Abstract
Objective
To develop a simple imaging rule to predict neurodevelopmental outcomes at 4.5 years in a cohort of preterm neonates with white matter injury (WMI) based on lesion location and examine whether clinical variables enhance prediction.
Methods
Sixty-eight preterm neonates born 24–32 weeks' gestation (median 27.7 weeks) were diagnosed with WMI on early brain MRI scans (median 32.3 weeks). 3D T1-weighted images of 60 neonates with 4.5-year outcomes were reformatted and aligned to the posterior commissure–eye plane and WMI was classified by location: anterior or posterior-only to the midventricle line on the reformatted axial plane. Adverse outcomes at 4.5 years were defined as Wechsler Preschool and Primary Scale of Intelligence full-scale IQ <85, cerebral palsy, or Movement Assessment Battery for Children, second edition percentile <5. The prediction of adverse outcome by WMI location, intraventricular hemorrhage (IVH), bronchopulmonary dysplasia (BPD), and retinopathy of prematurity (ROP) was assessed using multivariable logistic regression.
Results
Six children had adverse cognitive outcomes and 17 had adverse motor outcomes. WMI location predicted cognitive outcomes in 90% (area under receiver operating characteristic curve [AUC] 0.80) and motor outcomes in 85% (AUC 0.75). Adding IVH, BPD, and ROP to the model enhances the predictive strength for cognitive and motor outcomes (AUC 0.83 and 0.88, respectively). Rule performance was confirmed in an independent cohort of children with WMI.
Conclusions
WMI on early MRI can be classified by location to predict preschool age outcomes in children born preterm. The predictive value of this WMI classification is enhanced by considering clinical factors apparent by term-equivalent age.
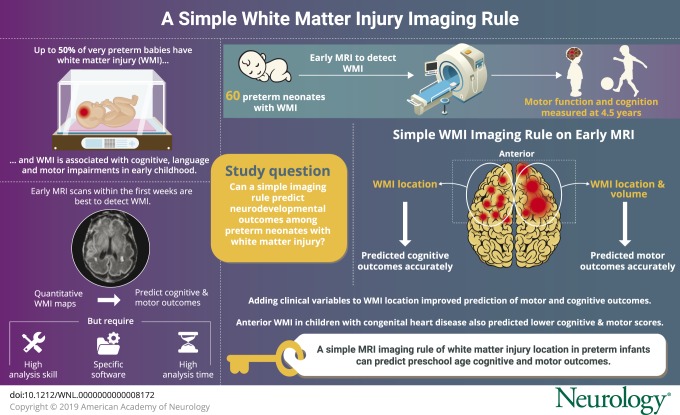
Despite advances in neonatal care, white matter injury (WMI) is still common among very preterm neonates, with reported incidence rates of up to 50% in this population.1–3 The nature of WMI has evolved over time, with a decline in the incidence of cystic periventricular leukomalacia and moderate to severe noncystic injury4 and a concomitant rise in more subtle patterns of white matter abnormalities, such as focal punctate white matter lesions and diffuse volume loss.5 Studies of the long-term effect of these patterns of injury have demonstrated associations with cognitive, language, and motor impairments in early childhood.6 The severity of punctate WMI is most apparent on MRI scans acquired in the first weeks of life, relative to MRI at term-equivalent age.7,8 Using manual segmentation and quantitative lesion mapping, we previously found that neonatal WMI lesion location and extent on early MRI in very preterm neonates predicts cognitive and motor outcomes at 18 months of age.9 These quantitative imaging maps suggest that frontal lesions are most predictive of adverse outcomes.
While quantitative WMI maps provided useful information to facilitate the identification of neonates at risk who would benefit from timely intervention or those more likely to have optimal outcomes, this approach may not be directly applicable to day-to-day clinical use. Calculation of brain lesion volumes requires skill, time, and software, limiting its accessibility to most clinicians who counsel families. Until automated methods to accurately segment WMI volume become widely accessible,10 it will remain impractical for most centers. Based on this, we aimed to develop a simple imaging rule for clinical use on early MRI scans to predict neurodevelopmental outcomes at 4.5 years in preterm neonates with WMI. We then sought to examine whether including clinical variables previously established as predictors of neurodevelopmental outcome in this population,11 together with the imaging rule, enhances prediction of adverse outcomes.
Methods
Study participants and imaging
Over a 7-year period (2006–2012), 234 very preterm neonates (122 male, 52.1%) born between 24 and 32 weeks' gestation (median 27.7 weeks) and admitted to the neonatal intensive care unit (NICU) at British Columbia's Women's Hospital, Vancouver, Canada, were enrolled prospectively. Exclusion criteria for the overall cohort consisted of large parenchymal hemorrhagic infarctions (>2 cm), congenital malformations/syndromes, or antenatal infections.
A total of 221 early (median postmenstrual age: 32.1 weeks, interquartile range [IQR] 30.4–33.9) nonsedated MRIs were acquired on a 1.5T Siemens Avanto scanner (Erlangen, Germany). Detailed imaging measures were reported previously.12 An experienced neuroradiologist blinded to the neonates' medical history assessed the images for brain injury, including WMI.13 WMI severity on each image was rated as none (0), minimal (1: ≤3 lesions of <2 mm), moderate (2: >3 lesions or lesions >2 mm and <5% hemispheric involvement), or severe (3: >5% of the hemisphere).7,13 Sixty-eight neonates were identified with WMI on their early MRI; 2 children did not survive to follow-up and 60 had follow-up at 4.5 years of age. The WMI volumes of these 60 children were measured previously; these 60 children were included in subsequent analyses.9 Infants with follow-up did not differ meaningfully from the 6 neonates not evaluated at 4.5 years (table 1).
Table 1.
Characteristics of children who were evaluated vs not evaluated at 4.5 years
Standard protocol approvals, registration, and patient consents
The Clinical Research Ethics Board at the University of British Columbia and BC Children's and Women's Hospitals reviewed and approved the study protocol. A parent or legal guardian provided written informed consent.
Clinical data collection
Detailed clinical information about the pregnancy, delivery, and perinatal course was collected through systematic detailed prospective chart reviews. In this study, clinical factors previously associated with impaired neurodevelopmental outcomes were examined including the presence of moderate to severe intraventricular hemorrhage (IVH grade 2–3; maximum severity over NICU course based on clinical ultrasound), bronchopulmonary dysplasia (BPD) (defined as the need for supplemental oxygen at 36 weeks' postmenstrual age), and retinopathy of prematurity (ROP). Maternal level of education was divided into 2 categories: “primary/secondary school,” defined as education up to and including the completion of high school, and “undergraduate/postgraduate,” which included mothers with at least 1 year of college or university studies.
Neurodevelopmental outcomes
The Wechsler Primary and Preschool Scale of Intelligence, fourth edition (WPPSI-IV) was used to evaluate cognitive function of the study participants at 4.5 years of age. The overall full-scale IQ (FSIQ) has a mean of 100 and SD of 15. FSIQ less than 85 was considered to reflect adverse cognitive outcome at 4.5 years. Motor function was evaluated with the Movement Assessment Battery for Children, second edition (MABC-2), or a neurologic examination for cerebral palsy (CP). Participants with MABC-2 scores of less than the 5th percentile or the presence of CP were considered to have adverse motor outcome at 4.5 years. Of the 66 survivors with WMI on early MRI, 4.5-year motor outcomes were available for 60 children and cognitive outcomes for 49.
Neuroimaging rule of WMI to predict outcomes
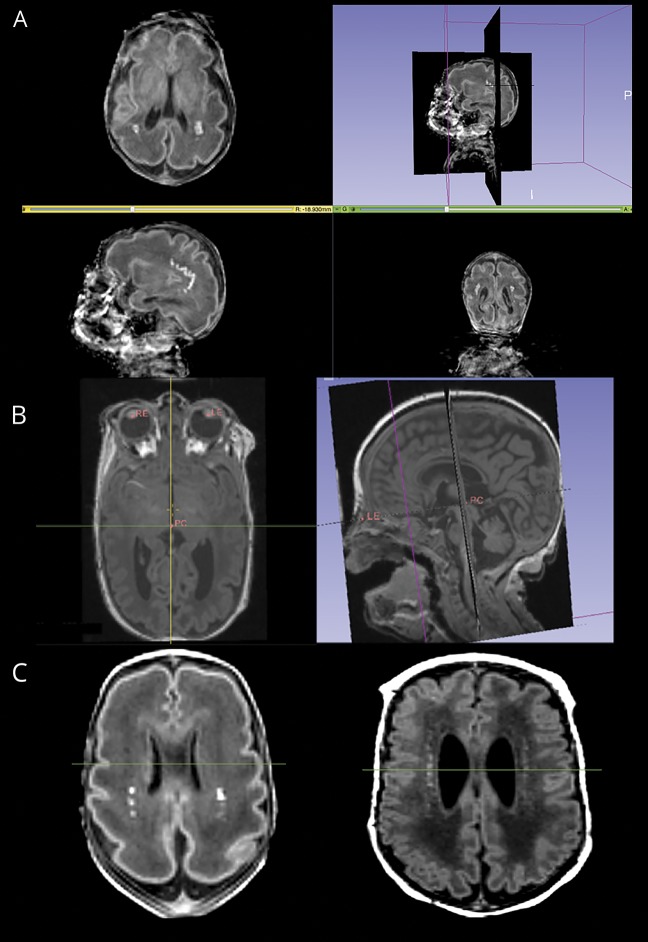
The quantitative odds ratio (OR) maps of WMI demonstrated that, rather than lesion volume, the spatial location of lesions was more predictive of outcome and adverse neurodevelopmental outcomes were associated with anterior lesions.9 In order to evaluate whether this finding can be implemented in clinical practice, we applied a simple and robust imaging rule to assist clinicians to predict the neurodevelopmental outcomes of very preterm neonates based on the location of WMI lesions on the 3D T1-weigthed MRI. Punctate WMI lesions were seen as areas of abnormal T1 hyperintensity on the early T1-weighted images. This 3-step imaging method (figure 1) was developed and tested using an open source software platform, 3DSlicer (slicer.org), which allows simultaneous display of axial, coronal, and sagittal planes, 3D visualization of the image volume, and interactive image processing and measurements. Initial alignment to the bicommissural line proved unreliable, as the anterior commissure was difficult to delineate clearly on the early-life MRI. Therefore, a more reliable plane was chosen with 3 easily identifiable landmarks. First, the posterior commissure (PC) and left and right eyes were identified and marked on the original 3D T1-weighted image. Second, the 3D T1-weighted MRI was reformatted to the PC–eye space to provide a common orientation for visualizing the brain. In the PC–eye space, the 3 landmarks were aligned on one axial plane (PC–eye plane) with the 2 landmarks of the eyes aligned on the perpendicular coronal plane. Following the image reformatting, a specific axial plane superior and parallel to the PC–eye plane was used to perform the WMI assessment. This plane was determined according to the shape of the lateral ventricles when viewing the brain on axial planes along the superior/inferior direction in the reformatted image. The plane immediately superior to the one on which the shape of the lateral ventricles changes from straight to curved form was chosen. Third, on this WMI identification plane, WMI lesion location was determined in relation to the midventricle line dividing the anterior half and the posterior half of the lateral ventricles, after measuring the length of each ventricle. The lesions were then classified into 2 groups, anterior or posterior-only, according to the location of WMI. Images with any WMI anterior to the midventricle line on this plane were considered anterior. Presence of WMI lesion anterior to the midventricle line was considered as a predictor of adverse outcome in subsequent analyses.
Figure 1. White matter injury (WMI) identification method.
(A) Bilateral punctate WMI in an unformatted image seen in the axial, sagittal, and coronal planes. (B) Identification and alignment of the posterior commissure (PC), left eye (LE), and right eye (RE) on the same plane in the axial T1-weighted image and sagittal T1-weighted image. (C) Axial images at the level immediately above the curving of the ventricles, with punctate WMI located posterior to the midventricle line in green (left) and located both anterior and posterior to the midventricle line (right).
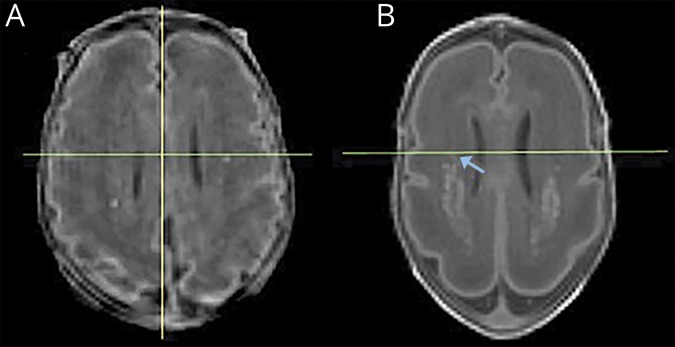
Blinded to the medical history and outcomes of each neonate, a pediatric neurologist (D.C.-R.) and a neuroimaging researcher (T.G.) applied the 3-step approach on each of the 60 early T1-weighted images and classified the images according to the criterion defined above independently. Following review of the 4 cases of disagreement by a third rater (S.P.M.), the classification of the primary rater was used. In addition, the pediatric neurologist (D.C.-R.) performed WMI identification/image classification on all 60 scans on a second occasion more than 1 month from the first session to examine intrarater reliability. To illustrate applicability of the method, representative “borderline” cases of WMI adjacent to the midventricle line are shown in figure 2.
Figure 2. Borderline cases.
Reformatted axial T1-weighted images at the level immediately above the curving of the ventricles, showing punctate white matter injury (WMI) adjacent to the midventricle line (green). The image on the left (A) was classified by both raters as posterior-only. It is important to note that although only a few punctate lesions are seen on this slice, total WMI volume for this subject was 480.69 mm3. This child had normal cognitive and motor outcomes. The image on the right (B) was a case of disagreement among raters due to the right-sided hyperintensity (arrow) abutting the midventricle line and was classified as posterior-only after measurement of ventricle length. This child had a normal cognitive and an adverse motor outcome.
Statistical analyses
Intrarater and interrater reliability on image classification results defined as with or without anterior WMI on the WMI identification plane was evaluated quantitatively using kappa and the prevalence-adjusted and bias-adjusted kappa (PABAK).14 The latter index accounts for the bias that occurs with the high or low prevalence of a given response and was calculated for each measure. PABAK scores were interpreted using established methods15 as follows: <0, less than the probability agreement; 0.01–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, good agreement; and 0.81–0.99, very good agreement.
Clinical characteristics and demographic variables were compared using Pearson χ2 test or Fisher exact test when the expected frequency was less than 5 for categorical data and the Student t test or Mann-Whitney U test for continuous data variables. Logistic regression was used when comparing a dichotomous variable with a qualitative variable with more than 2 categories.
To verify the robustness and reliability of the WMI rule in predicting neurodevelopmental outcomes, we calculated the sensitivity, specificity, positive and negative predictive values, and accuracy of the rule. In addition, the prediction value of this imaging rule in combination with total WMI volume and other clinical variables was assessed using multivariable logistic regression analysis. Statistical analysis was performed using Stata 15.0 software (StataCorp, College Station, TX).
Independent cohort validation
For the purpose of validation, we examined a cohort of 31 neonates (20 male, 64.5%) born at term (median gestational age 39.3 weeks, IQR 39.0–40.3) with congenital heart disease (CHD) and punctate WMI: 23 with transposition of the great arteries, 6 with single ventricle physiology, and 2 with interrupted aortic arch. As described previously, these neonates from 3 cardiac units had WMI with similar total WMI volumes as the preterm cohort.16 A total of 27 of these children with CHD (87.10%) underwent cognitive assessments at 4.5 years of age using WPPSI-III (5 with the German version). A uniform standardized assessment of motor outcome was not available in these children.
Data availability
Anonymized data from the current study will be made available at the request of qualified investigators if approved by our Research Ethics Board at the end of the overall study period.
Results
Clinical characteristics and outcomes of study participants
Among 60 patients who had WMI on their early MRI, 10 were classified to anterior and 50 to posterior-only groups according to the WMI location in relation to the midventricle line on the PC–eye plane. All but one patient with anterior lesions also had posterior lesions. Clinical characteristics did not differ between the neonates with anterior WMI lesions and those with posterior-only WMI, except for volume of WMI and outcomes (table 2). Neonates with anterior lesions had significantly larger WMI volumes than those with posterior-only lesions. Children with anterior WMI lesions on early MRI had both lower cognitive scores (p = 0.01) and lower motor scores (p = 0.001) (table 2). Of 49 children with WMI who underwent cognitive assessments (median FSIQ: 103, IQR 94–111) at 4.5 years' corrected age, 6 (12.2%) had FSIQ scores under 85, with 5 below 70. Of 60 children who underwent motor assessments (median MABC-2 scores: 25th percentile) at 4.5 years' corrected age, 17 (28.3%) had CP or MABC-2 scores below the 5th percentile. Of those, 12 had CP: 5 with spastic hemiplegia, 3 with spastic diplegia, 1 with spastic triplegia, 1 with spastic quadriplegia, and 2 with mixed forms; an additional 5 children had MABC-2 scores under the 5th percentile, in the absence of CP and in the presence of normal intellectual abilities. Of note, among the children diagnosed with CP, 3 had normal intelligence, 5 had intellectual impairment, and 4 were not evaluated with the WPPSI-IV. One child with spastic hemiplegic CP had a normal MABC-2 percentile score (25th percentile).
Table 2.
Characteristics of the cohort classified according to lesion location
Reliability of the simple imaging rule in WMI identification/image classification
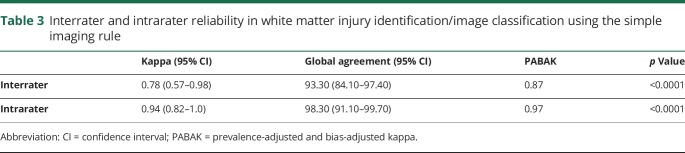
As shown in table 3, the interrater agreement of the imaging rule was good and improved to very good when optimized with the PABAK to account for the small number of scans with anterior lesions. The intrarater agreement was very good.
Table 3.
Interrater and intrarater reliability in white matter injury identification/image classification using the simple imaging rule
Predicting cognitive outcomes at 4.5 years
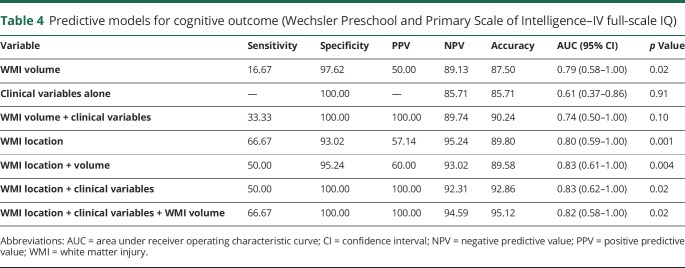
Greater accuracy of predicting cognitive outcomes at 4.5 years was achieved using the simple imaging rule of WMI location in comparison to the methods based on WMI volume alone, clinical variables alone, or the combination of WMI volume and clinical variables. WMI volume had limited value when added to location of injury in predicting cognitive outcomes (table 4).
Table 4.
Predictive models for cognitive outcome (Wechsler Preschool and Primary Scale of Intelligence–IV full-scale IQ)
Adding clinical variables apparent by term-equivalent age that were previously established as predictors of adverse outcome11 improved the positive predictive value of the simple WMI location imaging rule (table 4). These clinical variables alone were not significant predictors of outcomes at 4.5 years of age (p = 0.91).
Predicting motor outcomes at 4.5 years
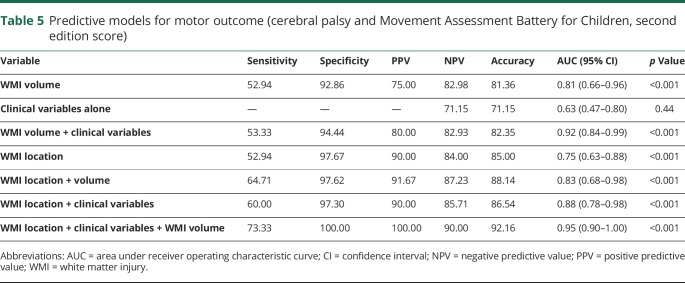
Both volume and location of WMI were significantly predictive of adverse motor outcomes at 4.5 years of age for very preterm neonates (table 5). With a positive predictive value of 90% and a negative predictive value of 84%, the simple imaging rule of using WMI location alone predicted motor outcomes. Although employing only the clinical variables was not significant in predicting motor outcomes (p = 0.44), adding them to the rule of WMI location improved prediction (table 5). Using the combined information of WMI location and clinical variables after adjusting for WMI volume allowed the most accurate prediction of motor outcomes (table 5).
Table 5.
Predictive models for motor outcome (cerebral palsy and Movement Assessment Battery for Children, second edition score)
Further adjustment of these models for gestational age at birth and maternal level of education did not meaningfully alter the area under receiver operating characteristic curve (AUC) of the models for cognitive or motor outcomes.
Comparison with other scores
Using the severity score previously established to predict neurodevelopmental outcomes at 18 months of age,13 where severe WMI is defined qualitatively as over 5% of the hemisphere involved, a score of severe WMI had similar prediction as location for motor outcomes (AUC 0.74, 95% confidence interval [CI] 0.62–0.87, p < 0.001) but was inferior for cognitive outcomes (AUC 0.69, 95% CI, 0.47–0.92, p = 0.04).
Validation
When we applied the anterior location rule to a cohort of infants with CHD and WMI, as previously reported,16 it was predictive of cognitive outcomes (FSIQ <85) with an AUC of 0.74 (95% CI 0.52–0.96, p = 0.046).
Discussion
In this prospective cohort of very preterm neonates with WMI, we applied a simple imaging rule to predict neurodevelopmental outcomes based on location of WMI lesions. Lesions anterior to the midventricle line on reformatted images were predictive of impaired cognitive and motor outcomes at preschool age. In this study, WMI location was a more accurate predictor of preschool cognitive outcome than WMI volume and was equally predictive as volume for motor outcomes. Adding the clinical variables of BPD, ROP, and IVH to the model enhanced its predictive strength for both motor and cognitive outcomes.
The higher rate of adverse cognitive outcomes among children with anterior lesions may be explained by altered connectivity in frontal lobe areas responsible for learning, memory, and cognition, as demonstrated in studies of functional MRI,17,18 magnetoencephalography,19 and tractography20 among preterm children. However, it may also reflect the severity of WMI. All but one of the patients with anterior WMI had additional posteriorly located lesions, suggesting that anterior location is a marker of severity of WMI. This is reinforced by the fact that those with anterior lesions have a higher overall volume of total WMI compared with those with posterior injury alone. Notably, in multivariable analysis, it is lesion location and not volume that is a better predictor of cognitive scores, while both were equally predictive of motor outcomes. The distribution of lesions is consistent with the maturation of the oligodendrocyte lineage with central regions being most vulnerable, followed by posterior and then anterior brain regions.9
White matter abnormalities, including punctate lesions, seen on term-equivalent MRI have been associated with short and long-term adverse neurodevelopmental outcomes in several studies,21–23 with the number of lesions negatively correlated with outcome. Clinically accessible imaging scoring systems that also include extent of WMI as a component of a global score have been developed for MRI at term-equivalent age24–26 and applied to early in life scans,27 however, none take into account location of the lesions. Kersbergen et al.28 analyzed patterns of punctate WMI and their sequential evolution on early-life and term equivalent age scans, classifying them according to their position in relation to the lateral ventricles, and found no significant association with outcomes at 15 months of age. However, in the present study, location classification is based on OR maps previously shown to be predictive of outcomes.9
Adding clinical variables to the prediction model for adverse cognitive outcome modestly augmented the predictive value of the WMI location rule. The lack of sizable improvement with the addition of these factors may be explained by the effect of these factors on neurodevelopment via white matter pathways. This suggestion is supported by studies linking IVH,29 BPD,30 and ROP31 to impaired white matter development, tempering their effect after WMI has been taken into account. Given the ready availability of these clinical variables and the importance of prognosis conversations, even the modest enhancement is worth pursuing.
Consistent with other studies, we have found an increased rate of motor abnormalities, in the absence of CP and intellectual disability, among children with WMI.25,32 A range of motor impairments from developmental coordination disorder to CP has been described in the literature; however, these studies examined WMI seen on MRI at term-equivalent age. The present study shows the predictive value of WMI seen on the early-life MRI, when focal WMI is most easily visible as T1 hyperintense lesions and reinforces the importance of sequential imaging.8,33
The value of early MRIs in predicting motor outcomes has recently been studied. In a meta-analysis and systematic review, George et al.34 found that global scores on early MRI had a high sensitivity for predicting CP and adverse motor outcomes but that white matter scores were less sensitive. Punctate white matter lesions were not associated with motor outcome in some studies.2,35 This systematic review examined adverse outcomes defined by the presence of CP or Bayley motor scores that were 1 or 2 SDs under the mean. The present study uses MABC-2 percentile scores, shown to be predictive of motor functioning in preterm-born children up to middle childhood36 and often used to help diagnose developmental coordination disorder.
Our finding of an almost equal number of children with adverse motor outcomes in the anterior and posterior-only groups may reflect the high predictive value of WMI volume alone for motor outcomes and the highest predictive accuracy when location, volume, and clinical factors are considered together. This study also reaffirmed the association between preschool motor impairment with BPD and severe ROP found in prior studies.37,38
A primary strength of this study is the simplicity of applying the rule. Although the images need to be reformatted, the software is free and the graphical interface is user-friendly. The anatomical landmarks (posterior commissure and eyes) are easy to locate, making it practical for clinicians. Furthermore, alignment to the PC–eye plane and lesion location classification can potentially be automated and applied during image acquisition, making the prediction strategy standardized for interpretation. The WMI location rule is robust across cohorts and remains accurate when assessing frontal WMI among infants with WMI and CHD, another population at high risk of this pattern of brain injury. Another strength is the preschool age outcomes, when standardized cognitive tests are more reliable and tend to remain stable, reducing the risk of bias in classifying adverse outcomes. Some higher cognitive skills will only emerge at school age and more subtle behavioral issues may only appear later, making longer-term follow-up necessary to examine the full spectrum of neurodevelopmental outcomes impacted by WMI. Regarding motor outcomes, developmental coordination disorder and other subtle motor abnormalities can be diagnosed at the preschool-age assessment and long-term functional motor impairments found at this age tend to last until adolescence.39 Children with poor motor outcomes not explained by CP, even in the absence of intellectual disability, need to be better characterized through longer-term follow-up.
Given our sample size of children with adverse outcome, there is overlap in the area under the curve measures of prediction and we are cautious not to dismiss clinical measures as important predictors of neurodevelopmental outcome in this population. A limitation of this study is the use of standardized test scores, which do not necessarily reflect children's functioning in their individual environments. However, the predictive values can be helpful to identify children at risk of an adverse outcome for referral to early intervention and rehabilitation services. Although several studies looking at the predictive value of MRI apply more global scores to predict outcomes, the aim of this study was to make a clinically accessible tool for clinicians to use when treating and counseling children with WMI. For early-life counseling, prior to the appearance of BPD and ROP, our WMI location rule may ostensibly be used alone. By term-equivalent age, when additional clinical variables are apparent, the imaging findings may provide more accurate prognoses when synthesized with clinical measures.
Classifying WMI by lesion location, anterior or posterior-only to the midventricle line, can predict preschool age cognitive and motor outcomes among preterm children. This rule can be easily applied by clinicians when evaluating WMI identified on early MRI in babies born preterm and is potentially amenable to automation, allowing for more accurate classification of WMI volume and location in the future. Importantly, findings from MRI scans can be integrated with clinical information from the child's NICU course. Considering clinical variables that appear by term-equivalent age may enhance the prediction of neurodevelopmental outcome when considered with imaging findings. Our findings provide an opportunity for clinicians to reassure families whose children have WMI when a favorable outcome is likely.
Acknowledgment
The authors thank the families that participated in this research, Dr. Shabnam Peyvandi for contributing data on children with congenital heart disease, Janet Rigney for data collection, and Cecil Chau for database development.
Glossary
- AUC
area under receiver operating characteristic curve
- BPD
bronchopulmonary dysplasia
- CHD
congenital heart disease
- CI
confidence interval
- CP
cerebral palsy
- FSIQ
full-scale IQ
- IQR
interquartile range
- IVH
intraventricular hemorrhage
- MABC-2
Movement Assessment Battery for Children, second edition
- NICU
neonatal intensive care unit
- OR
odds ratio
- PABAK
prevalence-adjusted and bias-adjusted kappa
- PC
posterior commissure
- ROP
retinopathy of prematurity
- WMI
white matter injury
- WPPSI-IV
Wechsler Primary and Preschool Scale of Intelligence, fourth edition
Appendix. Authors

Footnotes
Editorial, page 569
Study funding
Study funded by the Canadian Institutes of Health Research operating grants MOP-79262 (S.P.M.), MOP-93780 (S.P.M.), and MOP-86489 (R.E.G.) and NIH NINDS P01 NS082330 (P.M.) and grants from the Kids Brain Health Network (S.P.M.), a Networks of Centres of Excellence of Canada, the Mӓxi Foundation (B.L.), and the Ted Arison Family Foundation (D.C.-R.). S.P.M. supported by the Bloorview Children's Hospital Chair in Paediatric Neuroscience.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed 2008;93:F153–F161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dyet LE, Kennea N, Counsell SJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics 2006;118:536–548. [DOI] [PubMed] [Google Scholar]
- 3.Volpe JJ. Cerebral white matter injury of the premature infant: more common than you think. Pediatrics 2003;112:176–180. [DOI] [PubMed] [Google Scholar]
- 4.Gano D, Andersen SK, Partridge JC, et al. Diminished white matter injury over time in a cohort of premature newborns. J Pediatr 2015;166:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev 2006;12:129–140. [DOI] [PubMed] [Google Scholar]
- 6.Shah DK, Doyle LW, Anderson PJ, Bear AJ, Hunt RW, Inder TE. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr 2008;153:170-e1–175-e171. [DOI] [PubMed] [Google Scholar]
- 7.Miller SP, Ferriero DM, Leonard C, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr 2005;147:609–616. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Biarge M, Groenendaal F, Kersbergen KJ, et al. MRI based preterm white matter injury classification: the importance of sequential imaging in determining severity of injury. PLoS One 2016;11:e0156245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo T, Duerden EG, Adams E, et al. Quantitative assessment of white matter injury in preterm neonates: association with outcomes. Neurology 2017;88:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukherjee S, Cheng I, Miller S, Guo T, Chau V, Basu A. A fast segmentation-free fully automated approach to white matter injury detection in preterm infants. Med Biol Eng Comput 2019;57:71–87. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt B, Roberts RS, Davis PG, et al. Prediction of late death or disability at age 5 years using a count of 3 neonatal morbidities in very low birth weight infants. J Pediatr 2015;167:982-e2. [DOI] [PubMed] [Google Scholar]
- 12.Chau V, Poskitt KJ, McFadden DE, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol 2009;66:155–164. [DOI] [PubMed] [Google Scholar]
- 13.Chau V, Synnes A, Grunau RE, Poskitt KJ, Brant R, Miller SP. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology 2013;81:2082–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrt T, Bishop J, Carlin JB. Bias, prevalence and kappa. J Clin Epidemiol 1993;46:423–429. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Faris P, Hemmelgarn B, Walker RL, Quan H. Measuring agreement of administrative data with chart data using prevalence unadjusted and adjusted kappa. BMC Med Res Methodol 2009;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo T, Chau V, Peyvandi S, et al. White matter injury in term neonates with congenital heart diseases: topology & comparison with preterm newborns. Neuroimage 2018;185:742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smyser CD, Snyder AZ, Shimony JS, Blazey TM, Inder TE, Neil JJ. Effects of white matter injury on resting state fMRI measures in prematurely born infants. PLoS One 2013;8:e68098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He L, Parikh NA. Aberrant executive and frontoparietal functional connectivity in very preterm infants with diffuse white matter abnormalities. Pediatr Neurol 2015;53:330–337. [DOI] [PubMed] [Google Scholar]
- 19.Moiseev A, Doesburg SM, Herdman AT, Ribary U, Grunau RE. Altered network oscillations and functional connectivity dynamics in children born very preterm. Brain Topogr 2015;28:726–745. [DOI] [PubMed] [Google Scholar]
- 20.Batalle D, Hughes EJ, Zhang H, et al. Early development of structural networks and the impact of prematurity on brain connectivity. Neuroimage 2017;149:379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Bruïne FT, van den Berg-Huysmans AA, Leijser LM, Rijken SJ, van der Grond J, van Wezel-Meijler G. Clinical implications of MR imaging findings in the white matter in very preterm infants: a 2-year follow-up study. Radiology 2011;261:899–906. [DOI] [PubMed] [Google Scholar]
- 22.Tusor N, Benders MJ, Counsell SJ, et al. Punctate white matter lesions associated with altered brain development and adverse motor outcome in preterm infants. Sci Rep 2017;7:13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodward LJ, Clark CA, Bora S, Inder TE. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS One 2012;7:e51879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol 2013;34:2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 2006;355:685–694. [DOI] [PubMed] [Google Scholar]
- 26.Hintz SR, Barnes PD, Bulas D, et al. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics 2015;135:e32-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George JM, Fiori S, Fripp J, et al. Validation of an MRI brain injury and growth scoring system in very preterm infants scanned at 29- to 35-week postmenstrual age. AJNR Am J Neuroradiol 2017;38:1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kersbergen KJ, Benders MJ, Groenendaal F, Koopman-Esseboom RA, van Haastert IC, de Vries LS. Different patterns of punctate white matter lesions in serially scanned preterm infants. PLoS One 2014;9:e108904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ou X, Glasier CM, Ramakrishnaiah RH, et al. Impaired white matter development in extremely low-birth-weight infants with previous brain hemorrhage. AJNR Am J Neuroradiol 2014;35:1983–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anjari M, Counsell SJ, Srinivasan L, Allsop JV, Rutherford MA, Edwards AD. The association of lung disease with cerebral white matter abnormalities in preterm infants. Pediatrics 2009;124:268–276. [DOI] [PubMed] [Google Scholar]
- 31.Glass TJA, Chau V, Gardiner J, et al. Severe retinopathy of prematurity predicts delayed white matter maturation and poorer neurodevelopment. Arch Dis Child Fetal Neonatal Ed 2017;102:F532–F537. [DOI] [PubMed] [Google Scholar]
- 32.Spittle AJ, Cheong J, Doyle LW, et al. Neonatal white matter abnormality predicts childhood motor impairment in very preterm children. Dev Med Child Neurol 2011;53:1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vries LS, Volpe JJ. Value of sequential MRI in preterm infants. Neurology 2013;81:2062–2063. [DOI] [PubMed] [Google Scholar]
- 34.George JM, Pannek K, Rose SE, Ware RS, Colditz PB, Boyd RN. Diagnostic accuracy of early magnetic resonance imaging to determine motor outcomes in infants born preterm: a systematic review and meta-analysis. Dev Med Child Neurol 2018;60:134–146. [DOI] [PubMed] [Google Scholar]
- 35.Cornette LG, Tanner SF, Ramenghi LA, et al. Magnetic resonance imaging of the infant brain: anatomical characteristics and clinical significance of punctate lesions. Arch Dis Child Fetal Neonatal Ed 2002;86:F171–F177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffiths A, Morgan P, Anderson PJ, Doyle LW, Lee KJ, Spittle AJ. Predictive value of the movement assessment Battery for children, second edition at 4 years, for motor impairment at 8 years in children born preterm. Dev Med Child Neurol 2017;59:490–496. [DOI] [PubMed] [Google Scholar]
- 37.Zwicker JG, Yoon SW, Mackay M, Petrie-Thomas J, Rogers M, Synnes AR. Perinatal and neonatal predictors of developmental coordination disorder in very low birthweight children. Arch Dis Child 2013;98:118–122. [DOI] [PubMed] [Google Scholar]
- 38.Goyen TA, Lui K. Developmental coordination disorder in "apparently normal" schoolchildren born extremely preterm. Arch Dis Child 2009;94:298–302. [DOI] [PubMed] [Google Scholar]
- 39.Cantell MH, Smyth MM, Ahonen TP. Two distinct pathways for developmental coordination disorder: persistence and resolution. Hum Mov Sci 2003;22:413–431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data from the current study will be made available at the request of qualified investigators if approved by our Research Ethics Board at the end of the overall study period.