Abstract
Context: Hesperidin (HSP), a flavanoglycone found in citrus fruits, has antioxidant, anti-inflammatory and neuroprotective properties.
Objective: This study evaluates the protective effect of HSP on l-methionine-induced hyperhomocysteinemia (HHcy) in rats.
Materials and methods: Male Wistar rats were randomly divided into seven groups as DMSO, l-methionine, HSP (25, 50 and 100 mg/kg), HSP-per se (100 mg/kg) and donepezil (0.1 mg/kg). HHcy was induced by oral administration of l-methionine (1.7 g/kg) for 32 days. From the 14th day of study HSP (25, 50 and 100 mg/kg) and donepezil was administered orally to l-methionine-treated rats. Cognitive impairment induced by HHcy was determined using the Morris water maze (MWM) and Y-maze on video tracking system (28th–32nd day). Different biomarkers of HHcy in serum and brain and vascular reactivity were evaluated and histopathology (thoracic aorta and brain) was done.
Results: HSP (100 mg/kg) treatment in l-methionine-treated rats exhibited significant (p < 0.001) dose-dependent activity and reduced behavioural deficits, brain acetylcholinesterase (25.99 ± 2.36 versus 10.73 ± 1.26 μmoles/mg), brain lipid peroxidation (15.25 ± 1.65 versus 6.18 ± 0.74 nM/mg), serum homocysteine (Hcy) (22.37 ± 0.30 versus 11.01 ± 1.01 μg/mL) and serum cholesterol (182.7 ± 2.15 versus 101.5 ± 2.76 mg/dL) and increased brain antioxidant levels. HSP significantly (p < 0.001) reduced endothelial dysfunction (ED) by abolishing the effect of l-methionine on acetylcholine-induced endothelial-dependent relaxation and increased serum nitrite and vascular nitric oxide bioavailability along with the restoration of histological aberrations.
Conclusion: HSP exerts a protective effect on HHcy by abrogating oxidative stress, ED and neurotoxicity.
Keywords: Acetylcholinesterase, Morris water maze, video tracking system, L-NAME, thoracic aorta, hippocampus, donepezil
Introduction
Vascular dementia (VaD) is a disorder of cognitive impairment along with dysfunction of the vascular endothelium. Similar to Alzheimer’s disease, VaD results in progressive stepwise deteriorating impairments in higher functions of the brain, such as memory, new learning, recognition, motor functions and planning. Oxidative stress has been implicated as an important risk factor in the neuropathology of several neurodegenerative disorders characterized by cognitive deficits (Coyle & Puttfarcken 1993; Olanow 1993).
Acute oral administration of l-methionine or subcutaneous administration of dl-homocysteine in rats induces hyperhomocysteinemia (HHcy), which further cause VaD-like syndrome (Shah & Singh 2007). Homocysteine (Hcy) is an excitatory amino acid, which markedly enhances the vulnerability of neuronal cells to excitotoxic and oxidative injury (Hankey & Eikelboom 1999). Literature survey shows a positive, dose-dependent relationship between mild to moderate increase in plasma total Hcy concentrations and the risk of neurodegenerative diseases, such as VaD, cognitive impairment or stroke (Herrmann & Obeid 2011). Elevated total plasma Hcy more than 14 μM is termed as HHcy (Seshadri et al. 2002). Several studies reported that HHcy inhibits the expression of antioxidant enzymes and also produces complex changes in blood vessels that include ED, oxidative stress, pro-inflammatory effects such as expression of tumour necrosis factor and inducible nitric oxide (NO) synthase (Heinecke 1988; Hankey & Eikelboom 1999; Lentz et al. 2000).
Flavonoids are phenolic compounds that are most abundant in plants, especially in fruits and vegetables. It has been reported that most of them are effective antioxidants (Rice-Evans et al. 1996). Hesperidin (HSP, 30, 5, 9-dihydroxy-40-methoxy-7-orutinosyl flavanone) is a pharmacologically active flavonoglycone abundantly found in sweet oranges and lemons. It has been reported that large amounts of HSP is present in immature orange of Citrus aurantium L., C. sinensis, C. unshiu (Rutaceae) species (Emim et al. 1994; Kawaguchi et al. 1997). Clinical and experimental data showed antihypertensive, lipid-lowering, insulin-sensitizing, antioxidative and anti-inflammatory properties of HSP (Suarez et al. 1998; Chanet et al. 2012; Parhiz et al. 2015). In addition, HSP and its aglycone HSP are reported for their role in protecting neuronal cells and osteoblasts from oxidative stress by functioning as a potent radical scavenger (Kim et al. 2004; Hirata et al. 2005; Cho, 2006). HSP is moreover found to be an effective supplement in the treatment of several neurodegenerative diseases (Kumar & Kumar 2010). However, there is no literature report of HSP against HHcy-associated VaD and oxidative stress, induced by the administration of l-methionine in rats. Hence, this study is designed to investigate the protective role of HSP against HHcy-induced oxidative damage, memory impairment and endothelial dysfunction in rats. The potential effect of HSP was determined by behavioural, biochemical and histopathological analysis using donepezil as a positive control.
Materials and methods
Reagents and chemicals
HSP, l-methionine, acetylthiocholine iodide, reduced glutathione, sodium nitroprusside, l-NAME and indomethacin were purchased from Sigma-Aldrich (St. Louis, MO). Donepezil was a gift sample from Hetero Labs Ltd., Medak district, Telangana, India. All other reagents are of analytical grade were purchased from SD Fine Chemicals Ltd., India. Double-distilled water was used throughout the experiments for biochemical assays.
Animals
Male Wistar rats of 180–220 g procured from the National Center for Laboratory Animal Sciences, National Institute of Nutrition, Hyderabad, India was used for the study. All animals had free access to water ad libitum and standard laboratory pellet chow diet under constant temperature (22–24 °C, humidity 45–50%) on a 12 h light/dark cycle. The experiments were conducted between 9.00 h and 18.00 h under optimal conditions. The experimental protocol was approved by the institutional animal ethics committee of Anurag Group of Institutions (Formerly Lalitha College of Pharmacy), and care about the animals was taken as per guidelines of the committee for the purpose of control and supervision of experiments on animals (Protocol No: I/IAEC/LCP/034/2013/15).
Induction of HHcy and ED
Rats were treated with l-methionine (1.7 g/kg/day, per oral (P.O.) for 32 days) to induce HHcy-associated endothelial dysfunction and VaD (Shah & Singh 2007; Koladiya et al. 2008, 2009).
Experimental design
Wistar rats were divided into seven groups, and each group consists of 10 animals (n = 10). HSP and l-methionine were freshly prepared using 0.1% w/v dimethyl sulphoxide (DMSO) and 0.5% w/v carboxymethylcellulose (CMC), respectively and donepezil was suspended in distilled water before administration. Low, intermediate and high doses of HSP were selected as per the previously published work by Balakrishnan and Menon (2007). All drugs were administered by oral route.
Group I: 0.1% w/v DMSO was administered once daily for 4 weeks, and then they were subjected to MWM test. During acquisition trial, conducted from day 1 to day 4 and retrieval trial conducted on day 5, DMSO was administered before 45 min of initiation of the study.
Group II: l-Methionine (1.7 g/kg/day) was administered for 4 weeks. l-Methionine was also administered, 45 min before every time during the acquisition trial. The animals were treated with 0.5% w/v CMC, 10 mL/kg, po 45 min before retrieval trial.
Group III: HSP is administered at 25 mg/kg/day from the 14th day of l-methionine treatment for two weeks. The treatment was also continued during acquisition trial. The animals were administered only with vehicle (0.1% DMSO) during retrieval trial.
Group IV and V: HSP was administered at 50 and 100 mg/kg/day from the 14th day of l-methionine treatment for 2 weeks, and rest of the protocol was same as mentioned in group III.
Group VI: HSP was administered at 100 mg/kg/day for 4 weeks, and rest of the protocol was same as mentioned in group III.
Group VII: Donepezil (0.1 mg/kg/day) was administered from the 14th day of l-methionine treatment for 2 weeks. The treatment was continued during acquisition trial. The animals were administered with distilled water 10 mL/kg, po, during retrieval trial.
Behavioural testing
Y-maze and Morris water maze (MWM) tests were carried out at the end of the fourth week after the induction of HHcy (28th day). The experiment was performed between 9.00 and 16.00 h in the neuroscience laboratory at established optimal conditions.
Video tracking on Y-maze
The Y-maze test is used for measuring spatial working memory in rodents (Reddy, 1997). Each rat was placed at the one end of the arm and allowed to move freely through the maze for 8 min. When all four paws of the mouse were within any of three arms, an arm entry was registered. Entries into all three arms on consecutive choices (i.e., ABC, CAB, or BCA but not BAB) were defined as alternations. The series of arm entries, including possible returns into the same arm, is recorded using maze master software on the video tracking system (VJ Instruments, Washim, Maharashtra, India). The percentage of alteration is calculated using the formula:
Video tracking on MWM
Spatial learning and memory performance were assessed using the MWM test (Morris 1984; Vorhees & Williams 2006). The water maze consists of circular (180 cm diameter, 60 cm depth) tank filled with water (22 °C) up to a depth of 30 cm. A circular, 10 cm diameter escape platform made of black Plexiglas was placed into the pool 1 cm below the surface of the water and was maintained in left quadrant. The acquisition trial was carried out from 28th day to 31st day for four times with a rest period of 1 h between each trial. The starting position was randomly assigned in a counterbalanced manner as mentioned below. The target quadrant (Q1) remained constant throughout the trial.
| Day 1 | Q1 | Q2 | Q3 | Q4 |
| Day 2 | Q2 | Q3 | Q4 | Q1 |
| Day 3 | Q3 | Q4 | Q1 | Q2 |
| Day 4 | Q4 | Q1 | Q2 | Q3 |
Mean escape latency time (ELT) was calculated for each day during the acquisition trials and day 4 ELT was used as an index of acquisition. The retrieval trial was performed on the 32nd day. The platform was removed and the rats were placed in MWM and allowed to explore the maze for 120 s. Mean time spent in all three quadrants (TSTQ) i.e., Q2, Q3 and Q4 were recorded and the time spent in the target quadrant i.e., Q1 in search of the missing platform was considered as an index for retrieval. ELT and TSTQ were recorded and analyzed using maze master software on the video tracking system (VJ Instruments, Washim, Maharashtra, India).
Biochemical analysis
At the end of the experiment (32nd day), blood samples were collected from retro-orbital venipuncture and centrifuged at 3500 rpm for 15 min at room temperature for the separation of a clear, non-hemolyzed supernatant sera for Hcy, nitrite and cholesterol estimations. Later, all rats were sacrificed by cervical dislocation and immediately brains and thoracic aorta (n = 6) were isolated for biochemical analysis and aortic ring assay. Brain homogenate (10% w/v) was used for acetylcholinesterase, LPO and antioxidant estimations.
Estimation of serum biochemical parameters
Serum Hcy was determined according to the method described earlier (Kemse et al. 2014) using Chemiluminescent Microparticle Immunoassay (Abbott Laboratory, Abbott Park, Chicago, IL). Serum nitrite was determined using the earlier described method (Sastry et al. 2002) with slight modifications, and total cholesterol was estimated using commercially available kits (Biosystems, Costa Brava, Barcelona, Spain) on a semi-automated analyzer (Optima-S, LAB INDIA Healthcare Pvt. Ltd., Gurgaon, Haryana, India).
Estimation of brain biochemical parameters
Brain AChE was measured using the earlier described method with slight modifications (Ellman et al. 1961). The solution containing eserine solution was used as a blank and change in absorbance was read spectrophotometrically at 420 nm (UV-1800 spectrophotometer; Shimadzu Co., Kyoto, Japan). Malondialdehyde, a lipid peroxidation end product in brain homogenate, was measured according to the method described by Wills (1969) with some modifications. The absorbance of the pink coloured extract in n-butanol was measured spectrophotometrically at 532 nm. The amount of MDA was calculated using a molar extinction coefficient of 1.56 × 105 M−1 cm−1 and expressed as nmoles of MDA formed per gram wet weight of tissue. SOD activity was determined by the pyrogallol oxidation method (Marklund & Marklund 1974). The reaction is initiated by adding pyrogallol, and the change in optical density is measured at 420 nm. GSH levels were measured according to the earlier described method (Ellman 1959) and absorbance was measured spectrophotometrically at 412 nm. Catalase activity was determined using the earlier method (Chance & Maehly 1955) with some modifications, and the breakdown of hydrogen peroxide (H2O2) is measured at 240 nm.
Determination of total brain protein
Brain total protein was determined by Lowry et al. (1951) method with slight modifications. The absorbance was measured spectrophotometrically at 750 nm.
Vascular reactivity measurements
After decapitation and the thoracic aorta was isolated and cut into ring segments of 4 to 5 mm width, and mounted in an organ bath containing Kreb’s-bicarbonate buffer bubbled with carbonated oxygen (95% O2:5% CO2), and maintained at 37.8 °C. Isometric tension developed in the vasculature was recorded using a force transducer, which was connected to a power lab data acquisition system (AD Instruments, Australia). Aortic rings were allowed to equilibrate for 30 min at a resting tension of 2 g, with the bath medium changed every 10 min. After the equilibration period, aortic rings were precontracted with l-phenylephrine (PHE) (1 × 10−6 M) and exposed to (i) acetylcholine (Ach) (1 × 10−8 to 1 × 10−5 M) to assess the endothelium-dependent relaxation, (ii) sodium nitroprusside (SNP) (1 × 10−8 to 1 × 10−5 M) to assess the endothelium-independent relaxation (Pieper 1997; Shah & Singh 2006). To characterize the involvement of endothelial-NO, aortic nitric oxide bioavailability was measured as an NOS inhibitor (NG-nitro-l-arginine methyl ester) l-NAME (100 mM) induced contraction of isolated rat aorta after submaximal precontraction of the vessel with l-phenylephrine. To prevent synthesis and influence of prostaglandins, the experiment was conducted in the presence of 10 mM indomethacin (Unger & Patil 2009).
Histopathological examination
Thoracic aorta and brain tissues (n = 4) were fixed in 10% phosphate-buffered formalin, processed, embedded in paraffin and sectioned to approximately 5 μm thickness using a microtome (Leica, Bensheim, Germany). The aorta and brain sections were then processed and stained with haematoxylin and eosin (H&E) for light microscopy (Axiovision software, Axioplan 2 Imaging, Zeiss microscope). Histological examination and quantification were performed by an investigator blinded to the interventions.
Statistical analysis
All the results were expressed as mean ± standard error of mean (SEM). Data for isolated aortic ring preparation were statistically analyzed, using repeated measures of analysis of variance ANOVA followed by Newman Keul’s test. All other data were analyzed using one-way ANOVA followed by Tukey’s multiple range tests. The statistical significance of difference was taken as p < 0.05. All the study results were statistically analyzed using Graph Pad prism 5.0 software (San Diego, CA).
Results
Behavioural estimations
Morris water maze
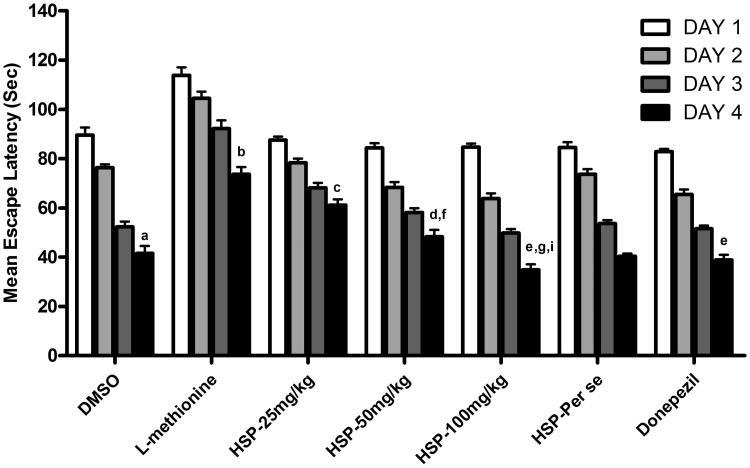
DMSO-treated animals have shown a downward trend in their ELT and there was a significant fall in the day 4 ELT when compared to day 1 ELT of these rats (p < 0.001), reflecting normal learning ability. Further, on day 5, a significant rise in TSTQ was observed, when compared to time spent in other quadrants (p < 0.001) reflecting normal retrieval as well. However, l-methionine-treated animals showed a significant increase in the day 4 ELT when compared to day 4 ELT of DMSO group (p < 0.001) indicating impairment of acquisition (Figure 1). Further, l-methionine administration also produced a significant decrease in the day 5 TSTQ, when compared to day 5 TSTQ of DMSO group (p < 0.001); indicating impairment of memory. Administration of HSP at 25 mg/kg has shown a less significant effect (p < 0.05) on day 4 ELT along with decrease in the day 5 TSTQ (p < 0.01) when compared to l-methionine. Further administration of HSP (50 and 100 mg/kg) and donepezil there is a significant prevention of rise in the day 4 ELT (p < 0.001) and decrease in the day 5 TSTQ in a significant manner (p < 0.001) indicating a reversal of l-methionine-induced impairment of memory (Table 1). p < 0.01 indicates comparison of HSP-100 mg/kg with HSP-50 mg/kg. HSP (100 mg/kg) treatment exhibited a significant dose-dependent effect (p < 0.001, p < 0.01) on comparison with the HSP-25 mg/kg- and HSP-50 mg/kg-treated groups and p < 0.05 on comparison of HSP-50 mg/kg with HSP-25 mg/kg, respectively (Table 2).
Figure 1.
Effect of HSP administration on ELT OF MWM. Values are expressed as mean ± SEM of 10 animals Superscript letters represent the statistical significance done by ANOVA, followed by Tukey’s multiple comparison tests. ap < 0.001 indicates comparison of Day 4 ELT with Day 1 ELT of DMSO group; bp < 0.001 indicates comparison of L-methionine with DMSO; cp < 0.05; dp < 0.01; ep < 0.001 indicates comparison with HSP-25 mg/kg, HSP-50 mg/kg, HSP-100 mg/kg and donepezil groups with L-methionine; fp < 0.05, gp < 0.001 indicates comparison of HSP-50 and 100 mg/kg with HSP-25 mg/kg; Ip < 0.01 indicates comparison of HSP-100 mg/kg with HSP-50 mg/kg.
Table 1.
Effect of HSP administration on serum Hcy, nitrite and cholesterol levels.
| Groups | Serum Hcy (μg/ml) | Serum nitrite (Μm/ml) | Serum cholesterol (mg/dl) |
|---|---|---|---|
| DMSO | 6.71 ± 0.89 | 14.21 ± 1.25 | 90.83 ± 1.53 |
| L-Methionine | 22.37 ± 0.30a | 4.43 ± 0.32a | 182.7 ± 2.15a |
| HSP-25 mg/kg | 18.91 ± 0.31f | 8.96 ± 0.75f | 127.8 ± 2.80b |
| HSP-50 mg/kg | 12.89 ± 1.07c,g | 10.96 ± 0.66c | 106.2 ± 1.75c,g |
| HSP-100 mg/kg | 11.01 ± 1.01d,h | 11.17 ± 0.60d | 101.5 ± 2.76d,h |
| HSP-Per se | 7.63 ± 0.38 | 14.27 ± 1.27 | 90.17 ± 2.44 |
| Donepezil | 7.27 ± 0.86e | 13.75 ± 1.39e | 98.67 ± 2.01e |
Values are expressed as mean ± SEM of 6 animals. Superscript letters represent the statistical significance done by ANOVA, followed by Tukey’s multiple comparison tests.
ap < 0.001 indicates comparison of L-methionine with DMSO.
b,c,d,ep < 0.001 indicates comparison of HSP-25, 50 and 100 mg/kg, donepezil with L-methionine.
fp < 0.05 indicates comparison of HSP-25 mg/kg with L-methionine.
g,hp < 0.001 indicates dose-dependent activity on comparison of HSP-50 and 100 mg/kg with HSP-25 mg/kg.
Table 2.
Effect of HSP administration on TSTQ of MWM and Percentage alterations in Y-maze.
| MWM Y-maze |
||
|---|---|---|
| Groups | Target time spent in target quadrant (s) | Percentage alterations |
| DMSO | 61.60 ± 1.53 | 60.41 ± 1.71 |
| L-Methionine | 20.90 ± 1.26a | 22.32 ± 1.22a |
| HSP-25 mg/kg | 31.20 ± 1.91b | 32.58 ± 1.72b |
| HSP-50 mg/kg | 56.40 ± 2.43c,f | 51.55 ± 2.76c,f |
| HSP-100 mg/kg | 62.30 ± 2.59d,f | 53.77 ± 2.48d,f |
| HSP-Per se | 64.20 ± 1.37 | 59.06 ± 1.78 |
| Donepezil | 69.00 ± 1.67e | 60.02 ± 0.72d |
Values are expressed as mean ± SEM of 10 animals. Superscript letters represent the statistical significance done by ANOVA, followed by Tukey’s multiple comparison tests.
ap < 0.001 indicates comparison of L-methionine with DMSO.
bp < 0.01.
c,d,ep < 0.0001 indicates comparison of HSP-25 mg/kg, HSP-50, 100 mg/kg, donepezil with L-methionine.
fp < 0.001 indicates comparison of HSP-50 and 100 mg/kg with HSP-25 mg/kg.
Y-maze
In l-methionine-treated animals, there is a significant (p < 0.001) decrease of percentage alteration in the Y-maze when compared with DMSO group. However, HSP at all doses (25, 50 and 100 mg/kg) and donepezil administration has shown significant improvement (p < 0.01, p < 0.001) in the percentage alteration when compared to l-methionine group. Significant improvement in percentage alteration (p < 0.001) indicates the dose-dependent activity on comparison of HSP-50 and 100 mg/kg with HSP-25 mg/kg (Table 2).
Biochemical studies
Serum Hcy, nitrite and cholesterol
In l-methionine group, there is a significant (p < 0.001) increase in Hcy, and cholesterol levels with significant (p < 0.001) reduction in nitrite levels when compared with DMSO group. There was a significant (p < 0.05; p < 0.001) decrease in Hcy and cholesterol levels with a significant (p < 0.001) increase in the nitrite levels in all HSP, and donepezil-treated groups when compared with l-methionine. Dose-dependent effect was seen with the significance of p < 0.001 on a comparison of intermediate and high dose with a low dose of HSP in the case of Hcy and cholesterol levels (Table 1).
Brain AChE, MDA and antioxidants
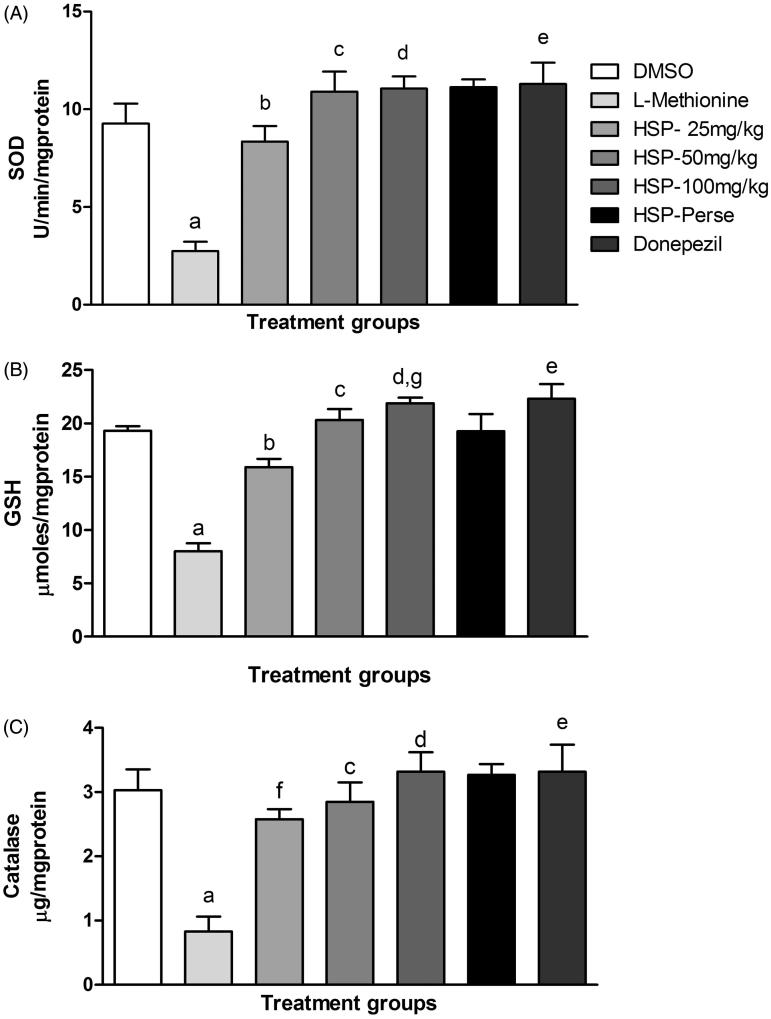
L-Methionine administration significantly (p < 0.001) increased the AChE and MDA along with significant (p < 0.001) reduction in the GSH, SOD and CAT levels when compared with DMSO group. There was a significant (p < 0.05; p < 0.001) decrease in AChE and MDA levels along with significant (p < 0.05; p < 0.001) increase in GSH, SOD and CAT levels in HSP and donepezil-treated groups (Figure 2) when compared with l-methionine group. Dose-dependent effect (p < 0.01; p < 0.001) was seen when a high dose is compared with the low dose in case of brain AChE and GSH (Table 3 and Figure 2).
Figure 2.
Effect of HSP administration on brain antioxidants. Values are expressed as mean ± SEM of six animals. Superscript letters represent the statistical significance done by ANOVA, followed by Tukey’s multiple comparison tests. ap < 0.001 indicates comparison of L-methionine with DMSO; fp < 0.05 indicates comparison of HSP-25 mg/kg with L-methionine; b,c,d,ep < 0.001 indicates comparison of HSP-25 mg/kg, HSP-50 mg/kg, HSP-100 mg/kg and donepezil with L-methionine; gp < 0.01 indicates dose-dependent activity on comparing HSP-100 mg/kg with HSP-25 mg/kg.
Table 3.
Effect of HSP administration on brain AChE and MDA.
| Groups | AChE (μmoles/min/mg protein) | MDA (nM/mg protein) |
|---|---|---|
| DMSO | 9.36 ± 1.12 | 6.38 ± 0.82 |
| L-Methionine | 25.99 ± 2.36a | 15.25 ± 1.65a |
| HSP-25 mg/kg | 19.17 ± 1.57f | 9.16 ± 0.78b |
| HSP-50 mg/kg | 13.70 ± 0.73c | 6.65 ± 0.70c |
| HSP-100 mg/kg | 10.73 ± 1.26d,g | 6.18 ± 0.74d |
| HSP-Per se | 9.32 ± 0.32 | 6.11 ± 0.46 |
| Donepezil | 5.25 ± 1.07e | 5.75 ± 0.37e |
Values are expressed as mean ± SEM of six animals. Superscript letters represent the statistical significance done by ANOVA, followed by Tukey’s multiple comparison tests.
ap < 0.001 indicates comparison of L-methionine with DMSO.
b,c,d,ep < 0.001 indicates comparison of HSP-25, 50 and 100 mg/kg, donepezil with L-methionine.
fp < 0.05 indicates comparison of HSP-25 mg/kg with L-methionine.
gp < 0.001 indicates dose-dependent activity on comparison of HSP-100 mg/kg with HSP-25 mg/kg.
Vascular endothelial function
Endothelium-dependent and independent relaxation
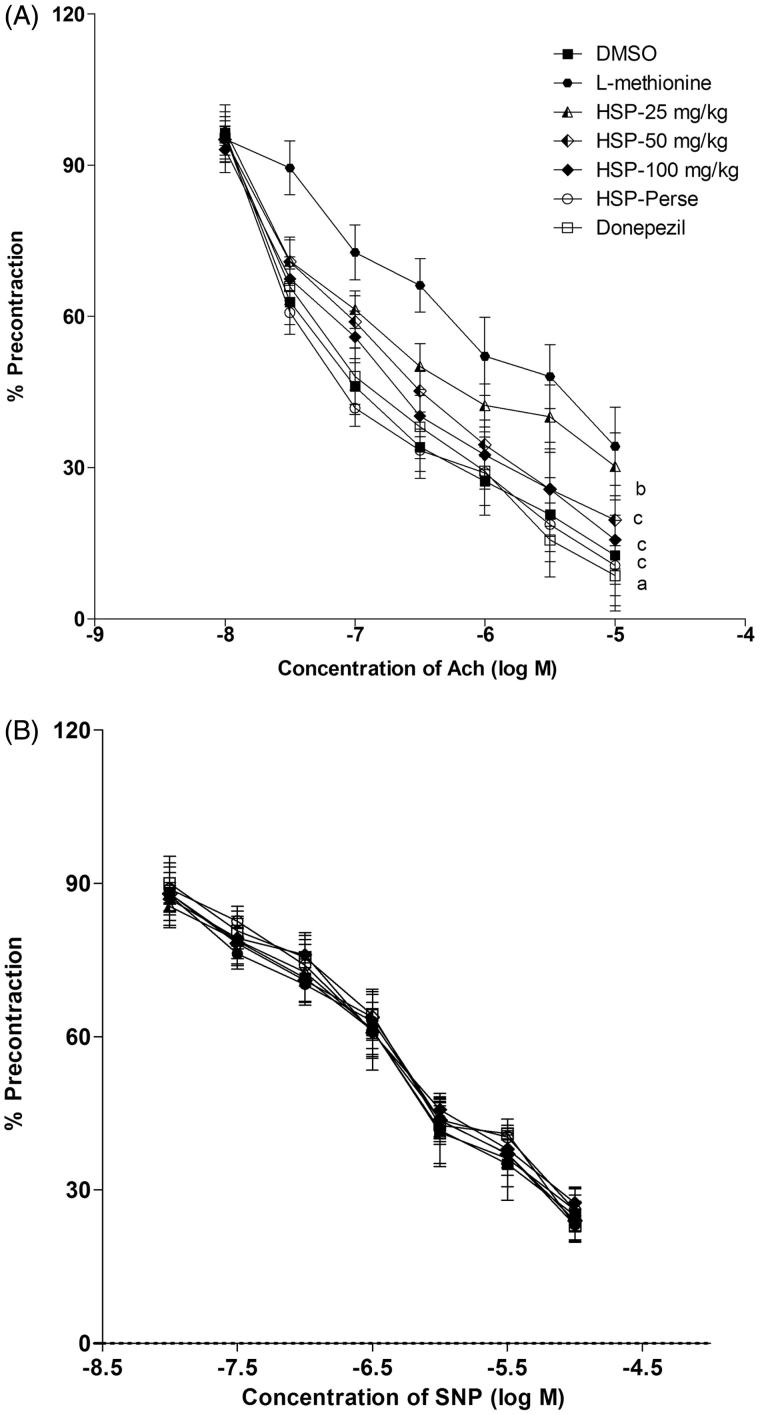
ACh and SNP in a dose-dependent manner produced endothelium-dependent and independent relaxation in PHE precontracted isolated rat aortic ring preparation. l-Methionine administration significantly (p < 0.001) attenuated acetylcholine-induced endothelium-dependent relaxation. ED in the aorta was significantly (p < 0.01; p < 0.001) protected by HSP (25, 50 and 100 mg/kg) and donepezil by abolishing the effect of l-methionine on Ach-induced endothelial-dependent relaxation (Figure 3). On the other hand, l-methionine, HSP (25, 50 and 100 mg/kg) and donepezil did not show any significant effect on the endothelium-independent relaxation produced by SNP.
Figure 3.
Effect of hesperidin administration on acetylcholine-induced endothelium-dependent relaxation and sodium nitroprusside-induced endothelium-independent relaxation using aortic ring preparation. (A) Responses are expressed as a percentage of precontraction induced by phenylephrine. Results are mean ± SEM (n = 6); Repeated measure ANOVA followed by Newman–Keul’s test. ap < 0.001 indicates comparison of L-methionine with DMSO; bp < 0.01 indicates comparison of HSP-25 mg/kg with L-methionine; cp < 0.001 indicates comparison of HSP-50 mg/kg, HSP-100 mg/kg and donepezil with L-methionine. (B) Responses are expressed as percentage of precontraction induced by phenylephrine. Results are mean ± SEM (n = 6); Repeated measures of ANOVA followed by Newman–Keul’s test. Ach: acetylcholine; DMSO: Dimethyl sulfoxide; HSP: Hesperidin; SNP: sodium nitroprusside.
Vascular NO bioavailability
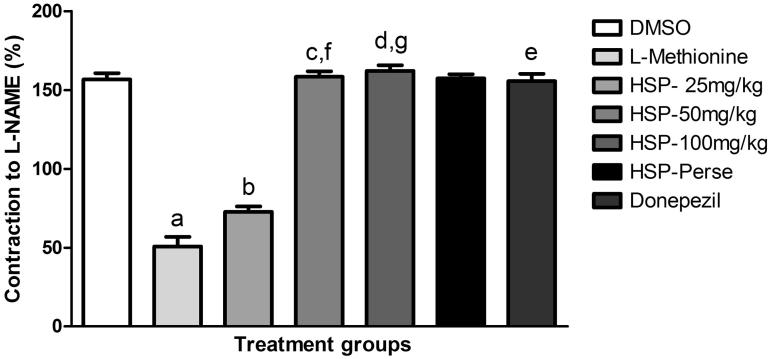
A significant decrease in L-NAME induced contraction of the isolated rat aorta preconstricted with PHE revealed a marked decrease in vascular NO bioavailability in the aorta isolated from l-methionine-treated rats compared with DMSO at p < 0.001. There was a significant prevention of decrease in contraction (p < 0.01; p < 0.001) in HSP and donepezil-treated groups. Dose-dependent effect (p < 0.001) was seen with HSP (50 and 100 mg/kg) on comparison with a low dose (Figure 4).
Figure 4.
Nitric oxide level measured as an L-NAME-induced contraction of aortic ring preconstricted with phenylephrine. Values are expressed as mean ± SEM of six animals. Symbol represents the statistical significance done by ANOVA, followed by Tukey’s multiple comparison tests. ap < 0.001 indicates comparison of L-methionine with DMSO; bp < 0.01 indicates comparison of HSP-25 mg/kg with L-methionine, c,d,ep < 0.001 indicates comparison of HSP-50 mg/kg, HSP-100 mg/kg and donepezil with L-methionine; f,gp < 0.001 indicates dose-dependent activity on comparision of HSP-50 mg/kg and HSP-100 mg/kg with HSP-25 mg/kg.
Histological evaluation
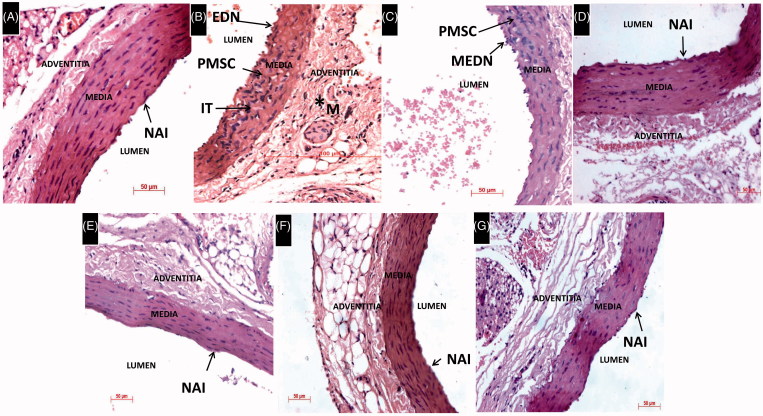
Histological evaluation of the thoracic aorta revealed that l-methionine treatment in rats caused endothelial denuding (EDN), intimal thickening (IT) and proliferation of smooth muscle cells (PSMC). The proliferation of connective tissue and disruption of normal fibrillar pattern in adventitia layer of the aorta is also noticed. However, HSP-25 mg/kg treated rat aorta showed mild endothelial denuding (MEDN), PSMC and a decrease in the proliferation of connective tissue when compared to l-methionine-treated group. HSP (50, 100 mg/kg and Per se) and donepezil-treated groups showed normal aortic intima (NAI) with the regular morphology of the aorta same as that of the DMSO-treated group (Figure 5).
Figure 5.
Histological examination of the thoracic aorta, H&E 400×. (A) DMSO-treated rat thoracic aorta showing normal architecture with regular vascular morphology. (B) L-Methionine-treated group showing EDN IT and PSMC. Proliferation of connective tissue, disruption of normal fibrillar pattern and increase of matrix (M) tissue is also seen in adventitia layer of the aorta. (C) HSP-25 mg/kg group showing MEDN and PSMC similar to L-methionine group. (D–G) HSP-50,100 mg/kg, HSP-Per se and donepezil-treated groups showing NAI with regular morphology of the aorta same as the control group.
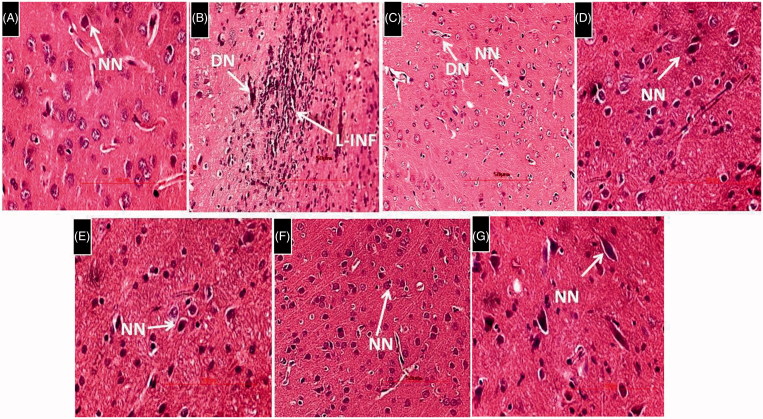
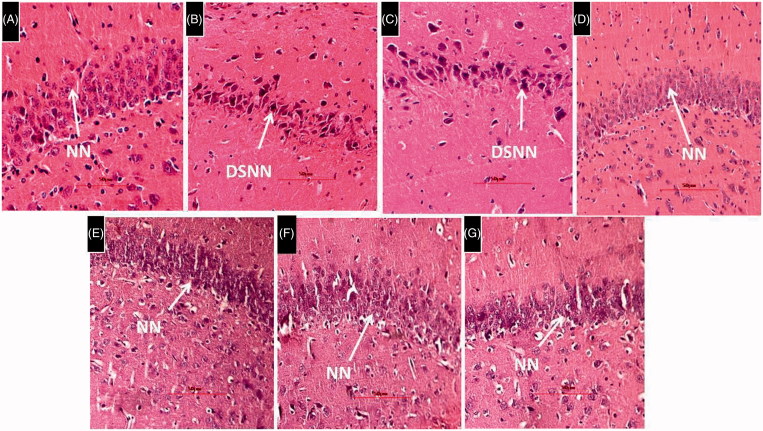
Histological evaluation of brain tissues revealed the presence of a larger area of necrotic foci in the brain parenchyma along with lymphocyte infiltration and degenerating neurons (DN) in the cortex and deeply stained necrotic neurons (DSNN) in the hippocampus. HSP-25 mg/kg showed no sign of infiltrated lymphocytes and less number of DN in the cortex. However, DSNN were noticed in the hippocampus similar to l-methionine-treated group. HSP-50,100 mg/kg. HSP-Per se and donepezil-treated rat cortex and hippocampus regions showed the cytoarchitecture of neurons similar to the DMSO-treated group. All the histological data of brain was represented in the Figures 6 and 7.
Figure 6.
Histological examination of rat brain cortex. H&E 400 × (A) DMSO-treated rat brain cortex showing cytoarchitecture of normal neurons (NN). (B) L-Methionine-treated rat cortical region showing a larger area of necrotic foci in brain parenchyma along with lymphocyte infiltration (L-INF) and DN. (C) HSP-25 mg/kg-treated showing rat cortical region with no signs of necrotic foci and L-INF in brain parenchyma. However, DN similar to L-methionine-treated group were noticed along with normal neurons. (D–G) HSP (50,100 mg/kg), HSP-Per se and donepezil-treated rat cortex regions showing many neurons with normal structure and the cytoarchitecture of neurons are similar to the control group.
Figure 7.
Histological examination of rat brain hippocampus. H&E 400 × (A) DMSO-treated rat brain showing normal cytoarchitecture of neurons having nuclei and properly arranged in a row. (B) L-Methionine-treated rat showing nerve cells and their nuclei lost normal structure and outlines and there are number of DSNN. (C) HSP-25 mg/kg group of rat showing DSNN similar to L-methionine-treated group indicating nil effects of HSP at a low dose. (D,E,F&G) HSP-50 mg/kg, HSP-100 mg/kg, HSP-Per se and donepezil groups of rat hippocampus showing many neurons with normal cytoarchitecture of neurons(NN) similar to DMSO group.
HSP per se effect
HSP alone treatment (100 mg/kg) in rats did not show any significant per se effect (p > 0.05) on all study parameters (behavioural activity, serum, and brain biochemical parameters, endothelial function and histopathology) when compared with DMSO-treated animals.
Discussion
Flavonoids were widely distributed in a number of fruits and vegetables and have a broad range of biological and pharmacological effects. The diet rich in flavonoids is also found effective in the regulation of brain function (Maher et al. 2006). Therefore, with support from earlier literature review, the present study is designed to investigate the role of HSP in preventing cognitive decline, oxidative damage, ED and neuropathological alterations in hyperhomocysteinemic rats. The l-methionine-treated rats are mostly used for the assessment of HHcy and its secondary complications, including ED and VaD (Koladiya et al. 2008; Sain et al. 2011). HHcy has been reported to produce cognitive dysfunction (Streck et al. 2004; Baydas et al. 2005) and increase in the brain AChE activity (Stefanello et al. 2007). HHcy has been reported to induce endothelial dysfunction by decreasing the bioavailability of NO and by increasing vascular oxidative stress (Abahji et al. 2007). Our findings are also relevant to earlier reports as l-methionine administration for 32 days in rats raised serum Hcy and cholesterol with a significant decrease in the serum nitrite and marked attenuation of acetylcholine-induced endothelium-dependent relaxation, therefore, reflecting ED. In l-methionine-treated animals there is a significant impairment of acquisition and retrieval of memory which is shown by decreased MWM and y-maze performance. Moreover, AChE, MDA, levels were enhanced and a decrease in GSH, SOD and CAT levels were observed. HHcy is reported to produce a change in anatomical and physiological characteristics of cerebral blood vessels along with oxidative stress, which further leads to cerebral vascular dysfunction (Dayal et al. 2005). Several reports have strongly suggested a direct relationship between vascular endothelial dysfunction and dementia better known as VaD (Atkinson 2001; Zhu et al. 2007).
The altered behaviour of rodents in Y-maze indicates operation of spatial working or short-term memory (Hughes 2004). Significant increase in the percentage of alteration in HSP and donepezil-treated animals was also observed during Y-maze test indicating HSP’s role on the improvement of spatial working memory. The MWM test (Morris 1984) used in the current study is one of the widely accepted models for evaluation of spatial learning and memory. In the present investigation, HSP and donepezil showed significant improvement in spatial learning and memory, which is evident as an increase in the time spent in the target quadrant and decrease in ELT on MWM when compared to l-methionine-treated animals. These observations are in concurrence with reports from the present study and another laboratory (Sharma & Singh 2011) and the dose-dependent effect is seen with HSP treatment. The improvement in spatial memory as measured by the two different maze tasks is dependent on hippocampus-mediated learning and memory, which is related to the NMDA receptor/Ca2 + influx signalling pathway (Obeid & Herrmann 2006).Recent reports have revealed that flavonoids and other small molecules or drugs affect long-term potentiation and consequently, memory and cognitive performance through their interactions with these signalling pathways (Maher 2008; Mans et al. 2010).
The degree of ED was assessed by estimating endothelial-dependent and independent relaxation in aortic strips. HHcy has been reported to induce endothelial dysfunction by decreasing the bioavailability of NO and by increasing vascular oxidative stress (Abahji et al. 2007). In the present study, serum Hcy, nitrite and cholesterol levels were estimated as an index of ED. Earlier studies have reported that HHcy suppressed endogenous bioavailable NO, secondary to enhanced formation of superoxide, and attenuated vascular relaxation through impaired endothelial nitric oxide synthase (eNOS) activity in endothelial cells (Jiang et al. 2005). NO bioavailability could be diminished by the reaction of NO with superoxide anion (O2−) or by the reaction of thiol groups in the Hcy molecule with NO. Exposure of endothelial cells to Hcy led to the formation of S-nitroso-homocysteine, decreasing the bioactivity of NO (Stamler et al. 1993). In the present study, HSP treatment for 2 weeks has shown significant endothelial protection by the relaxation of aortic ring preparation and we also further determined the aortic contraction in the presence of L-NAME. NO, as the most important endogenous vasodilator agent is generated by the catalysis of l-arginine by eNOS, which is a critical regulator of vascular tone (Landmesser et al. 2003). Employment of l-NAME indicated the participation of eNOS in vasorelaxation in HSP, and donepezil-treated groups. This is further confirmed by a significant dose-dependent decrease in serum Hcy and cholesterol along with significant increase in nitrite levels on treatment with HSP and donepezil in l-methionine treated rats.
Moreover, significant attenuation of elevated brain AChE after HSP treatment may be due to its acetylcholinesterase inhibition. HSP showed potent antioxidant property by causing a significant increase in the levels of GSH, SOD, CAT and amelioration of oxidative stress by decreasing the MDA levels. Our findings revealed that HSP had shown significant multiple dose-dependent protective effects in all parameters against HHcy and associated complications when administered at 50 and 100 mg/kg but not at 25 mg/kg after l-methionine administration in rats. Treatment (100 mg/kg) of HSP alone did not show any significant per se effect on all our study parameters when compared with DMSO-treated rats.
Donepezil is a well-known acetylcholinesterase inhibitor, which is commonly prescribed for the management of cognitive disorders. Earlier literature on donepezil suggests that in addition to its usefulness in dementia of Alzheimer’s disease, it also exerts a beneficial effect in different animal models of VaD (Koladiya et al. 2009; Sharma & Singh 2011). Present study data with donepezil, as positive control, is in concurrence with earlier reports from another laboratory (Sharma & Singh 2011). All our study results were well documented by histopathological examination of the thoracic aorta, brain cortex and hippocampus tissues. HSP administration (50 and 100 mg/kg) showed significant endothelial protection along with a marked reduction in the neurodegeneration with observable improvement in neuronal and regeneration features when compared to hyperhomocysteinemic rats.
Conclusions
Our present findings indicate that HSP has offered significant neuroprotection in hyperhomocysteinemic rats in a dose-dependent manner. Further, we found that the reduction in Hcy, cholesterol, nitrite, MDA and AChE with increased levels of antioxidants remarkably played a central role in cognitive improvement on neurotoxicity and memory deficit induced by HHcy. This hypothesis revealed that HSP exerts specific endothelial and neuroprotective effects and could be used as a potential dietary supplement for the therapeutic benefit in HHcy-induced ED and VaD.
Acknowledgements
The authors thank Dr. P. Rajeshwar Reddy (Chairman, Anurag Group of Institutions, Hyderabad, India), Dr. Jerald Mahesh Kumar (Animal House Facility, CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India) for their constant support and encouragement. Authors also thank Hetero Labs Pvt Ltd, Hyderabad, India for providing gift sample of donepezil.
Disclosure statement
The authors report no declarations of interest.
References
- Abahji TN, Nill L, Ide N, Keller C, Hoffmann U, Weiss N.. 2007. Acute hyperhomocysteinemia induces microvascular and macrovascular endothelial dysfunction. Arch Med Res. 38:411–416. [DOI] [PubMed] [Google Scholar]
- Atkinson J. 2001. Cerebrovascular structure and dementia: new drug targets . Trends Pharmacol Sci. 22:630–635. [DOI] [PubMed] [Google Scholar]
- Balakrishnan A, Menon VP.. 2007. Protective effect of hesperidin on nicotine induced toxicity in rats. Indian J Exp Biol. 45:194–202. [PubMed] [Google Scholar]
- Baydas G, Ozer M, Yasar A, Tuzcu M, Koz ST.. 2005. Melatonin improves learning and memory performances impaired by hyperhomocysteinemia in rats. Brain Res. 1046:187–194. [DOI] [PubMed] [Google Scholar]
- Chance B, Maehly AC.. 1955. Assay of catalase and peroxidases. Methods Enzymol. 11:764–775. [Google Scholar]
- Chanet A, Milenkovic D, Manach C, Mazur A, Morand C.. 2012. Citrus flavanones: what is their role in cardiovascular protection? J Agric Food Chem. 60:8809–8822. [DOI] [PubMed] [Google Scholar]
- Cho J. 2006. Antioxidant and neuroprotective effects of hesperidin and its aglycone hesperetin. Arch Pharm Res. 29:699–706. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P.. 1993. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 262:689–695. [DOI] [PubMed] [Google Scholar]
- Dayal S, Devlin AM, McCaw RB, Liu ML, Arning E, Bottiglieri T, Shane B, Faraci FM, Lentz SR.. 2005. . Cerebral vascular dysfunction in methionine synthase-deficient mice. Circulation. 112:737–744. [DOI] [PubMed] [Google Scholar]
- Ellman GL. 1959. Tissue sulfhydryl groups. Arch Biochem Biophys. 82:70–77. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V Jr., Feather-Stone RM.. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7:88–95. [DOI] [PubMed] [Google Scholar]
- Emim JA, Oliveira AB, Lapa AJ.. 1994. Pharmacological evaluation of the anti-inflammatory activity of a citrus bioflavonoid, hesperidin, and the isoflavonoids, duartin and claussequinone, in rats and mice. J Pharm Pharmacol. 46:118–122. [DOI] [PubMed] [Google Scholar]
- Hankey GJ, Eikelboom JW.. 1999. Homocysteine and vascular disease. Lancet. 354:407–413. [DOI] [PubMed] [Google Scholar]
- Heinecke JW. 1988. Superoxide mediated oxidation of low density lipoprotein by thiols In: Cerutti PA, Fridovich I, Mc Cord JM, editors. Oxy-radicals in molecular biology and pathology. New York (NY): Alan R Liss; pp. 443–457. [Google Scholar]
- Herrmann W, Obeid R.. 2011. . Homocysteine: a biomarker in neurodegenerative diseases. Clin Chem Lab Med. 49:435–441. [DOI] [PubMed] [Google Scholar]
- Hirata A, Murakami Y, Shoji M, Kadoma Y, Fujisawa S.. 2005. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res. 25:3367–3374. [PubMed] [Google Scholar]
- Hughes RN. 2004. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 28:497–505. [DOI] [PubMed] [Google Scholar]
- Jiang X, Yang F, Tan H, Liao D, Bryan RM Jr., Randhawa JK, Rumbaut RE, Durante W, Schafer AI, Yang X, et al. . 2005. Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler Thromb Vasc Biol. 25:2515–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi K, Mizuno T, Aida K, Uchino K.. 1997. Hesperidin as an inhibitor of lipases from porcine pancreas and Pseudomonas. Biosci Biotechnol Biochem. 61:102–104. [DOI] [PubMed] [Google Scholar]
- Kemse NG, Kale AA, Joshi SR.. 2014. A combined supplementation of omega-3 fatty acids and micronutrients (folic acid, vitamin B12) reduces oxidative stress markers in a rat model of pregnancy induced hypertension. PLoS One. 9:e111902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Jung KJ, Choi JS, Chung HY.. 2004. Hesperetin: a potent antioxidant against peroxynitrite. Free Radic Res. 38:761–769. [DOI] [PubMed] [Google Scholar]
- Koladiya RU, Jaggi AS, Singh N, Sharma BK.. 2008. Ameliorative role of atorvastatin and pitavastatin in l-methionine induced vascular dementia in rats. BMC Pharmacol. 8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koladiya RU, Jaggi AS, Singh N, Sharma BK.. 2009. Beneficial effects of donepezil on vascular endothelial dysfunction-associated dementia induced by l-methionine in rats. J Health Sci. 55:215–225. [Google Scholar]
- Kumar P, Kumar A.. 2010. Protective effect of hesperidin and naringin against 3-nitropropionic acid induced Huntington's like symptoms in rats: possible role of nitric oxide. Behav Brain Res. 206:38–46. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG.. 2003. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 111:1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz SR, Erger RA, Dayal S, Maeda N, Malinow MR, Heistad DD, Faraci FM.. 2000. Folate dependence of hyperhomocysteinemia and endothelial dysfunction in cystathionine-synthase-deficient mice. Am J Physiol Heart Circ Physiol. 279:H970–H975. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ.. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–275. [PubMed] [Google Scholar]
- Maher P. 2008. The flavonoid fisetin promotes nerve cell survival from trophic factor withdrawal by enhancement of proteasome activity. Arch Biochem Biophys. 476:139–144. [DOI] [PubMed] [Google Scholar]
- Maher P, Akaishi T, Abe K.. 2006. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory . Proc Natl Acad Sci USA. 103:16568–16573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans A, Chowdhury N, Cao D, McMahon LL, Li L.. 2010. Simvastatin enhances hippocampal long-term potentiation in C57BL/6 mice. Neuroscience. 166:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S, Marklund G.. 1974. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 47:469–474. [DOI] [PubMed] [Google Scholar]
- Morris RGM. 1984. Developments of a water maze producer for studying spatial learning in the rats. J Neurosci Methods. 11:47–60. [DOI] [PubMed] [Google Scholar]
- Obeid R, Herrmann W.. 2006. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 580:2994–3005. [DOI] [PubMed] [Google Scholar]
- Olanow CW. 1993. A rationale for monoamine oxidase inhibition as neuroprotective therapy for Parkinson's disease. Mov Disord. 8:S1–S7. [DOI] [PubMed] [Google Scholar]
- Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M.. 2015. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res. 29:323–331. [DOI] [PubMed] [Google Scholar]
- Pieper GM. 1997. . Diabetic-induced endothelial dysfunction in rat aorta: role of hydroxyl radicals. Cardiovasc Res. 34:145–156. [DOI] [PubMed] [Google Scholar]
- Reddy DS. 1997. Assessment of nootropic and amnesic activity of centrally acting agents. Indian J Pharmacol. 29:208–221. [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G.. 1996. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 20:933–956. [DOI] [PubMed] [Google Scholar]
- Sain H, Sharma B, Jaggi AS, Singh N.. 2011. Pharmacological investigations on potential of peroxisome proliferator-activated receptor-gamma agonists in hyperhomocysteinemia induced vascular dementia in rats. Neuroscience. 192:322–333. [DOI] [PubMed] [Google Scholar]
- Sastry KVH, Moudgal RP, Mohan J, Tyagi JS, Rao GS.. 2002. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal Biochem. 306:79–82. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, Wilson PW, Wolf PA.. 2002. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 346:476–483. [DOI] [PubMed] [Google Scholar]
- Shah DI, Singh M.. 2006. . Inhibition of protein tyrosin phosphatase improves vascular endothelial dysfunction. Vascul Pharmacol. 44:177–182. [DOI] [PubMed] [Google Scholar]
- Shah DI, Singh M.. 2007. Possible role of Akt to improve vascular endothelial dysfunction in diabetic and hyperhomocysteinemic rats. Mol Cell Biochem. 295:65–74. [DOI] [PubMed] [Google Scholar]
- Sharma B, Singh N.. 2011. Behavioral and biochemical investigations to explore pharmacological potential of PPAR-gamma agonists in vascular dementia of diabetic rats. Pharmacol Biochem Behav. 100:320–329. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Osborne JA, Jaraki O, Rabbani LE, Mullins M, Singel D, Loscalzo J.. 1993. Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J Clin Invest. 91:308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanello FM, Monteiro SC, Matté C, Scherer EB, Netto CA, Wyse AT.. 2007. Hypermethioninemia increases cerebral acetylcholinesterase activity and impairs memory in rats. Neurochem Res. 32:1868–1874. [DOI] [PubMed] [Google Scholar]
- Streck EL, Bavaresco CS, Netto CA, Wyse AT.. 2004. Chronic hyperhomocysteinemia provokes a memory deficit in rats in the Morris water maze task. Behav Brain Res. 153:377–381. [DOI] [PubMed] [Google Scholar]
- Suarez J, Herrera MD, Marhuenda E.. 1998. . In vitro scavenger and antioxidant properties of hesperidin and neohesperidin dihydrochalcone. Phytomedicine. 5:469–473. [DOI] [PubMed] [Google Scholar]
- Unger BS, Patil BM.. 2009. Apocynin improves endothelial function and prevents the development of hypertension in fructose fed rat. Indian J Pharmacol. 41:208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT.. 2006. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 1:848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills ED. 1969. . Lipid peroxide formation in microsomes. Relationship of hydroxylation to lipid peroxide formation. Biochem. J. 113:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Smith MA, Honda K, Aliev G, Moreira PI, Nunomura A, Casadesus G, Harris PL, Siedlak SL, Perry G.. 2007. Vascular oxidative stress in Alzheimer disease. J Neurol Sci. 257:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]