Abstract
Context: Adiantum capillus-veneris L. (Adiantaceae) hypocholesterolemic activity is therapeutically praised.
Objectives: Pharmacological modulation of pancreatic triacylglycerol lipase (PL) and α-amylase/α-glucosidase by A. capillus-veneris are evaluated.
Materials and methods: Using positive controls (acarbose, orlistat, guar gum, atorvastatin, glipizide and metformin) as appropriate, crude aqueous extracts (AEs) of A. capillus-veneris aerial parts were tested via a combination of in vitro enzymatic (0.24–100 mg/mL), acute in vivo carbohydrate tolerance tests (125, 250 or 500 mg/kg body weight [b.wt]) and chronic in vivo studies (500 mg/kg b.wt) in high cholesterol diet (HCD) fed Wistar rats.
Results: Like acarbose, A. capillus-veneris as well as chlorogenic acid, with respective IC50 values (mg/mL) of 0.8 ± 0.0 and 0.2 ± 0.0, were identified as in vitro potent dual inhibitors of α-amylase/α-glucosidase. Unlike guar gum, A. capillus-veneris had no glucose diffusion hindrance capacity. Equivalent to orlistat, A. capillus-veneris and its phytoconstituents inhibited PL in vitro with an ascending order of PL- IC50 values (μg/mL): ferulic acid; 0.48 ± 0.06 < ellagic acid; 13.53 ± 1.83 < chlorogenic acid; 38.4 ± 2.8 < A. capillus-veneris; 1600 ± 100. Incomparable to acarbose or metformin and glipizide, A. capillus-veneris (125, 250 and 500 mg/kg b.wt) lacked antihyperglycaemic efficacies in acute starch- or glucose-evoked postprandial hyperglycaemia increments in normoglycaemic overnight fasting rats. Superior to atorvastatin; A. capillus-veneris exerted significant antiobesity (p < 0.001) with marked triacylglycerol-reducing capacities (p < 0.001) in comparison to rats fed with HCD for 10 weeks.
Discussion and conclusion: A. capillus-veneris, modulating pancreatic digestive enzymes, may be advocated as a combinatorial diabesity prevention/phytotherapy agent.
Keywords: Adiantaceae, pancreatic lipase and amylase, high cholesterol diet, phenolics
Introduction
Type 2 diabetes and obesity, referred to as diabesity, comprise global health threats (Tschop & DiMarchi 2012) with rising prevalence rates of cardiovascular diseases, especially in people with metabolic syndrome and diabetes (Xanthakis et al. 2015). Recently, the high prevalence of dyslipidemia, obesity and diabetes in Jordan is being alarmingly worrisome (Al-Kloub & Froelicher 2009; Al-Nsour et al. 2012). Plants have been long used for the ethnomedical integrative/complementary treatment of obesity-diabetes in various systems of medicine (Hasani-Ranjbar et al. 2008, 2009). Rosmarinus officinalis L. (Lamiaceae) (Al-Jamal & Alqadi 2011; Al-Jamal et al. 2012; Al Sheyab et al. 2012), Apium graveolens (Mill.) Pers. (Apiaceae) (Mansi et al. 2009), a combination of prickly pear (Opuntia ficus-indica L. Mill. [Cactaceae]), artichoke (Cynara scolymus L. [Asterraceae]), turmeric (Curcuma longa L. [Zingiberaceae]) and garlic extracts (Allium sativum L. [Amaryllidaceae]) (Qinna et al. 2012), Crataegus aronia L. (DC.) (Rosaceae) (Al-Hallaq et al. 2012), and Lavandula angustifolia L. (Lamiaceae) (Issa et al. 2011) have been investigated locally for hypolipidemic/hypocholesterolemic benefits. Keeping that in mind, additional comprehensive reviews of native ethnomedicinal plants with antidiabetic therapeutic values were detailed by Afifi and Kasabri (2013). Natural inhibitors of hydrolysing enzymes in carbohydrates and lipids digestion and absorption can offer an attractive combinatorial therapeutic strategy for the management/prevention of postprandial dysglycaemia and diabesity. Hence, diverse studies were conducted to explore medicinal plants as potential therapeutic agents for dual management of diabetes and hyperlipidemia via digestive enzyme inhibition, namely pancreatic α-amylase, intestinal α-glucosidase and pancreatic lipase (PL) (Li et al. 2009a, 2011; Etoundi et al. 2010; Adisakwattana & Chanathong 2011; Rani et al. 2012; Sun et al. 2012). Given the comprehensive hypocholesterolemic propensities and HPLC-MS-based phytochemical characterization of Adiantum capillus-veneris L. (Adiantaceae) (Al-Hallaq et al. 2015), this study investigates the inhibitory effects of crude aqueous extracts (AEs) of A. capillus-veneris on these extrapancreatic digestive enzymes in vitro. Thus, more detailed investigations to elucidate the dual anti-diabetic antiobesity pharmacotherapeutic effects of A. capillus-veneris on cell-free in vitro systems of carbohydrate and lipid enzymatic digestion and absorption were undertaken. Additionally, acute as well chronic in vivo effects in high fat fed rats were investigated.
Materials and methods
Chemicals, biochemicals and instruments
Unless stated otherwise, all reference drugs (orlistat, metformin, glipizide, acarbose and atorvastatin with purities >95%) reagents, and chemicals (chlorogenic acid, ellagic acid and ferulic acid with purities >95%) were procured from Sigma (Dorset, UK). Dialysis tubing Spectra/Por® 7 Biotech Regenerated Cellulose (RC) membranes, MWCO 2000, was purchased from Spectrum Europe B.V (Breda, Netherlands). Shaking incubator was from LabTech®, Daihan LabTech Co., LTD. (Hwado-eup, Korea). The Glucose GOD-PAP kit was obtained from BIOLABO Reagents (Maizy, France). In the UV determinations, the UV–VIS spectrophotometer from SpectroScan 80D (Sedico Ltd., Nicosia, UK) was used. The sonicator (Bandelin Sonorex, BANDELIN electronic GmbH & Co., Berlin, Germany) and rotary evaporator (Laborota 4000-efficient, Heidolph Instruments GmbH & Co., Schwabach, Germany) were also used.
Plant material and preparation of the Adiantum capillus-veneris aqueous extracts (AEs)
During the spring of 2013, dried aerial parts of A. capillus-veneris from northern Jordan were provided and identified by Prof. K. Abdul-Razzak using a descriptive reference (Zohary 1966) and in comparison with the herbarium specimens of the Herbarium of the Department of Biological Sciences, Faculty of Science, The University of Jordan. Voucher specimens of the plant material were deposited at the Department of Pharmaceutical Sciences, The University of Jordan (Reference No. 127). AEs were prepared as described by Kasabri et al. (2014). In brief, AEs were prepared by gentle boiling each 10 g of the dried coarsely powdered plant material with 100 mL tap water for 15 min. The overnight kept extracts were filtered twice through filter paper and the volume of the filtered solution was increased to 100 mL with tap water to obtain 10% (equivalent to 100 mg/mL) crude aqueous solutions. Sonication of the stock crude extract or testing concentrations was performed before implementation of investigations. For PL experimentation, water was evaporated under vacuum at 40 °C using a rotary evaporator. The solid residues were collected and stored in dry conditions until analysis.
Preparation of A. capillus-veneris crude AEs and its phytoconstituents for in vitro pancreatic triacylglycerol lipase activity assay
The tested AEs were initially dissolved in Tris–HCl buffer (2.5 mM [Promega Biotechnology company, Madison, WI], pH 7.4 with 2.5 mM NaCl) to give five different stock solutions with a concentration range of 12–192 mg/mL. Subsequently, a 20 μL aliquot of each stock solution was used in the reaction mixture to give a final concentration range of 240–3840 μg/mL. Extracts were prepared according to the traditional indications of use. Thus, DMSO or any other organic solvent, even to the minimum concentration, was avoided (Gurbuz et al. 2003). Also ellagic acid, caffeic acid (in DMSO, separately) and chlorogenic acid (in absolute ethanol) isolated from A. capillus-veneris were prepared into five different stock solutions with an initial chlorogenic acid concentration range of 0.625–100 mg/mL, initial ellagic acid concentration range of 0.086–5.5 mg/mL and initial ferulic acid concentration range of 6.10–390.63 μg/mL. Thereafter, a 20-μL aliquot of each stock solution was used in the reaction mixture to give a final chlorogenic acid concentration range of 12.5–2000 μg/mL, a final ellagic acid concentration range of 1.72–110 μg/mL, and a final ferulic acid concentration range of 0.12–7.81 μg/mL. Finally, orlistat (Sigma-Aldrich, St. Louis, MO), the reference drug (in DMSO; 1 mg/mL), was prepared into six different stock solutions with a concentration range of 0.625–20 μg/mL (Habtemariam 2012). Thereafter, a 20-μL aliquot of each stock solution was used in the reaction mixture to give a final concentration range of 0.0125–0.4 μg/mL.
Spectrophotometric quantification of pancreatic lipase (PL) activity and assaying PL inhibition by crude AEs and phytochemicals
According to Bustanji et al. (2011), in vitro enzymatic PL activity was assayed. Subsequent determinations were undertaken for the tested extracts/phytoconstituents in comparison to the control evaluations to calculate the concentration value required for PL 50% inhibition (IC50).
In vitro α-amylase/α-glucosidase assay
In vitro enzymatic starch digestion was assayed with acarbose as the reference drug (Kasabri et al. 2014, 2015). The extent of polysaccharide breakdown into glucose was evaluated in a concentration range of different parts of plant AE 1, 5, 10, 12.5, 25, 50 and 100 mg/mL. The chlorogenic acid tested concentration gradient was 0.0625, 0.125, 0.25, 0.5, 1 and 2 mg/mL. The effects of acarbose at 1000 μg/mL concentration were evaluated as well. Control (tap water only) samples did not contain acarbose, plant extract or chlorogenic acid.
Glucose movement in vitro assay
In vitro glucose movement was assayed according to Kasabri et al. (2015). To imitate the viscosity-based diffusion hindrance of gel-forming dietary fibres, and hence, their postprandial glucose lowering efficacies in vitro, guar gum 50 mg/mL was used as a classical positive control, and 10, 25 and 50 mg/mL of A. capillus-veneris AEs in 0.22 M glucoses in triplicates were dialysed against 0.15 M NaCl overnight at 37 °C with gentle shaking and a parallel plant-free (negative) control was included (Gallagher et al. 2003; Butt et al. 2007).
In vivo confirmatory studies
Oral starch tolerance test (OSTT) and oral glucose tolerance test (OGTT)
With the treatment plant administered in doses 125, 250 and 500 mg/kg b.wt; OSTT and OGTT were conducted according to Kasabri et al. (2015) in the Experimental Animal Laboratory of the School of Medicine, The University of Jordan. All animals were housed, fed and treated in accordance with the University of Jordan ethical guidelines for animal protection and experimental approval (registration number 218/2007–2008) obtained from the Scientific Research Council at the Deanship of Academic Research and the School of Pharmacy.
Body weight and triglycerides determinations
Experimental animal groups
At the Experimental Animal Laboratory of the Department of Biological Science, Faculty of Science, The University of Jordan, the study was conducted as briefed in Al-Hallaq et al. (2015). All animals were housed, fed and treated in accordance with the University of Jordan ethical guidelines for animal protection and experimental approval. It also fulfils the accepted international requirements in this field. Throughout the experimental period, animals were kept in single cages. Locally inbred male Wistar rats of 212.5 ± 1.9 g average body weight (b.wt) were used in the experiments. Rats were provided with normal diet chow (called basal diet hereafter) and water ad libitum for the duration of the experiment except during the 12–13 h fasting period preceding cholesterol administration and blood collection. Rats were divided into the following groups (n = 6). Control: The control group was given only the control diet ad libitum for 10 weeks. HCD: The hypercholesterolemic control group was given the control diet ad libitum. HCD was given once daily for 10 weeks. HCD + A. capillus-veneris (500 mg/kg b.wt): The treated group was given the control diet ad libitum. HCD was administered once daily for 10 weeks, while A. capillus-veneris 500 mg/kg b.wt was fed in the last four weeks once daily (week 7–10). HCD + Atorvastatin (10 mg/kg b.wt): This group was given the control diet ad libitum. HCD was fed once daily for 10 weeks. Atorvastatin (positive control) 10 mg/kg b.wt was administered once daily for the last four weeks (week 7–10). LD50 determinations are reported in Al-Hallaq et al. (2015). Thereafter, A. capillus-veneris AEs were selected based on the obtained LD50 values and administered to animals of groups 3 and 4 by gavage at 6:30 am daily. Body weight was measured daily; doses of different treatment and plant extract were calculated and given according to b.wt.
Statistical analysis
The values are presented as mean ± S.E.M. (standard error of the mean) for the 3–6 independent experiments. Statistical differences between the control and different treatment groups and A.U.Cs (incremental area under the glucose curve) were determined using GraphPad Prism version 3.02 for Windows (GraphPad Software, San Diego, CA) one-way analysis of variance (ANOVA) followed by Dunnett’s post-test whenever appropriate. Values were considered significantly different if p < 0.05, p < 0.01 and p < 0.001.
Results, discussion and future directives
In vitro extrapancreatic inhibitory effects ofA. capillus-veneris crude AEs and its bioactive phytoconstituents on PL activity
PL inhibition is one of the most widely studied mechanisms to determine the potential efficacy of natural products and ethnomedicinal botanicals as obesity modulating agents (Mansi et al. 2009; Habtemariam 2012; Marrelli et al. 2013, 2014). In this study, the pancreatic triacylglycerol anti-lipase activity profiles of the crude AEs of A. capillus-veneris and its bioactive phytoconstituents (chlorogenic acid, ellagic acid and ferulic acid) are shown in Figure 1. Orlistat PL IC50 value of 114.0 ± 4.0 ng/mL, equivalent to 0.2 ± 0.0 μM, is comparable to reported PL IC50 values elsewhere (Habtemariam 2012) (Table 1). Comparable to orlistat performance, a marked concentration dependent PL inhibition trend was obtained per tested extracts (Figure 1) and their pure phytoconstituents PL-IC50 values obtained for a minimum of triple separate determinations are also illustrated (Table 1).
Figure 1.
In vitro inhibitory effects of Adiantum capillus-veneris AE, its bioactive phytoconstituents; chlorogenic acid, ellagic acid and ferulic acid, and orlistat concentrations on pancreatic triacylglycerol lipase activity. Results are mean ± SEM (n = 3 independent replicates).
Table 1.
In vitro pancreatic triacylglycerol lipase IC50 values for increasing concentrations of different parts of Adiantum capillus-veneris AEs, its bioactive phytoconstituent; chlorogenic acid, ellagic acid and ferulic acid, and orlistat. Results are mean ± SEM (n = 3 independent replicates).
| Tested extract/compound | IC50 (ng/mL–mg/mL) | IC50 (μM–mM) |
|---|---|---|
| Orlistat | 114.0 ± 4.0 ng/mL | 0.23 ± 0.0 μM |
| Adiantum capillus-veneris AEs | 1.6 ± 0.1 mg/mL | – |
| Chlorogenic acid | 38.4 ± 2.8 μg/mL | 0.11 ± 0.0 mM |
| Ellagic acid | 13.53 ± 1.83 μg/mL | 44.78 ± 6.07 μM |
| Ferulic acid | 0.48 ± 0.06 μg/mL | 2.49 ± 0.29 μM |
These significant anti-lipase effects may be solidly related to the effect of the major compounds identified in the crude extract (Habtemariam 2012; Al-Hallaq et al. 2013). Caffeic acid and chlorogenic acid contributed to the PL and α-amylase inhibitory potential of Clematis vitalba L. (Ranunculaceae) and Echium vulgare L. (Boraginaceae) extracts (Marrelli et al. 2013, 2014). Evidently, chlorogenic acid could modulate glucose and lipid metabolism in vivo (Li et al. 2009b; Karthikesan et al. 2010) and in vitro (Sakulnarmrat & Konczak 2012). Ellagic acid could attenuate the metabolism characteristic changes in association with high carbohydrate and high fat diet induced metabolic syndrome in rats (Panchal et al. 2013). Furthermore, ellagic acid could inhibit the fatty acid synthase and adipogenesis of 3T3L1 adipocytes (Dan et al. 2013), and it was linked to porcine lipase inhibitory potential of pomegranate peel extracts (Hadrich et al. 2014). Besides that, A. capillus-veneris content of rutin and quercetin were recognized for their in vitro antilipolytic properties (Al-Hallaq et al. 2014). In effect, the results indicate that the pancreatic triacylglycerol lipase (PL) inhibitory efficacy of A. capillus-veneris may be attributable to its multiple components acting additively or synergistically in optimal ratio (Bansal et al. 2011). Overall, pharmacological inhibition of dietary lipid digestion and absorption can induce favourable amelioration of dyslipidemia, atherosclerosis and obesity. Impressively, PL natural inhibitors offer the utility for adjuvant or alternative treatment to statins or orlistat as likely synergies can exist between new and established lipid-lowering drugs (Wierzbicki et al. 2012).
In vitro extrapancreatic inhibitory effects of A. capillus-veneris crude extracts and its bioactive phytoconstituent on extrapancreatic enzymatic starch digestion
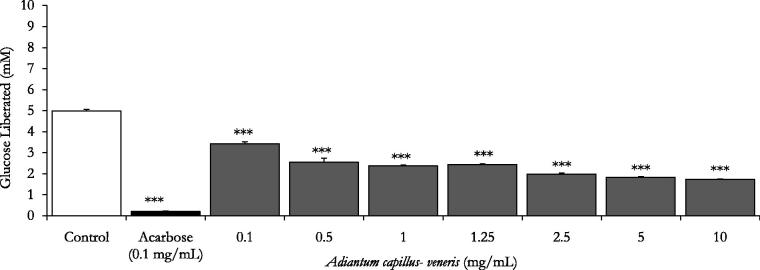
With acarbose (0.1 mg/mL) as the reference drug, glucose liberation from starch was inhibited by 97.6% (p < 0.001 vs. drug-free control incubation, n = 3, Figures 2 and 3). Furthermore, Figure 2 demonstrates that A. capillus-veneris AE concentrations 0.1–10 mg/mL had pronounced dose-related reductions in aldohexose release from culinary polymeric corn starch (p < 0.05–0.001 vs. plant-free control determinations, n = 3). With an IC50 value of 0.8 ± 0.0 mg/mL, the highly significant dose related (p < 0.05–.001) percentage decreases in enzymatic starch hydrolysis by A. capillus-veneris dosage gradient (1–10 mg/mL) are summarized in Table 2. Additionally, chlorogenic acid, A. capillus-veneris bioactive phyto-principle, exerted highly significant (p < 0.001 vs. basal control determinations, n = 3) concentration dependent inhibitions of polymeric starch enzymatic digestion, with an IC50 value of 0.2 ± 0.0 mg/mL (0.5 ± 0.1 mM) over a dose range of 6.25–200 μg/mL (Figure 3). Further details of percentage decreases in polysaccharide hydrolysis are tabulated (Table 2). Importantly, the exquisite dual anti-α-amylase/anti-α-glucosidase activity of A. capillus-veneris can be strongly attributed to its phytochemicals, mainly chlorogenic acid (Sakulnarmrat & Konczak 2012). Suggestively, co-incubations A. capillus-veneris with acarbose can reduce the therapeutic concentration required for its effective dual amylase and glucosidase inhibitions. Such synergic interactions can impact/substitute the clinical prescriptions of acarbose for type-2 diabetics’ postprandial glycaemia management (Adisakwattana & Chanathong 2011; Grussu et al. 2011). Taken together, as pancreatic enzymes elevation in type 2 diabetes has recently been marked (Maolly et al. 2012), gastrointestinal enzyme inhibition serves multiple pharmacotherapeutic targets in the treatment of diabetes, obesity and hyperlipoproteinemia (Raju et al. 2010).
Figure 2.
In vitro inhibitory effects of Adiantum capillus-veneris AE (mg/mL) on enzymatic starch digestion. Results are mean ± SEM (n = 3 independent replicates). *p < 0.05 and ***p < 0.001 compared to control (drug-free or plant-free) incubations, as determined by ANOVA followed by Dunnett’s post-test.
Figure 3.
In vitro inhibitory effects of chlorogenic acid, A. capillus-veneris phytoconstituent (μg/mL) on enzymatic starch digestion. Results are mean ± SEM (n = 3 independent replicates). ***p < 0.001 compared to control (drug-free or plant-free) incubations, as determined by ANOVA followed by Dunnett’s post-test.
Table 2.
Effects of ascending concentrations of different parts of A. capillus-veneris AE (mg/mL) and its bioactive phytoconstituent chlorogenic acid (μg/mL) on % reduction of enzymatic starch digestion in vitro. Results expressed as % decrease in control values are mean ± SEM (n = 3 independent determinations).
| Plant AE (mg/mL) | 0.1 | 0.5 | 1 | 1.25 | 2.5 | 5 | 10 |
|---|---|---|---|---|---|---|---|
| Adiantum capillus-veneris | 31.3 ± 2.0 | 49.0 ± 4.3a | 51.3 ± 1.2a | 51.0 ± 0.8a | 60.2 ± 1.3a | 63.3 ± 0.6a | 64.0 ± 0.3a |
| Chlorogenic acid (μg/mL) | 6.25 | 12.5 | 25 | 50 | 100 | 200 | |
| Chlorogenic acid | 30.6 ± 0.9a | 31.4 ± 1.9a | 32.6 ± 1.3a | 36.8 ± 1.4a | 43.7 ± 1.4a | 57.2 ± 0.9a |
p < 0.001 compared to control (drug-free or plant-free) incubations as determined by ANOVA followed by Dunnett’s post-test.
Acute in vivo studies
OSTT
At the 30-min time point, the administration of acarbose 3 mg/kg b.wt reduced highly significantly the starch induced postprandial hyperglycaemia at 45, 90 and 135 min post corn starch load at 0 min, thus evoking a highly substantial reduction (p < 0.001 vs. untreated animals, n = 6) of the overall glycaemic excursion AUC compared to controls (Figure 4). Unlike acarbose, A. capillus-veneris at concentrations 125, 250 and 500 mg/kg b.wt, AE could not diminish overall glycaemic excursions AUCs (Figure 4). Except for A. capillus-veneris 250 mg/kg b.wt eliciting a significant decrease in culinary starch-related postprandial hyperglycaemia at 45 min (p < 0.05 vs. control rats, Figure 4). A. capillus-veneris tested doses at the determination time points lacked acute anti-hyperglycaemic efficacies. These outcomes did not correspond with those obtained in vitro.
Figure 4.
In vivo modulatory postprandial antihyperglycaemic effects of A. capillus-veneris AE (mg/kg b.wt) on oral starch tolerance over 165 min and AUC in normoglycaemic overnight fasting rats. *p < 0.05 and ***p < 0.001 compared to control untreated animals, as determined by ANOVA followed by Dunnett’s post-test.
OGTT
Thirty-min pre-glucose-load treatments with metformin (300 mg/kg b.wt) or glipizide (0.6 mg/kg b.wt) significantly minimized (p < 0.001 compared to control rats, n = 6) the overall glycaemic excursions in OGTTs (Figure 5(A)). The same figure demonstrates the highly substantial anti-hyperglycaemic efficacies of both oral anti-diabetic therapeutics 45 min (p < 0.001), 90 min (p < 0.001) and 135 min (p < 0.001) following sugar load. Oral administration of A. capillus-veneris AEs did not evoke any marked improvement of glucose tolerance AUCs in comparison to control determinations respective AUCs contrary to metformin and glipizide therapeutic propensities (Figure 5(A)). In parallel terms, none of A. capillus-veneris AEs exhibited any postprandial acute antihyperglycaemic activity in glucose fed rats at any determination point (Figure 5(A)). This was further ascertained by the lack of in vitro efficacies of A. capillus-veneris AEs on the impedance of glucose movement in vitro. Using the diffusion model described in Kasabri et al. (2015), viscous water-soluble gel-forming guar gum (50 mg/mL) significantly decreased the mean AUC (area under 24 h glucose curve) by 30.8 ± 2.5% (p < 0.001) (n = 3, vs. overnight negative control, Figure 5(B)). A. capillus-veneris extracts (10, 25 and 50 mg/mL), nevertheless, lacked any marked glucose diffusion hindrances in external solution across dialysis membrane (with respective 1.4 ± 0.8, 1.0 ± 0.0 and 1.0 ± 0.1% AUC reductions, p > 0.05 vs. plant free basal control, Figure 5(B)).
Figure 5.
(A). Lack of in vivo modulatory postprandial antihyperglycaemic effects of A. capillus-veneris AE (mg/kg b.wt) on oral glucose tolerance over 165 min and AUC in normoglycaemic overnight fasting rats. **p < 0.01 and ***p < 0.001 compared to control untreated animals, as determined by ANOVA followed by Dunnett’s post-test. (B). Lack of modulatory in vitro effects of A. capillus-veneris AE (mg/mL) on the incremental AUC of 24-h glucose movement. ***p < 0.001 compared to control (drug-free or plant-free) incubations, as determined by ANOVA followed by Dunnett’s post-test.
Chronic effects of A. capillus-veneris on body weight in HCD rats
Weights were standardized by considering the starting weight for each animal as 100%. Body weights of all groups increased with time. Figure 6 illustrates the effect of A. capillus-veneris on b.wt over 7–10 weeks in 10-week-HCD fed rats. The b.wt. AUC10 weeks in the HCD group and in atorvastatin of the HCD group were significantly increased compared to the normal diet control animals (with respective 1200.3 ± 77.3 and 1122.5 ± 51 vs. 941.9 ± 28.6, n = 6 rats/group, p < 0.05–0.01, Figure 6). Most interestingly, the b.wt AUC10 weeks in A. capillus-veneris (500 mg/k b.wt) of the HCD rats was highly substantially reduced in comparison to the HCD fatty rats (870.7 ± 38.8 vs. 1200.3 ± 77.3, n = 6 rats/group, p < 0.001, Figure 6). Impressively, the b.wt AUC10 in the HCD group A. capillus-veneris 500 mg/kg b.wt was normalized to negative controls (870.7 ± 38.8 vs. 941.9 ± 28.6, n = 6 rats/group, p > 0.05, Figure 6). Strikingly, over a four-week intervention period, daily plant treatment proved markedly more effective in weight reduction (p < 0.01, n = 6) than atorvastatin, the reference hypocholesterolemic drug (Figure 6).
Figure 6.
In vivo chronic effects of A. capillus-veneris AE (500 mg/kg b.wt) on the incremental AUC of 10-week treatment on the body weight. Results are expressed as mean ± SEM of area under curve (AUC) of body weight for 10-week intervention using ANOVA followed by Dunnett’s post-test, where *p < 0.05 and **p < 0.01 compared to control group, ΔΔΔp < 0.001 compared to HCD group, and ∇∇p < 0.01 compared to atorvastatin group.
Chronic effects of A. capillus-veneris on plasma triglycerides (TAG) in HCD rats
Figure 7 demonstrates the effect of A. capillus-veneris on plasma triacylglycerols (TAG) over 7–10 weeks of treatment in the 10-week-HCD fed rats. TAG AUC10 weeks in HCD fed-animals is significantly greater than the control rats’ (648.5 ± 33.3 vs. 552.3 ± 22, n = 6 rats/group, p < 0.05, Figure 7). TAG AUC10 weeks in atorvastatin-HCD fed rats is substantially less than the HCD-fed rats (548.6 ± 17.8 vs. 648.5 ± 33.3, n = 6 rats/group, 0.05). Also, it has impressively been normalized as in control’s (548.6 ± 17.8 vs. 552.3.5 ± 22, n = 6 rats/group, p > 0.05, Figure 7). In A. capillus-veneris 500 mg/kg b.wt HCD fed rats, TAG AUC10 weeks is significantly less than HCD-fed animals’ (443.1 ± 18 vs. 648.5 ± 33.3, n = 6 rats/group, p < 0.001, Figure 7) and is proven substantially less than controls’ (443.1 ± 18.0 vs. 552.3 ± 22, n = 6 rats/group, p < 0.01); thus, it is brought to levels pronouncedly less than the reference hypocholesterolemic drug atorvastatin (443.1 ± 18.0 vs. 548.6 ± 17.8, p < 0.01, n = 6 rats/group, Figure 7). These outcomes are perfectly aligned with the in vitro PL inhibitory effects of A. capillus-veneris. In a study performed by Mnafgui et al. (2012), it was found that the inhibitory action of PL leads to a decrease in lipid profiles which agrees with our in vitro and in vivo findings combined. As the occurrence of obesity is on the rise, various studies were accomplished on obesity treatment through the suppression of triglycerides accumulation by inhibiting the digestion of dietary lipids and minimizing intestinal fat absorption (Bustanji et al. 2011; Jeong et al. 2012).
Figure 7.
In vivo chronic effects of A. capillus-veneris AE (500 mg/kg b.wt) on the incremental AUC of 10 weeks treatment on triglycerides (TAG) concentrations. Results are expressed as mean ± SEM of AUC (area under curve) of TAG for 10-week intervention using ANOVA followed by Dunnett’s post-test where *p < 0.05 and **p < 0.01 compared to control group, Δp < 0.05 and ΔΔΔp < 0.001 compared to HCD group and ∇∇p < 0.01 compared to atorvastatin group.
Conclusions
Succinctly, A. capillus-veneris phytochemicals in an optimal ratio can inhibit crucial gastrointestinal enzymes involved in carbohydrate and lipid digestion and absorption, thus advocating a dual-target phytotherapeutic/preventive strategy in glycaemia control of obesity-diabetes (diabesity).
Acknowledgements
Professor Yusuf Al-Hiari, The University of Jordan is gratefully thanked for the kind gift of ferulic acid. The technical assistance of Dana AlQudah, Esra Fodah, Haneen Ramadan and Hazar Shawash is acknowledged.
Disclosure statement
The authors declare that they have no conflict of interest concerning this article.
Funding
We are grateful for the Scientific Research Fund, Ministry of Higher Education [grant No. MPH/1/05/2014] and the Deanship of Academic Research, The University of Jordan [grant No. 1347, 1348] for funding this work.
References
- Adisakwattana S, Chanathong B.. 2011. alpha-Glucosidase inhibitory activity and lipid-lowering mechanisms of Moringa oleifera leaf extract. Eur Rev Med Pharmacol Sci. 15:803–808. [PubMed] [Google Scholar]
- Afifi FU, Kasabri V.. 2013. . Pharmacological and phytochemical appraisal of selected medicinal plants from Jordan with claimed antidiabetic activities. Sci Pharma. 81:889–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hallaq EK, Afifi FU, Abdalla SS.. 2012. Evaluation of the hypocholesterolemic effect and phytochemical screening of the hydroethanolic extract of Crataegus aronia from Jordan. Nat Prod Commun. 7:35–38. [PubMed] [Google Scholar]
- Al-Hallaq EK, Kasabri V, Abdalla SS, Bustanji YK, Afifi FU.. 2013. Anti-obesity and antihyperglycemic effects of Crataegus aronia extracts: In vitro and in vivo evaluations. Food Nutr Sci. 4:972–983. [Google Scholar]
- Al-Hallaq EK, Litescu SC, Kasabri V, Abdul-Razzak KK, Afifi F.. 2015. Hypocholesterolemic effects of Adiantum capillus veneris L. aqueous extract in high cholesterol diet-fed rats and HPLC-MS determination of its polyphenolics. Rev Roum Chim. 60:357–365. [Google Scholar]
- Al-Jamal AR, Ibrahim A, Al-Fararjeh MA, Alqadi T.. 2012. Effects of rosemary on lipid profile in diabetic rats. Afr J Plant Sci. 6:222–225. [Google Scholar]
- Al-Jamal AR, Alqadi T.. 2011. Effects of rosemary (Rosmarinus officinalis) on lipid profile of diabetic rats. Jordan J Biol Sci. 4:199–204. [Google Scholar]
- Al-Kloub M, Froelicher ES.. 2009. Factors contributing to adolescent obesity. Saudi Med J. 30:737–749. [PubMed] [Google Scholar]
- Al-Nsour M, Zindah M, Belbeisi A, Hadaddin R, Brown DW, Walke H.. 2012. Prevalence of selected chronic, noncommunicable disease risk factors in Jordan: results of the 2007 Jordan behavioral risk factor surveillance survey. Prev Chronic Dis. 9:E25. [PMC free article] [PubMed] [Google Scholar]
- Al Sheyab FM, Abuharfeil N, Salloum L, Bani Hani R, Awad DS.. 2012. The effect of rosemary (Rosmarinus officinalis L) plant extracts on the immune response and lipid profile in mice. J Biol Life Sci. 3:37–58. [Google Scholar]
- Bansal P, Paul P, Shankar G, Munjal D, Nayek PG, Priyadarsini KI, Unnikrishnan MK.. 2011. Flavonoid rich fraction of Pilea microphylla (L.) attenuates metabolic abnormalities and improves pancreatic function in C57BL/KsJ-db-db mice. Biomed Prev Nutr. 1:268–272. [Google Scholar]
- Bustanji Y, AlMasri I, Mohammad M, Hudaib M, Tawaha K, Tarazi H, Alkhatib HS.. 2011. . Pancreatic lipase inhibition activity of trilactone terpenes of Ginkgo biloba. J Enzyme Inhib Med Chem. 26:453–459. [DOI] [PubMed] [Google Scholar]
- Butt MS, Ahmad A, Sharif MK.. 2007. Influence of pectin and guar gum composite flour on plasma biochemical profile of streptozocin-induced diabetic male albino rats. Int J Food Propert. 10:345–361. [Google Scholar]
- Dan W, Xiaofeng M, Weixi T.. 2013. Pomegranate husk extract, punicalagin and ellagic acid inhibit fatty acid synthase and adipogenesis of 3T3L1 adipocytes. J Funct Food. 5:633–641. [Google Scholar]
- Etoundi CB, Kuate D, Ngondi JL, Oben J.. 2010. Anti-amylase, anti-lipase and anti-oxidant effects of aqueous extracts of some Cameroonian spices. J Nat Prod. 3:165–171. [Google Scholar]
- Gallagher AM, Flatt PR, Duffy G, Abdel-Wahab YHA.. 2003. The effects of traditional antidiabetic plants on in vitro glucose diffusion. Nutr Res. 23:413–424. [Google Scholar]
- Grussu D, Stewart D, McDoagall GJ.. 2011. Berry polyphenols inhibit α-amylase in vitro: identifying active components in rowanberry and raspberry. J Agric Food Chem. 59:2324–2331. [DOI] [PubMed] [Google Scholar]
- Gurbuz I, Ustun O, Yesilada E, Sezik E, Kutsal O.. 2003. Anti-ulcerogenic activity of some plants used as folk remedy in Turkey. J Ethnopharmacol. 88:93–97. [DOI] [PubMed] [Google Scholar]
- Habtemariam S. 2012. . The anti-obesity potential of sigmoidin A. Pharm Biol. 50:1519–1522. [DOI] [PubMed] [Google Scholar]
- Hadrich F, Cherif S, Gargouri YT, Sayari A.. 2014. Antioxidant and lipase inhibitory activities and essential oil composition of pomegranate peel extracts. J Oleo Sci. 63:515–525. [DOI] [PubMed] [Google Scholar]
- Hasani-Ranjbar S, Larijani B, Abdollahi M.. 2008. A systematic review of Iranian plants useful in diabetes mellitus. Arch Med Sci. 4:285–292. [Google Scholar]
- Hasani-Ranjbar S, Nayebi N, Larijani B, Abdollahi M.. 2009. A systematic review of the efficacy and safety of herbal medicines used in the treatment of obesity. World J Gastroenterol. 15:3073–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa A, Mohammad M, Hudaib M, Tawah K, Aburjai T, Oran S, Bustanji Y.. 2011. A potential role of Lavendula angustifolia in the management of diabetic dyslipidemia. J Med Plant Res. 5:3876–3882. [Google Scholar]
- Jeong SM, Kang MJ, Choi HN, kim JH, Kim JI.. 2012. . Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr Res Pract. 6:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikesan K, Pari L, Menon VP.. 2010. Antihyperlipidemic effect of chlorogenic acid and tetrahydrocurcumin in rats subjected to diabetogenic agents. Chem Biol Interact. 188:643–650. [DOI] [PubMed] [Google Scholar]
- Kasabri V, Afifi FU, Abu Dahab F, Mhaidat N, Bustanji YK, Abaza IF, Mashallah S.. 2014. In vitro modulation of metabolic syndrome enzymes and proliferation of obesity related-colorectal cancer cell line panel by Salvia species from Jordan. Rev Roum Chim. 59:693–705. [Google Scholar]
- Kasabri V, Afifi FU, Bustanji Y, Mashallah S, Naffa R, Mehdi HS.. 2015. In vitro enhancement of pancreatic β cells MIN6 proliferation by insulinotropic Gymnema sylvestre aqueous extracts: evidence-based regenerative therapeutic capacity of a medicinal herb. Brit J Med Medic Res. 7:180–194. [Google Scholar]
- Li Y, Gao F, Shan F, Bian J, Zhao C.. 2009a. Study on the interaction between 3 flavonoid compounds and alpha-amylase by fluorescence spectroscopy and enzymatic kinetics. J Food Sci. 74:C199–C203. [DOI] [PubMed] [Google Scholar]
- Li SY, Chang CQ, Ma FY, Yu CL.. 2009b. Modulating effects of chlorogenic acid on lipids and glucose metabolism and expression of hepatic peroxisome proliferator-activated receptor-alpha in golden hamsters fed on high fat diet. Biomed Environ Sci. 22:122–129. [DOI] [PubMed] [Google Scholar]
- Li YQ, Yang P, Gao F, Zhang ZW, Wu B.. 2011. Probing the interaction between 3 flavonoids and pancreatic lipase by methods of amylase by fluorescence spectroscopy and enzymatic kinetics. Eur Food Res Technol. 233:63–69. [Google Scholar]
- Mansi K, Abushoffa AM, Disi A, Aburjai T.. 2009. Hypolipidemic effects of seed extract of celery (Apium graveolens) in rats. Pharmcog Mag. 5:301–305. [Google Scholar]
- Maolly J, Gurney K, Shan K, Yan P, Chen S.. 2012. Increased variability and abnormalities in pancreatic enzyme concentrations in otherwise asymptomatic subjects with type 2 diabetes. Diabetes Metab Syndr Obes. 5:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelli M, Loizzo MR, Nicoletti M, Menichini F, Conforti F.. 2013. Inhibition of key enzymes linked to obesity by preparations from Mediterranean dietary plants: effects on α-amylase and pancreatic lipase activities. Plant Foods Hum Nutr. 68:340–346. [DOI] [PubMed] [Google Scholar]
- Marrelli M, Loizzo MR, Nicoletti M, Menichini F, Conforti F.. 2014. In vitro investigation of the potential health benefits of wild Mediterranean dietary plants as anti-obesity agents with α-amylase and pancreatic lipase inhibitory activities. J Sci Food Agric. 94:2217–2224. [DOI] [PubMed] [Google Scholar]
- Mnafgui K, Hamden K, Ben Salah H, Kchaou M, Nasri M, Slama S, Derbali F, Allouche N, Elfeki A.. 2012. Inhibitory activities of Zygophyllum album: a natural weight-lowering plant on key enzymes in high-fat diet fed rats. Evid Based Complement Altern Med. 8:620384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal SK, Ward L, Brown L.. 2013. . Ellagic acid attenuates high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur J Nutr. 52:559–568. [DOI] [PubMed] [Google Scholar]
- Qinna NA, Kamona BS, Alhussainy TM, Taha H, Badwan AA, Matalka KZ.. 2012. Effects of prickly pear dried leaves, artichoke leaves, turmeric and garlic extracts, and their combinations on preventing dyslipidemia in rats. ISRN Pharmacol. 2012:167979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju SN, Kumar DS, Banji D, Harani A, Shankar P, Kumar PA, Reddy VR.. 2010. Inhibitory effects of ethanolic extract of Piper trioicum on amylase, lipase and α-glucosidase. Der Pharmacia Lettre J. 2:237–244. [Google Scholar]
- Rani N, Sharma SK, Vasudeva N.. 2012. Assessment of antiobesity potential of Achyranthes aspera Linn. seed. Evid Based Complement Alternat Med. 2012:715912. ID 715912, pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakulnarmrat K, Konczak I.. 2012. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chem. 134:1011–1019. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhang K, Ji X, Wang Y, Jeffrey Z, Tong Y, Gao H, Zhang J, Wang Z.. 2012. Screening of pancreatic lipase and alpha-glucosidase inhibitors from Chinese dietary herbs. Zhongguo Zhong Yao Za Zhi. 37:1319–1323. [PubMed] [Google Scholar]
- Tschop MH, DiMarchi RD.. 2012. Outstanding scientific achievement award lecture 2011: defeating diabesity: the case for personalized combinatorial therapies. Diabetes. 61:1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicki AS, Hardman TC, Viljoen A.. 2012. New lipid-lowering drugs: an update. Int J Clin Pract. 66:270–280. [DOI] [PubMed] [Google Scholar]
- Xanthakis V, Sung JH, Samdarshi TE, Hill AN, Musani SK, Sims M, Ghraibeh KA, Liebson PR, Taylor HA, Vasan RS, Fox ER.. 2015. Relations between subclinical disease markers and type 2 diabetes, metabolic syndrome, and incident cardiovascular disease: the Jackson heart study. Diabetes Care. 38:1082–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary M. 1966. Flora Palaestina. 1st ed Jerusalem: The Israel Academy of Sciences and Humanities; p. 7. [Google Scholar]