Abstract
Purpose of review
Physiological assessment of coronary artery disease (CAD) is an essential component of the interventional cardiology toolbox. However, despite long-term data demonstrating improved outcomes, physiology-guided percutaneous coronary intervention (PCI) remains underutilized in current practice. This review outlines the indications and technical aspects involved in evaluating coronary stenosis physiology, focusing on the latest developments in the field.
Recent findings
Beyond fractional flow reserve (FFR), non-hyperemic pressure ratios (NHPR) that assess coronary physiology at rest without hyperemia now abound. Additional advances in other alternative FFR approaches, including non-invasive coronary CT (FFRCT), invasive angiography (FFRangio), and optical coherence tomography (FFROCT), are being realized. Artificial intelligence algorithms and robust tools that enable detailed pre-procedure “virtual” intervention are also emerging.
Summary
The benefits of coronary physiological assessment to determine lesion functional significance are well established. In addition to stable CAD, coronary physiology can be especially helpful in clinical scenarios such as left main and multivessel CAD, serial lesions, non-infarct-related arteries in acute coronary syndromes, and residual ischemia post-PCI. Today, coronary physiological assessment remains an indispensable tool in the catheterization laboratory, with an exciting technological future that will further refine clinical practice and improve patient care.
Keywords: Coronary artery disease, Physiology, Angiography, Fractional flow reserve, Instantaneous wave-free ratio
Introduction
Angiographic assessment of coronary artery disease (CAD) severity is limited in providing reliable information regarding the physiological significance of coronary lesions [1]. Angiography is particularly problematic in coronary stenoses of intermediate severity (40–70% obstruction), where it predicts functional significance in < 50% of lesions [2, 3]. The rationale behind why coronary angiography fails to adequately determine lesion significance is intuitive, in that lumen narrowing is only one variable out of many that influence the flow limitation of coronary lesions [4]. Other important factors that are not readily assessed by coronary angiography include lesion length, collateral flow, and the amount and health of the downstream myocardial bed supplied [5, 6].
Invasive physiology of coronary lesions with pressure-sensing wires overcomes angiographic ambiguity by simultaneously accounting for these disparate factors through measurement of the transstenotic pressure gradient across the lesion, a concept that has been in clinical use for decades [7]. In this way, compared with angiography alone, invasive physiology measures can more precisely guide clinical decision-making regarding the need for coronary revascularization [8, 9], as well as provide objective metrics on the success of the final result [10••]. Coronary physiology–guided percutaneous coronary intervention (PCI) has been shown to be safe and effective, with improved clinical outcomes and resource utilization in stable angina and acute coronary syndromes [2, 8, 9, 10••, 11••].
While, traditionally, hyperemia-dependent fractional flow reserve (FFR) has been the primary modality employed for coronary physiological assessment, a host of invasive non-hyperemic techniques that evaluate resting coronary flow have been developed recently, reducing procedure time, cost, and potential patient discomfort [12••, 13••]. Furthermore, advances in noninvasive coronary CT imaging physiology and other novel techniques continue to emerge [14••, 15, 16•, 17•].
In the modern cardiac catheterization laboratory, becoming facile with techniques for coronary lesion physiological assessment is essential. Coronary physiology–guided PCI not only ensures that functionally significant lesions are treated, but perhaps even more critical, that non-significant lesions are appropriately deferred. However, despite a myriad of studies with long-term data demonstrating safety, efficacy, and improved patient outcomes, physiology-guided PCI remains woefully underutilized in current clinical practice. In a recent study, FFR was employed in a meager 6.1% of intermediate severity coronary lesions undergoing PCI [18]. Many factors may be responsible for the disconnect between the robustness of physiology data and its practical use, among them cost, a perceived negative impact on procedure time, and potentially a lack of system availability or understanding of the technology. This review outlines the indications for use and technical aspects for physiology evaluation of coronary stenoses, and highlights the growing menu of options available with a focus on the latest developments in the field.
Technical aspects
Fractional flow reserve
Coronary physiological assessment is achieved by measuring pressures within the coronary tree in order to infer the relative amount of myocardial blood flow supplied by a stenotic coronary artery, and expressed as a fraction of the expected maximum blood flow. Fractional flow reserve (FFR) can easily be accomplished during coronary angiography using specialized coronary guidewires with built-in pressure sensors located near the wire tip. FFR is defined as the ratio of the pressure measured proximal (aortic pressure, Pa) and distal (Pd) to the stenosis, and the calculated Pd/Pa represents the relative fraction of total blood flow across the lesion(s) of interest.
Accurate FFR determination requires pressure measurements to be performed at steady state (i.e., no pressure fluctuation with time) during maximal coronary blood flow (hyperemia). Hyperemia, a physiologic state where coronary microvascular resistance is minimized, is typically achieved by administration of intravenous continuous infusion (140 mcg/kg/min) or intracoronary bolus (right coronary artery 50–100 mcg, left coronary artery 100–200 mcg) adenosine. In general, a more consistent hyperemic effect occurs with intravenous adenosine, although this approach has potential drawbacks such as increased systemic side effects. In a normal, non-diseased coronary artery, an FFR of 1.0 is expected, indicating the absence of epicardial coronary artery obstruction to blood flow. In diseased vessels, FFR values that reach a threshold of ≤ 0.80 identify ischemia-provoking coronary stenoses with greater than 90% accuracy [19, 20].
Intracoronary injection of iodinated contrast media also induces hyperemia, and therefore may offer a natural extension to derive physiology data during routine coronary angiography. Contrast-based hyperemic FFR (cFFR) using a binary threshold of cFFR ≤ 0.83 discriminates ischemia-producing stenoses with an accuracy > 85% [21]. A microcatheter-based FFR (FFRMC) device has also been recently studied, demonstrating an optimal ischemic FFRMC cutoff value of 0.78 based on receiver operating curves [22]. However, due to its larger physical profile (maximum 0.036″ microcatheter versus 0.014″ pressure wire), in certain cases, FFRMC variability may overestimate stenosis functional severity, potentially inadvertently leading to revascularization of non-significant lesions.
Non-hyperemic pressure ratios
Non-hyperemic pressure ratios (NHPR) are able to interrogate coronary stenosis functional significance without the need for administration of hyperemic agents such as adenosine. Among NHPR options, a simple resting Pd/Pa measurement offers a non-proprietary assessment of coronary flow that correlates well with FFR and iFR at a binary threshold ≤ 0.91 [23]. Several device manufacturers have created additional NHPR using proprietary algorithms with different ischemic threshold cutoffs (Table 1). One well-studied NHPR metric, the instantaneous wave-free ratio (iFR), exploits the wave-free diastolic period in which resting intracoronary resistance is similar to that measured during hyperemic FFR [24]. In two large clinical trials, iFR demonstrated non-inferiority compared with FFR [12••, 13••]. Newer NHPR such as the diastolic hyperemia-free ratio (DFR), resting full-cycle ratio (RFR), and diastolic pressure ratio (DPR/dPR) have also been validated against FFR and iFR and found to be essentially interchangeable [25, 26, 27•].
Table 1.
Non-hyperemic pressure ratios (NHPR) used in clinical practice
| NHPR | Company | Definition | Ischemia cutoff |
|---|---|---|---|
| Pd/Pa | Generic | Average Pd/Pa during the entire cardiac cycle | ≤0.91 |
| cFFR | Generic | Average Pd/Pa during contrast-induced hyperemia | ≤0.83 |
| iFR | Phillips | Average Pd/Pa during the WFP | ≤0.89 |
| DFR | Boston Scientific | Average Pd/Pa during diastolic period when Pa < mean Pa | |
| RFR | Abbott Vascular | Lowest mean Pd/Pa during the entire cardiac cycle | |
| DPR | Opsens Medical | Average Pd/Pa during the entire diastolic period | |
| dPR | Erasmus MC | Pd/Pa during the flat period (identified using dP/dt) of WFP |
NHPR, non-hyperemic pressure ratio; WFP, wave-free diastolic period; Pd/Pa, pressuredistal/pressureaorta; cFFR, contrast FFR; DFR, diastolic hyperemia-free ratio; RFR, resting full-cycle ratio; DPR, diastolic pressure ratio; MC, medical center; dP/dt, change in pressure/change in times
Indications and guidelines for coronary physiological assessment
According to the most recent American College of Cardiology (ACC)/American Heart Association (AHA)/Society for Cardiovascular Angiography and Interventions (SCAI) guidelines on coronary revascularization, physiological assessment is reasonable for the evaluation of intermediate severity lesions and can be helpful in guiding revascularization decisions (class IIa, level A) [28]. A recent SCAI consensus statement on the use of coronary physiology recommends expanded utilization of physiological assessment when non-invasive stress imaging is uncertain, non-diagnostic, or unavailable [29]. Furthermore, physiologic lesion assessments may justify PCI in stenoses of intermediate angiographic severity, or when there is angiographic uncertainty [29]. The European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS) also make strong recommendations for physiological testing to assess the hemodynamic relevance of intermediate-grade stenoses [30]. In support, the appropriate use criteria on coronary revascularization for stable CAD endorse the use of physiological assessment in both single and multivessel CAD [31].
Correlation with intravascular imaging
Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) intracoronary imaging offer high-resolution tomographic views of the lumen and vessel wall, providing extensive anatomical detail that is beneficial for guiding stent implantation over angiography alone [32–34]. However, intracoronary imaging performs relatively poorly when compared with invasive physiology for determining lesion functional significance. In a prospective registry of 350 patients with 367 intermediate severity lesions, IVUS minimal luminal area (MLA) showed only a moderate correlation with FFR [35]. Similarly, an OCT cutoff MLA of 4 mm2 had a strong negative correlation with FFR, but a poor positive correlation (approximately 50%). Taken together, intracoronary imaging is more helpful in exonerating non-significant lesions [36], and in general physiology measures should be the primary method used to evaluate coronary lesion functional significance over IVUS or OCT.
Evidence for utilization
The DEFER, FAME, and FAME-2 randomized, prospective trials have established that an FFR-guided strategy to defer or perform PCI in patients with stable CAD is safe, is economically advantageous, and reduces long-term major adverse cardiac events including the need for urgent revascularization [2, 37–40, 41••]. Extending these data to NHPR metrics, the iFR SWEDEHEART and DEFINE-FLAIR trials demonstrated that iFR is non-inferior to FFR with respect to predicting clinical outcomes [12••, 13••]. Furthermore, without the need to induce hyperemia in the iFR group, adverse procedural reactions were lower and procedural times shorter with iFR compared with FFR [13••]. A recent meta-analysis of patients who were deferred revascularization in iFR SWEDEHEART and DEFINE-FLAIR trials revealed that PCI deferral is equally safe with both iFR and FFR, with a low (~ 4%) MACE rate in both groups at 1-year follow-up [11••]. Interestingly, a sub-group analysis of the patients with left anterior descending (LAD) lesions in DEFINE-FLAIR demonstrated lower MACE when deferral was based on iFR (2.44%) as compared with FFR (5.26%) [42••]. This difference is hypothesized to be related to greater correlation between iFR and coronary flow reserve [43, 44], a strong predictor of MACE [45]. Given the solid evidence base supported by society guidelines recommending its use, we strongly recommend the liberal use of invasive physiology to gauge the functional significance of coronary stenoses (Fig. 1), particularly in angiographically intermediate lesions.
Fig. 1.
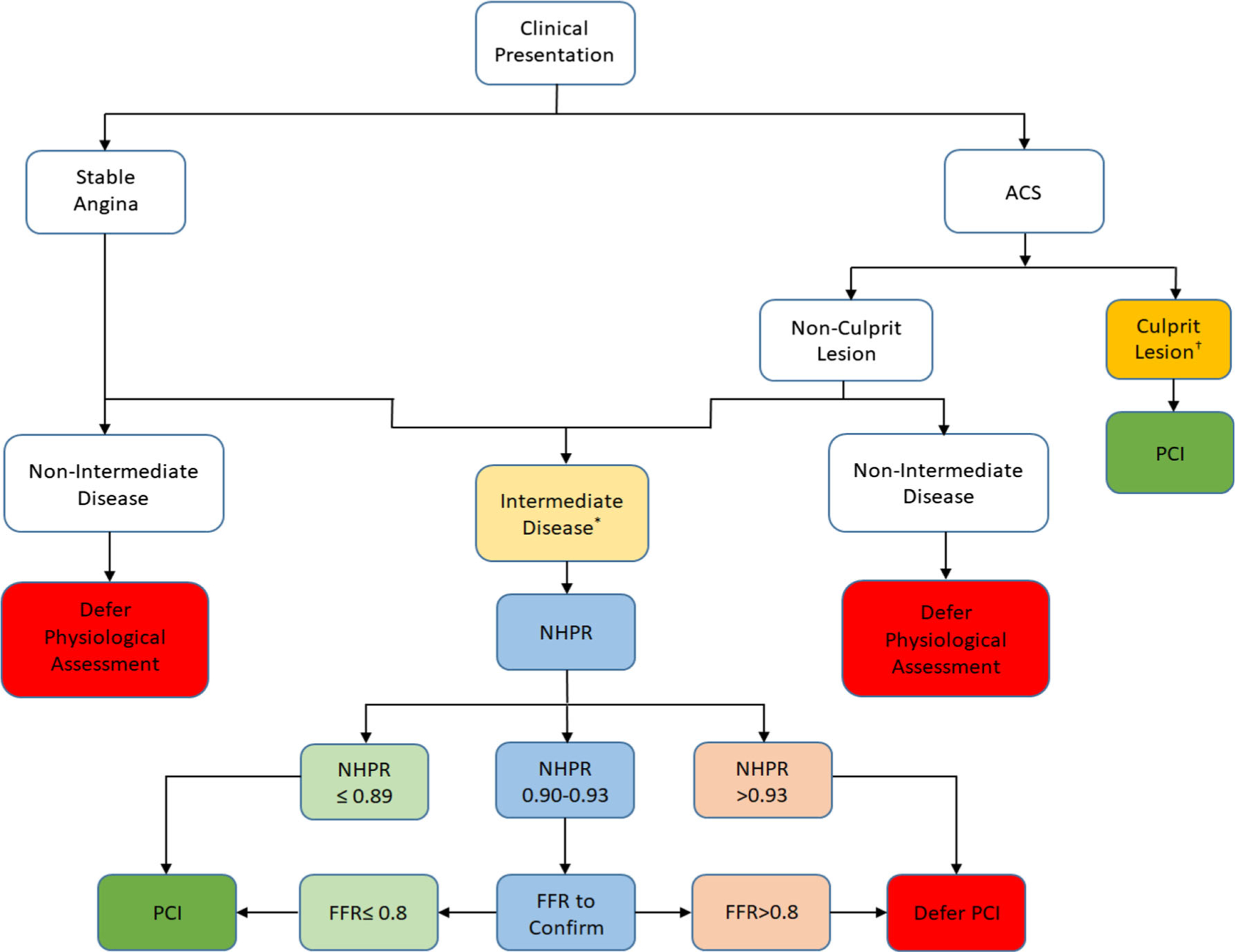
A recommended approach to pre-PCI coronary physiological assessment.
NHPR, non-hyperemic pressure ratio; PCI, percutaneous coronary intervention; FFR, fractional flow reserve
* Intermediate disease is defined as stenosis severity of 40–70% † Physiological assessment should be avoided early after MI in infarct-related arteries
Special clinical scenarios
Acute coronary syndromes
Following myocardial infarction (MI), not only is the amount of distal myocardial bed compromised to a variable degree, but also a concomitant increase in left ventricular end-diastolic pressure (LVEDP) can precipitate heightened microvascular resistance that falsely increases the measured FFR in the culprit vessel [46]. With time after the inciting event, as the remaining viable myocardium in the affected region recovers, hyperemic flow may then increase in the infarct-related artery leading to a subsequent FFR reduction. Thus, during the initial hours after MI, physiological assessment of stenoses within the infarct-related artery can be unreliable, reporting a false negative result when the lesion is in fact hemodynamically significant. Invasive physiology of the culprit artery should therefore generally be avoided until at least 4 days after presentation with an acute coronary syndrome [47].
In contrast to culprit vessels, physiological investigation can reliably play a role in the assessment of non-infarct-related arteries. In the FAMOUS-NSTEMI randomized trial, subjects presenting with non-ST-segment elevation myocardial infarction (NSTEMI) had a lower rate of revascularization in the FFR-guided group as compared with angiography guidance alone, with otherwise no detectable difference in health outcomes and quality of life between the two populations [48]. In ST-segment elevation myocardial infarction (STEMI) patients, complete revascularization improved cardiovascular outcomes when FFR-guided revascularization decisions were implemented for non-culprit lesions [49••, 50••, 51, 52••].
Left main coronary artery stenosis
Left main coronary artery (LMCA) stenosis, a high-risk lesion subset, remains among the most difficult coronary locations to evaluate angiographically due to its size and often lack of a clear non-diseased segment for reference. In addition, LMCA disease can involve a range of locations that impact diagnostic and interventional decision-making, including the ostium, mid-body, or distal vessel with or without involvement of the LAD/left circumflex (LCx) origins. When performing physiological assessment of an ostial LMCA stenosis, additional care must be taken to avoid guide catheter damping during system equalization and physiological measurements by actively disengaging the guide from the coronary artery.
Numerous studies have demonstrated that LMCA lesions are accurately evaluated by FFR when there is isolated LMCA disease and no significant stenoses located downstream [53, 54]. However, if the isolated LMCA disease involves the distal LMCA abutting the bifurcation, invasive physiology measures should be undertaken with the wire sequentially placed in the LAD and LCx, and the lower value used for decision-making. In FFR situations that involve LMCA stenosis plus disease in either the LAD or LCx, there are theoretical concerns that reductions in flow in the downstream diseased vessel may result in an underestimation of stenosis severity (i.e., falsely elevated FFR) and inappropriate deferral of LMCA revascularization [55•]. While this interaction is recognized, the impact of downstream disease on flow-mediated FFR variation in human studies was found to likely be clinically insignificant if the pressure wire is placed in the non-diseased vessel, except perhaps in rare situations where the diseased vessel is extremely severe (FFR < 0.45) [56]. In this scenario, and in cases with left main disease and concomitant disease in LAD and LCx, IVUS minimal lumen area assessment is recommended [57].
Aortic stenosis
Coronary hemodynamics are influenced by aortic stenosis as evident by FFR variations after transcatheter aortic valve replacement [58]. Aortic stenosis can lead to an underestimation of the functional severity of intermediate lesions, with a reported misclassification incidence of 6–8% [58]. Furthermore, in severe aortic stenosis patients, the conventional iFR cutoff has a lower diagnostic agreement with FFR [59], and FFR may be preferred over iFR due to iFR exhibiting greater false positives in a small study employing the typical iFR ischemic threshold [60]. Given that the available data in this population is currently limited, there remains significant uncertainty in the accuracy of coronary physiological assessment in aortic stenosis, and therefore, caution should be taken interpreting invasive physiology measures in these patients.
Multivessel disease
The FAME-2 trial showed that FFR-guided PCI plus guideline-directed medical therapy was superior to medical management alone in patients with stable, multivessel CAD [8, 10••]. In this trial, a lower primary end point of all-cause death, nonfatal MI, and repeat revascularization in the FFR-guided PCI group (4.3% versus 12.7%) was observed, with the difference driven primarily by a greater need for urgent revascularization due to acute coronary syndrome in subjects treated with medical therapy alone [8, 10••]. The ongoing FAME-3 randomized trial will investigate whether an FFR-guided PCI approach using contemporary drug-eluting stents in patients with multivessel CAD is a non-inferior revascularization strategy compared with coronary artery bypass graft (CABG) surgery [61].
Assessment prior to CABG
There is limited data regarding the utility of performing physiological assessment of intermediate severity lesions in patients referred for CABG surgery. An observational study of 627 patients demonstrated similar MACE but a lower rate of angina in the FFR-guided revascularization group compared with angiography alone (31% versus 47%) at 3-year follow-up [62]. A small study also reported a higher rate of subsequent graft closure when bypass grafts were implanted on hemodynamically non-significant stenoses as assessed by FFR [63]. While additional investigations are needed, the available data imply that physiology-guided CABG revascularization may have benefit over the current clinical standard approach of grafting all available vessels of sufficient size with lesions deemed angiographically significant. New and emerging physiology techniques such as FFRCT and FFRangio that can assess lesion functional significance without the need for invasive wire-based physiology measures may eventually change the calculus for multivessel and pre-CABG assessment.
Diffuse disease and serial lesions
The clinical significance of moderate diffuse disease and long lesions can be uncertain. As these lesions may not be associated with a visually severe stenosis, in-depth physiological assessment with a pressure wire pullback from distal to proximal within the vessel can more precisely identify the location(s) of hemodynamically significant pressure loss. In diffuse atherosclerosis, the pressure gradient may be gradual and mild in some segments, while other regions may demonstrate a sharp pressure change across specific areas that contain the most functionally significant lesions. The information obtained from a pressure wire pullback can be very useful for pre-PCI planning in diffuse disease, such that stents can be positioned strategically in a parsimonious manner by intervening on only the functionally significant areas that will maximize post-PCI blood flow.
Given that FFR measurement relies upon maximal hyperemia, in cases where there is a need to interrogate individual areas within diffuse disease or isolate the relative significance of focal serial lesions, FFR is suboptimal as it is challenging to deconstruct the relative contribution of individual lesions in a state of maximal hyperemia. On the other hand, NHPR, which by definition measure physiology in the resting state, are attractive options for pullback studies to create an accurate physiological map of lesion severity. The availability of iFR pullback data has been shown to alter revascularization procedural planning in nearly one-third of patients [64••, 65].
Application of co-registration technology that combines NHPR pullback information with angiographic images can enable overlay of the physiological maps directly onto the coronary anatomy for precise localization of significant lesions. Hyperemic pullback pressure gradients (PPGs), during which a motorized pressure pullback device mechanically withdraws the pressure wire at a predefined speed (1 mm/s), are currently being investigated as adjunctive physiologic tools. PPGs can be used to establish patterns of coronary artery disease as focal, diffuse, or a combination of both phenotypes. A recent prospective multicenter study demonstrated that addition of PPGs led to vessel disease pattern reclassification in > 30% [66].
Discrepancies between hyperemic and non-hyperemic indices
A meta-analysis of 23 studies comparing iFR with FFR found a good correlation (correlation coefficient 0.80 (0.78–0.82), p < 0.001) between the two indices [67]. However, resting and hyperemic discordance has been reported in approximately 14–22% of cases, with the majority of these being iFR negative discordant (FFR+/iFR−) [43, 68, 69]. LMCA or proximal LAD stenosis, diffuse disease, more severe lesions, younger age, and a slower heart rate were predictors of a negative discordant iFR [69, 70]. FFR+/iFR− discordance has been explained by high coronary flow rates enhancing trans-lesional flow, which can result in lower measured FFR values [43].
In cases of a borderline NHPR value that falls within the gray zone of a specific NHPR metric, we recommend performing a hyperemic assessment with FFR and proceeding with intervention if the subsequent FFR is positive (Fig. 1). In this way, FFR can be utilized as an arbiter for ambiguous NHPR values, as FFR has been extensively validated in a wide variety of clinical settings with long-term data available. Nevertheless, as long as one index is negative, there may not be a clinical penalty to deferring intervention of lesions with discordant FFR/iFR results and pursuing an upfront treatment strategy of guideline-directed medical therapy. In a recent study of 840 lesions in which PCI was deferred, where 109 lesions had discordant FFR/iFR results with 40 of these being iFR negative discordant, there was no significant difference in all-cause death, myocardial infarction, or need for revascularization at 5-year follow-up [71••].
Assessment of residual ischemia post-PCI
There is an unacceptably high prevalence (approximately 20–30%) of persistent angina in patients within the first year after PCI [2, 72, 73]. With the use of physiology guidance, post-PCI angina can be improved by at least 50% [2, 74]. However, post-PCI physiology is rarely performed in clinical practice, even when physiology was utilized for pre-PCI determination of lesion functional significance. Conceptually, coronary physiology performed after PCI should increase above the ischemic threshold, where a greater physiological improvement corresponds to a larger amount of patient symptomatic relief and improved clinical outcomes [75]. In contrast, a low residual post-PCI physiology value has been linked to a higher risk of target vessel failure [76•], and a higher incidence of MACE [77••]. The potential magnitude of this problem is suggested by a recent study in which 24% of patients left the cardiac catheterization lab with residual ischemia (iFR ≤ 0.89) despite operator-assessed angiographically successful PCI [78••]. Notably, 82% of these cases with residual pressure gradients identified by invasive physiology were focal, and thus potentially amenable to treatment optimization with additional intervention. This data highlights the potential role of post-intervention physiology guidance to improve procedural and clinical outcomes, although additional studies are needed to define optimal targets.
Future directions
Non-invasive FFR
Non-invasive technologies, most notably FFRCT, that utilize three-dimensional coronary reconstructions and computational fluid dynamics models are able to solve the hemodynamic profile within the coronary arteries and determine FFR measures. FFRCT has been shown to have good accuracy when compared with invasively measured FFR [14••, 79–81]. Although limited by one in four nonevaluable studies, FFRCT had slightly higher diagnostic accuracy compared with non-invasive coronary CTA and SPECT, but not PET imaging [82, 83]. As FFRCT holds potential to reduce the need for invasive coronary physiology assessment in patients with suspected CAD symptoms and improve pre-PCI planning for those that require intervention, there has been substantial interest in developing “wireless” FFRCT physiological assessment tools and clinical algorithms [15]. A negative FFRCT may hold important prognostic implications, according to recently published 1-year outcomes from the ADVANCE FFRCT Registry [84]. Compared with patients with abnormal FFRCT values, those with a negative FFRCT had lower revascularization rates and a trend toward lower overall MACE including MI and cardiovascular death [84]. Furthermore, in patients with LMCA or multivessel disease, the SYNTAX III Revolution trial showed that treatment decisions between CABG and PCI were feasible based on coronary CTA with FFRCT alone, as compared with conventional invasive coronary angiography [85, 86]. In a significant step forward for FFRCT applications, the U.S. Food and Drug Administration recently approved the HeartFlow® Planner (HeartFlow, Redwood City, CA), a non-invasive FFRCT virtual modeling tool that enables non-invasive exploration and simulation of PCI treatment options prior to embarking on the intervention.
Angiography-derived FFR
FFR derived from routine coronary angiography (FFRangio), a technique based on quantitative coronary angiography, offers the potential to reduce the need for invasive guidewire placement in order to evaluate the physiological significance of coronary stenoses. A recent international FFRangio study of 319 vessels in 301 patients demonstrated that FFRangio (as compared with conventional FFR) had a high sensitivity (94%), specificity (91%), and diagnostic accuracy (92%) [87]. FFRangio performed after successful PCI has also shown ability to predict adverse events, where vessels with FFRangio < 0.89 exhibited a threefold greater risk [88]. Similar to FFRangio, other angiography-based FFR platforms are being developed such as virtual FFR (vFFR). A proof-of-concept 54-patient study (59 vessels) showed that vFFR and invasive FFR closely tracked together with high accuracy before and after PCI (± 0.02–0.05 variation) [16•]. Assuming that appropriate angiographic views can be obtained of the coronary region of interest, and with continued improvements in data processing speeds, angiography-derived FFR may provide a simple, rapid method for lesion physiological assessment before and after PCI, thus improving the diagnostic yield of information gained during coronary angiography.
Hybrid assessment
A major limitation of currently available technologies is that physiological and anatomic coronary evaluations require separate assessments with separate tools. Integration of physiology and anatomy into single hybrid devices thus holds significant potential to improve the efficiency and clinical assessment of coronary pathology. Optical coherence tomography (OCT)–based FFR (FFROCT) has shown promise in this regard, where, in a single catheter pullback, lesion anatomy can be assessed with high-resolution, and then the tomographic data utilized to reconstruct physiology metrics. Compared with invasive FFR, a recent study (118 patients, 125 vessels) demonstrated that FFROCT exhibited good sensitivity (87%), specificity (92%), and positive (92%) and negative predictive values (88%), with an overall accuracy of 90% [17•]. FFROCT has also shown promise in assessing tandem lesion physiology, with early results revealing good agreement with wire-based invasive physiology [89].
Artificial intelligence
Interpretation of large volumes of coronary physiology data can be complex, and in certain cases subjective and dependent on operator expertise. As evident presently across many clinical areas, artificial intelligence (AI) applications offer potential data handling and automated analysis solutions to improve decision-making for invasive physiology studies. An AI-centered approach was examined in the CEREBRIA-1 study, which included 1008 iFR pullbacks evaluated by both AI and a panel of interventional cardiologists [90•]. In this investigation, AI was found to be non-inferior to expert consensus decision for both PCI appropriateness and determining treatment strategy [90•]. While AI offers substantial promise as a physiological co-pilot guiding interventional decisions, additional studies are required to support future clinical use of this emerging concept.
Conclusion
Coronary physiological assessment is an essential interventional tool that overcomes the ambiguity of coronary angiography by quantitatively determining lesion functional significance. Supported by strong clinical evidence and guideline recommendations, a physiology-guided approach to CAD management informs lesion selection for intervention or deferral, leading to greater appropriateness of revascularization for only those lesions that truly cause ischemia. In addition, coronary physiology data provides crucial information to help formulate an optimal intervention strategy and enables objective evaluation of the final PCI result. Continued technological advances have led to the development of new invasive physiology techniques, notable the family of NHPR measures, as well as growing data supporting non-invasive physiology modalities such as FFRCT. Overall, these developments and more are spawning a golden age for coronary physiology, in which physiological CAD assessment is poised to enable improved safety, efficiency, and successful outcomes for patients.
Footnotes
Conflict of InterestEric A. Osborn reports personal fees from Abbott Vascular and Dyad Medical, outside the submitted work. Mohsin Chowdhury declares that he has no conflict of interest.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55(25):2816–21. 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 2.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–24. 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 3.Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56(3):17–84. 10.1016/j.jacc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92(8):2333–42. 10.1161/01.cir.92.8.2333. [DOI] [PubMed] [Google Scholar]
- 5.Katritsis D, Webb-Peploe M. Limitations of coronary angiography: an underestimated problem? Clin Cardiol. 1991;14(1):20–4. 10.1002/clc.4960140106. [DOI] [PubMed] [Google Scholar]
- 6.Halon DA. Can angiography predict physiology? Int J Cardiol. 2018;270:74–5. 10.1016/j.ijcard.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Anderson HV, Roubin GS, Leimgruber PP, Cox WR, Douglas JS Jr, King SB 3rd, et al. Measurement of transstenotic pressure gradient during percutaneous transluminal coronary angioplasty. Circulation. 1986;73(6):1223–30. 10.1161/01.cir.73.6.1223. [DOI] [PubMed] [Google Scholar]
- 8.De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991–1001. 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 9.De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371(13):1208–17. 10.1056/NEJMoa1408758. [DOI] [PubMed] [Google Scholar]
- 10.••.Xaplanteris P, Fournier S, NHJ P, Fearon WF, Barbato E, Tonino PAL, et al. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med. 2018;379(3):250–9. 10.1056/NEJMoa1803538. [DOI] [PubMed] [Google Scholar]; This study demonstrated that an FFR-guided PCI strategy along with optimal medial therapy for multi-vessel stable coronary artery disease decreased composite outcome of death, MI, and urgent revascularization at 5-year follow-up as compared with optimal medical therapy alone.
- 11.••.Escaned J, Ryan N, Mejia-Renteria H, Cook CM, Dehbi HM, Alegria-Barrero E, et al. Safety of the deferral of coronary revascularization on the basis of instantaneous wave-free ratio and fractional flow reserve measurements in stable coronary artery disease and acute coronary syndromes. JACC Cardiovasc Interv. 2018;11(15):1437–49. 10.1016/j.jcin.2018.05.029. [DOI] [PubMed] [Google Scholar]; A meta-analysis of patients who were deferred revascularization in the iFR SWEDEHEART and DEFINE-FLAIR trials showed that PCI deferral is equally safe with both iFR and FFR, with a low rate of adverse events at 1-year follow-up.
- 12.••.Gotberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med. 2017;376(19):1813–23. 10.1056/NEJMoa1616540. [DOI] [PubMed] [Google Scholar]; The iFR SWEDEHEART study established the non-inferiority of an iFR-guided revascularization strategy in patients with stable angina and acute coronary syndrome as compared with FFR-guided revascularization.
- 13.••.Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer SS, et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;376(19):1824–34. 10.1056/NEJMoa1700445. [DOI] [PubMed] [Google Scholar]; This large study (DEFINE-FLAIR) demonstrated that iFR-guided PCI is non-inferior to FFR-guided revascularization.
- 14.••.Norgaard BL, Terkelsen CJ, Mathiassen ON, Grove EL, Botker HE, Parner E, et al. Coronary CT angiographic and flow reserve-duided management of patients with stable ischemic heart disease. J Am Coll Cardiol. 2018;72(18):2123–34. 10.1016/j.jacc.2018.07.043. [DOI] [PubMed] [Google Scholar]; This important clinical study assessed the accuracy of noninvasive coronary CT physiology assessment (FFRCT) as compared with invasive physiological modalities.
- 15.Colleran R, Douglas PS, Hadamitzky M, Gutberlet M, Lehmkuhl L, Foldyna B, et al. An FFRCT diagnostic strategy versus usual care in patients with suspected coronary artery disease planned for invasive coronary angiography at German sites: one-year results of a subgroup analysis of the PLATFORM (Prospective Longitudinal Trial of FFRCT: Outcome and Resource Impacts) study. Open Heart. 2017;4(1):e000526 10.1136/openhrt-2016-000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.•.Gosling RC, Morris PD, Silva Soto DA, Lawford PV, Hose DR, Gunn JP. Virtual coronary intervention: a treatment planning tool based upon the angiogram. JACC Cardiovasc Imaging. 2019;12(5):865–72. 10.1016/j.jcmg.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; This proof-of-concept study demonstrated that virtual FFR (vFFR) utilizing angiography with 3-dimensional (3D) reconstructions can assess physiology with high accuracy compared with invasive FFR.
- 17.•.Yu W, Huang J, Jia D, Chen S, Raffel OC, Ding D, et al. Diagnostic accuracy of intracoronary optical coherence tomography-derived fractional flow reserve for assessment of coronary stenosis severity. EuroIntervention. 2019;15(2):189–97. 10.4244/EIJ-D-19-00182. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that a hybrid FFR modality employing intracoronary optical coherence tomography (FFROCT) has a good overall accuracy as compared with traditional invasive FFR.
- 18.Dattilo PB, Prasad A, Honeycutt E, Wang TY, Messenger JC. Contemporary patterns of fractional flow reserve and intravascular ultrasound use among patients undergoing percutaneous coronary intervention in the United States: insights from the National Cardiovascular Data Registry. J Am Coll Cardiol. 2012;60(22):2337–9. 10.1016/j.jacc.2012.08.990. [DOI] [PubMed] [Google Scholar]
- 19.Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JKJJ, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334(26):1703–8. 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 20.Pijls NH, Van Gelder B, Van der Voort P, Peels K, Bracke FA, Bonnier HJ, et al. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995;92(11):3183–93. 10.1161/01.cir.92.11.3183. [DOI] [PubMed] [Google Scholar]
- 21.Johnson NP, Jeremias A, Zimmermann FM, Adjedj J, Witt N, Hennigan B, et al. Continuum of vasodilator stress from rest to contrast medium to adenosine hyperemia for fractional flow reserve assessment. JACC Cardiovasc Interv. 2016;9(8):757–67. 10.1016/j.jcin.2015.12.273. [DOI] [PubMed] [Google Scholar]
- 22.Demir OM, Mitomo S, Mangieri A, Ancona MB, Regazzoli D, Lanzillo G, et al. Diagnostic accuracy of microcatheter derived fractional flow reserve. Am J Cardiol. 2019;124(2):183–9. 10.1016/j.amjcard.2019.04.038. [DOI] [PubMed] [Google Scholar]
- 23.Leone AM, Scalone G, De Maria GL, Tagliaferro F, Gardi A, Clemente F, et al. Efficacy of contrast medium induced Pd/Pa ratio in predicting functional significance of intermediate coronary artery stenosis assessed by fractional flow reserve: insights from the RINASCI study. EuroIntervention. 2015;11(4):421–7. 10.4244/EIJY14M07_02. [DOI] [PubMed] [Google Scholar]
- 24.Sen S, Escaned J, Malik IS, Mikhail GW, Foale RA, Mila R, et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol. 2012;59(15):1392–402. 10.1016/j.jacc.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Johnson NP, Li W, Chen X, Hennigan B, Watkins S, Berry C, et al. Diastolic pressure ratio: new approach and validation vs. the instantaneous wave-free ratio. Eur Heart J. 2019;40(31):2585–94. 10.1093/eurheartj/ehz230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svanerud J, Ahn JM, Jeremias A, van’t Veer M, Gore A, Maehara A, et al. Validation of a novel non-hyperaemic index of coronary artery stenosis severity: the Resting Full-cycle Ratio (VALIDATE RFR) study. EuroIntervention. 2018;14(7):806–14. 10.4244/EIJ-D-18-00342. [DOI] [PubMed] [Google Scholar]
- 27.•.Van’t Veer M, Pijls NHJ, Hennigan B, Watkins S, Ali ZA, De Bruyne B, et al. Comparison of different diastolic resting indexes to iFR: are they all equal? J Am Coll Cardiol. 2017;70(25):3088–96. 10.1016/j.jacc.2017.10.066. [DOI] [PubMed] [Google Scholar]; This study established that existing diastolic NHPR resting indices are not significantly different compared with iFR and correlate well with FFR.
- 28.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2013;82(4):E266–355. 10.1002/ccd.23390. [DOI] [PubMed] [Google Scholar]
- 29.Lotfi A, Davies JE, Fearon WF, Grines CL, Kern MJ, Klein LW. Focused update of expert consensus statement: use of invasive assessments of coronary physiology and structure: a position statement of the society of cardiac angiography and interventions. Catheter Cardiovasc Interv. 2018;92(2):336–47. 10.1002/ccd.27672. [DOI] [PubMed] [Google Scholar]
- 30.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Kardiol Pol. 2018;76(12):1585–664. 10.5603/KP.2018.0228. [DOI] [PubMed] [Google Scholar]
- 31.Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease : a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Nucl Cardiol. 2017;24(5):1759–92. 10.1007/s12350-017-0917-9. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Gao X, Kan J, Ge Z, Han L, Lu S, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: the ULTIMATE trial. J Am Coll Cardiol. 2018;72(24):3126–37. 10.1016/j.jacc.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Choi KH, Song YB, Lee JM, Lee SY, Park TK, Yang JH, et al. Impact of intravascular ultrasound-guided percutaneous coronary intervention on long-term clinical outcomes in patients undergoing complex procedures. JACC Cardiovasc Interv. 2019;12(7):607–20. 10.1016/j.jcin.2019.01.227. [DOI] [PubMed] [Google Scholar]
- 34.Jones DA, Rathod KS, Koganti S, Hamshere S, Astroulakis Z, Lim P, et al. Angiography alone versus angiography plus optical coherence tomography to guide percutaneous coronary intervention: outcomes from the Pan-London PCI Cohort. JACC Cardiovasc Interv. 2018;11(14):1313–21. 10.1016/j.jcin.2018.01.274. [DOI] [PubMed] [Google Scholar]
- 35.Waksman R, Legutko J, Singh J, Orlando Q, Marso S, Schloss T, et al. FIRST: Fractional Flow Reserve and Intravascular Ultrasound Relationship Study. J Am Coll Cardiol. 2013;61(9):917–23. 10.1016/j.jacc.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalo N, Escaned J, Alfonso F, Nolte C, Rodriguez V, Jimenez-Quevedo P, et al. Morphometric assessment of coronary stenosis relevance with optical coherence tomography: a comparison with fractional flow reserve and intravascular ultrasound. J Am Coll Cardiol. 2012;59(12):1080–9. 10.1016/j.jacc.2011.09.078. [DOI] [PubMed] [Google Scholar]
- 37.Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103(24):2928–34. 10.1161/01.cir.103.24.2928. [DOI] [PubMed] [Google Scholar]
- 38.Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van’t Veer M, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49(21):2105–11. 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 39.van Nunen LX, Zimmermann FM, Tonino PA, Barbato E, Baumbach A, Engstrom T, et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomised controlled trial. Lancet. 2015;386(10006):1853–60. 10.1016/S0140-6736(15)00057-4. [DOI] [PubMed] [Google Scholar]
- 40.Marso SP, Mercado N, Maehara A, Weisz G, Mintz GS, McPherson J, et al. Plaque composition and clinical outcomes in acute coronary syndrome patients with metabolic syndrome or diabetes. J Am Coll Cardiol Img. 2012;5(3 Suppl):S42–52. 10.1016/j.jcmg.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 41.••.Fearon WF, Nishi T, De Bruyne B, Boothroyd DB, Barbato E, Tonino P, et al. Clinical outcomes and cost-effectiveness of fractional flow reserve-guided percutaneous coronary intervention in patients with stable coronary artery disease: three-year follow-up of the FAME 2 Trial (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation). Circulation. 2018;137(5):480–7. 10.1161/CIRCULATIONAHA.117.031907. [DOI] [PubMed] [Google Scholar]; This trial demonstrated the safety and effectiveness of FFR-guided multi-vessel PCI in stable coronary artery disease.
- 42.••.Sen S, Ahmad Y, Dehbi HM, Howard JP, Iglesias JF, Al-Lamee R, et al. Clinical events after deferral of LAD revascularization following physiological coronary assessment. J Am Coll Cardiol. 2019;73(4):444–53. 10.1016/j.jacc.2018.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study investigated the safety of physiology-guided deferral of left anterior descending (LAD) coronary artery disease.
- 43.Cook CM, Jeremias A, Petraco R, Sen S, Nijjer S, Shun-Shin MJ, et al. Fractional flow reserve/instantaneous wave-free ratio discordance in angiographically intermediate coronary stenoses: an analysis using Doppler-derived coronary flow measurements. JACC Cardiovasc Interv. 2017;10(24):2514–24. 10.1016/j.jcin.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petraco R, van de Hoef TP, Nijjer S, Sen S, van Lavieren MA, Foale RA, et al. Baseline instantaneous wave-free ratio as a pressure-only estimation of underlying coronary flow reserve: results of the JUSTIFY-CFR Study (Joined Coronary Pressure and Flow Analysis to Determine Diagnostic Characteristics of Basal and Hyperemic Indices of Functional Lesion Severity-Coronary Flow Reserve). Circ Cardiovasc Interv. 2014;7(4):492–502. 10.1161/CIRCINTERVENTIONS.113.000926. [DOI] [PubMed] [Google Scholar]
- 45.Lee JM, Choi KH, Hwang D, Park J, Jung JH, Kim HY, et al. Prognostic implication of thermodilution coronary flow reserve in patients undergoing fractional flow reserve measurement. JACC Cardiovasc Interv. 2018;11(15):1423–33. 10.1016/j.jcin.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Van Herck PL, Carlier SG, Claeys MJ, Haine SE, Gorissen P, Miljoen H, et al. Coronary microvascular dysfunction after myocardial infarction: increased coronary zero flow pressure both in the infarcted and in the remote myocardium is mainly related to left ventricular filling pressure. Heart. 2007;93(10):1231–7. 10.1136/hrt.2006.100818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samady H, Lepper W, Powers ER, Wei K, Ragosta M, Bishop GG, et al. Fractional flow reserve of infarct-related arteries identifies reversible defects on noninvasive myocardial perfusion imaging early after myocardial infarction. J Am Coll Cardiol. 2006;47(11):2187–93. 10.1016/j.jacc.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 48.Layland J, Oldroyd KG, Curzen N, Sood A, Balachandran K, Das R, et al. Fractional flow reserve vs. angiography in guiding management to optimize outcomes in non-ST-segment elevation myocardial infarction: the British Heart Foundation FAMOUS-NSTEMI randomized trial. Eur Heart J. 2015;36(2):100–11. 10.1093/eurheartj/ehu338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.••.Smits PC, Abdel-Wahab M, Neumann FJ, Boxma-de Klerk BM, Lunde K, Schotborgh CE, et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376(13):1234–44. 10.1056/NEJMoa1701067. [DOI] [PubMed] [Google Scholar]; This randomized controlled trial demonstrated that FFR-guided complete revascularization with PCI of the non-infarct related artery reduces major adverse cardiac events.
- 50.••.Lonborg J, Engstrom T, Kelbaek H, Helqvist S, Klovgaard L, Holmvang L, et al. Fractional flow reserve-guided complete revascularization improves the prognosis in patients with ST-segment-elevation myocardial infarction and severe nonculprit disease: a DANAMI 3-PRIMULTI Substudy (Primary PCI in Patients With ST-Elevation Myocardial Infarction and Multivessel Disease: Treatment of Culprit Lesion Only or Complete Revascularization). Circ Cardiovasc Interv. 2017;10(4). 10.1161/CIRCINTERVENTIONS.116.004460. [DOI] [PubMed] [Google Scholar]; In this trial, the benefits of an FFR-guided complete revascularization strategy were assessed in patients presenting with ST-segment elevation myocardial infarciton (STEMI).
- 51.Engstrom T, Kelbaek H, Helqvist S, Hofsten DE, Klovgaard L, Holmvang L, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665–71. 10.1016/s0140-6736(15)60648-1. [DOI] [PubMed] [Google Scholar]
- 52.••.Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019;381(15):1411–21. 10.1056/NEJMoa1907775. [DOI] [PubMed] [Google Scholar]; This recent study also demonstrates the benefits of pursuing complete revascularization in patients presenting with myocardial infarction.
- 53.Mallidi J, Atreya AR, Cook J, Garb J, Jeremias A, Klein LW, et al. Long-term outcomes following fractional flow reserve-guided treatment of angiographically ambiguous left main coronary artery disease: a meta-analysis of prospective cohort studies. Catheter Cardiovasc Interv. 2015;86(1):12–8. 10.1002/ccd.25894. [DOI] [PubMed] [Google Scholar]
- 54.Hamilos M, Muller O, Cuisset T, Ntalianis A, Chlouverakis G, Sarno G, et al. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation. 2009;120(15):1505–12. 10.1161/CIRCULATIONAHA.109.850073. [DOI] [PubMed] [Google Scholar]
- 55.•.Modi BN, van de Hoef TP, Piek JJ, Perera D. Physiological assessment of left main coronary artery disease. EuroIntervention. 2017;13(7):820–7. 10.4244/EIJ-D-17-00135. [DOI] [PubMed] [Google Scholar]; This study highlights the challenges associated with physiological assessment of left main coronary artery disease.
- 56.Fearon WF, Yong AS, Lenders G, Toth GG, Dao C, Daniels DV, et al. The impact of downstream coronary stenosis on fractional flow reserve assessment of intermediate left main coronary artery disease: human validation. JACC Cardiovasc Interv. 2015;8(3):398–403. 10.1016/j.jcin.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 57.Ramadan R, Boden WE, Kinlay S. Management of left main coronary artery disease. J Am Heart Assoc. 2018;7(7). 10.1161/JAHA.117.008151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scarsini R, Pesarini G, Zivelonghi C, Piccoli A, Ferrero V, Lunardi M, et al. Physiologic evaluation of coronary lesions using instantaneous wave-free ratio (iFR) in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. EuroIntervention. 2018;13(13):1512–9. 10.4244/EIJ-D-17-00542. [DOI] [PubMed] [Google Scholar]
- 59.Pesarini G, Scarsini R, Zivelonghi C, Piccoli A, Gambaro A, Gottin L, et al. Functional assessment of coronary artery disease in patients undergoing transcatheter aortic valve implantation: influence of pressure overload on the evaluation of lesions severity. Circ Cardiovasc Interv. 2016;9(11). 10.1161/CIRCINTERVENTIONS.116.004088. [DOI] [PubMed] [Google Scholar]
- 60.Scarsini R, Cantone R, Venturi G, De Maria GL, Variola A, Braggio P, et al. Correlation between intracoronary physiology and myocardial perfusion imaging in patients with severe aortic stenosis. Int J Cardiol. 2019;292:162–5. 10.1016/j.ijcard.2019.04.050. [DOI] [PubMed] [Google Scholar]
- 61.Zimmermann FM, De Bruyne B, Pijls NH, Desai M, Oldroyd KG, Park SJ, et al. Rationale and design of the Fractional Flow Reserve versus Angiography for Multivessel Evaluation (FAME) 3 Trial: a comparison of fractional flow reserve-guided percutaneous coronary intervention and coronary artery bypass graft surgery in patients with multivessel coronary artery disease. Am Heart J. 2015;170(4):619–26 e2. 10.1016/j.ahj.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 62.Toth G, De Bruyne B, Casselman F, De Vroey F, Pyxaras S, Di Serafino L, et al. Fractional flow reserve-guided versus angiography-guided coronary artery bypass graft surgery. Circulation. 2013;128(13):1405–11. 10.1161/CIRCULATIONAHA.113.002740. [DOI] [PubMed] [Google Scholar]
- 63.Botman CJ, Schonberger J, Koolen S, Penn O, Botman H, Dib N, et al. Does stenosis severity of native vessels influence bypass graft patency? A prospective fractional flow reserve-guided study. Ann Thorac Surg. 2007;83(6):2093–7. 10.1016/j.athoracsur.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 64.••.Kikuta Y, Cook CM, ASP S, Salinas P, Kawase Y, Shiono Y, et al. Pre-angioplasty instantaneous wave-free ratio pullback predicts hemodynamic outcome in humans with coronary artery disease: primary results of the International Multicenter iFR GRADIENT Registry. JACC Cardiovasc Interv. 2018;11(8):757–67. 10.1016/j.jcin.2018.03.005. [DOI] [PubMed] [Google Scholar]; In serial lesions and diffuse coroanry disease, this study revealed that an iFR pullback accurately predicts the physiological outcome after PCI.
- 65.Kern MJ, Seto AH. Instantaneous wave-free ratio pressure pullback with virtual percutaneous coronary intervention planning: seeing the future of coronary interventions? JACC Cardiovasc Interv. 2018;11(8):768–70. 10.1016/j.jcin.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 66.Collet C, Sonck J, Vandeloo B, Mizukami T, Roosens B, Lochy S, et al. Measurement of hyperemic pullback pressure gradients to characterize patterns of coronary atherosclerosis. J Am Coll Cardiol. 2019;74(14):1772–84. 10.1016/j.jacc.2019.07.072. [DOI] [PubMed] [Google Scholar]
- 67.De Rosa S, Polimeni A, Petraco R, Davies JE, Indolfi C. Diagnostic performance of the instantaneous wave-free ratio: comparison with fractional flow reserve. Circ Cardiovasc Interv. 2018;11(1):e004613 10.1161/CIRCINTERVENTIONS.116.004613. [DOI] [PubMed] [Google Scholar]
- 68.Jeremias A, Maehara A, Genereux P, Asrress KN, Berry C, De Bruyne B, et al. Multicenter core laboratory comparison of the instantaneous wave-free ratio and resting Pd/Pa with fractional flow reserve: the RESOLVE study. J Am Coll Cardiol. 2014;63(13):1253–61. 10.1016/j.jacc.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 69.Warisawa T, Cook CM, Howard JP, Ahmad Y, Doi S, Nakayama M, et al. Physiological pattern of disease assessed by pressure-wire pullback has an influence on fractional flow reserve/instantaneous wave-free ratio discordance. Circ Cardiovasc Interv. 2019;12(5):e007494 10.1161/CIRCINTERVENTIONS.118.007494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Derimay F, Johnson NP, Zimmermann FM, Adjedj J, Witt N, Hennigan B, et al. Predictive factors of discordance between the instantaneous wave-free ratio and fractional flow reserve. Catheter Cardiovasc Interv. 2019. 10.1002/ccd.28116. [DOI] [PubMed] [Google Scholar]
- 71.••.Lee SH, Choi KH, Lee JM, Hwang D, Rhee TM, Park J, et al. Physiologic characteristics and clinical outcomes of patients with discordance between FFR and iFR. JACC Cardiovasc Interv. 2019. 10.1016/j.jcin.2019.06.044. [DOI] [PubMed] [Google Scholar]; This study demonstrated that the most common type of FFR/iFR discordance was iFR+/FFR−, and that regardless of the modality of discordance there was no significant penalty for deferring revascularization for FFR/iFR discordant lesions.
- 72.Cohen DJ, Van Hout B, Serruys PW, Mohr FW, Macaya C, den Heijer P, et al. Quality of life after PCI with drug-eluting stents or coronary-artery bypass surgery. N Engl J Med. 2011;364(11):1016–26. 10.1056/NEJMoa1001508. [DOI] [PubMed] [Google Scholar]
- 73.Abdallah MS, Wang K, Magnuson EA, Spertus JA, Farkouh ME, Fuster V, et al. Quality of life after PCI vs CABG among patients with diabetes and multivessel coronary artery disease: a randomized clinical trial. JAMA. 2013;310(15):1581–90. 10.1001/jama.2013.279208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Agarwal SK, Kasula S, Hacioglu Y, Ahmed Z, Uretsky BF, Hakeem A. Utilizing post-intervention fractional flow reserve to optimize acute results and the relationship to long-term outcomes. JACC Cardiovasc Interv. 2016;9(10):1022–31. 10.1016/j.jcin.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 75.Fournier S, Ciccarelli G, Toth GG, Milkas A, Xaplanteris P, Tonino PAL, et al. Association of improvement in fractional flow reserve with outcomes, including symptomatic relief, after percutaneous coronary intervention. JAMA Cardiol. 2019;4(4):370–4. 10.1001/jamacardio.2019.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.•.Lee JM, Hwang D, Choi KH, Rhee TM, Park J, Kim HY, et al. Prognostic implications of relative increase and final fractional flow reserve in patients with stent implantation. JACC Cardiovasc Interv. 2018;11(20):2099–109. 10.1016/j.jcin.2018.07.031. [DOI] [PubMed] [Google Scholar]; This study established that a low post-PCI FFR is associated with a higher risk of subsequent target vessel failure.
- 77.••.Azzalini L, Poletti E, Demir OM, Ancona MB, Mangieri A, Giannini F, et al. Impact of post-percutaneous coronary intervention fractional flow reserve measurement on procedural management and clinical outcomes: the REPEAT-FFR Study. J Invasive Cardiol. 2019;31(8):229–34. [PubMed] [Google Scholar]; This study highlighted the high proportion of patients with suboptimal physiological outcomes post-PCI that were associated with a greater frequency of adverse events.
- 78.••.Jeremias A, Davies JE, Maehara A, Matsumura M, Schneider J, Tang K, et al. Blinded physiological assessment of residual ischemia after successful angiographic percutaneous coronary intervention: the DEFINE PCI Study. JACC Cardiovasc Interv. 2019;12(20):1991–2001. 10.1016/j.jcin.2019.05.054. [DOI] [PubMed] [Google Scholar]; With blinded physiological assessement, this study demonstrated that almost 25% of patients had residual ischemia post-PCI despite a good visual angiographic result.
- 79.Norgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol. 2014;63(12):1145–55. 10.1016/j.jacc.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 80.Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011;58(19):1989–97. 10.1016/j.jacc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 81.Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK, van Mieghem C, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA. 2012;308(12):1237–45. 10.1001/2012.jama.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Driessen RS, Danad I, Stuijfzand WJ, Raijmakers PG, Schumacher SP, van Diemen PA, et al. Comparison of coronary computed tomography angiography, fractional flow reserve, and perfusion imaging for ischemia diagnosis. J Am Coll Cardiol. 2019;73(2):161–73. 10.1016/j.jacc.2018.10.056. [DOI] [PubMed] [Google Scholar]
- 83.Danad I, Raijmakers PG, Driessen RS, Leipsic J, Raju R, Naoum C, et al. Comparison of coronary CT angiography, SPECT, PET, and hybrid imaging for diagnosis of ischemic heart disease determined by fractional flow reserve. JAMA Cardiol. 2017;2(10):1100–7. 10.1001/jamacardio.2017.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patel MR, Norgaard BL, Fairbairn TA, Nieman K, Akasaka T, Berman DS, et al. 1-year impact on medical practice and clinical outcomes of FFRCT: the ADVANCE Registry. JACC Cardiovasc Imaging. 2019. 10.1016/j.jcmg.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 85.Collet C, Onuma Y, Andreini D, Sonck J, Pompilio G, Mushtaq S, et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur Heart J. 2018;39(41):3689–98. 10.1093/eurheartj/ehy581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Modolo R, Collet C, Onuma Y, Serruys PW. SYNTAX II and SYNTAX III trials: what is the take home message for surgeons? Ann Cardiothorac Surg. 2018;7(4):470–82. 10.21037/acs.2018.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fearon WF, Achenbach S, Engstrom T, Assali A, Shlofmitz R, Jeremias A, et al. Accuracy of fractional flow reserve derived from coronary angiography. Circulation. 2019;139(4):477–84. 10.1161/CIRCULATIONAHA.118.037350. [DOI] [PubMed] [Google Scholar]
- 88.Biscaglia S, Tebaldi M, Brugaletta S, Cerrato E, Erriquez A, Passarini G, et al. Prognostic value of QFR measured immediately after successful stent implantation: the international multicenter prospective HAWKEYE study. JACC Cardiovasc Interv. 2019;12(20):2079–88. 10.1016/j.jcin.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 89.Okuya Y, Seike F, Yoneda K, Takahashi T, Kishi K, Hiasa Y. Functional assessment of tandem coronary artery stenosis by intracoronary optical coherence tomography-derived virtual fractional flow reserve: a case series. Eur Heart J Case Rep. 2019;3(2). 10.1093/ehjcr/ytz087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.•.Cook CM, Warisawa T, Howard JP, Keeble TR, Iglesias JF, Schampaert E, et al. Algorithmic versus expert human interpretation of instantaneous wave-free ratio coronary pressure-wire pull back data. JACC Cardiovasc Interv. 2019;12(14):1315–24. 10.1016/j.jcin.2019.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examined the use of artificial intelligence in the interpretation of coronary physiology data.


