Abstract
Cardiac resynchronization therapy (CRT) benefits have been firmly established in patients with heart failure and reduced left ventricular ejection fraction (HFrEF), who remain in New York Heart Association (NYHA) functional classes II and III, despite optimal medical therapy, and have a wide QRS complex. An important and consistent finding in published systematic reviews and in subgroup analyses is that the benefits of CRT are maximum for patients with a broader QRS durations, typically described as QRS duration > 150 ms, and for patients with a typical left bundle branch block (LBBB) QRS morphology. It remains uncertain whether patients with non-LBBB QRS complex morphology clearly benefit from CRT or only modestly respond.
Keywords: Non-LBBB, RBBB, Typical LBBB, HFrEF: Cardiac resynchronization therapy, QRS duration
Introduction
Cardiac resynchronization therapy (CRT) benefits have been firmly established in patients with heart failure and reduced left ventricular ejection fraction (HFrEF), who remain in New York Heart Association (NYHA) functional classes II and III despite optimal medical therapy, and have a wide QRS complex [1]. An important and consistent finding in published systematic reviews and in subgroup analyses is that the benefits of CRTs are maximum for patients with a broader QRS durations, typically described as QRS duration > 150 ms, and for patients with a typical left bundle branch block (LBBB) QRS morphology [2]. It remains uncertain whether patients with non-LBBB QRS complex morphology clearly benefit from CRT or only modestly respond [3-6].
In this article, we reviewed the major trials that enriched the most recent international guidelines for CRT implantation focusing on the available data about the outcome of using CRT in non-LBBB cohort. Furthermore, we conferred the current guidelines, including the comprehensive update of the Canadian Cardiovascular Society (CCS) guidelines for the management of heart failure (HF) 2017 [2], the European Society of Cardiology (ESC) Heart Failure Association guidelines for the diagnosis and treatment of acute and chronic HF 2016 [7], the National Institute of Health and Care Excellence (NICE) guidelines for ICD (implantable cardioverter defibrillator) and CRT for arrhythmia and heart failure 2014 [8], the American College of Cardiology Foundation/American Heart Association guideline for the management of heart failure 2013 [9], the ESC European Heart Rhythm Association guidelines on cardiac pacing and cardiac resynchronisation therapy 2013 [10], and the update to National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand guidelines for the prevention, detection and management of chronic HF in Australia 2011 [11].
The Non-LBBB Wide QRS Complex Electrocardiogram (ECG) Criteria
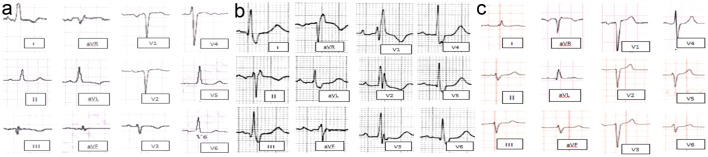
Non-LBBB wide QRS complex patterns include the following four groups are represented in Figure 1 as follow: 1) Atypical LBBB represent “QRS duration greater than or equal to 120 ms in adults, broad notched or slurred R wave in leads I, aVL, V5, and V6, and an occasional RS pattern in V5 and V6 attributed to displaced transition of QRS complex, absent q waves in leads I, V5, and V6, and R peak time greater than 60 ms in leads V5 and V6” with atypical feature such as Q wave in I and aVL, larger R wave in V1 and V2, or V6 QRS complex morphology which is different from those in I and aVL (Fig. 1a). 2) Complete (typical) right bundle branch block (RBBB) is described as QRS duration ≥ 120 ms in adults, rsr′, rsR′, or rSR′ in leads V1 or V2, R or r deflection is usually wider than the initial R wave patients, S wave of greater duration than R wave or greater than 40 ms in leads I and V6 in adults, and normal R peak time in leads V5 and V6 but > 50 ms in lead V (Fig. 1b). 3) Interventricular conduction delay (IVCD) which characterized by wide QRS morphology that does not resemble either typical LBBB or RBBB. The definition may also be applied to a pattern with RBBB criteria in the precordial leads and LBBB criteria in the limb leads, and vice versa (Fig. 1c). 4) Atypical RBBB may represent underlying delay in left ventricular (LV) activation as well. RBBB masks the underlying co-existent LBBB in broader QRS indicating advanced grade of dyssynchrony (Fig. 2) [12].
Figure 1.
Different ECG morphological pattern of non-LBBB wide QRS complex. (a) Atypical LBBB. (b) Typical RBBB. (c) Nonspecific interventricular conduction block. ECG: electrocardiogram; LBBB: left bundle branch block; RBBB: right bundle branch block.
Figure 2.
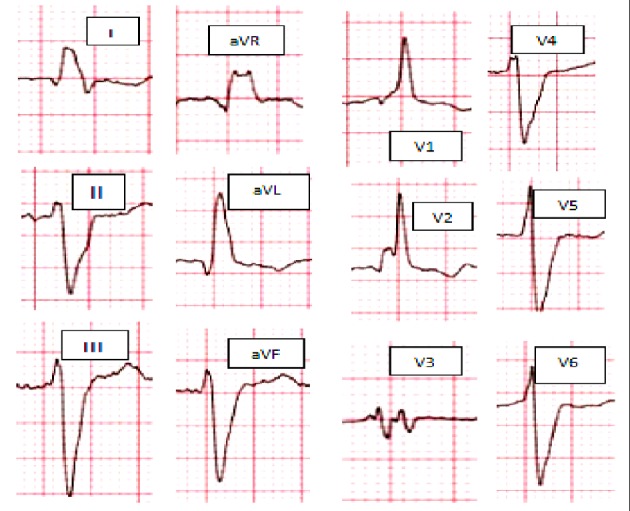
Atypical RBBB: broad, slurred, and notched R wave on leads I and aVL, together with a leftward axis deviation. RBBB: right bundle branch block.
The ECG morphological patterns of atypical LBBB, typical RBBB and IVCD ECGs are illustrated in Figure 1. The atypical RBBB ECG is illustrated in Figure 2.
Indications of CRT in Non-LBBB QRS Morphology in the Landmark Clinical Trials
Over last decade, 13 major studies, involving the outcomes of CRT use in patients with HFrEF, were conducted between 2002 and 2018 (Table 1, [13-25]). Remarkably, from 2002 to 2010, the ECG selection criteria were based solely on prolonged QRS duration without differentiation between types of bunch branch block morphology [13-23]. On the other hand, the ENHANCE CRT pilot study (2018) was conducted solely in such “non-LBBB” patients to investigate the advantage of using an electrophysiologic measure of left ventricular (LV) delay to guide lead placement when implanting the CRT’s biventricular lead system [25].
Table 1. Summary of the CRT Landmark Clinical Trials.
| Study | Aim | Patients and randomization | QRS complex pattern | Results |
|---|---|---|---|---|
| Path CHF, Auricchio et al, 2002 [13] | Compare the short- and long-term clinical effects of atrial synchronous, pre-excitation of univentricular or biventricular therapy with cardiac CRT. | N = 42; randomized to biventricular CRT (24)/univentricular CRT (17); followed for 9 months | QRS ≥ 120 ms; LBBB, 39 (93%)/ RBBB, 3 (7%) | CRT produces a long-term improvement in the clinical symptoms of patients with HF who have significant IVCD. |
| MIRACLE, Abraham et al, 2002 [14] | Evaluate the clinical benefit of CRT in symptomatic heart failure with IVCD. | N = 453; randomized to CRT group (228)/control (225); followed for 6 months | QRS ≥ 130ms | Significant clinical improvement in moderate to severe heart failure with IVCD. |
| CONTAK CD, Higgins et al, 2003 [15] | Assess the safety and effectiveness of cardiac CRT when combined with an ICD. | N = 490; randomized to CRT (245)/control (245); followed for 6 months | QRS ≥ 120 ms; CRT group: LBBB 50%/NSIVCD 32%/RBBB 18%; non-CRT group: LBBB 54%/NSIVCD 34%/RBBB 12% | CRT implant has improved the functional status in all patients that were indicated for ICD and have HFrEF and IVCD. |
| MIRACLE ICD, Young et al, 2003 [16] | Examine the efficacy and safety of combined CRT and ICD therapy in patients with NYHA class III or IV CHF despite appropriate medical management. | N = 369; randomized to CRT on (187)/ CRT off (182); followed for 6 months | QRS ≥ 130 ms; CRT group: LBBB 87%/RBBB 13%; control group: LBBB 86%/RBBB 14% | CRT improved quality of life, functional status, and exercise capacity in patients with moderate to severe HF, a wide QRS interval, and life-threatening arrhythmias. CRT effect on QOL score and NYHA functional class was not influenced by morphology of the BBB (R vs. L) |
| MIRACLE ICD II, Abraham et al, 2004 [17] | Assess the efficacy and safety of combined CRT and ICD therapy in patients with NYHA class II CHF despite appropriate medical management. | N = 186; randomized to CRT on (86)/control (101); followed for 6 months | QRS ≥ 130 ms; CRT group: LBBB 88%/RBBB 12%; non-CRT group: LBBB 79%/RBBB 21% | Significant improvement in cardiac structure and function over 6 months. CRT did not alter exercise capacity. |
| CARE HF, Cleland et al, 2005 [18] | Evaluation of CRT on morbidity and mortality in patients with NYHA class III or IV. | N = 813; randomized to CRT group (409)/control (404); followed for 18 months | QRS ≥ 120 ms | CRT improves symptoms, the QOL and reduces complications and improves mortality. The broader the QRS in general the overall better results. |
| REVERSE, Linde et al, 2008 [19] | Assess the effects of CRT use in patients with NYHA functional class I and II. | N = 610; randomized to CRT group (419)/control (191); followed for 12 months | QRS ≥ 120 ms | CRT in combination with optimal medical therapy (+/-defibrillator), reduces the risk for HF hospitalization and improves ventricular structure and function in NYHA I and II. |
| MADIT CRT, Breithardt et al, 2009 [20] | Determine whether CRT with biventricular pacing would reduce the risk of death or HF events in patients with NYHA I or II, reduced EF of ≤ 30% and QRS duration ≥ 130 ms. | N = 1,820; randomized to CRT (CRT and ICD on) group (1,089)/control (CRT off and ICD on) (731); followed for up of 2.4 years | QRS ≥ 130 ms; CRT group: LBBB (761)/RBBB (136); control: LBBB (520)/RBBB (92) | CRT combined with ICD decreased the risk of HF events in relatively asymptomatic patients with a low ejection fraction and wide QRS complex. |
| REVERSE, Daubert et al, 2009 [21] | Evaluate the long-term effects of CRT in the European cohort of patients enrolled in the REVERSE trial. | N = 262, randomized to CRT group (ICD activated, CRT on) (180)/control (ICD activated, CRT off) (82); followed for 24 months | QRS ≥ 120 ms | Clinical functional outcomes improved and LV end systolic volume decreased by a greater mean in CRT on than CRT off. First HF hospitalization or death was significantly delayed by CRT (HR: 0.38; P = 0.003). |
| COMPANION, Anand et al, 2009 [22] | Assess the use of CRT as a treatment of CHF on mortality and hospitalization. | N = 1,520; randomized in 1:2:2 ratios for optimal medical management (308)/CRT-p (617)/CRT-d (595); followed for 15 months | QRS ≥ 120 ms | CRT pacing with or without ICD capability was associated with a significant 1-year relative risk reduction of about 20% for all-cause death or hospitalization. |
| RAFT, Tang et al, 2010 [23] | Evaluate whether CRT benefits patients with LV systolic dysfunction and a wide QRS. | N = 1,798; randomized to CRT group (ICD activated, CRT on) (894)/control (ICD activated, CRT off) (904); followed up for 40 months | QRS ≥ 120 ms; CRT group: LBBB72.9%/NIVCD 11.9%/RBBB 7.6%; control group: LBBB71.1%/NIVCD11.2%/RBBB 7.4% | The combined use of CRT with ICD has reduced the mortality and hospitalization for HF patients. |
| BLOCK HF, Curtis et al, 2016 [24] | Assess biventricular pacing against primary end points of reduce mortality, morbidity, and adverse left ventricular remodeling in patients with high grade AV block; and NYHA class I, II, or III; and a LVEF of 50% or less. | N = 691; randomized to Biventricular pacing (349)/ RV pacing (342); followed for 24 months | QRS ≥ 120 ms; biventricular pacing: 1st AV block (68)/2nd AV block (119)/3rd AV block 162/LBBB (123)/RBBB (73); RV pacing: 1st AV block (66)/2nd AV block (108)/3rd AV block (167)/LBBB (102)/RBBB (74) | Biventricular pacing was superior to conventional right ventricular pacing alone in patients with AV block and left ventricular systolic dysfunction with NYHA class I, II, or III HF. |
| ENHANCE CRT, Singh et al, 2018 [25] | Evaluate the effect of a non-traditional LV lead implant strategy on the clinical composite score in a non-LBBB patient population. | N = 248; randomized to QLV implant strategy (161)/standard of care (81); followed up for 12 months | QRS ≥ 120 ms; QLV study arm: IVCD (55)/RBBB (86)/RBBB and LAFB (15)/RBBB and LPFB (2)/others (3); standard of care study arm: IVCD (33)/RBBB (36)/RBBB and LAFB (9)/RBBB and LPFB (1)/others (2) | CRT is an effective therapy in patients with non-LBBB. No apparent variation was documented in responses by subgroups analysis (i.e. RBBB vs. IVCD, QRS interval, sex, HF cause, or LVEF). |
The table summarized all landmark trials influencing CRT guidelines since 2002. Most of these trials do not have any subgroup analysis of patients with non-LBBB. The trials consist of patients of varying classes of NYHA, using different endpoints such as rehospitalization or mortality, the cohort however is primarily LBBB or non-specified QRS prolongation. CHF: congestive heart failure; CRT: cardiac resynchronization therapy; NYHA: New York Heart Association; ICD: implantable cardioverter defibrillator; IVCD: intraventricular conduction delay; NSIVCD: nonspecific interventricular conduction delay; LVEF: left ventricular ejection fraction; LBBB: left bundle branch block; RBBB: right bundle branch block; QOL: quality of life; HFrEF: heart failure with reduced ejection fraction; LBFB: left posterior fascicular block; LAFB: left anterior fascicular block; LV: left ventricular; RV: right left ventricular; AV: atrioventricular.
Until 2015, the major trials lacked the evidence that non-LBBB patients as a group would benefit from CRT implantation. The MIRACLE ICD trial stated that the benefit of CRT was positive regardless of QRS morphology although they admit they may have been underpowered in this regard [16].
Investigators of the COMPANION trial did a subgroup univariate analysis on factors associated with hospitalization risk for all patients in RBBB and/or IVCD and compared to LBBB, which produced clear evidence that the benefit of CRT was mainly observed in patients with LBBB (hazard ratio (HR) of 1.26). Similarly, IVCD was compared to RBBB or LBBB yielding a similar outcome (HR of 1.24) [22]. However, RAFT trial had comparable outcomes (HR = 1) [23].
The MADIT-CRT trial stated that the benefits from CRT among the trial’s patients without LBBB were not the same as LBBB patients, and in fact it suggested CRT might increase their mortality [20]. However, recently in 2018, the ENHANCE CRT study, the first head-to-head comparison of additional LV lead placement guided by electrical delay versus the standard of care, concluded that CRT is an effective therapy in patients with non-LBBB with no apparent distinction seen in responses by subgroups, including RBBB vs. IVCD, QRS interval, sex, HF cause, or left ventricular ejection fraction (LVEF). In addition, there were no significant differences between the two interventional arms in quality of life or LVEF [25]. The earlier trials finding of possible harm in non-LBBB are less relevant to this study as the included patients were in softer indications (i.e. NYHA class I to II in MADIT-CRT versus III to IV in ENHANCE CRT).
Guidelines and Recommendations for CRT in Non-LBBB QRS Morphology
ACC/AHA/HRS, ESC, and CCS guidelines agree that if a patient has a QRS duration > 150 ms and is in NYHA functional class III or ambulatory IV, then a CRT “better to be considered” (class IIa). When QRC duration is < 150 ms, there is considerable inconsistency in the guidelines. Both ACC/AHA/HRS and ESC guidelines favor the CRT (class IIb), however the CCS guidelines do not provide a formal recommendation for this patient group; instead, they simply state that there is no clear evidence of benefit with CRT among patients with QRS duration < 150 ms because of non-LBBB conduction.
NICE guidelines recommend CRT device insertion in patients with non-LBBB QRS morphology, who have QRS duration ≥ 150 ms and in NYHA functional classes II, III, and IV. CRT pacemaker without ICD insertion is indicated in patients with non-LBBB QRS morphology who have a QRS between 120 and 149 ms and in NYHA functional class IV. NICE guidelines also provide a clear guidance on whether to implant a cardiac resynchronization therapy with pacemaker (CRT-P) or a cardiac resynchronization therapy defibrillator (CRT-D). In addition, NICE does not provide classes of recommendation or levels of evidence.
Finally, the guidelines published by the National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand in 2011, do not distinguish between LBBB and non-LBBB in their recommendations for CRT in patients in sinus rhythm. In Table 2, we summarize the different international guidelines of indications of CRT in patients with non-LBBB wide QRS complex.
Table 2. Summary of the CRT Landmark Clinical Trials.
| Guideline | Recommendation |
|---|---|
| American College of Cardiology Foundation/American Heart Association 2013, ESC European Heart Rhythm Association 2013 | CRT can be useful for patients who have LVEF less than or equal to 35%, sinus rhythm, a non-LBBB pattern with a QRS duration greater than or equal to 150 ms, and NYHA class III/ambulatory class IV symptoms on GDM. Class IIa, level of evidence A. |
| CRT may be considered for patients who have LVEF less than or equal to 35%, sinus rhythm, a non-LBBB pattern with QRS duration 120 to 149 ms, and NYHA class III/ambulatory class IV on GDM. Class IIb, level of evidence B. | |
| National Institute of Health and Care Excellence (NICE) guidelines for ICD and CRT for arrhythmia and HF 2014 | CRT device insertion is indicated in patients with non-LBBB QRS morphology, who have QRS duration ≥ 150 ms and in NYHA functional classes II, III, and IV. |
| CRT pacemaker without ICD insertion is indicated in patients with non-LBBB QRS morphology who have a QRS between 120 and 149 ms and in NYHA functional class IV. | |
| ESC Heart Failure Association guidelines for the diagnosis and treatment of acute and chronic HF 2016 | CRT should be considered for symptomatic patients with HF in sinus rhythm with QRS duration ≥ 150 ms and non-LBBB QRS morphology and with LVEF ≤ 35% despite OMT in order to improve symptoms and reduce morbidity and mortality. Class IIa, level of evidence B. |
| CRT may be considered for symptomatic patients with HF in sinus rhythm with QRS duration of 130 to 149 ms and non-LBBB QRS morphology and with LVEF ≤ 35% despite OMT in order to improve symptoms and reduce morbidity and mortality. Class IIb, level of evidence B. | |
| Comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure 2017 | CRT may be considered for patients in sinus rhythm with NYHA class II, III, or ambulatory class IV HF despite optimal medical therapy, LVEF ≤ 35% and QRS duration ≥ 150 ms with non-LBBB (weak recommendation; low-quality evidence). |
| There is no clear evidence of benefit with CRT among patients with QRS durations < 150 ms because of non-LBBB conduction. |
The table showed the summary of different international guidelines on indications of CRT in patients with non-LBBB wide QRS complex pattern. ESC: European Society of Cardiology; GDM: guideline-directed medical therapy; OMT: optical medical therapy; HF: heart failure; CRT: cardiac resynchronization therapy; NYHA: New York Heart Association; ICD: implantable cardioverter defibrillator; LBBB: left bundle branch block; RBBB: right bundle branch block.
Evidence for CRT Efficacy in RBBB (Typical vs. Atypical RBBB Responders)
Since the introduction of CRT in the treatment of patients with HF, an increasing number of patients with RBBB QRS morphology or long-drawn-out IVCD have been treated. The reason for that is QRS duration ≥ 120 ms had been considered initially as the only ECG selection criterion for CRT [26, 27]. Angelo et al recently reviewed the past observational studies that assessed the effect of CRT on some surrogate end points of mortality/morbidity and mortality directly. The results of two large US registries including patients with LBBB, IVCD, and RBBB were also included in the review. Neither the observational studies nor the meta-analysis demonstrated any significant benefit in CRT implant in patients with non-LBBB QRS complex pattern including typical RBBB. Moreover, the evidence of excess in mortality in RBBB CRT-treated patients than in LBBB CRT-treated patients is observed in both registries. The straightforward application of CRT in patients with typical RBBB was accordingly discouraged [28].
Although RBBB typically reflect delayed right ventricular (RV) activation, some patients with HF and RBBB pattern on ECG have concomitant superimposed delay in LV activation as well. RBBB commonly masks the underlying co-existent LBBB in broader QRS, the theory that was confirmed by electroanatomic mapping data, which demonstrated that not only RV activation is abnormally delayed but also LV activation delayed [29]. Rosenbaum et al [30] described atypical RBBB pattern as broad, slurred, sometimes bifid R wave on leads I and aVL, together with a leftward axis deviation frequently noted in LBBB QRS morphology patients (Fig. 2).
A recent review of several studies, that considered CRT in the subset of atypical RBBB, stated that acute response to CRT is clinically relevant and has positive values. Additional studies should be valued also as to whether a subset of patients with RBBB may benefit from CRT [28]. Subsequently, a study evaluated 66 patients with RBBB (31 with typical RBBB and 35 with atypical RBBB) treated with CRT and followed up for almost 2 years. The target end points of reduction in LV end-systolic volume index (ESVI) ≥ 15% or reduction in the NYHA class ≥ 1 or Packer score variation (NYHA response with no HF-related hospitalization events or death) were considered. This showed 71.4% ESVI responders in atypical RBBB group in comparison with only 19.4% in typical RBBB group (P = 0.001). Furthermore, 74.3% of patients in atypical RBBB group were NYHA responders compared with 32.3% in typical RBBB group (P = 0.002). Similarly, in the atypical and typical RBBB groups, respectively 71.4% and 29.0% of patients exhibited a 2-year Packer score of 0 (P = 0.002) [31].
We have represented the comparative number of patients studied with specified non-LBBB versus LBBB and unspecified groups in a line graph as shown in Figure 3. This graph clearly demonstrates the much greater numbers of subjects in the LBBB or unspecified IVCD arms of each study. We can see that only from 2016 onwards does the discrepancy of patients’ numbers between the two begin to narrow and increase data for non-LBBB patients.
Figure 3.
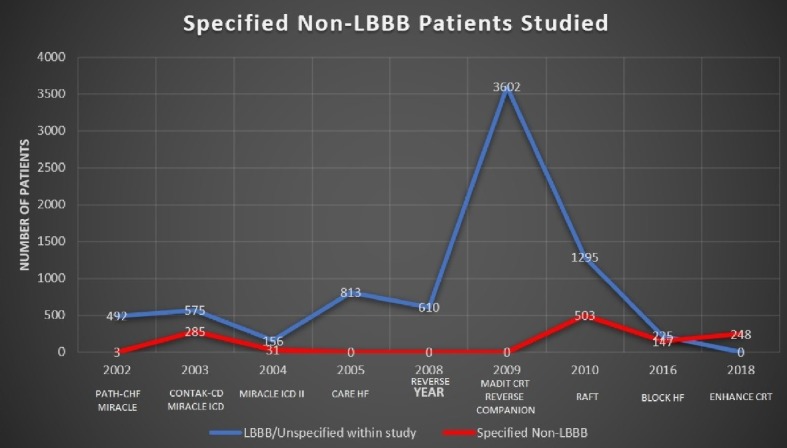
Line-graph representing the volume of patients studied over time, both LBBB/unspecified (blue) and specified non-LBBB (red). Only since 2016 can we see the gap beginning to narrow.
Conclusions and Recommendations
Non-LBBB (including atypical RBBB) in symptomatic HF patients may benefit from CRT implants. While the ESC task forces guidelines were directed towards symptomatic HF with EF < 35% patients with broad QRS > 150 ms in non-LBBB patients, yet QRS 130 - 149 may respond with modest expectations of a good response. The American guidelines have the same considerations. However, it is clear that the Canadian guidelines still weakly support non-LBBB/CRT implants if QRS > 150 ms, and in fact, it discourages CRT implants in QRS duration less than 150 ms in non-LBBB patients. Finally, NICE recommendation of non-LBBB with QRS 120 - 149 ms is only indicated in disabling HF (NYHA IV).
Non-LBBB CRT implants remain an area of debate. The previous support to CRT in those patients was on the basis of atypical features of RBBB and great IVCD. It remains a valid clinical decision to consider CRT implant in symptomatic patients (despite of optimized medical therapy) in non-LBBB with QRS duration ≥ 150 ms. Multidisciplinary approaches (e.g. cardiac electrophysiologists, HF cardiologists, physiologist and specialists liaison HF nurses) and new techniques of multipoint pacing are promising in such difficult group of patients with debated indication and expected poor responders. The data are not encouraging in regards to typical RBBB with QRS duration less than 150 ms.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
Maged Henin contributed to study design, manuscript writing and references check. Hany Ragy involved in manuscript review and re editing; James Mannion contributed to creating the tables of the major trials and edited the summary for each and language check. Santhosh David contributed to manuscript review and re-editing. Beshoy Refila contributed to study design, editing the manuscript and review. Usama Boles, the main supervisor, involved in study design, review, editing, and pre-submission check.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH. et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Ezekowitz JA, O'Meara E, McDonald MA, Abrams H, Chan M, Ducharme A, Giannetti N. et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can J Cardiol. 2017;33(11):1342–1433. doi: 10.1016/j.cjca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Al-Majed NS, McAlister FA, Bakal JA, Ezekowitz JA. Meta-analysis: cardiac resynchronization therapy for patients with less symptomatic heart failure. Ann Intern Med. 2011;154(6):401–412. doi: 10.7326/0003-4819-154-6-201103150-00313. [DOI] [PubMed] [Google Scholar]
- 4.Wells G, Parkash R, Healey JS, Talajic M, Arnold JM, Sullivan S, Peterson J. et al. Cardiac resynchronization therapy: a meta-analysis of randomized controlled trials. CMAJ. 2011;183(4):421–429. doi: 10.1503/cmaj.101685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods B, Hawkins N, Mealing S, Sutton A, Abraham WT, Beshai JF, Klein H. et al. Individual patient data network meta-analysis of mortality effects of implantable cardiac devices. Heart. 2015;101(22):1800–1806. doi: 10.1136/heartjnl-2015-307634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldenberg I, Kutyifa V, Klein HU, Cannom DS, Brown MW, Dan A, Daubert JP. et al. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med. 2014;370(18):1694–1701. doi: 10.1056/NEJMoa1401426. [DOI] [PubMed] [Google Scholar]
- 7.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 8.Colquitt JL, Mendes D, Clegg AJ, Harris P, Cooper K, Picot J, Bryant J. Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure: systematic review and economic evaluation. Health Technol Assess. 2014;18(56):1–560. doi: 10.3310/hta18560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM. et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61(3):e6–75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J. et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA) Eur Heart J. 2013;34(29):2281–2329. doi: 10.1093/eurheartj/eht150. [DOI] [PubMed] [Google Scholar]
- 11.Krum H, Jelinek MV, Stewart S, Sindone A, Atherton JJ, National Heart Foundation of A, Cardiac Society of A. et al. 2011 update to National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand Guidelines for the prevention, detection and management of chronic heart failure in Australia, 2006. Med J Aust. 2011;194(8):405–409. doi: 10.5694/j.1326-5377.2011.tb03031.x. [DOI] [PubMed] [Google Scholar]
- 12.Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, Hancock EW. et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119(10):e235–240. doi: 10.1161/CIRCULATIONAHA.108.191095. [DOI] [PubMed] [Google Scholar]
- 13.Auricchio A, Stellbrink C, Sack S, Block M, Vogt J, Bakker P, Huth C. et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol. 2002;39(12):2026–2033. doi: 10.1016/S0735-1097(02)01895-8. [DOI] [PubMed] [Google Scholar]
- 14.Abraham W, Fisher W, Smith A. Cardiac resynchronization in chronic heart failure. ACC Current Journal Review. 2002;11(6):75. doi: 10.1016/S1062-1458(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 15.Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, Boehmer JP. et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42(8):1454–1459. doi: 10.1016/S0735-1097(03)01042-8. [DOI] [PubMed] [Google Scholar]
- 16.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, Canby RC. et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289(20):2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 17.Abraham WT, Young JB, Leon AR, Adler S, Bank AJ, Hall SA, Lieberman R. et al. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation. 2004;110(18):2864–2868. doi: 10.1161/01.CIR.0000146336.92331.D1. [DOI] [PubMed] [Google Scholar]
- 18.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 19.Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C. REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction) Study Group. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52(23):1834–1843. doi: 10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 20.Breithardt G. MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy): cardiac resynchronization therapy towards early management of heart failure. Eur Heart J. 2009;30(21):2551–2553. doi: 10.1093/eurheartj/ehp383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daubert C, Gold MR, Abraham WT, Ghio S, Hassager C, Goode G, Szili-Torok T. et al. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the European cohort of the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J Am Coll Cardiol. 2009;54(20):1837–1846. doi: 10.1016/j.jacc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Anand IS, Carson P, Galle E, Song R, Boehmer J, Ghali JK, Jaski B. et al. Cardiac resynchronization therapy reduces the risk of hospitalizations in patients with advanced heart failure: results from the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) trial. Circulation. 2009;119(7):969–977. doi: 10.1161/CIRCULATIONAHA.108.793273. [DOI] [PubMed] [Google Scholar]
- 23.Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH. et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363(25):2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 24.Curtis AB, Worley SJ, Chung ES, Li P, Christman SA, St John Sutton M. Improvement in Clinical Outcomes With Biventricular Versus Right Ventricular Pacing: The BLOCK HF Study. J Am Coll Cardiol. 2016;67(18):2148–2157. doi: 10.1016/j.jacc.2016.02.051. [DOI] [PubMed] [Google Scholar]
- 25.Singh JP, Berger RD, Doshi RN, Lloyd M, Moore D, Daoud EG. for the ENHANCE CRT Study Group. Rationale and design for ENHANCE CRT: QLV implant strategy for non-left bundle branch block patients. ESC Heart Fail. 2018;5(6):1184–1190. doi: 10.1002/ehf2.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hara H, Oyenuga OA, Tanaka H, Adelstein EC, Onishi T, McNamara DM, Schwartzman D. et al. The relationship of QRS morphology and mechanical dyssynchrony to long-term outcome following cardiac resynchronization therapy. Eur Heart J. 2012;33(21):2680–2691. doi: 10.1093/eurheartj/ehs013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kandala J, Upadhyay GA, Altman RK, Parks KA, Orencole M, Mela T, Kevin Heist E. et al. QRS morphology, left ventricular lead location, and clinical outcome in patients receiving cardiac resynchronization therapy. Eur Heart J. 2013;34(29):2252–2262. doi: 10.1093/eurheartj/eht123. [DOI] [PubMed] [Google Scholar]
- 28.Auricchio A, Lumens J, Prinzen FW. Does cardiac resynchronization therapy benefit patients with right bundle branch block: cardiac resynchronization therapy has a role in patients with right bundle branch block. Circ Arrhythm Electrophysiol. 2014;7(3):532–542. doi: 10.1161/CIRCEP.113.000628. [DOI] [PubMed] [Google Scholar]
- 29.Fantoni C, Kawabata M, Massaro R, Regoli F, Raffa S, Arora V, Salerno-Uriarte JA. et al. Right and left ventricular activation sequence in patients with heart failure and right bundle branch block: a detailed analysis using three-dimensional non-fluoroscopic electroanatomic mapping system. J Cardiovasc Electrophysiol. 2005;16(2):112–119. doi: 10.1046/j.1540-8167.2005.40777.x. discussion 120-111. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum MB, Yesuron J, Lazzari JO, Elizari MV. Left anterior hemiblock obscuring the diagnosis of right bundle branch block. Circulation. 1973;48(2):298–303. doi: 10.1161/01.CIR.48.2.298. [DOI] [PubMed] [Google Scholar]
- 31.Pastore G, Morani G, Maines M, Marcantoni L, Bolzan B, Zanon F, Noventa F. et al. Patients with right bundle branch block and concomitant delayed left ventricular activation respond to cardiac resynchronization therapy. Europace. 2018;20(11):e171–e178. doi: 10.1093/europace/eux362. [DOI] [PubMed] [Google Scholar]
- 32. Relias media, K.G. Internal Medicine Alert. [Online]. 2019. Available from: https://www.reliasmedia.com.
- 33.Ferry DR. ECG in 10 days. United States of America: McGraw-Hill Companies, Inc.; 2007. [Google Scholar]
- 34.Drezner JA, Fischbach P, Froelicher V, Marek J, Pelliccia A, Prutkin JM, Schmied CM. et al. Normal electrocardiographic findings: recognising physiological adaptations in athletes. Br J Sports Med. 2013;47(3):125–136. doi: 10.1136/bjsports-2012-092068. [DOI] [PubMed] [Google Scholar]
- 35.Yanowitz FG. ECG Learning centre. [Online]. 2019. Available from: https://ecg.utah.edu/lesson/6#RBBB.