Abstract
Context: Linalool oxide (OXL) (a monoterpene) is found in the essential oils of certain aromatic plants, or it is derived from linalool. The motivation for this work is the lack of psychopharmacological studies on this substance.
Objective: To evaluate OXL’s acute toxicity, along with its anticonvulsant and antinociceptive activities in male Swiss mice.
Material and methods: OXL (50, 100 and 150 mg/kg, i.p.) was investigated for acute toxicity and in the Rota-rod test. Antinociceptive activity was evaluated by the acetic acid-induced writhing test, and by formalin testing. Anticonvulsant effects were demonstrated by testing for pentylenetetrazol (PTZ)-induced seizures and by Maximum Electroshock headset (MES) test. OXL was administered to the animals intraperitoneally 30 min before for pharmacological tests.
Results: OXL showed an LD50 of ∼721 (681–765) mg/kg. In the Rota-rod test, it was observed that OXL caused no damage to the animal’s motor coordination. OXL significantly reduced (p < .001) the number of writhings. OXL also significantly decreased (p < .05, p < .01 or p < .001) paw-licking time in the two phases of the formalin test. OXL significantly reduced (p < .01 or p < .001) the duration of tonic seizures in the MES test, and at the dose 150 mg/kg, significantly increased (p < .01) the latency to first seizure in the PTZ test.
Conclusion: The tested doses of OXL were safe, with no motor impairment, and show clear antinociceptive and anticonvulsant potential. Future investigations with this monoterpene may lead to the development of a new molecule with even higher potency and selectivity.
Keywords: Pain, essential oil, nociception
Introduction
Linalool is a monoterpene, and is often found as major component in essential oils of various species of aromatic plants such as: Jasminum subtriplinerve Blume (Oleaceae), Lavandula angustifolia Mill. (Lamiaceae), Melissa officinalis L. (Lamiaceae), Rosmarinus officinalis L. (Lamiaceae), Cymbopogon citratus (DC.) Stapf (Poaceae) and Ocimum sanctum L. (Lamiaceae) (Elisabetsky et al. 1995; Re et al. 2000; Batista et al. 2008; Linck et al. 2009, 2010; Cheng et al. 2012; Khan et al. 2014). It is widely used by industry as an essence in the manufacture of perfumes, shampoos, soaps and detergents (Mitić-Ćulafić et al. 2009).
Some linalool-producing species are used in traditional medicine as sedatives, analgesics, anxiolytics and hypnotics to both alleviate symptoms and treat diseases (Peana et al. 2002; Linck et al. 2010)., To increase our knowledge about its safety for therapeutic use, many studies with this monoterpene have been undertaken (Elisabetsky et al. 1995, 1999; Miyashita & Sadzuka 2013).
Linalool oxide (OXL) can be found in certain essential oils of aromatic herbs, however, as a minor component (Alcântara et al. 2010). It is a monocyclic alcohol formed from linalool by either natural oxidation or by synthetic processes, and to the contrary of linalool, there are surprisingly few published studies (Demyttenaere & Willemen 1998). A previous study has noted that OXL has an anxiolytic effect when inhaled in animal models (Souto-Maior et al. 2011).
The dearth of research on OXL’s possible psychopharmacological activities, and the need for new and more effective drugs, serve as motivations for this study which aimed to evaluate the antinociceptive and anticonvulsant effects of OXL in animal models.
Materials and methods
Animals
Male Swiss mice (25–35 g), 2–3 months old, from the Prof. Dr. Thomas George vivarium of the UFPB Health Science Center were used in the study. The animals were housed in plastic cages and maintained under controlled conditions of temperature (21 ± 2 °C), with free access to water and pellet type food (Purina, Brazil). The animals were kept in a 12 h dark/light cycle, with lights on at 6:00 a.m. All experimental procedures were previously analyzed and approved by the Ethics in Animal Research Committee (UFPB) under certification No. 0405/07.
Drugs
OXL, diazepam and phenytoin were purchased from Sigma (St. Louis, MO, USA). Glacial acetic acid and formaldehyde 37% were purchased from Vetec (São Paulo, Brazil). Morphine and Tween 80 were purchased from Merck (Darmstadt, Germany). All administrations were via intraperitoneal (i.p.) in volumes of 0.1 mL/10 g body weight.
Acute toxicity
For the experiment, OXL was administered i.p. at doses of 500, 750, 850, 1000 and 1250 mg/kg, in groups of 10 mice. Signs of toxicity and mortality were registered for a period of 72 h. The LD50 was calculated by the probit method, using the percentage mortality, and the logarithms of the doses followed by linear regression (Litchfield & Wilcoxon 1949).
Rota-rod test
The procedure was first described by Dunham and Miya (1957), and is a suitable methodology for the detection of impairment. Initially, the mice were subjected to pre-selection and those considered able to participate were submitted to the test in the Rota-rod device. The motor performance of the animals (n = 8) was evaluated (for a maximum period of 3 min) at 30, 60 and 120 min after i.p. treatment; with vehicle (10 mL/kg), OXL (50, 100 and 150 mg/kg), and diazepam (2 mg/kg,) used as a positive control.
Test by acetic acid-induced writhing
The experiment was conducted based on the methodology described by Huang et al. (2010). The mice were divided into five groups (n = 8) received (i.p.) vehicle (10 mL/kg), OXL (50, 100 and 150 mg/kg), and morphine (10 mg/kg); administered 30 min before acetic acid 0.85% (0.1 mL/10 g). Five minutes after injection of acetic acid, the number of writhings was registered for 10 min.
Formalin test
The mice were divided into five groups (n = 8) pretreated (i.p.) with vehicle (10 mL/kg), OXL (50, 100 and 150 mg/kg) or morphine (10 mg/kg). After 30 min of treatment, 20 μL of formalin 2.5% was injected into the dorsal surface of the right hind paw of each rat. Immediately, they were placed individually in observation boxes. The paw licking time was recorded during the first 5 min (neurogenic phase), and later, during the interval between 15 and 30 min (inflammatory phase).
Induced seizures maximum electroshock headset testing
The animals were divided into 5 groups (n = 8) and were treated (i.p.) with vehicle (10 mL/kg), OXL (50, 100 and 150 mg/kg), and phenytoin (25 mg/kg). At 30 min from treatment, the animals were submitted to a headset shock at 150 pulses/s for 0.5 s (Electrostimulator ECT UNIT 7801). The observed parameters were duration of tonic convulsions, and deaths (Putnam & Merritt 1937).
Induced seizures testing with pentylenetetrazole (PTZ)
The animals were divided into 5 groups (n = 8) and were treated (i.p.) with vehicle (10 mL/kg), OXL (50, 100 and 150 mg/kg), and diazepam (4 mg/kg). At 30 min from the respective treatments, the mice received pentylenetetrazol (60 mg/kg) (i.p.) and were immediately observed for a period of 20 min. The registered parameter latency was represented as the elapsed time to the first recorded seizure (Everett & Richards 1944).
Statistical analysis
Results are expressed as the mean ± S.E.M. Differences between the means of the groups were evaluated by one-way ANOVA, and followed by Dunnett’s test. The p < .05 was considered statistically significant. LD50 was calculated by the probit method, followed by linear regression.
Results
Acute toxicity
From the results obtained, the LD50 was calculated for OXL in mice (estimated at 721 mg/kg), presenting confidence limits between 681 and 765 mg/kg body weight.
Effect of linalool oxide on the Rota-rod test results
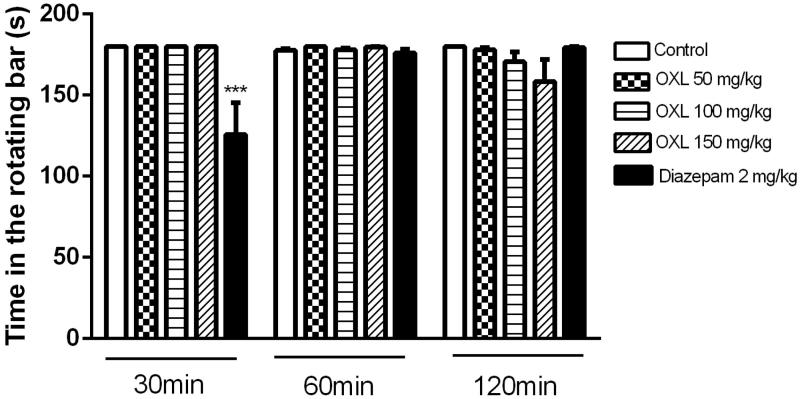
No significant changes were observed in the performance of animals treated with OXL during the Rota-rod device evaluation (50, 100 and 150 mg/kg, ip) at 30 min (180.0 ± 0.0, 180.0 ± 0.0, 180, 0 ± 0.0), 60 min (180.0 ± 0.0, 178.0 ± 1.0, 179.5 ± 0.5) or 120 min (178.0 ± 1.3, 170.5 ± 6.3, 158.4 ± 13.5), as compared to the control group for the same periods of evaluation being 30 (180.0 ± 0.0), 60 (177.6 ± 1.2) and 120 (180.0 ± 0.0) min (Figure 1).
Figure 1.
Effect of OXL (50, 100 and 150 mg/kg, i.p.) on mice for permanence time on the swivel bar in seconds. Values are expressed as mean ± S.E.M. ANOVA ‘one way’ followed by Dunnet's test. ***p < .001 versus control group (vehicle).
Effect of linalool oxide on abdominal contortions in the acetic acid induced writhing test
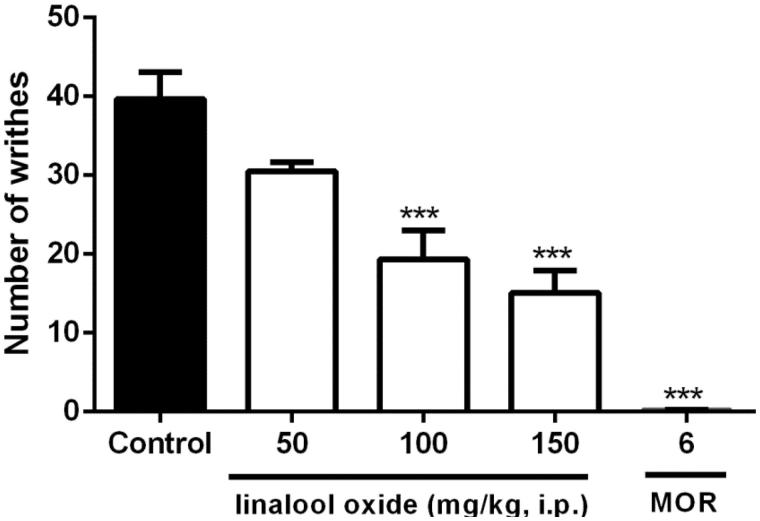
The results obtained in this test show a significant decrease in the number of writhes for the animals treated with OXL at: 100 (19.3 ± 3.6) and 150 mg/kg (15.1 ± 2.8) when compared to the control (39.6 ± 3.4). As expected, the morphine group also showed a significant decrease in the number of contortions (0.1 ± 0.1) (Figure 2).
Figure 2.
Effect of OXL (50, 100 and 150 mg/kg; i.pon the number of writhes induced by acetic acid in mice. Values are expressed as mean ± S.E.M. ANOVA ‘one way’ followed by Dunnet's test. ***p < .001 versus control group (vehicle).
Effect of linalool oxide in the formalin test
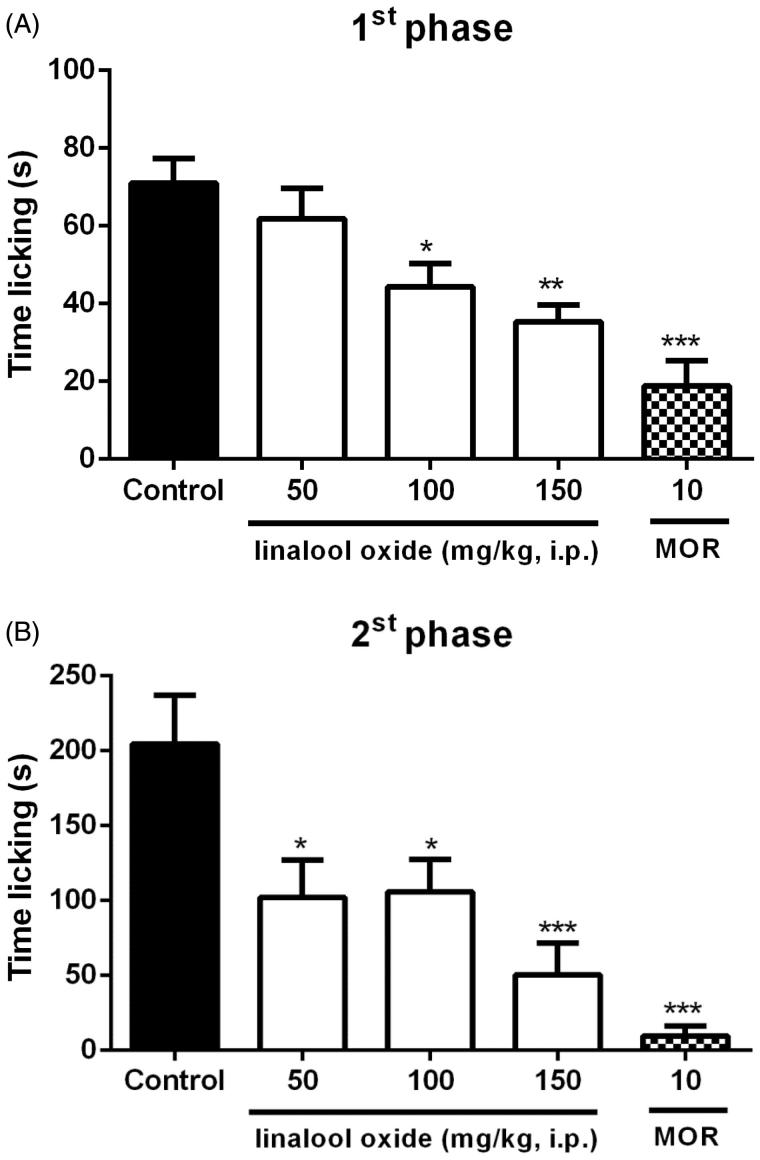
In the first phase of the formalin test (Figure 3(A)), the groups that received OXL revealed decreased paw licking times at doses of 100 (44.3 ± 6.1s) and 150 (35.3 ± 4.4s) mg/kg in relation to the control group (71.0 ± 6.3s). In the second phase of the formalin test (Figure 3(B)), OXL (50, 100 and 150 mg/kg) significantly decreased respective paw-licking times (102.3 ± 24.7s; 105.8 ± 21.8s; 73.5 ± 29.6s) as compared to the control group (204.5 ± 32.5s). Morphine (10 mg/kg, i.p.) significantly decreased paw-licking time in the first and second test phase (9.4 ± 6.9s and 18.9 ± 6.5s), respectively.
Figure 3.
Effect of OXL (50, 100 and 150 mg/kg, i.p.) on paw-licking time in the first (A), and second (B) phases of the formalin test in mice. Values are expressed as mean ± S.E.M. ANOVA ‘one way’ followed by Dunnet’s test. *p < .05; **p < .01; ***p <.001 versus the control group (vehicle).
Effect of linalool oxide on seizures induced in the maximum electroshock headset test
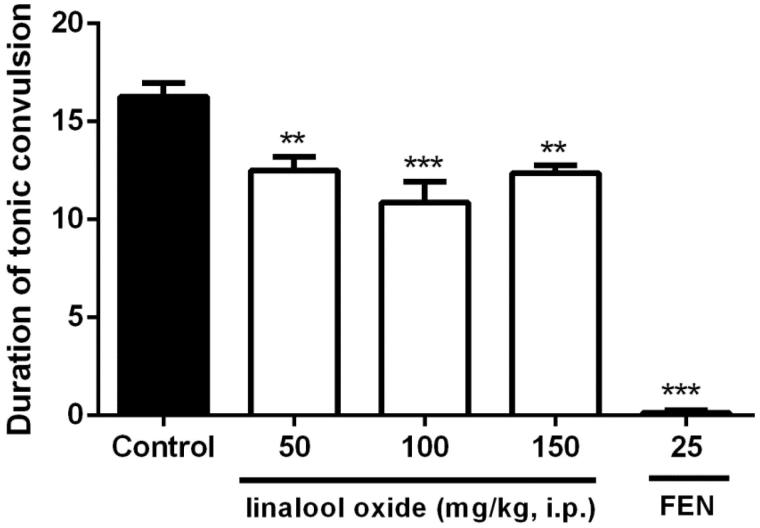
OXL at doses of 50 (12.5 ± 0.7s), 100 (10.9 ± 1.1s) and 150 mg/kg (12.4 ± 0.4s) significantly reduced the duration of tonic seizures (Figure 4) as compared to the control group (16.3 ± 0.7s). The same result can be observed for phenytoin at a dose of 25 mg/kg (0.1 ± 0.1s). There was no significant difference in the number of deaths for all groups tested.
Figure 4.
Effect of OXL (50, 100 and 150 mg/kg; i.p.) on the duration of tonic convulsions in the Maximum Electroshock test in mice. Values are expressed as mean ± S.E.M. ANOVA ‘one way’ followed by Dunnet's test. **p < .01; ***p < .001 versus the control group (vehicle).
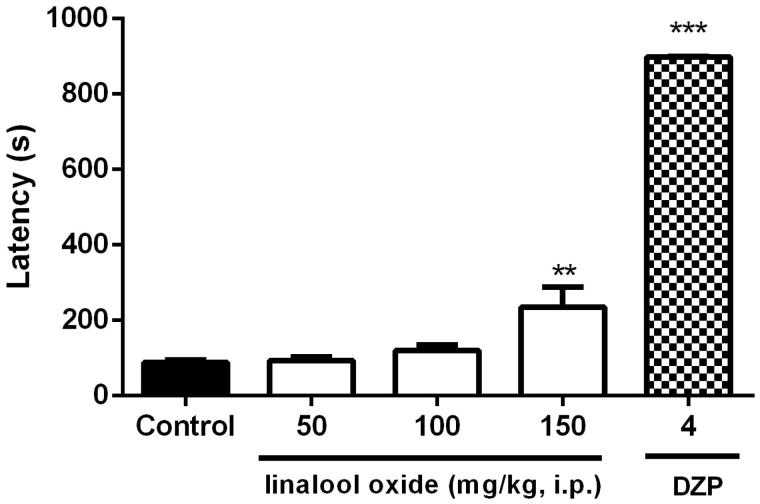
Effect of linalool oxide on PTZ-induced seizures
OXL at a dose of 150 mg/kg (235.4 ± 53.1s) significantly increased latency to first seizure onset (Figure 5) as compared to the control group (87.5 ± 7.5s). The standard drug diazepam (4 mg/kg) also proved effective in increasing this parameter (900.0 ± 0.1s).
Figure 5.
Effect of OXL (50, 100 and 150 mg/kg, i.p.) on the onset latency to the first seizure in the pentylenetetrazol induced seizures test in mice. Values are expressed as mean ± S.E.M. ANOVA ‘one way’ followed by Dunnet's test. **p < .01; ***p < .001 versus control group (vehicle).
Discussion
The pharmacological activities of linalool and linalool acetate are found as major components of essential oils in various aromatic plants (Jirovetz et al. 1991; Barocelli et al. 2004; Koto et al. 2006). OXL, however, has not yet been investigated for its antinociceptive and anticonvulsant effects. This may be related to the occurrence of OXL isomers, usually at lower concentrations in essential oils.
In the initial stage of this OXL study, acute toxicity was tested, which represents a first step in the toxicological investigation of an unknown substance, with the determination of LD50, being the indicator of acute toxicity (Lorke 1983). This step is extremely important as it can help to avoid or prevent potential organ damage during treatments of animals in testing (Cunha et al. 2008). The LD50 of OXL stipulated at 721 mg/kg, with confidence limits between 681 and 765 mg/kg body weight provides a good safety margin for standardization of doses of 50, 100, and 150 mg/kg to be used in mice, by intraperitoneal route, in subsequent testing.
The Rota-rod test is a widely used method at this stage of research since it can detect physical disabilities caused by pharmacological agents such as muscle relaxants and CNS depressants (Sen & Chaudhuri 1992; Pultrini et al. 2006). OXL did not cause muscle relaxation or motor coordination deficits; the mice did not decrease their lengths of stay on the swivel bar, compared to the control group. Similarly, a study by Linck et al. (2009) found that the monoterpene linalool, (by inhalation) did not alter the motor coordination of the animals tested with the same methodology. Linalool and OXL are structurally similar, and these findings show that small differences are not likely to promote neurotoxicity or changes in motor coordination. This is considered advantageous in the design of new drugs, since locomotion changes feature as a negative effect associated with toxicity.
The writhings test was the first to be conducted for evaluation of possible OXL antinociception, being a widely employed model for the study of pain when screening analgesic drugs and useful in evaluating agents that act both at central and peripheral levels (Meotti et al. 2007; Santos et al. 2007; Huang et al. 2010). The results obtained during this step, suggest that OXL exerts antinociceptive activity (decreases in the number of writhings presented by mice). Other studies confirm the analgesic effect of monoterpenes, such as linalool (Peana et al. 2006; Batista et al. 2008, 2010), citral (Nishijima et al. 2014), and p-cymene (Quintans et al. 2013; Oliveira et al. 2015). The similar chemical structures of these substances may well justify similar pharmacological effects.
Continuing the investigations on the antinociceptive effects of OXL, we carried out the formalin test, which is a well-established model, and valid for the study of various specific analgesic substances (Elhabazi et al. 2006; Huang et al. 2010).
The formalin test may be divided into two distinct stages involving different mechanisms of nociception (Azevedo et al. 2007). The first is due to the direct effect of formalin on nociceptors, and represents the neurogenic phase pain (acute pain or tonic) (Lee & Jeong 2002; Santos et al., 2007; Rozisky et al. 2011). The second phase appears due to inflammation of the peripheral tissues of the initial injury, and represents the inflammatory response to pain (inflammatory local pain), it is often persistent (Lee & Jeong 2002; Santos et al. 2007; Rozisky et al. 2011). It was observed that OXL presented an antinociceptive profile in the formalin test, shortening the animal paw-licking time during both phases of observation. Peana et al. (2006) also observed the antinociceptive effects of linalool in the formalin test, in both phases; this confirms that the two substances have similar activity. This result suggests that OXL operates at the central level, similar to morphine, yet the need for further study remains to elucidate the possible mechanism of action involved.
Researchers still seek drugs for the treatment of epilepsy that are either more effective and/or less toxic. The Maximum Electroshock Headset test (MES), and Seizures Induced by PTZ are the two most used animal models in the search for new antiepileptic drugs. The Maximum Electroshock Test is regarded as a predictive model for generalized tonic-clonic epilepsy in humans, while the PTZ test is known as a model of absence epilepsy. Although having limitations, these models can reveal drugs with potential clinical usefulness (Smith et al. 2007). OXL was effective in reducing the duration of seizures induced by Maximum Electroshock, and in increasing the latency of seizures induced by PTZ. In a literature review, Almeida et al. (2011) showed that of 30 essential oils with anticonvulsant activity, the principal chemical constituents were monoterpenes, including linalool. Other studies using linalool and its isomers, demonstrated anticonvulsant effects using the same animal models, as in this study, and also demonstrated that the (S)-(+) and (R)-(−) isomers of linalool display different pharmacological potentials (De Sousa et al. 2010; Elisabetsky et al. 1995). Studies suggest that the anticonvulsant mechanism of linalool involves interaction with the GABAA receptor, the competitive antagonism of glutamate (Elisabetsky et al. 1995, 1999) and direct interaction with NMDA receptors, by noncompetitive and dose-dependent inhibition (Brum et al. 2001). Further, linalool has shown inhibitory effects on the release of acetylcholine and on the opening time of ion channels in the mouse neuromuscular junction (Re et al. 2000). The findings concerning linalool are interesting for our study since OXL obtained from linalool retains structural similarity, and thus suggests the same research lines were used for the precursor.
In summary, from the results obtained in this study, OXL presents antinociceptive and anticonvulsant activities, which open perspectives for new studies to elucidate the mechanisms involved.
References
- Alcântara JM, Yamaguchi KKL, Veiga Junior VF.. 2010. Essential oils composition from Aniba and Licaria species and their antioxidant and antiplatelet activities. Quim Nova. 33:141–145. [Google Scholar]
- Almeida RN, Agra MF, Maior FN, Sousa DP.. 2011. Essential oils and their constituents: anticonvulsant activity. Molecules. 16:2726–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo AO, Campos JJ, Galdino GS, Braga FC, Duarte ID, Perez AC.. 2007. Antinociceptive effect from Davilla elliptica hydroalcoholic extract. J Ethnopharmacol. 113:354–356. [DOI] [PubMed] [Google Scholar]
- Barocelli E, Calcina F, Chiavarini M, Impicciatore M, Bruni R, Bianchi A, Ballabeni V.. 2004. Antinociceptive and gastroprotective effects of inhaled and orally administered Lavandula hybrida Reverchon “Grosso” essential oil. Life Sci. 76:213–223. [DOI] [PubMed] [Google Scholar]
- Batista PA, Werner MFP, Oliveira EC, Burgos L, Pereira P, Brum LF, Story GM, Santos AR.. 2010. The antinociceptive effect of (−)-linalool in models of chronic inflammatory and neuropathic hypersensitivity in mice. J Pain. 11:1222–1229. [DOI] [PubMed] [Google Scholar]
- Batista PA, Werner MFP, Oliveira EC, Burgos L, Pereira P, Brum LF, Santos AR.. 2008. . Evidence for the involvement of ionotropic glutamatergic receptors on the antinociceptive effect of (−)-linalool in mice. Neurosci Lett. 440:299–303. [DOI] [PubMed] [Google Scholar]
- Brum LFS, Elisabetsky E, Souza D.. 2001. . Effects of linalool on [3H]MK801 and [3H] muscimol binding in mouse cortical membranes. Phytother Res. 15:422–425. [DOI] [PubMed] [Google Scholar]
- Cheng B, Lin C, Yeh T, Chang ST.. 2012. Potential source of S-(+)-linalool from Cinnamomum osmophloeum ct. linalool leaf: essential oil profile and enantiomeric purity. J Agric Food Chem. 60:7623–7628. [DOI] [PubMed] [Google Scholar]
- Cunha LC, Paula JR, Sá VA, da Paixão e Amorim ME, Barros ICM, Brito LAB, da Silveira N.. 2008. Acute toxicity of Brosimum gaudichaudii Trécul. root extract in mice: Determination of both approximate and median lethal doses. Braz J Pharmacogn. 18:532–538. [Google Scholar]
- De Sousa DP, Nóbrega FF, Santos CC, de Almeida RN.. 2010. Anticonvulsant activity of the linalool enantiomers and racemate: Investigation of chiral influence. Nat Prod Commun. 5:1847–1851. [PubMed] [Google Scholar]
- Demyttenaere JCR, Willemen HM.. 1998. Biotransformation of linalool to furanoid and pyranoid linalool oxides by Aspergillus niger. Phytochemistry. 47:1029–1036. [DOI] [PubMed] [Google Scholar]
- Dunham NW, Miya TS.. 1957. . A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc. 46:208–209. [DOI] [PubMed] [Google Scholar]
- Elhabazi K, Aboufatima R, Benharref A, Zyad A, Chait A, Dalal A.. 2006. Study on the antinociceptive effects of Thymus broussonetii Boiss extracts in mice and rats. J Ethnopharmacol. 107:406–411. [DOI] [PubMed] [Google Scholar]
- Elisabetsky E, Brum LF, Souza DO.. 1999. . Anticonvulsant properties of linalool in glutamate-related seizure models. Phytomedicine. 6:107–113. [DOI] [PubMed] [Google Scholar]
- Elisabetsky E, Marschner J, Souza DO.. 1995. Effects of linalool on glutamatergic system in the rat cerebral cortex. Neurochem Res. 20:461–565. [DOI] [PubMed] [Google Scholar]
- Everett GM, Richards RK.. 1944. Comparative anticonvulsive action of 3,5,5-trimethyloxazolidine-2,4-dione (Tridione), dilantin and phenobarbital. J Pharmacol Exp Ther. 81:402–407. [Google Scholar]
- Huang GJ, Huang SS, Lin SS, Shao YY, Chen CC, Hou WC, Kuo YH.. 2010. . Analgesic effects and the mechanisms of anti-inflammation of ergostatrien-3beta-ol from Antrodia camphorata submerged whole broth in mice. J Agric Food Chem. 58:7445–7452. [DOI] [PubMed] [Google Scholar]
- Jirovetz L, Jäger W, Buchbauer G, Nikiforov A, Raverdino V.. 1991. Investigations of animal blood samples after fragrance drug inhalation by gas chromatography/mass spectrometry with chemical ionization and selected ion monitoring. Biol Mass Spectrom. 20:801–803. [DOI] [PubMed] [Google Scholar]
- Khan A, Ahmad A, Khan LA, Mansoor N.. 2014. Ocimum sanctum (L.) essential oil and its lead molecules induce apoptosis in Candida albicans. Res Microbiol. 165:411–419. [DOI] [PubMed] [Google Scholar]
- Koto R, Imamura M, Watanabe C, Obayashi S, Shiraishi M, Sasaki Y, Azuma H.. 2006. Linalyl acetate as a major ingredient of lavender essential oil relaxes the rabbit vascular smooth muscle through dephosphorylation of myosin light chain. J Cardiovasc Pharmacol. 48:850–856. [DOI] [PubMed] [Google Scholar]
- Lee IO, Jeong YS.. 2002. Effects of different concentrations of formalin on paw edema and pain behaviors in rats. J Korean Med Sci. 17:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linck VM, Silva AL, Figueiró M, Caramão EB, Moreno PR, Elisabetsky E.. 2010. Effects of inhaled linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine. 17:679–683. [DOI] [PubMed] [Google Scholar]
- Linck VM, Silva AL, Figueiró M, Piato AL, Herrmann AP, Dupont Birck F, Caramão EB, Nunes DS, Moreno PR, Elisabetsky E.. 2009. Inhaled linalool-induced sedation in mice. Phytomedicine. 16:303–307. [DOI] [PubMed] [Google Scholar]
- Litchfield JT, Wilcoxon F.. 1949. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 96:99–113. [PubMed] [Google Scholar]
- Lorke D. 1983. . A new approach to practical acute toxicity testing. Arch Toxicol. 54:275–287. [DOI] [PubMed] [Google Scholar]
- Meotti FC, Fachinetto R, Maffi LC, Missau FC, Pizzolatti MG, Rocha JB, Santos AR.. 2007. Antinociceptive action of myricitrin: involvement of the K+ and Ca2+ channels. Eur J Pharmacol. 567:198–205. [DOI] [PubMed] [Google Scholar]
- Mitić-Ćulafić D, Žegura B, Nikolić B, Vuković-Gacić B, Knezević-Vukcević J, Filipic M.. 2009. Protective effect of linalool, myrcene and eucalyptol against t-butyl hydroperoxide induced genotoxicity in bacteria and cultured human cells. Food Chem Toxicol. 47:260–266. [DOI] [PubMed] [Google Scholar]
- Miyashita M, Sadzuka Y.. 2013. Effect of linalool as a component of Humulus lupulus on doxorubicin-induced antitumor activity. Food Chem Toxicol. 53:174–179. [DOI] [PubMed] [Google Scholar]
- Nishijima CM, Ganev EG, Mazzardo-Martins L, Martins DF, Rocha LR, Santos AR, Hiruma-Lima CA.. 2014. Citral: A monoterpene with prophylactic and therapeutic anti-nociceptive effects in experimental models of acute and chronic pain. Eur J Pharmacol. 736:16–25. [DOI] [PubMed] [Google Scholar]
- Oliveira TM, Carvalho RB, Costa IHF, de Oliveira GA, de Souza AA, de Lima SG, de Freitas RM.. 2015. Evaluation of p-cymene, a natural antioxidant. Pharm Biol. 53:423–428. [DOI] [PubMed] [Google Scholar]
- Peana AT, Marzocco S, Popolo A, Pinto A.. 2006. (−)-Linalool inhibits in vitro NO formation: Probable involvement in the antinociceptive activity of this monoterpene compound. Life Sci. 78:719–723. [DOI] [PubMed] [Google Scholar]
- Peana AT, D'aquila PS, Panin F, Serra G, Pippia P, Moretti MD.. 2002. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine. 9:721–726. [DOI] [PubMed] [Google Scholar]
- Pultrini AM, Galindo LA, Costa M.. 2006. Effects of the essential oil from Citrus aurantium L. in experimental anxiety models in mice. Life Sci. 78:1720–1725. [DOI] [PubMed] [Google Scholar]
- Putnam TJ, Merritt HH.. 1937. Experimental determination of the anticonvulsant properties of some phenyl derivatives. Science. 85:525–526. [DOI] [PubMed] [Google Scholar]
- Quintans JSS, Menezes PP, Santos MRV, Bonjardim LR, Almeida JR, Gelain DP, Araújo AA, Quintans-Júnior LJ.. 2013. Improvement of p-cymene antinociceptive and anti-inflammatory effects by inclusion in β-cyclodextrin. Phytomedicine. 20:436–440. [DOI] [PubMed] [Google Scholar]
- Re L, Barocci S, Sonnino S, Mencarelli A, Vivani C, Paolucci G, Scarpantonio A, Rinaldi L, Mosca E.. 2000. . Linalool modifies the nicotinic receptor-ion channel kinetics at the mouse neuromuscular junction. Pharmacol Res. 42:177–181. [DOI] [PubMed] [Google Scholar]
- Rozisky JR, Medeiros LF, Adachi LS, Espinosa J, de Souza A, Neto AS, Bonan CD, Caumo W, Torres IL.. 2011. Morphine exposure in early life increases nociceptive behavior in a rat formalin tonic pain model in adult life. Brain Res. 1367:122–129. [DOI] [PubMed] [Google Scholar]
- Santos TC, Marques MS, Menezes IAC, Dias KS, Silva AB, Mello IC, Carvalho AC, Cavalcanti SC, Antoniolli AR, Marçal RM.. 2007. Antinociceptive effect and acute toxicity of the Hyptis suaveolens leaves aqueous extract on mice. Fitoterapia. 78:333–336. [DOI] [PubMed] [Google Scholar]
- Sen T, Chaudhuri KN.. 1992. Studies on the neuropharmacological aspects of Pluchea indica root extract. Phytother Res. 6:175–179. [Google Scholar]
- Smith M, Wilcox KS, White HS.. 2007. Discovery of antiepileptic drugs. Neurotherapeutics. 4:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto-Maior FN, Carvalho FL, de Morais LCSL, Netto SM, de Sousa DP, de Almeida RN.. 2011. Anxiolytic-like effects of inhaled linalool oxide in experimental mouse anxiety models. Pharmacol Biochem Behav. 100:259–263. [DOI] [PubMed] [Google Scholar]