Abstract
Objectives
To assess whether plasma biomarkers of oxidative stress predict diffusion-perfusion mismatch in patients with acute ischemic stroke (AIS).
Methods
We measured plasma levels of oxidative stress biomarkers such as F2-isoprostanes (F2-isoPs), total and perchloric acid Oxygen Radical Absorbance Capacity (ORACTOT and ORACPCA), urinary levels of 8-oxo-7,8-dihydro-2′-deoxyguoanosine, and inflammatory and tissue-damage biomarkers (high-sensitivity C-reactive protein, matrix metalloproteinase-2 and -9) in a prospective study of patients with AIS presenting within 9 hours of symptom onset. Diffusion-weighted (DWI) and perfusion-weighted (PWI) MRI sequences were analyzed with a semiautomated volumetric method. Mismatch was defined as baseline mean transit time volume minus DWI volume. A percent mismatch cutoff of >20% was considered clinically significant. A stricter definition of mismatch was also used. Mismatch salvage was the region free of overlap by final infarction.
Results
Mismatch >20% was present in 153 of 216 (70.8%) patients (mean [±SD] age 69.2 ± 14.3 years, 41.2% women). Patients with mismatch >20% were more likely to have higher baseline plasma levels of ORACPCA (p = 0.020) and F2-isoPs (p = 0.145). Multivariate binary logistic regression demonstrated that lnF2-isoP (odds ratio [OR] 2.44, 95% confidence interval [CI] 1.19–4.98, p = 0.014) and lnORACPCA (OR 4.18, 95% CI 1.41–12.41, p = 0.010) were independent predictors of >20% PWI-DWI mismatch and the stricter mismatch definition, respectively. lnORACTOT significantly predicted mismatch salvage volume (>20% mismatch p = 0.010, stricter mismatch definition p = 0.003).
Conclusions
Elevated hyperacute plasma levels of F2-isoP and ORAC are associated with radiographic evidence of mismatch and mismatch salvage in patients with AIS. If validated, these findings may add to our understanding of the role of oxidative stress in cerebral tissue fate during acute ischemia.
Ischemic stroke is a leading cause of mortality and disability in United States.1 Thrombolytic therapy with IV tissue-type plasminogen activator (tPA) and, more recently, endovascular treatment showed consistent efficacy in acute ischemic stroke (AIS) also outside the clinical trials.2–4 The time window for acute reperfusion/recanalization therapies is narrow, representing a therapeutic challenge to stroke physicians in that many patients with acute stroke are not eligible for these treatments because of delayed presentation.3 The concept of ischemic penumbra provides hope in this regard. The ischemic penumbra is an area characterized by extremely dynamic biochemical processes occurring during the acute phase of cerebral ischemia and not yet irreversibly impaired, which can be generally detected by appropriate methods.5 Timely identification of this brain tissue at risk for infarction might allow the selection of patients who are most likely to benefit from reperfusion/revascularization treatments even beyond the current therapeutic time window.6
Advanced neuroimaging techniques such as the MRI sequences of diffusion (diffusion-weighted imaging [DWI]) and perfusion (perfusion-weighted imaging [PWI]) have been used to identify a mismatch between the regions of abnormalities. This imaging mismatch has been used as a surrogate marker of the ischemic penumbra,6 although specific radiologic criteria defining mismatch have not yet been standardized.6–8 However, these imaging techniques are not widely available in the emergency setting. Hence, more practical, less expensive, and time-efficient biomarkers of salvageable brain tissue within the first hours of symptom onset could become very helpful for clinical decision-making.
There is much evidence to support a major role for oxidative stress in the pathogenesis of ischemic and reperfusion-related brain injury.9 Such injury is mediated through free radicals and lipid peroxidation.10,11 In experimental models of cerebral ischemia, decreases of enzymatic and nonenzymatic antioxidants12,13 and, in general, of the total plasma antioxidant capacity14 have been found. It might be expected that increased oxidative stress will correlate with larger infarct volumes and more severe neurologic impairment.15
However, there are limited data on oxidative stress biomarkers in the hyperacute phase of AIS in humans. F2-isoprostanes (F2-isoPs) are products of noncyclooxygenase free radical–induced neuronal arachidonic acid peroxidation of membrane phospholipids and lipoproteins.16 In humans, they are detectable in plasma, urine, and CSF. We previously reported an increase of plasma levels of F2-isoPs in patients with AIS, particularly in the first 8 hours, but not at 24 hours and later time points, suggesting early oxidative stress activation after stroke onset.17 Moreover, we found that elevated hyperacute plasma F2-isoP concentrations independently predict the occurrence of infarct growth and infarct growth volume in patients with AIS enrolled within 9 hours of symptom onset.18 Because mitochondrial DNA is more susceptible to oxidative stress than nuclear DNA, biomarkers of oxidative DNA damage in brain mitochondrial DNA such as 8-oxo-7,8-dihydro-2′-deoxyguoanosine (8-OHdG) have been studied in an exploratory manner.19,20 Recently, assays evaluating the total plasma antioxidant capacity have been developed as further methods for studying oxidative stress. The Oxygen Radical Absorbance Capacity (ORAC) assay is one of the most commonly used.21,22
Because oxidative stress is one of the earliest responses after an acute cerebral ischemic injury, its markers might increase before irreversible energy failure and cell death occur. Therefore, such biomarkers might indicate the presence of salvageable brain tissue. Hence, in this study, we sought to evaluate whether plasma biomarkers of oxidative stress predict perfusion-diffusion mismatch in patients with AIS.
Methods
Patients
This study is a retrospective analysis of data collected prospectively. Over a period of 3 years, we prospectively measured the plasma levels of oxidative stress biomarkers (F2-isoP and ORAC), inflammatory and tissue damage biomarkers (high-sensitivity C-reactive protein [hs-CRP] and matrix metalloproteinase [MMP] -2 and -9), and urinary levels of 8-OHdG in a prospective study of patients with AIS who were ≥18 years of age and presented within 9 hours of stroke symptom onset in 2 large academic centers (Massachusetts General Hospital and Brigham and Women's Hospital). If unwitnessed or unknown, stroke onset was defined as the midway point between the last seen well and first seen abnormal times. Patients with any degree of neurologic severity (NIH Stroke Scale [NIHSS] score ≥1) were eligible. Patients with intracranial hemorrhage, stroke believed to be due to vasculitis, endocarditis or venous infarction, or TIA and stroke mimics were excluded from the cohort, as were patients with active infection (body temperature >38°C or white blood cell count >15 th/mm3), systemic inflammatory disorders, dialysis-dependent renal failure or end-stage hepatic dysfunction, active metastatic malignancy at the time of stroke, or history of stroke, myocardial infarction, major thromboembolic event, or surgery within 30 days of the index stroke onset.
Data collection
Demographic and clinical data were collected. Stroke severity was assessed with the NIHSS at baseline and 48 hours. Relevant baseline and follow-up neuroimaging data were also recorded.
Stroke subtypes were assigned by stroke neurologists based on the Causative Classification of Stroke System criteria.23,24 Follow-up assessment was performed at 3 months with the modified Rankin Scale (mRS) score and Barthel Index assessed by staff certified in administering in-person or by-telephone interview of the patient or caregiver.25
Laboratory methods
All patients underwent venous blood sampling at admission (<9 hours after stroke onset). For logistical reasons and to avoid delays in patients eligible for IV thrombolytic treatment, all blood samples were obtained after the treatment was initiated. A butterfly needle was used to reduce endothelial membrane shear stress. Biomarker levels were analyzed by investigators blinded to clinical information.
To assay F2-isoPs, plasma samples were drawn in a 10-mL ethylenediaminetetraacetic acid tube. Plasma was frozen at −80°C before processing. F2-isoPs were quantified with an 8-Isoprostane Enzyme Immunoassay Kit (Cayman Chemical, Ann Arbor, MI) (intra-assay and interassay variability 7.2 and 15.5, respectively) (see e-methods available from Dryad, doi.org/10.5061/dryad.4j07c14, for details on F2-isoP stability).
The ORAC assay reflects the capacity of compounds in the sample to retard the free radical–induced loss of fluorescence of the protein probe phycoerythrin.21,22 The ORAC was quantified according to methods previously described.26 Total ORAC (ORACTOT) represents the antioxidant capacity of plasma; precipitation of plasma proteins by 0.5 M perchloric acid (ORACPCA) in a 1:1 ratio with the sample reflects the antioxidant capacity of the remaining small-molecular-weight compounds (intra-assay and interassay variability: ORACTOT 3.0 and 7.3, ORACPCA 4.1 and 9.8, respectively). The tubes were kept on ice until centrifugation and wrapped in foil to protect them from light. Samples were then centrifuged at 13,000 rpm with a microplate centrifuge for 15 minutes and frozen at −80°C before processing.
Laboratory methods used for measuring 8-OHdG, MMPs, and hs-CRP are reported in the supplemental material (available from Dryad, doi.org/10.5061/dryad.4j07c14).
Neuroimaging analysis
CT or MRI was performed on admission and at follow-up as part of the routine clinical stroke workup and on the basis of clinical need. In this analysis, we included only patients with both DWI and PWI (cerebral blood flow, cerebral blood volume, and mean transit time [MTT])27 MRI, mostly performed approximately at the time of plasma sampling at admission (in any case, within 24 hours of stroke onset) to assess baseline infarct volume, mismatch volume, and percentage mismatch.6 PWI deficit was measured with MTT sequences. DWI or CT scans performed at 48 hours (with a range of 24–96 hours) from symptom onset were considered to assess final infarct size and mismatch salvage volume because both can reliably and consistently define infarct limits 24 hours after stroke onset.28,29
Mismatch volume was defined as baseline MTT volume minus baseline DWI volume (DWIV).5 Percentage mismatch volume was calculated by using the formula ([mismatch volume/baseline DWIV] × 100). A percentage mismatch cutoff of >20% was primarily used because this is the most commonly used cutoff in clinical practice and in most therapeutic trials.30–32 In secondary analyses, we also used stricter mismatch definitions, which exclude too small DWI lesions and PWI deficit and were found to be associated with improved clinical outcomes such as those adopted in the Diffusion and perfusion imaging Evaluation for Understanding Stroke Evolution (DEFUSE) and Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) trials (PWI-DWI ≥ 10 cm3, PWI ≥ 10 cm3).29,33 Mismatch salvaged volume was calculated for those patients with DWI and PWI MRI at follow-up according to the following formula: (mismatch volume − final infarct volume). Mismatch salvaged percentage was calculated as follows: ([mismatch salvage volume/mismatch volume] × 100).
Evidence of large vessel occlusion was another neuroimaging characteristic that was included in the analysis. All lesion volumes were corrected for differences in overall brain size with midsagittal cross-sectional intracranial area used as a surrogate measure of the intracranial volume.34 All volumetric data were collected with a validated semiautomated protocol.35 Imaging analyses were performed by trained readers blinded to clinical data, including biomarker values. The intraclass correlation coefficient for the volumetric lesion analysis was 0.99 for both DWIV and final infarct volume.36
Standard protocol approvals, registrations and patient consents
All patients or their health care proxies provided informed consent. The study was approved by our Institutional Review Board.
Statistical analysis
Continuous variables were expressed as medians (interquartile range [IQR]) except for age, systolic blood pressure (SBP), diastolic blood pressure (DBP), and white blood cell count, which were expressed as mean ± SD. Categorical variables were expressed as counts, and proportions were calculated by dividing the number of events by the total number of patients, excluding missing or unknown cases.
The Student t test or Mann-Whitney U test and χ2 or Fisher exact test were used for univariate analyses, as appropriate. Correlation coefficients (Spearman ρ) were derived to quantify the association between biomarkers and mismatch salvage volume. Natural logarithm transformation of continuous variables was used if appropriate in case of nonnormal distribution such as for the biomarkers.
Multivariate binary logistic regression and linear regression modeling was used to adjust for the effects of potential confounders and to evaluate whether plasma biomarkers measured within 9 hours of symptom onset independently predicted mismatch (both >20% mismatch definition as primary analysis and the stricter mismatch definition as secondary analysis) used as both a dichotomous and a continuous variable. Area under the curve (AUC) and cutoff levels of biomarkers with corresponding sensitivity and specificity were also obtained for both definitions of mismatch. As further secondary analysis, a linear regression multivariate analysis for mismatch salvage volume was performed by selecting either patients with >20% mismatch or those with the stricter mismatch definition. Covariates with a univariate association with mismatch variables at a value of p ≤ 0.15 were included in the multivariate models, plus other potential predictors of mismatch such as age and NIHSS score. However, a more conservative approach was also used by including in the multivariate models either the variables with a univariate nominal p value (p < 0.05) or those with a univariate value of p ≤ 0.10, i.e., closer to the nominal p value. Because in patients treated with thrombolysis blood draws were performed after the therapy was given, treatment with tPA was included in the models as a confounder for the association between biomarkers and mismatch. Furthermore, we tested in the resultant multivariate models all of the biomarkers independently of their univariate significant association with mismatch and one by one to avoid model overfitting and spurious associations. Because only a subgroup of patients had MRI and blood samples, the inverse propensity score method was used to adjust for this potential selection bias. Statistical significance level was set at p < 0.05 for all analyses. All statistical analyses were performed with SPSS statistical package (SPSS 22 Inc., Chicago, IL).
Data availability
Anonymized individual participant data and the study protocol will be shared with qualified parties on request to the corresponding author.
Results
Overall, we enrolled 525 patients in 2 large academic hospitals. Of these, 489 patients had a definite diagnosis of ischemic stroke. Both biomarkers and a baseline DWI/PWI study were available in 216 (44.2%) patients (figure). Compared to these, the remaining 273 patients without both biomarkers and baseline DWI/PWI MRI not included in the analysis differed for a lower proportion of whites (89.7% vs 94.9%, p = 0.035) and alcohol consumers (35.5% vs 50.7%, p = 0.001), lower functional independence (mRS score 0–1) before the index stroke (77.4% vs 87.4%, p = 0.005), and smaller baseline DWI lesion volume (median 4.3 vs 8.5 cm3, p = 0.002). They were more likely to have a history of diabetes mellitus (26.8% vs 18.5%, p = 0.030), lacunar stroke (9.5% vs 4.2%, p = 0.023) more than atherothrombotic stroke (13.6% vs 21.3%, p = 0.024), and higher median baseline plasma levels of hs-CRP (4.3 vs 3.4 mg/L, p = 0.033) and ORACPCA (1,700.4 vs 1,415.3 μmol troloxequivalents (TE)/L, p < 0.001).
Figure. Flowchart of patient selection.
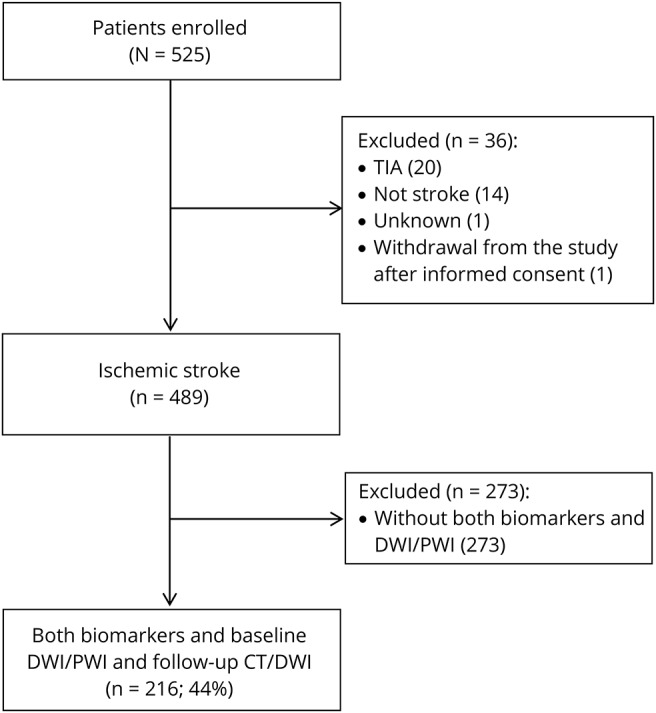
From January 2007 to April 2010, 525 patients were enrolled in 2 large academic hospitals. Of these, 489 patients had a definite diagnosis of ischemic stroke. Both biomarkers and a baseline diffusion-weighted imaging (DWI)/perfusion-weighted imaging (PWI) study were available in 216 (44%) patients.
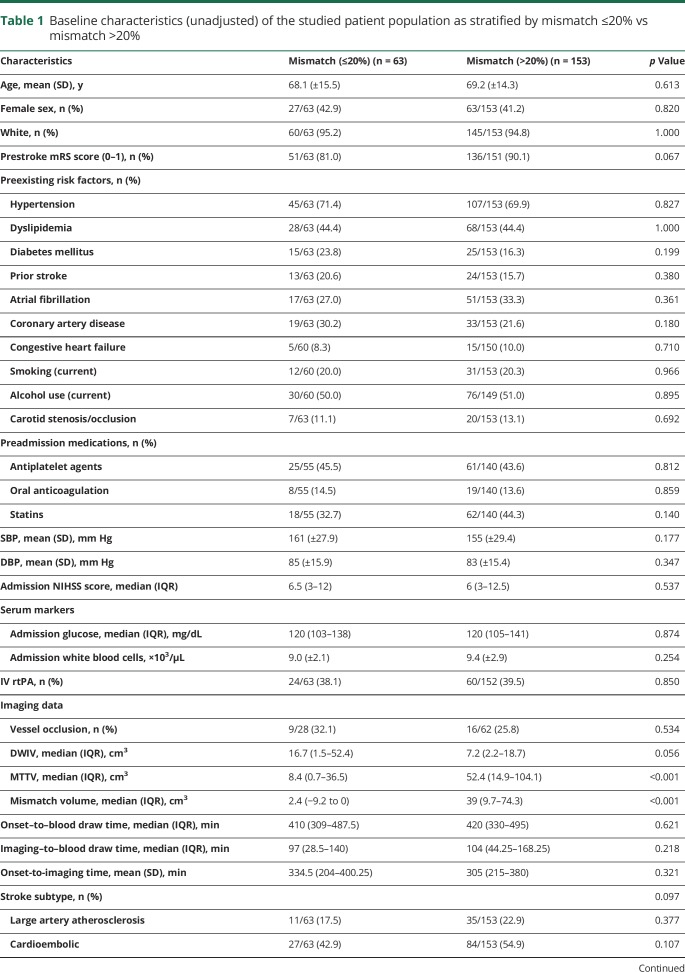
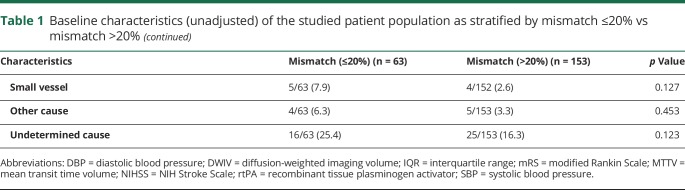
Mismatch of >20% was found in 153 of 216 (70.8%) patients (mean [±SD] age 69 ± 14.3 years, 41.2% women). Mismatch using a stricter definition was found in 116 of 216 (53.7%). Baseline clinical and imaging characteristics and univariate associations with mismatch are reported in table 1. Approximately 39% of patients received IV thrombolysis, and only 1 patient was also treated with thrombectomy. Table 2 shows the distribution of biomarker levels based on mismatch percentage. Compared with patients with mismatch ≤20%, those with >20% mismatch had significantly higher median levels of baseline ORACPCA (1,473.5 vs 1,330.4, p = 0.020) and a trend toward higher median levels of baseline F2-isoPs, although nominal statistical significance was not reached (57.7 vs 52.4 pg/mL, p = 0.145, Mann-Whitney U test) (e-results and table e-1 available from Dryad, doi.org/10.5061/dryad.4j07c14, provide further details on univariate analysis).
Table 1.
Baseline characteristics (unadjusted) of the studied patient population as stratified by mismatch ≤20% vs mismatch >20%
Table 2.
Baseline plasma biomarkers of the studied patient population as stratified by mismatch ≤20% vs mismatch >20%
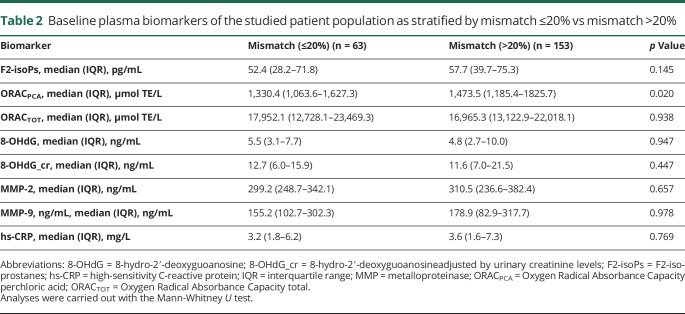
After adjustment for confounding variables with prespecified univariate value of p ≤0.15, multivariate analysis, despite the reduction of the number of patients due to the fact that variables with missing values were included in the analysis, demonstrated that lnF2-isoPs (odds ratio [OR] 2.44, 95% confidence interval [CI] 1.19–4.98, p = 0.014) were a predictor of >20% mismatch, as well as lnDWIV (OR 0.57, 95% CI 0.38–0.84, p = 0.005), and small vessel stroke subtype (OR 0.12, 95% CI 0.20–0.74, p = 0.023) (table 3) (e-results and tables e-2 and e-3 available from Dryad, doi.org/10.5061/dryad.4j07c14, give further details on multivariate analysis). Of note, it should be taken into account that the number of small vessel stroke subtypes was very low and the concept of DWI-PWI mismatch is appropriate for territorial infarctions but not really helpful in small vessel, i.e., lacunar, strokes.
Table 3.
Multivariate logistic regression analysis for mismatch >20% including variables with the prespecified univariate p ≤ 0.15
lnF2-isoPs remained statistically significant even when small vessel stroke subtype (OR 2.15, 95% CI 1.10–4.18, p = 0.025) or other variables showing potential interaction with F2-isoPs such as lnDWIV (OR 1.90, 95% CI 1.01–3.57, p = 0.048) or lnORACPCA (OR 1.90, 95% CI 1.01–3.59, p = 0.047) were excluded from the model (e-results and table e-4 available from Dryad, doi.org/10.5061/dryad.4j07c14, provide further details, and figure e-1 available from Dryad reports the relationship between baseline levels of oxidative stress biomarkers and baseline infarct volume when no nominal significant correlation was observed). Similarly, lnF2-isoPs were still an independent predictor of >20% mismatch when the inverse propensity score methods were applied (OR 2.98, 95% CI 1.48–5.99, p = 0.002) and after the inclusion in the model of time intervals such as onset–to–blood draw, imaging–to–blood draw, and onset-to-imaging times as continuous variables (2.13, 95% CI 1.01–4.48, p = 0.046) (see e-results and table e-5 available from Dryad for further details) or other biomarkers such as hs-CRP (OR 2.45, 95% CI 1.20–5.01, p = 0.014), MMP-2 (OR 2.48, 95% CI 1.19–5.14, p = 0.015), MMP-9 (OR 2.35, 95% CI 1.14–4.82, p = 0.021), and ORACTOT (OR 2.34, 95% CI 1.15–4.75, p = 0.019). No statistically significant association with any of the other biomarkers investigated in this study was found (see e-results and table e-6 available from Dryad for further details). The model using 8-OHdG or 8-OHdG adjusted by urinary creatinine levels (8-OHdG_cr) was not reliable due to missing values in this patient cohort. Even when mismatch was considered as a continuous dependent variable in a linear regression model, baseline lnF2-isoPs were confirmed as a predictor of ln of mismatch (B = 0.64, 95% CI 0.071–1.210, p = 0.028). The r2 and adjusted r2 of the model were 0.47 and 0.42, respectively.
More conservative approaches including only variables with a univariate nominal value of p < 0.05 or p ≤ 0.10 in the multivariate model showed similar results (see e-results available from Dryad, doi.org/10.5061/dryad.4j07c14).
Both oxidative stress biomarkers F2-isoP and ORACPCA showed a better predictive performance compared with the other relevant independent predictor of >20% mismatch, i.e., baseline DWI infarct volume (ORACPCA: AUC 0.61, 95% CI 0.52–0.70, p = 0.026; F2isop: AUC 0.58, 95% CI 0.49–0.68, p = 0.083; baseline DWI: AUC 0.42, 95% CI 0.31–0.52, p = 0.086). Cutoff levels of 52.7 pg/mL for F2-isoPs had a sensitivity of 62% and a specificity of 51%, and cutoff levels of 1,350.2 μmol TE/L for ORACPCA had a sensitivity of 61% and a specificity of 55%.
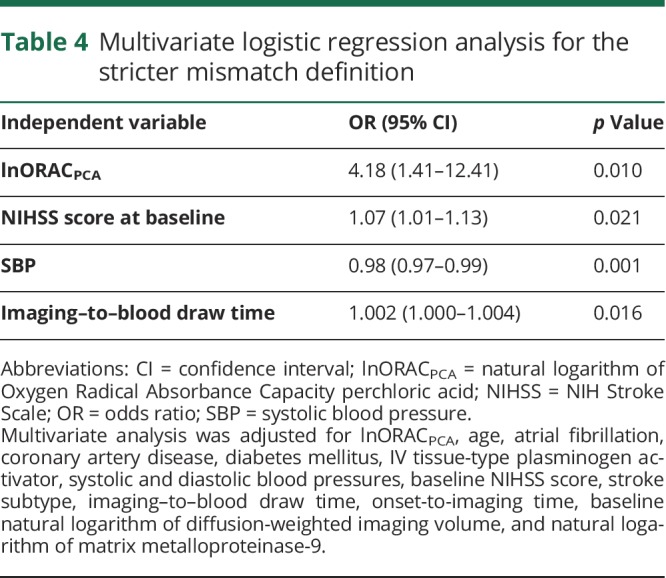
When the stricter definition of mismatch was used, lnORACPCA independently predicted mismatch (OR 4.18, 95% CI 1.41–12.41, p = 0.010) in a model adjusted for age, atrial fibrillation, coronary artery disease, diabetes mellitus, IV tPA, SBP, DBP, baseline NIHSS score, stroke subtype, imaging–to–blood draw and onset-to-imaging interval times, baseline lnDWIV, and lnMMP-9 (table 4). None of the other biomarkers investigated in this study resulted as independent predictor of the stricter mismatch definition, except for a borderline statistical significance for lnMMP-9 (OR 1.48, 95% CI 1.02–2.13, p = 0.036), which was not confirmed in the multivariate forward stepwise logistic regression analysis (table e-7 available from Dryad, doi.org/10.5061/dryad.4j07c14). Overall, ORACPCA had a statistically significant predictive performance for the stricter mismatch definition similar to that of baseline NIHSS score and better than that of the other variables that resulted as independent predictors in the multivariate analysis (ORACPCA AUC 0.59, 95% CI 0.51–0.68, p = 0.032 vs baseline NIHSS score 0.64, 95% CI 0.55–0.72, p = 0.002; SBP AUC 0.39, 95% CI 0.31–0.47, p = 0.013; imaging–to–blood draw time AUC 0.58, 95% CI 0.50–0.67, p = 0.064). Cutoff levels of 1,362.3 μmol TE/L had a sensitivity of 61% and a specificity of 53%.
Table 4.
Multivariate logistic regression analysis for the stricter mismatch definition
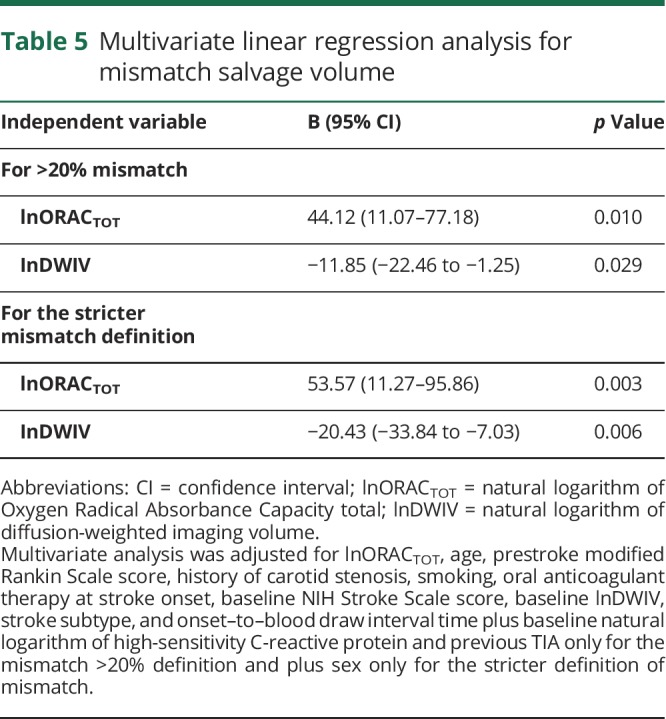
Moreover, in all patients and the subgroup with >20% mismatch, ORACTOT, as a marker of total plasma antioxidant capacity, significantly correlated with mismatch salvage volume (Spearman ρ = 0.18, p = 0.049 and ρ = 0.30, p = 0.006, respectively) and mismatch salvage percentage (ρ = 0.21, p = 0.025 and ρ = 0.25, p = 0.020, respectively). Linear regression multivariate analyses for mismatch salvage volume, performed by selecting either patients with >20% mismatch or those with the stricter definition of mismatch, confirmed lnORACTOT as an independent predictor of mismatch salvage volume (B = 44.12, 95% CI 11.07–77.18, p = 0.010 and B = 53.57, 95% CI 11.27–95.86, p = 0.003, respectively) (table 5). No statistically significant association with any of the other biomarkers investigated in this study was found (tables e-8 and e-9 available from Dryad, doi.org/10.5061/dryad.4j07c14). ORACTOT had a better predictive performance for the presence of mismatch salvage compared with the other variables that resulted as independent predictors in the multivariate analysis (for the stricter mismatch definition: AUC for ORACTOT 0.65, 95% CI 0.53–0.76, p = 0.023 and AUC for DWIV 0.59, 95% CI 0.48–0.71, p = 0.142; for >20% mismatch: AUC for ORACTOT 0.59, 95% CI 0.49–0.70, p = 0.086 and AUC for DWIV 0.58, 95% CI 0.48–0.68, p = 0.148). Cutoff levels of ORACTOT of 14,982.2 μmol TE/L for the stricter mismatch definition had a sensitivity of 67% and a specificity of 53%, and cutoff levels of 14,949.8 μmol TE/L for 20% mismatch had a sensitivity of 66% and a specificity of 52%.
Table 5.
Multivariate linear regression analysis for mismatch salvage volume
Of note, even when a dichotomous variable indicating whether blood draws were performed before imaging was included in multivariate models for mismatch >20%, for the stricter definition of mismatch, and for mismatch salvage volume, the results were consistent and showed that this variable did not have any effect on the predictive value of oxidative stress biomarkers for ischemic penumbra (see e-results available from Dryad, doi.org/10.5061/dryad.4j07c14, regarding time variables for further details).
Overall, a significant association has been found between mismatch salvage volume and functional outcome at 3 months as per an mRS score of 2 to 6 (overall: OR 0.989, 95% CI 0.982–0.996, p = 0.002; in patients with >20% mismatch: OR 0.988, 95% CI 0.979–0.997, p = 0.011; in patients with stricter mismatch definition: OR 0.986, 95% CI 0.976–0.996, p = 0.007) or an mRS score of 3 to 6 (overall: OR 0.986, 95% CI 0.979–0.994, p < 0.001; in patients with >20% mismatch: OR 0.987, 95% CI 0.977–0.996, p = 0.007; in patients with stricter mismatch definition: OR 0.984, 95% CI 0.973–0.994, p = 0.003). A smaller mismatch salvage volume was also statistically significant associated with a higher mortality at 3 months (OR 0.993, 95% CI 0.987–0.999, p = 0.017). Regarding the association between biomarkers and 3-month functional outcome and mortality, this will be the topic of another study on the overall biomarker dataset because the current analysis was focused mainly on the associations with neuroimaging findings (see e-results available from Dryad, doi.org/10.5061/dryad.4j07c14, for preliminary results).
Discussion
In this study, we found that plasma levels of F2-isoPs and ORACPCA, the oxidative stress biomarkers, are independent molecular predictors of radiographic evidence of ischemic penumbra in patients with AIS evaluated within 9 hours of symptom onset. The ischemic penumbra was defined as PWI-DWI mismatch >20% alone or on the basis of the stricter definition including PWI-DWI and PWI cutoff of ≥10 cm3. Moreover, ORACTOT, a biomarker of the total plasma antioxidant capacity, significantly correlates with mismatch salvage volume and percentage and independently predicts mismatch salvage volume. Therefore, these oxidative stress biomarkers might contribute to our understanding of the pathophysiology of ischemic penumbra.
Experimental models have shown that the ischemic cascade is a series of complex biochemical events that recruit excitotoxic, oxidative, and inflammatory pathways. These events are triggered at the onset of stroke and are responsible for evolution from normal brain tissue to salvageable penumbra and eventually irreversibly infarcted tissue (see also e-references 1–5 available from Dryad, doi.org/10.5061/dryad.4j07c14). Neuroimaging methods, particularly MRI-based mismatch between PWI and DWI lesion volumes, may allow us to identify the ischemic penumbra and predict brain tissue viability in patients with AIS, although criteria to define clinically relevant mismatch are not yet standardized.7,8 Even if reliable neuroimaging criteria have been established, MRI is not widely available in the emergency setting, restricting the clinical application of these methods to primarily major academic institutions. Therefore, there is a need for more affordable, time-efficient, and widely available methods for evaluating the presence of salvageable brain tissue.
There is little reported research correlating molecular biomarkers and mismatch identified by neuroimaging in acute stroke. A study focused on the association between early serum levels of biomarkers and clinical-diffusion mismatch.37 The authors found that high levels of excitotoxic biomarkers such as interleukin-10, tumor necrosis factor-α, and glutamate and low levels of neuronal damage and blood-brain barrier disruption biomarkers (neuron-specific enolase, interleukin-6, and active MMP-9) were associated with clinical-diffusion mismatch.37,38 In our study, we used a well-defined radiographic PWI-DWI mismatch protocol, and we examined a different set of biomarkers, those of oxidative stress, that demonstrated potential to identify ischemic brain before irreversible infarction.
Evidence on the contribution of oxidative stress biomarkers in the hyperacute phase of an ischemic stroke and on their association with specific radiologic findings in humans is not so extensive.9 Studies have found an increase of free radical formation and lipid peroxidation and a decrease of enzymatic and nonenzymatic antioxidants and plasma antioxidant capacity in association with a larger infarct volume and more severe neurologic deficit.9–15 This oxidative stress–induced release of molecules is not limited to the necrotic core but extends to the ischemic penumbra.39
Previous studies have evaluated the role of oxidative biomarkers such as malondialdehyde, myeloperoxidase, and urate40,41 (see also e-reference 6 available from Dryad, doi.org/10.5061/dryad.4j07c14) but did not focus on F2-isoPs or ORAC. F2-isoPs are stable, sensitive, and specific markers of oxidative stress–induced lipid peroxidation.16 They are detectable in plasma, urine, and CSF, making them clinically applicable. Recently, F2-isoP levels were shown to be significantly higher in patients with stroke at presentation and at day 3 up to day 7.42 To the best of our knowledge, this is the first study to demonstrate an association between oxidative stress biomarkers and mismatch in patients with ischemic stroke. In this analysis, F2-isoPs were independently predictive of mismatch after adjustment for confounding variables. This is consistent with the results of another recently published study from our biomarker dataset showing that baseline F2-isoP levels independently predict infarct growth occurrence (OR 2.57, 95% CI 1.37–4.83, p = 0.007) and infarct growth volume (B = 0.38, 95% CI 0.04–0.72, p = 0.03).18 A further analysis showed that infarct growth volume positively correlates with mismatch volume (ρ = 0.17, p = 0.041), i.e., the larger the mismatch volume, the higher the chance to have infarct growth. Overall, these findings suggest that baseline plasma levels of F2-isoP are likely to have a role in predicting that part of ischemic penumbra that is prone to evolve toward infarction in the absence of an efficient spontaneous or pharmacologic/mechanical recanalization/reperfusion. Indeed, baseline DWI infarct volume and small vessel stroke subtype resulted as the other independent predictors of >20% mismatch in terms of lower likelihood of having >20% mismatch in case of larger lesions on DWI or small vessel stroke subtype, as expected on the basis of biological plausibility. In any case, F2-isoPs remained statistically significant even when small vessel stroke subtype or baseline DWIV was excluded from the model.
When a stricter definition of mismatch excluding too small stroke lesions and perfusion deficit were applied, ORACPCA was an independent predictor of mismatch. In addition, we found a correlation between a higher total plasma antioxidant capacity (ORACTOT) and a larger mismatch salvage volume.
This study was limited by the retrospective analysis of the neuroimaging variables, which were not prespecified in the protocol. PWI deficit was quantified with the use of the MTT sequence, which can result in overestimation of mismatch. Stroke severity in our cohort, as indicated by the median NIHSS score, was moderate, which limits the generalizability of these findings to patients with severe strokes. Moreover, generalizability of this study findings could be further limited by the exclusion criteria that are usually adopted in biomarker research studies so as not to include all those conditions that, for their underlying pathophysiologic characteristics, could be associated with the increase of inflammatory or oxidative stress biomarkers and could be confounding for the evaluation of association between biomarker levels and mismatch. In this analysis, we had a wide range of missing variables for the biomarkers investigated and measured at baseline: hs-CRP, 1.4% (3 of 216); MMP-2 and MMP-9, 8.3% (18 of 216) each; F2-isoPs, 12% (26 of 216); ORACTOT and ORACPCA, 16.2% (35 of 216) each; and 8-OHdG and 8-OHdG_cr, 57.9% and 57.4% (125 and 124 of 216), respectively. However, we think that most of these percentages can be considered acceptable for an analysis of biomarkers in the hyperacute phase of stroke like this, particularly for those biomarkers that have been less investigated so far such as F2-isoPs, compared with others in acute stroke in humans. Moreover, no difference in the baseline characteristics was found between patients with missing values of F2-isoPs at admission and those with no such missing values, except for a lower alcohol consumption (p = 0.046), a lower use of antiplatelet therapy before stroke onset (p = 0.006), and higher baseline NIHSS scores (p = 0.010) in the group of patients with missing admission F2-isoP levels. Therefore, we are of the opinion that the percentage of missing values for F2-isoPs did not affect the main results of our study. It is acknowledged that the percentage of missing values for 8-OHdG and 8-OHdG adjusted by creatinine levels is relatively high. The reason is that this biomarker was measured in urine samples, and in an emergency setting, it was not always possible to collect these data. However, we think that the number of measurements for this biomarker, which to the best of our knowledge has never been studied in the hyperacute phase of stroke in humans, can still be considered valuable in order to evaluate in an exploratory manner the presence of an association with mismatch. The AUC, specificity, and sensitivity of F2-isoPs and ORACPCA for >20% mismatch and the stricter mismatch definition, respectively, and of ORACTOT for mismatch salvage are not so optimal; therefore, further specific and prospective studies, probably with larger sample size and without the above-mentioned limitations, are needed to confirm the promise of these tests for acute stroke.
This study is strengthened by the prospective collection of biomarker samples, by the investigation of molecular biomarkers that are novel compared to those usually considered in studies on acute stroke in humans, and by the analysis of DWI and PWI MRI sequences with a semiautomated volumetric method. The samples were collected in the hours shortly after onset of cerebral ischemia, making our data applicable to the initial pathophysiologic processes involved in ischemic stroke. The relatively large sample size allowed adjustment for variables that might have an influence on the plasma levels of biomarkers and their associations with mismatch.
In conclusion, this study shows that oxidative stress biomarkers can help in predicting the presence of tissue at risk for infarction in patients with AIS. High plasma levels of baseline F2-isoPs and ORACPCA were independently associated with clinically significant mismatch definitions, and ORACTOT, as a marker of plasma antioxidant capacity, significantly correlated with more mismatch salvage. However, at this stage, it is not clear whether any of these markers will add a clinical meaningful incremental value for identifying patients with tissue at risk. Nevertheless, biomarkers have the potential to serve the purpose of rapid detection of salvageable brain tissue. This study provides a platform to formulate future trials using the full potential of biomarkers. As an example, after validation of the sensitivity and specificity of these biomarkers for predicting mismatch in a larger patient population, such a trial would aim to evaluate whether these biomarkers can measure the effectiveness of revascularization (pharmacologic or mechanical) methods being currently used in the clinical management of patients with AIS.
Acknowledgment
The authors thank all the patients who participated in this Specialized Program of Transitional Research in Acute Stroke (SPOTRIAS) project. They also thank the NIH/National Institute of Neurological Disorders and Stroke (NINDS) SPOTRIAS program, the Massachusetts General Hospital, and the Brigham and Women's Hospital. In addition, they thank Harvard Catalyst, the Harvard Clinical and Translational Science Center, Harvard University.
Glossary
- AIS
acute ischemic stroke
- AUC
area under the curve
- CI
confidence interval
- DBP
diastolic blood pressure
- DEFUSE
Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution
- DWI
diffusion-weighted imaging
- DWIV
DWI volume
- 8-OHdG
8-oxo-7,8-dihydro-2′-deoxyguoanosine
- EPITHET
Echoplanar Imaging Thrombolytic Evaluation Trial
- F2-isoP
F2-isoprostane
- hs-CRP
high-sensitivity C-reactive protein
- IQR
interquartile range
- MMP
matrix metalloproteinase
- mRS
modified Rankin Scale
- MTT
mean transit time
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- ORAC
Oxygen Radical Absorbance Capacity
- PWI
perfusion-weighted imaging
- SBP
systolic blood pressure
- TE
troloxequivalents
- TPA
tissue-type plasminogen activator
Authors contributions
S. Lorenzano conceptualized and designed the study, collected the data, analyzed and interpreted the data, performed the statistical analysis, drafted the manuscript, reviewed the manuscript for intellectual content, and revised the manuscript. H. Li, L.M. Batista, A. Chutinet, R.E. Green, T.K. Thankachan, B. Thornell, A.T. Som, and L.-D.D. Pham collected the data and reviewed the manuscript for intellectual content. N.S. Rost, M. Khan, A. Muzikansky, K. Arai, O. Wu, G.J. Harris, E.H. Lo, J.B. Blumberg, P.E. Milbury, S.K. Feske, and K.L. Furie, interpreted the data and reviewed the manuscript for intellectual content.
Study funding
This study was supported by the NIH/NINDS SPOTRIAS grant P50-NS051343.
Disclosure
S. Lorenzano was supported by NIH/NINDS SPOTRIAS grant P50-NS051343; she served as expert consultant for Boehringer Ingelheim; she received 2 travel grants from Boehringer Ingelheim and 1 travel grant from Bayer, Quintiles IMS, and Daichii Sankyo. N.S. Rost was supported by NIH-NINDS K23NS064052 and R01 NS082285. M. Khan, H. Li, L.M. Batista, A. Chutinet, R.E. Green, T.K. Thankachan, B. Thornell, A.T. Muzikansky, K. Arai, A.T. Som, and L.-D.D. Pham report no disclosures relevant to the manuscript. O. Wu was supported in part by NIH/NINDS P50NS051343, R01NS059775, and R01NS063925. She is the coinventor of a patent on “Delay-Compensated Calculation of Tissue Blood Flow,” US Patent 7,512,435, and the patent has been licensed to General Electric, Siemens, Imaging Biometrics, and Olea Medical. G.J. Harris, E.H. Lo, J.B. Blumberg, P.E. Milbury, S.K. Feske, and K.L. Furie report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014;129:e28-e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 3.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010;375:1695–1703. [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 5.Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg 2009;111:483–495. [DOI] [PubMed] [Google Scholar]
- 6.Fisher M, Bastan B. Identifying and utilizing the ischemic penumbra. Neurology 2012;79:S79–S85. [DOI] [PubMed] [Google Scholar]
- 7.Kane I, Sandercock P, Wardlaw J. Magnetic resonance perfusion diffusion mismatch and thrombolysis in acute ischaemic stroke: a systematic review of the evidence to date. J Neurol Neurosurg Psychiatry 2007;78:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wintermark M, Albers GW, Broderick JP, et al. Acute stroke imaging research roadmap II. Stroke 2013;44:2628–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kontos HA. Oxygen radicals in cerebral ischemia: the 2001 Willis Lecture. Stroke 2001;32:2712–2716. [DOI] [PubMed] [Google Scholar]
- 10.Zini I, Tomasi A, Grimaldi R, Vannini V, Agnati LF. Detection of free radicals during brain ischemia and reperfusion by spin trapping and microdialysis. Neurosci Lett 1992;138:279–282. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto A, Ohnishi ST, Ohnishi T, Ogawa R. Relationship between free radical production and lipid peroxidation during ischemia-reperfusion injury in the rat brain. Brain Res 1991;554:186–192. [DOI] [PubMed] [Google Scholar]
- 12.Cherubini A, Polidori MC, Bregnocchi M, et al. Antioxidant profile and early outcome in stroke patients. Stroke 2000, 31:2295–2300. [DOI] [PubMed] [Google Scholar]
- 13.Polidori MC, Cherubini A, Stahl W, Senin U, Sies H, Mecocci P. Plasma carotenoid and malondialdehyde levels in ischemic stroke patients: relationship to early outcome. Free Radic Res 2002;36:265–268. [DOI] [PubMed] [Google Scholar]
- 14.Gariballa SE, Hutchin TP, Sinclair AJ. Antioxidant capacity after acute ischaemic stroke. QJM 2002;95:685–690. [DOI] [PubMed] [Google Scholar]
- 15.Leinonen JS, Ahonen JP, Lönnrot K, Jehkonen P, Molnár G, Alho H. Low plasma antioxidant activity is associated with high lesion volume and neurological impairment in stroke. Stroke 2000;31:33–39. [DOI] [PubMed] [Google Scholar]
- 16.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol 2005;25:279–286. [DOI] [PubMed] [Google Scholar]
- 17.Kelly PJ, Morrow JD, Ning M, et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: the Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) study. Stroke 2008;39:100–104. [DOI] [PubMed] [Google Scholar]
- 18.Lorenzano S, Rost NS, Khan M, et al. Oxidative stress biomarkers of brain damage: hyperacute plasma F2-Isoprostane predicts infarct growth in stroke. Stroke 2018;49:630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui J, Holmes EH, Greene TG, Liu PK. Oxidative DNA damage precedes DNA fragmentation after experimental stroke in rat brain. FASEB J 2000;14:955–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Souza-Pinto NC, Hogue BA, Bohr VA. DNA repair and aging in mouse liver: 8-oxodG glycosylase activity increase in mitochondrial but not in nuclear extracts. Free Radic Biol Med 2001;30:916–923. [DOI] [PubMed] [Google Scholar]
- 21.Cao G, Giovanoni M, Prior RL. Antioxidant capacity in different tissues of young and old rats. Proc Soc Exp Biol Med 1996;211:359–365. [DOI] [PubMed] [Google Scholar]
- 22.Cao G, Prior RL. Measurement of oxygen radical absorbance capacity in biological samples. Meth Enzymol 1999;299:50–62. [DOI] [PubMed] [Google Scholar]
- 23.Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol 2005;58:688–697. [DOI] [PubMed] [Google Scholar]
- 24.Arsava EM, Ballabio E, Benner T, et al. The Causative Classification of Stroke system: an international reliability and optimization study. Neurology 2010;75:1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruno A, Akinwuntan AE, Lin C, et al. Simplified modified Rankin Scale questionnaire: reproducibility over the telephone and validation with quality of life. Stroke 2011;42:2276–2279. [DOI] [PubMed] [Google Scholar]
- 26.Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Prior RL. High-throughput assay of Oxygen Radical Absorbance Capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J Agric Food Chem 2002;50:4437–4444. [DOI] [PubMed] [Google Scholar]
- 27.Wu O, Østergaard L, Weisskoff RM, Benner T, Rosen BR, Sorensen AG. Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med 2003;50:164–174. [DOI] [PubMed] [Google Scholar]
- 28.Mohr JP, Biller J, Hilal SK, et al. Magnetic resonance versus computed tomographic imaging in acute stroke. Stroke 1995;26:807–812. [DOI] [PubMed] [Google Scholar]
- 29.Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomized trial. Lancet Neurol 2008;7:299–309. [DOI] [PubMed] [Google Scholar]
- 30.Butcher KS, Parsons M, MacGregor L, et al. Refining the perfusion-diffusion mismatch hypothesis. Stroke 2005;36:1153–1159. [DOI] [PubMed] [Google Scholar]
- 31.Hacke W, Albers G, Al-Rawi Y, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 2005;36:66–73. [DOI] [PubMed] [Google Scholar]
- 32.Furlan AJ, Eyding D, Albers GW, et al. Dose Escalation of Desmoteplase for acute ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke 2006;37:1227–1231. [DOI] [PubMed] [Google Scholar]
- 33.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance Imaging profile predict clinical response to early reperfusion: the Diffusion and perfusion imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study. Ann Neurol 2006;60:508–517. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson KJ, Wardlaw JM, Edmond CL, Deary IJ, Maclullich AM. Intracranial area: a validated method for estimating intracranial volume. J Neuroimaging 2005;15:76–78. [DOI] [PubMed] [Google Scholar]
- 35.Gurol ME, Irizarry MC, Smith EE, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology 2006;66:23–29. [DOI] [PubMed] [Google Scholar]
- 36.Ay H, Arsava EM, Vangel M, et al. Interexaminer difference in infarct volume measurements on MRI: a source of variance in stroke research. Stroke 2008;39:1171–1176. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez-Yáñez M, Sobrino T, Arias S, et al. Early biomarkers of clinical-diffusion mismatch in acute ischemic stroke. Stroke 2011;42:2813–2818. [DOI] [PubMed] [Google Scholar]
- 38.Dávalos A, Blanco M, Pedraza S, et al. The clinical-DWI mismatch: a new diagnostic approach to the brain tissue at risk of infarction. Neurology 2004;62:2187–2192. [DOI] [PubMed] [Google Scholar]
- 39.Sairanen T, Ristimaki A, Karjalainen-Lindsberg ML, Paetau A, Kaste M, Lindsberg PJ. Cyclooxygenase-2 is induced globally in infarcted human brain. Ann Neurol 1998;43:738–747. [DOI] [PubMed] [Google Scholar]
- 40.Domínguez C, Delgado P, Vilches A, et al. Oxidative stress after thrombolysis-induced reperfusion in human stroke. Stroke 2010;41:653–660. [DOI] [PubMed] [Google Scholar]
- 41.Brouns R, Wauters A, Van De Vijver G, De Surgeloose D, Sheorajpanday R, De Deyn PP. Decrease in uric acid in acute ischemic stroke correlates with stroke severity, evolution and outcome. Clin Chem Lab Med 2010;48:383–390. [DOI] [PubMed] [Google Scholar]
- 42.Seet RC, Lee CY, Chan BP, et al. Oxidative damage in ischemic stroke revealed using multiple biomarkers. Stroke 2011;42:2326–2329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized individual participant data and the study protocol will be shared with qualified parties on request to the corresponding author.