Abstract
Context: Despite some studies related to Juniperus phoenicea L. (Cupressaceae), phytochemical and biological investigations of this plant remain unexplored.
Objective: This work is the first report dealing with the identification and characterization of volatile components and flavonoids in hexane and methanol extracts from J. phoenicea leaves
Materials and methods: Antioxidant activity of hexane, and methanol extracts from J. phoenicea leaves were determined by DPPH-radical scavenging assay. α-Amylase inhibitory activity was evaluated by enzyme inhibition using in vitro assay (each extract was dissolved in DMSO to give concentrations of 50, 100 and 200 mg/mL). The chemical composition of fractions (Fr1-Fr3) from methanol extract was determined by high-performance liquid chromatography coupled with mass spectroscopy (HPLC-MS) analysis.
Results and discussion: The hexane extract was analyzed by GC-MS technique which allowed the identification of 32 compounds. The main constituents were α-humulene (16.9%), pentadecane (10.2%) and α-cubebene (9.7%). Fraction Fr 2 exhibited a strong DPPH radical-scavenging activity (IC50 = 20.1 μg/mL) compared to that of BHT as well as the highest α-amylase inhibitory activity (IC50 = 28.4 μg/mL). Three flavonoids were identified in these fractions using HPLC-MS analysis: Quercetin 3-O-glucoside, isoscutellarein 7-O-pentoside and quercetin 3-O-pentoside. In addition, the more active fraction (Fr 2) was purified with semi-preparative HPLC affording one pure compound (amentoflavone) using 1H NMR analysis. This compound exhibited powerful DPPH radical-scavenging (IC50 = 14.1 μg/mL) and α-amylase inhibition (IC50 = 20.4 μg/mL) effects.
Conclusion: This study provides scientific support to some medicinal uses of J. phoenicea found in North Africa.
Keywords: HPLC-DAD–ESI/MS, semi-prep HPLC, biological activities
Introduction
Among the natural products found in plants, flavonoids and their glycosides constitute one of the largest classes of natural compounds known. Flavonoids are very common and widespread secondary plant metabolites. They have a wide range of biological and physiological activities and serve as chemotaxonomic marker compounds (Cuyckens & Claeys 2004). Flavonoids are diphenylpropanes (C6C3C6) with different numbers of hydroxyl groups attached to the ring structures. They occur primarily in conjugated form, with one or more sugar residues linked to hydroxyl groups. Associations with other compounds, such as carboxylic acids, amines and lipids as well as linkages with other phenols are also common. The growing interest in flavonoids is primarily due to their antioxidant activity, which is closely linked to their structure (Cook & Samman 1996; Bravo 1998; Moure et al. 2001). Flavonoids can act as reducing agents, hydrogen-donating antioxidants and singlet oxygen quenchers.
Searching for new compounds, and also for quality control, there is a need to have reliable methodology for the analysis of flavonoids in medicinal plants. Juniperus phoenicea L. (Cupressaceae) is considered as an important medicinal plant largely used in the Tunisian traditional medicine. Its leaves are used in the form of a decoction to cure many diseases such as diarrhoea, bronchitis, rheumatism (Madar 1989; Bellakhder 1997), and diabetes (Amer et al. 1994; Allali et al. 2008). Most of the works reported in the literature to date have focused on the chemical composition determination of J. phoenicea essential oil (Adams et al. 1996; Rezzi et al. 2001; Cavaleiro et al. 2001; Barrero et al. 2006; Keskes et al. 2014), whereas only limited data are reported on the nonvolatile components of leaves and/or twigs of other juniper varieties (Nakanishi et al. 2002, 2005, 2004). These studies have shown that the main identified classes of metabolites are flavonoids and biflavonoids.
In our previous study, we evaluated the antioxidant property of various extracts from J. phonicea leaves and the in vitro inhibitory activity against α-amylase and lipase (Keskes et al. 2014). This work investigates, for the first time, the chemical composition of hexane extract from J. phonicea leaves and of active fractions obtained from the methanol extract fractionation using gas chromatography coupled with mass spectroscopy (GC-MS) and high-performance liquid chromatography coupled with mass spectroscopy (HPLC-MS) techniques, respectively. In addition, we were interested in the purification of the active fraction obtained from methanol extract chromatography as well as in the identification and the α-amylase inhibition activity evaluation of the purified compound.
Materials and methods
Collection of plant material
Leaves of J. phoenicea were collected in October 2011, from local Ain Silisla El Oyoun Company in Kasserine Governorate in west-central Tunisia. The collected plant was identified by Professor Mohamed Chaieb of the Botany and Plant Ecology Department. A voucher specimen of J. phoenicea (LCSN 126) was deposited at the Laboratory of Chemistry of Natural Substances, Faculty of Sciences, Sfax.
Chemicals
2-Chloro-4-nitrophenyl-α-d-maltotrioside (CNPG3) was purchased from Biolabo Reagents (Nord-Pas-de-Calais-Picardie, France), acarbose from Pharmaghreb (Tunisia), dimethylsulfoxide (DMSO) from Merck (Darmstadt, Germany) and porcine pancreatic α-amylase (PPA) was procured from Sigma-Aldrich (St. Louis, MO). Methanol, acetic acid, and acetonitrile HPLC-grade solvents were purchased from Riedel-de Haen (Seelze, Germany). The solvents were of appropriate purity. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) was purchased from Sigma-Aldrich (Chemie Gmbh, Steinhein, Germany). Double-distilled water was used in the HPLC mobile phase. All the other chemicals used were of analytical grade.
Extraction of plant material
The dried powdered leaves of J. phoenicea (130 g) were extracted sequentially by maceration in hexane (4 × 600 mL), ethyl acetate (EtOAc) (4 × 600 mL) and MeOH (4 × 600 mL) at room temperature and concentrated under vacuum at 40 °C to afford 6.25, 6.88 and 21.8 g, respectively. The methanol extract (4 g) was subjected to column chromatography on Sephadex LH-20 (Amersham Pharmacia Biotech AB, Uppsala, Sweden) (MeOH) to give 3 fractions (Fr 1-Fr 3).
Gas chromatography/mass spectrometry (GC/MS) analysis
GC/MS analysis of J. phoenicea leaves’ hexane extract was performed using an Agilent 7890A mass spectrometer coupled to an Agilent 7000 gas chromatograph. An aliquot of extract (1 mL) was then injected into the GC/MS apparatus. Next, the data were displayed on a HP5MS column, 30 m in length, 0.25 mm i.d. and 0.25 μm in thickness (Agilent Technologies, J&W Scientific Products, Santa Clara, CA). The carrier gas was helium. GC oven temperature started at 100 °C and was held for 1 min at 260 °C and then for 10 min with program rate 4 °C/min. The injector and detector temperatures were set at 250 and 230 °C, respectively. The mass range was scanned from 50 to 550 amu. The identification of components was based on the comparison of their mass spectra with those of NIST mass spectral library (Davies 1990; Massada 1996).
High-performance liquid chromatography/mass spectrometry (HPLC/MS) analysis
Flavonoids compositions of fractions (Fr1–Fr3) obtained from the methanol extract fractionation were determined using a Hewlett-Packard 1100 chromatograph (Agilent Technologies, Santa Clara, CA) with a quaternary pump and a diode array detector (DAD). The column is coupled with an MSD Ion Trap XCT mass spectrometer (Agilent Technologies, Santa Clara, CA) equipped with an electrospray ionization interface (ESI). Fractions were injected onto a C-18 column (4.6 × 25 cm, 5 μm; Phenomenex UK, Macclesfield, UK). The solvents used were 90% acetic acid–water (A) and 10% MeOH (B). The elution gradient was isocratic 10% B for 5 min, 10–100% B over 20 min, 100% B for 6 min, and re-equilibration of the column, using a flow rate of 200 μL/min. Spectra were recorded in negative and positive ionization mode between m/z 50 and 1200.
Semi-preparative high-performance liquid chromatography (semi-prep HPLC)
The active fraction (Fr 2) recovered from the methanol extract fractionation was purified with semi-prep HPLC using an Agilent 1260 LC system (Agilent, Waldbronn, Germany) equipped with a 5 μm Agilent Eclips XDB-C18 column (9.4 × 250 mm i.d.; Dikma Technologies, Beijing, China). The following solvent and gradient systems were used. Solvent system: A: water; B: acetonitrile; gradient: linear gradient from 80% A to 20% B within 5 min; followed by a 30 min linear gradient 20 to 100% B, then 100% B for 5 min. The flow rate was 1 mL/min and the injection volume was 100 μL.
DPPH radical-scavenging effect assay
DPPH (1,1-diphenyl-2-picrylhydrazyl) radical-scavenging effect was evaluated following the procedure described in a previous study (Feki et al. 2005). Various concentrations of extracts and fractions (5 μL) dissolved in methanol were added to 5 mL of a 0.004% methanol solution of DPPH. After a 30 min incubation period at room temperature, the absorbance was read against a blank at 517 nm. Inhibition of free radical, DPPH, in percent (I%) was calculated as follows:
where Ablank is the absorbance of the control reaction (containing all reagents except the studied sample) and Asample is the absorbance of the studied sample. Extract concentration providing 50% inhibition (IC50) was calculated from the graph plotted of inhibition percentage against extract concentration. The synthetic antioxidant reagents BHT and vitamin E were used as positive control and all tests were carried out in triplicate.
α-Amylase inhibition assay by CNPG3 method
The in vitro α-amylase inhibition activity of extracts and fractions from J. phoenicea leave was determined based on the spectrophotometric assay using acarbose as the reference compound (Gella et al. 1997). The studied sample was dissolved in DMSO to give concentrations of 50, 100, and 200 mg/mL. The enzyme solution was prepared by mixing α-amylase in 100 mL of 40 mM phosphate buffer, pH 6.9. The assays were measured by mixing 80 mL of the studied sample, 20 mL of α-amylase solution, and 1 mL of 2-chloro-4-nitrophenol-α-d-maltotrioside (CNPG3). The mixture was incubated at 37 °C for 5 min. The absorbance was measured at 405 nm spectrophotometrically (Jenway 6405 UV/Visible, Staffordshire, UK). Similarly, a control reaction was carried out without the studied sample or acarbose. Percentage inhibition was calculated by the following expression and all tests were carried out in triplicate:
Statistical analyses
The values were expressed as mean standard deviation of three parallel measurements. The data were evaluated by a one-way analysis of variance using Microsoft Excel 2000 (Microsoft Corporation, Syracuse, NY), and differences between the means were determined using Student’s t-test. Values were considered statistically significant when p < 0.05.
Results and discussion
Chemical composition of hexane extract
GC/MS analysis of the hexane extract from J. phoenicea leaves enabled the identification of 36 compounds (Table 1) belonging to different chemical families. The hexane extract contained 56.5% of sesquiterpene hydrocarbons: α-humulene (16.9%), pentadecane (10.2%), α-cubebene (9.7%) and β-cadinene (4.9%) were the major compounds in this fraction. The monoterpene hydrocarbons represented 18.4% consisting mainly of δ-3-carene (5.8%). The oxygenated sesquiterpene fraction represented 3.3% along with other minor constituents. According to our knowledge, the chemical composition of hexane extract from J. phoenicea leaves, investigated by gas chromatography coupled with mass spectroscopy (GC/MS), was performed for the first time in this study. The hexane extract from plants may contain some fatty acids/methyl esters and straight chain alkanes (Kumar et al. 2010; Abad et al. 2012). It was reported that the secondary metabolites and bioactive phyto-constituents identified by GC/MS in hexane extracts from plants possess antimicrobial, anti-inflammatory, antioxidant and antidiabetic activities (Kumar et al. 2010; Marzoug et al. 2011; Abad et al. 2012; Keskes et al. 2014).
Table 1.
Chemical composition of hexane extract from Juniperus phoenicea leaves analyzed by GC-MS.
| RT (min) | Compounds | KI | % |
|---|---|---|---|
| 4.16 | β-Phellandrene | 1027 | 10.8 |
| 8.75 | α-Humulene | 1452 | 16.9 |
| 9.96 | Sabinene | 969 | 0.1 |
| 10.43 | β-Myrcene | 988 | 0.1 |
| 10.98 | δ-3-Carene | 1008 | 5.8 |
| 11.54 | δ-Limonene | 1032 | 0.1 |
| 14.87 | α-Thujene | 924 | 0.2 |
| 15.06 | trans-Verbenol | 1144 | 0.1 |
| 17.54 | β-Citronellol | 1228 | 0.1 |
| 18.21 | Vetiverol | 1238 | 0.1 |
| 20.77 | Cyclohexene | 1197 | 1.5 |
| 21.73 | β-Bourbonene | 1387 | 0.8 |
| 21.86 | Γ-Muurolene | 1518 | 0.5 |
| 22.63 | γ-Elemene | 1436 | 2.7 |
| 23.49 | β-Cadinene | 1476 | 4.9 |
| 24.17 | Germacrene D | 1441 | 0.5 |
| 24.30 | β-Selinene | 1488 | 1.2 |
| 24.46 | β-Cubebene | 1391 | 1.5 |
| 25.03 | Cedreanol | 1692 | 1.2 |
| 25.25 | Epizonarene | 1450 | 1.8 |
| 25.40 | Cadina-1,4-diene | 1535 | 0.4 |
| 25.80 | Elemol | 1549 | 0.3 |
| 26.0 | Germacrene B | 1533 | 0.2 |
| 27.67 | τ-Murolol | 1650 | 1.7 |
| 27.95 | α-Cubebene | 1355 | 9.7 |
| 34.54 | Hexadecanoic acid | 1957 | 0.2 |
| 37.0 | Citronellyl acetate | 1351 | 0.4 |
| 39.49 | Benzenepropanoic acid | 1412 | 0.7 |
| 40.01 | 4-epi-Abietal | 2303 | 1.7 |
| 41,10 | Ferruginol | 1970 | 0.5 |
| 41.95 | Pentadecane | 1500 | 10.2 |
| 42.0 | 4-epi-Palustric acid | – | 3.6 |
| Total | 80.5 |
RT: Retention time; KI: Kovats Index on DB-5MS column in reference to n-alkanes.
Chemical composition of fractions from methanol extract
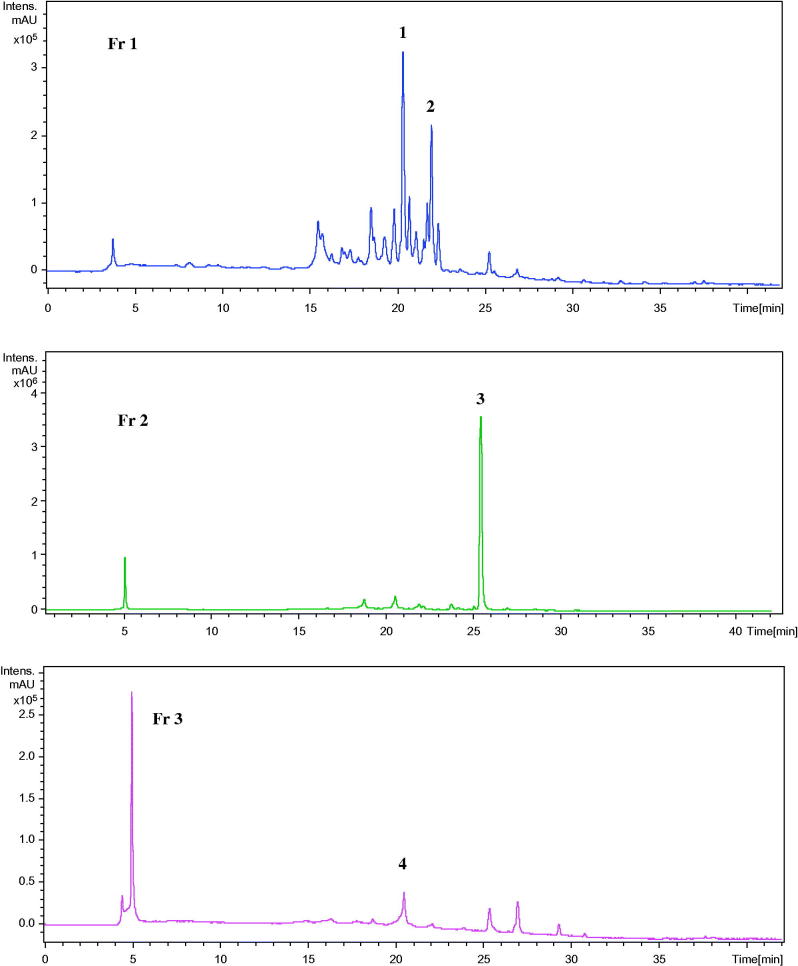
The methanol extract from J. phoenicea leaves was subjected to a column chromatography on Sephadex LH-20 eluted with MeOH to afford three fractions (Fr1–Fr3) according to their thin-layer chromatography (TLC) profile. The chemical composition of these fractions was determined using the HPLC-MS-UV technique. The obtained chromatograms are shown in Figure 1. Accordingly, all the detected peaks present flavonoid-type UV spectra composed of two bands: λmax values of the first and the second band ranges between 320–370 nm and 250–280 nm, respectively. Table 2 lists the identified compounds in fractions Fr1–Fr3. Structure assignment of flavonoids, for which no standards were available, was based on a systematic search for molecular ions using extracted ion mass chromatograms and comparing them with data in the literature (Grayer et al. 2000; Bouaziz et al. 2010; Heneidak et al. 2006). Our discussion focuses on the identification of the most interesting compounds. Flavonoids are generally part of a complex mixture isolated from plant extracts, making a purification step necessary for adequate analysis. Also, since the amount of plant extracts is often limited, on-line techniques are to be preferred as they have the additional advantage of providing faster analysis. GC-MS technique is not widely used in flavonoid analysis owing to the limited volatility of flavonoid glycosides. Hence, derivatization is needed, making the analysis more time consuming, and the fragmentation patterns of the derivatives are often difficult to interpret. Since the development of atmospheric pressure ionization (API) sources, HPLC-MS technique has become more efficient and easy to use, making it by far the most popular technique for flavonoid analysis nowadays.
Figure 1.
Chromatographic profiles at 330 nm of the fractions (Fr2, Fr3, and Fr4) of methanol extract. The numeration is that of Table 2.
Table 2.
Identification of the principal compounds in fractions Fr 1-3 by HPLC-MS-UV.
| Peaks | Compounds | RT(min) | λmax(nm) | [M − H]−;fragmentions |
|---|---|---|---|---|
| 1 | Quercetin 3-O-glycoside | 20.4 | 256; 350 | 463; 301 |
| 2 | Isoscutellarein 7-O-pentoside | 22.5 | 275; 303; 328 | 417; 285 |
| 3 | Amentoflavone | 25.9 | 230; 269; 332 | 537; 375 |
| 4 | Quercetin 3-O-pentoside | 20.9 | 257; 362 | 433; 301 |
RT: Retention time.
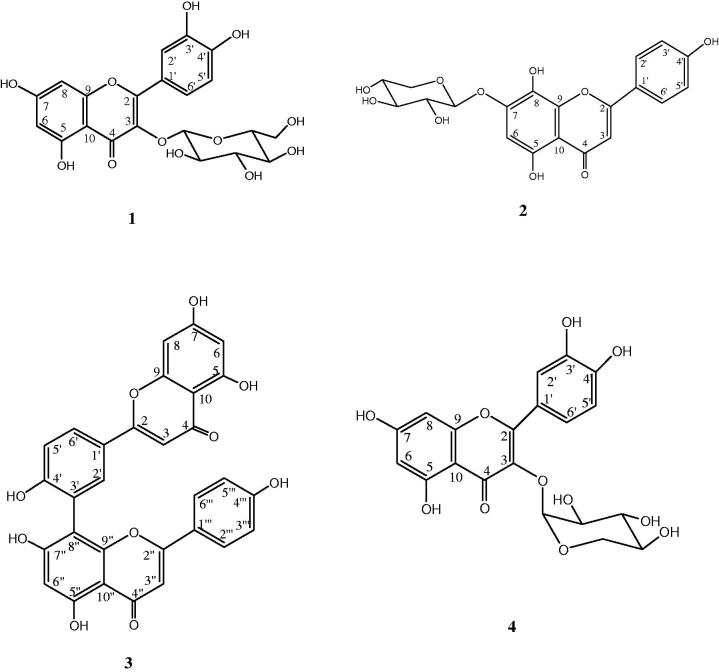
Compound 1 in Fr 1 (Figures 1 and 2) exhibited a base peak [M − H]− at m/z 463 and a strong fragment related to an aglycon ion at m/z 301. The loss of 162 amu from the pseudomolecular is related to the sugar glucose. The λmax in the UV spectrum at 256 and 350 nm suggests that compound 1 is a quercetin 3-O-glycoside (Boukhris et al. 2013). The identification of this compound was confirmed by comparing its HPLC retention time and UV and mass spectra with those obtained for standard, analyzed in the same conditions.
Figure 2.
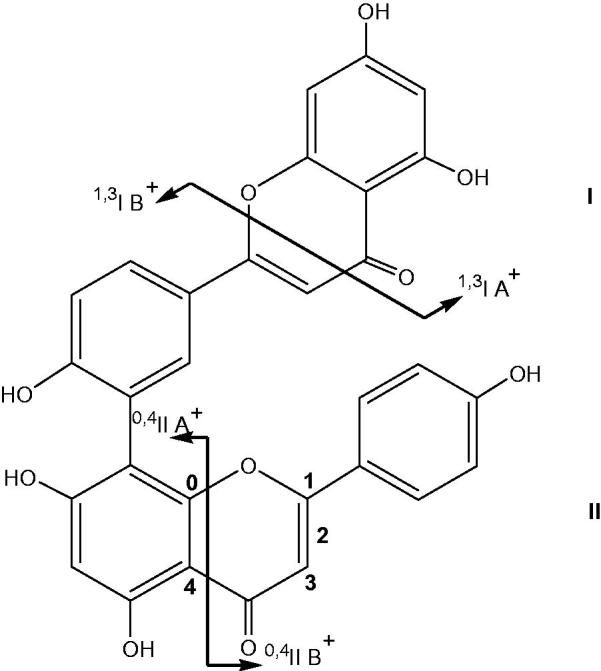
Nomenclature adopted for the retrocyclization fragments of biflavone (illustrated on amentoflavone).
Compound 2 in Fr 1 (Figures 1 and 2) showed a base peak [M − H]− at m/z 417 and an MS2 fragment at m/z 285 corresponding to an aglycone. These data suggested that compound 2 would be identified to either luteolin or isoscutellarein. The loss of 132 amu is attributed to mono-O-pentosyl moiety. On the other hand, the characteristic UV spectrum with maxima at 275, 303 and 328 nm was in agreement with those described for isoscutellarein glycosides in Veronica L. (Scrophulariaceae) species (Saracoglu et al. 2004). Furthermore, the λmax at 303 nm in the UV spectrum related to compound 2 would support that this compound derives from isoscutellarein rather than from luteolin having λmax around 350 nm in its UV spectrum (João et al. 2014). Besides, the sugar moiety (mono-O-pentosyl) would be linked to the isoscutellarein hydroxyl group either in the 8- or in the 7-position. Owing to the absence of pure standards helpful for attribution of the sugar position, comparison of compound 2 UV spectrum with literature data (Grayer-Barkmeijer & Tomás-Barberán 1993; Upson et al. 2000) was diagnostic. Due to a certain disagreement in the literature on this matter, we selected as reference a paper that describes a structural characterization of isolated isoscutellarein 7-O-pentoside. The λmax values (275, 303, 328 nm) reported for this compound are nearly superimposable in compound 2. Accordingly, compound 2 was identified as isoscutellarein 7-O-pentoside.
Compound 3 in Fr 2 (Figures 1 and 2) exhibited a base peak [M − H]− at m/z 537 and MS2 fragment at m/z 375. The λmax values of absorption (230, 269, 332 nm) in its UV spectrum are characteristic of a biflavonoid. Furthermore, the overlapping of the UV spectrum of apigenin with those of the biflavonoid derivatives confirmed the presence of biapigenin compounds (Natalizia et al. 2009). HPLC-MS is rarely used for full structure characterization, but it provides molecular mass of the different constituents. With this knowledge, HPLC-MS-MS and MS-MS analysis can be pursued for further structure characterization. Additionally, it can be used to determine the occurrence of previously identified compounds, and hence minimizes the effort lost in their isolation (Constant & Beecher 1995). It is also employed for quantitative analysis or suited to the identification of labile compounds in solution, such as acylated flavonoid. The highest sensitivity is obtained using ESI in the negative ion mode with an eluent system consisting of an acidic ammonium acetate buffer (Rauha et al. 2001).
The major fragmentations helpful for flavonoid aglycone identification are the 0/2, 0/4, 1/3 and 1/4 cleavages represented in Figure 2 and their occurrence depends on the substituent’s flavonoid skeleton. We also denote the biflavonoid Retro–Diels–Alder (RDA) fragments similar to the way that flavonoid aglycone is named, taking flavonoid part whose B‐ring is connected to the other flavonoid as part I and the other as part II (Figure 2).
In a previous study, it was reported that the fragmentation routes involving C‐ring cleavage of flavonoid part I at positions 1/3 and part II at position 0/4 are the primary pathways of amentoflavone‐type biflavonoids, whereas the chances are greater that C‐ring cleavage fragmentation occurs on flavonoid part I at positions 1/3 and 1/4 for robustaflavonoids (Zhang et al. 2011). The ESI-MS fragmentation routes of robustaflavone-type biflavonoids had strong similarities and differences compared with amentoflavone-type biflavonoids. Hence, in order to confirm the structure of compound 3 (amentoflavone or robustaflavone) we have purified fraction Fr 2 by a semi-preparative HPLC. This purification led to the isolation of a pure compound (m = 7 mg). The comparison of the 1H NMR spectroscopic data given in Table 3 of compound 3 with those of amentoflavone and robustaflavone reported in the literature (Lin & Chen 1974; Zhang et al. 2011) allowed the identification of compound 3 as amentoflavone.
Table 3.
Comparison of the 1H NMR spectroscopic data of compound 3 with those of amentoflavone and robustaflavone.
| Carbon | Compound 3 | Amentoflavone | Robustaflavone |
|---|---|---|---|
| number | δH (m, J Hz) | δH (m, J Hz) | δH (m, J Hz) |
| 1 | – | – | – |
| 2 | – | – | – |
| 3 | 6.76 (s) | 6.76 (s) | 6.77 (s) |
| 4 | – | – | – |
| 5 | – | – | – |
| 6 | 6.21 (d, 2.0) | 6.21 (d, 2.0) | 6.19 (d, 1.8) |
| 7 | – | – | – |
| 8 | 6.45 (d, 2.0) | 6.46 (d, 2.0) | 6.48 d (1.8) |
| 9 | – | – | – |
| 1' | – | – | – |
| 2' | 7.99 (s) | 7.99 (s) | 7.79 (d, 1.8) |
| 3' | – | – | – |
| 4' | – | – | – |
| 5' | 7.17 (d, 8.8) | 7.17 (d, 8.8) | 7.05 (d, 8.6) |
| 6' | 7.95 (m) | 7.97 (dd, 2.0, 8.8) | 7.91 (dd, 1.8, 8.6) |
| 1' | – | – | – |
| 2'' | – | – | – |
| 3'' | 6.72 (s) | 6.72 (s) | 6.80 (s) |
| 4'' | – | – | – |
| 5'' | – | – | – |
| 6'' | 6.42 (s) | 6.42 (s) | – |
| 7'' | – | – | – |
| 8'' | – | – | 6.65 (s) |
| 9'' | – | – | – |
| 1''' | – | – | – |
| 2''' | 7.57 (d, 8.8) | 7.57 (d, 8.8) | 7.95 (d, 8.8) |
| 3''' | 6.75 (d, 8.8) | 6.74 (d, 8.8) | 6.95 (d, 8.8) |
| 4''' | – | – | – |
| 5''' | 6.75 (d, 8.8) | 6.74 (d, 8.8) | 6.95 (d, 8.8) |
| 6''' | 7.57 (d, 8.8) | 7.57 (d, 8.8) | 7.95 (d, 8.8) |
Compound 4 in Fr 3 presented a base peak [M − H]− at m/z 433 and a significant fragment at m/z 301 in its mass spectrum. Its UV spectrum showed two absorption bands at λmax 257 and 362 nm. These data suggested that compound 4 would be the flavonoid quercetin 3-O-pentosides. The identification of this compound was confirmed by co-chromatography with a standard.
Compounds 1, 2, 3 and 4 (Figure 3) were identified as quercetin 3-O-glycosides, isoscutellarein 7-O-pentoside, amentoflavone and quercetin 3-O-pentosides, respectively. To the best of our knowledge, these compounds were identified for the first time in J. phoenicea leaves.
Figure 3.
Chemical structures of the main identified compounds.
Antioxidant activity
The hexane, methanol extracts and fractions (Fr1–3) as well as the purified amentoflavone were tested for their antioxidant property using the DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging assay (Table 4). Amentoflavone is endowed with a powerful antioxidant activity (IC50 = 14.1 ± 0.4) higher than that of butylate hydroxytoluene (BHT) (IC50 = 18.1 ± 0.2 μg/mL). In addition, fraction Fr2 (IC50 = 20.1 ± 0.2 μg/mL) exhibited a strong scavenging activity comparable to that of BHT, whereas Fr1 and Fr3 were less active (IC50 = 40 ± 1.2, IC50 = 55 ± 1.5 μg/mL, respectively). Hexane extract did not show any activity with the DPPH method. However, methanol extract has a potent antioxidant activity (IC50 = 28.0 ± 0.1). This activity could in part be attributed to the presence of flavonoid and biflavone compounds: amentoflavone, quercetin 3-O-glycosides, isoscutellarein 7-O-pentoside and quercetin 3-O-pentosides identified in fractions from J. phoenicea methanol extract. In this context, it was established that stable flavonoid radicals are obtained when the compounds are acting as antioxidants. The flavonoids function acts as terminator of free radicals by rapid donation of a hydrogen atom affording a phenoxy radical intermediate that is relatively stable (Torel et al. 1986; Bors et al. 1990). In addition, the antioxidant activity of flavonoids is also linked to their function as chelators of metal ions that are capable of catalyzing lipid peroxidation (Arora & Nair 1998). Several studies of the relationship between the antioxidant activity of flavonoids, as hydrogen-donating free radical scavengers, and their chemical structures have been reported (Arora & Nair 1998). Mora et al. showed that maximum effectiveness of radical scavenging activity apparently requires the 3-hydroxyl (OH) group attached to the 2,3-double bond adjacent to the 4-carbonyl in the C-ring and an o-diphenolic group in ring B. The flavonol quercetin possesses all these structural requirements and is therefore a potent radical scavenger. O-Glycosylation has a slight negative influence at C-7, suggesting a partial role for the free hydroxyl at this position, while at C-3 it has no effect. Furthermore, previous studies reported the radical scavenging properties of aglicons quercetin and their glycosides and of amentoflavone (Ibrahim et al. 2007; Mariani et al. 2008). Therefore, the determined antioxidant activity of fractions from methanol extract could be related, almost in part, to the presence of the identified flavonoids.
Table 4.
DPPH-radical scavenging and α-amylase inhibition activities of hexane and methanolic extracts, fractions Fr1–3 and amentoflavone.
| Fractions | α-Amylase IC50 (μg/ml) | DPPH IC50 (μg/mL) |
|---|---|---|
| Hexane extract | 30.15 ± 0.72 | inactif |
| MeOH extract | 53.76 ± 1.23 | 28.0 ± 0.1 |
| Fr1 | 29.2 ± 1.4 | 40.0 ± 1.2 |
| F 2 | 28.4 ± 1.3 | 20.1 ± 0.2 |
| F 3 | 34.8 ± 1.7 | 55.0 ± 1.5 |
| Amentoflavone | 20.4 ± 1.2 | 14.1 ± 0.4 |
| Acarbose | 14.9 ± 1.0 | – |
| BHT | – | 18.1 ± 0.2 |
α-Amylase inhibitory activity
Inhibition of α-amylase is important for the resolution of type II diabetes (Mariani et al. 2008). In our previous studies (Keskes et al. 2014) we have established that the hexane and methanol extracts from J. phoenicea leaves are endowed with a potent α-amylase inhibition activity (IC50 = 30.15 and 53.76 μg/mL, respectively) (Table 4). The powerful activity of hexane extract may be explained by its richness in terpenic compounds (74.9%), including monoterpens and sesquiterpens. This result is in full agreement with the finding of Basak and Candan (2010) who proved that terpenic compounds cause a decrease in the rate of glucose and postprandial-glucose absorption. These researchers tested the α-amylase inhibition capacity of Eucalyptus camaldulensis Dehnh (Myrtaceae) essential oil and its three major compounds: p-cymene (68.43%), 1,8-cineole (13.92%) and α-pinene (3.45%). They found that α-amylase inhibition effect caused by the essential oil is superior to that of the three pure compounds tested. These results suggest that these compounds synergy each other or with other components of the essential oil.
On the other hand, Table 4 indicates that the purified amentoflavone is endowed with a powerful inhibition activity of α-amylase (IC50 = 20.4 μg/mL) compared to that of acarbose (IC50 = 14.9 μg/mL). In addition, Fr1, Fr2 and Fr3 exhibited significant inhibitory effects against α-amylase (IC50 = 29.2, 28.4 and 34.8 μg/mL, respectively). This result could be explained by the presence of O-glycosylated flavonoids and biflavone in these fractions. In this context, previous studies have used a Roman ethnobotanic approach and identified species of medicinal plants used to treat symptoms of diabetes (Hamdan & Afifi 2004; Basak & Candan 2010). Screening studies of the antidiabetic activity of extracts from these species have revealed that quercetin and quercetin-O-glycosides are the primary causes for the decrease of glucose levels in the blood. Moreover, Kim et al. (2000) have found that amentoflavone with the free OH groups at C-7, C-4” and C-4''' have a relatively important inhibitory activity against α-glucosidase, an enzyme involved in diabetes.
Conclusion
This work is the first report dealing with the identification and characterization of volatile components and flavonoids in hexane and methanol extracts from J. phoenicea leaves, respectively. The biological activities obtained for these extracts suggest that this species justifies further study. The results of this study also provide a scientific support to some medicinal uses of J. phoenicea in North Africa. The antioxidant and antidiabetic activities could be accredited to the high content of flavonoids and volatile components identified for the first time in J. phoenicea leaves by HPLC-MS and GC-MS, respectively.
Disclosure statement
The authors report that they have no conflicts of interest.
Funding
This research was supported by the Ministry of Higher Education and Scientific Research, Tunisia.
References
- Abad MJ, Bedoya LM, Apaza L, Bermejo P.. 2012. The Artemisia L. genus: a review of bioactive essential oils. Molecules. 17:2542–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RP, Barrero AF, Lara A.. 1996. Comparisons of the leaf essential oils of Juniperus phoenicea, Juniperus phoenicea subsp. eu-mediterranea Lebr. & Thiv. and J. phoenicea var. turbinata (Guss.) Parl. J Essent Oil Res. 8:367–371. [Google Scholar]
- Allali H, Benmehdi H, Dib MA, Ghalem TS, Benabadji N.. 2008. Phytotherapy of diabetes in West Algeria. Asian J Chem. 20:2701–2710. [Google Scholar]
- Amer MM, Wasif MM, Abo-Aytta AM, Gabr FAA.. 1994. Chemical and biological evaluation of Juniperus phoenicea as a hypoglycemic agent. Zagazig J Agric Res. 21:1077–1091. [Google Scholar]
- Arora A, Nair MG.. 1998. Structure-activity relationships for antioxidant activities of a series of flavonoids in a liposomal system . Free Radic Biol Med. 24:1355–1365. [DOI] [PubMed] [Google Scholar]
- Barrero AF, Herrador MM, Arteaga P, Quílez del Moral E, Sánchez-Fernández E, Akssira M, Aitigri M, Mellouki F, Akkad S.. 2006. Chemical composition of the essential oil from the leaves of Juniperus phoenicea L. from North Africa. J Essent Oil Res. 18:168–169. [Google Scholar]
- Basak SS, Candan F.. 2010. Chemical composition and in vitro antioxidant and antidiabetic activities of Eucalyptus Camaldulensis Dehnh. essential Oil. J Iran Chem Soc. 7:216–226. [Google Scholar]
- Bellakhder J. 1997. Traditional Moroccan pharmacopeia. Paris, France: Ibis Press French. [Google Scholar]
- Bors W, Heller W, Michel C, Saran M.. 1990. Flavonoids as antioxidants: determination of radical-scavenging efficiencies . Meth Enzymol. 186:343–355. [DOI] [PubMed] [Google Scholar]
- Bouaziz M, Feki I, Ayadi M, Jemai H, Sayadi S.. 2010. Stability of refined olive oil and olive-pomace oil added by phenolic compounds from olive leaves. Eur J Lipid Sci Tech. 112:894–905. [Google Scholar]
- Boukhris M, Simmonds MSJ, Sayadi S, Bouaziz M.. 2013. Chemical composition and biological activities of polar extracts and essential oil of rose-scented geranium, Pelargonium graveolens. Phytother Res. 27:1206–1213. [DOI] [PubMed] [Google Scholar]
- Bravo L. 1998. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 56:317–333. [DOI] [PubMed] [Google Scholar]
- Cavaleiro C, Rezzi S, Salgueiro L, Bighelli A, Casanova J, da Cunha AP.. 2001. Infraspecific chemical variability of the leaf essential oil of Juniperus phoenicea var. turbinata from Portugal. Biochem Syst Ecol. 29:1175–1183. [DOI] [PubMed] [Google Scholar]
- Constant HL, Beecher CWW.. 1995. A method for the dereplication of natural product extracts using electrospray HPLC/MS. Nat Prod Lett. 6:193–196. [Google Scholar]
- Cook NC, Samman S.. 1996. Flavonoids—chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem 7:66–76. [Google Scholar]
- Cuyckens F, Claeys M.. 2004. Mass spectrometry in the structural analysis of flavonoids. J Mass Spectrom. 39:1–15. [DOI] [PubMed] [Google Scholar]
- Davies NW. 1990. Gas chromatographic retention indices of monoterpenes on methyl silicone and Carbowax 20M phases. J Chromatogr. 503:1–24. [Google Scholar]
- Feki I, Allouche N, Sayadi S.. 2005. The use of polyphenolic extract, purified hydroxytyrosol and 3, 4-dihydroxypheny l acetic acid from olive mill wastewater for the stabilization of refined oils: a potential alternative to synthetic antioxidants. Food Chem. 93:197–204. [Google Scholar]
- Gella FJ, Gubern G, Vidal R, Canalias F.. 1997. . Determination of total and pancreatic alpha-amylase in human serum with 2-chloro-4-nitrophenyl-alpha-d-maltotrioside as substrate. Clin Chim Acta. 259:147–160. [DOI] [PubMed] [Google Scholar]
- Grayer RJ, Kite GC, Abou-Zaid M, Archer LJ.. 2000. The application of atmospheric pressure chemical ionization liquid chromatography mass-spectrometry in the chemotaxonomic study of flavonoids: characterization of flavonoids from Ocimum gratissimum var. gratissimum. Phytochem Analys. 11:257–267. [Google Scholar]
- Grayer-Barkmeijer RJ, Tomás-Barberán FA.. 1993. 8-Hydroxylated flavone O-glycosides and other flavonoids in chemotypes of Gratiola officinalis. Phytochemistry. 34:205–210. [Google Scholar]
- Hamdan II, Afifi FU.. 2004. Studies on the in vitro and in vivo hypoglycemic activities of some medicinal plants used in treatment of diabetes in Jordanian traditional medicine. J Ethnopharmacol. 93:117–121. [DOI] [PubMed] [Google Scholar]
- Heneidak S, Grayer RJ, Kite GC, Simmonds MSJ.. 2006. Flavonoid glycosides from Egyptian species of the tribe Asclepiadeae (Apocynaceae, subfamily Asclepiadoideae). Biochem Syst Ecol. 34:575–584. [Google Scholar]
- Ibrahim NA, El-Seedi HR, Mohammed MM.. 2007. Phytochemical investigation and hepatoprotective activity of Cupressus sempervirens L. leaves growing in Egypt. Nat Prod Res. 21:857–866. [DOI] [PubMed] [Google Scholar]
- João CMB, Maria ID, Jelena Ž, Stojković D, Soković M, Santos-Buelgab C, Ferreira ICFR.. 2014. . Phenolic profiling of Veronica spp. grown in mountain, urban and sandy soil environments. Food Chem. 163:275–283. [DOI] [PubMed] [Google Scholar]
- Keskes H, Mnafgui K, Hamden K, Damak M, El Feki A, Allouche N.. 2014. In vitro anti-diabetic, anti-obesity and antioxidant proprieties of Juniperus phoenicea L. leaves from Tunisia. Asian Pac J Trop Biomed. 4:789–795. [Google Scholar]
- Kim JS, Kwon CS, Son KH.. 2000. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem. 64:2458–2461. [DOI] [PubMed] [Google Scholar]
- Kumar P, Kumaravel S, Lalitha C.. 2010. Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr J Biochem Res. 4:191–195. [Google Scholar]
- Lin YM, Chen FC.. 1974. Robustaflavone from the seed-kernels of Rhus succedanea. Phytochemistry. 13:1916–1919. [Google Scholar]
- Madar Z. 1989. The effect of acarbose and miglitol (BAY-M-1099) on postprandial glucose levels following ingestion of various sources of starch by nondiabetic and streptozotocin-induced diabetic rats. J Nutr. 119:2023–2029. [DOI] [PubMed] [Google Scholar]
- Mariani C, Braca A, Vitalini S, De Tommasi D, Visiolid F, Fico G.. 2008. Flavonoid characterization and in vitro antioxidant activity of Aconitum anthora L. (Ranunculaceae). Phytochemistry. 69:1220–1226. [DOI] [PubMed] [Google Scholar]
- Marzoug HNB, Romdhane M, Lebrihi A, Mathieu F, Couderc F, Abderraba M, Khouja ML, Bouajila J.. 2011. Eucalyptus oleosa essential oils: Chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits). Molecules. 16:1695–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massada Y. 1996. Analysis of essential oils by gas chromatography and, mass spectrometry. New York, NY: John Wiley and Sons. [Google Scholar]
- Moure A, Cruz JM, Franco D, Domínguez JM, Sineiro J, Domínguez H, Núñez MJ, Parajó JC.. 2001. Natural antioxidants from residual sources. Food Chem. 72:145–171. [Google Scholar]
- Nakanishi T, Iida N, Inatomi Y, Murata H, Inada A, Murata J, Lang FA, Iinuma M, Tanaka T.. 2004. Neolignan and flavonoid glycosides in Juniperus communis var. depressa. Phytochemistry. 65:207–213. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Iida N, Inatomi Y, Murata H, Inada A, Murata J, Lang FA, Iinuma M, Tanaka T, Sakagami Y.. 2005. A monoterpene glucoside and three megastigmane glycosides from Juniperus communis var. depressa. Chem Pharm Bull. 53:783–787. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Inatomi Y, Murata H, Iida N, Inada A, Lang FA, Murata J.. 2002. Phytochemical study on American plants I. Two new phenol glucosides, together with known biflavones and diterpene, from leaves of Juniperus occidentalis Hook. Chem Pharm Bull. 50:1358–1361. [DOI] [PubMed] [Google Scholar]
- Natalizia M, Ada T, Paola D, Cacciola F, Donato P, Marino A, Bellinghieri La Barbera TM, Güvenç A, Taviano MF.. 2009. Comparative analysis of flavonoid profile, antioxidant and antimicrobial activity of the berries of Juniperus communis L. var. communis and Juniperus communis L. var. saxatilis Pall. from Turkey. J Agric Food Chem. 57:6570–6577. [DOI] [PubMed] [Google Scholar]
- Rauha JP, Vuorela H, Kostiainen R.. 2001. Effect of eluent on the ionization efficiency of flavonoids by ion spray, atmospheric pressure chemical ionization, and atmospheric pressure photoionization mass spectrometry. J Mass Spectrom. 36:1269–1280. [DOI] [PubMed] [Google Scholar]
- Rezzi S, Cavaleiro C, Bighelli A, Salgueiro L, da Cunha AP, Casanova J.. 2001. Intraspecific chemical variability of the leaf essential oil of Juniperus phoenicea subsp. turbinata from Corsica. Biochem Syst Ecol. 29:179–188. [DOI] [PubMed] [Google Scholar]
- Saracoglu I, Harput US, Ogihara Y.. 2004. Acylated flavone glycosides from Veronica pectinata var. glandulosa and V. persica. Turkish J Chem. 28:751–759. [Google Scholar]
- Torel J, Cillard J, Cillard P.. 1986. Antioxidant activity of flavonoids and reactivity with peroxy radical. Phytochemistry. 25:383–385. [Google Scholar]
- Upson TM, Grayer RJ, Greenham JR, Williams CA, Al-Ghamdi G, Chen FH.. 2000. Leaf flavonoids as systematic characters in the genera Lavandula and Sabaudia. Biochem Syst Ecol. 28:991–1007. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Yue LQ, Yan LL, Shi Y.. 2011. Structural characterization and identification of biflavones in Selaginella tamariscina by liquid chromatography-diode-array detection/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 25:2173–2186. [DOI] [PubMed] [Google Scholar]