ABSTRACT
Inflammatory myofibroblastic tumor (IMT) is a neoplasm of intermediate biological potential. Few cases of spermatic cord IMT have been reported in the literature. Inflammatory myofibroblastic tumor is a consequence of the proliferation of fibroblasts and inflammatory cells. Despite its benign nature, the tumor often clinically mimics intrascrotal malignancy and usually remains undiagnosed preoperatively. The diagnosis of spermatic IMT is difficult preoperatively due to the non-specific findings. Therefore, if testicular tumors cannot be precisely excluded, radical orchiectomy should be performed for the diagnosis and treatment. However, it mainly occurs in children and young adults; spermatic IMT may also be seen among elderly men. Here, we report two cases of inflammatory myofibroblastic tumor involving the spermatic cord.
SIMILAR CASES PUBLISHED:
There are seven cases entitled “inflammatory myofibroblastic tumor of spermatic cord” in the literature. In our study we present two cases that had a spermatic cord IMT. Furthermore, one of these cases was 82 years of age and is the oldest patient presented in the literature.
INTRODUCTION
Mesenchymal neoplasms of the spermatic cord are uncommon tumors, and inflammatory myofibroblastic tumor (IMT) is one of the rarest among them.1,2 IMT is a mass-forming disease that can be observed in any part of the body. It is commonly confused with many mass forming diseases. In most cases it is diagnosed by pathologists after resection of the mass. Only a few cases of IMT have been reported in the spermatic cord.2 Here we report two cases of inflammatory myofibroblastic tumor involving the spermatic cord.
CASE 1
An 82-year-old male presented with a slowly growing painless swelling in the left inguinal region of one-year duration. The patient reported no other complaints such as anorexia, fever or weight loss. Physical examination revealed an approximately 13×8 cm, firm nodular, oval-shaped swelling, which was recumbent in the left inguinal canal, separate from the testis and firm in consistency. The physical examination of the external genitalia including the right testis was normal. There was no inguinal lymphadenopathy. The patient had no known medical problem previously except for hypertension. His personal medical history did not include trauma, recurrent urinary tract infection, and tuberculosis. Abdominal examination was normal. The hematological and biochemical findings were within normal ranges.
Ultrasonography with colour Doppler examination showed a 4.5×6 cm sized heterogeneous and moderately vascular, multilocular-solid mass, with a clear margin from the surrounding structures, which was visualized in the left inguinal region. Contrast-enhanced CT revealed a nodular mass lesion of 5×6 cm in the left inguinal canal adjacent to the left testis in addition to a 7-cm hypodense mass lesion, extending from the lateral towards the inferior of the left femur. MR imaging showed a non-homogeneous mass, situated at the upper part of the left hemiscrotum and causing compression onto the corpus cavernosum. They were hypointense on T1 and hyperintense on T2 compared with the surrounding tissues (Figure 1).
Figure 1.
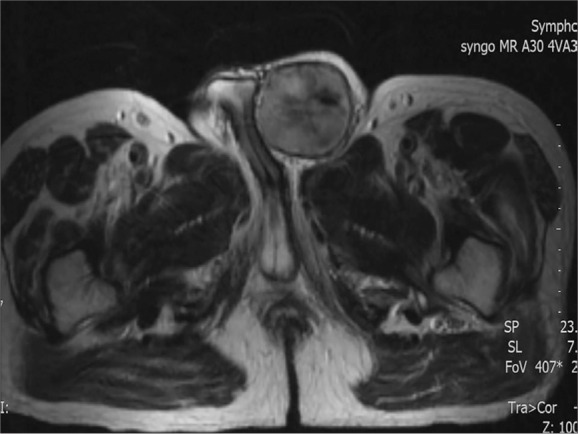
Preoperative MR image of a contrasting mass lesion, situated at the upper part of the left hemiscrotum, causing compression on the corpus cavernosum.
The patient underwent left inguinal exploration after having given informed consent for a left radical orchiectomy. A lobulated tumor originating from the spermatic cord was seen adjoining, but separate from the testis. The mass was removed along with the total spermatic cord and the left testis (Figure 2). On macroscopic evaluation, the tumor tissue was in the form of three nodular, well-circumscribed masses located in the spermatic cord, adjacent to each other and of dimensions 7×6.8×6 cm, 4.7×3.8×3.5 cm and 2×2×1 cm. The cut surface of growth showed a multinodular fibrous tumor, firm to hard, that was gray-white in color. The testis and epididymis were normal.
Figure 2.
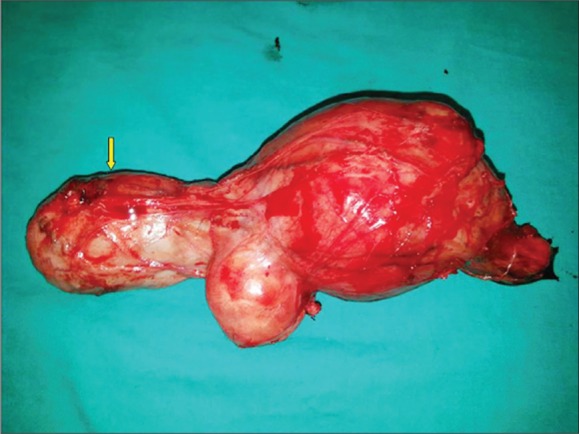
Surgical specimen and unaffected left testis (yellow arrow).
Histologically, the tumoral tissue comprised fusiform fibroblastic/myofibroblastic cells with round/oval normochromic nuclei and narrow eosinophilic cytoplasms with indistinct cell borders and mixed type inflammatory cells comprised of lymphocytes that formed local lymphoid aggregates, plasma cells, eosinophil leukocytes and histiocytes. In the tumor, there were also cells with large vesicular nuclei, distinct nucleolus, large eosinophilic cytoplasm, polygonal-shaped, ganglion-like cells and cells (Figure 3). There was no necrosis. Immunohistochemically, the tumor tissue stained with vimentin. With actin and desmin, the vascular walls and some tumor cells, and with CD68, the histiocytes were stained. With CD34 vascular endothelium and some of ganglion-like cells/Reed Sternberg-like cells stained (Figure 4). There was no staining of tumoral cells with CK (AE1/AE3), CD15, CD30, S-100 and ALK (anaplastic lymphoma kinase). The Ki-67 proliferation index was below 1%. During the follow up, the patient was free of recurrence or metastasis within 46 months.
Figure 3.
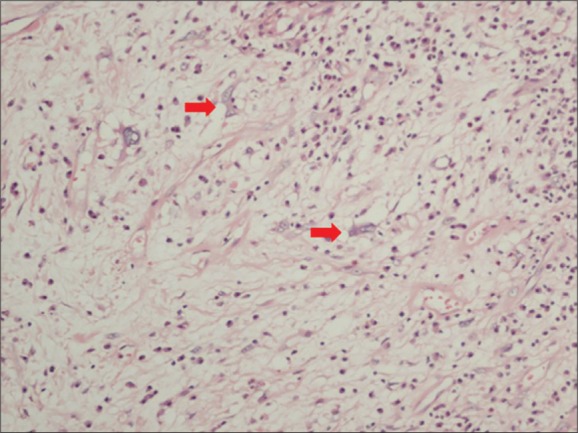
Tumoral tissue cells with large vesicular nuclei, distinct nucleolus, large eosinophilic cytoplasm polygonal-shaped, ganglio-like cells and Reed Sternberg-like cells (red arrow) (H&E ×200).
Figure 4.
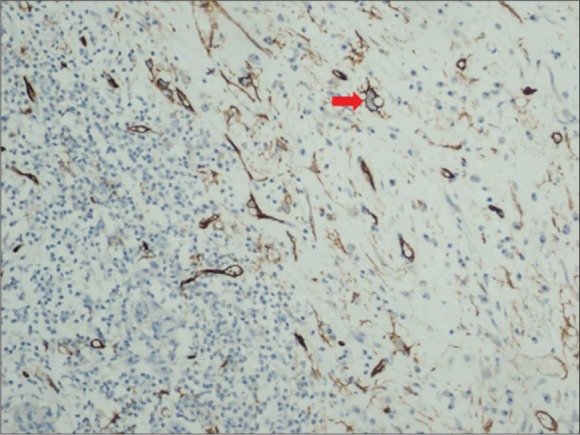
Immunohistochemistry: vascular endothelium and some of ganglion-like cells, Reed Sternberg-like cells immunostained with CD34 (red arrow). (CD34 ×200).
CASE 2
A 56-year-old male patient presented with a painless scrotal mass that had been present for 4 years. His past medical history was unremarkable. Physical examination revealed a firm to hard, irregular, oval-shaped swelling of approximately 3×3 cm dimensions in the right side of the scrotum, separate from the testis. Abdominal and external genitalia examination was normal. The biochemical results were within normal limits. Scrotal ultrasonography revealed a 2.5×2.0 cm ovoid heterogeneous mass separate from testis. The patient underwent right inguinal exploration after having given his informed consent for a right radical orchiectomy. After inguinal exploration, radical orchiectomy was performed.
On the macroscopic evaluation, the tissue mass was capsulated on the external surface, a cream to gray-colored cut surface and firm consistency in the testis that was well-circumscribed and located in the distal spermatic cord, with a congruent border and measuring 3.5×3×2.7 cm in dimensions. The testis and epididymis were normal.
Histologic sections showed fibroblastic/myofibroblastic cell proliferation; the cells proliferated without a clear string pattern, and the tumoral tissue was composed of mixed type inflammatory cells comprising lymphocytes, eosinophil leukocytes and plasma cells (Figure 5). In the tumor, there were also cells with large vesicular nuclei, distinct nucleolus, large eosinophilic cytoplasm, polygonal-shaped, ganglion like cells and Reed Sternberg-like cells. Atypia and mitosis were not observed in the tumoral cells. There was no necrosis. Immunohistochemically, the tumoral cells stained with vimentin and actin, and some of the cells stained with CD34. CK (AE1 / AE3) and desmin were negative. Anaplastic lymphoma kinase (ALK) was negative (Figure 6). Histopathological and immunohistochemical evaluations confirmed the mass as an IMT. During the follow-up, the patient was free of recurrence or metastasis within 36 months.
Figure 5.

Sections indicate tumoral formation consisting of fusiform fibroblastic/myofibroblastic cells with normochromic nucleus and narrow eosinophilic cytoplasm with indistinct cell borders and mixed type inflammatory cells comprising lymphocytes, eosinophil leukocytes and plasma cells (HE ×400).
Figure 6.
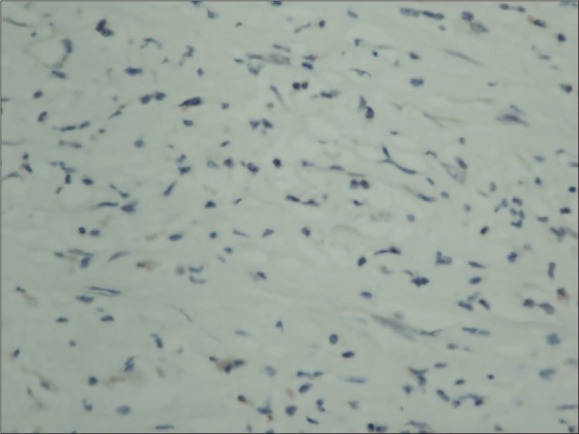
No immunostaining with ALK in tumoral tissue. (ALK×400)
DISCUSSION
IMTs are rare lesions that can be seen in multiple organs. Although their etiology is uncertain, there are some factors attributed to their occurrence such as infection, trauma and radiation.3 They can be seen in any age group.1,4 Very few cases of spermatic cord IMT have been reported.1,5-7 Like our patient, spermatic cord IMT patients usually present with a painless scrotal mass of variable duration.5 The differential diagnosis of paratesticularly located IMT's includes a variety of neo-plastic and inflammatory formations of the testis and the epididymis.8
Diagnosis of IMT with ancillary diagnostic methods is considerably difficult as they mimic malignant tumors in imaging studies. Ultrasonography is the initial imaging modality of choice in a case of scrotal mass.4 MRI and CT imaging helps if ultrasonography is not conclusive.4,9,10 Immunohistochemistry helps exclude similar tumors.4,5 Histologically, IMTs are characterized with a variably cellular spindle cell proliferation in a myxoid to collagenous stroma with a prominent inflammatory infiltrate composed primarily of an inflammatory infiltrate of plasma cells, lymphocytes, and/or eosinophils.11 Approximately 50% of IMT patients have anaplastic lymphoma kinase gene (ALK) rearrangements on chromosome 2p23.11,12 Gene rearrangements are seen more frequently in younger patients and in more aggressive tumors.13 In our two cases, ALK was negative. In our cases, which were suggestive of malignant tumor mitosis, pleomorphism and necrosis was not observed. In addition, CD34 positivity was observed in some of the tumoral cells and was thought to be related to the clone of the antibody used.
The preferred treatment for paratesticular IMT is extensive surgical excision.4 Radical orchiectomy is performed in most of the paratesticular IMTs. Paratesticular IMT has a good prognosis after surgical excision.4,14 Patients should be followed-up after incomplete resection. Complete surgical resection is difficult in multiple intra-abdominal tumors and recurrence is more common in such cases. IMTs are known to be benign tumors with no potential for metastasis. However, these tumors are known to infiltrate the surrounding tissues and to recur frequently due to their severe proliferative capacity, even after resection.15 In a study involving 44 patients Kovach et al demonstrated 8% recurrence rates with the primary surgical approach and proposed surgical resection of all lesions if there are no contraindications related to the anatomy or morbidity.16 Distant metastases appear mainly in ALK-negative IMTs, but local recurrence occurs regardless of ALK expression.15
IMT was diagnosed after the presence of histomorphologic findings and other tumors in the differential diagnosis had been eliminated. Inflammatory malignant fibrous histiocytoma included in the differential diagnosis consists of bizarre pleomorphic cells containing numerous mitotic figures, mixed with xanthomatous and inflammatory cells. Atypia, mitosis and pleomorphism were not observed in our cases. There were also no xanthomatous cells.
When compared to inflammatory leiomyosarcoma, IMT enables a differential diagnosis due to its composition of elongated fusiform cells with eosinophilic cytoplasm and focal fascicular growth pattern. However, in leiomyosarcoma, the cell nuclei are cigarette-shaped and demonstrate a more regular fascicular growth pattern.
IgG4-related disease (IgG4-RD), another pathology involved in the differential diagnosis, is a systemic disease known to respond well to steroids, with elevated serum IgG4 levels and typical histopathological findings in two thirds of the cases.17 Its distinctive feature is the presence of tumor-like growths in the involved organs, consisting of lymphoplasmacytic infiltration rich in IgG4-positive plasma cells, obliterative phlebitis, mild-to-moderate eosinophilic infiltration, and variable amounts of fibrosis forming a characteristic storiform pattern. In our cases, there was fibroblastic/myofibroblastic proliferation that did not produce a histologically significant pattern, and obliterative phlebitis was absent. In addition, the serum IgG4 levels were normal in both patients.
Patients suffering from local recurrence after surgery should be followed by appropriate imaging techniques and physical examination. Although there is no evidence supporting the routine use of chemotherapy and radiotherapy, a limited number of publications in the literature support administration of chemotherapy and radiotherapy in these patients.18,19 Chemotherapy and/or radiotherapy should be considered in patients whose surgical resection is incomplete, in those with postoperative recurrences, and in those with evidence of malignant (or “sarcomatoid”) transformation that can be identified histologically.16,20 In our cases, we did not give any additional treatment. Cases reported in the literature are summarized in Table 1.4-7,14,21,22
Table 1.
Cases reported in the literature.
| Reference | Age | Presentation | Immunmarkers | Treatment | Follow-up (months) | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Orosz et al. 21 | 63 | Left scrotal mass | SMA, vimentin, kappa and lambda chain | Desmin, S-100, factor VIII-related antigen | Radical orchiectomy | – |
| Chakrabarti et al.6 | 64 | Right scrotal mass | Vimentin, SMA and desmin | myoglobin S100 protein | Radical orchiectomy | 36 |
| Rico et al.22 | 46 | Left inguinoscrotal mass | Vimentin, SMA, ALK | Desmin, PAX-8, cytokeratin | Radical orchiectomy | 48 |
| Rafeek et al.4 | 22 | Left scrotal mass | Vimentin, CD34, epithelial membrane antigen | ALK, demsin, SMA, S100 protein cytokeratin | Excision of the mass | – |
| Yee et al.7 | 40 | Left lower quadrant mass | SMA | Desmin, ALK 1, CD34, CD21, cytokeratins (AE1/AE3), S100 protein | Radical orchiectomy | 30 |
| Kapur et al.14 | 36 | Left scrotal mass | Vimentin SMA | CD34, ALK, inhibin, cytokeratin (AE1/AE3), desmin | Radical orchiectomy | – |
| Megremis et al.5 | 45 | Left scrotal mass | SMA, desmin | CD34, S-100, cytokeratin (AE1/AE3), ALK | Radical orchiectomy | 36 |
| Our case 1 | 82 | Left inguinoscrotal mass | Actin, desmin, vimentin, CD68 | CD30, CD15, S-100, cytokeratin (AE1/AE3), ALK | Radical orchiectomy | 46 |
| Our case 2 | 56 | Right scrotal mass | Vimentin, actin, CD34 | Desmin, cytokeratin (AE1/AE3), ALK | Radical orchiectomy | 36 |
It is difficult to distinguish the origin of a paratesticular mass and to differentiate a benign or malignant nature with physical examination and radiological intervention. The most common treatment option is extensive surgical excision. Immunohistochemical study helps differentiate IMT from similar tumors. Long-term follow-up is proposed, although recurrence is rare following appropriate treatment.
Funding Statement
None.
REFERENCES
- 1.Coffin CM, Fletcher JA. In: World Health Organization classification of tumours: pathology and genetics of tumours of soft tissue and bone. Fletcher CD, Unni KK, Mertens F, editors Lyon: IARC Press; 2002. pp. 91–93. [Google Scholar]
- 2.Dagur G, Gandhi J, Kapadia K, Inam R, Smith NL, Joshi G, et al. Neoplastic diseases of the spermatic cord: an overview of pathological features, evaluation, and management. Transl Androl Urol. 2017;6(1):101-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussong JW, Brown M, Perkins SL, Dehner LP, Coffin CM.. Comparison of DNA ploidy, histologic, and immunohistochemical findings with clinical outcome in inflammatory myofibroblastic tumors. Mod Pathol. 1999;12:279-86. [PubMed] [Google Scholar]
- 4.Rafeek N, Joseph LD, Rajendiran S, Narayanan CD.. Inflammatory myofibroblastic tumor of spermatic cord. Int J Surg Case Rep. 2012;3:618-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Megremis S, Papamitsaki E, Ieromonachou P, Zois E.. Inflammatory myofibroblastic tumor of the paratestis: Sonographic appearance with pathologic correlation. J Ultrasound Med. 2007;26(9):1227-30. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti N, Shetty R.. Inflammatory myofibroblastic sarcoma of the spermatic cord. Indian J Surg. 2010;72:152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yee CH, To KF, Hou SM, Ng CF. Inflammatory myofibroblastic tumor of spermatic cord in undescended testis. Urology. 2009;73:9-12. [DOI] [PubMed] [Google Scholar]
- 8.Tunuguntla H, Mishra A, Jorda M, Gosalbez R.. Inflammatory myofibroblastic tumor of the epididymis: case report and review of the literature. Urology. 2011;78:183-5. [DOI] [PubMed] [Google Scholar]
- 9.Narla LD, Newman B, Spottswood SS, Narla S, Kolli R.. Inflammatory pseudotumor. Radiographics. 2003;23:719-29. [DOI] [PubMed] [Google Scholar]
- 10.Akbar SA, Sayyed TA, Jafri SZ, Hasteh F, Neill JS.. Multimodality imaging of paratesticular neoplasms and their rare mimics. Radiographics. 2003;23:1461-76. [DOI] [PubMed] [Google Scholar]
- 11.Gleason BC, Hornick JL.. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008;61(4):428-37. [DOI] [PubMed] [Google Scholar]
- 12.Lovly CM, Gupta A, Lipson D, Otto G, Brennan T, Chung CT, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. 2014;4(8):889-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel Sugganth, Gay Laurie M., Vergilio Jo-Anne, Ross Jeffrey S.. A clinical and genomic profile of inflammatory myofibroblastic tumors. Journal of Clinical Oncology. 2017;35:15_suppl, 1538. [Google Scholar]
- 14.Kapur P, Treat K, Chuang AT, Hoang MP.. Pathologic quiz case: paratesticular mass in a young man. Inflammatory myofibroblastic tumor of the paratestis. Arch Pathol Lab Med. 2004;128:58990. [DOI] [PubMed] [Google Scholar]
- 15.Coffin CM, Watterson J, Priest JR, Dehner LP.. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol.1995;19:859-872. [DOI] [PubMed] [Google Scholar]
- 16.Kovach SJ, Fischer AC, Katzman PJ, Salloum RM, Ettinghausen SE, Madeb R, et al. Inflammatory myofibroblastic tumors. J Surg Oncol. 2006;94:385-91. [DOI] [PubMed] [Google Scholar]
- 17.Masaki Y, Kurose N, Umehara H.. IgG4-related disease: a novel lymphoproliferative disorder discovered and established in Japan in the 21st century. J Clin Exp Hematop. 2011;51(1):13-20. [DOI] [PubMed] [Google Scholar]
- 18.Dishop MK, Warner BW, Dehner LP, Kriss VM, Greenwood MF, Geil JD, et al. Successful treatment of inflammatory myofibroblastic tumor with malignant transformation by surgical resection and chemotherapy. J Pediatr Hematol Oncol. 2003;25:153-8. [DOI] [PubMed] [Google Scholar]
- 19.Chen M, Zhang L, Cao G, Zhu W, Chen X, Fang Q.. Partial response to chemotherapy in a patient with retroperitoneal inflammatory myofibroblastic tumor. Mol Clin Oncol. 2016;5(4):463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang T, Yuan Y, Ren C, DU S, Chen J, Sun Q, Liu Z.. Recurrent inflammatory myofibroblastic tumor of the inguinal region: A case report and review of the literature. Oncol Lett. 2015;10(2):675-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orosz Z., Besznyák I.. Diffuse inflammatory pseudotumor of the testis, the epididymis and the spermatic cord. Pathology & Oncology Research. 1995;1(1):75–9. [DOI] [PubMed] [Google Scholar]
- 22.Rico L, López FM, Vitagliano G, Carlos A.. Inflammatory myofibroblastic genitourinary tumor: The challenge of controlling and monitoring an non frequent oncological disease. Arch Esp Urol. 2017;70(2):306-10. [PubMed] [Google Scholar]


