strength and motor function throughout life in a cross-sectional cohort of 180 patients with spinal muscular atrophy types 1c-4. Eur J Neurol 2018;25:512–518. [DOI] [PubMed] [Google Scholar]
- 10.O'Hagen JM, Glanzman AM, McDermott MP, et al. An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscul Disord 2007;17:693–697. [DOI] [PubMed] [Google Scholar]
- 11.Mercuri E, Finkel RS, Muntoni F, et al. Diagnosis and management of spinal muscular atrophy, part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord 2018;28:103–115. [DOI] [PubMed] [Google Scholar]
- 12.Chiriboga CA, Swoboda KJ, Darras BT, et al. Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy. Neurology 2016;86:890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med 2018;378:625–635. [DOI] [PubMed] [Google Scholar]
- 14.Pera MC, Coratti G, Forcina N, et al. Content validity and clinical meaningfulness of the HFMSE in spinal muscular atrophy. BMC Neurol 2017;17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouault F, Christie-Brown V, Broekgaarden R, et al. Disease impact on general well-being and therapeutic expectations of European type II and type III spinal muscular atrophy patients. Neuromuscul Disord 2017;27:428–438. [DOI] [PubMed] [Google Scholar]
- 16.Qian Y, McGraw S, Henne J, Jarecki J, Hobby K, Yeh WS. Understanding the experiences and needs of individuals with spinal muscular atrophy and their parents: a qualitative study. BMC Neurol 2015;15:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Main M, Kairon H, Mercuri E, Muntoni F. The Hammersmith Functional Motor Scale for children with spinal muscular atrophy: a scale to test ability and monitor progress in children with limited ambulation. Eur J Paediatr Neurol 2003;7:155–159. [DOI] [PubMed] [Google Scholar]
- 18.Mercuri E, Signorovitch JE, Swallow E, et al. Categorizing natural history trajectories of ambulatory function measured by the 6-minute walk distance in patients with Duchenne muscular dystrophy. Neuromuscul Disord 2016;26:576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goemans N, Vanden Hauwe M, Signorovitch J, Swallow E, Song J; Collaborative Trajectory Analysis Project (cTAP). Individualized prediction of changes in 6-minute walk distance for patients with Duchenne muscular dystrophy. PLoS One 2016;11:e0164684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual details of all the patients are fully shown in figures 1 and 2. Methods and the statistical plan are also fully reported in the Methods section.
Figure 1. Details of the HFMSE distribution.
Hammersmith Functional Motor Scale–Expanded (HFMSE) score distribution of the whole cohort (black circle) and longitudinal cohort (gray circle). Interpolation line represents the HFMSE progression subdivided by whole cohort (black line) and longitudinal cohort (gray line).
Figure 2. Details of progression.
(A–D) Individual details of the overall progression in all the 28 patients followed up longitudinally and in the subgroups divided according to their Hammersmith Functional Motor Scale–Expanded (HFMSE) scores at baseline.