ABSTRACT
Earlier, we demonstrated that transcript levels of METAL TOLERANCE PROTEIN2 (MTP2) and of HEAVY METAL ATPase2 (HMA2) increase strongly in roots of Arabidopsis upon prolonged zinc (Zn) deficiency and respond to shoot physiological Zn status, and not to the local Zn status in roots. This provided evidence for shoot-to-root communication in the acclimation of plants to Zn deficiency. Zn-deficient soils limit both the yield and quality of agricultural crops and can result in clinically relevant nutritional Zn deficiency in human populations. Implementing Zn deficiency during cultivation of the model plant Arabidopsis thaliana on agar-solidified media is difficult because trace element contaminations are present in almost all commercially available agars. Here, we demonstrate root morphological acclimations to Zn deficiency on agar-solidified medium following the effective removal of contaminants. These advancements allow reproducible phenotyping toward understanding fundamental plant responses to deficiencies of Zn and other essential trace elements.
KEYWORDS: zinc deficiency, chelator, EDTA, HBED, iron, root morphology, primary root length, Arabidopsis, mineral nutrition, root biomass, zinc limitation
We demonstrated that Zn deficiency activates shoot-to-root signaling, which acts in the acclimation of plants to Zn-deficient conditions in addition to local root-mediated responses.1 We used both hydroponic and agar-solidified plant media of controlled composition. Omitting Zn from the growth medium for prolonged periods of time allows the assessment of responses to Zn deficiency. Long-term experiments are possible in hydroponic culture under short-day conditions that maintain Arabidopsis in the vegetative phase of its lifecycle. Implementing Zn-deficient conditions on agar-solidified media had remained difficult prior to our study. Substantial impurities in commercially available agars and agaroses supply sufficient amounts of Zn to growing seedlings.2 Consequently, morphological consequences of Zn deficiency remain to be described in wild-type plants on agar-solidified medium. To generate Zn-deficient conditions on agar-solidified medium, we adapted a protocol that was first used for obtaining copper (Cu)-deficient agar-solidified medium3 and chelator-independent iron (Fe) deficiency.4 We performed three sequential washes of agar in a solution of EDTA (e.g. 5 L of 10 mM EDTA for 50 g of agar) for 10 to 12 hours per wash, followed by 6 washes in ultra-pure water to remove all EDTA. The resulting EDTA-washed agar was then mixed with a modified Hoagland’s nutrient solution in order to produce agar-solidified plant cultivation media of a defined composition.5 Zn was omitted from the modified Hoagland’s solution for Zn deficiency treatments, or included to prepare the corresponding control medium. As shown by measurements of shoot and root Zn content of 17-day-old Arabidopsis seedlings grown on Zn-deficient and control agar-solidified media, our strategy was highly effective. Seedlings cultivated on Zn-deficient plates showed a ~ 3-fold decrease in shoot Zn concentration, a ~ 1.8-fold decrease in root Zn, as well as a 2-fold decrease in shoot fresh biomass compared to Zn-sufficient controls.1 Such clear differences after only 18 days of growth additionally allowed the analysis of root morphology. Root dry biomass of seedlings grown on Zn-deficient agar-solidified medium was significantly reduced by about 40% compared to seedlings grown on control medium, whereas primary root growth was stimulated 1.6-fold under Zn deficiency (Figure 1). Moreover, lateral root density, i.e. the number of lateral roots per length unit of primary root, was lowered under Zn-deficient growth conditions from the 10th day of plant cultivation onwards, varying from a 22% decrease (on d 12) to a 37% decrease (on d 17) (Figure 1). Our observations indicate that the acclimation of seedlings to Zn-deficient conditions includes an increase in primary root elongation. This suggests that Arabidopsis evolved to explore the soil solution to a greater depth in pursuit of Zn, despite an overall decreased root biomass production. The morphological Zn deficiency response thus contrasts with responses to deficiencies of other nutrients. For example, during the phosphate starvation response primary root growth is impaired at the benefit of increased lateral root density and length.6 This novel aspect of the response to Zn-deficiency implies that when breeding crops for optimal growth and Zn content in Zn-deficient soils, varieties with longer primary roots should be prioritized.
Figure 1.
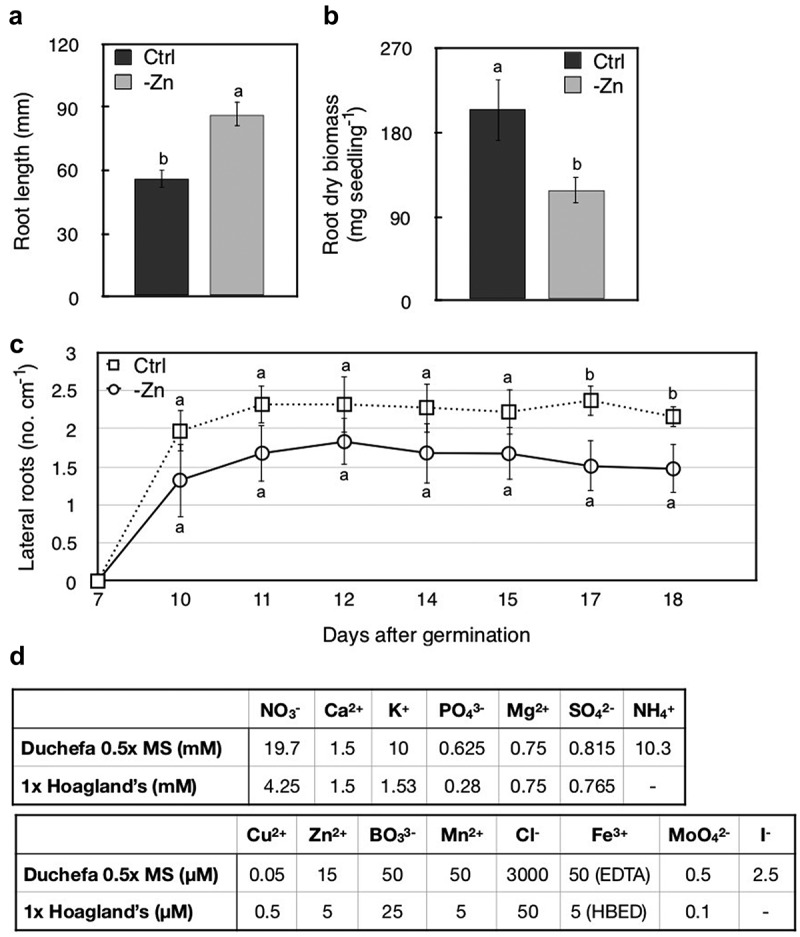
Quantification root morphology in Zn deficiency. (a) Primary root length in 18-day-old Col-0 seedlings grown on control or Zn-deficient agar-solidified modified Hoagland’s media. (b) Dry root biomass in 18-day-old Col-0 seedlings grown on control or Zn-deficient agar-solidified modified Hoagland’s media. (c) Lateral root density (number of lateral roots per centimetre of primary root length) over 18 d in Col-0 seedlings grown on control or Zn-deficient agar-solidified modified Hoagland’s media.(d) Comparison of macronutrient (upper) and micronutrient (lower) concentrations in plant growth media. In (a-c), error bars represent SD (n = 15). Distinct letters indicate significant differences (p < 0.05, Bonferroni-corrected Student’s t-test). In (d) comparison is between 1x Hoagland’s1 and 0.5x MS (Duchefa, M0254).
No Zn deficiency-dependent changes in root morphology were observed in past attempts on agar-solidified 0.5x Murashige and Skoog (MS) medium over a similar cultivation period as employed in our experiments.2 We propose that minimizing contaminant Zn by using either highly pure7 or washed1 agar is of key importance in such experiments. In addition, our modified Hoagland’s medium3,4 approximates the nutrient conditions experienced by plants outside the laboratory more closely than the commonly used Murashige and Skoog (MS) medium.8 For example, the concentrations of nitrate, chloride and ammonium in MS medium are very high compared to the modified Hoagland’s medium we employed (Figure 1(d)). Ammonium, for example, has a strong negative impact on root growth that may obscure important phenotypes.9 MS medium contains iodide anions and usually (not always) cobalt cations, both of which are not essential for Arabidopsis.
Another relevant aspect of the modified Hoagland’s medium used in this study is the replacement of the general cation chelator EDTA by the more specific Fe3+ chelator N,N’-Bis(2-hydroxybenzyl)ethylenediamine-N,N’-diacetic acid (HBED). The stability constants of EDTA complexes of metals follows the sequence Fe3+ > Cu2+ > Zn2+ > Co2+ > Fe2+ > Mn2+ Ca2+ > Mg2+.10 In MS medium containing FeIII-EDTA, a proportion of the EDTA will necessarily form complexes with other cations instead of FeIII.11 Thus, altering the concentrations of any cations in MS medium will secondarily alter the proportions of cations interacting with EDTA, and this can free up cations to form other complexes. In addition, metal cations associated with phosphate, oxide, hydroxide or carbonate anions can precipitate as salts in the growth medium, which strongly reduces their bioavailability. Consequently, the manipulation of cation concentrations in media containing FeIII-EDTA and comparably high levels of phosphate, such as MS medium, may result in unexpected secondary changes in the bioavailability of other micronutrients. An unusually high level of FeIII-EDTA combined with an unusually low Cu content was shown to render MS media Cu-deficient.3
By removing cations from agar through washing in EDTA solution, we were able to reproducibly generate micronutrient metal-deficient growth conditions in agar-solidified medium without the addition of excess chelator. Our agar-solidified modified Hoagland’s medium, in which trace amounts of Zn2+ can be effectively minimized, employs a specific Fe3+ chelator and resembles more closely the nutrient profile of natural soils. Using this medium, we identified a variety of plant acclimations to physiological Zn deficiency.1 Here we report a root morphological acclimation to Zn deficiency that deserves exploration in crop plants. Given that Zn deficiency affects 50% of the land on which cereal grains are cultivated, understanding plant acclimation to such conditions is vital for the improvement of both crop yields and crop quality on such soils.12 Our work demonstrates that eliminating potentially confounding effects (trace element contamination in agar, non-physiological levels of other ions, inappropriate iron chelator) allows the effective analysis and quantification of plant responses to nutrient imbalances.
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft [DFG, grants Kr1967/3-3 and Kr1967/15-1].
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Sinclair SA, Senger T, Talke IN, Cobbett CS, Haydon MJ, Krämer U.. Systemic upregulation of MTP2- and HMA2-mediated Zn partitioning to the shoot supplements local Zn deficiency responses. Plant Cell. 2018;30:1–3. doi: 10.1105/tpc.18.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gruber BD, Giehl RFH, Friedel S, von Wirén N.. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013;163:161–179. doi: 10.1104/pp.113.218453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernal M, Casero D, Singh V, Wilson GT, Grande A, Yang H, Dodani SC, Pellegrini M, Huijser P, Connolly EL, et al. Transcriptome sequencing identifies SPL7-regulated copper acquisition genes FRO4/FRO5 and the copper dependence of iron homeostasis in Arabidopsis. Plant Cell. 2012;24:738–761. doi: 10.1105/tpc.111.090431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haydon MJ, Kawachi M, Wirtz M, Hillmer S, Hell R, Krämer U. Vacuolar nicotianamine has critical and distinct roles under iron deficiency and for zinc sequestration in Arabidopsis. Plant Cell. 2012;24(2):724–737. doi: 10.1105/tpc.111.095042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talke IN, Hanikenne M, Krämer U. Zinc-dependent global transcriptional control, transcriptional deregulation, and higher gene copy number for genes in metal homeostasis of the hyperaccumulator Arabidopsis halleri. Plant Physiol. 2006;142:148–167. doi: 10.1104/pp.105.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Péret B, Desnos T, Jost R, Kanno S, Berkowitz O, Nussaume L. Root architecture responses: in search of phosphate. Plant Physiol. 2014;166:1713–1723. doi: 10.1104/pp.114.244541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka N, Fujiwara T, Tomioka R, Krämer U, Kawachi M, Maeshima M. Characterization of the histidine-rich loop of arabidopsis vacuolar membrane zinc transporter AtMTP1 as a sensor of zinc level in the cytosol. Plant Cell Physiol. 2015;56:510–519. doi: 10.1093/pcp/pcu194. [DOI] [PubMed] [Google Scholar]
- 8.Murashige T, Skoog F, Revised A. Medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;3:473–479. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 9.Cao Y, Glass ADM, Crawford NM. Ammonium inhibition of Arabidopsis root growth can be reversed by potassium and by auxin resistance mutations aux7, axr7, and axr2. Plant Physiol. 1993;102:983–989. doi: 10.1104/pp.102.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderegg D. Critical survey of stability constants of EDTA complexes. UK: Pergamon. 1977;1–36. [Google Scholar]
- 11.Chaney RL. Plants can utilize iron form Fe‐N,N’‐di‐(2‐hydroxybenzoyl)‐ethylenediamine‐N,N’‐diacetic acid, a ferric chelate with 106 greater formation constant than Fe‐EDDHA. J Plant Nutr. 1988;11:1033–1050. doi: 10.1080/01904168809363867. [DOI] [Google Scholar]
- 12.Alloway BJ. Soil factors associated with zinc deficiency in crops and humans. Environ Geochem Health. 2009;31(5):537–548. doi: 10.1007/s10653-009-9255-4. [DOI] [PubMed] [Google Scholar]


