ABSTRACT
Tumor-associated macrophages (TAMs), which generally exhibit an M2-like phenotype, play a critical role in tumor development. Triptolide exerts a unique bioactive spectrum of anticancer activities. The aim of this study was to determine whether triptolide has any effect on the activation of TAMs and the production of tumor-promoting mediators. ICR-1 mice with azoxymethane/dextran sulfate sodium (AOM/DSS)-induced colon tumors and BALB/c mice co-inoculated with 4T1 cells and M2-polarized RAW264.7 cells were used to examine whether the inhibitory effect of triptolide on tumor progression was mediated by the targeting of TAMs. Real-time PCR, Western blot, immunofluorescence staining, and flow cytometry assays were performed to determine the expression of cell surface markers and cytokine production. The results showed that triptolide inhibited macrophage differentiation toward the M2 phenotype and abolished M2 macrophage-mediated tumor progression. Furthermore, triptolide inhibited the expression of M2 markers, such as CD206, Arginase 1, and CD204, and inhibited the secretion of anti-inflammatory cytokines. Thus our study indicated that triptolide selectively inhibited the functions of M2-polarized macrophages and TAMs, and this inhibitory effect of triptolide on TAM viability, differentiation, and cytokine production might elucidate the major mechanisms underlying its antitumor activity. Our findings provide important information for the potential clinical application of triptolide in cancer therapy.
KEYWORDS: Tumor-associated macrophages, M2-polarized, triptolide, tumor growth, colitis-associated colon cancer, breast cancer
Introduction
Macrophages play significant roles in the immune response to various pathogens and in tissue homeostasis, and can be divided into classically activated (M1) and alternatively activated (M2) phenotypes.1 Tumor-associated macrophages (TAMs), which are derived from circulating monocytes,2,3 have been identified as one of the main components of the tumor microenvironment. Furthermore, according to several clinical studies, the increase observed in TAMs around the tumor microenvironment is closely associated with a poor prognosis in cancer patients.4–8 TAMs generally exhibit an M2-like phenotype induced by T helper 2 (Th2) cytokines such as interleukin (IL) −4 and IL-139,10 and play a critical role in tumor progression, angiogenesis, invasion and metastasis.11–14 Recent studies have suggested that the inhibition of macrophage polarization toward an M2 phenotype in an inflammatory microenvironment represents a novel preventive strategy for pancreatic cancer patients.15
Triptolide, an active component of the Chinese medicinal herb Tripterygium wilfordii Hook F (TWHF), exerts notable immunosuppression and anti-inflammatory effects and induces severe multiorgan toxicities.16–18 Triptolide is effective for the treatment of several autoimmune diseases,19 and its anti-tumor effect has attracted extensive attention.20 Several preclinical studies have indicated that this natural product is more effective than other anticancer agents; specifically, triptolide shows strong anticancer activities at nanomolar concentrations even in highly resistant malignant cells.18 The underlying anti-tumor mechanisms, particularly those related to apoptosis, autophagy, angiogenesis and associated molecular pathways, have been summarized.21 Our previous studies showed that triptolide selectively inhibits the production of cytokines/chemokines in macrophages.22 Some studies have also indicated that triptolide inhibits the expression of the cytokine IL-10 (Th2) in mouse primary splenocytes, and that this immunosuppressive activity is partially attributed to its inhibition of monocyte activation.23,24 However, the effect of triptolide on TAMs remains unknown. In the present study, we examined the effects of triptolide on colon and breast cancer models, the activation of TAMs and the production of proinflammatory cytokines and other tumor-promoting mediators.
Results
Triptolide exhibited a selective cytotoxic effect on macrophage viability in vitro
First, the selective cytotoxic effect of triptolide on macrophages derived from bone marrow monocytes (in vitro TAMs model), neutrophils, lymphocytes purified from BALB/c mice and 4T1 mammary carcinoma cells was assessed through a CCK-8 assay. Macrophages derived from bone marrow monocytes were highly susceptible to the cytotoxic effects of triptolide. Specifically, triptolide exerted a dose-dependent cytotoxic effect on macrophages with a 50% inhibitory concentration (IC50) of 25.7 nM. Purified neutrophils were significantly less susceptible to triptolide, with an IC50 of 37.7 nM, whereas freshly isolated lymphocytes and 4T1 cells were the least susceptible (IC50 > 40 nM) (Figure 1(a)).
Figure 1.
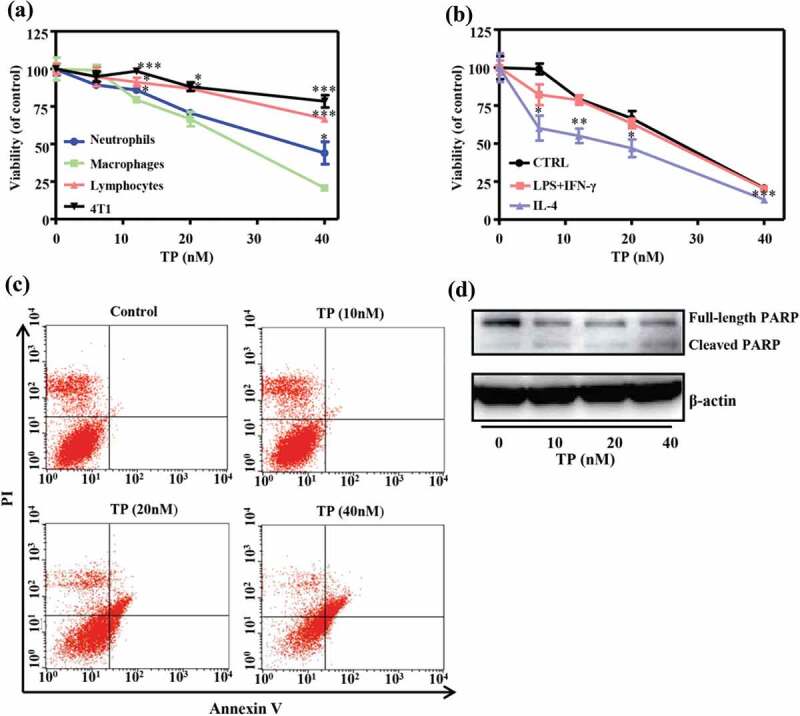
Triptolide affects the viability of leukocyte subsets and tumor cells in vitro. (a) The cell viability of macrophages, neutrophils, lymphocytes and 4T1 mammary tumor cells after treatment with triptolide for 48 h was assessed by CCK8 analysis. The results are presented as the means from three different experiments. (b) The in vitro-differentiation of macrophages was stimulated with LPS (100 ng/mL) + IFN-γ (500 IU/mL) (M1) and IL-4 (20 ng/mL) (M2) in the presence or absence of triptolide for 48 h. (c) Macrophages derived from blood monocytes and treated with different concentrations of triptolide were stained with Annexin V and PI and analyzed by flow cytometry. (d) Effect of triptolide on the level of the apoptosis-related protein poly (ADP-ribose) polymerase (PARP) in macrophages derived from blood monocytes and treated with different concentrations of triptolide. The levels of PARP were assessed by Western blotting. *P < .05, **P < .01 and ***P < .001 compared with the macrophage (a) or CTRL group (b) (as determined by student’s t-test).
The cytotoxic effect of triptolide on differentiated macrophages was then evaluated. Although polarized subsets of macrophages activated by LPS and IFN-γ (M1) or by IL-4 (M2) were both susceptible to triptolide in vitro, M2-polarized macrophages showed significantly higher susceptibility to the cytotoxic effect of triptolide (Figure 1(b)).
The effects of triptolide on the viability of macrophages derived from bone marrow monocytes were examined by flow cytometry, and the results showed that triptolide induced macrophage apoptosis. Specifically, higher concentrations of triptolide led to increased numbers of Annexin V+/PI+ cells (Figure 1(c)). Furthermore, the levels of apoptosis-related protein poly (ADP-ribose) polymerase (PARP) were assessed by Western blotting. The ratio of cleaved PARP to full-length PARP increased after triptolide treatment, which revealed that triptolide induced apoptosis of the differentiated macrophages (Figure 1(d)).
Triptolide inhibited macrophage differentiation
We subsequently studied the effect of triptolide on the differentiation of macrophages in vitro. Specifically, THP-1 cells with PMA for 12 h and then with 20 ng/mL recombinant IL-4 for 24 h to obtain M2-polarized THP-1 macrophages, and RAW264.7 cells (M0) were treated with 20 ng/mL recombinant IL-4 for 24 h to obtain M2-polarized RAW264.7 cells.25
At a low dose range (5 nM, 10 nM, or 20 nM for 24 h), triptolide exerted no significant effect on the viability of normally cultured PMA-induced-THP-1 or RAW264.7 cells and did not induce a significant degree of cell apoptosis (Figure 2(a)). To examine the role of triptolide on the regulation of the macrophage phenotype, we treated PMA-induced THP-1 cells with non-cytotoxic concentrations of triptolide (5 nM, 10 nM, or 20 nM) in combination with 20 ng/mL recombinant IL-4 and found that treatment with triptolide downregulated the mRNA expression levels of the M2 markers Arginase 1, CD206, CD204 and CD163 (Figure 2(b)). Moreover, the protein levels of mannose receptor CD206 (Figure 2(C–E)) were also decreased after treatment with triptolide. Thus, the cellular level assays revealed that triptolide treatment is sufficient to alter the M2 phenotype of macrophages in the presence of IL-4.
Figure 2.
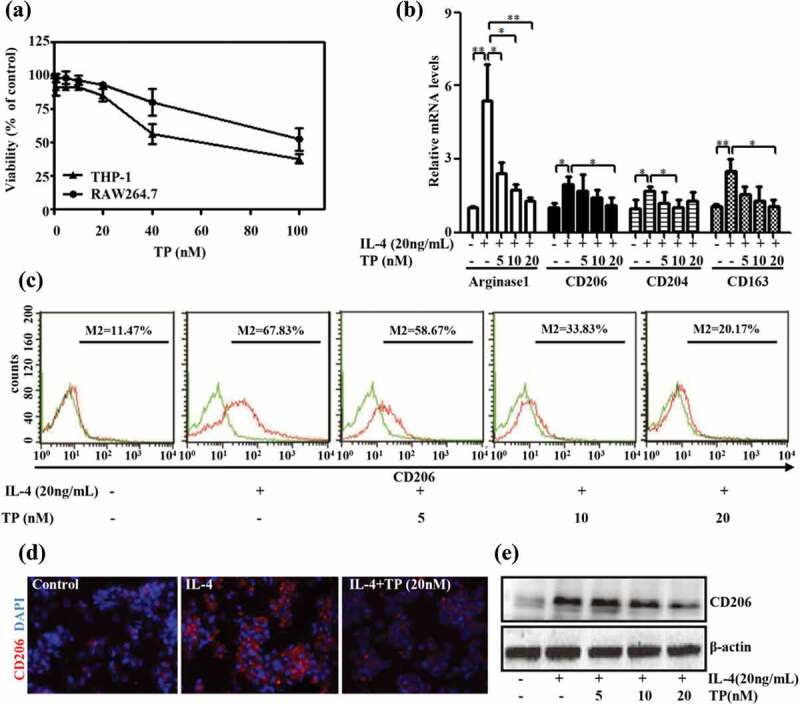
Triptolide suppresses M2-type macrophage polarization. (a) The effect of various concentrations of triptolide (1–100 nM) on cell viability as measured by the CCK8 assay. Data from at least three independent experiments were used to assess each condition. The values show the means ± SEMs. PMA-induced THP-1 cells were incubated with different concentrations of triptolide in the presence or absence of IL-4 (20 ng/mL) and then subjected to qRT-PCR (b), flow cytometry (c), immunofluorescence (d), and Western blot (e) analyses. The extracellular marker CD206 was stained red, and the nuclei were stained with DAPI (blue). The data are expressed as the means ± SEMs from three repeated experiments. *P < .05 and **P < .01 compared with the IL-4 group (as determined by student’s t-test).
Antitumor activity of triptolide and selective depletion of TAMs
ICR-1 mice with AOM/DSS-induced colon tumors were used to elucidate the role of macrophage targeting in the antitumor activity of triptolide in vivo. Triptolide was administered to the mice after the last DSS cycle (Figure 3(a)).26,27 After four weeks of triptolide treatment, the antitumor efficacy and toxicity of triptolide were analyzed. The results revealed that triptolide treatment did not exert any statistically significant effect on the body weight of the animals by the end of the study period (Figure 3(b)). However, triptolide treatment reduced the size and number of macroscopic tumors compared with those of the vehicle-treated mice (Figure 3(c,d)). Additionally, the life span curve indicated that triptolide, particularly at 0.2 mg/kg/day, increased mouse survival at 3 months (90% survival) compared to the model group (55% survival) (Figure 3(e)). These data indicate that triptolide effectively suppresses tumor progression in AOM/DSS-induced (colitis-associated) tumors.
Figure 3.
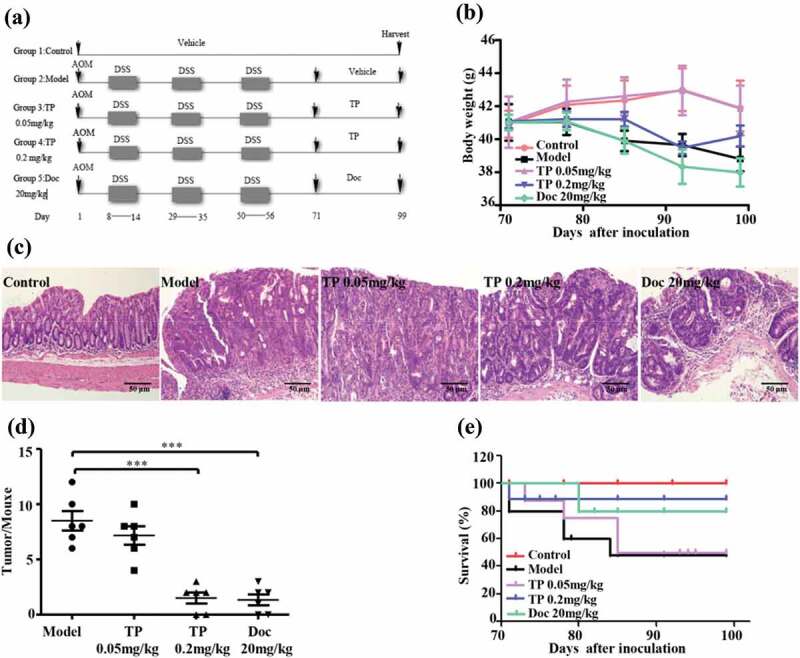
Triptolide protects against tumor development in an AOM/DSS-induced model of CACC. (a) Experimental protocol for investigating the effects of triptolide in a mouse model of CACC. (b) AOM/DSS mice were treated with different doses of triptolide as described in (a) and weighed once per week, and their body weight loss was calculated. The data represent the means ± SEMs from n = 5–11 mice/group. (c) Histological sections (hematoxylin-eosin staining) of colon samples obtained from the different groups described in (a). Scale bars, 50 μm. (d) Tumor incidence induced by AOM+DSS in the different groups. (e) The survival differences were determined through a Kaplan-Meier analysis. The data will statistical analyzed with Student’s t test. ***P < .001 compared with the model group.
We subsequently identified the leukocyte infiltrate in the blood at the end of the treatment period (Figure 4(a)). The number of leukocytes and the proportion of monocytes were increased in the model group compared with the control group and, were significantly reduced in the triptolide-treated group compared with the model group. CD68 has been used for the identification of TAMs in many studies,28,29 and some studies have indicated that stromal MCP-1 is responsible for the recruitment of macrophages into the tumor area.30 The mRNA expression levels of CD68 and MCP-1 in the mouse colons were increased, which was consistent with previously reported results. However, the mRNA expression levels of CD68 and MCP-1 were significantly downregulated after treatment with triptolide (Figure 4(b)), which implies that triptolide might inhibit the infiltration of TAMs into the mouse intestinal tumor stroma by interrupting the MCP-1 pathway.
Figure 4.
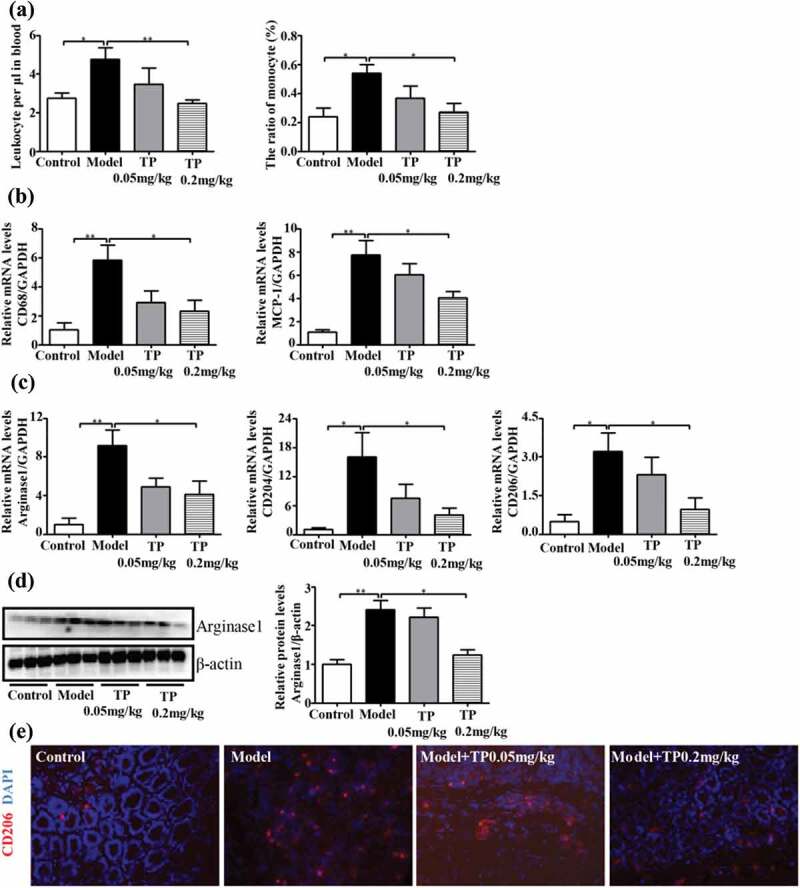
Triptolide alters the TAM phenotypes of M2 macrophages in CACC model mice. (a) Triptolide is directly cytotoxic to blood monocytes and leukocytes. The data are shown as absolute numbers per microlitre of blood (means ± SEMs, n = 6 mice/group). (b, c) mRNA expression levels in colon cells. (d) Representative Western blots of total protein isolated from colons were probed with anti-arginase 1. Anti-β-actin was used as a loading control. (e) Immunofluorescence analysis for detection of the extracellular marker CD206 (red), and nuclei (stained with DAPI) (blue). The statistical analysis was performed using Student’s t test; *P < .05 and, **P < .01 compared with the model group.
We then assessed the macrophage phenotypes in the tumors and found that the mRNA expression levels of representative M2 genes (Arginase 1, CD204 and CD206) were significantly increased in the colon sections from the model group (Figure 4(c)). A Western blot analysis showed that the colon sections from mice belonging to the model group exhibited markedly increased protein levels of Arginase 1 compared with those of the control group (Figure 4(d)). These results indicate that in mice with AOM/DSS-induced tumors, TAMs that infiltrate colon sections are polarized to the M2 phenotype. Subsequently, we investigated the effect of triptolide on TAM polarization in the AOM/DSS mouse colon and found that mRNA expression levels of Arginase 1, CD204 and CD206 were significantly downregulated in the groups treated with triptolide, particularly at a dose of 0.2 mg/kg/day. Furthermore, the expression of the M2 marker CD206 in TAMs isolated from the the mice with AOM/DSS-induced tumors was detected by immunofluorescence, and as expected, the CD206 protein level in colon sections from the triptolide-treated mice was reduced (Figure 4(e)). These results indicate that triptolide inhibits macrophage differentiation toward the M2 phenotype, and this finding is correlated with the reduced tumor burdens observed in AOM/DSS-treated mice.
Triptolide downregulated the expression of Th2 cytokines in M2 macrophages
As immunosuppressive macrophages, TAMs suppress immunological responses by secreting many anti-inflammatory cytokines, of which IL-10 and TGF-β1 are the most important.31 The effects of triptolide at concentrations of 5, 10 and 20 nM on the production of IL-10 and TGF-β1 by M2-poralized THP-1 macrophages were further examined. Real-time PCR and ELISA assays showed that IL-4 facilitated PMA-induced THP-1 expression and the release of IL-10 and TGF-β1. Triptolide treatment decreased both the mRNA expression and the protein secretion of IL-10 and TGF-β1 stimulated by IL-4 (Figure 5(a,b)).
Figure 5.
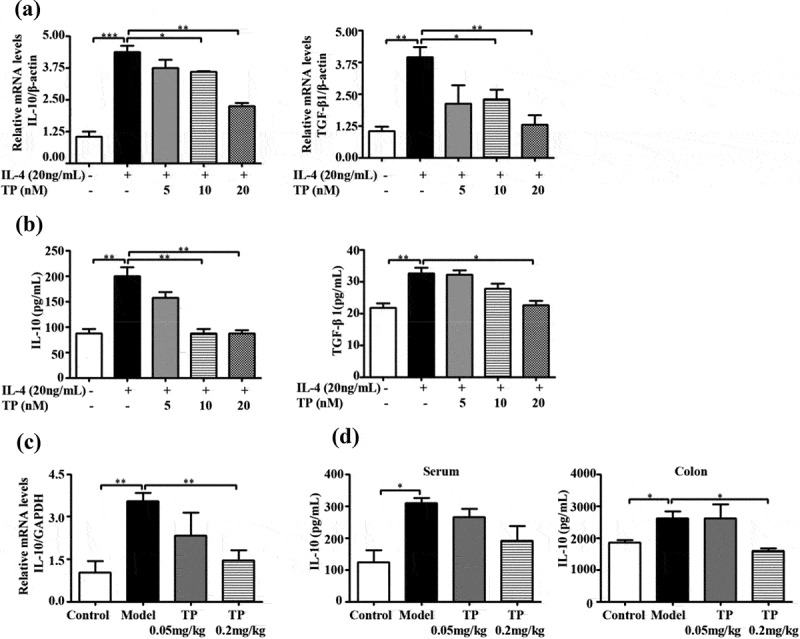
Triptolide inhibits Th2-predominant cytokines in the tumor microenvironment. RT-PCR (a) and ELISA (b) analyses were performed to determine the Th2 cytokine milieu in M2-poralized THP-1 macrophages. RT-PCR (c) and ELISA (d) analyses were performed to determine the level of IL-10 in the AOM/DSS mouse serum and colon tissue. Colon tissue with a normal histology was used as a control . *P < .05, **P < .01, and ***P < .001 compared to model group.
The cytokine expression profile of the AOM/DSS mouse colon was consistent with that of the M2-poralized THP-1 macrophages. The colon sections from AOM/DSS mice showed higher mRNA expression of IL-10 than normal colon sections (Figure 5(c)), and the secretion of IL-10 in the colon and serum was also increased (Figure 5(d)). Subsequently, we investigated the effect of triptolide on IL-10 expression in the AOM/DSS mouse colon. As shown in Figure 5(c,d), the mRNA expression and secretion of IL-10 were significantly decreased in the colons of groups treated with triptolide, particularly at the dose of 0.2 mg/kg/day. Collectively, these findings indicate that triptolide inhibits the secretion of anti-inflammatory cytokines both in vitro and in vivo.
Triptolide abrogated the positive effect of M2-polarized macrophages on tumor growth
Our results with murine macrophage RAW264.7 cells were similar to those obtained with M2-poralized THP-1 macrophages. Triptolide inhibited the M2 activation status of RAW264.7 cells (Figure 6(a,b)). Subsequently, BALB/c mice co-inoculated with 4T1 cells and M2-polarized RAW264.7 cells (control or pretreated with triptolide) were used to further examine whether the inhibitory effect of triptolide on tumor progression is mediated by the targeting of TAMs. The tumor growth curves were monitored, and an increased growth rate was found in the 4T1+ M2-induced tumor syngeneic graft model compared with the 4T1 induced syngeneic graft model (Figure 6(c-e)). At the end of the study, the tumor volumes and weights of the tumors in the 4T1+ M2 mice were increased by 13% and 34%, respectively, compared with those in the control 4T1-inoculated mice (P < .05 for both). This promotion of tumor growth in the mice was inhibited by co-inoculation of triptolide-treated M2 macrophages with 4T1 cells, as demonstrated by the finding that the tumor volumes and weights of these mice were decreased by 16% (P < .01) and 26% (P < .05), respectively, compared with those of the mice that were co-inoculated with 4T1 tumors plus M2-polarized macrophages. These results demonstrate the ability of M2-polarized macrophages (TAMs) to promote tumor growth in vivo, and reveal that triptolide-treated M2-polarized macrophages inhibit this growth-promoting effect.
Figure 6.
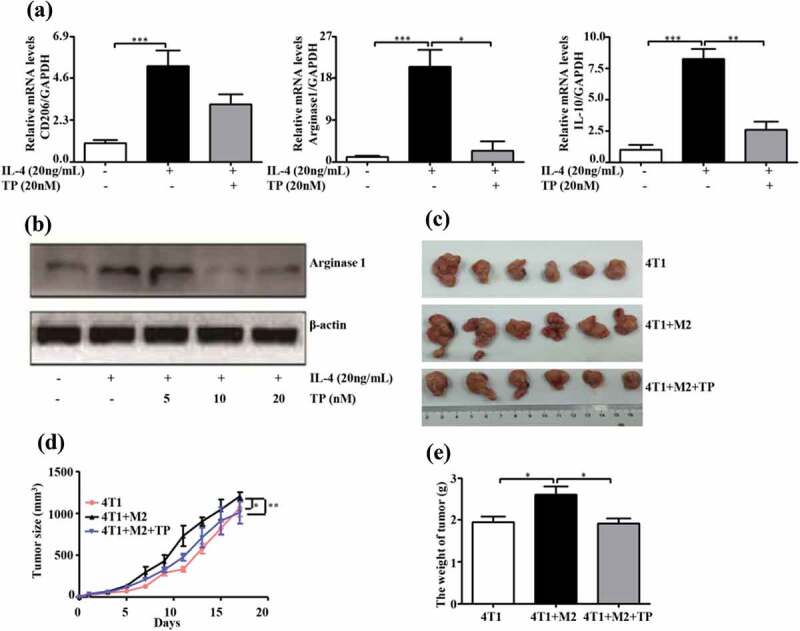
Triptolide abrogates the positive effect of M2-polarized RAW264.7 macrophages on tumor growth of 4T1 cells in vivo. (a) mRNA expression levels of CD206, Arginase 1 and IL-10 and protein expression of Arginase 1 (b) in RAW264.7 cells treated with IL-4 and triptolide. The change in volume (c, d) and weight (e) of the tumors in mice inoculated with 4T1 cells with or without RAW264.7 cells were measured. The tumor volume was measured twice per week until day 19, and the tumor weight was measured on day 19. The weight values indicate the means ± SEMs (n = 6). *P < .05, **P < .01, and ***P < .001 compared with the control (as determined by student’s t test).
Discussion
Triptolide exerts potent anticancer effects on apoptosis, angiogenesis, metastasis and drug resistance.18,32,33 Many in vitro and in vivo studies have attempted to elucidate the potential antitumor mechanism of triptolide, but the conclusions from these studies are inconsistent.33–35 In this study, we demonstrated that triptolide exerts direct effects on the tumor microenvironment and the selective depletion of TAMs. This conclusion is based on the following observations: First, macrophages were more susceptible to triptolide than neutrophils, lymphocytes and tumor cells, and M2-polarized macrophages showed increased susceptibility compared with M0/M1-polarized macrophages. Second, triptolide inhibited the expression of M2 markers, such as CD206, Arginase 1 and CD204, and inhibited the secretion of the anti-inflammatory cytokines TGF-β1 and IL-10 both in vitro and in vivo. Third, triptolide inhibited macrophage differentiation toward the M2 phenotype, and this inhibition was correlated with a reduction of the tumor burden in AOM/DSS-treated mice. In addition, triptolide abolished M2 macrophage-mediated tumor progression in cancer co-inoculated syngeneic graft mouse models.
The clinical applications of triptolide are limited by its narrow therapeutic window,36 and our data show that triptolide might affect not only neoplastic cells but also the tumor microenvironment and induce the selective depletion of TAMs. These findings unveil different perspectives for the exploitation of triptolide in cancer, for instance, in combination with antiangiogenic therapies. Considering the dose-dependent toxicity of triptolide, the combination of a low dose of triptolide with other anticancer therapeutics is a good choice for improving the antitumor activities and reducing the toxicity of triptolide.
Two different polarization statuses have been identified for the macrophage population, and these depend on different cytokines and microbial products.25 Macrophages activated by Th1 cytokines, such as LPS and IFN-γ, are categorized as having the “classical” activation profile (M1) and exhibit antitumor functions. Alternatively, Th2 cytokines (e.g., IL-4, and IL-10), induce an “alternative” activation phenotype (M2) that supports tissue repair and tumor growth.9,10 The functional plasticity of macrophages allows switching from an inflammatory M1 phenotype to an M2 profile and vice versa in response to the cytokine milieu.37 TAMs exhibit a polarized M2 phenotype and stimulate tumor growth and metastasis,13,38 and recent studies have suggested that the inhibition of macrophage polarization toward an M2 phenotype in an inflammatory microenvironment represents a novel preventive strategy for cancer patients.15
To investigate the role of targeting the differentiation of M2 macrophages by triptolide in cancer growth, we established a syngeneic graft model in which 4T1 breast cancer cells were co-inoculated with the M2-polarized RAW264.7 macrophage cell line. Prior to co-inoculation, the M2-polarized RAW264.7 cells were pretreated with 20 ng/mL triptolide for 24 h. Interestingly, the inoculation of macrophages together with breast tumor cells into BALB/c mice promoted tumor growth, whereas the inoculation of breast tumor cells together with macrophages pretreated with triptolide eliminated this growth-promoting effect. Increasing evidence suggests that TAMs play a role in the regulation of metastasis in human breast cancer.39–41
In summary, our study indicates that triptolide selectively inhibits the functions of M2-polarized macrophages and TAMs. The inhibitory effect of triptolide on TAM viability, differentiation, and cytokine production might elucidate the mechanisms underlying its antitumor activity. Our findings provide important information for the potential clinical application of triptolide in cancer therapy.
Materials and methods
Chemicals and animals
Triptolide (purity >98%, HPLC grade) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Virus-free, six-week-old BALB/c mice, five-week-old Crj: CD-1 (ICR-1) mice and six-week-old C57BL/6 mice were purchased from the Shanghai SLAC Laboratory Animal Co. Ltd (Shanghai, China). The animals were maintained in a well-ventilated and pathogen-free environment at 23 ± 2°C with a 12-h light/12-h dark photoperiod. The animal experiments were conducted in strict accordance with the standard ethical guidelines and approved by the Ethical Committee of the China Pharmaceutical University. All efforts were made to minimize mice suffering.
Cell culture
4T1 mammary tumor cells and murine macrophage RAW264.7 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin and 100 mg/mL streptomycin, and the human monocytic leukemia cell line THP-1 was maintained in Roswell Park Memorial Institute (RPMI)-1640 medium. All the cells were purchased from the American Type Culture Collection (ATCC, USA), and culture media and additives were acquired from Invitrogen (Carlsbad, CA, USA). The differentiation of THP-1 cells toward macrophages was triggered by treatment with 100 nM phorbol myristate acetate (PMA, Sigma-Aldrich) for 12 h.25 M2-polarized macrophages were obtained by treating RAW264.7 cells (M0) or PMA-induced-THP-1 cells (M0) with 20 ng/mL recombinant IL-4 for 24 h.
Cell preparation and treatment
BALB/c mouse bone marrow-derived monocytes (>80% CD14+ cells) were isolated and purified by Ficoll-Hypaque density gradient centrifugation (TBD, Tianjin, China).23,42 Mouse spleen lymphocytes (>95% CD3+) and bone marrow-derived neutrophils (>75% CD11b+) were purified and obtained by centrifugation using Percoll gradients (TBD, Tianjin, China) according to the manufacturer’s instructions.
The in vitro differentiation of macrophages was induced by treating purified bone marrow-derived monocytes (106 cells/mL) for five days with 20 ng/mL macrophage colony-stimulating factor (M-CSF, PeproTech, London, United Kingdom). The cells were cultured with lipopolysaccharides (LPS, 100 ng/mL; Sigma Aldrich, St. Louis, MO, USA) and interferon gamma (IFN-γ, 500 IU/mL; PeproTech, London, United Kingdom) 24 h to promote the M1 phenotype, whereas IL-4 (20 ng/mL; PeproTech) was used to drive cells toward the M2 phenotype.43
Cell viability assay
The cell viability was analyzed via a CCK-8 assay; and calculated as the percentage of cell viability compared with the control using the following equation: (optical density (OD) after treatment/OD of the control)*100.
Differentiated macrophages that were treated or not treated with triptolide for 48 h were harvested, washed with cold phosphate-buffered saline (PBS) and stained with the double dye Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) according to the manufacturer’s recommended protocol. Subsequently, the apoptotic cells were measured via flow cytometry (BD, FACSCalibur) using Cell Quest software.
Mouse model of colon carcinogenesis
The azoxymethane (AOM, Sigma–Aldrich, St. Louis, MO, USA)/dextran sulfate sodium (DSS, molecular weight of 40,000, MP Biomedicals, Solon, OH, USA) evoked colon tumor model was used to investigate the influence of triptolide on tumor incidence (see Figure 3(a)). ICR-1 mice were injected with a single dose (12 mg/kg) of AOM on day 1. Starting on day 8, DSS was added to the drinking water at a concentration of 2.5%, and after one week of DSS administration, the mice were administered regular water for two weeks. This cycle was then repeated twice with 2% DSS. Starting on day 71, the mice received daily administrations of triptolide (0.05 mg/kg and 0.2 mg/kg) by gavage, and weekly intravenous injection of 20 mg/kg docetaxel (used as the positive control) based on their body weight.
The mice were sacrificed on day 99. The colons were removed, and the tumors were counted. Portions of the distal colon were fixed with 10% neutral buffered formalin overnight at 4°C and embedded in paraffin.
TAMs from colon tumors were isolated by enzymatic digestion and the Ficoll density gradient method44 and were further purified by plastic adherence (RPM1 1640 w/o FBS, 1 h, 37°C), and the adherent cells were assessed by flow cytometry.
Tumor syngeneic graft model
BALB/c mice at 6 to 8 weeks of age were separated randomly into four groups and subcutaneously inoculated with a total of 5 × 104 4T1 cells combined with M2-polarized RAW264.7 cells (M2) or M2-polarized RAW264.7 cells pretreated with triptolide (20 ng/mL) for 24 h (triptolide + M2) into their flank. After 19 days, all the mice were euthanized, and the tumor tissues were removed and weighed.
Phenotype analysis
Immunofluorescence was performed to investigate the expression of cell membrane markers, and the cells were analyzed by flow cytometry (Calibrate; Becton Dickinson, Palo Alto, CA, USA). Anti-mouse CD14 (Sa14-2, Bio Legend, San Diego, CA, USA), anti-mouse CD11b (M1/70, Bio Legend), anti-mouse CD3 (17A2, Bio Legend), anti-mouse CD68 (FA-11, Bio Legend), anti-human CD68 (eBioY1/82A, eBioscience, San Diego, CA, USA) and anti-human CD206 (19.2, BD Pharmingen, San Diego, CA, USA) were used as the labeling antibodies for detection. At least 10,000 cells were analyzed. The following isotype controls were used: IgG2b (eBMG2b, eBioscience) conjugated to phycoerythrin (PE) and IgG1 (P3.6.2.8.1, eBioscience). The cells were centrifuged and suspended in PBS. The data were analyzed using Cell Quest software (Becton Dickinson).
Cytokine production
The supernatants of the M2 macrophages were tested for the presence of cytokines using commercially available enzyme-linked immunosorbent assays (ELISAs) for TGF-β1 and IL-10 (Neo Bioscience) following the manufacturers’ instructions. The serum and colon IL-10 levels were measured by ELISA (Raybio), according to the manufacturers’ recommended protocol.
RNA extraction and real-time PCR
Total RNA from the cells or colon sections was isolated using the TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and then used for first-strand cDNA synthesis using a HiScript® First Strand cDNA Synthesis Kit (Vazyme Biotech Co., Ltd). The assays were performed in triplicate, and the mRNA levels were normalized to the expression levels of the housekeeping genes β-actin or GAPDH. The primer sequences are presented in Table 1.
Table 1.
Primers for real-time PCR.
| Species | Gene | Sequence (5′→3′) |
|---|---|---|
| Human | Arginase 1 | Forward TAACTCGAACAGTGAACACAGCAG Reverse TAGGTGGGTTAAGGTAGTCAATAGG |
| Human | CD163 | Forward GCAAGTGGCCTCTGTAATCT Reverse AGCACTTTCTTCTGGAATGG |
| Human | CD204 | Forward CTCCCCTTTTCCCCTTTCTG Reverse ATCGAGGTCCCACTGGAGAAAGT |
| Human | CD206 | Forward CTGCTACTGAACCCCCACAAC Reverse GGAAACCAGAGAGGAACCCAT |
| Human | IL-10 | Forward GTGAAGAATGCCTTTAATAAGCTCC Reverse TTCATTGTCATGTAGGCTTCTATGT |
| Human | TGF-β1 | Forward ACCAACTATTGCTTCAGCTC Reverse TTATGCTGGTTGTACAGG |
| Human | β-actin | Forward GCGTGACATTAAGGAGAAG Reverse GAAGGAAGGCTGGAAGAG |
| Mouse | Arginase 1 | Forward GGCAACCTGTGTCCTTTCTCCT Reverse CCCAGCTTGTCTACTTCAGTCATG |
| Mouse | CD204 | Forward TGGAGGAGAGAATCGAAAGCA Reverse CTGGACTGACGAAATCAAGG AA |
| Mouse | CD206 | Forward ATCCTGGTGGAAGAAGAAGTAGCCT Reverse GAGTAGTGGTTGGAGAAACAGGCAG |
| Mouse | CD68 | Forward TGTCTGATCTTGCTAGGACCG Reverse AGAGTAACGGCCTTTTTGTGA |
| Mouse | GAPDH | Forward AGAAGGTGGTGAAGCAGGCATC Reverse CGAAGGTGGAAGAGTGGGAGTTG |
| Mouse | MCP-1 | Forward TTAAAAACCTGGATCGGAACCAA Reverse GCATTAGCTTCAGATTTACGGGT |
| Mouse | IL-10 | Forward GGAAGACAATAACTGCACCCACT Reverse CAACCCAAGTAACCCTTAAAGTCC |
| Mouse | TGF-β1 | Forward CTCCCGTGGCTTCTAGTGC Reverse GCCTTAGTTTGGACAGGATCTG |
Western blot analysis
Whole cell lysate were prepared using lysis buffer (150 mM NaCl, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris, pH 8.0) containing protease and phosphatase inhibitors (Roche, Nutley, NJ, USA), and the protein concentrations in the samples were determined using a BCA protein assay kit (Beyotime, Shanghai, China). Equal protein concentrations were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the proteins were then transferred to Immobilon polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). The membranes were incubated with primary antibodies against Arginase 1, CD206 (Abcam, Biotechnology), PARP (Cell Signaling Technology) and β-actin (Santa Cruz, Biotechnology) overnight at 4°C. The immunoreactive bands were detected using horseradish peroxidase (HRP)-conjugated secondary antibodies with the Western Lightning Chemiluminescence Plus reagent.
Complete blood count
Blood samples were collected in vacuum blood collection tubes (Improve Medical Instruments Co., Ltd, Guangzhou, China). The number of leukocytes and the ratio of monocytes in complete blood were analyzed using an automatic hematology analyzer (XE-2100, SYSMEX Ltd., Tokyo, Japan).
Histopathology
For histological observations, colon tissues were fixed in 4% formalin, embedded in paraffin, and serially sectioned at 5 μm. The sections were then deparaffinized, rehydrated and stained with hematoxylin and eosin for microscopic observation. The histological sections were used for qualitative tumor evaluation, and the samples were viewed under a confocal microscope with a 40× objective.
Statistical analysis
The differences between two groups were analyzed using Student’s t-test, whereas the differences among more than two groups were assessed by analysis of variance (ANOVA). The data are presented as the means ± standard errors of the mean (SEMs). The Kaplan-Meier method was used for the survival analyzes. * P < .05, ** P < .01, *** P < .001.
Funding Statement
This study was supported by the National Natural Science Foundation of China [81573690, 81274146, 81320108029, 81803784, 81773995, 81673684, and 81703626], the Specific Fund for Public Interest Research of Traditional Chinese Medicine, the Ministry of Finance [201507004-002], the National “Major Scientific and Technological Special Project for Significant New Drugs Creation” project [2015ZX09501004-002-004], the Beijing Nova Program [No. Z141107001814061], the Beijing Municipal Science & Technology Commission [No. Z181100001718150], and “Double First-Class” University projects [CPU2018GY06].
Abbreviations
- AOM
azoxymethane
- ATCC
American Type Culture Collection
- CACC
Colitis-associated colon cancer
- DAPI
4’,6-diamidino-2-phenylindole
- DMEM
Dulbecco’s modified Eagle’s medium
- Doc
docetaxel
- DSS
Dextran sulfate sodium
- ELISA
Enzyme linked immunosorbent assay
- FBS
Fetal bovine serum
- FITC
Fluorescein isothiocyanate
- IFN-γ
Interferon γ
- ICR-1
Crj:CD-1
- IL
Interleukin
- LPS
Lipopolysaccharide
- M-CSF
Macrophage colony-stimulating factor
- PI
Propidium iodide
- PMA
Phorbol 12-myristate 13-acetate
- RPMI
Roswell Park Memorial Institute
- RT-PCR
Real-time PCR
- TAM
Tumor-associated macrophages
- TP
Triptolide
- TGF β1
Transforming growth factor β1
- TWHF
Tripterygium wilfordii Hook F
- PBS
Phosphate buffered saline
Disclosure of Potential Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, Li J.. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192–197. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Pechkovsky DV, Prasse A, Kollert F, Engel KMY, Dentler J, Luttmann W, Friedrich K, Müller-Quernheim J, Zissel G.. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin Immunology. 2010;137:89–101. doi: 10.1016/j.clim.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Madsen DH, Jurgensen HJ, Siersbaek MS, Kuczek DE, Grey Cloud L, Liu S, Behrendt N, Grontved L, Weigert R, Bugge TH. Tumor-associated macrophages derived from circulating inflammatory monocytes degrade collagen through cellular uptake. Cell Rep. 2017;21:3662–3671. doi: 10.1016/j.celrep.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sunakawa Y, Stintzing S, Cao S, Heinemann V, Cremolini C, Falcone A, Yang D, Zhang W, Ning Y, Stremitzer S, et al. Variations in genes regulating tumor-associated macrophages (TAMs) to predict outcomes of bevacizumab-based treatment in patients with metastatic colorectal cancer: results from TRIBE and FIRE3 trials. Ann Oncol. 2015;26:2450–2456. doi: 10.1093/annonc/mdv474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X. TIPE2 regulates tumor-associated macrophages in skin squamous cell carcinoma. Tumour Biol. 2016;37:5585–5590. doi: 10.1007/s13277-015-4388-9. [DOI] [PubMed] [Google Scholar]
- 6.Wolf GT, Chepeha DB, Bellile E, Nguyen A, Thomas D, McHugh J, University of Michigan H, Neck SP. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: a preliminary study. Oral Oncol. 2015;51:90–5. doi: 10.1016/j.oraloncology.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee C, Kim GR, Yoon J, Kim SE, Yoo JS, Piao Y. In vivo delineation of glioblastoma by targeting tumor-associated macrophages with near-infrared fluorescent silica coated iron oxide nanoparticles in orthotopic xenografts for surgical guidance. Sci Rep. 2018;8:11122. doi: 10.1038/s41598-018-29424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang M, Li Z, Ren M, Li S, Zhang L, Zhang X, Liu F. Stromal infiltration of tumor-associated macrophages conferring poor prognosis of patients with basal-like breast carcinoma. J Cancer. 2018;9:2308–2316. doi: 10.7150/jca.25155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q, Atsuta I, Liu S, Chen C, Shi S, Shi S, Le AD. IL-17–mediated M1/M2 macrophage alteration contributes to pathogenesis of bisphosphonate-related osteonecrosis of the jaws. Clin Cancer Res. 2013;19:3176–3188. doi: 10.1158/1078-0432.CCR-13-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sprinzl MF, Puschnik A, Schlitter AM, Schad A, Ackermann K, Esposito I, Lang H, Galle PR, Weinmann A, Heikenwälder M. Sorafenib inhibits macrophage-induced growth of hepatoma cells by interference with insulin-like growth factor-1 secretion. J Hepatol. 2014 [DOI] [PubMed] [Google Scholar]
- 11.Ke X, Zhang S, Wu M, Lou J, Zhang J, Xu T, Huang L, Huang P, Wang F, Pan S. Tumor-associated macrophages promote invasion via toll-like receptors signaling in patients with ovarian cancer. Int Immunopharmacol. 2016;40:184–195. doi: 10.1016/j.intimp.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 12.Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol. 2014;5:1–13. doi: 10.3389/fphys.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma R, Ji T, Chen D, Dong W, Zhang H, Yin X, Ma J, Liang X, Zhang Y, Shen G, et al. Tumor cell-derived microparticles polarize M2 tumor-associated macrophages for tumor progression. Oncoimmunology. 2016;5. e1118599. doi: 10.1080/2162402X.2015.1118599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wenes M, Shang M, Di Matteo M, Goveia J, Martin-Perez R, Serneels J, Prenen H, Ghesquiere B, Carmeliet P, Mazzone M. Macrophage metabolism controls tumor blood vessel morphogenesis and metastasis. Cell Metab. 2016;24:701–715. doi: 10.1016/j.cmet.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 15.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L, Jiang Z, Liu J, Huang X, Wang T, Liu J, Zhang Y, Zhou Z, Guo J, Yang L. Sex differences in subacute toxicity and hepatic microsomal metabolism of triptolide in rats. Toxicol. 2010;271:57–63. doi: 10.1016/j.tox.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Sun L, Li H, Huang X, Wang T, Zhang S, Yang J, Huang S, Mei H, Jiang Z, Zhang L. Triptolide alters barrier function in renal proximal tubular cells in rats. Toxicol Lett. 2013;223:96–102. doi: 10.1016/j.toxlet.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Hou ZY, Tong XP, Peng YB, Zhang BK, Yan M. Broad targeting of triptolide to resistance and sensitization for cancer therapy. Biomed Pharmacother. 2018;104:771–780. doi: 10.1016/j.biopha.2018.05.088. [DOI] [PubMed] [Google Scholar]
- 19.Guo X, Xue M, Li CJ, Yang W, Wang SS, Ma ZJ, Zhang XN, Wang XY, Zhao R, Chang BC, et al. Protective effects of triptolide on TLR4 mediated autoimmune and inflammatory response induced myocardial fibrosis in diabetic cardiomyopathy. J Ethnopharmacol. 2016;193:333–344. doi: 10.1016/j.jep.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Lu Q, Xie W, Wang Y, Wang G. Anti-tumor effects of triptolide on angiogenesis and cell apoptosis in osteosarcoma cells by inducing autophagy via repressing Wnt/β-Catenin signaling. Biochem Biophys Res Commun. 2018;496:443–449. doi: 10.1016/j.bbrc.2018.01.052. [DOI] [PubMed] [Google Scholar]
- 21.Li XJ, Jiang ZZ, Zhang LY. 2014. Triptolide: progress on research in pharmacodynamics and toxicology. J Ethnopharmacol. 155:67–79. doi: 10.1016/j.jep.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Sun L, Zhang S, Jiang Z, Huang X, Wang T, Huang X, Li H, Zhang L. Triptolide inhibits COX-2 expression by regulating mRNA stability in TNF-alpha-treated A549 cells. Biochem Biophys Res Commun. 2011;416:99–105. doi: 10.1016/j.bbrc.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Zhou G-S, Hu Z, Fang H-T, Zhang F-X, Pan X-F, Chen X-Q, Hu A-M, Xu L, Zhou G-B. Biologic activity of triptolide in t(8;21) acute myeloid leukemia cells. Leuk Res. 2011;35:214–218. doi: 10.1016/j.leukres.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Ku CM, Lin JY. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013;141:1104–1113. doi: 10.1016/j.foodchem.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 25.Freytes DO, JWK IM-C, Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114:220–229. doi: 10.1002/jcb.24357. [DOI] [PubMed] [Google Scholar]
- 26.Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda I, Tomimoto A, Wada K, Fujisawa T, Fujita K, Yonemitsu K, Nozaki Y, Endo H, Takahashi H, Yoneda M, et al. 5-aminosalicylic acid given in the remission stage of colitis suppresses colitis-associated cancer in a mouse colitis model. Clin Cancer Res. 2007;13:6527–6531. doi: 10.1158/1078-0432.CCR-07-1208. [DOI] [PubMed] [Google Scholar]
- 28.Wu SQ, Xu R, Li XF, Zhao XK, Qian BZ. Prognostic roles of tumor associated macrophages in bladder cancer: a system review and meta-analysis. Oncotarget. 2018;9:25294–25303. doi: 10.18632/oncotarget.25334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buldakov M, Zavyalova M, Krakhmal N, Telegina N, Vtorushin S, Mitrofanova I, Riabov V, Yin S, Song B, Cherdyntseva N, et al. CD68+, but not stabilin-1+ tumor associated macrophages in gaps of ductal tumor structures negatively correlate with the lymphatic metastasis in human breast cancer. Immunobiology. 2017;222:31–38. doi: 10.1016/j.imbio.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto H, Sangai T, Ishii G, Ikehara A, Nagashima T, Miyazaki M, Ochiai A. Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer. 2009;125:1276–1284. doi: 10.1002/ijc.24378. [DOI] [PubMed] [Google Scholar]
- 31.Sawa-Wejksza K, Kandefer-Szerszen M. Tumor-associated macrophages as target for antitumor therapy. Arch Immunol Ther Exp. 2018;66:97–111. doi: 10.1007/s00005-017-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang CY, Lin CK, Lin GJ, Hsieh CC, Huang SH, Ma KH, Shieh YS, Sytwu HK, Chen YW. Triptolide represses oral cancer cell proliferation, invasion, migration, and angiogenesis in co-inoculation with U937 cells. Clin Oral Investig. 2017;21:419–427. doi: 10.1007/s00784-016-1808-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong J, Su T, Qu Z, Yang Q, Wang Y, Li J, Zhou S. Triptolide has anticancer and chemosensitization effects by down-regulating Akt activation through the MDM2/REST pathway in human breast cancer. Oncotarget. 2016;7:23933–23946. doi: 10.18632/oncotarget.v7i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang C, Fang X, Zhang H, Wang X, Li M, Jiang W, Tian F, Zhu L, Bian Z. Triptolide inhibits the growth of osteosarcoma by regulating microRNA-181a via targeting PTEN gene in vivo and vitro. Tumour Biol. 2017;39:1–10. doi: 10.1177/1010428317697556. [DOI] [PubMed] [Google Scholar]
- 35.Li R, Zhang X, Tian X, Shen C, Zhang Q, Zhang Y, Wang Z, Wang F, Tao Y. Triptolide inhibits tumor growth by induction of cellular senescence. Oncol Rep. 2017;37:442–448. doi: 10.3892/or.2016.5258. [DOI] [PubMed] [Google Scholar]
- 36.Xi C, Peng S, Wu Z, Zhou Q, Zhou J. Toxicity of triptolide and the molecular mechanisms involved. Biomed Pharmacother. 2017;90:531–541. doi: 10.1016/j.biopha.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:122. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Hollmen M, Karaman S, Schwager S, Lisibach A, Christiansen AJ, Maksimow M, Varga Z, Jalkanen S, Detmar M. G-CSF regulates macrophage phenotype and associates with poor overall survival in human triple-negative breast cancer. Oncoimmunology. 2016;5:e1115177–17. doi: 10.1080/2162402X.2015.1115177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu T, Larionova I, Litviakov N, Riabov V, Zavyalova M, Tsyganov M, Buldakov M, Song B, Moganti K, Kazantseva P, et al. Tumor-associated macrophages in human breast cancer produce new monocyte attracting and pro-angiogenic factor YKL-39 indicative for increased metastasis after neoadjuvant chemotherapy. Oncoimmunology. 2018;7:e1436922–17. doi: 10.1080/2162402X.2018.1436922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mou W, Xu Y, Ye Y, Chen S, Li X, Gong K, Liu Y, Chen Y, Li X, Tian Y, et al. Expression of Sox2 in breast cancer cells promotes the recruitment of M2 macrophages to tumor microenvironment. Cancer Lett. 2015;358:115–123. doi: 10.1016/j.canlet.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Xu J, Ke X, Zhang S, Huang P, Xu T, Huang L, Lou J, Shi X, Sun R. Expression and function of toll-like receptors in peripheral blood mononuclear cells from patients with ovarian cancer. Cancer Immunol ImmunoTher. 2015;64(3):275-286. doi: 10.1007/s00262-014-1632-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makita N, Hizukuri Y, Yamashiro K, Murakawa M, Hayashi Y. IL-10 enhances the phenotype of M2 macrophages induced by IL-4 and confers the ability to increase eosinophil migration. Int Immunol. 2015;27:131–141. doi: 10.1093/intimm/dxu090. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Li J, Shi Z, Yang Y, Xie X, Lee SM, Wang Y, Leong KW, Chen M. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities. Acta Biomater. 2017;58:349–364. doi: 10.1016/j.actbio.2017.04.029. [DOI] [PubMed] [Google Scholar]


