Abstract
Introduction
We investigated risk of coronary heart disease and heart failure in phenotypes of obstructive airway disease.
Methods
Among 91 692 participants in the Copenhagen General Population Study, 42 058 individuals were classified with no respiratory disease, and 11 988 individuals had different phenotypes of obstructive airways disease: asthma with early onset or late-onset, chronic obstructive pulmonary disease (COPD) with forced expiratory volume in one second (FEV1) above or below 50% of predicted value (%p) or asthma-COPD overlap (ACO).
Results
During a mean follow-up of 5.7 years we registered 3584 admissions for coronary heart disease and 1590 admissions for heart failure. Multivariable Cox regression analyses of time to first admission were used with a two-sided p value of 0.05 as significance level. Compared with no respiratory disease the highest risks of coronary heart disease and heart failure were observed in ACO with late-onset asthma and FEV1 <50% p, HR=2.2 (95% CI 1.6 to 3.0), and HR=2.9 (95% CI 2.0 to 4.3), respectively. In COPD with FEV1 above 50% p the HRs were 1.3 (95% CI 1.2 to 1.5) for coronary heart disease and 1.9 (95% CI 1.6 to 2.3) for heart failure. Asthma associated with increased risks of coronary heart disease and heart failure, however, in asthma without allergy the HR was 1.1 (95% CI 0.7 to 1.6) for coronary heart disease while individuals with allergy had an HR of 1.4 (95% CI 1.1 to 1.6).
Conclusions
Risks of coronary heart disease and heart failure were increased in asthma, COPD and ACO. In asthma, the risk of coronary heart disease depended on presence of allergy. We suggest that cardiovascular risk factors should be assessed systematically in individuals with obstructive airway disease with the potential to facilitate targeted treatments.
Keywords: clinical epidemiology, COPD epidemiology
Key messages.
Do different phenotypes of obstructive airway disease associate with increased risk of coronary heart disease and heart failure?
Several population-based studies have shown an association between chronic obstructive pulmonary disease (COPD) and cardiovascular disease, however, most studies lack adjustment for important clinical characteristics and prospective designs. Similarly, previously observed associations between asthma and cardiovascular disease are poorly understood, and recently it has been proposed that these associations could depend on age of asthma onset or presence of allergy. Furthermore, there are to date few studies investigating the association between asthma-COPD overlap and cardiovascular disease.
Obstructive airway disease phenotypes increase risk of cardiovascular disease, but could depend on allergy presence in individuals with asthma.
Background
Asthma and chronic obstructive pulmonary disease (COPD) are prevalent chronic inflammatory airway diseases and an increased risk of cardiovascular comorbidity has been described in both.1 2 Recent systematic reviews suggest that the risk of being diagnosed with coronary heart disease or heart failure is approximately twofold higher in individuals with asthma and even higher in COPD.3–5
In patients with asthma, observed associations with cardiovascular disease are poorly understood, inconsistent,6–8 and recently it has been proposed that the risk could depend on age of asthma onset.9–11 Furthermore, studies have indicated that allergy associates with risk of cardiovascular disease.12 Since allergy is associated with asthma13 this could influence observed associations between asthma and risk of cardiovascular comorbidity.
Other studies have shown an association between COPD and risk of cardiovascular disease. However, a recent systematic literature review points to inconsistencies in these associations with lack of adjustment for important and well-characterised clinical characteristics, and a paucity of significant prospective studies.4
Recently, asthma-COPD overlap (ACO) has been acknowledged as a clinical entity,14 that is, individuals with persistent airflow limitation and features usually associated with both COPD and asthma,15 but only few studies have focused on cardiovascular comorbidity in individuals with ACO.16 These studies have mostly been cross-sectional and based on registry data without comprehensive information on clinical and lifestyle characteristics16–18 but significant associations between ACO and both coronary heart disease and heart failure have been reported in retrospective study designs.19
We hypothesised that obstructive airway disease phenotypes, asthma, COPD and ACO are prospectively associated with risk of coronary heart disease and heart failure.
Methods
Participants
In the present study, we analysed data from The Copenhagen General Population Study. Written informed consent was obtained from all participants. The Copenhagen General Population Study is a prospective epidemiological study that has recruited more than 100 000 individuals representative of the general population and collected genotypical and phenotypical data of relevance to a wide range of health-related problems.20 Recruitment began in 2003 (response rate 45%), and a follow-up examination of all individuals was initiated in 2014 and is still ongoing. In the present study we had access to participants examined in the first examination from 2003 to 2014 and therefore the follow-up examination (the second round of the Copenhagen General Population Study) is not included in the present study. All participants completed a comprehensive questionnaire and underwent a physical examination including spirometry at recruitment. Only prebronchodilator measurements were available. Predicted values were calculated using internally derived reference values based on a subsample of healthy asymptomatic never-smokers, as reported earlier.21
Airway disease phenotypes
Among 91 692 participants aged above 40 years with spirometry, 2867 (3.1%) had missing data on at least one of the variables necessary for the stratification described below. Among the remaining participants, we identified 54 046 individuals defined by one of eight groups of different phenotypes of airway disease, based on the information obtained in the questionnaires and results of spirometry, and a reference group consisting of participants with no respiratory disease:
No respiratory disease: individuals with forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ≥0.70, no self-reported asthma, no prior admissions for asthma and COPD, and ≤10 pack-years of tobacco smoking.
Asthma with early onset: current self-reported asthma with onset before 40 years of age, ≤10 pack-years of tobacco smoking and FEV1/FVC≥0.70.
Asthma with late-onset: current self-reported asthma with onset after 40 years of age, ≤10 pack-years of tobacco smoking and FEV1/FVC≥0.70.
COPD with FEV1 ≥ 50% of predicted value: FEV1/FVC<0.70 with >10 pack-years of tobacco smoking, no self-reported asthma and FEV1 ≥50% of predicted value
COPD with FEV1 < 50% of predicted value: FEV1/FVC<0.70 with >10 pack-years of tobacco smoking, no self-reported asthma and FEV1<50% of predicted value.
ACO with early onset asthma and FEV1 ≥ 50% of predicted value: current self-reported asthma with onset before 40 years of age, FEV1/FVC<0.70 and FEV1≥50% of predicted value. There were no criteria regarding smoking for assignment into this group.
ACO with late-onset asthma and FEV1 ≥ 50% of predicted value: current self-reported asthma with onset after 40 years of age, FEV1/FVC<0.70 and FEV1≥50% of predicted value. There were no criteria regarding smoking for assignment into this group.
ACO with early onset asthma and FEV1 <50% of predicted value: current self-reported asthma with onset before 40 years of age, FEV1/FVC<0.70 and FEV1<50% of predicted value. There were no criteria regarding smoking for assignment into this group.
ACO with late-onset asthma and FEV1 < 50% of predicted value: current self-reported asthma with onset after 40 years of age, FEV1/FVC<0.70 and FEV1<50% of predicted value. There were no criteria regarding smoking for assignment into this group.
Individuals with missing data were excluded. In the study population of 91 692 individuals, only 3% had missing data on either smoking, body mass index, hypertension, family history of cardiovascular disease, diabetes, total cholesterol or physical activity in leisure time, hence imputation of missing data was not performed.
Hospitalisations for coronary heart disease and heart failure
Data on hospitalisations were available from the national Danish Patient Registry,22 23 until November 2014. The national Danish Patient Registry was established in 1977 and provides complete nationwide registry data without loss to follow-up. Data are available on permission for research purposes from 1977 and forward and the cardiovascular diagnoses have a high specificity and positive predictive value.24 25 Denmark used the International Classification of Diseases (ICD)-8 until 1994 and hereafter shifted directly to ICD-10. Participants with previous admissions for COPD (ICD-8: 491–492 and ICD-10: J41–J44) and asthma (ICD-8: 493 and ICD-10: J45–J46) were removed from the reference group of no respiratory disease. We recorded hospital admissions for coronary heart disease (ICD-8: 410–414 and ICD-10: I20–I25) and heart failure (ICD-8: 427.09–427.11 and ICD-10: I50), and in our main analysis individuals with previous admissions of coronary heart disease and heart failure were included in the prospective analysis, however, we also performed a sensitivity analysis excluding individuals with previous cardiovascular admissions from our analysis, as described below.
Patient and public involvement
Patients were not involved in the development of the research question or outcome measure, nor in the design of the study, recruitment to or conduct of the study. Results are not disseminated to the study participants and patient advisers were not involved.
Licence for publication
The corresponding author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group to permit this article to be published in BJO and any other BMJPGL
products and sublicences such use and exploit all subsidiary rights, as set out in our licence (http://group.bmj.com/products/journals/instructions-for-authors/licence-forms).
Statistical analysis
For demographics, continuous variables and categorical variables were compared using analysis of variance and χ2 tests as appropriate. The Kaplan-Meier estimator was used to estimate the percentage of events during follow-up after the initial examination, accounting for censoring of data. Hence, percentages presented are 100% minus the Kaplan-Meier estimate of being event-free. Kaplan-Meier curves were included to describe the clinical prognosis for groups of individuals with obstructive airway disease phenotypes, thereby indicating which patients are at increased risk. To estimate the confounder adjusted risks of hospital admissions we used a Cox regression model. Time since examination was the underlying timescale and all Cox models were adjusted for age. In a base model, age and gender were included as covariates, and in a full multivariable model we also included established cardiovascular risk factors: family history of cardiovascular disease, smoking, body mass index, hypertension, diabetes mellitus, total cholesterol levels and physical activity in leisure time26 (details of all variables are provided in the online supplementary table S1). In our analysis, a two-sided p value less than 0.05 was considered significant. The assumption of proportionality in the Cox regression models were tested with the Lin, Wei and Ying Score process test by visual examination of plots of cumulative martingale residuals, and no violations of the model assumptions were found.27 No adjustments for multiple comparisons were made. All analyses were performed with R V.3.2.0 (R Foundation for Statistical Computing, Vienna, Austria).28
bmjresp-2019-000470supp001.pdf (634.4KB, pdf)
Sensitivity analyses
Asthma with or without allergy
Previous studies have indicated that there is an association between allergy and risk of cardiovascular disease.12 Due to the rather low numbers of participants in our main analysis in some of the phenotype subgroups, we disregarded age of onset in our asthma definition and divided asthma into two other groups: asthma with or without allergy. Presence of allergy was defined by answering yes to the question ‘Do foods, medicine, grass, flowers, animal hair or other factors cause asthma, hay fever or eczema in you?’. In the reference group (‘No respiratory disease’) individuals reporting presence of allergy were excluded explaining a smaller reference group.
Furthermore, we also did a subgroup sensitivity analysis by analysing the association between presence of allergy compared with no presence of allergy in the ‘No respiratory disease’ subgroup, and prospective risk of coronary heart disease and heart failure.
Age of asthma onset
Early onset asthma and late-onset asthma were defined based on a previous study using 40 years of age as the cut point.29 However, this choice could be discussed, and we did a second sensitivity analysis with 20 years of age as the cut point defining early onset versus late-onset asthma.
No history of admissions with coronary heart disease or heart failure
In our third sensitivity analysis, acknowledging the presence of coronary heart disease and heart failure in some individuals with obstructive airway disease at baseline, we excluded these individuals from our study population and repeated our analysis.
Adjusting for pack-years
Smoking cessation is a strong predictor of cardiovascular disease30 and cumulated tobacco consumption measured in pack-years was used to define some of our airway disease phenotype subgroups. Therefore, we initially adjusted our main analysis for smoking status as current/former/never. However, in our fourth and final sensitivity analysis acknowledging that smoking is a possible strong confounder we adjusted our main analysis for cumulated smoking exposure calculated in pack-years, as this variable could have a finer resolution and provide better confounder control than smoking status.
Results
Characteristics of participants with different phenotypes of airway disease
The distribution of the 54 046 participants and their characteristics are shown in the table 1. There were 11 988 individuals with any airway disease and 1901 of these were classified as having ACO. Individuals with ACO constituted 2.1% of all participants, 15.9% of those in the airway disease phenotype groups and 19.3% of all those with airflow limitation.
Table 1.
Baseline characteristics* of 54 046 individuals according to different phenotypes of obstructive airway disease
| No respiratory disease (n=42 058) |
Asthma with early onset (n=1215) |
Asthma with late-onset (n=904) |
COPD GOLD stage 1+2 (n=7447) |
COPD GOLD stage 3+4 (n=521) |
ACO with GOLD stage 1+2 and early asthma onset (n=780) |
ACO with GOLD stage 1+2 and late asthma onset (n=813) |
ACO with GOLD stage 3+4 and early asthma onset (n=101) |
ACO with GOLD stage 3+4 and late asthma onset (n=207) |
|
| General characteristics | |||||||||
| Men, % | 16598/42058 (39) | 460/1215 (38) | 235/904 (26) | 4151/7447 (56) | 305/521 (59) | 359/780 (46) | 351/813 (43) | 57/101 (56) | 83/207 (40) |
| Age, years | 58 (11) | 51 (9) | 61 (10) | 66 (11) | 70 (9) | 58 (11) | 68 (10) | 62 (11) | 69 (9) |
| Respiratory characteristics | |||||||||
| FEV1, litres | 3.14 (0.81) | 3.11 (0.79) | 2.62 (0.74) | 2.37 (0.72) | 1.10 (0.31) | 2.41 (0.73) | 2.03 (0.67) | 1.15 (0.34) | 0.98 (0.28) |
| FEV1, % of predicted | 104 (13) | 95 (13) | 96 (15) | 83 (17) | 41 (7) | 78 (14) | 77 (16) | 39 (9) | 39 (7) |
| FEV1/FVC, % | 79 (5) | 78 (5) | 78 (5) | 64 (6) | 50 (10) | 63 (6) | 62 (6) | 50 (8) | 49 (9) |
| mMRC ≥2, % | 1881/41844 (4) | 121/1206 (10) | 176/899 (20) | 1152/7393 (16) | 298/520 (57) | 136/774 (18) | 235/810 (29) | 59/100 (59) | 147/206 (71) |
| At least five respiratory infections during preceding 10 years, % | 270/41529 (1) | 106/1206 (9) | 104/902 (12) | 233/7348 (3) | 68/506 (13) | 93/774 (12) | 100/805 (12) | 32/101 (32) | 58/205 (28) |
| Medication for airway disease, % | 470/41527 (1) | 729/1209 (60) | 672/898 (75) | 449/7346 (6) | 194/504 (38) | 589/772 (76) | 674/808 (83) | 89/101 (88) | 194/207 (94) |
| Asthma, hay fever or eczema as child, % | 5081/41906 (12) | 739/1206 (61) | 202/894 (23) | 616/7413 (8) | 31/517 (6) | 511/775 (66) | 130/800 (16) | 54/100 (54) | 27/204 (13) |
| Current allergy | 11371/41926 (27) | 1109/1213 (91) | 660/899 (73) | 1329/7417 (18) | 65/516 (13) | 693/779 (89) | 486/804 (60) | 76/101 (75) | 100/203 (49) |
| Cardiovascular risk factors | |||||||||
| Smoking, % | |||||||||
| Never | 27711/41813 (66) | 799/1210 (66) | 584/900 (65) | 0/7326 (0) | 0/509 (0) | 275/748 (37) | 199/797 (25) | 27/97 (28) | 12/201 (6) |
| Former | 12358/41813 (30) | 375/1210 (31) | 288/900 (32) | 3915/7326 (53) | 261/509 (51) | 333/748 (45) | 444/797 (56) | 36/97 (37) | 105/201 (52) |
| Current | 1744/41813 (4) | 36/1210 (3) | 28/900 (3) | 3411/7326 (47) | 248/509 (49) | 140/748 (19) | 154/797 (19) | 34/97 (35) | 84/201 (42) |
| Pack-years, years | 2 (3) | 2 (3) | 2 (3) | 36 (23) | 46 (26) | 13 (18) | 22 (26) | 28 (31) | 36 (23) |
| Systolic blood pressure, mm Hg | 137 (21) | 134 (20) | 139 (21) | 142 (21) | 146 (24) | 137 (21) | 144 (21) | 149 (22) | 145 (20) |
| Blood pressure medication, % | 7485/41706 (18) | 165/1209 (14) | 237/896 (26) | 2166/7385 (29) | 174/511 (34) | 139/773 (18) | 275/809 (34) | 29/101 (29) | 72/206 (35) |
| Diabetes, % | 1198/41899 (3) | 30/1207 (2) | 28/902 (3) | 412/7416 (6) | 37/518 (7) | 30/778 (4) | 58/808 (7) | 9/101 (9) | 18/204 (9) |
| Body mass index, kg/m2 | 26 (4) | 27 (5) | 27 (5) | 26 (4) | 26 (5) | 26 (4) | 26 (4) | 27 (6) | 26 (5) |
| Family history of CVD, % | 2192/41748 (5) | 61/1211 (5) | 53/898 (6) | 381/7375 (5) | 20/510 (4) | 40/773 (5) | 41/802 (5) | 6/100 (6) | 10/204 (5) |
| Total cholesterol, mmol/l | 5.6 (1.0) | 5.6 (1.1) | 5.9 (1.1) | 5.6 (1.1) | 5.6 (1.2) | 5.6 (1.0) | 5.8 (1.2) | 5.6 (1.1) | 5.7 (1.1) |
| HDL cholesterol, mmol/l | 1.68 (0.51) | 1.60 (0.47) | 1.72 (0.52) | 1.61 (0.54) | 1.68 (0.57) | 1.66 (0.55) | 1.77 (0.58) | 1.66 (0.52) | 1.83 (0.61) |
| Physical activity in leisure time, % | |||||||||
| Low (sedentary) | 1816/41819 (4) | 84/1211 (7) | 46/894 (5) | 665/7365 (9) | 83/516 (16) | 60/776 (8) | 57/800 (7) | 16/96 (17) | 44/203 (22) |
| Moderate | 16914/41819 (40) | 471/1211 (39) | 408/894 (46 | 3421/7365 (46) | 292/516 (57) | 343/776 (44) | 364/800 (46) | 58/96 (60) | 104/203 (51) |
| High | 20261/41819 (48) | 560/1211 (46) | 389/894 (44) | 2907/7365 (39) | 125/516 (24) | 322/776 (41) | 331/800 (41) | 21/96 (22) | 51/203 (25) |
| Very high | 2828/41819 (7) | 96/1211 (8) | 51/894 (6) | 372/7365 (5) | 16/516 (3) | 51/776 (7) | 48/800 (6) | 1/96 (1) | 4/203 (2) |
| Previous admissions CHD or heart failure, % | 1746/42058 (4) | 37/1215 (3) | 75/904 (8) | 891/7447 (12) | 103/521 (20) | 43/780 (6) | 106/813 (13) | 18/101 (18) | 46/207 (22) |
*Continuous variables are presented as mean (SD).
ACO, asthma-COPD overlap; CHD, coronary heart disease; CVD, cardiovascular disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HDL, high-density lipoprotein; mMRC, modified Medical Research Council Dyspnoea Scale.
General characteristics
As shown in the table, severity of lung function impairment in individuals with COPD and ACO was related to presence of dyspnoea (ie, mMRC ≥2) whereas a high frequency of previous respiratory infections was particularly present in ACO with FEV1<50% p, regardless of age of asthma onset. Prevalence of treatment with medication for respiratory disease was highest in participants with ACO, followed by those with asthma, and then those with COPD and FEV1<50% p, whereas only 6% of those with COPD and FEV1≥50% p were on medical treatment. Current smoking was most prevalent in those with COPD. Individuals with asthma and those with ACO with early onset asthma more often reported having experienced asthma, hay fever or eczema in childhood.
Cardiovascular disease
The prevalence of cardiovascular disease was highest in participants with FEV1<50% p in both COPD and ACO as shown in the table. There was a trend towards higher blood pressure and a higher prevalence of diabetes mellitus in COPD and ACO with FEV1<50% p, and more individuals with these obstructive airway disease phenotypes reported a low physical activity level in leisure time. Furthermore, family history of cardiovascular disease, total cholesterol, and HDL cholesterol levels seemed largely similar across different phenotypes of airway disease.
Hospitalisations for coronary heart disease and heart failure
During an average follow-up of 5.7 years (maximum: 11.0 years), 3584 admissions for coronary heart disease and 1590 admissions for heart failure were recorded.
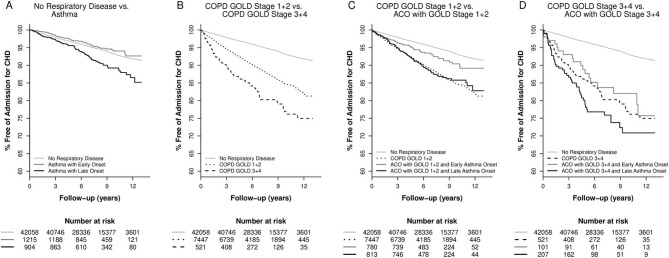
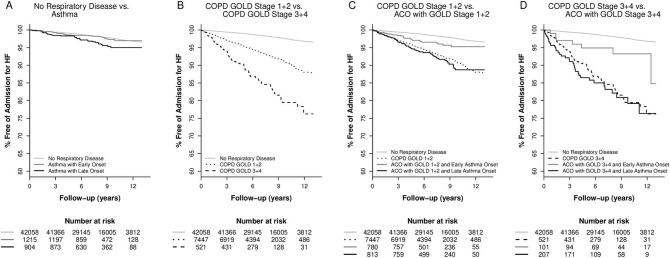
Figure 1 shows Kaplan-Meier curves with regard to admissions for coronary heart disease while figure 2 shows heart failure. These unadjusted analyses illustrate the increased risks of coronary heart disease and heart failure in real life groups of individuals with different phenotypes of obstructive airway disease.
Figure 1.
Kaplan-Meier curves comparing risk of coronary heart disease for different phenotypes of obstructive airway disease in the Copenhagen General Population Study. (A) Asthma with early onset or late-onset versus no respiratory disease. (B) COPD with FEV1<50%, COPD with FEV1≥50%, versus no respiratory disease. (C) ACO with late-onset asthma and FEV1≥50%, ACO with early onset asthma and FEV1≥50%, COPD with FEV1≥50%, vs no respiratory disease. (D) ACO with late-onset asthma and FEV1<50%, ACO with early onset asthma and FEV1<50%, COPD with FEV1<50%, versus no respiratory disease. ACO, asthma-COPD overlap; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Figure 2.
Kaplan-Meier curves comparing risk of heart failure for different phenotypes of obstructive airway disease in the Copenhagen General Population Study. (A) Asthma with early onset or late-onset versus no respiratory disease. (B) COPD and FEV1<50%, COPD and FEV1≥50%, versus no respiratory disease. (C) ACO with late-onset asthma and FEV1≥50%, ACO with early onset asthma and FEV1≥50%, COPD with FEV1≥50%, versus no respiratory disease. D) ACO with late-onset asthma and FEV1<50%, ACO with early onset asthma and FEV1<50%, COPD with FEV1<50%, versus no respiratory disease. ACO, asthma-COPD overlap; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
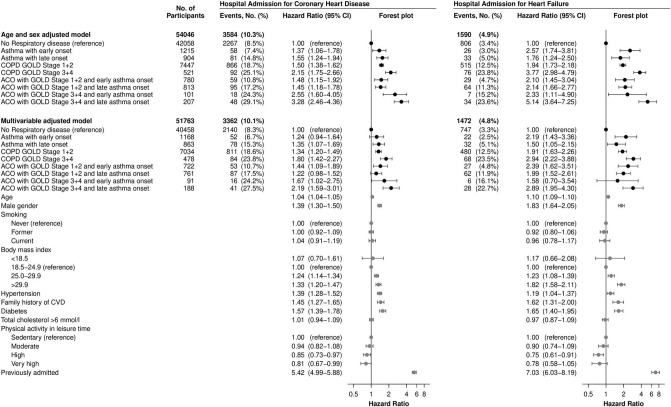
Figure 3 shows adjusted HRs for hospitalisations due to coronary heart disease in the left panel and heart failure in the right panel. In full multivariable models, early onset asthma did not associate with coronary heart disease with an HR of 1.2 (95% CI 0.9 to 1.6, p=0.13) but was associated with heart failure HR=2.2 (95% CI 1.4 to 3.4, p<0.001). Late-onset asthma was associated with both coronary heart disease and heart failure, HR=1.4 (95% CI 1.1 to 1.7, p=0.01) and HR=1.5 (95% CI 1.1 to 2.2, p=0.02), respectively.
Figure 3.
Risk of hospitalisations for coronary heart disease or heart failure in the Copenhagen General Population Study, by eight phenotypes of airway disease with no respiratory disease as reference. Percentages are Kaplan-Meier estimates. Individuals with asthma are divided by early onset versus late-onset using 40 years of age as cut point. Left part shows coronary heart disease and right part shows heart failure. Top-down: base model or multivariable model (‘Age and sex adjusted model‘ or ‘Multivariable adjusted model’). Left to right: the number of participants included (‘No. of Participants’), number of events recorded during follow-up (“Events No.’), HR from Cox-regression analyses with 95% CIs (“Hazard Ratio (95% CI)"). Online supplementary table S1 shows details of variables included in multivariable modelling. ACO, asthma-COPD overlap; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
The highest risks of coronary heart disease and heart failure were observed in COPD with FEV1<50% p and in ACO with late-onset asthma and FEV1<50% p, and the HR was 1.8 (95% CI 1.4 to 2.3, p<0.001) and 2.2 (95% CI 1.6 to 3.0, p<0.001), respectively, for coronary heart disease, and 2.9 (95% CI 2.2 to 3.9, p<0.001) and 2.9 (95% CI 2.0 to 4.3, p<0.001), respectively, for heart failure, compared with no respiratory disease. Interestingly, COPD with FEV≥50% p also associated with coronary heart disease as well as heart failure even when adjusting for established cardiovascular risk factors, HR=1.3 (95% CI 1.2 to 1.5, p<0.001) and HR=1.9 (95% CI 1.6 to 2.3, p<0.001), respectively.
Sensitivity analyses
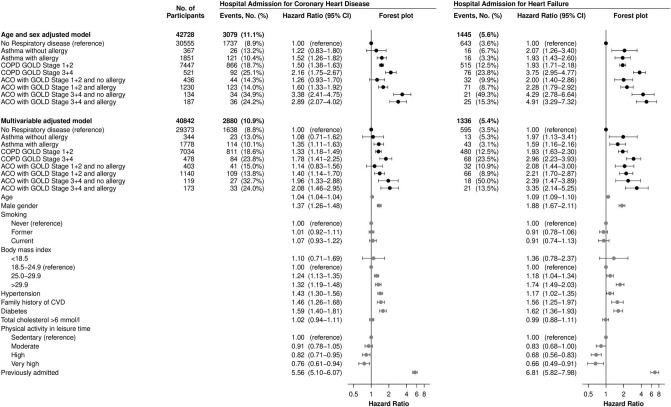
Asthma with or without allergy
Online supplementary table S2 shows characteristics of individuals with asthma with or without allergy and ACO with or without allergy. As shown in figure 4, asthma without allergy did not associate with risk of coronary heart disease, HR=1.1 (95% CI 0.7 to 1.6, p=0.73), while asthma with presence of allergy was significantly associated and the HR was 1.4 (95% CI 1.1 to 1.6, p=0.002) for coronary heart disease compared with no respiratory disease. Similarly, ACO with Global Initiative for Chronic Obstructive Lung Disease (GOLD) 1+2 COPD without allergy did not associate with risk of coronary heart disease, HR=1.1 (95% CI 0.8 to 1.6, p=0.41) while ACO with GOLD 1+2 COPD and allergy had an HR of 1.4 (95% CI 1.1 to 1.7, p<0.001) for coronary heart disease compared with no respiratory disease. Asthma with or without allergy and ACO with or without allergy associated significantly with risk of heart failure.
Figure 4.
Risk of hospitalisations for coronary heart disease or heart failure in the Copenhagen General Population Study, by eight phenotypes of airway disease with no respiratory disease as reference. Percentages are Kaplan-Meier estimates. Individuals with asthma are divided by presence of allergy. Left part shows coronary heart disease and right part shows heart failure. Top-down: base model or multivariable model (‘Age and sex adjusted model’ or ‘Multivariable adjusted model’). Left to right: the number of participants included (‘No. of Participants’), number of events recorded during follow-up (‘Events No.’), HR from Cox-regression analyses with 95% CIs (‘Hazard Ratio (95% CI)’). Online supplementary table S1 shows details of variables included in multivariable modelling. ACO, asthma-COPD overlap; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Furthermore, there were no associations between allergy and coronary heart disease (multivariable adjusted HR=1.03 (95% CI 0.93 to 1.13, p=0.60)) nor heart failure (multivariable adjusted HR=0.94 (95% CI 0.79 to 1.12, p=0.51)) in the ‘No respiratory disease’ subgroup.
Asthma age of onset
In our second sensitivity analysis, defining early versus late asthma onset by using 20 years as the age cut point, we observed only minor changes and most HRs and p values were largely unchanged compared with our main analyses, as shown in online supplementary figure S1. Importantly, though, using this cut point, asthma with early onset was significantly associated with risk of coronary heart disease, HR=1.5 (95% CI 1.0 to 2.2, p=0.03).
No history of admissions with coronary heart disease or heart failure
Excluding individuals with previous admissions of coronary heart disease or heart failure from our study population, we observed similar results as in our main analyses. However, statistical power was reduced by the somewhat smaller study population and fewer events recorded during follow-up, as shown in online supplementary figure S2. However, late-onset asthma was not statistically associated with heart failure, HR=1.5 (95% CI 1.0 to 2.3) while early onset asthma remained associated with an HR of 2.3 (95% CI 1.4 to 3.7).
Adjusting for pack-years
After adjustment for pack-years, we observed similar estimates between obstructive airway disease phenotypes and prospective risk of coronary heart disease and heart failure as shown in online supplementary figure S3.
Discussion
In this large prospective study of the general population we observed that risks of coronary heart disease and heart failure were increased in COPD, even in mild-to-moderate disease. We also observed a similar increased risk in individuals with ACO with observed associations largely explained by severity of lung function impairment. Asthma also associated with risks of coronary heart disease and heart failure, with increased risk of coronary heart disease only in individuals who reported presence of allergy. We suggest that cardiovascular risk factors should be assessed systematically in obstructive airway disease with the potential to facilitate targeted treatments.
The burden of cardiovascular comorbidity in obstructive airway disease is increasingly acknowledged, and there is a need of identifying which patients are at increased risk31 to facilitate optimal treatment and prevention. To our knowledge, this is the first large prospective population-based study showing that individuals with ACO have an increased risk of coronary heart disease and heart failure, and that this risk is of similar magnitude, or perhaps even slightly higher, than the risk observed in individuals with COPD. Recently, we have observed a poor survival in individuals with ACO in another Copenhagen cohort, The Copenhagen City Heart Study,29 and therefore the present study suggests that cardiovascular comorbidity is not a major part of the explanation for the poorer prognosis observed in ACO compared with COPD. The prevalence of ACO among those with airflow limitation in the present study was approximately 19%, which is in line with the prevalence reported in other cohorts.16 17 29 32 33 Our definition of ACO is based on objectively verified airflow limitation and self-reported asthma and our main findings of a higher risk of cardiovascular events in ACO are in line with findings of van Boven et al, although these investigators used a register-based diagnosis in their main analyses.16
Ageing in itself is a common risk factor to both obstructive airway disease and cardiovascular disease. However, in a recent study of ACO using the Copenhagen City Heart Study, we reported that age of asthma onset significantly influenced lung function decline and survival.29 Our present findings suggest that the age of asthma onset in ACO does not seem to play a major role with regard to future risk of cardiovascular comorbidity, which is somewhat surprising as previous research has indicated that early onset asthma and late-onset asthma could represent different pathological processes. However, in line with such a hypothesis, our main asthma analysis suggested that an increased risk of coronary heart disease might only be present in individuals with late-onset asthma. Similar results were observed in the Wisconsin Sleep Cohort9 and among women in the Atherosclerosis Risk in Communities Study.10 Importantly, in our sensitivity analysis using 20 years as the cut point, both early onset and late-onset asthma associated with risk of coronary heart disease. Thus, the lack of association with early onset asthma in our main analysis could depend on the choice of age cut point. Furthermore, limitations regarding our asthma definition should be acknowledged. Both heavy smoking history and airflow limitation were exclusion criteria in this group, thereby excluding individuals with a major cardiovascular risk factor or with obstructive lung function impairment.3 7 34 35 We thus recognise that our results may not apply to individuals with asthma and presence of airflow limitation, although the majority of individuals with asthma in the general population do not show presence of obstruction on spirometry.
Previous studies have indicated that presence of allergy could be associated with increased risk of cardiovascular disease.12 Therefore, we did a sensitivity analysis dividing asthmatic individuals by presence of allergy. In this analysis, we observed that only asthma with presence of allergy associated significantly with the risk of coronary heart disease. A possible explanation could be cellular pathways such as mast cell release linking allergy in asthma with coronary heart disease.36 The hypothesis of inflammatory pathways as a possible common mechanism is compatible with at recent finding of an increased risk only present in individuals with active asthma37 and also in line with observed associations between atopic dermatitis and myocardial infarction.38 Furthermore, the observed association between presence of allergy in asthma and coronary heart disease could be dependent on the late-phase reaction and chronic allergic inflammation involving mediators such as transforming growth factor α,39 a cytokine that has been associated with coronary heart disease.40 However, many complex mechanisms and mediators are involved in chronic inflammation in allergic asthma,39 and future studies are needed to investigate whether there could be particular inflammatory links between presence of allergy in asthma and cardiovascular comorbidity since our subgroup sensitivity analysis showed no association between presence of allergy without presence of asthma and prospective risk of cardiovascular disease.
As expected, we observed that diabetes and hypertension were strongly associated with the prospective risk of cardiovascular disease. These risk factors can be easily assessed at regular pulmonary examinations in an ambulatory setting by measuring glycated haemoglobin and blood pressure in other to facilitate targeted treatment. Our analyses also showed reduction in risk of coronary heart disease and heart failure associated with increased physical activity in leisure time, also when adjusting for several other important covariates. Considering that many previous studies have corroborated the importance of pulmonary rehabilitation in COPD, physical activity seems particularly important in patients with presence of both obstructive airway disease and comorbid cardiovascular disease.
In addition, while increased body mass index, as expected, associated with increased risk of both coronary heart disease and heart failure, previous research has shown that an increased body mass index is, in fact, associated with a lower risk of exacerbations in COPD.41 This seems like a paradox as several studies have shown a strong association between increased risk of exacerbations in COPD and increased risk of cardiovascular events.42 We therefore also call for future studies of associations between body mass index, weight changes, and body composition and risk of exacerbations in individuals with obstructive airway disease and cardiovascular comorbidity.
Our study seems to corroborate and supplement previous findings on the strong association between COPD, disease severity assessed by lung function impairment, and future risk of coronary heart disease and heart failure.4 5 A major strength of our study was inclusion of important clinical respiratory and cardiovascular characteristics. After adjusting for established risk factors, we observed that even mild-to-moderate COPD was associated with coronary heart disease and heart failure. Severity of FEV1 impairment seemed important in our study, thus, individuals with FEV1 below 50% of predicted had a higher risk than those with FEV1 above 50% p. A potential limitation is that our spirometries are prebronchodilator only. However, we believe that our findings support the notion that severity of lung function impairment plays the most important role in the risk of hospitalisations with coronary heart disease and heart failure in COPD. These results are in line with a previous study from the Copenhagen City Heart Study,43 and also in line with a study using the UK-based General Practice Research Database, where the investigators used COPD treatment as a proxy for disease severity and observed a higher risk among those patients with COPD with most severe disease.44
Most individuals with COPD have a history of smoking and therefore, we included pack-years in our definition of COPD phenotype. This explains why smoking was a weakly associated variable in multivariable modelling. We are aware of the fact that the way we defined our subgroups of airway disease phenotypes may significantly affect our findings and interpretation such as exclusion of individuals with light smoking COPD and heavy smoking asthma. However, our classification used both elements from the Global Initiative for Asthma and GOLD documents, and also elements commonly employed to define inclusion criteria for drug trials in asthma and COPD.45 46
It should also be acknowledged that we lack further characterisation of types of airway inflammatory process in our individuals with different phenotypes of obstructive airway disease. Furthermore, as another possible limitation, we only had access to information on self-reported asthma, and a physician-based diagnosis would likely be more reliable. Nevertheless, the use of self-reported asthma is well established for identification of asthma in population-based settings,47 48 as well as reported age of asthma onset.49
Strengths to the present study include the high number of enrolled participants and a complete follow-up in nationwide registries of diagnoses with a high specificity.24 25 We also consider the quite comprehensive information on both established cardiovascular and respiratory risk factors and an objectively verified presence of airflow limitation an important advantage that allows new clinical information on the associations between obstructive airway disease phenotypes and prospective risk of coronary heart disease and heart failure.
Despite our findings, we acknowledge that whether obstructive airway disease phenotypes should be considered as genuine cardiovascular risk factors is still a matter of dispute. Recently, a large randomised trial of treatment with inhaled corticosteroids and long-acting β2-agonists, the SUMMIT Study, aiming at reducing risk of cardiovascular mortality in individuals with COPD, failed to show any effect.50 Considering the importance of low lung function as a major risk factor both for cardiovascular disease and mortality, and considering that the SUMMIT Study did not show an effect of inhaled maintenance medications on mortality in subjects with COPD at increased cardiovascular risk, we suggest that cardiovascular as well as metabolic concomitant diseases should be not only carefully searched for in subjects with impaired lung function, but also appropriately treated with cardiovascular and metabolic agents. All together, we therefore suggest that, presence of asthma, COPD and ACO should lead to an increased focus on established cardiovascular risk factors in this population and result in relevant interventions if indicated.
In conclusion, risks of coronary heart disease and heart failure were increased in COPD, even in mild-to-moderate disease. We also observed similar increased risks in individuals with ACO, that seemed largely explained by the severity of lung function impairment. Asthma also associated with risks of coronary heart disease and heart failure, with an increased risk of coronary heart disease only present in individuals who also reported presence of allergy. We suggest that cardiovascular risk factors should be assessed systematically in obstructive airway disease with the potential to facilitate targeted treatments.
Footnotes
Contributors: Study concept and design: PL, TSI, JLM, JV, BGN; Acquisition of data: BGN, PL, JLM; Analysis and interpretation of data: TSI, PL, JLM, JV; First drafting of the manuscript: PL and TSI; Critical revision of the manuscript for important intellectual content: All authors; Statistical analysis: JLM, TSI; Obtained funding: BGN, PL; Administrative, technical and material support: BGN, PL; Study supervision: PL, BGN; Data access and responsibility: PL, TSI, JLM. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: Capital Region of Copenhagen, Danish Lung Foundation, Velux Foundation, Herlev Hospital, GlaxoSmithKline (unrestricted grant: EPI 115882 – EUPharmaLocal). JV is supported by the NIHR Manchester BRC. The funding sources had no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Competing interests: TSI has received fee for speaking from AstraZeneca. JV has received fee for speaking from AstraZeneca, Boehringer Ingelheim, Chiesi and Novartis; received fee for consulting from AstraZeneca, Boehringer Ingelheim, Chiesi and Novartis. PL has received research grants from Astra Zeneca, Boehringer Ingelheim and GlaxoSmithKline; received fee for speaking from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithCline; received fee for consulting from AstraZeneca, Boehringer Ingelheim and GlaxoSmithKline.
Patient consent for publication: Not required.
Ethics approval: The study was approved by institutional review boards and the local Danish ethics committees (H-KF01-144/01), and was conducted according to the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available. For this research project a protocol was approved by the board of the Copenhagen General Population Study. Researchers were allowed access to the data in the study through encrypted access.
References
- 1. Boulet L-P, Boulay Marie-Ève, Boulay ME. Asthma-Related comorbidities. Expert Rev Respir Med 2011;5:377–93. 10.1586/ers.11.34 [DOI] [PubMed] [Google Scholar]
- 2. Patel ARC, Hurst JR. Extrapulmonary comorbidities in chronic obstructive pulmonary disease: state of the art. Expert Rev Respir Med 2011;5:647–62. 10.1586/ers.11.62 [DOI] [PubMed] [Google Scholar]
- 3. Su X, Ren Y, Li M, et al. . Prevalence of comorbidities in asthma and Nonasthma patients. Medicine 2016;95:e3459 10.1097/MD.0000000000003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mullerova H, Agusti A, Erqou S, et al. . Cardiovascular comorbidity in COPD: systematic literature review. Chest 2013;144:1163–78. [DOI] [PubMed] [Google Scholar]
- 5. Chen W, Thomas J, Sadatsafavi M, et al. . Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med 2015;3:631–9. 10.1016/S2213-2600(15)00241-6 [DOI] [PubMed] [Google Scholar]
- 6. Tattersall MC, Guo M, Korcarz CE, et al. . Asthma predicts cardiovascular disease events: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol 2015;35:1520–5. 10.1161/ATVBAHA.115.305452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schanen JG, et al. Asthma and incident cardiovascular disease: the Atherosclerosis risk in Communities study. Thorax 2005;60:633–8. 10.1136/thx.2004.026484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knoflach M, Kiechl S, Mayr A, et al. . Allergic rhinitis, asthma, and atherosclerosis in the Bruneck and army studies. Arch Intern Med 2005;165:2521–6. 10.1001/archinte.165.21.2521 [DOI] [PubMed] [Google Scholar]
- 9. Tattersall MC, Barnet JH, Korcarz CE, et al. . Late‐Onset asthma predicts cardiovascular disease events: the Wisconsin sleep cohort. J Am Heart Assoc 2016;5 10.1161/JAHA.116.003448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Onufrak SJ, Abramson JL, Austin HD, et al. . Relation of adult-onset asthma to coronary heart disease and stroke. Am J Cardiol 2008;101:1247–52. 10.1016/j.amjcard.2007.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang L, Gao S, Yu M, et al. . Association of asthma with coronary heart disease: a meta analysis of 11 trials. PLoS One 2017;12:e0179335 10.1371/journal.pone.0179335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim J, Purushottam B, Chae YK, et al. . Relation between common allergic symptoms and coronary heart disease among NHANES III participants. Am J Cardiol 2010;106:984–7. 10.1016/j.amjcard.2010.05.029 [DOI] [PubMed] [Google Scholar]
- 13. Leynaert B, Neukirch F, Demoly P, et al. . Epidemiologic evidence for asthma and rhinitis comorbidity. J Allergy Clin Immunol 2000;106:S201–5. 10.1067/mai.2000.110151 [DOI] [PubMed] [Google Scholar]
- 14. Postma DS, Rabe KF. The Asthma–COPD overlap syndrome. N Engl J Med 2015;373:1241–9. 10.1056/NEJMra1411863 [DOI] [PubMed] [Google Scholar]
- 15. Miravitlles M. Diagnosis of asthma–COPD overlap: the five commandments. Eur Respir J 2017;49 10.1183/13993003.00506-2017 [DOI] [PubMed] [Google Scholar]
- 16. van Boven JFM, Román-Rodríguez M, Palmer JF, et al. . Comorbidome, pattern, and impact of Asthma-COPD overlap syndrome in real life. Chest 2016;149:1011–20. 10.1016/j.chest.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 17. Izquierdo-Alonso JL, Rodriguez-GonzálezMoro JM, de Lucas-Ramos P, et al. . Prevalence and characteristics of three clinical phenotypes of chronic obstructive pulmonary disease (COPD). Respir Med 2013;107:724–31. 10.1016/j.rmed.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 18. Pleasants RA, Ohar JA, Croft JB, et al. . Chronic obstructive pulmonary disease and asthma-patient characteristics and health impairment. COPD 2014;11:256–66. 10.3109/15412555.2013.840571 [DOI] [PubMed] [Google Scholar]
- 19. Yeh J-J, Wei Y-F, Lin C-L, et al. . Association of asthma–chronic obstructive pulmonary disease overlap syndrome with coronary artery disease, cardiac dysrhythmia and heart failure: a population-based retrospective cohort study. BMJ Open 2017;7:e017657 10.1136/bmjopen-2017-017657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lange P, Marott JL, Vestbo J, et al. . Prediction of the clinical course of chronic obstructive pulmonary disease, using the new gold classification. Am J Respir Crit Care Med 2012;186:975–81. 10.1164/rccm.201207-1299OC [DOI] [PubMed] [Google Scholar]
- 21. Løkke A, Marott JL, Mortensen J, et al. . New Danish reference values for spirometry. Clin Respir J 2013;7:153–67. 10.1111/j.1752-699X.2012.00297.x [DOI] [PubMed] [Google Scholar]
- 22. Andersen TF, Madsen M, Jørgensen J, et al. . The Danish national Hospital register. A valuable source of data for modern health sciences. Dan Med Bull 1999;46:263–8. [PubMed] [Google Scholar]
- 23. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health 2011;39:30–3. 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 24. Kümler T, Gislason GH, Kirk V, et al. . Accuracy of a heart failure diagnosis in administrative registers. Eur J Heart Fail 2008;10:658–60. 10.1016/j.ejheart.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 25. Sundbøll J, Adelborg K, Munch T, et al. . Positive predictive value of cardiovascular diagnoses in the Danish national patient registry: a validation study. BMJ Open 2016;6:e012832 10.1136/bmjopen-2016-012832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schnohr P, Marott JL, Kristensen TS, et al. . Ranking of psychosocial and traditional risk factors by importance for coronary heart disease: the Copenhagen City heart study. Eur Heart J 2015;36:1385–93. 10.1093/eurheartj/ehv027 [DOI] [PubMed] [Google Scholar]
- 27. Lin DY, Wei LJ, Ying Z. Checking the COX model with cumulative sums of martingale-based residuals. Biometrika 1993;80:557–72. 10.1093/biomet/80.3.557 [DOI] [Google Scholar]
- 28. R Core Team R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2013. www.R-project.org [Google Scholar]
- 29. Lange P, Çolak Y, Ingebrigtsen TS, et al. . Long-Term prognosis of asthma, chronic obstructive pulmonary disease, and asthma-chronic obstructive pulmonary disease overlap in the Copenhagen City heart study: a prospective population-based analysis. Lancet Respir Med 2016;4:454–62. 10.1016/S2213-2600(16)00098-9 [DOI] [PubMed] [Google Scholar]
- 30. Metz L, Waters DD. Implications of cigarette smoking for the management of patients with acute coronary syndromes. Prog Cardiovasc Dis 2003;46:1–9. 10.1016/S0033-0620(03)00075-6 [DOI] [PubMed] [Google Scholar]
- 31. Roversi S, Fabbri LM, Sin DD, et al. . Chronic obstructive pulmonary disease and cardiac diseases. An urgent need for integrated care. Am J Respir Crit Care Med 2016;194:1319–36. 10.1164/rccm.201604-0690SO [DOI] [PubMed] [Google Scholar]
- 32. Cosio BG, Soriano JB, López-Campos JL, et al. . Defining the Asthma-COPD overlap syndrome in a COPD cohort. Chest 2016;149:45–52. 10.1378/chest.15-1055 [DOI] [PubMed] [Google Scholar]
- 33. Hardin M, Cho M, McDonald M-L, et al. . The clinical and genetic features of COPD-asthma overlap syndrome. Eur Respir J 2014;44:341–50. 10.1183/09031936.00216013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iribarren C, Tolstykh IV, Miller MK, et al. . Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: a prospective study of 2 matched cohorts. Am J Epidemiol 2012;176:1014–24. 10.1093/aje/kws181 [DOI] [PubMed] [Google Scholar]
- 35. Agarwal SK, Heiss G, Barr RG, et al. . Airflow obstruction, lung function, and risk of incident heart failure: the Atherosclerosis risk in communities (ARIC) study. Eur J Heart Fail 2012;14:414–22. 10.1093/eurjhf/hfs016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Triggiani M, Patella V, Staiano RI, et al. . Allergy and the cardiovascular system. Clin Exp Immunol 2008;153:7–11. 10.1111/j.1365-2249.2008.03714.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bang DW, Wi C-I, Kim EN, et al. . Asthma status and risk of incident myocardial infarction: a population-based case-control study. J Allergy Clin Immunol Pract 2016;4:917–23. 10.1016/j.jaip.2016.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yuan M, Cao W-F, Xie X-F, et al. . Relationship of atopic dermatitis with stroke and myocardial infarction. Medicine 2018;97:e13512 10.1097/MD.0000000000013512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature 2008;454:445–54. 10.1038/nature07204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ridker PM, Rifai N, Pfeffer M, et al. . Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation 2000;101:2149–53. 10.1161/01.CIR.101.18.2149 [DOI] [PubMed] [Google Scholar]
- 41. Wei Y-F, Tsai Y-H, Wang C-C, et al. . Impact of overweight and obesity on acute exacerbations of COPD - subgroup analysis of the Taiwan Obstructive Lung Disease cohort. Int J Chron Obstruct Pulmon Dis 2017;12:2723–9. 10.2147/COPD.S138571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reilev M, Pottegård A, Lykkegaard J, et al. . Increased risk of major adverse cardiac events following the onset of acute exacerbations of COPD. Respirology 2019;24:1183–90. 10.1111/resp.13620 [DOI] [PubMed] [Google Scholar]
- 43. Lange P, Mogelvang R, Marott JL, et al. . Cardiovascular morbidity in COPD: a study of the general population. COPD 2011;7:5–10. 10.3109/15412550903499506 [DOI] [PubMed] [Google Scholar]
- 44. Schneider C, Bothner U, Jick SS, et al. . Chronic obstructive pulmonary disease and the risk of cardiovascular diseases. Eur J Epidemiol 2010;25:253–60. 10.1007/s10654-010-9435-7 [DOI] [PubMed] [Google Scholar]
- 45. Travers J, Marsh S, Williams M, et al. . External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax 2007;62:219–23. 10.1136/thx.2006.066837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kruis AL, Ställberg B, Jones RCM, et al. . Primary care COPD patients compared with large pharmaceutically-sponsored COPD studies: an unlock validation study. PLoS One 2014;9:e90145 10.1371/journal.pone.0090145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oksanen T, Kivimäki M, Pentti J, et al. . Self-Report as an indicator of incident disease. Ann Epidemiol 2010;20:547–54. 10.1016/j.annepidem.2010.03.017 [DOI] [PubMed] [Google Scholar]
- 48. Torèn K, Brisman J, Järvholm B. Asthma and asthma-like symptoms in adults assessed by questionnaires. Chest 1993;104:600–8. 10.1378/chest.104.2.600 [DOI] [PubMed] [Google Scholar]
- 49. Torén K, Palmqvist M, Löwhagen O, et al. . Self-Reported asthma was biased in relation to disease severity while reported year of asthma onset was accurate. J Clin Epidemiol 2006;59:90–3. 10.1016/j.jclinepi.2005.03.019 [DOI] [PubMed] [Google Scholar]
- 50. Vestbo J, Anderson JA, Brook RD, et al. . Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (Summit): a double-blind randomised controlled trial. Lancet 2016;387:1817–26. 10.1016/S0140-6736(16)30069-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2019-000470supp001.pdf (634.4KB, pdf)