Abstract
Context: Increasing incidence and impact of inflammatory diseases have encouraged the search of new pharmacological strategies to face them. Licorice has been used to treat inflammatory diseases since ancient times in China.
Objective: To summarize the current knowledge on anti-inflammatory properties and mechanisms of compounds isolated from licorice, to introduce the traditional use, modern clinical trials and officially approved drugs, to evaluate the safety and to obtain new insights for further research of licorice.
Methods: PubMed, Web of Science, Science Direct and ResearchGate were information sources for the search terms ‘licorice’, ‘licorice metabolites’, ‘anti-inflammatory’, ‘triterpenoids’, ‘flavonoids’ and their combinations, mainly from year 2010 to 2016 without language restriction. Studies were selected from Science Citation Index journals, in vitro studies with Jadad score less than 2 points and in vivo and clinical studies with experimental flaws were excluded.
Results: Two hundred and ninety-five papers were searched and 93 papers were reviewed. Licorice extract, 3 triterpenes and 13 flavonoids exhibit evident anti-inflammatory properties mainly by decreasing TNF, MMPs, PGE2 and free radicals, which also explained its traditional applications in stimulating digestive system functions, eliminating phlegm, relieving coughing, nourishing qi and alleviating pain in TCM. Five hundred and fifty-four drugs containing licorice have been approved by CFDA. The side effect may due to the cortical hormone like action.
Conclusion: Licorice and its natural compounds have demonstrated anti-inflammatory activities. More pharmacokinetic studies using different models with different dosages should be carried out, and the maximum tolerated dose is also critical for clinical use of licorice extract and purified compounds.
Keywords: Glycyrrhiza uralensis Fisch., Glycyrrhiza inflata Bat., Glycyrrhiza glabra L., glycyrrhizin, glycyrrhetinic acid, flavonoid
Introduction
The applications of natural compounds and medicinal plants to diseases are novel trends in clinical medicine research. Licorice is a very famous ancient herb, which is most frequently used in traditional Chinese medicine (TCM). In Chinese Pharmacopoeia, three original plants from the family Leguminosae, Glycyrrhiza uralensis Fisch., G. inflata Bat. and G. glabra L. are prescribed as licorice. The licorice cuts from the dry roots and rhizomes of licorice are widely used in clinical prescriptions (Figure 1). The pharmaceutical importance of licorice lies in their capacity to produce a great variety of secondary metabolites. Depending on the modern studies, the most important bioactive compounds in licorice are triterpenes, flavonoids and polysaccharides (Seki et al. 2011; Zhu et al. 2016). They have been reported with antitumor (Wang KL et al. 2013; Li et al. 2014), antimicrobial (Ahn et al. 2012; Long et al. 2013), antiviral (Kwon et al. 2010; Feng et al. 2013), anti-inflammatory (Chandrasekaran et al. 2011; Wu et al. 2011), antidiabetic (Mae et al. 2003; Li et al. 2010), immunoregulatory (Hong et al. 2009; Li et al. 2012), hepatoprotective (Abe et al. 2008; Sharifzadeh et al. 2008), neuro-protective activities (Zhao et al. 2008; Michel et al. 2013) and adrenal cortical hormone kind functions (Kageyama et al. 2004; Raikkonen et al. 2010).
Figure 1.
The licorice cuts.
In recent years, inflammation responses with Celsus’ four cardinal signs, namely calor (heat), dolor (pain), rubor (redness) and tumour (swelling) have attracted increasing attention (Fullerton & Gilroy 2016). Inflammation responses play an important role in multiple diseases with a high prevalence among population, such as hepatitis (Matsuzaki et al. 2007), lung disease (Yang H et al. 2013) and Alzheimer’s disease (Jayaraman et al. 2014). And, they are also centrally related to the pathogenesis of a large number of acute and chronic diseases, such as rheumatoid arthritis (Yang CLH et al. 2013), colonic inflammatory response (Takhshid et al. 2012) and periodontitis (Farhad et al. 2013). However, the conventional therapies for inflammation, including steroids and nonsteroid anti-inflammatory drugs (NSAID) (Sostres et al. 2010; Parikh & Scadding 2014; Carrasco-Pozo et al. 2016), have shown many side effects and deficiencies. Considering this, licorice is an excellent alternative choice, due to the fact that it causes minimal disorders in the physiological functions of organism, has a nonspecific action and exerts a therapeutic action regardless of the direction of the pathological state. Furthermore, it is especially suitable for children, since glycyrrhizin (GC), a compound isolated from licorice, is 50 times sweeter than sugar that makes it much easier for children to accept (Liu et al. 2011).
The present review aims to summarize the anti-inflammatory properties and mechanisms of licorice and its natural compounds, introduce the related clinical drugs, evaluate the safety and obtain new insights for further research of licorice.
Literature search
The present review is intended to discuss past and current research on the anti-inflammatory activities of licorice and its natural products. With this objective, an extensive collection of scientific literature was examined by considering all highlighted research articles and reviews on the issue. Four main databases, PubMed, Web of Science, Science Direct and ResearchGate were used as information sources by the inclusion of the search terms ‘licorice’, ‘licorice metabolites’, ‘anti-inflammatory’, ‘triterpenoids’, ‘flavonoids’ and their combinations, mainly from the years 2010 to 2016 without language restriction. All the references were selected from Science Citation Index journals, in vitro studies with the Jadad score less than 2 points and in vivo and clinical studies with experimental flaws were excluded. As a result, we searched 295 papers and a total of 93 references were included in the present work.
Licorice applications in TCM therapeutics to treat inflammation
In TCM therapeutics, licorice has been used to strengthen the function of digestive system, eliminate phlegm, relieve coughing and alleviate pain since ancient times (Guo et al. 2014). Licorice is honoured as the ‘excellent coordinator’ for harmonizing different ingredients, and regarded as ‘guide drug’ for helping the rapid absorption into bloodstream, organs and target cells (Wang X et al. 2013). In authoritative medical formulary in ancient China, it has been applied to treat respiratory, gastric and liver diseases, and also used to alleviate the toxicity of other drugs.
Sanao decoction, which consists of licorice, ephedra (the stem of Ephedra sinica Stapf, Mahuang in Chinese) and apricot seeds (the seeds of Prunusarmeniaca L. var. ansu Maxim, Xingren in Chinese), and Jiegeng decoction, which consists of licorice and Platycodon grandiflorum (the roots of Platycodon grandiflorum (Jacq.) A. DC., Jiegeng in Chinese), are used to treat the phlegm retention, cough and asthma. When acting as an agent for relaxing spasm, relieving pain and recovering the gastric ulcer, licorice is always combined with peony (the roots of Paeonia lactiflora Pall., Shaoyao in Chinese) in Shaoyaogancao decoction, with ginseng (the roots of Panax ginseng C. A. Mey., Renshen in Chinese), white atractylodes (the rhizome of Atractylodes macrocephala Koidz., Baizhu in Chinese) and poria (the sclerotium of Poriacocos (Schw.) Wolf, Fuling in Chinese) in Sijunzi decoction, and with scutellaria baicalensis (the roots of Scutellaria baicalensis Georgi, Huangqin in Chinese), peony and fructus ziziphi jujubae (the fructus of Ziziphus jujuba Mill., Dazao in Chinese) in Huangqin decoction. Above all, licorice has functions of eliminating phlegm, relieving cough, preventing asthma and recovering the gastric ulcer due to TCM theories for thousands of years.
With the development of Chinese traditional medicine modernization, the pharmacological mechanisms of Chinese medicine formula containing licorice were also investigated. The Chinese herbal formula Sini Tang decreased the expression of atrial natriuretic peptide levels in plasma and increased the vascular active marker nitric oxide (NO), which limited vascular inflammation (Liu et al. 2014). Shaoyaogancao decoction suppressed clozapine metabolism in human liver microsomal system principally associated with the inhibition of related CYP activity (Wang et al. 2015). When applied to HaCaT human keratinocyte cell line, it attenuated tumour necrosis factor-α (TNF-α) and interferon-γ-mediated chemokine production by targeting the STAT1 and nuclear factor-kappa B (NF-κB) signalling in keratinocytes (Jeong et al. 2015). In the 2,4,6-trinitrobenzenesulfonic acid-induced colitis mice models, the level of colonic myeloperoxidase (MPO) activity and the tissue levels of TNF-α, interleukin-1β (IL-1β) and IL-6 were markedly decreased after the gavage of Huangqin decoction (Bi et al. 2014). All the above suggest that many Chinese formulas containing licorice could serve as a therapeutic drug candidate for the treatment of inflammatory diseases.
Anti-inflammatory activities of licorice extracts
Thus far, reports about the anti-inflammatory activity of licorice extracts concentrated mainly on G. glabra and G. uralensis (Table 1). Glycyrrhiza glabra has been used to treat gastric ulcer, oral ulcer (Liu et al. 2011) and ulcerative colitis (Samadnejad et al. 2012). Glycyrrhiza glabra reduced the ulcer zone, and is a good choice for children who do not like taking bitter medicines (Liu et al. 2011). It attenuated macroscopic damage, improved the microscopic structure of the colonic mucosa, and effectively increased superoxide dismutase (SOD) enzymatic defence system to treat acetic acid-induced ulcerative colitis. Furthermore, TNF-α, NO and IL-6 levels were also diminished dose-dependently (p < 0.05) (Samadnejad et al. 2012).
Table 1.
The anti-inflammatory activities of licorice extracts.
| Species | Solvent | Inflammation tissue/disease | Model formation | Extract concentration | Inhibition rate | Toxic signs/mortality | Reference |
|---|---|---|---|---|---|---|---|
| G. glabra | Acetone | LPS (0.1 μg·mL−1)- induced J774A.1 murine macrophage cell line | Stimulation with LPS (0.1μg·mL−1). | 20–40 μg·mL−1 | Dose-dependently inhibit IL-1β, up to 47.8% | (Thiyagarajan et al. 2011) | |
| G. uralensis | Ethanol | The murine RAW264.7 macrophage cells | Stimulation with LPS (1 μg·mL−1) | 25 μg·mL−1 | Inhibit LPS-induced NO production (p<0.001) by 48% | (Wu et al. 2011) | |
| G. uralensis | Ethanol | Human colon cancer cells HT-29 (HT-29-N9) | Stimulation with LPS (1 μg·mL−1) | 25 μg·mL−1 | Suppress the LPS-induced NF-κB luciferase activity (p<0.05) | (Wu et al. 2011) | |
| G. uralensis | Ethanol | Human hepatoma HepG2 cell (HepG2-C8) | Stimulation with LPS (1 μg·mL−1) | 25 μg·mL−1 | Induce the luciferase activity in HepG2C8 cells by fourfolds (p<0.001) | (Wu et al. 2011) |
Glycyrrhiza uralensis has been applied to lipopolysaccharide (LPS)-treated Raw264.7 macrophages and mouse skin treated with 12-O-tetradecanoylphorbol-13-acetate (TPA) in vitro. In LPS-treated Raw264.7 macrophages model, G. uralensis reduced NO and prostaglandin E2 (PGE2) release, the secretion and mRNA levels of TNF-α, IL-6, cyclooxygenase-2 (COX-2) and IL-1β, the protein expression and transcriptional activity of inducible nitric oxide synthase (iNOS) and phospholipase A2 (PLA2) (Wu et al. 2011). It also prevented the inhibitor of NF-κB α (IκBα) degradation and p65 nuclear translocations. In the mouse inflammation model, it suppressed skin swelling and the expression of iNOS and COX-2 (Cho HJ et al. 2010).
Anti-inflammatory active compounds of licorice
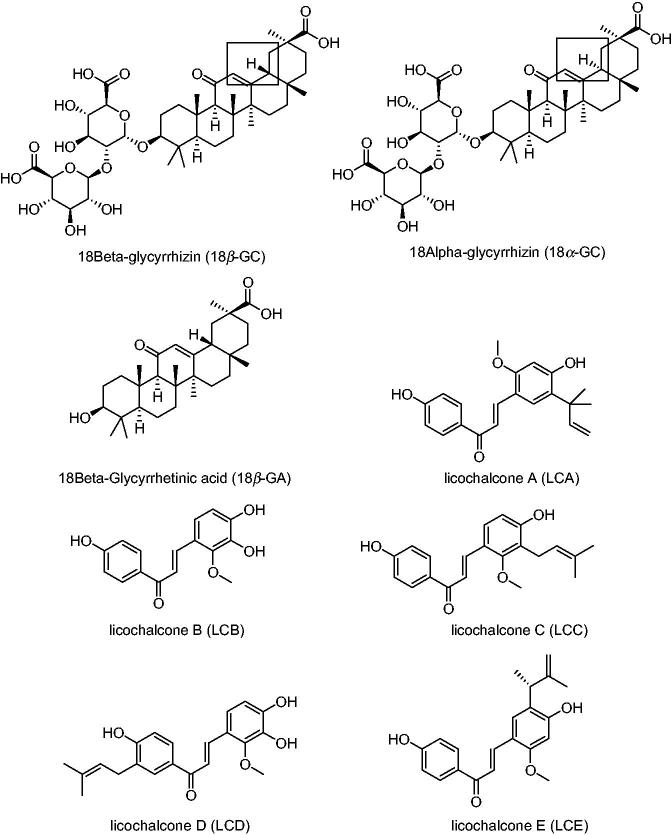
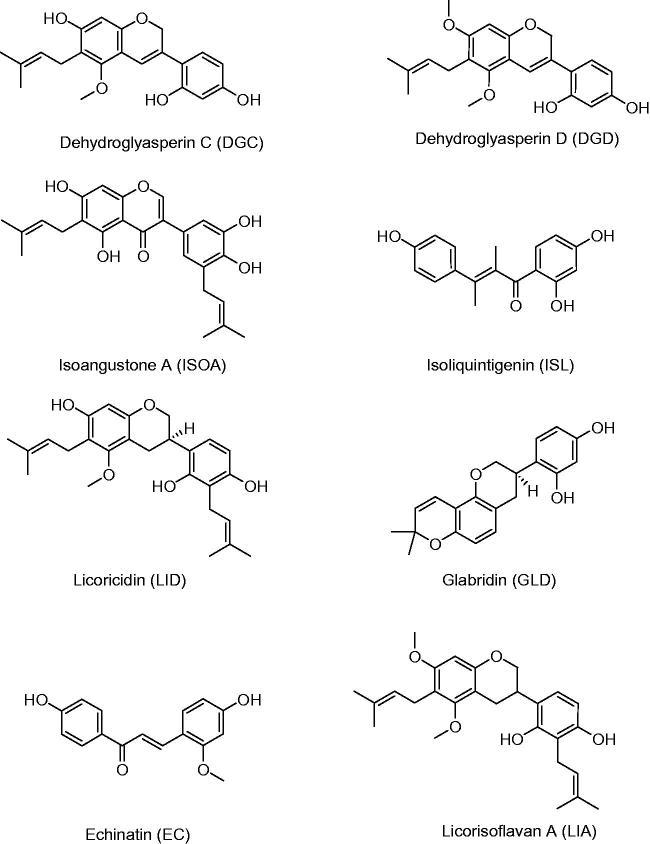
The three original plants of licorice are G. uralensis, G. inflata and G. glabra. They contain many natural active compounds, including more than 20 triterpenes and 300 flavonoids. Seventy-three bioactive compounds and 91 potential targets are identified for this medicinal herb (Li et al. 2011; Liu et al. 2013). Among them, 3 triterpenes, 18β-GC, 18α-GC and 18β-glycyrrhetinic acid (18β-GA), and 13 flavonoids, licochalcone A (LCA), licochalcone B (LCB), licochalcone C (LCC), licochalcone D (LCD), licochalcone E (LCE), isoliquiritigenin (ISL), echinatin (EC), glabridin (GLD), isoangustone A (ISOA), licoricidin (LID), licorisoflavan A (LIA), dehydroglyasperin C (DGC) as well as dehydroglyasperin D (DGD), all have been reported to possess anti-inflammatory activity. The large number of metabolites indicated that licorice was an ideal option for obtaining anti-inflammation compounds. The chemical structure formulas of the above compounds are shown in Figure 2. Furthermore, in order to have a full appreciation of these active compounds, all available data related to in vitro anti-inflammatory activities referring to 16 compounds in 52 assays are shown in Table 2. Similarly, in Table 3, we focussed on the anti-inflammatory activities of these natural compounds in vivo, thus, recent investigations of 6 compounds and 10 assays have been collected. The inflammation tissues, cell lines and animal models, dosage of drugs, inhibition rates, detective methods and the toxic signs are all listed in detail.
Figure 2.
The chemical structure formulas of compounds with anti-inflammatory activity in licorice.
Table 2.
The anti-inflammatory properties of licorice compounds in vitro.
| Compounds | Inflammation tissue/disease | Cell | Concentration | Inhibition rate | Method | Toxic signs/mortality | Reference |
|---|---|---|---|---|---|---|---|
| 18β-GC | LPS (1 μg·mL−1)-induced murine RAW 264.7 cells | RAW 264.7 cells | 75 μM | 51% reduction in NO | ELISA | Do not affect the viability of the RAW 264.7 cells at the concentration lower than 200 μM | (Wang et al. 2011) |
| 18β-GC | LPS (1 μg·mL−1)-induced murine RAW 264.7 cells | RAW 264.7 cells | 75 μM | 49% reduction in PGE2 | ELISA | (Wang et al. 2011) | |
| 18β-GC | LPS (1 μg·mL−1)-induced murine RAW 264.7 cells | RAW 264.7 cells | 75 μM | 46% reduction in TNF-α | ELISA | (Wang et al. 2011) | |
| 18β-GC | LPS (1 μg·mL−1)-induced murine RAW 264.7 cells | RAW 264.7 cells | 75 μM | 42% reduction in IL-6. | ELISA | (Wang et al. 2011) | |
| 18β-GC | LPS (1 μg·mL−1)-induced murine RAW 264.7 cells | RAW 264.7 cells | 75 μM | 51% reduction in IL-1β | ELISA | (Wang et al. 2011) | |
| 18β-GC | Leishmania donovani-infected macrophages | Peritoneal macrophages of Leishmania donovani-infected BALB/c mice (4–6 weeks old) | 50 mg·mL−1 | 90.94% reduction in the parasite load | ELISA | Optimal viability at mg·mL−1 showing 88% survival | (Bhattacharjee et al. 2012) |
| 18α-GC | Ischaemia/reperfusion in L02 cells | The human hepatic L02 cell line | 10 mg·mL−1 | Increase the activities of SOD and GSH-Px | SOD and GSH-Px Detection Kits | (Huang et al. 2014) | |
| 18α-GC | LPS (1 μg·mL−1)-induced murine RAW 264.7 cells | RAW264.7 macrophages | 0.5 mg·mL−1 or 1 mg·mL−1 | Suppress PGE2, PGI2, TXB2 and LTB4 | ELISA | (Xie et al. 2015) | |
| 18β-GA | Indomethacin-induced small intestinal damage | Complex compound of 18β-GA and hydroxypropyl-γ-cyclodextrin | Reduce mRNA expressions of TNF-α, IL-1β and IL-6 | (Ishida 2013) | |||
| 18β-GA | LPS (1 μg·mL−1)-induced murine RAW 264.7 cells | RAW 264.7 cells | 75 μM | 34% reduction in NO | ELISA | Do not affect the viability of the RAW 264.7 cells at the concentration lower than 150 μM | (Wang et al. 2011) |
| 18β-GA | 75 μM | 58% reduction in PEG2 | ELISA | (Wang et al. 2011) | |||
| 18β-GA | 75 μM | 34% reduction in TNF-α | ELISA | (Wang et al. 2011) | |||
| 18β-GA | 75 μM | 35% reduction in IL-6 | ELISA | (Wang et al. 2011) | |||
| 18β-GA | 75 μM | 42% reduction in IL-1β | ELISA | (Wang et al. 2011) | |||
| LCA | TNFα (10 ng·mL−1)-induced NF-κB activation | NIH-3T3 cells | 10/20/30 μM | Inhibit in a dose-dependent manner | EMSA | (Funakoshi-Tago et al. 2010) | |
| LCA | LPS (1 μg·mL−1)-induced mouse peritoneal macrophage cells | Mouse peritoneal macrophage cells | 0.1/0.5/1 μg·mL−1 | Decrease PGE2 by 31.1, 58.3 and 80.3% | PGE2 kit | (Cui et al. 2008) | |
| LCA | LPS (1 μg·mL−1)-induced murine RAW 264.7 cells | RAW 264.7 cells | 10 μM. | The PGE2 inhibition rates exceed 80% | DCFH-DA fluorometric assay | (Fu et al. 2013) | |
| LCA | LPS (1 μg·mL−1)-induced murine RAW 264.7 cells | RAW 264.7 cells | 12.8 ± 1.45 μM. | The effective concentration of ABTS+ radicals are scavenged by 50% | ABTS + radical scavenging capacity assay | (Fu et al. 2013) | |
| LCA | LPS (1 μg·mL−1)-induced murine RAW 264.7 cells | RAW 264.7 cells | 11.6 ± 1.84 μM | Inhibitory activity on lipid peroxidation EC50 | Fe2+-ascorbic acid system | (Fu et al. 2013) | |
| LCB | LPS (1 μg·mL−1)-induced murine RAW 264.7 cells | RAW 264.7 cells | 3 μM. | The inhibition rate of NO exceeds 50%. | DCFH-DA fluorometric assay | ||
| LCB | LPS (1 μg·mL−1)-induced murine RAW 264.7 cells | RAW 264.7 cells | 2.68 ± 0.09 μM. | The concentration of ABTS+ radicals are scavenged by 50% | ABTS+ radical scavenging capacity assay | (Fu et al. 2013) | |
| LCB | LPS (1 μg·mL−1)-induced murine RAW 264.7 cells | RAW 264.7 cells | 3.92 ± 0.12 μM | Inhibitory activity on lipid peroxidation EC50 | Fe2+-ascorbic acid system | (Fu et al. 2013) | |
| LCC | RBL-2H3 cells sensitized with anti-DNP IgE (100 ng·mL−1) | RBL-2H3 cells | 24 μM | Inhibition of β-hexosaminidase release | β-hexosaminidase release assay and trypan blue exclusion assay | 30% cytotoxicity: > 30 μM | (Tanifuji et al. 2010) |
| LCD | RBL-2H3 cells sensitized with anti-DNP IgE (100 ng·mL−1) | RBL-2H3 cells | 21 μM | Inhibition of β-hexosaminidase release | β-hexosaminidase release assay and trypan blue exclusion assay | 30% cytotoxicity: > 30 μM | (Tanifuji et al. 2010) |
| LCE | LPS-stimulated RAW 264.7 murine macrophage | RAW 264.7 murine macrophage | 2.5–7.5 μmol·L−1 | Dose-dependently inhibit NO, PGE2; markedly suppress the expression of iNOS and COX-2 proteins; and the secretion of IL-6, IL-1β and TNF-α | ELISA | (Lee et al. 2013) | |
| Echinatin | LPS (1 μg·mL−1)-induced murine RAW 264.7 cells | RAW 264.7 cells | 2.95 ± 0.11 μM | The effective concentration of ABTS+ radicals are scavenged by 50% | ABTS+ radical scavenging capacity assay | (Fu et al. 2013) | |
| Echinatin | 47.2 ± 2.64 μM | Inhibitory activity on 50% lipid peroxidation | Fe2+-ascorbic acid system | (Fu et al. 2013) | |||
| ISL | LPS (0.1 μg·mL−1)-induced J774A.1 murine macrophage cell line | J774A.1 murine macrophage cell line | 2.5–10 μg·mL−1 | NO levels with 50% inhibition attain at 7.5 μg·mL−1 (29 μM). | ELISA | (Thiyagarajan et al. 2011) | |
| ISL | LPS (0.1 μg·mL−1)-induced J774A.1 murine macrophage cell line | J774A.1 murine macrophage cell line | 1.85 μg·mL−1 (7.2 μM) | IL-1 levels with 50% inhibition | ELISA | (Thiyagarajan et al. 2011) | |
| ISL | LPS (0.1 μg·mL−1)-induced J774A.1 murine macrophage cell line | J774A.1 murine macrophage cell line | 1.92 μg·mL−1 (7.16 μm) | IL-6 levels with 50% inhibition | ELISA | (Thiyagarajan et al. 2011) | |
| ISL | PMA (50 ng·mole−1)-exposed human umbilical vein endothelial cells | Human umbilical vein endothelial cells | 10 μM | Nearly abolish the expression of MMP-2 mRNA | MTT | Nontoxic concentrations showed up 25 ≤ μM for 24h serum-free culture experiments | (Kang et al. 2010) |
| GLD | LPS (0.1 μg·mL−1)-induced J774A.1 murine macrophage cell line | J774A.1 murine macrophage cell line | 10 μg·mL−1 | 33% inhibition in NO levels | ELISA | (Thiyagarajan et al. 2011) | |
| GLD | LPS (0.1 μg·mL−1)-induced J774A.1 murine macrophage cell line | J774A.1 murine macrophage cell line | 10 μg·mL−1 (30.8 μM) | IL-1 levels with 50% inhibition | ELISA | (Thiyagarajan et al. 2011) | |
| LIA | LPS (0.1 μg·mL−1)-induced U937 cells line | U937 cells (ATCC CRL-1593.2; human monoblastic leukaemia cell line | 0.1, 0.5, 1 μg·mL−1 | Decreased the secretion of IL-6 | No obvious cytotoxic effects were detected at 1mg·mL−1 with the cell viability of 85% | (La et al. 2011) | |
| LIA | LPS (0.1 μg·mL−1)-induced U937 cells line | U937 cells (ATCC CRL-1593.2; human monoblastic leukaemia cell line | 1 μg·mL−1 | Decreased the secretion of CCL5 | (La et al. 2011) | ||
| LIA | LPS (0.1 μg·mL−1)-induced U937 cells line | U937 cells (ATCC CRL-1593.2; human monoblastic leukemia cell line | 0.1, 0.5, 1 μg·mL−1 | Decreased the secretion of MMP-8 | (La et al. 2011) | ||
| LIA | LPS (0.1 μg·mL−1)-induced U937 cells line | U937 cells (ATCC CRL-1593.2; human monoblastic leukaemia cell line | 0.5, 1 μg·mL−1 | Decreased the secretion of MMP-7 | (La et al. 2011) | ||
| LIA | LPS (0.1 μg·mL−1)-induced U937 cells line | U937 cells (ATCC CRL-1593.2; human monoblastic leukaemia cell line | 1 μg·mL−1 | Decreased the secretion of MMP-9 | (La et al. 2011) | ||
| LID | LPS (0.1 μg·mL−1)-induced U937 cells line | U937 cells (ATCC CRL-1593.2; human monoblastic leukaemia cell line | 0.1, 0.5, 1 μg·mL−1 | Decreased the secretion of IL-6 | No obvious cytotoxic effects were detected at 1mg·mL−1 with the cell viability of 85% | (La et al. 2011) | |
| LID | LPS (0.1 μg·mL−1)-induced U937 cells line | U937 cells (ATCC CRL-1593.2; human monoblastic leukaemia cell line | 0.1, 0.5, 1 μg·mL−1 | Decreased the secretion of MMP-7 and MMP-8 | (La et al. 2011) | ||
| LID | LPS (0.1 μg·mL−1)-induced U937 cells line | U937 cells (ATCC CRL-1593.2; human monoblastic leukaemia cell line | 0.5, 1 μg·mL−1 | Decreased the secretion of MMP-9 | (La et al. 2011) | ||
| DGC | 0.5 mM | Ferric reducing antioxidant power 855.07 ± 84.14 μmole·L−1 | Ferric reducing antioxidant power assay | (Kim HJ et al. 2012b) | |||
| DGC | 0.205 ± 0.005 mM | IC50 for DPPH | DPPH radical scavenging assay | (Kim HJ et al. 2012b) | |||
| DGC | 0.465 ± 0.081 mM | IC50 value for ABTS+ | ABTS+ radical cation-decolourization assay | (Kim HJ et al. 2012b) | |||
| DGC | Glutamate (5 mM)-induced HT22 cells | HT22 cells | 2 μM | Dose-dependently inhibit ROS production | 2,7-dichlorofluorescein (DCF) assay and western-blot | (Kim HJ et al. 2012a) | |
| DGD | 0.5 mM | Ferric reducing antioxidant power 812.04 ± 40.35 μmole·L−1 | Ferric reducing antioxidant power assay | (Kim HJ et al. 2012b) | |||
| DGD | 0.309 ± 0.002 mM | IC50 for DPPH | DPPH radical scavenging assay | (Kim HJ et al. 2012b) | |||
| DGD | 0.635 ± 0.035 mM | IC50 value for ABTS+ | ABTS+ radical cation-decolourization assay | (Kim HJ et al. 2012b) | |||
| ISOA | 0.5 mM | Ferric reducing antioxidant power 231.57 ± 24.44 μmole·L−1 | Ferric reducing antioxidant power assay | (Kim HJ et al. 2012b) | |||
| ISOA | 0.418 ± 0.015 mM | IC50 for DPPH | DPPH radical scavenging assay | (Kim HJ et al. 2012b) | |||
| ISOA | 0.655 ± 0.042 mM | IC50 value for ABTS+ | ABTS+ radical cation-decolourization assay | (Kim HJ et al. 2012b) |
Table 3.
The anti-inflammatory properties of licorice compounds in vivo.
| Compounds | Inflammation tissue/disease | Models | Treatment | Outcomes | Reference |
|---|---|---|---|---|---|
| 18β-GC | An intratracheal instillation of LPS (1 mg·kg−1) | Male BALB/C mice weighing 20–25 g | Intraperitoneal injection of 10, 25 and 50 mg·kg−1 | Markedly decrease the MPO activity and NO concentrations | (Ni et al. 2011) |
| Injection of 0.94 nmole (0.2 μg) of kainic acid (KA)-induced neuronal death model | Male BALB/c mice (25-30 g) | Intraperitoneal injection of 10 or 50 mg·kg−1 | Iβ-1-positive cells are almost completely suppressedby 50 mg·kg−118β-GC | (Luo et al. 2013) | |
| 18α-GC | 20% paraquat poisoning solution at 15 mg·kg−1 dose | 30 male Sprague Dawley rats from 180 g to 200 g | Intraperitoneal injection of 30 mg·kg−1 | Significantly decrease intercellular adhesion molecule-1 (ICAM-1) and matrix metalloproteinase-9 (MMP-9) | (Xiao et al. 2014) |
| LCA | Noninfectious mouse model of asthma | BALB/c mice | 50 mg·kg−1 | Inhibit the increase in T-helper type 2 cytokines, reduce serum levels of ovalbumin-specific IgE and IgG | (Chu et al. 2013) |
| Topical inflammation was instantly induced on the posterior surface of the same ear by the application of xylene (0.05 mL) | Kunming mice 20–25 g and Wistar rats (150–200 g) | 5 mg·kg−1 | Decrease the ear oedema rate by30.3% | (Cui et al. 2008) | |
| 0.1 mL freshly prepared carrageenan was injected into the right hind paw | Kunming mice 20–25 g and Wistar rats (150–200 g) | 2.5, 5 and 10 mg·kg−1 body weight | Dose-dependentreduce the paw oedema rateby 41.3, 39.7 and 30.7%, respectively | (Cui et al. 2008) | |
| LCE | 5 nmoles of TPA 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse ear oedema | ICR mice | 0.5–2 mg | Dose-dependently reduce the TPA-induced increase in ear weight and ear thickness | (Lee et al. 2013) |
| ISL | Male, 5-week-old C57BL/6 mice were fed a HFD containing 60% fat | C57BL/6 mice | 10 μM | Inhibit HFD-induced IL-1 and caspase-1 production | (Honda et al. 2014) |
| GLD | 5% dextran sulphate sodium-induced BALB/c mice | BALB/c mice | 10 or 50 mg·kg−1 | Attenuate mortality, loss of body weight, shortening of the colon and severe clinical symptoms. | (Kwon et al. 2008) |
Triterpenes and related possible mechanisms for inflammation prevention
More than 20 triterpenes have been isolated from the roots of licorice, but only 18β-GC, 18α-GC and 18β-GA, have been reported to possess the anti-inflammatory activity. The possible mechanisms for the inflammation prevention of the three triterpenes and the inflammatory types were investigated as follows.
18β-Glycyrrhizin
18β-GC is regarded as the marker compound in licorice. It has been demonstrated that 18β-GC suppressed MPO activity (Ni et al. 2011) and phosphorylation and secretion of high mobility group protein 1 (Kim SW et al. 2012). It also decreased the levels of cholesterol of lipid rafts, the translocation of toll-like receptor 4 to lipid rafts and the interferon regulating factor 3 activation (Fu et al. 2014). Furthermore, it attenuated the production of PGE2, intracellular reactive oxygen species (ROS), TNF-α, COX-2 and iNOS (Luo et al. 2013). Moreover, 18β-GC also activated ATP-binding cassette transporter A1, which induced cholesterol efflux from lipid rafts (Fu et al. 2014).
Thus far, 18β-GC has been applied to LPS-stimulated macrophage models (Wang et al. 2011), mouse mammary epithelial cells (Fu et al. 2014) and Leishmania donovani-infected macrophages (Bhattacharjee et al. 2012) in vitro, and been applied to the postischaemic brain rats models (Kim SW et al. 2012; Luo et al. 2013;), LPS-induced mastitis rat models (Fu et al. 2014) and LPS-induced acute lung injury (ALI) rat models in vivo. It can also suppress microglia activation, the mammary gland histopathological changes and LPS-induced alveolar haemorrhage (Ni et al. 2011).
18α-Glycyrrhizin
18α-GC and 18β-GC is a pair of epimers, differed only in the C18-H. The anti-inflammatory activities of 18α-GC have been affirmed. It suppressed PLA2/arachidonic acid pathway metabolites, such as PGE 2, prostacyclin 2, thromboxane 2 and leukotrienes B4 (Xie et al. 2015). It significantly reduced the content of intercellular adhesion moledule-1 and MMP-9 (Xiao et al. 2014). What’s more, it increased the activities of SOD and GSH-Px, and the expression of p-Akt and p-ERK (Huang et al. 2014).
It has been reported that the protective and anti-inflammatory effects of 18α-GC were better than 18β-GC (Zeng et al. 2006). It has been applied to RAW264.7 macrophages (Xie et al. 2015), human ischaemia/reperfusion injury hepatic L02 cells (Huang et al. 2014) in vitro, and paraquat poisoning-induced lung injury rat models (Xiao et al. 2014) in vivo.
18β-Glycyrrhetinic acid
18β-GA is a hydrolyzed metabolite of 18β-GC. Since 18β-GC can generate 18β-GA through metabolic processes in the human body, the pharmacological effects of 18β-GA are essentially the same as 18β-GC. 18β-GA exerted its anti-inflammatory activities via inducing antioxidant defence systems, decreasing lipid peroxidations, ameliorated oxidative and histological damage. It also significantly reduced the generation of excessive NO, PGE2 and ROS, inhibited the protein and mRNA levels of iNOS and COX-2 and suppressed the release of LPS-induced TNF-α, IL-6 and IL-1β in a dose-dependent manner (Wang et al. 2011; Ishida et al. 2013). It has been studied in indomethacin-induced small intestinal damage (Ishida et al. 2013), LPS-induced macrophages (Wang et al. 2011) in vitro and neuronal damage caused by global cerebral ischaemia/reperfusion in C57BL/J6 mouse models (Oztanir et al. 2014) in vivo, and the anti-inflammatory actions were significantly affirmed.
Flavonoids and related mechanisms for inflammation prevention
About 300 polyphenols have been isolated from licorice, including phenolic acids, flavonoids, flavans, chalcones, isoflavan and isoflavonoids. Thus far, the main anti-inflammatory active polyphenols in licorice are chalcones, isoflavan and isoflavonoids. Among them, chalcones, such as LCA, LCB, LCC, LCD, LCE, ISL and EC, isoflavonoids, such as ISOA, and isoflavan, such as GLD, LID, LIA, DGC and DGD have shown the potential as anti-inflammatory drugs.
Chalcones
Chalcones include LCA, LCB, LCC, LCD, LCE, ISL and EC. The special scaffold of chalcones was regarded as the key factor for their broad biological activities (Karthikeyan et al. 2015). It is believed that the fixed structure of LCA is necessary for its anti-inflammatory activity, since α,β-unsaturated ketone reduced LCA, which lacks a double bond, failed to inhibit TNFα-induced NF-κB activation. Furthermore, LCA markedly inhibited acute carrageenan-induced paw oedema in mice while the reduced LCA failed (Funakoshi-Tago et al. 2009, 2010).
The mechanisms for the anti-inflammatory activities of chalcones have been fully investigated. LCA, LCB, ISL and EC all inhibited the production of NO, IL-6 and PGE2, while LCA, LCB and LCD all exhibited potent inhibition of lipid peroxidation (Haraguchi et al. 1998; Thiyagarajan et al. 2011; Fu et al. 2013; Honda et al. 2014). LCB and EC both showed strong scavenging activity towards the 2, 2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) (+) radical. LCB and LCD both strongly inhibited superoxide anion production in the xanthine oxidase system, showed potent scavenging activity on DPPH radical and inhibited phosphorylation of NF-κB p65 (Haraguchi et al. 1998; Furusawa et al. 2009). Furthermore, LCA significantly inhibited the release of cytokines, such as IL-4, IL-5 and IL-13, and serum levels of ovalbumin-specific immunoglobulin E (IgE), IgG. It also reduced the mRNA expression of acidic mammalian chitinase, chitinase 3-like protein 4, E-selectin, Muc5ac, CCl11 and CCR3 in lung tissues (Chu et al. 2013). LCC decreased the expression and activity of iNOS, and modulated the antioxidant network activity of SOD, catalase and glutathione peroxidase (Wang et al. 2013). LCD inhibited the mast cell degranulation through the inhibition of both extracellular Ca2+ influx and activation of the MEK-ERK pathway (Tanifuji et al. 2010; Kim & Jun 2013). LCE effectively inhibited PKC/JNK, ERK1/2, reduced the expression of iNOS, COX-2, IL-6, IL-1β, IL-12p40, TNF-α, AKT and p38 mitogen-activated protein kinase (MAPK), and attenuated IκBα degradation and NF-κB activities, as well as transcriptional activity of activator protein AP-1 (Cho et al. 2010; Lee et al. 2013). ISL dampened the expression of MMP-2 mRNA in a dose-response manner, and at ≥10 μM, the expression was nearly abolished (Kang et al. 2010).
For studies in vitro, LCA, LCB, LCC, LCD, LCE and EC has been applied to LPS-induced RAW264.7 mouse macrophage cell models and rat liver microsomes (Cui et al. 2008; Fu et al. 2013). LCD has been studied in rat baso-leukaemia (RBL)-2H3 cells, and ISL has been applied to LPS-induced J774A.1 murine macrophages cells models and human umbilical vein endothelial cells (Kang et al. 2010; Thiyagarajan et al. 2011).
For studies in vivo, LCA attenuated allergic airway inflammation in a murine model of asthma (Chu et al. 2013), inhibited xylene-induced mice ear oedema and carrageenan-induced paw oedema (Cui et al. 2008). LCE has been studied in TPA-induced mouse ear oedema models and oxazolone-induced chronic allergic contact dermatitis mouse skin models (Cho YC et al. 2010; Lee et al. 2013;). In vivo analyses also revealed that ISL potently attenuated high-fat-diet-induced obesity, hypercholesterolaemia and insulin resistance, which indicated that ISL could be useful for the treatment of NLRP3 inflammasome-associated diseases (Honda et al. 2014).
Depending on some clinical studies, LCA had a similar effect to moderate childhood atopic dermatitis in comparison with 1% hydrocortisone. The transepidermal water loss was significantly lower than baseline, and the use of LCA for four weeks could maintain clinical improvement (Wananukul et al. 2013).
Other flavonoids
Besides chalcones, other flavonoids in licorice, including DGC, DGD, ISOA, GLD, LID and LIA, also showed excellent anti-inflammatory activities. DGC, DGD and ISOA all showed strong ferric reducing activities and effectively scavenged DPPH, ABTS + and singlet oxygen radicals (Kim HJ et al. 2012b). Furthermore, DGC increased the expression of haemeoxygenase-1 and MAPK phosphatase-1, suppressed the inflammation-mediated neurodegeneration, production of TNF-α, NO, ROS, NF-κB and phosphorylation of p38 MAPKs, ERK1/2, IκB-α and p65 (Kim HJ et al., 2012a; Kim et al. 2013). GLD significantly inhibited NO and IL-1β release (Thiyagarajan et al. 2011), attenuated colonic inflammation in mice with dextran sulphate sodium-induced colitis (Kwon et al. 2008), and decreased the iNOS mRNA expression under high-glucose levels, which indicated that GLD could be applied to diabetes-related vascular dysfunction (Yehuda et al. 2015). LID and LIA inhibited the secretion of IL-6, chemokine (C-C motif) ligand 5, MMP-7, -8 and -9. The suppression of cytokine and MMP secretion by LID and LIA was associated with the reduced activation of NF-κB p65 in periodontitis treatment (La et al. 2011).
In vitro, the anti-inflammatory activities of DGC, DGD and ISOA have been demonstrated in glutamate-induced mouse hippocampal HT22 cells models (Kim HJ et al. 2012a). DGC, GLD, LID and LIA have been used in LPS-treated Raw264.7 macrophages models (La et al. 2011; Thiyagarajan et al. 2011). DGC has also been applied to LPS-stimulated BV-2 microglia models (Kim et al. 2013). And GLD has been applied in macrophage-like cells models under chronic glucose stress (Yehuda et al. 2015). In vivo, GLD has been used in dextran sulphate sodium-induced colitis mice models (Kwon et al. 2008).
The summary of main anti-inflammatory mechanisms of licorice
Depending on previous studies, we found that decreasing the inflammatory factors was the key strategy for licorice to treat inflammation-related disease, such as rheumatoid arthritis (Yang et al. 2013), liver oxidative injury (Huo et al. 2011), colonic inflammatory response (Takhshid et al. 2012) and periodontitis (Farhad et al. 2013). Tumour necrosis factor, MMPs, PGE2 and free radicals are four main factors most widely reported among numerous studies related to licorice’s anti-inflammatory mechanisms.
Tumour necrosis factor
The role of TNF-α played in the progress of inflammation has been explored deeply. TNF-α is an autocrine stimulator as well as a potent paracrine inducer of pro-inflammatory mediators including IL-1, IL-6, IL-8 (Suzuki et al. 2000) and granulocyte-macrophage colony-stimulating factor (Haworth et al. 1991). Additionally, TNF-α stimulates chondrocytes to release MMPs in rheumatoid arthritis and periodontitis patients (Sorsa et al. 2006). Furthermore, TNF-α also induces NO production and releases PGE2 by synovial cells, which in turn causes tissue destruction (Nagy et al. 2008). Recently, treatment of ulcerative colitis with TNF-α antibody has achieved encouraging results in the clinic (Takhshid et al. 2002). In the progressive accumulation of liver fibrosis, the progress is triggered by a series of chemical mediators, with a prominent role played by the TNF-β (Poli 2000). Depending on the findings of licorice and its isolated pure compounds, G. glabra extracts (Samadnejad et al. 2012) inhibited the formation of TNF in acetic acid-induced ulcerative colitis animal model, 18β-GA (Ishida. 2013) exerted the activity in indomethacin-induced small intestinal damage, G. uralensis extracts (Wu et al. 2011), 18β-GC (Wang et al. 2011), LCE (Lee et al. 2013) and DGC (Kim HJ et al. 2012a) inhibited the formation of TNF in LPS-treated Raw264.7.
MMPs
The pathogenic MMPs may lead to joint destruction. In the process of liver fibrosis, the expressions of MMPs are activated by reactive oxygen species and lipid peroxidation products (Poli 2000). In periodontal inflammation, MMPs form a family of enzymes that mediate multiple functions both in the tissue destruction and immune responses. The expression and activity of MMPs in noninflamed periodontium is low but is drastically enhanced to pathologically elevated levels due to the dental plaque and infection-induced periodontal inflammation (Sorsa et al. 2006). 18α-GC, ISL, LID and LIA all showed up inhibition activities towards MMPS in paraquat poisoning-induced lung injury rat models (Xiao et al. 2014), PMA-exposed human umbilical vein endothelial cells (Kang et al. 2010) and LPS-induced U937 cells line (La et al. 2011) separately.
PGE2
Prostaglandins are potent eicosanoid lipid mediators derived from phospholipase-released arachidonic acid that are involved in numerous homeostatic biological functions and inflammation. They are generated by cyclooxygenase isozymes. The prime mode of prostaglandin is through specific G protein-coupled receptors (Funk 2001). In TCM therapeutics, licorice has been used to strengthen the function of digestive system and alleviate pain for thousands of years. G. uralensis extract (Wu et al. 2011), 18β-GC (Wang et al. 2011), 18α-GC (Xie et al. 2015), 18β-GA (Wang et al. 2011), LCA (Cui et al. 2008), LCB (Fu et al. 2013), and EC (Fu et al. 2013) were all reported to suppress the generation of PGE2 in LPS-treated Raw264.7 macrophages model. PGE2 was reported to activate sensitizing pain receptors and induce fever (Ferreira 1972). The inhibition of PGE 2 could explain licorice’s ancient characteristics of alleviating pain.
Free radicals
Free radicals, including reactive oxygen species, such as the hydroxyl radical, superoxide anion, and hydrogen peroxide, and reactive nitrogen species, such as NO, are all associated with pathology and cell damage, which have been reported to attack nucleic acids and proteins, as well as unsaturated fatty acids in the cell membrane (Fernández-Moriano et al. 2016). In the rheumatoid arthritis, NO has been reported to be an important mediator in the progression of cartilage and bone destruction and induce the production of pathogenic cytokines and chemokines. In liver models, involvement of reactive oxygen species and lipid peroxidation products can be clearly demonstrated in other fundamental events of hepatic fibrogenesis (Poli 2000). Glycyrrhiza uralensis extract (Wu et al. 2011), 18β-GC, 18β-GA (Wang et al. 2011), LCA, LCB (Fu et al. 2013), LCC (Lee et al. 2013), ISL, EC, GLD (Thiyagarajan et al. 2011) and DGC (Kim HJ et al. 2012b), all significantly inhibited the production of free radicals in LPS-treated Raw264.7 macrophages model.
Thus, the underlying anti-inflammatory mechanisms for targeting the related pathogenic factors could explain the extraordinary inhibition properties of licorice.
Drugs that include compounds of licorice
Drugs came from GC have been successfully used in China and Japan for many years to treat inflammation diseases. Five hundred and fifty-four kinds of drugs containing GC have been approved by the China Food and Drug Administration (CFDA), and four generations of GC preparations have been developed so far, from GC tablets to ammonium glycyrrhizinate, diammonium glycyrrhizinate and magnesium isoglycyrrhizinate (MgIG). The dosage forms are quite abundant, such as extractum, tablet, capsula, injection, granule and oral solution, the main active compounds and preparations have been listed in Table 4. Depending on the clinical researches, MgIG, mainly containing 18α-GC, had a better lipotropy, a higher targeting and fewer adverse reactions, and was regarded as a safer and more effective drug compared with preparations mainly containing 18β-GC (Zeng et al. 2006; Xu et al. 2013).
Table 4.
The preparations from licorice approved by CFDA.
| China Approved Drug Names (CADN) | Component | Dosage forms | Batch number | Drug standard code |
|---|---|---|---|---|
| Licorice extract powder | Licorice extract | Powder | H65020417 | 86905972000020 |
| Bismuth glycyrrhetate powder | Bismuth glycyrrhetate | Powder | H62021142 | 86905894000351 |
| Trisodium glycyrrhetate | Trisodium glycyrrhetate | Raw material | H65020217 | 86906000000098 |
| Glycyrrhetinic acid | Glycyrrhetinic acid | Raw material | H15021234 | 86904264000083 |
| MgIG | MgIG | Raw material | H20051941 | 86901523001461 |
| Glycyrrhizic acid A | Glycyrrhizic acid A | Raw material | H65020210 | 86906000000081 |
| Licorzine | Licorzine | Raw material | H15021235 | 86904264000168 |
| Diammonium glycyrrhizinate | Diammonium glycyrrhizinate | Raw material | H20065456 | 86901375000735 |
| Dipotassium glycyrrhetate | Dipotassium glycyrrhetate | Raw material | H65020215 | 86906000000111 |
| Monopotassium glycyrrhiznate A | Monopotassium glycyrrhiznate A | Raw material | H61022582 | 86902362000219 |
| Monoammonium glycyrrhizinate S | Monoammonium glycyrrhizinate S | Raw material | H20057930 | 86901498000025 |
| Potassium glycyrrhefate M | Potassium glycyrrhefate M | Raw material | H20057335 | 86901498000018 |
| Mono potassium glycyrrhizinate tablets | Mono potassium glycyrrhizinate | Tablets | Z15021799 | 86903911000452 |
| Compound Licorice tablets | Licorice extract, opioid, camphor, star anise oil, sodium benzoate | Tablets | Z53020718 | 86905614002672 |
| Ephedrine Hydrochloride and Glycyrrhizia Extract Tablets | Ephedrine hydrochloride, glycyrrhizia extract | Tablets | H61023525 | 86902503000504 |
| Compound Glycyrrhizin tablets | Glycyrrhizin, glycine and cysteine hydrochloride | Tablets | H20073723 | 86903094000386 |
| Compound licorice Aluminium and Magnesium tablets | Aluminium hydroxide, magnesium trisilicate, magnesium oxide, calcium carbonate, Bletilla striata, Radix aucklandiae, Extractum glycyrrhizae liquidum, Belladonna liquid extract | Tablets | H42022362 | 86901984002199 |
| Diammonium glycyrrhizinate enteric-coated tablets | Diammonium glycyrrhizinate | Enteric-coated tablets | H20150025 | 86904797000703 |
| Extractum glycyrrhizae | Licorice extract | Extractum | Z61021679 | 86902331000028 |
| Licorzine granules | Licorzine | Granules | H32022277 | 86901474000070 |
| Mono potassium glycyrrhizinate capsule | Mono potassium glycyrrhizinate | Capsule | Z20060085 | 86904313000217 |
| Licorzine capsule | Licorzine | Capsule | H31022339 | 86900727000478 |
| Diammonium glycyrrhizinate capsules | Diammonium glycyrrhizinate | Capsules | H20093489 | 86901651001531 |
| Compound glycyrrhizin capsules | Glycyrrhizin, glycine and cysteine hydrochloride | Capsules | H20110056 | 86904152003899 |
| Compound glycyrrhiza oral solution | Extractum glycyrrhizae liquidum, paregoric, glycerinum, guaiamar, concentrated ammonia solution | Oral solution | H46020470 | 86905840001623 |
| Diammonium glycyrrhizinate for injecton | Diammonium glycyrrhizinate | Injection | H20052225 | 86900151000082 |
| Compound glycyrrhizin for injection | Glycyrrhizin, glycine and cysteine hydrochloride | Injection | H20070217 | 86900234000039 |
| MgIG injection | MgIG | Injection | H20051942 | 86901523001478 |
| Compound monoammonium glycyrrhizinate S for injection | Glycyrrhizin, glycine and cysteine hydrochloride | Injection | H20041998 | 86900356001242 |
| Diammonium glycyrrhizinate and glucose injection | Diammonium glycyrrhizinate and glucose | Injection | H20030421 | 86901523001126 |
| Diammonium glycyrrhizinate and sodium chloride injection | Diammonium glycyrrhizinate and sodium chloride | Injection | H20010630 | 86901523001218 |
| Monoammonium glycyrrhizinate and cysteine and sodium chloride injection | Monoammonium glycyrrhizinate, cysteine and sodium | Injection | H22026458 | 86903282000280 |
Safety of licorice
Although licorice is considered to be a nontoxic herb in TCM, the safety use of licorice still attached much attention. The mechanisms have been fully evaluated. Licorice was reported to be a competitive inhibitor of 11β-hydroxysteroid dehydrogenases (11β-HSDs), the most important enzymes in the systemic regulation of glucocorticoids and mineralocorticoid (Whorwood et al. 1993). There are two 11β-HSDs, 11β-HSD1 and 11β-HSD2. 11β-HSD1 is a bidirectional enzyme that preferred activation of cortisol from cortisone, expressed in liver, adipose, bone and other inflamed tissues. 11β-HSD2 converts active cortisol to inactive cortisone, expressed in the kidney, pancreas and other mineralocorticoid sensitive tissues (Ma et al. 2011). GC administration to rats in vivo (75 mg·kg−1, day for 5 days) resulted in the inhibition of 11β-HSD mRNA levels and 11β-HSD activity in both predominantly mineralocorticoid (kidney and distal colon) and glucocorticoid (liver and pituitary) target tissues, and the inhibition was in a dose-dependent manner in vitro (Whorwood et al. 1993). In a study conducted in 12 healthy volunteers, the ingestion of 100 g licorice daily for 8 weeks increased the plasma atrial natriuretic peptide concentration and the mean body weight, and decreased the plasma concentrations of antidiuretic hormone, aldosterone and plasma renin activity, which reflected retention of sodium and fluid volume, and the effects were probably due to the mineralocorticoid properties of licorice (Forslund et al. 1989). In another case, a 51-year-old lady was diagnosed as acquired apparent mineralocorticoid excess and severe hypertension after eating considerable amounts of salted licorice, while her blood pressure quickly normalized after stopping the intake of the salted licorice (Ruiz-Granados et al. 2012).
All of the above reports showed that the hormonal-like effects of licorice might be the main reason for its side effects; hence the particular attention should be attached to the large doses or long-term ingestion of licorice (Wang & Nixon 2001). Furthermore, the genetic difference between individuals was also an important reason for different sensitivity, the 11β-HSD2 gene mutation led to lower 11β-HSD2 enzyme activity, and the patients with mutation would be more sensitive than the general population for licorice-induced hypertension. Therefore, the herbal medicine containing licorice may be contraindicated in patients with an 11β-HSD2 mutation (Harahap et al. 2011). Although the intake of licorice may have some side effects in humans, all of these side effects were reversible and the health benefits outweigh its side effects with proper control. Instead of raw licorice extract, the compounds isolated from licorice may reduce the GC-induced side effects and improve the therapeutical action.
Conclusions and perspectives
Licorice has been used in TCM for thousands of years to treat inflammatory diseases. The results of this paper showed that 3 triterpenes and 13 flavonoids were mainly responsible for the anti-inflammatory activity of licorice through a variety of mechanisms, especially downregulation of mediators, such as TNF-α, MMPs, PGE2 and oxidative stress on the progression of inflammation-related diseases. In this report, we also reflected the available data on in vitro anti-inflammatory activities of licorice and purified compounds on cellular substrates and in vivo on animal models. So far, 554 drugs containing natural compounds and derivatives of licorice have been approved by CFDA. As for safety evaluation, licorice was regarded as a competitive inhibitor of 11β-HSDs, long time intake of licorice may lead to acquired apparent mineralocorticoid excess and severe hypertension, furthermore, the genetic difference between individuals was also an important reason for different sensitivity. All the above suggest that licorice could serve as a therapeutic candidate sources for the treatment of inflammatory diseases with a kind consideration of licorice’s hormonal-like effects.
A series of licorice compounds have been indicated possessing anti-inflammatory effects. So far, studies focusing on licorice extracts are rather limited, and the active compounds in the extracts are not clear. The single compound, such as 18β-GC has attracted considerably more studies. However, the studies about the interactions of different active compounds are restrained. More importantly, dosage in different models are quite different, more pharmacokinetic studies on licorice using different models should be carried out, and the maximum tolerated dose is also critical for clinical use of licorice and its purified compounds.
Our previous studies showed that the contents of triterpenes and flavonoids varied a lot among three licorice original plants, hence a quite difference will be made among their anti-inflammatory activities, which is worthy of further studies. In addition, total contents of phenols, flavonoids and tannins in licorice varied a lot at different harvest times, the samples obtained during from May and November showed the most favourable free radical scavenging and antioxidant effects, whereas the best gastroprotective effect was observed in the sample obtained during May (Cheel et al. 2013). Many compounds, especially the triterpenes, have been developed to the registered drugs of CFDA so far, the side effects of triterpenes have also been investigated for many years. While, the flavonoids of licorice has not been studied deeply, and the large sample, randomized, double-blind and controlled chemoprevention clinical trials about flavonoids are very limited, which require more attention. We can conclude that licorice is a potential source of natural anti-inflammatory agent. However, at the same time, it still needs deeper researches for evaluating its pharmaceutical potentialities and better understanding of its pharmacological mechanisms.
Disclosure statement
All authors declare that they have no competing interests.
References
- Abe K, Ikeda T, Wake K, Sato T, Sato T, Inoue H.. 2008. Glycyrrhizin prevents of lipopolysaccharide/d-galactosamine-induced liver injury through down-regulation of matrix metalloproteinase-9 in mice. J Pharm Pharmacol. 60:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SJ, Cho EJ, Kim HJ, Park SN, Lim YK, Kook JK.. 2012. The antimicrobial effects of deglycyrrhizinated licorice root extract on Streptococcus mutans UA159 in both planktonic and biofilm cultures. Anaerobe. 18:590–596. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S, Bhattacharjee A, Majumder S, Majumdar SB, Majumdar S.. 2012. . Glycyrrhizic acid suppresses Cox-2-mediated anti-inflammatory responses during Leishmania donovani infection . J Antimicrob Chemother. 67:1905–1914. [DOI] [PubMed] [Google Scholar]
- Bi X, Gong M, Di L.. 2014. Review on prescription compatibility of shaoyaogancao decoction and reflection on pharmacokinetic compatibility mechanism of traditional Chinese medicine prescription based on in vivo drug interaction of main efficacious compounds. Evid Based Complement Alternat Med. 2014:208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco-Pozo C, Castillo RL, Beltrán C, Miranda A, Fuentes J, Gotteland M.. 2016. Molecular mechanisms of gastrointestinal protection by quercetin against indomethacin-induced damage: role of NF-κB and Nrf2. J Nutr Biochem. 27:289–298. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran CV, Deepak HB, Thiyagarajan P, Kathiresan S, Sangli GK, Deepak M, Agarwal A.. 2011. Dual inhibitory effect of Glycyrrhiza glabra (GutGard™) on COX and LOX products. Phytomedicine. 18:278–284. [DOI] [PubMed] [Google Scholar]
- Cheel J, Tůmová L, Areche C, Antwerpen PV, Neve J, Zouaoui-boudjeltia K, Martin AS, Vokral I, Wsol V, Neugebauerova J.. 2013. Variations in the chemical profile and biological activities of licorice (Glycyrrhiza glabra L.), as influenced by harvest times. Acta Physiol Plant. 35:1337–1349. [Google Scholar]
- Cho HJ, Lim SS, Lee YS, Kim JS, Lee CH, Kwon DY, Park JHY.. 2010. Hexane/ethanol extract of Glycyrrhiza uralensis licorice exerts potent anti-inflammatory effects in murine macrophages and in mouse skin. Food Chem. 121:959–966. [Google Scholar]
- Cho YC, Lee SH, Yoon G, Kim HS, Na JY, Choi HJ, Cho CW, Cheon SH, Kang BY.. 2010. . Licochalcone E reduces chronic allergic contact dermatitis and inhibits IL-12p40 production through down-regulation of NF-kappa B. Int Immunopharmacol. 10:1119–1126. [DOI] [PubMed] [Google Scholar]
- Chu X, Jiang L, Wei M, Yang X, Guan M, Xie X, Wei J, Liu D, Wang D.. 2013. Attenuation of allergic airway inflammation in a murine model of asthma by licochalcone A. Immunopharmacol Immunotoxicol. 35:653–661. [DOI] [PubMed] [Google Scholar]
- Cui Y, Ao M, Li W, Hu J, Yu L.. 2008. . Anti-inflammatory activity of licochalcone A isolated from Glycyrrhiza inflata . Z Naturforsch C J Biosci. 63:361–365. [DOI] [PubMed] [Google Scholar]
- Farhad SZ, Aminzadeh A, Mafi M, Barekatain M, Naghney M, Ghafari MR.. 2013. The effect of adjunctive low-dose doxycycline and licorice therapy on gingival crevicular fluid matrix metalloproteinase-8 levels in chronic periodontitis. Dent Res J. 10:624–629. [PMC free article] [PubMed] [Google Scholar]
- Feng YC, Wang KC, Chiang LC, Shieh DE, Yen MH, San CJ.. 2013. Water extract of licorice had anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J Ethnopharmacol. 148:466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SH. 1972. Prostaglandins, aspirin-like drugs and analgesia. Nature New Biol. 240:200–203. [DOI] [PubMed] [Google Scholar]
- Fernández-Moriano C, Gómez-Serranillos MP, Crespo A.. 2016. Antioxidant potential of lichen species and their secondary metabolites. A systematic review. Pharm Biol. 54:1–17. [DOI] [PubMed] [Google Scholar]
- Forslund T, Fyhrquist F, Frøseth B, Tikkanen I.. 1989. . Effects of licorice on plasma atrial natriuretic peptide in healthy volunteers. J Intern Med. 225:95–99. [DOI] [PubMed] [Google Scholar]
- Fu Y, Chen J, Li YJ, Zheng YF, Li P.. 2013. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 141:1063–1071. [DOI] [PubMed] [Google Scholar]
- Fu Y, Zhou E, Wei Z, Liang D, Wang W, Wang T, Guo M, Zhang N, Yang Z.. 2014. . Glycyrrhizin inhibits the inflammatory response in mouse mammary epithelial cells and a mouse mastitis model. FEBS J. 281:2543–2557. [DOI] [PubMed] [Google Scholar]
- Fullerton JN, Gilroy DW.. 2016. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 15:551–567. [DOI] [PubMed] [Google Scholar]
- Funakoshi-Tago M, Nakamura K, Tsuruya R, Hatanaka M, Mashino T, Sonoda Y, Kasahara T.. 2010. . The fixed structure of licochalcone A by alpha, beta-unsaturated ketone is necessary for anti-inflammatory activity through the inhibition of NF-kappaB activation. Int Immunopharmacol. 10:562–571. [DOI] [PubMed] [Google Scholar]
- Funakoshi-Tago M, Tanabe S, Tago K, Itoh H, Mashino T, Sonoda Y, Kasahara T.. 2009. . Licochalcone A potently inhibits tumor necrosis factor alpha-induced nuclear factor-kappaB activation through the direct inhibition of IkappaB kinase complex activation . Mol Pharmacol. 76:745–753. [DOI] [PubMed] [Google Scholar]
- Funk CD. 2001. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 294:1871–1875. [DOI] [PubMed] [Google Scholar]
- Furusawa J, Funakoshi-Tago M, Mashino T, Tago K, Inoue H, Sonoda Y, Kasahara T.. 2009. Glycyrrhiza inflata-derived chalcones, licochalcone A, licochalcone B and licochalcone D, inhibit phosphorylation of NF-κB p65 in LPS signaling pathway. Int Immuno Pharmacol. 9:499–507. [DOI] [PubMed] [Google Scholar]
- Furusawa J, Funakoshi-Tago M, Tago K, Mashino T, Inoue H, Sonoda Y, Kasahara T.. 2009. . Licochalcone A significantly suppresses LPS signaling pathway through the inhibition of NF-kappaB p65 phosphorylation at serine 276 . Cell Signal. 21:778–785. [DOI] [PubMed] [Google Scholar]
- Guo J, Shang E, Zhao J, Fan X, Duan J, Qian D, Tao W, Tang Y.. 2014. Data mining and frequency analysis for licorice as a “on Chinese Formulae Database” herb in Chinese Formulae based on Chinese Formulae. Phytomedicine. 21:1281–1286. [DOI] [PubMed] [Google Scholar]
- Haraguchi H, Ishikawa H, Mizutani K, Tamura Y, Kinoshita T.. 1998. Antioxidative and superoxide scavenging activities of retrochalcones in Glycyrrhiza inflata . Bioorg Med Chem. 6:339–347. [DOI] [PubMed] [Google Scholar]
- Harahap IS, Sasaki N, Gunadi Yusoff S, Lee MJ, Morikawa S, Nishimura N, Sasaki T, Usuki S, Hirai M, et al. 2011. Herbal medicine containing licorice may be contraindicated for a patient with an HSD11B2 mutation. Evid Based Complement Alternat Med. doi: 10.1093/ecam/nep211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth C, Brennan FM, Chantry D, Turner M, Maini RN, Feldmann M.. 1991. . Expression of granulocyte-macrophage colony-stimulating factor in rheumatoid arthritis: regulation by tumor necrosis factor-alpha. Eur J Immunol. 21:2575–2579. [DOI] [PubMed] [Google Scholar]
- Hong YK, Wu HT, Ma T, Liu WJ, He XJ.. 2009. Effects of Glycyrrhiza glabra polysaccharides on immune and antioxidant activities in high-fat mice. Int J Biol Macromol. 45:61–64. [DOI] [PubMed] [Google Scholar]
- Honda H, Nagai Y, Matsunaga T, Okamoto N, Watanabe Y, Tsuneyama K, Hayashi H, Fujii I, Ikutani M, Hirai Y, et al. 2014. Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. J Leukoc Biol. 96:1087–1100. [DOI] [PubMed] [Google Scholar]
- Huang X, Qin J, Lu S.. 2014. Magnesium isoglycyrrhizinate protects hepatic L02 cells from ischemia/reperfusion induced injury. Int J Clin Exp Pathol. 7:4755–4764. [PMC free article] [PubMed] [Google Scholar]
- Huo HZ, Wang B, Liang YK, Bao YY, Gu Y.. 2011. Hepatoprotective and antioxidant effects of licorice extract against CCl4-induced oxidative damage in rats. Int J Mol Sci. 12:6529–6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Miki I, Tanahashi T, Yagi S, Kondo Y, Inoue J, Kawauchi S, Nishiumia S, Yoshida M, Maeda H, et al. 2013. . Effect of 18β-glycyrrhetinic acid and hydroxypropyl γcyclodextrin complex on indomethacin-induced small intestinal injury in mice . Eur J Pharmacol. 714:125–131. [DOI] [PubMed] [Google Scholar]
- Jayaraman A, Lent-Schochet D, Pike CJ.. 2014. Diet-induced obesity and low testosterone increase neuroinflammation and impair neural function. J Neuroinflammation. 11:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SJ, Lim HS, Seo CS, Kim JH, Jin SE, Yoo SR, Shin HK.. 2015. Traditional herbal formula Jakyakgamcho-tang (Paeonia lactiflora and Glycyrrhiza uralensis) impairs inflammatory chemokine production by inhibiting activation of STAT1 and NF-κB in HaCa T cells. Phytomedicine. 22:326–332. [DOI] [PubMed] [Google Scholar]
- Kageyama K, Takayasu S, Moriyama T, Sakihara S, Suda T.. 2004. A case of pseudoaldosteronism, accompanied with hypocalcemia and exaggerated ACTH response. Endocr J. 51:83–87. [DOI] [PubMed] [Google Scholar]
- Kang SW, Choi JS, Choi YJ, Bae JY, Li J, Kim DS, Kim JL, Shin SY, Lee YJ, Kwun IS, et al. 2010. Licorice isoliquiritigenin dampens angiogenic activity via inhibition of MAPK-responsive signaling pathways leading to induction of matrix metalloproteinases. J Nutr Biochem. 21:55–65. [DOI] [PubMed] [Google Scholar]
- Karthikeyan C, Moorthy NS, Ramasamy S, Vanam U, Manivannan E, Karunagaran D, Trivedi P.. 2015. Advances in chalcones with anticancer activities. Recent Pat Anticancer Drug Discov. 10:97–115. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lim SS, Park IS, Lim JS, Seo JY, Kim JS.. 2012a. . Neuroprotective effects of dehydroglyasperin C through activation of heme oxygenase-1 in mouse hippocampal cells . J Agric Food Chem. 60:5583–5589. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Seo JY, Suh HJ, Lim SS, Kim JS.. 2012b. Antioxidant activities of licorice-derived prenylflavonoids. Nutr Res Pract. 6:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim J, Shim J, Lee S, Kim J, Lim SS, Lee KW, Lee HJ.. 2013. Licorice-derived dehydroglyasperin C increases MKP-1 expression and suppresses inflammation-mediated neurodegeneration. Neurochem Int. 63:732–740. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Jun JG.. 2013. First total synthesis of highly anti-inflammatory active licochalcone D through water-accelerated [3, 3]-sigmatropicre arrangement. Bull Korean Chem Soc. 34:54–58. [Google Scholar]
- Kim SW, Jin Y, Shin JH, Kim ID, Lee HK, Park S, Han PL, Lee JK.. 2012. Glycyrrhizic acid affords robust neuroprotection in the postischemic brain via anti-inflammatory effect by inhibiting HMGB1 phosphorylation and secretion . Neurobiol Dis. 46:147–156. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Kim HH, Ryu YB, Kim JH, Jeong HJ, Lee SW, Chang JS, Cho KO, Rho MC, Park SJ, et al. 2010. In vitro anti-rotavirus activity of polyphenol compounds isolated from the roots of Glycyrrhiza uralensis. Bioorg Med Chem. 18:7668–7674. [DOI] [PubMed] [Google Scholar]
- Kwon HS, Oh SM, Kim JK.. 2008. . Glabridin, a functional compound of liquorice, attenuates colonic inflammation in mice with dextran sulphate sodium-induced colitis. Clin Exp Immunol. 151:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La VD, Tanabe S, Bergeron C, Gafner S, Grenier D.. 2011. Modulation of matrix metalloproteinase and cytokine production by licorice isolates licoricidin and licorisoflavan A: potential therapeutic approach for periodontitis. J Periodontol. 82:122–128. [DOI] [PubMed] [Google Scholar]
- Lee HN, Cho HJ, Lim DY, Kang YH, Lee KW, Park JHY.. 2013. Mechanisms by which licochalcone E exhibits potent anti-inflammatory properties: studies with phorbol ester-treated mouse skin and lipopolysaccharide-stimulated murine macrophages. Int J Mol Sci. 14:10926–10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lee YS, Choi JS, Sung HY, Kim JK, Lim SS, Kang YH.. 2010. Roasted licorice extracts dampen high glucose-induced mesangial hyperplasia and matrix deposition through blocking Akt activation and TGF-beta signaling. Phytomedicine. 17:800–810. [DOI] [PubMed] [Google Scholar]
- Li S, Zhu JH, Cao LP, Sun Q, Liu HD, Li WD, Li JS, Hang CH.. 2014. Growth inhibitory in vitro effects of glycyrrhizic acid in U251 glioblastoma cell line. Neurol Sci. 35:1115–1120. [DOI] [PubMed] [Google Scholar]
- Li XB, He XJ, Liu B, Xu L, Lu C, Zhao HY, Niu XY, Chen SL, Lu AP.. 2012. Maturation of murine bone marrow-derived dendritic cells induced by Radix Glycyrrhizae polysaccharide. Molecules. 17:6557–6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Chen J, Li Y, Li Q, Zheng YF, Fu Y, Li P.. 2011. Screening and characterization of natural antioxidants in four Glycyrrhiza species by liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J Chromatogr A. 1218:8181–8191. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang J, Zhou W, Wang Y, Yang L.. 2013. Systems approaches and polypharmacology for drug discovery from herbal medicines: an example using licorice. J Ethnopharmacol. 146:773–793. [DOI] [PubMed] [Google Scholar]
- Liu HL, Peng NH, Chen LW, Shu CC, Hwu YJ.. 2011. Effectiveness of licorice on aphthous ulcers in children: a systematic review. JBI Database Syst Rev Implement Rep. 9:S198–S214. [PubMed] [Google Scholar]
- Liu J, Peter K, Shi D, Zhang L, Dong G, Zhang D, Breiteneder H, Bauer R, Jakowitsch J, Ma Y.. 2014. Anti-inflammatory effects of the Chinese herbal formula sini tang in myocardial infarction rats. Evid Based Complement Alternat Med. 2014:309378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long DR, Mead J, Hendricks JM, Hardy ME, Voyich JM.. 2013. 18β-Glycyrrhetinic acid inhibits methicillin-resistant Staphylococcus aureus survival and attenuates virulence gene expression . Antimicrob Agents Chemother.57:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Jin Y, Kim ID, Lee JK.. 2013. Glycyrrhizin attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. Exp Neurobiol. 22:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Lian QQ, Dong Q, Ge RS.. 2011. . Environmental inhibitors of 11β-hydroxysteroid dehydrogenase type 2. Toxicology. 285:83–89. [DOI] [PubMed] [Google Scholar]
- Mae T, Kishida H, Nishiyama T, Tsukagawa M, Konishi E, Kuroda M, Mimaki Y, Sashida Y, Takahashi K, Kawada T, et al. 2003. A licorice ethanolic extract with peroxisome proliferators activated receptor gamma ligand binding activity affects diabetes in KKAy mice, abdominal obesity in diet induced obese C57BL mice and hypertension in spontaneously hypertensive rats. J Nutr. 133:3369–3377. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K, Murata M, Yoshida K, Sekimoto G, Uemura Y, Sakaida N, Kaibori M, Kamiyama Y, Nishizawa M, Fujisawa J, et al. 2007. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology. 46:48–57. [DOI] [PubMed] [Google Scholar]
- Michel HE, Tadros MG, Abdel-Naim AB, Khalifa AE.. 2013. Prepulse inhibition (PPI) disrupting effects of Glycyrrhiza glabra extract in mice: a possible role of monoamines. Neurosci Lett. 544:110–114. [DOI] [PubMed] [Google Scholar]
- Nagy G, Clark JM, Buzas E, Gorman C, Pasztoi M, Koncz A, Falus A, Cope AP.. 2008. . Nitric oxide production of T lymphocytes is increased in rheumatoid arthritis. Immunol Lett. 118:55–58. [DOI] [PubMed] [Google Scholar]
- Ni YF, Kuai JK, Lu ZF, Yang GD, Fu HY, Wang J, Tian F, Yan XL, Zhao YC, Wang YJ, et al. 2011. Glycyrrhizin treatment is associated with attenuation of lipopolysaccharide-induced acute lung injury by inhibiting cyclooxygenase-2 and inducible nitric oxide synthase expression. J Surg Res. 165:e29–e35. [DOI] [PubMed] [Google Scholar]
- Oztanir MN, Ciftci O, Cetin A, Durak MA, Basak N, Akyuva Y.. 2014. The beneficial effects of 18β-glycyrrhetinic acid following oxidative and neuronal damage in brain tissue caused by global cerebral ischemia/reperfusion in a C57BL/J6 mouse model. Neurol Sci. 35:1221–1228. [DOI] [PubMed] [Google Scholar]
- Parikh A, Scadding GK.. 2014. Topical nasal lysine aspirin in aspirin-sensitive and aspirin-tolerant chronic rhinosinusitis with nasal polyposis. Expert Rev Clin Immunol. 10:657–665. [DOI] [PubMed] [Google Scholar]
- Poli G. 2000. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 21:49–98. [DOI] [PubMed] [Google Scholar]
- Raikkonen K, Seckl JR, Heinonen K, Pyhala R, Feldt K, Jones A, Pesonen AK, Phillips DI, Lahti J, Javenpaa AL, et al. 2010. Maternal prenatal licorice consumption alters hypothalamic-pituitary-adrenocortical axis function in children. Psychoneuroendocrinology. 35:1587–1593. [DOI] [PubMed] [Google Scholar]
- Ruiz-Granados ES, Shouls G, Sainsbury C, Antonios T.. 2012. A salty cause of severe hypertension. BMJ Case Rep. doi: 10.1136/bcr.12.2011.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadnejad AZ, Mehrvarz S, Naeini SA, Tanideh N.. 2012. Healing effect of licorice extract in acetic acid-induced ulcerative colitis in rat. Res PharmSci. 7:S837–S845. [Google Scholar]
- Seki H, Sawai S, Ohyama K, Mizutani M, Ohnishi T, Sudo H, Fukushima EO, Akashi T, Aoki T, Saito K, et al. 2011. Triterpene functional genomics in licorice for identification of CYP72A154 involved in the biosynthesis of glycyrrhizin. Plant Cell Online. 23:4112–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifzadeh M, Shamsa F, Shiran S, Karimfar MH, Miri AH, Jalalizadeh H, Gholizadeh S, Salar F, Tabrizian K.. 2008. A time course analysis of systemic administration of aqueous licorice extract on spatial memory retention in rats. Planta Med. 74:485–490. [DOI] [PubMed] [Google Scholar]
- Sorsa T, Tjäderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, Golub LM, Brown DL, Mäntylä P.. 2006. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 38:306–321. [DOI] [PubMed] [Google Scholar]
- Sostres C, Gargallo CJ, Arroyo MT, Lanas A.. 2010. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, and coxibs) on upper gastrointestinal tract. Best Pract Res Cl Ga. 24:121–132. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Tetsuka T, Yoshida S, Watanabe N, Kobayashi M, Matsui N, Okamoto T.. 2000. . The role of p38 mitogen-activated protein kinase in IL-6 and IL-8 production from the TNF-alpha- or IL-1beta-stimulated rheumatoid synovial fibroblasts. FEBS Lett. 465:23–27. [DOI] [PubMed] [Google Scholar]
- Takhshid MA, Mehrabani D, Ai J.. 2012. The healing effect of licorice extract in acetic acid-induced ulcerative colitis in rat model. Comp Clin Pathol. 21:1139–1144. [Google Scholar]
- Tanifuji S, Aizu-Yokota E, Funakoshi-Tago M, Sonoda Y, Inoue H, Kasahara T.. 2010. Licochalcones suppress degranulation by decreasing the intracellular Ca2+ level and tyrosine phosphorylation of ERK in RBL-2H3 cells. Int Immunopharmacol. 10:769–776. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan P, Chandrasekaran CV, Deepak HB, Agarwal A.. 2011. Modulation of lipopolysaccharide-induced pro-inflammatory mediators by an extract of Glycyrrhiza glabra and its phytoconstituents. Inflammopharmacology. 19:235–241. [DOI] [PubMed] [Google Scholar]
- Wananukul S, Chatproedprai S, Chunharas A, Limpongsanuruk W, Singalavanija S, Nitiyarom R, Wisuthsarewong W.. 2013. Randomized, double-blind, split-side, comparison study of moisturizer containing licochalcone A and 1% hydrocortisone in the treatment of childhood atopic dermatitis. J Med Assoc Thailand. 96:1135–1142. [PubMed] [Google Scholar]
- Wang CY, Kao TC, Lo WH, Yen GC.. 2011. Glycyrrhizic acid and 18β-glycyrrhetinic acid modulate lipopolysaccharide-induced inflammatory response by suppression of NF-κB through PI3K p110δ and p110γ inhibitions. J Agr Food Chem. 59:7726–7733. [DOI] [PubMed] [Google Scholar]
- Wang KL, Hsia SM, Chan CJ, Chang FY, Huang CY, Bau DT, Wang PS.. 2013. Inhibitory effects of isoliquiritigenin on the migration and invasion of human breast cancer cells. Expert Opin Ther Targets. 17:337–349. [DOI] [PubMed] [Google Scholar]
- Wang W, Tian DD, Zheng B, Wang D, Tan QR, Wang CY, Zhang ZJ.. 2015. Peony-Glycyrrhiza decoction, an herbal preparation, inhibits clozapine metabolism via cytochrome P450s, but not flavin-containing monooxygenase in vitro models. Drug Metab Dispos. 43:1147–1153. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cao Y, Paudel S, Yoon G, Cheon SH.. 2013. Concise synthesis of licochalcone C and its regioisomer, licochalcone H. Arch Pharm Res. 36:1432–1436. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Nixon DW.. 2001. Licorice and cancer. Nutr Cancer. 39:1–11. [DOI] [PubMed] [Google Scholar]
- Whorwood CB, Sheppard MC, Stewart PM.. 1993. Licorice inhibits 11 beta-hydroxysteroid dehydrogenase messenger ribonucleic acid levels and potentiates glucocorticoid hormone action. Endocrinology. 132:2287–2292. [DOI] [PubMed] [Google Scholar]
- Wu TY, Khor TO, Saw CL, Loh SC, Chen AI, Lim SS, Park JH, Cai L, Kong AN.. 2011. Anti-inflammatory/anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensis. AAPS J. 13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao ZW, Zhang W, Ma L, Qiu ZW.. 2014. Therapeutic effect of magnesium isoglycyrrhizinate in rats on lung injury induced by paraquat poisoning. Eur Rev Med Pharmacol Sci. 18:311–320. [PubMed] [Google Scholar]
- Xie C, Li X, Wu J, Liang Z, Deng F, Xie W, Zhu M, Zhu J, Zhu W, Geng S, et al. 2015. Anti-inflammatory activity of magnesium isoglycyrrhizinate through inhibition of phospholipase A2/Arachidonic acid pathway. Inflammation. 38:1639–1648. [DOI] [PubMed] [Google Scholar]
- Xu R, Xiao Q, Cao Y, Yang J.. 2013. Comparison of the exposure of glycyrrhizin and its metabolites and the pseudoaldosteronism after intravenous administration of alpha- and beta-glycyrrhizin in rat. Drug Res (Stuttg). 63:620–624. [DOI] [PubMed] [Google Scholar]
- Yang CLH, Or TCT, Ho MHK, Lau ASY.. 2013. Scientific basis of botanical medicine as alternative remedies for rheumatoid arthritis. Clin Rev Allerg Immu. 44:284–300. [DOI] [PubMed] [Google Scholar]
- Yang H, Ko HJ, Yang JY, Kim JJ, Seo SU, Park SG, Choi SS, Seong JK, Kweon MN.. 2013. Interleukin-1 promotes coagulation, which is necessary for protective immunity in the lung against Streptococcus pneumoniae infection. J Infect Dis. 207:50–60. [DOI] [PubMed] [Google Scholar]
- Yehuda I, Madar Z, Leikin-Frenkel A, Tamir S.. 2015. Glabridin, an isoflavan from licorice root, downregulates iNOS expression and activity under high-glucose stress and inflammation. Mol Nutr Food Res. 59:1041–1052. [DOI] [PubMed] [Google Scholar]
- Zeng CX, Yang Q, Hu Q.. 2006. A comparison of the distribution of two glycyrrhizic acid epimers in rat tissues. Eur J Drug Metab Pharmacokinet. 31:253–258. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Wang W, Guo H, Zhou D.. 2008. Antidepressant-like effect of liquiritin from Glycyrrhiza uralensis in chronic variable stress induced depression model rats. Behav Brain Res. 194:108–113. [DOI] [PubMed] [Google Scholar]
- Zhu ZH, Tao WW, Li JP, Guo S, Qian DW, Shang EX, Su SL, Duan JA.. 2016. Rapid determination of flavonoids in licorice and comparison of three licorice species. J Sep Sci. 39:473–482. [DOI] [PubMed] [Google Scholar]