Abstract
Context: Indigofera suffruticosa Miller (Fabaceae) and I. truxillensis Kunth produce compounds, such as isatin (ISA) and indirubin (IRN), which possess antitumour properties. Their effects in mammalian cells are still not very well understood.
Objective: We evaluated the activities of ISA and/or IRN on cell viability and apoptosis in vitro, their genotoxic potentials in vitro and in vivo, and the IRN- and ISA-induced expression of ERCC1 or BAX genes.
Materials and methods: HeLa and/or CHO-K1 cell lines were tested (3 or 24 h) in the MTT, Trypan blue exclusion, acridine orange/ethidium bromide, cytokinesis-blocked micronucleus (CBMN) and comet (36, 24 and 72 h) tests after treatment with IRN (0.1 to 200 μM) or ISA (0.5 to 50 μM). Gene expression was measured by RT-qPCR in HeLa cells. Swiss albino mice received IRN (3, 4 or 24 h) by gavage (50, 100 and 150 mg/kg determined from the LD50 – 1 g/kg b.w.) and submitted to comet assay in vivo.
Results: IRN reduced the viability of CHO-K1 (24 h; 5 to 200 μM) and HeLa cells (10 to 200 μM), and was antiproliferative in the CBMN test (CHO-K1: 0.5 to 10 μM; HeLa: 5 and 10 μM). The drug did not induce apoptosis, micronucleus neither altered gene expression. IRN and ISA were genotoxic for HeLa cells (3 and 24 h) at all doses tested. IRN (100 and 150 mg/kg) also induced genotoxicity in vivo (4 h).
Conclusion: IRN and ISA have properties that make them candidates as chemotherapeutics for further pharmacological investigations.
Keywords: Indigofera suffruticosa, Indigofera truxillensis, HeLa cells, CHO-K1 cells, cytokinesis-blocked micronucleus assay, comet assay, apoptosis
Introduction
Naturally occurring compounds, including those extracted from medicinal plants, continue to play an essential role in primary health care, especially in developing countries, and are a promising source of novel bioactive compounds for drug development (Mothana et al. 2009; Cragg and Newman 2013). Specifically, the genus Indigofera (Fabaceae) is known to contain several biologically active compounds, such as flavonoid glycosides (Hasan et al. 1994; Calvo et al. 2011), nitro compounds (Garcez et al. 2003) and alkaloids (Chanayath et al. 2002; Calvo et al. 2011). Among Indigofera species, Indigofera suffruticosa Miller and Indigofera truxillensis Kunth, from which the compounds indoxyl and isatin can be isolated, are common plants found in Brazilian savannah (Calvo et al. 2011). Following dimerization, these substances are converted, respectively, into indigo and its isomer, indirubin (Cooksey 2001).
Since the 1980s, when researchers started testing indirubin in clinical trials for the treatment of chronic myelocytic leukaemia, studies have been conducted to evaluate the antitumour properties of this compound and its analogues, named indigoids (Blažević et al. 2015). Nowadays, several pieces of evidence suggest that these drugs present antiproliferative and proapoptotic activities against different types of tumour cell lines (Nam et al. 2005; Perabo et al. 2011; Singh et al. 2012; Cândido-Bacani et al. 2013; Song et al. 2013; Ichimaru et al. 2015).
Indigoids’ mechanisms of action are still under investigation, but several studies suggest that indirubins act as inhibitors of cyclin-dependent kinases (CDKS) and glycogen synthase kinase 3β (GSK3β) in tumour cells, resulting in an impairment of cell cycle progression. Also, these drugs induce apoptosis by inactivation of Stat3, a transcription factor that controls cell proliferation and survival (Polychronopoulos et al. 2004; Nam et al. 2005; Yu et al. 2016). Moreover, indirubin and its derivatives may induce antiproliferative effects through the regulation of growth factors pathways, interfering with the activity of protein kinase B (Akt), extracellular signal-regulated kinases (Erk), Notch1 and cytokines (Sethi et al. 2006; Zhen et al. 2007; Lee et al. 2008; Kim et al. 2011). In the same direction, isatin inhibits cell proliferation and induces apoptosis in mouse and human neuroblastoma cells by altering Erk signaling (Hou et al. 2008). Also, it is suggested that these drugs inhibit protooncogenes, such as c-myb (Liu et al. 1996), and activates Bax, a proapoptotic Bcl-2 family member (Shi and Shen 2008).
While there are some studies in the literature unveiling mechanisms that may mediate indigoids’ antitumour activities, so far the genotoxic and mutagenic potentials of indirubin in tumour cells remain poorly investigated. Also, for future clinical proposes, it is very important to assess possible toxic effects of the drug in non-tumour cell lines and in vivo. In a previous study, we have demonstrated that isatin did not show a significant mutagenic effect on either Chinese hamster ovary cells (CHO-K1) or human cervical cancer cells (HeLa). However, the drug did reduce cell proliferation and promoted apoptosis in both cell lines (Cândido-Bacani et al. 2013). In addition to these in vitro results, isatin was genotoxic and mutagenic in mice bone marrow and peripheral blood cells after 14 consecutive days of treatment, but not after acute injection (Cândido-Bacani et al. 2011).
Similarly, to better clarify some pharmacological effects and safety of indirubin, the present study aimed to verify whether acute treatment could induce cytotoxicity, mutagenicity and genotoxicity in cultured mammalian cells (CHO-K1 and HeLa cells) and in peripheral blood cells. Furthermore, to complement the studies previously performed using acute isatin treatment (Cândido-Bacani et al. 2011, 2013), we evaluated its genotoxic activity in vitro and its capacity to reduce cell viability in HeLa cells. Finally, we investigated indirubin- and isatin-induced expression of two genes critical for DNA repair and apoptosis, the enzyme excision repair cross-complementation group 1 (ERCC1) and Bcl-2 associated X (BAX), respectively, in HeLa cells.
Materials and methods
Chemicals
Aerial parts of plants were collected in 2006 in Rubião Junior, Botucatu City, São Paulo State, Brazil, and authenticated by Prof. Dr Jorge Yoshio Tamashiro. The voucher specimens of I. suffruticosa (HUEC 129598) and I. truxillensis (HUEC 131827) were deposited at the Herbarium of the State University of Campinas (Unicamp), Campinas, São Paulo, Brazil. The compounds were purified at the Institute of Organic Chemistry, UNESP, Campus of Araraquara, Brazil. Initially, indirubin was obtained from aerial parts (1.5 kg) of I. suffruticosa (5.0 mg) and I. truxillensis (8.0 mg). However, due to the low yield of indirubin isolated from Indigofera species (Calvo et al. 2011), it was synthesized in the laboratory to obtain enough compound for the bioassays. The indirubin was produced based on a modified methodology of Ferandin et al. (2006), where isatin reacted with 3-acetoxyindole in alkaline medium to give, in good yields, the bisindole indirubin selectively in the Z form.
General procedure for the preparation of indirubin is as follows: isatin (0.91 mmol) was dissolved in methanol (20 mL) and 3-acetoxyindole (0.61 mmol) was added, followed by Na2CO3 (155 mg). The mixture was stirred under an inert atmosphere (N2) for 4 h. The dark product obtained was washed with MeOH/H2O (1:1, v/v, 20.0 mL) and filtered. Drying overnight gave the corresponding Z-indirubin (126 mg, 80%). Its structure was identified on the basis of the mass spectra. 1H and 13 C NMR data were compared to those reported in the literature (Guengerich et al. 2004). Since the yield of isatin obtained from medicinal plants is very low, it was commercially acquired (P.A. ≥ 99%, Fluka, St. Louis, MO, CAS: 91-56-5).
Both compounds were diluted in phosphate buffer solution (PBS, 0.01 M) and used at a proportion of 1% of the total culture medium volume. The doses ranged from 0.1 to 200 μM for indirubin and from 0.5 to 50 μM for isatin. The last doses were defined as non-cytotoxic by the MTT assay (Cândido-Bacani et al. 2013). For the in vivo experiments, indirubin was diluted in dimethyl sulphoxide (DMSO, CAS: 67-68-5, Mallinckrodt, Phillipsburg, NJ) to 50% and PBS. The doses of indirubin were determined by the LD50 of indirubin (1 g/kg b.w.) using the acute oral toxicity test, according to the Organization for Economic Co-operation and Development (OECD) protocols (OECD 2001). The doses selected for the genotoxicity and mutagenicity tests (50, 100, and 150 mg/kg b.w.) correspond to 5, 10 and 15% of the estimated LD50. Cytochalasin B (C29H37NO5 – CAS: 14930-96-2, Sigma) was diluted in dimethyl sulphoxide (DMSO, CAS: 67-68-5, Mallinckrodt) to obtain a stock solution of 2 mg/mL, which was kept at 4 °C in the dark. From this stock solution, we prepared a working solution diluted in PBS (0.3 mg/mL) that was stored at 4 °C in the dark. Cytochalasin B was used in culture medium at a final concentration of 3 μg/mL.
Doxorubicin [DXR, 0.3 μM (Pharmacia & Upjohn, Milan, Italy)] and cyclophosphamide [CPA, 40 mg/kg b.w., (CAS: 50-18-0, Sigma, St. Louis, MO)] were used as positive controls in the in vitro and in vivo (intraperitoneally) experiments, respectively. PBS was used as a negative control.
In vitro experiments
Cell culture
CHO-K1 and HeLa cells were maintained in disposable culture flasks (25 cm2 of area, Nunc) at 37 °C in a BOD incubator (Fanem, Bangalore, Karnataka, India). Cells were maintained in complete culture medium (HAM – F10, Sigma + Dulbecco’s modified Eagle’s medium (DMEM), Gibco, Grand Island, NY, 1:1). The medium was supplemented with 10% of foetal calf serum (Gibco), sodium bicarbonate (Reagen, Paraná, Brazil, 1.20 g/L), antibiotics (penicillin 0.06 g/L and streptomycin 0.12 g/L, Sigma) and HEPES (2.38 g/L, Sigma-Aldrich, St. Louis, MO). CHO-K1 and HeLa cells were used between the 3rd and 8th culture passage after thawing. Under these conditions, the cell cycle was approximately 12 h for CHO-K1 cells and 20 h for HeLa cells.
Viability assay: MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide)
The MTT assay is commonly used to measure cell proliferation and survival. Metabolically active cells cleave the MTT solution within their mitochondria and produce a purple crystal product called formazan (Vellonen et al. 2004). This assay was performed to assess cell viability as described by Mosmann (1983), with some modifications. The concentrations of indirubin used were 0.1 to 200.0 μM. Cells were seeded independently in a 96-well plate with the final volume of 200 μL of complete culture medium containing 2 × 104 cells per well (CHO-K1) or 1 × 104 cells per well (HeLa). The medium was aspirated and cells were exposed to 10 concentrations of indirubin (7 wells for each concentration) and incubated for 3 or 24 h at 37 °C (CHO-K1) or 24 h at 37 °C (HeLa). After treatment, 100 μL of MTT solution (5 mg/mL) was added to each well and incubated at 37 °C for 4 h. After incubation, the MTT-containing medium was removed and 200 μL of DMSO was added to each well to dissolve the formazan crystals formed. The absorbances were measured using spectrophotometer (Uniscience) at 550 nm and the results were expressed as cell growth inhibition according to the following formula:
where the negative control group was considered as 100% of viable cells.
Trypan blue exclusion test of cell viability
The Trypan blue exclusion test is a common technique used to determine the number of viable cells in a cell suspension. Unviable cells have membrane damaged and thus stain blue due to the incorporation of the Trypan blue dye. In this test, HeLa cells were treated with indirubin (0.2 to 5.0 μM) or isatin (0.5 to 50 μM) for 3 and 24 h. Cells were then centrifuged (5 min, 1000 rpm) and the pellet was resuspended with culture medium and the Trypan blue dye (0.04%, Acrós Organics, Geel, Belgium). Immediately after, 10 μL was put in a haemocytometer with a coverslip and observed in an inverted optical microscope (Olympus). A total of 200 cells were counted per treatment and the results were expressed as a percentage of viable cells.
Cytokinesis-blocked micronucleus (CBMN) assay
To assess the mutagenic potential of indirubin, the CBMN test was used to observe the presence of micronucleus in binucleated cells as previously described by Fenech and Morley (1985), with some modifications. Three independent experiments were performed testing five concentrations of indirubin (0.1 to 10.0 μM). These concentrations were deemed appropriate by the MTT test. CHO-K1 cells were treated for 3 and 24 h (Aardema et al. 2006), and HeLa cells were treated for 24 h. After treatment, cultures were exposed to cytochalasin B for 20 h (cells CHO-K1) and 24 h (cells HeLa) to obtain binucleated cells. CHO-K1 cells were resuspended in chilled hypotonic solution of sodium citrate (1%) together with one drop of 1% formaldehyde. Cells were fixed with a 3:1 methanol/acetic acid solution. HeLa cells were resuspended in chilled hypotonic solution of potassium chloride (0.4%), together with one drop of 1% formaldehyde and fixed with a 4:1 methanol/acetic acid solution. Following fixation, the slides were prepared and dyed with Giemsa solution (3%) diluted in phosphate buffer (Na2HPO4 0.06 M and KH2PO4 0.06 M, pH =6.48) for 5 min, washed with water, dried and kept at 4 °C until microscopic analysis. Cells were analyzed using a microscope (Nikon, Brazil) with a magnification of 40×. The three parameters considered in the cytological analysis were as follows: (i) frequency of micronucleated binucleated cells in 3000 binucleated cells as based on established criteria (Titenko-Holland et al. 1997; Fenech 2000; Lorge et al. 2006), (ii) induction factor (IF) and (iii) nuclear division index (NDI) calculated as previously described by Eastmond and Tucker (1989) using the formula: NDI = [(1 × M1) + (2 × M2) + (3 × M3) + (4 × M4)]/N, where M1, M2, M3 and M4 indicate the number of cells with one, two, three and four nuclei, respectively, and N is the total number of viable cells. To determine NDI, 500 cells were analyzed by repetition.
Comet assay in vitro
The comet assay was performed using a modified methodology of the alkaline version as proposed by Singh et al. (1988). Cells were treated with indirubin (0.2 to 5.0 μM) or isatin (0.5 to 50 μM) for 3 and 24 h. Next, cells were centrifuged (1000 rpm, 5 min) and 20 μL of the cell suspension was added to 120 μL of low-melting point agarose (Invitrogen). This solution was spread in slides previously covered with normal melting point agarose (Invitrogen). Slides were maintained at 4 °C for 12 h in a membrane lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, 10% DMSO, 1% Triton-X, pH =10) and afterwards submitted to an horizontal electrophoresis (300 mA, 25 V, 20 min, 4 °C in the absence of light). Next, slides were neutralized with Tris 0.4 M (pH =7.5), fixed with absolute ethanol and stored at 4° until later analysis. Cells were stained using Gel Red (Uniscience, Miami, EUA) and analyzed using a fluorescence microscope (Nikon, Brazil) with a magnification of 40×, excitation filter of 515–560 nm and emission filter of 590 nm. A total of 100 cells were analyzed by slide. Visual criteria used to evaluate the size of the comets tail were applied as described by Kobayashi et al. (1995). Each treatment’s score was calculated using the following formula (Manoharan and Banerjee 1985):
where n(x) is the number of nucleoids of the x class and N is the total number of nucleoids evaluated.
Apoptosis test
A fluorescent mixture of acridine orange and ethidium bromide (AO/EB) was used to assess apoptosis (McGahon et al. 1995). To do this, 25 μL of the cell suspension obtained in the MN assay, prior to addition of the hypotonic solution, was added to 10 μL of the AO/EB dye (100 μg/mL). Two hundred cells were counted on each slide and classified as follows: (i) normal cells, (ii) apoptotic cells with the presence of apoptotic corpuscles and no change in the membrane (initial apoptosis), (iii) late apoptotic cells with the presence of apoptotic corpuscles and an altered membrane and (iv) necrotic cells. Slides were examined using a Nikon microscope (Brazil) 60 × magnification with excitation filter of 515–560 nm and emission filter of 590 nm. The results are shown as the mean percentage of apoptosis according to the following formula:
where IA = the number of cells in initial apoptosis and LA = the number of cells in late apoptosis.
Gene expression
Approximately 1 × 106 HeLa cells were pre-incubated in each experimental culture flask for 24 h. Subsequently, the cells were exposed to 5 μM of indirubin and 50 μM of isatin; PBS was used as a control. Total RNA was extracted using Trizol® reagent (Invitrogen, Life Technologies, Carlsbad, CA) and purified with RNA Miniprep Super Kit (Bio Basic Inc., Markham, Canada) according to the manufacturer’s instructions. RNA integrity was verified using 1% agarose gel electrophoresis. RNA quantification and purity were determined by spectrophotometry (Biophotometer – Eppendorf, Hamburg, Germany). Experiments were performed in duplicate.
cDNA synthesis was carried out in 20 μL reactions of containing 1 μg of total RNA, 40 pmol of oligo dT12–18 primer, 10 mM of dNTP Mix, 2 U of RNase out and 10 U of reverse transcriptase M-MLV (Invitrogen, Life Technologies).
Real-time PCR was performed in a PTC 200 DNA Engine Cycler (MJ Research, St. Bruno, Quebec, Canada) using the Chromo 4 detection system (Bio-Rad, Hercules, CA). The reactions were performed in triplicate in a final volume of 20 μL containing 10 μL of Platinum® SYBR® Green qPCR Supermix-UDG (Invitrogen, Life Technologies), 5 pmol of each primer (forward and reverse), and 2 μL of cDNA template. The PCR thermal cycling conditions included an initial step at 50 °C for 2 min and 96 °C for 5 min; 40 cycles at 94 °C for 20 s, 58 °C for 20 s and 72 °C for 20 s; and 72 °C for 2 min. The melting curve analysis was performed at the final stage of the reaction with temperatures ranging from 50 °C and 98 °C every 0.5 °C for 2 s.
The oligonucleotides were designed using the software Gene Runner version 3.05. The sequences are: forward 5′-GATGATTGCCGCCGTGGAC-3′ and reverse 5′-GCCTTGAGCACCAGTTTG-3′ for BAX gene; forward 5′-CGAGGAAGCTGGGCGGTA-3′ and reverse 5′-CAAATGTGGTCAGGAGGGTC-3′ for ERCC1 gene; and forward 5′-GGGCATCCTGGGCTACACT-3′ and reverse 5′-GGTCCAGGGGTCTTACTC-3′ for GAPDH gene.
In vivo experiments
Animals
Male Swiss albino mice (30 g, 7–8 weeks old) from the Central Animal Farm of the State University of Londrina (UEL) were single-housed (41 × 33 × 17 cm) in a temperature-controlled room (24 ± 2 °C) with a 12 × 12-h light-dark cycle. Female Swiss albino mice were maintained in the same conditions, but in groups of four per cage. Animals received water and food ad libitum throughout the study period. All experimental procedures were in accordance with the Ethical Principles in Animal Research adopted by the Brazilian College of Animal Experimentation and were approved by the Ethics Committee on Animal Use of State University of Londrina (CEUA/UEL).
Experimental design
To investigate the genotoxic and mutagenic effects of acute indirubin injections, mice were divided into groups of eight, each containing four males and four females for each treatment. Mice were treated with one of three different doses of indirubin: 50, 100 or 150 mg/kg b.w., by gavage [(0.1 mL/10 g of body weight (b.w.)]. After treatment time, animals were anesthetized and euthanized with 2% xylazine HCl combined with 10% ketamine HCl (1:1). All mice were weighted at the beginning and end of each treatment.
Micronucleus test of peripheral blood cells
The MN test of mice peripheral blood cells after acute exposure to the drug was used as described by Hayashi et al. (1990). Before treatment with indirubin [50, 100 and 150 mg/kg (time T0)] and after 36 h (T1), 48 h (T2) and 72 h (T3), mice blood samples were collected from the tail vein and dropped directly onto slides previously prepared with the dye acridine orange and then covered with cover slips. For each slide, 1000 reticulocytes were counted per animal with the 100 × objective (immersion) and the frequencies of micronucleated cells (MNRETs) were measured using a fluorescence microscope (Nikon, Brazil), combining blue light (488 nm) and yellow filter (515 nm).
Comet assay in vivo
The in vivo comet assay was performed using the alkaline version according to Singh et al. (1988) and Tice et al. (2000) with modifications introduced by da Silva et al. (2000). After 4 or 24 h of treatment with indirubin (50, 100 and 150 mg/kg), heparinized blood samples were collected from the tail vein of mice. Leukocytes were mixed with 0.5% low-melting-temperature agarose (120 μg) in phosphate-buffered saline (PBS) and applied to microscope slides pre-coated with 1.5% agarose in PBS. All subsequent steps were performed as described before (comet assay in vitro). After the procedure, slides were stained with ethidium bromide (20 μg/mL). One hundred cells per animal (two slides, 50 cells each) were analyzed under a fluorescence microscope (Nikon, Brazil) with a blue (488 nm) excitation filter and yellow (515 nm) emission (barrier). The visual criterion to evaluate the size of the comet tail was applied as described by Kobayashi et al. (1995), using the formula previously described (comet assay in vitro).
Statistical analysis
The mean frequency of micronuclei and NDI, scores of DNA damage and the mean percentage of apoptosis were calculated from three independent experiments. Statistically significant differences between control groups and treatments were determined in all experiments using the analysis of variance (ANOVA) test followed by the Tukey test at 95% of confidence. The mean of absorbance obtained in the MTT assay and the mean of viable cells were analyzed using the same statistical tests. All calculations were performed using the SPSS statistics program. The relative expressions of BAX and ERCC1 genes were calculated as described by Pfaffl (2001) using the relative expression software tool (REST) developed by Pfaffl et al. (2002). Data were normalized using the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Results
Cell viability tests
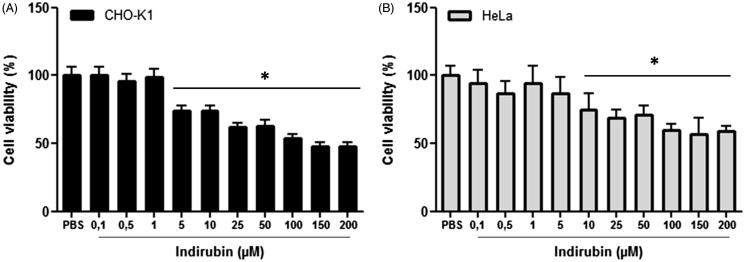
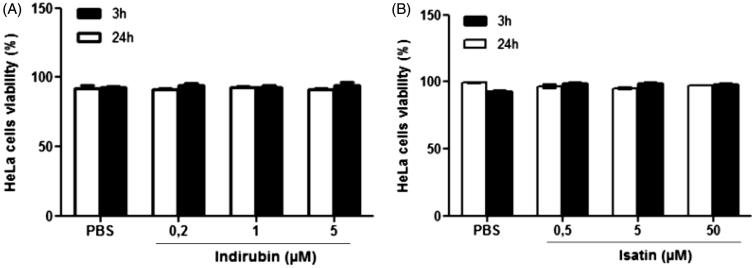
In the MTT test, a reduction in CHO-K1 cell viability was observed following a 24-h treatment at doses of 5 to 200 μM of indirubin (F11,72 = 17.34, p < 0.05, Tukey’s test) and in HeLa cells treated at 10 to 200 μM [Figure 1 (F11,70 = 51.85, p < 0.05, Tukey’s test)] in comparison with the negative control group. In the Trypan blue exclusion test of cell viability, neither indirubin (0.2, 1 and 5 μM, 3 h: F4,10 = 12.79; 24 h: F4,10 = 9.21, p > 0.05, Tukey’s test) nor isatin (0.5, 5 and 50 μM, 3 h: F4,10 = 8.10; 24 h: F4,10 = 43.37, p > 0.05, Tukey’s test) reduced the percentage of HeLa cell viability after 3 or 24 h of treatment (Figure 2).
Figure 1.
Percentage of CHO-K1 (A) and HeLa (B) cell viability obtained in the MTT test after 24 h of treatment with indirubin. The asterisk indicates significant difference compared to the negative control group (p < 0.05, ANOVA followed by Tukey’s test). Each bar represents the mean ± standard deviation of the mean (X ± SD).
Figure 2.
Percentage of HeLa cell viability obtained in the Trypan blue exclusion test after 3 and 24 h of treatment with indirubin (A) and isatin (B). The asterisk indicates significant difference compared to the negative control group (p < 0.05, ANOVA followed by Tukey’s test). Each bar represents the mean ± standard deviation of the mean (X ± SD).
Detection of apoptosis in cell culture
The percentage of apoptotic CHO-K1 and HeLa cells observed following treatment with indirubin (0.1 to 10.0 μM) were statistically equal to the negative control group (p > 0.05, Tukey’s test) and different from the positive control group after 24 h of treatment (CHO-K1: F6,14 = 21.04; HeLa: F6,14 = 6.94, p < 0.05, Tukey’s test, Table 1).
Table 1.
Mean apoptosis index obtained for CHO-K1 and HeLa cells after 24 h of treatment with indirubin.
| Apoptosis Index (%) Mean ± SD |
||
|---|---|---|
| Treatments | CHO-K1 | HeLa |
| PBS | 9.70 ± 0.02 | 6.17 ± 1.15 |
| DXR 0.3 μM | 32.30 ± 0.07* | 12.17 ± 1.44* |
| IRN 0.1 μM | 14.75 ± 0.03 | 7.50 ± 1.41 |
| IRN 0.5 μM | 11.17 ± 0.01 | 7.50 ± 1.41 |
| IRN 1.0 μM | 9.90 ± 0.02 | 7.33 ± 0.29 |
| IRN 5.0 μM | 9.80 ± 0.03 | 5.67 ± 1.04 |
| IRN 10.0 μM | 12.00 ± 0.04 | 8.17 ± 1.04 |
SD: standard deviation; PBS: phosphate-buffered saline, negative control; DXR: doxorubicin, positive control; IRN: indirubin.
Indicates significant difference compared to the negative control group (p < 0.05, ANOVA followed by Tukey’s test).
CBMN test in vitro
The frequencies of micronucleated CHO-K1 cells observed following treatment with five concentrations of indirubin (0.1 to 10.0 μM) were statistically equal to the negative control group (p > 0.05, Tukey’s test) and different from the positive control group after 3 and 24 h of treatment (3 h: F6,14 = 588.53; 24 h: F6,11 = 579.15 p < 0.05, Tukey’s test). HeLa micronucleated cells were statistically equal to the negative and positive control groups (F6,14 = 2.53, p > 0.05, Tukey’s test) after 24 h of treatment. Thus, indirubin did not induce mutagenicity in CHO-K1 and HeLa cells at the concentrations tested (Table 2).
Table 2.
Mean frequency of micronucleated binucleated cells (MNBC) in CHO-K1 and HeLa cells obtained in the MN test after 3 and 24 h of treatment with indirubin.
| CHO-K1 |
HeLa |
|||||
|---|---|---|---|---|---|---|
| MNBC (3 h) |
MNBC (24 h) |
MNBC T (24 h) |
||||
| Treatments | Mean ± SD | IF | Mean ± SD | IF | Mean ± SD | IF |
| PBS | 7.33 ± 1.53 | – | 8.00 ± 1.00 | – | 17.33 ± 6.43 | – |
| DXR 0.75 μg/mL | 171.00 ± 10.15* | 23.32 | 207.00 ± 13.90* | 25.88 | 26.00 ± 1.73 | 1.50 |
| IRN 0.1 μM | 9.67 ± 3.22 | 1.32 | 12.00 ± 2.65 | 1.50 | 14.00 ± 5.29 | 0.81 |
| IRN 0.5 μM | 11.00 ± 2.65 | 1.50 | 6.33 ± 0.58 | 0.79 | 28.33 ± 1.53 | 1.63 |
| IRN 1.0 μM | 9.33 ± 0.58 | 1.27 | 7.00 ± 1.00 | 0.88 | 21.00 ± 3.61 | 1.21 |
| IRN 5.0 μM | 9.33 ± 2.31 | 1.27 | 10.00 ± 1.00 | 1.25 | 25.33 ± 2.52 | 1.46 |
| IRN 10.0 μM | 11.00 ± 2.00 | 1.50 | 9.00 ± 1.00 | 1.13 | 23.67 ± 11.06 | 1.37 |
SD: standard deviation; PBS: phosphate-buffered saline, negative control; DXR: doxorubicin, positive control; IRN: indirubin; IF: induction factor.
Indicates significant difference compared to the negative control group (p < 0.05, ANOVA followed by Tukey’s test).
Nuclear division index (NDI)
The NDI calculated for CHO-K1 cells by the MN test following 3 h of treatment was not statistically significant at any of the concentrations tested (F6,14 = 2.57, p > 0.05, Tukey’s test). However, after a 24-h treatment, indirubin showed antiproliferative activity at concentrations ranging from 0.5 to 10.0 μM (F6,14 = 33.58, p < 0.05, Tukey’s test). Indirubin also showed antiproliferative activity in HeLa cells at 5.0 and 10.0 μM after 24 h of treatment (F6,14 = 5.65, Tukey’s test, p < 0.05, Table 3).
Table 3.
Nuclear division index (NDI) calculated in the MN test after treatment of CHO-K1 cells (3 and 24 h) and HeLa cells (24 h) with indirubin.
| CHO-K1 |
HeLa |
||
|---|---|---|---|
| Treatments | NDI (3 h) Mean ± SD |
NDI (24 h) Mean ± SD |
NDI (24 h) Mean ± SD |
| PBS | 1.83 ± 0.02 | 1.62 ± 0.02 | 1.73 ± 0.00 |
| DXR 0.3 μM | 1.83 ± 0.03 | 1.63 ± 0.03 | 1.71 ± 0.01 |
| IRN 0.1 μM | 1.85 ± 0.03 | 1.65 ± 0.04 | 1.68 ± 0.07 |
| IRN 0.5 μM | 1.79 ± 0.01 | 1.54 ± 0.04* | 1.63 ± 0.02 |
| IRN 1.0 μM | 1.78 ± 0.04 | 1.50 ± 0.02* | 1.61 ± 0.04 |
| IRN 5.0 μM | 1.82 ± 0.03 | 1.47 ± 0.02* | 1.57 ± 0.05* |
| IRN 10.0 μM | 1.84 ± 0.04 | 1.14 ± 0.03* | 1.55 ± 0.09* |
SD: standard deviation; PBS: phosphate-buffered saline, negative control; DXR: doxorubicin, positive control; IRN: indirubin; NDI: nuclear division index.
Indicates significant difference compared to the negative control group (p < 0.05, ANOVA followed by Tukey’s test).
CBMN test in vivo
No significant differences were observed between results for males and females (p > 0.05, Student’s t-test, data not shown); consequently, data for both sexes were combined (Table 4). The frequencies of micronucleated reticulocytes in mice peripheral blood observed following treatment with indirubin (50, 100 and 150 mg/kg) were statistically equal to the negative control group (p > 0.05, Tukey’s test) and different from the positive control group after 36, 48 and 72 h of treatment (0 h: F5,42 = 1.01, p > 0.05, Tukey’s test; 36 h: F5,42 = 145.65; 48 h: F5,42 = 170.39; 72 h: F5,42 = 67.51, p < 0.05, Tukey’s test, Table 4).
Table 4.
Frequency of micronucleated reticulocytes (MNRETs) in mice peripheral blood observed after 36, 48 and 72 h of treatment with indirubin.
| MNRET (Mean ± SD) |
||||
|---|---|---|---|---|
| Treatments (mg/kg b.w.) |
T0 | T1 | T2 | T3 |
| PBS | 2.50 ± 1.07 | 2.50 ± 1.31 | 1.75 ± 1.58 | 1.88 ± 1,13 |
| DMSO | 2.50 ± 1.20 | 22.00 ± 3.78* | 28.63 ± 5.15* | 10.25 ± 2.19 |
| CPA 40 | 3.13 ± 1.46 | 2.13 ± 0.64 | 2.88 ± 0.84 | 1.63 ± 1.06* |
| IRN 50 | 2.25 ± 1.91 | 2.88 ± 1.13 | 1.63 ± 0.74 | 1.00 ± 0.76 |
| IRN 100 | 1.63 ± 1.51 | 1.88 ± 1.56 | 2.25 ± 2.28 | 1.75 ± 0.70 |
| IRN 150 | 2.00 ± 1.31 | 2.13 ± 1.13 | 2.00 ± 1.07 | 1.38 ± 0.92 |
Mean ± SD: mean ± standard deviation; PBS: phosphate-buffered saline, negative control; DMSO: dimethyl sulphoxide, DMSO control; CPA: cyclophosphamide, positive control; IRN: indirubin; T0: before treatment; T1: 36 h; T2: 48 h; T3: 72 h.
Indicates significant difference compared to the negative control group (p < 0.05, ANOVA followed by Tukey’s test).
Comet assay
The damage scores obtained from the comet assay in HeLa cells after 3 h of treatment with indirubin (0.2, 1 and 5 μM) and isatin (0.5, 5 and 50 μM) were statistically different from the negative control group (indirubin: F4,10 = 364.40; isatin: F4,10 = 38.21, p < 0.05, Tukey’s test, Tables 5 and 6) and equal to the positive control group (p > 0.05, Tukey’s test) at all doses tested. After 24 h of treatment, indirubin did not induce genotoxicity at the dose of 0.2 μM (p > 0.05, Tukey’s test, Table 5), but increased the damage scores in relation to the negative control group at the doses of 1 and 5 μM (F4,10 = 77.29, p < 0.05, Tukey’s test). The values obtained by these doses, however, also differed from the positive control group (p < 0.05). Isatin induced genotoxicity after 24 h of treatment at all doses tested compared to the negative control group (F4,10 = 57.62, p < 0.05, Tukey’s test, Table 6) and equal to the positive control group only at 50 μM.
Table 5.
Mean frequency of damaged cells (DC), distribution of damage classes and scores obtained in the comet assay from HeLa cells after 3 and 24 h of treatment with indirubin.
| Damage levels |
|||||||
|---|---|---|---|---|---|---|---|
| Treatments | Time (h) | 0 | 1 | 2 | 3 | DC (Mean ± SD) | Score (Mean ± SD) |
| PBS | 3 | 70.33 ± 2.52 | 19.00 ± 1.00 | 10.33 ± 2.52 | 0.33 ± 0.58 | 0.30 ± 0.03 | 0.41 ± 0.06 |
| 24 | 38.66 ± 9.02 | 28.00 ± 4.00 | 21.33 ± 4.51 | 12.00 ± 1.00 | 0.61 ± 0.09 | 1.06 ± 0.14 | |
| DXR 0.3μM | 3 | 5.33 ± 5.51 | 12.67 ± 6.51 | 17.00 ± 7.94 | 65.33 ± 5.86 | 0.95 ± 0.06 | 2.43 ± 0.06* |
| 24 | 0.66 ± 1.15 | 10.33 ± 1.53 | 11.33 ± 1.53 | 77.66 ± 2.52 | 0.99 ± 0.01 | 2.66 ± 0.06* | |
| IRN 0.2 μM | 3 | 6.67 ± 3.51 | 61.33 ± 3.51 | 20.67 ± 2.52 | 11.33 ± 2.52 | 0.93 ± 0.04 | 1.37 ± 0.06* |
| 24 | 31.33 ± 7.57 | 17.33 ± 2.31 | 18.66 ± 8.14 | 32.66 ± 10.97 | 0.68 ± 0.07 | 1.52 ± 0.22# | |
| IRN 1 μM | 3 | 10.00 ± 1.00 | 71.00 ± 7.21 | 14.00 ± 3.46 | 5.00 ± 4.36 | 0.90 ± 0.01 | 1.14 ± 0.10* |
| 24 | 9.33 ± 2.52 | 14.00 ± 2.00 | 30.00 ± 5.29 | 46.66 ± 4.93 | 0.90 ± 0.02 | 2.14 ± 0.05*# | |
| IRN 5 μM | 3 | 1.67 ± 0.58 | 47.33 ± 4.04 | 36.33 ± 8.62 | 14.67 ± 5.51 | 0.98 ± 0.01 | 1.64 ± 0.04* |
| 24 | 5.66 ± 2.52 | 10.00 ± 2.00 | 23.00 ± 5.57 | 61.33 ± 5.51 | 0.94 ± 0.02 | 2.40 ± 0.07*# | |
SD: standard deviation; PBS: phosphate-buffered saline, negative control; DXR: doxorubicin, positive control; IRN: indirubin; DC: damaged cells.
Indicates significant difference compared to the negative control group.
#Indicates significant difference compared to the positive control group (p < 0.05, ANOVA followed by Tukey’s test).
Table 6.
Mean frequency of damaged cells (DC), distribution of damage classes and scores obtained in the comet assay from HeLa cells after 3 and 24 h of treatment with isatin.
| Damage levels |
|||||||
|---|---|---|---|---|---|---|---|
| Treatments | Time (h) | 0 | 1 | 2 | 3 | DC (Mean ± SD) | Score (Mean ± SD) |
| PBS | 3 | 97.00 ± 1.00 | 3.00 ± 1.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| 24 | 78.00 ± 1.00 | 18.00 ± 1.00 | 4.00 ± 2.00 | 0.00 ± 0.00 | 1.06 ± 0.14 | 1.06 ± 0.14 | |
| DXR 0.3 μM | 3 | 5.33 ± 2.52 | 4.33 ± 3.51 | 4.00 ± 2.65 | 86.33 ± 4.73 | 2.71 ± 0.08 | 2.71 ± 0.08* |
| 24 | 0.00 ± 0.00 | 9.33 ± 4.16 | 17.00 ± 0.00 | 73.66 ± 4.16 | 2.60 ± 0.06 | 2.60 ± 0.06* | |
| ISA 0.5 μM | 3 | 25.00 ± 12.53 | 8.00 ± 9.54 | 4.33 ± 3.21 | 62.67 ± 19.30 | 2.05 ± 0.53 | 2.05 ± 0.53* |
| 24 | 11.33 ± 3.78 | 27.00 ± 7.81 | 24.00 ± 4.36 | 37.66 ± 7.23 | 1.52 ± 0.22 | 1.52 ± 0.22*# | |
| ISA 5 μM | 3 | 16,00 ± 9.54 | 23.33 ± 11.02 | 6.33 ± 4.04 | 61.00 ± 17.05 | 2.19 ± 0.33 | 2.19 ± 0.33* |
| 24 | 10.33 ± 1.53 | 14.66 ± 4.16 | 21.00 ± 1.00 | 54.00 ± 4.58 | 2.14 ± 0.05 | 2.14 ± 0.05*# | |
| ISA 50 μM | 3 | 9.00 ± 2.00 | 10.67 ± 1.53 | 12.33 ± 6.81 | 71.33 ± 12.22 | 2.49 ± 0.25 | 2.49 ± 0.25* |
| 24 | 0.00 ± 0.00 | 11.33 ± 3.51 | 16.33 ± 2.08 | 72.33 ± 5.51 | 2.48 ± 0.19 | 2.48 ± 0.19* | |
SD: standard deviation; PBS: phosphate-buffered saline, negative control; DXR: doxorubicin, positive control; ISA: isatin; DC: damaged cells.
Indicates significant difference compared to the negative control group.
#Indicates significant difference compared to the positive control group (p < 0.05, ANOVA followed by Tukey’s test).
In the in vivo comet assay, indirubin induced genotoxicity in peripheral blood cells at the higher doses (100 and 150 mg/kg) after 4 h of treatment compared to both the negative and positive control groups (p < 0.05, Tukey’s test). However, after 24 h, indirubin failed to induce genotoxicity at all doses tested (p > 0.05, Tukey’s test). No significant differences were observed between males and females (Student’s t-test, p > 0.05, data not shown); consequently, data for both sexes were combined (Table 7).
Table 7.
Mean frequency of damaged cells (DC), distribution of damage classes and scores obtained in the comet assay from the peripheral blood after 4 and 24 h of treatment with indirubin.
| Damage levels |
|||||||
|---|---|---|---|---|---|---|---|
| Treatments (mg/kg b.w.) |
Time (h) | 0 | 1 | 2 | 3 | DC (Mean ± SD) | Score (Mean ± SD) |
| PBS | 4 | 90.25 ± 1.58 | 9.50 ± 1.31 | 0.13 ± 0.35 | 0.13 ± 0.35 | 9.63 ± 1.41 | 0.10 ± 0.02 |
| 24 | 87.14 ± 3.24 | 11.71 ± 1.89 | 0.86 ± 1.07 | 0.29 ± 0.76 | 12.86 ± 3.24 | 0.15 ± 0.06 | |
| CPA 40 | 4 | 61.86 ± 6.26 | 34.71 ± 4.50 | 2.14 ± 1.57 | 1.29 ± 0.95 | 38.14 ± 6.26 | 0.42 ± 0.09* |
| 24 | 61.17 ± 8.52 | 34.83 ± 7.57 | 3.33 ± 2.25 | 0.67 ± 0.82 | 38.83 ± 8.52 | 0.44 ± 0.10* | |
| DMSO | 4 | 90.63 ± 1.92 | 8.88 ± 1.46 | 0.50 ± 0.93 | 0.00 ± 0.00 | 9.36 ± 1.92 | 0.10 ± 0.03 |
| 24 | 85.75 ± 2.92 | 13.13 ± 2.17 | 1.13 ± 0.99 | 0.00 ± 0.00 | 14.25 ± 2.92 | 0.15 ± 0.04 | |
| IRN 50 | 4 | 90.14 ± 1.95 | 9.00 ± 1.29 | 0.71 ± 1.11 | 0.14 ± 0.38 | 9.86 ± 1.95 | 0.11 ± 0.03 |
| 24 | 87.29 ± 4.31 | 12.14 ± 4.18 | 0.63 ± 1.06 | 0.25 ± 0.71 | 12.71 ± 4.31 | 0.13 ± 0.04 | |
| IRN 100 | 4 | 80.43 ± 4.69 | 17.14 ± 4.71 | 1.86 ± 1.07 | 0.57 ± 0.79 | 19.57 ± 4.69 | 0.20 ± 0.04*# |
| 24 | 86.63 ± 3.16 | 12.25 ± 3.62 | 0.63 ± 1.06 | 0.25 ± 0.71 | 13.13 ± 3.36 | 0.14 ± 0.03 | |
| IRN 150 | 4 | 83.63 ± 4.78 | 15.75 ± 4.68 | 0.63 ± 0.92 | 0.00 ± 0.00 | 16.38 ± 4.78 | 0.17 ± 0.05*# |
| 24 | 87.13 ± 3.40 | 11.38 ± 3.42 | 1.25 ± 1.28 | 0.25 ± 0.07 | 12.88 ± 3.40 | 0.15 ± 0.04 | |
SD: standard deviation; PBS: phosphate-buffered saline, negative control; CPA: cyclophosphamide, positive control; DMSO: dimethyl sulphoxide, DMSO control; IRN: indirubin; DC: damaged cells.
Indicates significant difference compared to the negative control group.
Indicates significant difference compared to the positive control group (p < 0.05, ANOVA followed by Tukey’s test).
Gene expression
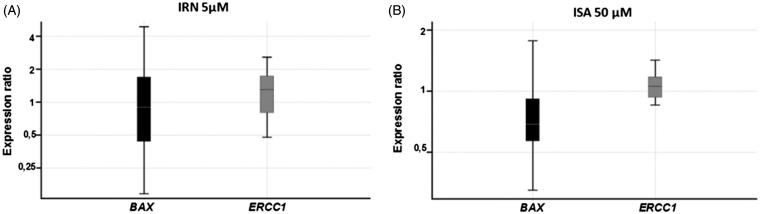
Neither indirubin nor isatin altered BAX (one-way ANOVA, p = 0.06) or ERCC1 (p = 0.06) gene expression in HeLa cells, respectively, in comparison with controls (Figure 3).
Figure 3.
Relative expression of BAX and ERCC1 genes after exposure of HeLa cells to (A) 5 µM of indirubin for 24 h and (B) to 50 µM of isatin for 24 h. Data represent mean values ± SD.
Discussion
Our data show that indirubin reduced cell viability in the MTT test in both CHO-K1 and HeLa cells without inducing apoptosis. Also, indirubin decreased the nuclear division index in the CBMN assay in both cell lines, indicating antiproliferative activity, and did not exhibit mutagenicity in vitro and in vivo. However, indirubin and isatin were genotoxic, suggesting that the DNA damage induced by the compounds could be repaired and thus not converted to a permanent mutation. Finally, although not statistically significant, treatment with indirubin and isatin induced a strong trend towards decreasing BAX and increasing ERCC1 gene expression, respectively.
Initially, we performed the MTT assay and the Trypan blue exclusion test to evaluate the capacity of indirubin in reducing cell viability and to define the range of concentrations to be used in the later experiments. In the MTT test, which measures the metabolic activity of the cell by producing colorimetric parameters for analysis (Ferandin et al. 2006), a 24-h treatment with indirubin produced cytotoxic effects in CHO-K1 (5 to 200 μM) and HeLa cells (10 to 200 μM). At lower doses (0.1 to 5 μM), no effect was found in the Trypan blue exclusion test in HeLa cells after treatment with indirubin or isatin. Accordingly, we have previously demonstrated that isatin reduces HeLa cells’ viability in the MTT test at doses higher than 500 μM (Cândido-Bacani et al. 2013). The NDI obtained in the CBMN assay indicated that indirubin induces antiproliferative activity in CHO-K1 cells after 24 h of treatment at concentrations ≥0.5 μM. In HeLa cells, this response was found at the same effective concentration observed in the MTT assay (10 μM), indicating that the decrease in cell viability observed could be due to an arrest in cell division.
The antiproliferative and cytotoxic activity of indirubin and its derivatives was reported in several studies using different cell lines and experimental approaches. Similar to our results, doses higher than 5 μM of indirubin-3′-monoxime, an indirubin analogue, reduced the viability of renal cancer cells after 24-h treatment (Perabo et al. 2006). The drug was also shown to induce cytotoxicity in Hep-2 human laryngeal carcinoma cells (Nam et al. 2005). A 24-h treatment with isatin-Schiff base copper (II) complexes did not alter the viability in the Trypan blue exclusion test in two tumour cell lines: the human neuroblastoma (SH-SY5Y) and promonocytic (U937) cells (Cerchiaro et al. 2005). Also, indirubin inhibited cell proliferation in several cancerous cell lines (Han 1994; Hoessel et al. 1999; Damiens et al. 2001; Marko et al. 2001; Nam et al. 2005; Moon et al. 2006; Perabo et al. 2006; Choi et al. 2010). However, other analogues generated by substitution at various positions of the bisindole skeleton usually require lower concentrations to induce antiproliferative effects, because of its higher solubility, better pharmacological properties and higher potency. Thus, most studies found in literature test synthetic substituted indirubin, such as indirubin-3′-monoxime, indirubin-5-sulphonic acid or 5-chloro-indirubin (Han 1994; Hoessel et al. 1999; Damiens et al. 2001; Marko et al. 2001; Nam et al. 2005; Moon et al. 2006; Perabo et al. 2006; Choi et al. 2010).
Although our results indicated that indirubin reduced cell viability and cell division, apoptosis/necrosis was not observed in HeLa and CHO-K1 cells. Similarly, indirubin-3′-monoxime (>5 μM) did not induce apoptosis in human transitional cell carcinoma (RT4 and T24 cells), although the same concentrations inhibited cell proliferation in a cytotoxicity assay (Perabo et al. 2006). In the present study, the doses of indirubin used to assess induction of apoptosis ranged from 0.1 to 10 μM. Most studies indicate that indirubin and its derivatives prevent cell cycle progression and induce cell death at concentrations above or equal to 10 μM. This effect was observed in SV-40 transformed human breast epithelial cells (HBL-100) (Choi et al. 2010), human transitional cell carcinoma RT112 (Kameswaran and Ramanibai 2009), human breast cancer cells [(MDA-MB-468 and MDA-MB-435 (Moon et al. 2006)], human mammary carcinoma [MCF-7 (Hoessel et al. 1999)] and renal cancer cells (Perabo et al. 2006, 2011). In contrast, indirubin-3′-monoxime caused programmed cell death in Hep-2 cells at lower concentrations (Nam et al. 2005). Differences found in the literature are probably because these activities seem to depend on the cell line used, the duration of treatment and the structure of the compound. On the other hand, a previous study found that 24-h treatment with isatin induced apoptosis at concentrations of 10 and 50 μM (Cândido-Bacani et al. 2013).
The mechanisms by which indigoids exert antiproliferative and cytotoxic effects still needs to be better understood. Several pieces of evidence suggest that indirubin derivatives selectively arrest cell division by inhibiting phosphorylase kinases that regulate the cell cycle, such as CDKs, Akt, Erk and GSK3β (Polychronopoulos et al. 2004; Nam et al. 2005; Lee et al. 2008; Kim et al. 2011; Begum et al. 2015; Yu et al. 2016). Also, these drugs induce apoptosis in tumour cells by blocking protein signal transducer and activator of transcription, particularly Stat3 protein, which is constitutively expressed in most cancerous cells but not in non-tumour cells (Nam et al. 2005; Zhang et al. 2015). Inactivation of Stat3 by indirubin-3′-monoxime (10 μM) and other derivative, E804, resulted in downregulation of anti-apoptotic proteins, such as Mcl-1 and survivin, and subsequent induction of programmed death in Cal-27 and HSC-3 oral cancer cell lines, as well as in human breast and prostate cancer cells (Nam et al. 2005; Lo and Chang 2013). In addition, it was shown that indirubin-3′-monoxime is an inhibitor of death-associated protein kinases (DAPKs) (Jung et al. 2016). Recently, a study using Hoechst 33342 and annexin-V-propidium iodide staining revealed that the indirubin derivative indirubin-3′-epoxide induces caspase-independent apoptosis in human neuroblastoma cells, probably due to a DNA fragmentation and impairment of DNA repair (Kurita et al. 2016). In addition to these mechanisms, indirubins can induce antiproliferative activities by binding the aryl hydrocarbon receptors (AhR), a ligand-activated transcription factor mainly involved in the regulation of xenobiotic-metabolizing enzymes (Adachi et al. 2001; Knockaert et al. 2004).
The magnitude of DNA damage induced by novel therapeutic targets is of great importance in order to determine its toxicity and safety. In the present study, the comet assay was used to evaluate the genotoxic potential of indirubin and isatin. We found that indirubin induced DNA damage in vitro and in vivo. Isatin was genotoxic in vitro at all doses tested after 3 and 24 h of treatment. However, the CBMN assay in vitro, using CHO-K1 and HeLa cells, and in vivo, in which we evaluated peripheral blood reticulocytes, revealed the absence of mutagenicity for indirubin at all concentrations tested. Thus, these results added to those obtained by Cândido-Bacani et al. 2011, 2013), in which mutagenicity was not detected in vitro, indicate that the cell damage detected could be repaired by the DNA repair system and thus not converted to permanent mutations. Thus, the absence of mutagenicity after indirubin treatment using CHO-K1 cells can serve as initial evidence that this compound may be safe for mammalian use, once it did not harm the genome. A recent study demonstrated that an aqueous extract of Perscicariae Rhizoma containing low concentrations of indirubin (0.009%) and indigo (0.043%) did not produce genotoxicity, chromosomal aberrations or micronucleated polychromatic erythrocytes from bone marrow (Lee et al. 2016).
Due to studies exploring the apoptotic activities of indirubin and isatin and considering the possibility that the damage caused by these compounds are repaired, we investigated the expression of two genes involved in these processes, the ERCC1 and BAX. ERCC1 has an important role in nucleotide excision repair (NER) pathway (De Laat et al. 1998). ERCC1 forms a heterodimeric complex with Xeroderma pigmentosum F (XPF), and the ERCC1-XPF complex has a structure-specific endonuclease activity, which cleaves the damaged strand at the 5′ end around the lesion (Sijbers et al. 1996; De Laat et al. 1999). BAX gene is important member that actively participates in the apoptotic mechanisms. It has been shown that the mitochondrial Bcl-2 protein family plays an essential role in this mechanism. This family includes anti-apoptotic proteins, e.g., Bcl-2, and proapoptotic proteins, such as Bax (Hou et al. 2008; Song et al. 2013).
Although indirubin and isatin produced a trend in decreasing BAX and increasing ERCC1 gene expression in HeLa cells after 24 h of treatment, respectively, we failed to detect any statistical significance. These results indicate that the absence of mutagenicity and the proapoptotic effect of isatin (Cândido-Bacani et al. 2013) may not be exclusively modulated by the NER pathway. A previous study showed that isatin increased the expression of the CASP-3 gene. This gene is involved in both intrinsic and extrinsic apoptosis pathways, encoding the protein caspase 3, which is responsible for activating endonucleases that initiate the process of DNA cleavage (Chang and Yang 2000; Grutter 2000; Hengartner 2000).
It has been shown that 48-h treatment with isatin (50, 100, 200 μmol/L) can exert proapoptotic activity in human neuroblastoma cells and mouse neuroblastoma cells (SH-SY5Y) in vitro (Song et al. 2013), accompanied by a decrease in the expression of BCL-2 mRNA. Moreover, while it did not modulate the expression of BAX mRNA, the BCL-2/BAX ratio was decreased (Song et al. 2013). Also, indirubin-3′-monoxime induced apoptosis in HeLa cells through the extrinsic pathway by modulating Bid and Bax proteins (Shi and Shen 2008).
However, in the present study indirubin did not induce significant changes in BAX gene expression and it was not found a proapoptotic effect in the acridine orange and ethidium bromide staining test. Thus, genes involved in other forms of cell death, as those regulated by death receptors in the extrinsic pathway, must be investigated. Also, as our results indicated that the DNA damage induced by indirubin and isatin in vitro and in vivo could be effectively repaired, more studies are needed to verify the genes involved in this process, such as the MMR, BER or DSB genes.
Conclusion
The results found in the present study showed that isatin and indirubin compounds might be candidates for further investigations directed at their use as chemotherapeutic substances, as results obtained in the present work and previous experiments suggest that these drugs display cytotoxic and antiproliferative activities and with a lack of mutagenicity.
Acknowledgements
This study was supported by CAPES-PROAP (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), CNPq (Conselho Nacional de Pesquisa) and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aardema MJ, Snyder RD, Spicer C, Divi K, Morita T, Mauthe RJ, Gibson DP, Soelter S, Curry PT, Thybaud V, et al. 2006. SFTG international collaborative study on in vitro micronucleus test III. Using CHO cells. Mutat Res. 607:61–87. [DOI] [PubMed] [Google Scholar]
- Adachi J, Mori Y, Matsui S, Takigami H, Fujino J, Kitagawa H, Miller CA, Kato T, Saeki K, Matsuda T.. 2001. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J Biol Chem. 276:31475–31478. [DOI] [PubMed] [Google Scholar]
- Begum J, Skamnaki VT, Moffatt C, Bischler N, Sarrou J, Skaltsounis AL, Leonidas DD, Oikonomakos NG, Hayes JM.. 2015. An evaluation of indirubin analogues as phosphorylase kinase inhibitors. J Mol Graph Model. 61:231–242. [DOI] [PubMed] [Google Scholar]
- Blažević T, Heiss EH, Atanasov AG, Breuss JM, Dirsch VM, Uhrin P.. 2015, Indirubin and indirubin derivatives for counteracting proliferative diseases. Evid Based Complement Alternat Med. 2015:654098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo TR, Cardoso CRP, Moura ACS, Dos Santos LC, Colus IM, Vilegas W, Varanda EA.. 2011. Mutagenic activity of Indigofera truxillensis and I. suffruticosa aerial parts. Evid Based Complement Alternat Med. 2011:323276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cândido-Bacani PM, Mori MP, Calvo TR, Vilegas W, Varanda EA, Cólus IM.. 2013. In vitro assessment of the cytotoxic, apoptotic and mutagenic potentials of isatin. J Toxicol Environ Health. 76:354–362. [DOI] [PubMed] [Google Scholar]
- Cândido-Bacani PM, Reis MB, Serpeloni JM, Calvo TR, Vilegas W, Varanda EA, Cólus IM.. 2011. Mutagenicity and genotoxicity of isatin in mammalian cells in vivo. Mutat Res. 719:47–51. [DOI] [PubMed] [Google Scholar]
- Cerchiaro G, Aquilano K, Filomeni G, Rotilio G, Ciriolo MR, Ferreira AM.. 2005. Isatin-Schiff base copper(II) complexes and their influence on cellular viability. J Inorg Biochem. 99:1433–1440. [DOI] [PubMed] [Google Scholar]
- Chanayath N, Lhieochaiphant S, Phutrakul S.. 2002. Pigment extraction techniques from the leaves of Indigofera tinctoria Linn and Baphicacanthus cusia Brem and chemical structure analysis of their major components. CMU J. 1:149–160. [Google Scholar]
- Chang HY, Yang X.. 2000. Proteases for cell suicide: functions and regulation of caspases. Microbiol Mol Biol Rev. 64:821–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S-J, Lee J-E, Jeong S-Y, Im I, Lee S-D, Lee E-J, Lee SK, Kwon S-M, Ahn S-G, Yoon J-H, et al. 2010. 5,5'-Substituted indirubin-3'-oxime derivatives as potent cyclin-dependent kinase inhibitors with anticancer activity. J Med Chem. 53:3696–3706. [DOI] [PubMed] [Google Scholar]
- Cooksey CJ. 2001. Tyrian purple: 6.6′-dibromoindigo and related compounds. Molecules. 6:736–769. [Google Scholar]
- Cragg GM, Newman DJ.. 2013. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 1830:3670–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiens E, Baratte B, Marie D, Eisenbrand G, Meijer L.. 2001. Anti-mitotic properties of indirubin-3'-monoxime, a CDK/GSK-3 inhibitor: induction of endoreplication following prophase arrest. Oncogene. 20:3786–3797. [DOI] [PubMed] [Google Scholar]
- da Silva J, de Freitas TRO, Marinho JR, Speit G, Erdtmann B.. 2000. An alkaline single-cell gel electrophoresis (comet) assay for environmental biomonitoring with native rodents. Genet Mol Biol. 23:241–245. [Google Scholar]
- De Laat WL, Appeldoorn E, Jaspers NG, Hoeijmakers JH.. 1998. DNA structural elements required for ERCC1-XPF endonuclease activity. J Biol Chem. 273:7835–7842. [DOI] [PubMed] [Google Scholar]
- De Laat WL, Jaspers NG, Hoeijmakers J.. 1999. Molecular mechanism of nucleotide excision repair. Genes Dev. 13:768–785. [DOI] [PubMed] [Google Scholar]
- Eastmond DA, Tucker JD.. 1989. Identification of aneuploidy inducing agents using cytokinesis-blocked human lymphocytes and an antikinetochore antibody. Environ Mol Mutagen. 13:34–43. [DOI] [PubMed] [Google Scholar]
- Fenech M, Morley A.. 1985. Solutions to the kinetic problem in the micronucleus assay. Cytobios. 43:233–246. [PubMed] [Google Scholar]
- Fenech M. 2000. The in vitro micronucleus technique. Mutat Res. 455:81–95. [DOI] [PubMed] [Google Scholar]
- Ferandin Y, Bettayeb K, Kritsanida M, Lozach O, Polychronopoulos P, Magiatis P, Skaltsounis AL, Meijer L.. 2006. 3'-Substituted 7-halogenoindirubins, a new class of cell death inducing agents. J Med Chem. 49:4638–4649. [DOI] [PubMed] [Google Scholar]
- Garcez WS, Garcez FR, Barison A.. 2003. Additional 3-nitropropanoyl esters of glucose from Indigofera suffruticosa (Leguminosae). Biochem Syst Ecol. 31:207–209. [Google Scholar]
- Grutter MG. 2000. Caspases: Key players in programmed cell death. Curr Opin Struct Biol. 10:649–655. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Sorrells JL, Schmitt S, Krauser JA, Aryal P, Meijer L.. 2004. Generation of new protein kinase inhibitors utilizing cytochrome P450 mutant enzymes for indigoid synthesis. J Med Chem. 47:3236–3241. [DOI] [PubMed] [Google Scholar]
- Han R. 1994. Highlight on the studies of anticancer drugs derived from plants in China. Stem Cells. 12:53–63. [DOI] [PubMed] [Google Scholar]
- Hasan A, Farman M, Ahmad I.. 1994. Flavonoid glycosides from Indigofera hebepetala. Phytochemistry. 35:75–276. [Google Scholar]
- Hayashi M, Morita T, Kodama Y, Sofuni T, Ishidate Jr M.. 1990. The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange-coated slides. Mutat Res. 245:245–249. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. 2000. The biochemistry of apoptosis. Nature. 407:770–776. [DOI] [PubMed] [Google Scholar]
- Hoessel R, Leclerc S, Endicott JA, Nobel ME, Lawrie A, Tunnah P, Leost M, Damiens E, Marie D, Marko D.. 1999. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1:60–67. [DOI] [PubMed] [Google Scholar]
- Hou L, Ju C, Zhang J, Song J, Ge Y, Yue W.. 2008. Antitumor effects of isatin on human neuroblastoma cell line (SH-SY5Y) and the related mechanism. Eur J Pharmacol. 589:27–31. [DOI] [PubMed] [Google Scholar]
- Ichimaru Y, Saito H, Uchiyama T, Metori K, Tabata K, Suzuki T, Miyairi S.. 2015. Indirubin 3′-(O-oxiran-2-ylmethyl)oxime: a novel anticancer agent. Bioorg Med Chem Lett. 25:1403–1406. [DOI] [PubMed] [Google Scholar]
- Jung ME, Byun BJ, Kim H-M, Lee JY, Park JH, Lee N, Son YH, Choi SU, Yang KM, Kim SJ.. 2016. Discovery of indirubin derivatives as new class of DRAK2 inhibitors from high throughput screening. Bioorg Med Chem Lett. 26:2719–2723. [DOI] [PubMed] [Google Scholar]
- Kameswaran TR, Ramanibai R.. 2009. Indirubin-3-monooxime induced cell cycle arrest and apoptosis in Hep-2 human laryngeal carcinoma cells. Biomed Pharmacother. 63:146–154. [DOI] [PubMed] [Google Scholar]
- Kim SA, Kwon SM, Kim JA, Kang KW, Yoon JH, Ahn SG.. 2011. 5′-Nitro-indirubinoxime, an indirubin derivative, suppresses metastatic ability of human head and neck cancer cells through the inhibition of Integrin β1/FAK/Akt signaling. Cancer Lett. 306:197–204. [DOI] [PubMed] [Google Scholar]
- Knockaert M, Blondel M, Bach S, Leost M, Elbi C, Hager GL, Nagy SR, Han D, Denison M, Ffrench M, et al. 2004. Independent actions on cyclin-dependent kinases and aryl hydrocarbon receptor mediate the antiproliferative effects of indirubins. Oncogene. 23:4400–4412. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Sugiyama C, Morikawa Y, Hayashi M, Sofuny T.. 1995. A comparison between manual microscopic analysis and computerized image analysis in the single cell gel electrophoresis assay. MMS Commun. 3:103–115. [Google Scholar]
- Kurita M, Hanada S, Ichimaru Y, Saito H, Tabata K, Asami S, Miyairi S, Suzuki T.. 2016. Indirubin 3'-epoxide induces caspase-independent cell death in human neuroblastoma. Biol Pharm Bull. 39:993–999. [DOI] [PubMed] [Google Scholar]
- Lee M-J, Kim M-Y, Mo J-S, Ann EJ, Seo MS, Hong JA, Kim YC, Park HS.. 2008. Indirubin-3′-monoxime, a derivative of a Chinese anti-leukemia medicine, inhibits Notch1 signaling. Cancer Lett. 265:215–225. [DOI] [PubMed] [Google Scholar]
- Lee WH, Choi SH, Kang SU, Song CH, Park SJ, Lee YJ, Ku SK.. 2016. Genotoxicity testing of Persicariae Rhizoma (Persicaria tinctoria H. Gross) aqueous extracts. Exp Ther Med. 12:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XM, Wang LG, Li HY, Ji XJ.. 1996. Induction of differentiation and down-regulation of c-myb gene expression in ML-1 human myeloblastic leukemia cells by the clinically effective anti-leukemia agent meisoindigo. Biochem Pharmacol. 51:1545–1551. [DOI] [PubMed] [Google Scholar]
- Lo WY, Chang NW.. 2013. An indirubin derivative, indirubin-3′-monoxime suppresses oral cancer tumorigenesis through the downregulation of survivin. PLoS One. 8:e70198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorge E, Thybaud V, Aardema MJ, Oliver J, Wakata A, Lorenzon G, Marzin D.. 2006. SFTG International Collaborative study on in vitro Micronucleus Test I: General conditions and overall conclusions of the study. Mutat Res. 607:13–16. [DOI] [PubMed] [Google Scholar]
- Manoharan K, Banerjee MR.. 1985. beta-Carotene reduces sister chromatid exchanges induced by chemical carcinogens in mouse mammary cells in organ culture. Cell Biol Int Rep. 9:783–789. [DOI] [PubMed] [Google Scholar]
- Marko D, Friedel A, Genzlinger A, Genzlinger A, Zankl H, Meijer L, Eisenbrand G.. 2001. Inhibition of cyclin-dependent kinase 1 (CDK1) by indirubin derivatives in human tumour cells. Br J Cancer. 84:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGahon AJ, Martin SJ, Bissonnette RP, Mahboubi A, Shi Y, Mogil RJ, Nishioka WK, Green DR.. 1995. The end of the (cell) line: methods for the study of apoptosis in vitro. Methods Cell Biol. 46:153–184. [DOI] [PubMed] [Google Scholar]
- Moon MJ, Lee S K, Lee J-W, Song WK, Kim SW, Kim JI, Cho C, Choi SJ, Kim Y-C.. 2006. Synthesis and structure-activity relationships of novel indirubin derivatives as potent anti-proliferative agents with CDK2 inhibitory activities. Bioorg Med Chem. 14:237–246. [DOI] [PubMed] [Google Scholar]
- Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63. [DOI] [PubMed] [Google Scholar]
- Mothana RA, Lindequist U, Gruenert R, Bednarski PJ.. 2009. Studies of the in vitro anticancer, antimicrobial and antioxidant potentials of selected Yemeni medicinal plants from the island Soqotra. BMC Complement Altern Med. 9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S, Buettner R, Turkson J, Kim D, Cheng JQ, Muehlbeyer S, Hippe F, Vatter S, Merz K.-H, Eisenbrand G, Jove R.. 2005. Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc Natl Acad Sci USA. 102:5998–6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization for Economic Co-operation and Development (OECD) 2001. Guideline for testing of chemicals no. 423: Acute Oral Toxicity Test. Adopted: 17th December 2001. Paris, France; p. 1–14. [Google Scholar]
- Perabo FGE, Frössler C, Landwehrs G, Schmidt DH, von Rücker A, Wirger A, Müller SC.. 2006. Indirubin-3′-monoxime, a CDK inhibitor induces growth inhibition and apoptosis-independent up-regulation of survivin in transitional cell cancer. Anticancer Res. 26:2129–2136. [PubMed] [Google Scholar]
- Perabo FGE, Landwehrs G, Frössler C, Schmidt DH, Mueller SC.. 2011. Antiproliferative and apoptosis inducing effects of indirubin-3-monoxime in renal cell cancer cells. Urol Oncol Semin Orig Invest. 29:815–820. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L.. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:2002–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polychronopoulos P, Magiatis P, Skaltsounis A-L, Myrianthopoulos V, Mikros E, Tarricone A, Musacchio A, Roe S M, Pearl L, Leost M, Greengard P, Meijer L.. 2004. Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. J Med Chem. 47:935–946. [DOI] [PubMed] [Google Scholar]
- Sethi G, Ahn KS, Sandur SK, Lin X, Chaturvedi MM, Aggarwal BB.. 2006. Indirubin enhances tumor necrosis factor-induced apoptosis through modulation of nuclear factor-kappa B signaling pathway. J Biol Chem. 281:23425–23435. [DOI] [PubMed] [Google Scholar]
- Shi J, Shen HM.. 2008. Critical role of Bid and Bax in indirubin-3′-monoxime-induced apoptosis in human cancer cells. Biochem Pharmacol. 75:1729–1742. [DOI] [PubMed] [Google Scholar]
- Sijbers AM, de Laat WL, Ariza RR, Biggerstaff M, Wei Y-F, Moggs JG, Carter KC, Shell BK, Evans E, de Jong MC, Rademakers S, de Rooij J.. 1996. Xeroderma pigmentosum Group F caused by a defect in a structure-specific DNA repair endonuclease. Cell. 86:811–822. [DOI] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL.. 1988. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 175:184–191. [DOI] [PubMed] [Google Scholar]
- Singh P, Sharma P, Anand A, Bedi PM, Kaur T, Saxena AK, Kumar V.. 2012. Azide-alkyne cycloaddition en route to novel 1H-1,2,3-triazole tethered isatin conjugates with in vitro cytotoxic evaluation. Eur J Med Chem. 55:455–461. [DOI] [PubMed] [Google Scholar]
- Song J, Hou L, Ju C, Zhang J, Ge Y, Yue W.. 2013. Isatin inhibits proliferation and induces apoptosis of SH-SY5Y neuroblastoma cells in vitro and in vivo. Eur J Pharmacol. 702:235–241. [DOI] [PubMed] [Google Scholar]
- Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu J-C, Sasaki YF.. 2000. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 35:206–221. [DOI] [PubMed] [Google Scholar]
- Titenko-Holland N, Windham G, Kolachana P, Reinisch F, Parvatham S, Osorio AM, Smith MT.. 1997. Genotoxicity of malathion in human lymphocytes assessed using the micronucleus assay in vitro and in vivo: a study of malathion-exposed workers. Mutat Res. 388:85–95. [DOI] [PubMed] [Google Scholar]
- Vellonen K-S, Honkakoski P, Urtti A.. 2004. Substrates and inhibitors of efflux proteins interfere with the MTT assay in cells and may lead to underestimation of drug toxicity. Eur J Pharm Sci. 23:181–188. [DOI] [PubMed] [Google Scholar]
- Yu J, Zheng J, Lin J, Jin L, Yu R, Mak S, Hu S, Sun H, Wu X, Zhang Z, Lee M, Tsim W.. 2016. Indirubin-3-Oxime prevents H2O2-induced neuronal apoptosis via concurrently inhibiting GSK3β and the ERK pathway. Cell Mol Neurobiol. 37:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X-M, Guo H, Li Z-S, Song FH, Wang WM, Dai HQ, Zhang LX, Wang JG.. 2015. Synthesis and evaluation of isatin-β-thiosemicarbazones as novel agents against antibiotic-resistant Gram-positive bacterial species. Eur J Med Chem. 101:419–430. [DOI] [PubMed] [Google Scholar]
- Zhen Y, Sørensen V, Jin Y, Suo Z, Wiedłocha A.. 2007. Indirubin-3′-monoxime inhibits autophosphorylation of FGFR1 and stimulates ERK1/2 activity via p38 MAPK. Oncogene. 26:6372–6385. [DOI] [PubMed] [Google Scholar]