ABSTRACT
There is still limited comprehensive genotyping data about young patients with lung adenocarcinoma. Herein, next generation sequencing (NGS) data of lung adenocarcinoma patients was retrospectively analyzed to evaluate the relationship between young age at diagnosis and the comprehensive molecular characteristics. The cBioPortal for Cancer Genomics database was queried for cancer genomic studies of lung adenocarcinoma and a cohort of 773 patients with complete cancer genomics data was selected from 2 of 11 studies. The relationship between age at diagnosis and frequency of targetable genotypes was analyzed and verified in another cohort composed of 177 Chinese lung adenocarcinoma patients undergoing NGS assay. Of the 773 eligible lung adenocarcinoma patients, younger age was associated with an increased likelihood of a targetable genotype (P < .001). Specifically, a higher prevalence of EGFR mutations (P = .005), ALK arrangements, ROS1 arrangements (P = .035) and RET arrangements (P < .001) were identified in younger patients. The frequency of KRAS mutations (P < .001) was significantly associated with older age at diagnosis and a similar trend existed for MET (P = .057) but not BRAF-V600E (P = .686) and ERBB2 (P = .083). Additionally, an age at diagnosis of 45 years was found to be a feasible cutoff point to differentiate the younger from the older patients by comprehensive molecular characteristics. These results indicated that younger patients with lung adenocarcinoma were associated with an increased likelihood of harboring a targetable genotype. Distinctive molecular characteristics were identified in patients younger than 45 years with lung adenocarcinoma, which highlights the importance of the NGS assay and personalized therapy in this subpopulation.
KEYWORDS: Lung adenocarcinoma, targetable genotypes, next generation sequencing, young patients
1. Introduction
Lung adenocarcinoma usually occurs in people at older age and patients diagnosed at younger age account for less than 5% of the total cases.1–3 Notably, the incidence rate of lung adenocarcinoma among young adults has shown an increasing trend in China.4,5 The prognosis of young patients with lung adenocarcinoma is poor especially for those with advanced-stage disease.1,2 Even when a targetable genotype was present, the survival of young patients with lung adenocarcinoma was unexpectedly poor, which suggested a more aggressive disease biology.6 Currently, the clinicopathological and molecular characteristics of young patients with lung adenocarcinoma have not been well studied. Given the great societal and economic effects caused by premature mortality, more investigations are needed for young cancer patients including those with lung adenocarcinoma.7
Previous studies have demonstrated that certain clinical characteristics could enrich driver mutations, such as EGFR mutations in Asian female nonsmokers.8,9 Younger age and no or light history of smoking were stated to be the primary clinical features of patients with ALK-positive lung adenocarcinoma.10 Interestingly, another study confirmed that ALK arrangement examinations could be more effectively performed by selecting young non-small cell lung cancer (NSCLC) patients, whereas selection based on a nonsmoking or adenocarcinoma history may not correctly identify the patient groups with potential ALK arrangements.11 Lung adenocarcinoma patients harboring ALK or ROS1 arrangements are more likely to be younger and present with highly aggressive disease.12–16 These findings suggest that young age might be an under-appreciated clinical marker of novel genomic subtypes to be discovered. Recently, several reports revealed that younger patients with lung adenocarcinoma had a distinctly unique prevalence of targetable genomic alterations, and younger age was associated with an increased likelihood of harboring a targetable genotype.6,17 Although controversial findings exist, multiple targetable genomic alterations, including EGFR, ALK, ROS1, RET, and HER2 were more prevalent in a young subpopulation, which underscores the necessity of comprehensive genomic profiling such as NGS in young patients with lung adenocarcinoma.3,6,17–22
Compared with the low-throughput assays used by the aforementioned research, the NGS assay could identify all the molecular alterations matching to the approved and emerging therapies.23 Due to the rarity of young patients with lung adenocarcinoma and the difficulty in acquiring enough tissue samples for genetic testing, there are few studies on the comprehensive profiling of young patients with lung adenocarcinoma. Via the targeted NGS assay, potentially targetable genomic alterations were detected in 90.8% of younger patients (≤45 years) with lung adenocarcinoma in our previous study.24 Young patients with lung adenocarcinoma harbored high prevalence of ALK arrangements, ERBB2-targeted alterations, and concurrent EGFR/TP53 mutations.24 In another study, whole genome sequencing was conducted to characterize the genomic alterations of 36 never-smoker Chinese patients who were diagnosed with lung adenocarcinoma at 45 years or younger. Lower mutation burden, fewer classic driver substitutions and higher frequency of several germline mutations were found, which provided insights into the molecular pathogenesis of this distinct subgroup.25 However, all these studies only contained small sample size and not all driver genes recommended for molecular detection by National Comprehensive Cancer Network (NCCN) guidelines were analyzed simultaneously.
In this study, the association between comprehensive targetable genotypes matching NCCN approved targeted therapies and young age were retrospectively analyzed in a large-scale study of lung adenocarcinoma genomic data derived from the cBioPortal for Cancer Genomics database. A high prevalence of targetable genotype and distinctive molecular characteristics were identified in patients younger than 45 years with lung adenocarcinoma. Rather than pooling all lung adenocarcinoma patients together, young patients should be recognized as a unique clinical subgroup to seek the greatest chance of deriving benefit from targeted treatments.
2. Material and methods
2.1. Definition of targetable genotypes
This study focused on an analysis of driver genes recommended for molecular detection by NCCN guidelines, for which approved targeted agents exist. The targetable genomic alterations of these genes were defined as EGFR mutations in the kinase domain, ALK rearrangements, ROS1 rearrangements, RET rearrangements, BRAF-V600E mutations, MET amplification or MET exon14 skipping mutations and ERBB2 mutations in the kinase domain. Mutations in KRAS have been associated with reduced responsiveness to EGFR tyrosine kinase inhibitors (EGFR-TKIs) and these were also analyzed as a single genotype. However, targetable genotype positivity was defined as harboring at least one targetable genomic alteration in the above genes, excluding KRAS. Pretreatment EGFRT790M found in rare NSCLC patients with concurrent EGFR-activating mutations was also defined as a targetable genomic alteration. Additionally, potentially targetable genomic alterations, including EGFR exon 20 insertions, BRAF non-V600E mutations and ERBB2 amplification, for which approved targeted therapies are still unavailable, were not studied.
2.2. Data source and patient selection
The cBioPortal for Cancer Genomics (http://www.cbioportal.org), originally developed at Memorial Sloan Kettering Cancer Center, is an open-access resource for the interactive exploration of multidimensional cancer genomics data sets.26,27 It can provide visualization, analysis and downloads of large-scale cancer genomics data sets. Eleven cancer genomic studies of lung adenocarcinoma were found in the cBioPortal for Cancer Genomics (Table S1). In the next analysis, the selected cohort was limited to patients with adenocarcinoma, data on age at diagnosis and complete analysis of mutation types (including single nucleotide variants, insertion/deletion, amplification, and arrangement). Those patients receiving prior treatment, selected to be treated with immunotherapy, diagnosed with multiple synchronous lung cancers and missing detailed sequencing data were excluded.
In addition, the targeted NGS assay data of 177 Chinese patients with lung adenocarcinoma were re-analyzed according to the previously mentioned criteria of a targetable genotype. The details of the NGS assay and clinical characteristics of these patients can be found in our previous report.24
2.3. Statistical analysis
The Mann–Whitney U test was used to analyze the gene mutation status and patient characteristics among age rank groups. Pearson’s chi-squared test and Fisher’s exact test were used to analyze the gene mutation status and patient characteristics between the two groups. The correlation between gene mutation status and age was analyzed using the linear-by-linear association chi-squared test. For all the tests, P values were two-tailed. Statistical significance was defined as P < .05.
3. Results
3.1. Patients characteristics
Patient selection for the study cohort obtained from the cBioPortal for Cancer Genomics database is depicted in Figure 1. The genotypes EGFR, ALK, ROS1, RET, BRAF-V600E, MET, ERBB2 and KRAS are exclusive of each other in the cohort except for five cases. These five concomitant genetic alterations included 3 MET/KRAS mutations, 1 KRAS/EGFR mutation and 1 EGFR/MET mutation (Table S2). According to the previously reported treatment response, these genotypes were classified into MET, KRAS and EGFR mutation types, respectively.28–30 Patients harboring no driver genomic alteration apart from these 8genes were considered to be wild-type.
Figure 1.
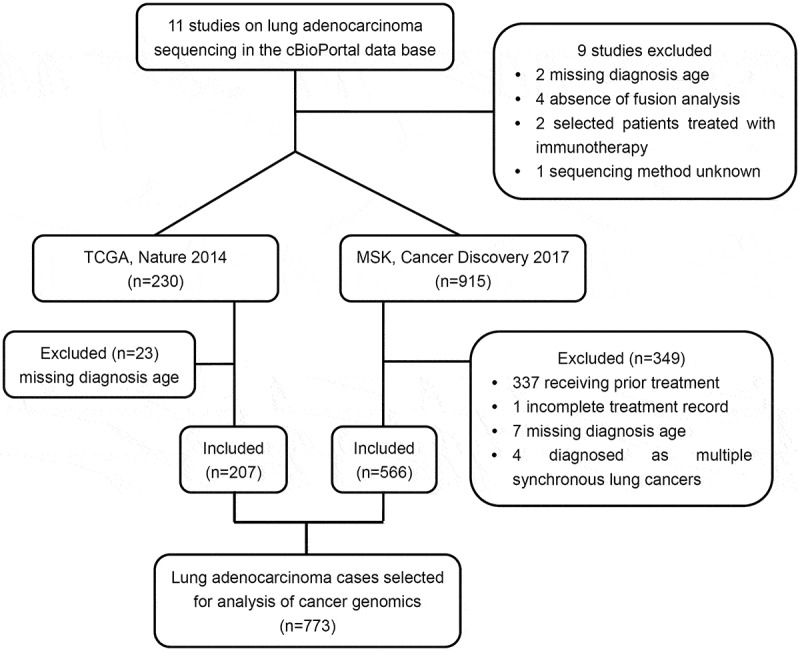
Profile summarizing the study cohort derivation.
A total of 773 eligible lung adenocarcinoma patients with complete NGS data and clinical information were selected from 2 of 11 studies in the cBioPortal for Cancer Genomics database.
A total of 773 eligible lung adenocarcinoma patients with complete cancer genomics data were identified from 2 of 11 studies (Table S2). Of the 773 patients, 442 (57.18%) were female patients, 188 (24.32%) had never smoked and 511 (66.11%) had stage IIIB/IV disease (Table 1). The median age of lung adenocarcinoma patients included in the study cohort was 64 years (range, 9–93 years). Younger age was associated with an increased likelihood of being female (P < .001), having never smoked (P = .028)and being initially diagnosed with stage IIIB/IV disease(P = 0 .001). Overall, 42 (5.43%) patients were diagnosed with lung adenocarcinoma at age 45 or younger (Table 1).
Table 1.
Clinical and molecular characteristics of lung adenocarcinoma patients.
| Diagnosis age groups (No.) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | ≤40 | 41-45 | 46-50 | 51-55 | 56-60 | 61-65 | 66-70 | 71-75 | 76-80 | >80 | Total | P value |
| Patients | 18 | 24 | 43 | 64 | 117 | 125 | 136 | 115 | 80 | 51 | 773 | / |
| Sex | ||||||||||||
| Male | 5 | 10 | 8 | 15 | 45 | 60 | 64 | 55 | 44 | 25 | 331 | <.001 |
| Female | 13 | 14 | 35 | 49 | 72 | 65 | 72 | 60 | 36 | 26 | 442 | |
| Smoking status | ||||||||||||
| Never | 9 | 7 | 13 | 19 | 30 | 27 | 32 | 19 | 17 | 15 | 188 | .043 |
| Former/current | 9 | 17 | 30 | 45 | 85 | 98 | 102 | 92 | 62 | 35 | 575† | |
| Stage | ||||||||||||
| I-IIIA | 0 | 6 | 8 | 19 | 38 | 42 | 57 | 43 | 22 | 22 | 257 | .005 |
| IIIB-IV | 18 | 18 | 35 | 45 | 78 | 83 | 77 | 71 | 57 | 29 | 511‡ | |
| Targetable genotype | ||||||||||||
| Positive | 13 | 12 | 16 | 28 | 40 | 51 | 41 | 39 | 16 | 16 | 272 | <.001 |
| Negative | 5 | 12 | 27 | 36 | 77 | 74 | 95 | 76 | 64 | 35 | 501 | |
| EGFR | ||||||||||||
| Mutant | 7 | 6 | 9 | 12 | 22 | 28 | 22 | 20 | 6 | 8 | 140 | .008 |
| Nonmutant | 11 | 18 | 34 | 52 | 95 | 97 | 114 | 95 | 74 | 43 | 633 | |
| ALK | ||||||||||||
| Mutant | 2 | 2 | 2 | 3 | 5 | 3 | 3 | 3 | 2 | 0 | 25 | .017 |
| Nonmutant | 16 | 22 | 41 | 61 | 112 | 122 | 133 | 112 | 78 | 51 | 748 | |
| ROS1 | ||||||||||||
| Mutant | 2 | 0 | 1 | 4 | 1 | 4 | 0 | 3 | 1 | 0 | 16 | .055 |
| Nonmutant | 16 | 24 | 42 | 60 | 116 | 121 | 136 | 112 | 79 | 51 | 757 | |
| RET | ||||||||||||
| Mutant | 2 | 2 | 0 | 3 | 3 | 1 | 1 | 0 | 0 | 0 | 12 | <.001 |
| Nonmutant | 16 | 22 | 43 | 61 | 114 | 124 | 135 | 112 | 80 | 51 | 758 | |
| BRAF-V600E | ||||||||||||
| Mutant | 0 | 1 | 0 | 1 | 2 | 3 | 5 | 1 | 2 | 1 | 16 | .686 |
| Nonmutant | 18 | 23 | 43 | 63 | 115 | 122 | 131 | 114 | 78 | 50 | 757 | |
| MET | ||||||||||||
| Mutant | 0 | 1 | 2 | 3 | 4 | 8 | 4 | 8 | 5 | 6 | 41 | .067 |
| Nonmutant | 18 | 23 | 41 | 61 | 113 | 117 | 132 | 107 | 75 | 45 | 732 | |
| ERBB2 | ||||||||||||
| Mutant | 0 | 0 | 2 | 2 | 3 | 4 | 6 | 4 | 0 | 1 | 22 | .720 |
| Nonmutant | 18 | 24 | 41 | 62 | 114 | 121 | 130 | 111 | 80 | 50 | 751 | |
| KRAS | ||||||||||||
| Mutant | 1 | 1 | 10 | 23 | 34 | 31 | 46 | 33 | 39 | 20 | 238 | <.001 |
| Nonmutant | 17 | 23 | 33 | 41 | 83 | 94 | 90 | 82 | 41 | 31 | 535 | |
Note: †10 patients with unknown smoking history were excluded from analysis. ‡5 patients with unknown stage at diagnosis were excluded from analysis. P < .05 was used to define statistical significance.
3.2. Association between targetable genomic alterations and age
Across the study cohort, 35.19% (272/773) of lung adenocarcinoma patients were found to harbor at least one targetable genomic alteration. The age categories of patients used in this analysis were 40 years or younger, 41 to 45, 46 to 50, 51 to 55, 56 to 60, 61 to 65, 66 to 70, 71 to 75, 76 to 80 and 80 years or older. The mutation prevalence of 8 driver genes is shown in Figure 2(a).The likelihood of harboring a targetable genomic alteration with approved agents was associated with age (P < .001) and increased with younger age at diagnosis (Figure 2(b)). Furthermore, association analysis of the relationship between the presence of an individual targetable genomic alteration and age was performed. Lung adenocarcinoma patients diagnosed at a younger age had increased likelihoods of EGFR kinase mutations(P = .005), ALK rearrangements (P = .009), ROS1 rearrangements (P = .035) and RET arrangements (P < .001) (Figure 3). No significant association with age at diagnosis was found for BRAF-V600E and ERBB2 mutations. Although an increasing trend toward older age at diagnosis was observed for MET, the result was no significant (P = .057). Finally, KRAS was significantly associated with older age at diagnosis (P < .001) (Figure 3).
Figure 2.
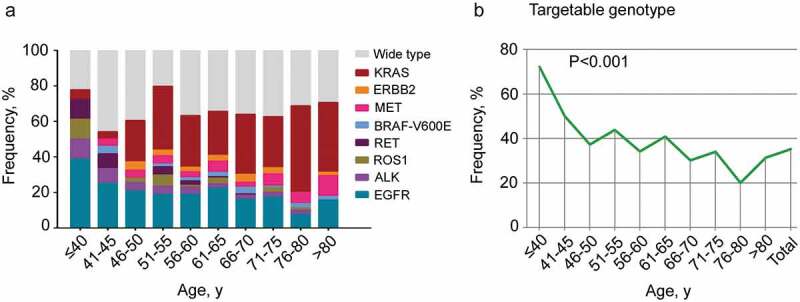
The prevalence of a targetable genotype in different age groups.
(a). The frequency of targetable genomic alterations in different age groups of patients with lung adenocarcinoma. (b). The correlation between the likelihood of harboring a targetable genotype and age was analyzed. Statistical significance was defined as P < .05.
Figure 3.
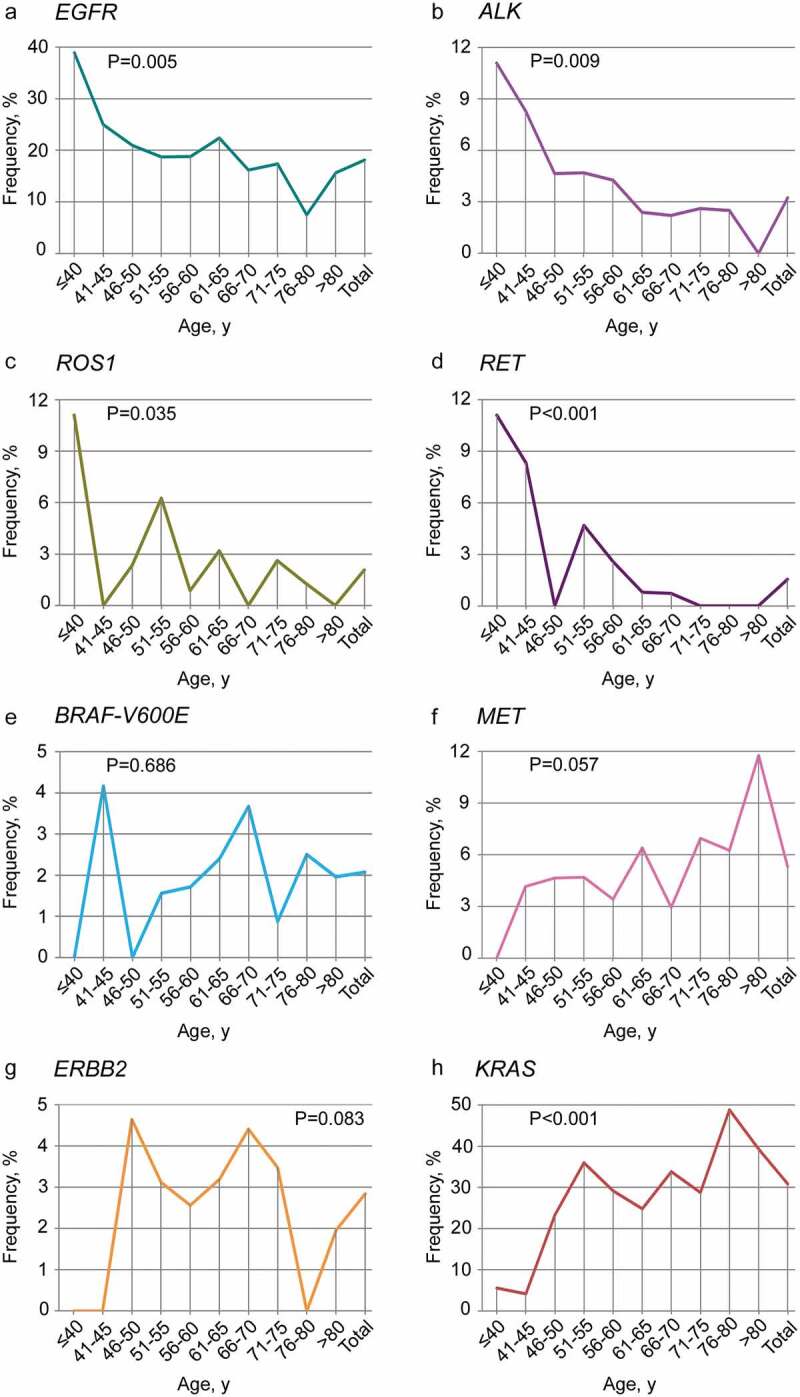
Frequency of eight targetable genomic alterations across age groups.
The frequencies of EGFR kinase mutations(a), ALK rearrangements (b), ROS1 rearrangements (c) and RET arrangements (d) gradually decrease with age. The frequencies of BRAF-V600E, MET, ERBB2 and KRAS across age groups are, respectively, shown in E, F, G and H. Notably, an increasing trend toward older age at diagnosis was observed for MET and KRAS. Statistical significance was defined as P < .05.
3.3. Defining a proper cutoff for young patients harboring a distinctive genomic type
To identify a proper age cutoff point for young patients with lung adenocarcinoma who have a unique genetic profile, the frequency of targetable genomic alterations across all age groups was studied. As shown in Figures 2(b) and 3, obvious turning points were found in the frequency of a targetable genotype, EGFR mutations, ALK arrangements, RET arrangements and KRAS mutations in patients younger than 45 years. We, therefore, studied whether an age at diagnosis of 45 years might be a cutoff point to differentiate the younger and older patients based on comprehensive molecular characteristics. As expected, the dramatic difference of targetable genotypes was found between the patients at age 45 or younger and the patients older than 45 years(P < .001) (Figure 4). Additionally, the above comparison analysis was confirmed in 177 Chinese lung adenocarcinoma patients, which also revealed a distinctive genomic genotype in patients diagnosed at 45 or younger (P < .001). Specifically, significant differences were found in EGFR mutations, ALK arrangements, RET arrangements and KRAS mutations between the younger and older patients derived from the cBioPortal database (Table 2), while in the Chinese lung adenocarcinoma subpopulation, a significant difference was only found in ALK arrangements and KRAS mutations (Table 2).
Figure 4.
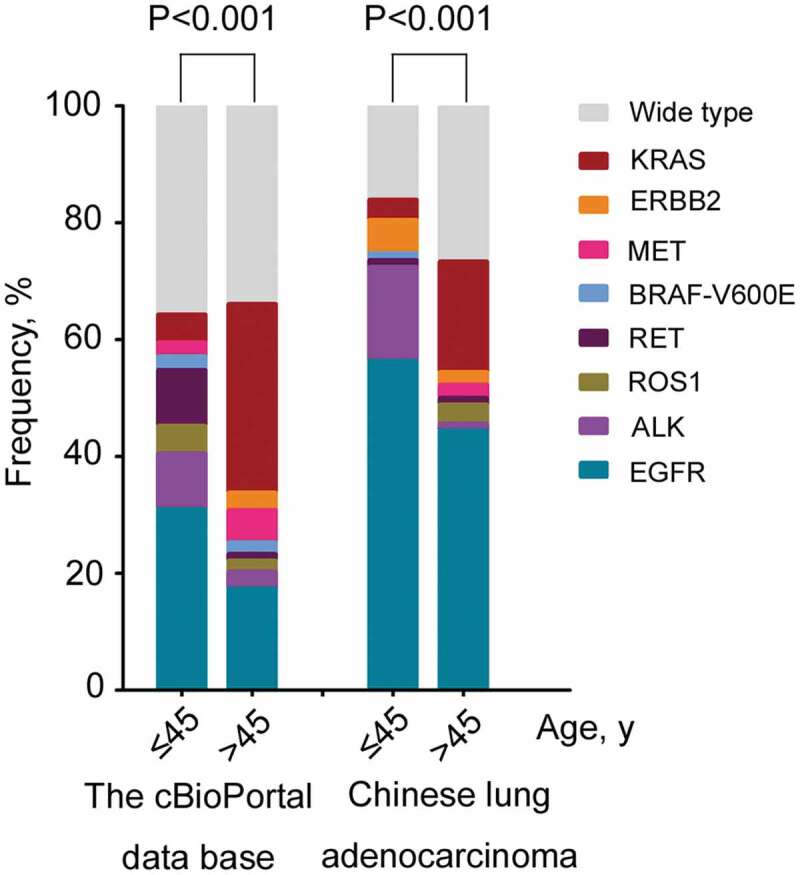
Targetable genotypes of different study cohorts.
Comparative analysis of the targetable genotypes between the patients aged 45 years or less and more than 45 years derived from different study cohorts.
Table 2.
The targetable genotype in different study populations.
| Targetable genomic alterations | The cBioportal data base (n = 773) |
Chinese lung adenocarcinoma (n = 177) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age groups | ≤45, y, No.(%) | >45, y, No.(%) | P | ≤45, y, No.(%) | >45, y, No.(%) | P | ||||
| Total | 42 | 731 | / | 87 | 90 | / | ||||
| EGFR | 13 | 30.95 | 127 | 17.37% | .037 | 49 | 56.32 | 40 | 44.44 | .134 |
| ALK | 4 | 9.52 | 21 | 2.87% | .041 | 14 | 16.09 | 1 | 1.11 | .001 |
| ROS1 | 2 | 4.76 | 14 | 1.92% | .214 | 0 | 0.00 | 3 | 3.33 | .246 |
| RET | 4 | 9.52 | 8 | 1.09% | .003 | 1 | 1.15 | 1 | 1.11 | 1.000 |
| BRAF-V600E | 1 | 2.38 | 15 | 2.05% | .595 | 1 | 1.15 | 0 | 0.00 | .492 |
| MET | 1 | 2.38 | 40 | 5.47% | .719 | 0 | 0.00 | 2 | 2.22 | .497 |
| ERBB2 | 0 | 0.00 | 22 | 3.01% | .626 | 5 | 5.75 | 2 | 2.22 | .272 |
| KRAS | 2 | 4.76 | 236 | 32.28% | <.001 | 3 | 3.45 | 17 | 18.89 | .002 |
| Wide type | 15 | 35.71 | 248 | 33.93% | .867 | 14 | 16.09 | 24 | 26.67 | .101 |
Note: P < .05 was used to define statistical significance.
4. Discussion
With the development of precision medicine, a young age at diagnosis has been increasingly reported to be associated with distinctive targetable genotypes in lung adenocarcinoma.6,17 However, these studies only focused on a few targetable genetic alterations and lacked a comprehensive analysis of molecular characteristics in young patients with lung adenocarcinoma. In this study, targeted genetic alterations of 8 genes recommended for molecular detection by the NCCN guidelines were retrospectively analyzed in773 lung adenocarcinoma patients derived from the cBioPortal for Cancer Genomics database. Younger age was identified to be significantly associated with an increased likelihood of a targetable genotype. Young patients with lung adenocarcinoma were a specific subpopulation who tended to have more targetable genomic alterations matching existing targeted therapies. This study provided knowledge on the comprehensive molecular characteristics of young patients with lung adenocarcinoma, laying the groundwork for personalized therapy of this subpopulation.
Multiple factors, especially the various genetic testing assays complicates the research on the molecular characteristics of young patients with lung adenocarcinoma. Targeted NGS could detect rare targetable alterations missed by those low throughput standard genotyping in lung adenocarcinoma, which suggests that comprehensive genomic profiling, such as NGS might be a better tool for studying molecular characteristics.31 Recently, the molecular characteristics of young patients with lung adenocarcinoma were studied using targeted NGS and whole genome sequencing.25,32 However, both studies only enrolled small numbers of patients, which limited the statistical power of the analyses. Here, we provided the first large-scale retrospective study on the comprehensive molecular characteristics of young patients with lung adenocarcinoma who underwent the NGS assay. Consistent with previous studies, we demonstrated an association between young age and specific targetable genomic alterations, including EGFR, ALK, and ROS1.6,12,17 It was noteworthy that a high prevalence of the rare genotype RET was identified in young patients in this study, as reported in previous studies.33–35 In contrast, the frequency of KRAS mutations was significantly associated with older age at diagnosis and a similar trend existed for MET but not BRAF-V600E and ERBB2. All these results revealed a comprehensive mutational profile of young patients with lung adenocarcinoma.
Due to the rarity of young patients with lung adenocarcinoma, it is difficult to define a proper age cutoff point for young patients with lung adenocarcinoma who have a unique genetic profile. Using a cohort of 2237 patients with NSCLC, researchers found that patients diagnosed at younger than 50 years have a 59%-increased likelihood of harboring a targetable genotype.6 Consequently, their findings suggested that the age of 50 years constitutes a useful age cutoff by which NSCLC in the young may be defined. However, given the obvious turning points found in the frequency of multiple targetable genomic alterations in patients younger than 45 years, we discovered that an age at diagnosis of 45 years might be a feasible cutoff point to distinguish the younger from older patients by molecular characteristics. This result was confirmed by one of our previous studies on the comprehensive molecular characterization of young Chinese patients with lung adenocarcinoma.24 The above controversy on defining the criteria for young age at diagnosis might result from pathology and age categories, which calls for a further prospective study. We also noted that the frequency of comprehensive targetable genotypes, such as EGFR and ERBB2, varied significantly between the younger and older patients derived from the cBioPortal database and Chinese patients.24,36 This implied that patients’ race is also an important factor to be considered in the future research.
It has been stated that the survival of young patients with NCSLC is unexpectedly poor compared with other age groups, despite harboring a higher frequency of potentially targeted genetic alterations.6 However, a survival analysis was not conducted due to incomplete follow-up data, which was a major limitation of this study. Additionally, other genetic alterations such as TP53 and CDKN2A, usually concurring with the aforementioned 8 genes, were not included in this analysis. These concomitant mutations might be involved in the resistance to targeted therapies and the prognosis of young patients with lung adenocarcinoma.24,37 Consequently, the unique characteristics and role of concurrent mutations in young patients with lung adenocarcinoma, except for the targetable genomic alterations calls for additional research.
5. Conclusions
In conclusion, our study further confirmed young age at diagnosis as a genomically enriched subtype of lung adenocarcinoma using NGS data from the cBioPortal for Cancer Genomics database. Young patients with lung adenocarcinoma are preferred candidates for precision cancer medicine, whom should not miss out on-tailored targeted treatments guided by NGS assay.
Funding Statement
This work was supported by Taishan Scholar Foundation of Shandong Province (No.tshw201502061 to Xiaochun Zhang), Qingdao People’s Livelihood Science and Technology Program (16-6-2-3-nsh to Xiaochun Zhang), Chinese Postdoctoral Science Foundation (2017M622143 to Helei Hou) and Qingdao Postdoctoral Application Research Funded Project (2016052 to Helei Hou).
Acknowledgments
The authors thank Dr. Jiahe Zhang for providing statistical guidance for this study.
Author Contributions
Helei Hou and Xiaochun Zhang conceived and designed the study; Chuantao Zhang, Xiaogai Qi, Lei Zhou, Hongying Lv, Tianjun Li, and Dantong Sun collected the study data; Helei Hou and Dong Liu analyzed and interpreted the genetic data; Helei Hou and Xiaochun Zhang wrote the manuscript; All authors reviewed and approved the final version of the paper.
Disclosure of Potential Conflict of interest
The authors declare no potential conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Arnold BN, Thomas DC, Rosen JE, Salazar MC, Blasberg JD, Boffa DJ, Detterbeck FC, Kim AW.. Lung cancer in the very young: treatment and survival in the national cancer data base. J Thorac Oncol. 2016;11:1121–1131. doi: 10.1016/j.jtho.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Gomes R, Dabo H, Queiroga H, Hespanhol V.. Non-small cell lung cancer in young patients–a retrospective analysis of 10 years in a tertiary university hospital. Rev Port Pneumol. 2006;2016(22):125–126. [DOI] [PubMed] [Google Scholar]
- 3.Corrales-Rodriguez L, Arrieta O, Mas L, Baez-Saldana R, Castillo-Fernandez O, Blais N, Martin C, Juarez M, Khanna P, Ramos-Esquivel A, et al. An international epidemiological analysis of young patients with non-small cell lung cancer (AduJov-CLICaP). Lung Cancer. 2017;113:30–36. doi: 10.1016/j.lungcan.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Li YF, Wang Y, Li JL, Hao XZ, Hu XS, Wang HY. Trend analysis and clinicopathological characteristics of 198 young patients with advanced lung adenocarcinoma. Zhonghua Zhong Liu Za Zhi. 2016;38:750–755. doi: 10.3760/cma.j.issn.0253-3766.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Chen SF, Zhen Y, Xiang J, Wu C, Bao P, Luketich J, Hu H, Zhou X, Zhang J, et al. Multicenter analysis of lung cancer patients younger than 45 years in Shanghai. Cancer Am Cancer Soc. 2010;116:3656–3662. [DOI] [PubMed] [Google Scholar]
- 6.Sacher AG, Dahlberg SE, Heng J, Mach S, Janne PA, Oxnard GR. Association between younger age and targetable genomic alterations and prognosis in non-small-cell lung cancer. Jama Oncol. 2016;2:313–320. doi: 10.1001/jamaoncol.2015.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-Foucher E, Bray F. Cancer incidence and mortality among young adults aged 20-39 years worldwide in 2012: a population-based study. Lancet Oncol. 2017;18:1579–1589. doi: 10.1016/S1470-2045(17)30677-0. [DOI] [PubMed] [Google Scholar]
- 8.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. SCIENCE. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 9.Wu YL, Zhong WZ, Li LY, Zhang XT, Zhang L, Zhou CC, Liu W, Jiang B, Mu XL, Lin JY, et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: a meta-analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol. 2007;2:430–439. doi: 10.1097/01.JTO.0000268677.87496.4c. [DOI] [PubMed] [Google Scholar]
- 10.Fukui T, Yatabe Y, Kobayashi Y, Tomizawa K, Ito S, Hatooka S, Matsuo K, Mitsudomi T. Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4-ALK fusion oncogene. Lung Cancer. 2012;77:319–325. doi: 10.1016/j.lungcan.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi M, Sakakibara T, Inoue A, Fukuhara T, Sasano H, Ichinose M, Nukiwa T. Effective enrichment strategy for EML4-ALK fusion gene screening in patients with non-small cell lung cancer. Respir Investig. 2014;52:49–56. doi: 10.1016/j.resinv.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Scarpino S, Rampioni VG, Di Napoli A, Fochetti F, Uccini S, Iacono D, Marchetti P, Ruco L. High prevalence of ALK+/ROS1+ cases in pulmonary adenocarcinoma of adoloscents and young adults. Lung Cancer. 2016;97:95–98. doi: 10.1016/j.lungcan.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Yang P, Kulig K, Boland JM, Erickson-Johnson MR, Oliveira AM, Wampfler J, Jatoi A, Deschamps C, Marks R, Fortner C, et al. Worse disease-free survival in never-smokers with ALK+ lung adenocarcinoma. J Thorac Oncol. 2012;7:90–97. doi: 10.1097/JTO.0b013e31823c5c32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catania C, Botteri E, Barberis M, Conforti F, Toffalorio F, De Marinis F, Boselli S, Noberasco C, Delmonte A, Spitaleri G, et al. Molecular features and clinical outcome of lung malignancies in very young people. Future Oncol. 2015;11:1211–1221. doi: 10.2217/fon.15.10. [DOI] [PubMed] [Google Scholar]
- 15.Kometani T, Sugio K, Osoegawa A, Seto T, Ichinose Y. Clinicopathological features of younger (aged </= 50 years) lung adenocarcinoma patients harboring the EML4-ALK fusion gene. Thorac Cancer. 2018;9(5):563–570. doi: 10.1111/1759-7714.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian HX, Zhang XC, Yang JJ, Guo WB, Chen ZH, Wang Z, Wu YL. Clinical characteristics and sequence complexity of anaplastic lymphoma kinase gene fusions in Chinese lung cancer patients. Lung Cancer. 2017;114:90–95. doi: 10.1016/j.lungcan.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka K, Hida T, Oya Y, Yoshida T, Shimizu J, Mizuno T, Kuroda H, Sakakura N, Yoshimura K, Horio Y, et al. Unique prevalence of oncogenic genetic alterations in young patients with lung adenocarcinoma. Cancer Am Cancer Soc. 2017;123:1731–1740. [DOI] [PubMed] [Google Scholar]
- 18.Wu SG, Chang YL, Yu CJ, Yang PC, Shih JY. Lung adenocarcinoma patients of young age have lower EGFR mutation rate and poorer efficacy of EGFR tyrosine kinase inhibitors. ERJ Open Res. 2017;3(3):00092–2016. doi: 10.1183/23120541.00092-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye T, Pan Y, Wang R, Hu H, Zhang Y, Li H, Wang L, Sun Y, Chen H. Analysis of the molecular and clinicopathologic features of surgically resected lung adenocarcinoma in patients under 40 years old. J Thorac Dis. 2014;6:1396–1402. doi: 10.3978/j.issn.2072-1439.2014.08.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagashima O, Ohashi R, Yoshioka Y, Inagaki A, Tajima M, Koinuma Y, Iwakami S, Iwase A, Sasaki S, Tominaga S, et al. High prevalence of gene abnormalities in young patients with lung cancer. J Thorac Dis. 2013;5:27–30. doi: 10.3978/j.issn.2072-1439.2012.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VandenBussche CJ, Illei PB, Lin MT, Ettinger DS, Maleki Z. Molecular alterations in non-small cell lung carcinomas of the young. Hum Pathol. 2014;45:2379–2387. doi: 10.1016/j.humpath.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Pan X, Lv T, Zhang F, Fan H, Liu H, Song Y. Frequent genomic alterations and better prognosis among young patients with non-small-cell lung cancer aged 40 years or younger. Clin Transl Oncol. 2018;20:1168–1174. doi: 10.1007/s12094-018-1838-z. [DOI] [PubMed] [Google Scholar]
- 23.Jordan EJ, Kim HR, Arcila ME, Barron D, Chakravarty D, Gao J, Chang MT, Ni A, Kundra R, Jonsson P, et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov. 2017;7:596–609. doi: 10.1158/2159-8290.CD-16-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou H, Zhu H, Zhao H, Yan W, Wang Y, Jiang M, Liu B, Liu D, Zhou N, Zhang C, et al. Comprehensive molecular characterization of young Chinese patients with lung adenocarcinoma identified a distinctive genetic profile. ONCOLOGIST. 2018;23:1008–1015. doi: 10.1634/theoncologist.2017-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo W, Tian P, Wang Y, Xu H, Chen L, Tang C, Shu Y, Zhang S, Wang Z, Zhang J, et al. Characteristics of genomic alterations of lung adenocarcinoma in young never-smokers. Int J Cancer. 2018;143:1696–1705. doi: 10.1002/ijc.31542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:l1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palma NA, Ali SM, O’Connor J, Dutta D, Wang K, Soman S, Palmer GA, Morosini D, Ross JS, Lipson D, et al. Durable response to Crizotinib in a MET-Amplified, KRAS-Mutated carcinoma of unknown primary. Case Rep Oncol. 2014;7:503–508. doi: 10.1159/000365326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda M, Okamoto I, Fujita Y, Arao T, Ito H, Fukuoka M, Nishio K, Nakagawa K. De novo resistance to epidermal growth factor receptor-tyrosine kinase inhibitors in EGFR mutation-positive patients with non-small cell lung cancer. J Thorac Oncol. 2010;5:399–400. doi: 10.1097/JTO.0b013e3181cee47e. [DOI] [PubMed] [Google Scholar]
- 30.Gainor JF, Niederst MJ, Lennerz JK, Dagogo-Jack I, Stevens S, Shaw AT, Sequist LV, Engelman JA. Dramatic response to combination erlotinib and crizotinib in a patient with advanced, EGFR-Mutant lung cancer Harboring De Novo MET amplification. J Thorac Oncol. 2016;11:e83–85. doi: 10.1016/j.jtho.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Drilon A, Wang L, Arcila ME, Balasubramanian S, Greenbowe JR, Ross JS, Stephens P, Lipson D, Miller VA, Kris MG, et al. Broad, hybrid capture-based next-generation sequencing identifies actionable genomic alterations in lung adenocarcinomas otherwise negative for such alterations by other genomic testing approaches. Clin Cancer Res. 2015;21:3631–3639. doi: 10.1158/1078-0432.CCR-14-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vavala T, Monica V, Lo IM, Mele T, Busso S, Righi L, Papotti M, Scagliotti GV, Novello S. Precision medicine in age-specific non-small-cell-lung-cancer patients: integrating biomolecular results into clinical practice-A new approach to improve personalized translational research. Lung Cancer. 2017;107:84–90. doi: 10.1016/j.lungcan.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Lin C, Wang S, Xie W, Chang J, Gan Y. The RET fusion gene and its correlation with demographic and clinicopathological features of non-small cell lung cancer: a meta-analysis. Cancer Biol Ther. 2015;16:1019–1028. doi: 10.1080/15384047.2015.1046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SE, Lee B, Hong M, Song JY, Jung K, Lira ME, Mao M, Han J, Kim J, Choi YL. Comprehensive analysis of RET and ROS1 rearrangement in lung adenocarcinoma. Mod Pathol. 2015;28:468–479. doi: 10.1038/modpathol.2014.107. [DOI] [PubMed] [Google Scholar]
- 35.Tsuta K, Kohno T, Yoshida A, Shimada Y, Asamura H, Furuta K, Kushima R. RET-rearranged non-small-cell lung carcinoma: a clinicopathological and molecular analysis. Br J Cancer. 2014;110:1571–1578. doi: 10.1038/bjc.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Sun Y, Pan Y, Li C, Shen L, Li Y, Luo X, Ye T, Wang R, Hu H, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res. 2012;18:1947–1953. doi: 10.1158/1078-0432.CCR-11-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradly DP, Gattuso P, Pool M, Basu S, Liptay M, Bonomi P, Buckingham L. CDKN2A (p16) promoter hypermethylation influences the outcome in young lung cancer patients. Diagn Mol Pathol. 2012;21:207–213. doi: 10.1097/PDM.0b013e31825554b2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


