Abstract
Context: Traditionally, Rhododendron arboreum Sm. (Ericaceae) is a very important medicinal plant having oxytocic, estrogenic, anti-inflammatory, analgesic and hepatoprotective activities; it also inhibits the prostaglandin synthetase.
Objectives: This study determines the cytotoxic potential of 15-oxoursolic acid isolated from R. arboreum against selected human cancer cell lines.
Materials and methods: Extraction from stem bark (5 kg) of R. arboreum was performed with methanol, which was successively partitioned into hexane, dichloromethane and ethyl acetate fractions, respectively. The new antitumor agent [15-oxoursolic acid (1)] was isolated from ethyl acetate fraction through column chromatography. Structure elucidation of new compound was performed through extensive spectroscopy i.e., IR, MS and 1D and 2D NMR. Cytotoxicity of isolated compound was determined at doses 5–100 μM for a period of 72 h on specified human cancer cell lines [renal cell carcinoma (A498), non-small cell lung (NCI-H226), squamous cell carcinoma (H157) and human ovarian carcinoma (MDR-2780AD)].
Results: Structure of isolated compound was characterized as 15-oxoursolic acid on the basis of various extensive spectroscopic techniques. 15-Oxoursolic acid revealed considerable anticancer activity with IC50 values of 2.3 ± 0.1 μM, 4.9 ± 0.2 μM, 9.2 ± 0.2 μM and 10.3 ± 0.1 μM against MDR 2780AD, Hep G2, H157 and NCI-H226, respectively, while in the case of A498, the activity was good (IC50 32.8 ± 1.2 μM).
Conclusions: This study highlighted the potential of 15-oxoursolic acid to be further explored as a new lead compound for cancer chemotherapy.
Keywords: spectroscopic techniques, anticancer, 2D NMR, H157
Introduction
Curing and prevention of diseases is a growing issue which needs greater consideration. Plants produce a number of phytopharmaceuticals that provide health benefits including prevention and treatment of diseases. Triterpenoids are a very important class of biologically active organic compounds. A number of biological activities have been reported about these triterpenes. Among them anti-HIV (Mayaux et al. 1994; Kashiwada et al. 2000; Zhu et al. 2001), HIV protease inhibition (Ma et al. 1999), antibacterial (Wolska et al. 2010) and anticancer activities against different cancer cell lines (Lee et al. 1988; Lin et al. 1990; Liu 1995) are the most important. Triterpenes have been identified as a potent and selective growth inhibitor of human melanoma (Pisha et al.1995; Zuco et al. 2002), neuroectodermal (Fulda and Debatin 2000) and malignant tumour cell having the basic mechanism of inducing apoptosis in these cells (Yogeeswari and Sriram 2005). Some of the triterpenes have been reported to possess inhibitory potential against nitric oxide production. This ability of triterpenes suggest they are good chemopreventive drugs for cancer, as over production of nitric oxide (NO) is mechanistically the root cause for carcinogenesis which can destroy normal functional tissues (Finlay et al. 1997; Honda et al. 1997, 2000a, 2000b).
In the search for a new cytotoxic drug, 15-oxoursolic acid (1), a new pentacyclic triterpene, was isolated from the stem bark of Rhododendron arboreum Sm. (Ericaceae) and was screened for cytotoxic potential against five human cancer lines, i.e., squamous cell carcinoma (H157), renal cell carcinoma (A498), human hepatocellular carcinoma (Hep G2), non-small cell lung (NCI-H226) and human ovarian carcinoma (MDR 2780AD).
Materials and methods
General experimental procedures
Optical rotation was recorded on JASCO DIP-360 digital polarimeter. Melting points were determined in glass capillaries using Buchi 535 melting point apparatus (BÜCHI, Essen, Germany). Mass spectrometery was performed using various ionization techniques including electron impact mass spectrum (EIMS), fast atom bombardment positive/negative spectrum (FAB + ve/FAB-ve), high-resolution fast atom bombardment mass spectrum (HRFABMS) and GCMS spectrum measurements were performed on Varian Mat 312 and Jeol JMS-600H with GC and Jeol JMS HX 110 mass spectrometer (Joel Inc., Tokyo, Japan). UV–visible spectra were recorded on a Perkin Elmer λ-EZ 201 spectrophotometer (Perkin Elmer Inc., Tempe, AZ). IR spectra were recorded in KBr disk on a Jasco 320-A spectrophotometer (Jasco, Jackson, MS). The 1H NMR (500 MHz, CDCl3), 13 C NMR (125 MHz, CDCl3) and related experiments were conducted on Bruker instrument (Bruker Corp., Billerica, MA), chemical shift value was presented in δ (ppm) and coupling constant (f) in Hz.
Collection of plant material
Rhododendron arboreum bark was collected from Seran valley Khyber Pakhtoonkhwa, Pakistan, in February 2011 and was authenticated by Dr. Abdul Rasheed, Department of Botany, University of Peshawar. A specimen was deposited in the herbarium under voucher number 7212/Bot.
Extraction and isolation
The air-dried bark (5 kg) of R. arboreum was repeatedly percolated with methanol (3 × 10 L) at room temperature. The methanol extract was filtered and concentrated by rotary evaporator under vacuum at 40 °C, to obtain a brownish crude methanol extract (650 g, 13.0% w/w). The methanol extract (650 g) was redissolved in distilled water and was successively extracted with hexane (9.5% w/w), chloroform (17.46% w/w), ethyl acetate (36.5% w/w), butanol (17.46% w/w) and finally with water (22.22% w/w) in order of increasing polarity to afford the respective fractions. The ethyl acetate fraction (20 g) was subjected to column chromatography using silica gel column (800 g, 200–300 mesh). The column was eluted with n-hexane:ethyl acetate with increasing polarity and finally with methanol:chloroform which yielded a total of 20 sub-fractions EA-EU. Sub-fraction EN (403 mg) was subjected to flash column chromatography (silica gel, 5 g) and was eluted with n-hexane:chloroform and finally with methanol: chloroform; (1:99), which resulted in the isolation of compound 1 (15 mg).
Physical data of compound 1
White amorphous powder with molecular formula C30H46O4 (15 mg), Rf value = 0.35 (methanol:chloroform; 2:8), m.p. = 288–290 °C, UV (λmax) 280 nm. IR (KBr) λmax cm−1: 3430, 1740, 1720 and 1650 cm−1. HREIMS m/z 470.0320 (calculated 470.3396 for C30H46O4), for 1H and 13 C NMR (Table 1).
Table 1.
1H and 13C NMR of 15-oxoursolic acid (1) [500 MHz (1H NMR) and 125 MHz (13C NMR), CDCl3, δ in ppm, J in Hz].
| C. No. | δC | δH/ppm (multi, integral, J/Hz) | HMBC |
|---|---|---|---|
| 1 | 38.61 | 1.37, 1.62 m | C3 |
| 2 | 28.01 | 1.84, 0.92 m | |
| 3 | 79.04 | 3.2 dd (5.0, 10.0) | C2, C24 |
| 4 | 38.75 | – | |
| 5 | 55.22 | 0.77 d (10.0) | |
| 6 | 18.2 | 1.35.1.53 m | |
| 7 | 30.6 | 1.65,1.71 m | |
| 8 | 39.5 | – | |
| 9 | 47.5 | 1.49m | |
| 10 | 37.1 | – | |
| 11 | 23.3 | 0.93 t (10), 1.91 dd (5,10) | C12, C13 |
| 12 | 125.9 | 5.24 s | C10, C11, C14 |
| 13 | 137.9 | – | |
| 14 | 42.0 | – | |
| 15 | 212.1 | – | |
| 16 | 36.7 | 1.65 m, 2.15 s | C15, C28 |
| 17 | 47.9 | – | |
| 18 | 52.7 | 2.18 d (15.0) | C13, C13, C28 |
| 19 | 38.8 | 0.84 d (5) | |
| 20 | 39.0 | 1.63 m | |
| 21 | 24.2 | 1.64, 1.89 m | |
| 22 | 32.4 | 1.36, 1.46 m | |
| 23 | 28.1 | 1.06 s | |
| 24 | 15.6 | 0.72 s | C3, C4, C5 |
| 25 | 15.4 | 0.87 s | |
| 26 | 17.1 | 0.80 s | |
| 27 | 23.5 | 1.06 s | C8, C14 |
| 28 | 181.4 | – | |
| 29 | 17.0 | 0.77 d (5) | |
| 30 | 21.1 | 0.84 d (10) |
In vitro cytotoxicity assay
Human cancer cell lines, i.e., HepG2, A498, NCI-H226, H157 and MDR-2780AD were purchased from American Type Culture Collection (ATCC). The cells were grown and maintained in RPMI 1640 medium supplemented with foetal bovine serum at 37 °C, 5% CO2 and 90% humidity.
The assay was performed in 96-well microtitre plates. The cells were seeded separately to 96-wells plates at a concentration of 1 × 105 cells/well. These cells were then treated with different concentrations of compound 1 (5–100 μM) with three replicates of each concentration. The control contained only the cells without test sample. The culture plates were then incubated at 37 °C for 72 h in a humidified incubator. After 72 h of incubation, the fraction of surviving cells was measured relative to the cell population in the control by colorimetric MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay (Mossmann 1983). A volume of 20 μL of MTT (5 mg/mL) in phosphate buffer solution was added to each well and incubated for 3–4 h. After this incubation, 100 μL of DMSO was added to dissolve the resulting MTT formazan crystals by pipetting up and down 10–20 times. The plates were left at room temperature for 15–30 min then the optical density (OD) was measured on an ELISA microplate reader at 570 nm. The percentage of cell viability was calculated using the following equation:
A plot of cell viability against the concentration of the sample gave a measure of cytotoxicity. The IC50 was then calculated from the plot.
Results and discussion
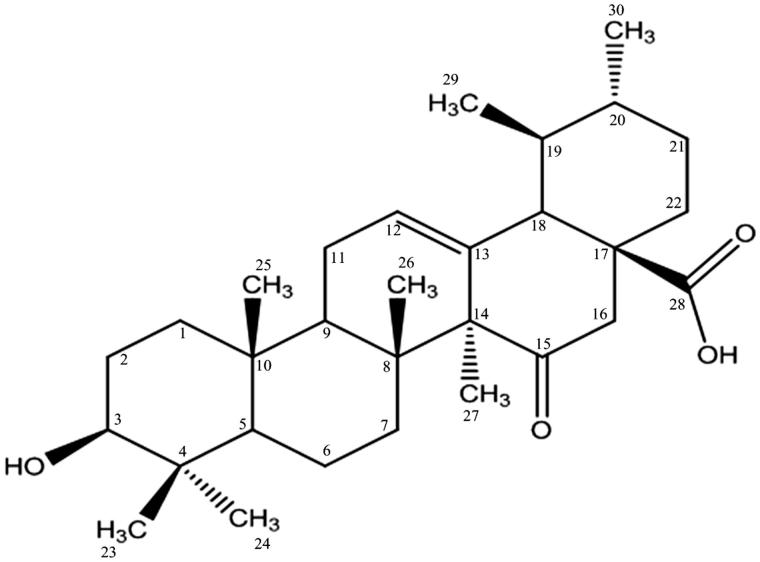
Compound 1 (15-oxoursolic acid) was obtained as white amorphous powder from the ethyl acetate fraction of bark. The structure of the title compound was elucidated by developing molecular formula C30H46O4 via high-resolution electron impact mass spectrometry, which showed molecular ion peak at m/z 470.0320 [M + H+] (calculated 470.3396). The IR spectrum showed strong absorption bands at 3430 cm−1, 1740 cm−1 and 1720 cm−1 indicating the presence of free hydroxyl, carboxyl and ketonic functionalities respectively, whereas three absorptions at 2998, 1650 and 815 cm−1 indicated the presence of trisubstituted double bond (Figure 1).
Figure 1.
Structure of compound 1.
The 1H NMR spectra (CDCl3, 500 MHz) of compound 1 distinctly showed the presence of five singlet resonating at δ 1.06, δ 0.72, δ 0.87, δ 0.80 and δ 1.06 which were assigned to C-23, C-24, C-25, C-26 and C-27 methyl protons, respectively. Two doublets resonating at δ 0.77 (d, J = 5 Hz) and δ 0.84 (d, J = 10 Hz) were assigned to C-29 and C-30 methyl protons which were indicative of ursane skeleton. A downfield doublet resonating at 3.2 (dd, J = 5.0 Hz, J = 10.0 Hz) was assigned to the H-3 methine proton indicating the presence of a geminal hydroxyl group. The chemical shift and the coupling constant of this proton suggested the configuration of hydroxyl group as β and equatorial at C-3. In addition, a downfield singlet resonating at δ 5.24 was assigned to C-12 olefinic methine proton. The detailed 1H NMR assignment of compound 1 is summarized in Table 1.
The 13C NMR spectra (CDCl3, 125 MHz) broad band decoupled and DEPT of compound 1 revealed the presence of 7 methyl, 8 methylene, 7 methine and 8 quaternary carbon atoms. The 13C chemical shift resonating at δ 212.1 was assigned to C-15 quaternary carbon atom having the ketonic group. Similarly, signal at δ 79.04 was ascribed to the C-3 methine carbon having the hydroxyl group. The resonances at δ 28.1, δ 15.6, δ 15.4, δ 17.1, δ 23.5, δ 17.0 and δ 21.1 were assigned to C-23, C-24, C-25, C-26, C-27, C-29 and C-30 methyl carbons, respectively. In addition, the resonances at δ 125.9 and δ 137.9 were ascribed to the olefinic carbons at C-12 and C-13, respectively. The detailed 13C NMR data of compound 1 are presented in Table 1.
The structure of compound 1 was further confirmed from 2D-NMR techniques (HMBC, COSY and NOESY). The important HMBC, COSY and NOESY interactions of compound 1 are presented in Figures 2 and 3, respectively.
Figure 2.
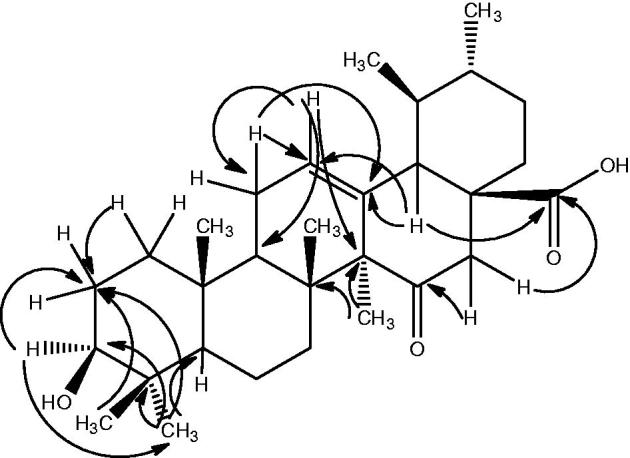
Important HMBC correlation of compound 1.
Figure 3.
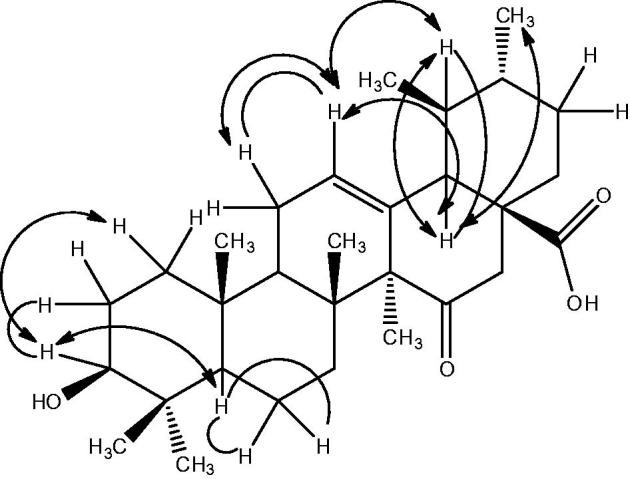
NOESY and COSY correlation.
In the HMBC spectrum, the C-3 methine proton (δ 3.2) showed relation with C-2 (δ 28.01) and C-24 (δ 15.6) methyl carbons. Similarly, the C-23 methyl protons (δ 1.06) exhibited interaction with C-2 (δ 28.01) methylene carbon. Furthermore, C-24 methyl protons (δ 0.72) exhibited interaction with C-4 (δ 38.75), C-3 (δ 79.04) and C-2 (δ 28.01), thus, confirming the position of the hydroxyl group at C-3. In addition, the C-12 olefinic methine proton (δ 3.2) exhibited heteronuclear interaction with C-11 (δ 23.3), C-9 (δ 47.5) and C-14 (δ 42.0). Likewise, C-11 methylene proton resonating at δ 1.91 showed interactions with C-12 (δ 125.9) and C-13 (δ 137.1), thus, confirming the position of double bond. Furthermore, of C-16 methylene proton, the one resonating at δ 2.15 (H-16) showed interaction with C-15 (δ 212.1) ketonic carbon, while the other one resonating at δ 1.65 (H-16) exhibited relationship with C-28 (δ 181.4) carboxylic carbon. Similarly, C-18 methine proton (δ 52.7) exhibited interaction with C-13 (δ 137.9), C-12 (δ 125.9) and C-28 (δ 181.4). In addition, the C-27 methyl protons (δ 1.06) showed interaction with C-14 (δ 42.0) and C-8 (δ 39.5), thus, confirming the position of different functionalities.
The magnitude of the coupling constant of proton geminal to hydroxyl group at C-3 suggested the assignment of β configuration to the hydroxyl group. The stereochemistry of the compound 1 was further confirmed from the NOESY experiment which showed the interaction of C-3 methine proton with C-1 methylene proton and C-5 methine proton. Similarly, the C-12 olefinic methine proton exhibited interaction with C-11 methylene proton, on one hand, and C-19 methine proton on the other hand. In addition, C-18 methine proton showed coupling interaction with C-19 methine proton and C-30 methyl protons. The important NOESY and COSY interactions are presented in Figure 3.
15-Oxoursolic acid 1 was screened against five human cancer cell lines: MDR 2780AD, Hep G2, H157, NCI-H226 and A498 cell lines for analyzing their cytotoxic potential. The results are expressed as the concentration inhibiting 50% of cells growth (IC50) (Table 2). Based on the IC50 values, interestingly, compound (1) exhibited considerable cytotoxic activity against MDR 2780AD, Hep G2, H157 and NCI-H226 cell line with IC50 values being 2.3 ± 0.1 μM, 4.9 ± 0.2 μM, 9.2 ± 0.2 μM and 10.3 ± 0.1 μM, respectively, while the effect was good against A498 cancer cell line with an IC50 value being 32.8 ± 1.2 μM. Taxol was used as a standard drug. The IC50 value of taxol against MDR 2780AD, Hep G2, H157, NCI-H226 and A498 cancer cell lines was 0.19 ± 0.08, 7.4 ± 0.31, 8.4 ± 0.31, 63.01 ± 0.03 and 95.98 ± 0.09 μM, respectively.
Table 2.
Anti-cancer activity of 15-oxoursolic acid (1).
| Cancer cell line | IC50 (μM) |
|---|---|
| Squamous cell carcinoma (H157) | 9.2 ± 0.23 |
| Renal cell carcinoma (A498) | 32.8 ± 1.54 |
| Human hepatocellular carcinoma (Hep G2) | 4.9 ± 0.02 |
| Non-small cell lung (NCI-H226) | 10.3 ± 0.01 |
| Human ovarian carcinoma (MDR 2780AD) | 2.3 ± 0.04 |
SEM: standard error mean of three experiments.
It is evident from the literature that the presence of hydroxyl group at C-3 and a carbonyl group at C-17 in triterpenoid is necessary for the cytotoxicity towards cancer cells. So the presence of both groups on triterpenes was essential for activity. Similarly, the configuration at C-3 was found to be much more important for cytotoxic activity. The compounds with a β-orientated hydrogen-bond forming group manifested significant cytotoxic activity than α-counterpart. The potential mechanisms behind the cytotoxicity of ursolic acid derivative may be either induction of apoptosis in cancer cell or blocking of the cell cycle progression in the G1 phase resulting in the triggering of apoptosis as determined by a DNA fragmentation assay (Hsu et al., 2004).
Conclusions
Phytochemical investigation of the bark of R. arboreum resulted in the isolation of a new potent triterpene. Cytotoxicity assays of the title compound 1 showed interesting results against different human cancer cell lines. Based on the cytotoxicity results, the compound 1 may be used as an important cytotoxic agent for the management of cancer; however, further investigation are needed to ascertain its efficacy, potency and safety for lead compound of long-term clinical utility.
Acknowledgements
The authors would like to thanks the director of H.E.J. Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, Karachi, for providing facilities to carry out this research work.
Disclosure statement
The authors of this article have no declaration of interest.
References
- Finlay HJ, Honda T, Gribble GW, Danielpour D, Benoit NE, Suh N, Williams C, Sporn MB.. 1997. Novel A-ring cleaved analogs of oleanolic and ursolic acids which affect growth regulation in NRP.152 prostate cells. Bioorg Med Chem Lett. 7:1769–1772. [Google Scholar]
- Fulda S, Debatin KM.. 2000. Betulinic acid induces apoptosis through a direct effect on mitochondria in neuroectodermal tumors. Med Pediatr Oncol. 35:616. [DOI] [PubMed] [Google Scholar]
- Hsu YL, Kuo PL, Lin CC.. 2004. Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci. 75:2303–2316. [DOI] [PubMed] [Google Scholar]
- Honda T, Finlay HJ, Gribble GW, Suh N, Sporn MB.. 1997. New enone derivatives of oleanolic acid and ursolic acid as inhibitore of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett. 7:1623–1628. [Google Scholar]
- Honda T, Gribble GW, Suh N, Finlay HJ, Rounds BV, Bore L, Favaloro FG Jr, Wang Y, Sporn MB.. 2000a. Novel synthetic oleanane and ursane triterpenoids with various enone functionalities in ring A as inhibitors of nitric oxide production in mouse macrophages. J Med Chem. 43:1866–1877. [DOI] [PubMed] [Google Scholar]
- Honda T, Rounds BV, Bore L, Finlay HJ, Favaloro FG Jr, Suh N, Wang Y, Sporn MB, Gribble GW.. 2000b. Synthetic oleanane and ursane triterpenoids with modified rings A and C: a series of highly active inhibitors of nitric oxide production in mouse macrophages. J Med Chem. 43:4233–4246. [DOI] [PubMed] [Google Scholar]
- Kashiwada Y, Nagao T, Hashimoto A, Ikeshiro Y, Okabe H, Cosentino LM, Lee KH.. 2000. Anti-AIDS agent 38. Anti-HIV activity of 3-O-acetyl ursolic acid derivatives. J Nat Prod. 63:1619–1622. [DOI] [PubMed] [Google Scholar]
- Lee KH, Lin YM, Wu TS, Zhang DC, Yamagishi T, Hayashi T, Hall IH, Chang JJ, Wu RY, Yang TH.. 1988. The cytotoxic principles of Prunella vulgaris, Psychotria serpens, and Hyptis capitata: ursolic acid and related derivatives. Planta Med. 54:308–311. [DOI] [PubMed] [Google Scholar]
- Lin CN, Lu CM, Cheng MK, Gan KH, Won SJ.. 1990. Alkylated flavanones from the bark of Cryptocarya chartacea as dengue virus NS5 polymerase inhibitors. J Nat Prod. 53:513–516. [DOI] [PubMed] [Google Scholar]
- Liu J. 1995. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 49:57–68. [DOI] [PubMed] [Google Scholar]
- Mayaux JF, Bousseau A, Pauwels R, Huet T, Henin Y, Dereu N, Evers M, Soler F, Poujade C, De Clercq E, et al. . 1994. Triterpene derivatives that block entry of human immuno-deficiency virus type I into cells. Proc Natl Acad Sci USA. 91:3564–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C-M, Nakamura N, Hattori M, Shimotohno K.. 1999. Inhibitory effects of constituents from Cynomorium songaricum and related triterpene derivatives on HIV-1 protease. Chem Pharm Bull. 47:141–145. [DOI] [PubMed] [Google Scholar]
- Mossmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63. [DOI] [PubMed] [Google Scholar]
- Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, Cordell GA, Beecher CW, Fong HH, Kinghorn AD, Brown DM, et al. . 1995. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med. 1:1046–1051. [DOI] [PubMed] [Google Scholar]
- Wolska KI, Grudnaik AM, Fiecek B, Dowjat AK, Kurek A.. 2010. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Cent Eur J Biol. 5:543–553. [Google Scholar]
- Yogeeswari P, Sriram D.. 2005. Betulinic acid and its derivatives: a review on their biological properties. Curr Med Chem. 12:657–666. [DOI] [PubMed] [Google Scholar]
- Zhu Y-M, Shen J-K, Wang H-K, Cosentino L-M, Lee K-H.. 2001. Synthesis and anti-HIV activity of oleanolic acid derivatives. Bioorg Med Chem Lett. 11:3115–3118. [DOI] [PubMed] [Google Scholar]
- Zuco V, Supino R, Righetti SC, Cleris L, Marchesi E, Gambacorti-Passerini C, Formelli F.. 2002. Selective toxicity of betulinic acid on tumor cell lines but not on normal cells. Cancer Lett. 175:17–25. [DOI] [PubMed] [Google Scholar]