ABSTRACT
EGISTs originating outside the gastrointestinal tract share some similarities with the GISTs regarding their immunohistochemical features including the positive expression of CD117 and CD34. The majority of EGISTs carry activating mutations of the C-KIT or PDGFRA genes. However, there is no precedent in the literature where the two mutations occur in one case of EGISTs to date. We describe herein, a 52-year-old female who presented as mesenteric and pelvic regions masses showing positive immunoreactivity for CD117, DOG-1, CD34. Mutation analysis identified two mutations that located in the exon 13 of C-KIT and in the exon 18 of PDGFRA. The patient was treated sequentially with imatinib, sunitinib, sorafenib, and regorafenib. However, the prognosis was undesirable. Previous research has shown that expression of members of Bcl-2 family may be helpful in predicting prognosis, the survival time, and the resistance to chemotherapeutic agents. IHC was performed to detect the expression of BCL-2 family. The results show that high BCL-2 expression and low BAX expression in both specimens. In conclusion, our case may suggest that the presence of both C-KIT and PDGFRA mutations in EGISTs patients may indicate a very poor prognosis; and the expression level of BCL-2 and BAX could predict clinical outcome.
KEYWORDS: EGIST, C- KIT, PDGFRA, CD117, CD34, DOG-1, BCL-2, BAX
Background
The most common mesenchymal tumors that grow in the gastrointestinal tract (GIT) are the gastrointestinal stromal tumors (GISTs), which are thought to originate the interstitial cells of Cajal (ICC) in the gastrointestinal muscle layer. But, GISTs are extremely infrequent, accounting for less than 2% of all neoplasm in the GIT.1–3 As another kind of rare tumors which are originated outside the GIT, such as omentum, mesentery, retroperitoneum, pelvic cavity, abdominal wall, and so forth, extragastrointestinal stromal tumors (EGISTs) share some similarities with GISTs regarding their immunohistochemical features including the positive expression of CD117 and CD34.4–7
CD117 showed a strongly and diffusely positive immunohistochemical response, which can support the diagnosis of GISTs/EGISTs.5 However, roughly 5% of the cases presented negative immunohistochemistry (IHC) for CD117,8 which is quite accessible for some GISTs/EGISTs to be misdiagnosed as other tumors.
The results of C-KIT and PDGFRA (platelet-derived growth factor) gene mutation analyses and immunohistochemical phenotypes of DOG-1 can be used as the basis for the diagnosis of GISTs/EGISTs, and guide disease management, especially when CD117 exhibits negative immunoreactivity. Here, we present a case of EGISTs in the mesentery with clinical manifestations of dysuria and recurrent mesenteric and pelvic masses, which was initially misdiagnosed as spontaneous-isolated fibromatosis of mesentery (SIFM).
Case presentation
A 52-year-old female patient with severe progressive dysuria for 2 months was admitted to The Second Affiliated Hospital of Fujian Medical University (Fujian, China). For her past history, she underwent abdominal surgery for spontaneous-isolated fibromatosis of mesentery (SIFM) 4 years ago in another hospital. Pathological analysis of the tumor (No.09085396) was positive for CD34, vimentin, BCL-2, while negative results were obtained for CD117, desmin, S-100, CD31, FN (Figure 1). On physical examination, she showed a slightly distended abdomen, obvious pain on superficial palpation of left lower quadrant, and palpable lesion approximately 15 cm wide in the lower abdominal. The findings of laboratory tests, such as routine blood test and tumor markers were all within the normal limits. Contrast-enhanced abdominal and pelvic computed tomography (CT) revealed a 10.2 × 12.0× 8.5 cm heterogeneously enhanced neoplasm with an unclear boundary and involved the uterus located in the lower abdomen and extended downward into the pelvic cavity and that compressed the rectum. Another 3.8 × 4.0 cm mass was demonstrated in the middle and lower part of the left paracolic sulcus that exhibited heterogeneous, following contrast enhancement (Figure 2). The patient also underwent esophagogastroduodenoscopy and colonoscopy; however, no abnormalities were informed in the gastrointestinal tract. No metastatic focus was observed on X-ray or ultrasound test.
Figure 1.
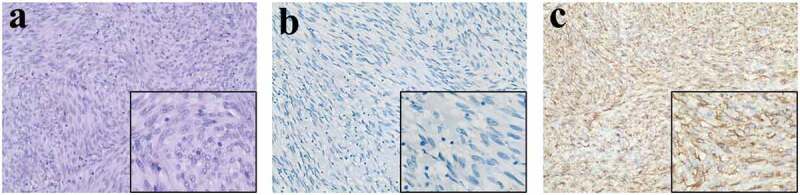
Postoperative histopathology of the first operation (No.09085396).
(a) Stained with H&E double stain for light microscopic observation. (magnification, ×200, ×400). (b) No tumor cells show positive immunoreactivity for CD117 (magnifications, ×200, ×400). (c) Extensively and moderately positivity within the cell membrane and cytoplasm for CD34 (magnifications, ×200, ×400).
Figure 2.
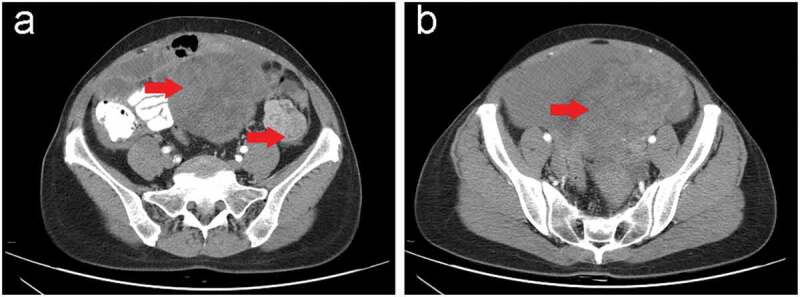
Contrast-enhanced abdominal and pelvic computed tomography (CT) scan in our hospital before the surgery.
(a,b) Computed tomography showed two heterogeneous masses (red arrow). (a) One was measured approximately 10.2 × 12.0 × 8.5 cm with an unclear boundary and involved the uterus located in the lower abdomen and extended downward into the pelvic cavity; and (b) the other was demonstrated approximately 3.8 × 4.0 cm in the middle and lower part of the left paracolic sulcus.
Subsequently, the patient experienced an exploratory laparotomy. Intraoperatively, a mass of approximately 20×15×15cm was demonstrated in the lower abdomen and extended downward into the pelvic cavity and had visible adhesion to the left ovary and broad ligament; another one measured 5x5x2.5cm located in the lower part of the left paracolic sulcus and associated with the adjacent mesentery. And no enlarged lymph nodes were found. Eventually, the tumors were resected entirely with partial adhesion tissue. On microscopically, the resected tissue composed of spindle-shaped cells with >5/50 high power fields (HPFs) of mitotic activity (high mitotic activity) (Figure 3(a)). The immunohistochemical analysis was positive for CD117 (focal) (Figure 3(b)), DOG-1 (diffuse) (Figure 3(c)), CD34 (diffuse) (Figure 3(d)), while negative results were obtained for smooth muscle actin (SMA), desmin, vimentin cytokeratin, S100 protein, CD10, ER, PR, NSE. The Ki-67 labeling was approximately 15%. Therefore, according to morphological and immunohistochemical features, this case (No.1304736) can be diagnosed as multiple malignant EGISTs occurring in the mesenteric and pelvic regions. After hospital discharge, the patient was treated with the tyrosine kinase inhibitor imatinib regularly.
Figure 3.
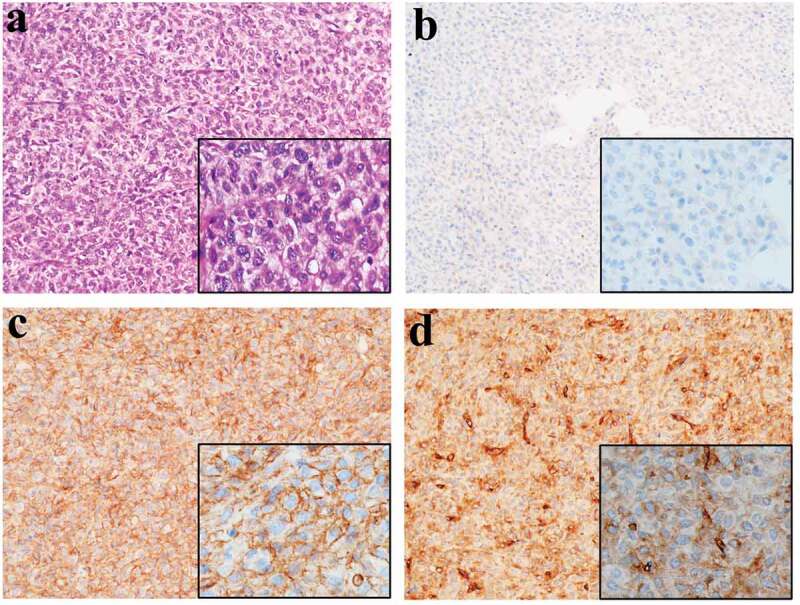
Postoperative histopathology of the second operation (No.1304736).
(a) Microscopy (histology) of the resected specimen showed that the tumor predominantly consisted of spindle cells (stain, H&E; magnification, ×200, ×400). (b) Focally and weakly cytoplasmic positivity for CD117 (magnifications, ×200, ×400). Extensively and strongly positivity within the cell membrane and cytoplasm for DOG-1(c) (magnifications, ×200, ×400) and CD34(d) (magnifications, ×200, ×400).
However, after a further 6 months, the patient was again admitted to our hospital for dysuria. CT revealed that the mesentery and pelvic cavity tumors recurred with multiple metastases (Figure 4(a–c)). That undoubtedly demonstrated the tumor resistant to imatinib and the disease progressed. Subsequently, the patient was treated with sunitinib as a second-line treatment. Three months later, a repeat CT scan revealed proof of a diffuse metastatic disease with numerous soft tissue nodules in the abdominal cavities and pelvic, and mass that has been previously discovered became larger (Figure 4(d–f)). That was considered to be the tumor resistance to sunitinib again. The previously postoperative specimens (No.09085396) from the other hospital were reexamined. The IHC was consistent with the diagnosis of the former hospital. Neoplasias DNA extracted from paraffin embed constitution (No.09085396, No.1304736) was used to detect C-KIT and PDGFRA gene genetic mutations. Polymerase chain reaction amplification and gene sequencing of exons 9, 11, 13, and 17 of C-KIT and exons 12, and 18 of PDGFRA were carried out. (The above steps were completed by the department of pathology of the Peking University Third Hospital). Sequence analysis showed that exon 13 of C-KIT underwent one missense mutation (NM_000222.2: c.1886C>G (p.A629G)) in previously postoperative specimens (No.09085396) (Figure 5(a)). And a missense mutation (NM_006206.4:c.2525A>T (p.D842V)) in exon 18 of PDGFRA was identified in the last surgical tissues of our hospital (No.1304736) (Figure 5(b)). Finally, based on these mutation analyses and immunohistochemical findings, the final diagnosis of the previously postoperative specimens (No.09085396) was that of a malignant EGIST of the mesentery rather than spontaneous-isolated fibromatosis of mesentery (SIFM). Hence, there is no doubt that the masses of mesenteric and pelvic of the last operation can be interpreted as multiple implantation metastases of the abdominal and pelvic cavity after surgery for malignant EGISTs of the mesentery.
Figure 4.
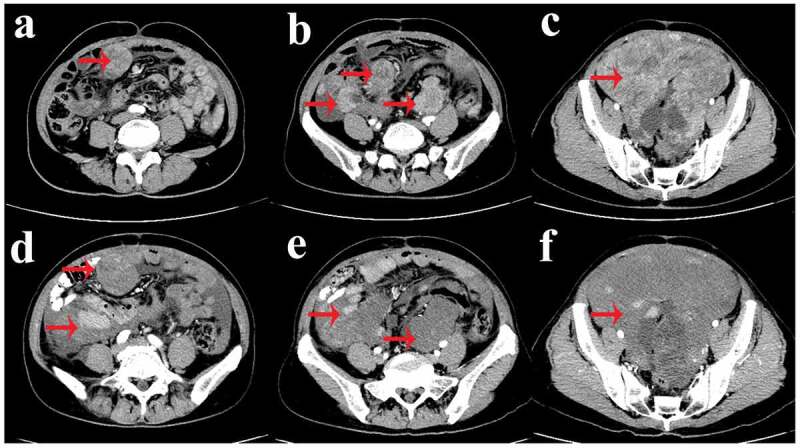
Contrast-enhanced abdominal and pelvic computed tomography (CT) scan after took imatinib for 6 months (a-c) and took sunitinib for 3 months (d-f).
(a-c) Computed tomography revealed that the mesentery and pelvic cavity tumors recurred with multiple metastases (red arrow). (d-f) Computed tomography revealed proof of the mass that had been previously discovered became larger (red arrow).
Figure 5.
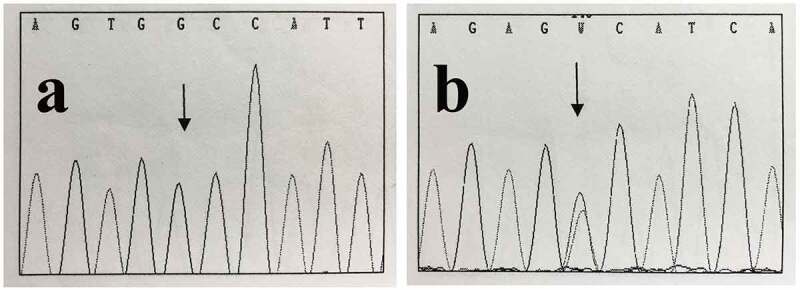
Genomic evaluations of C-KIT and PDGFRA mutation by sequencing.
(a) Sequence analysis showed that a missense mutation in the exon 13 of C-KIT (NM 000222.2: c.1886C>G (p.A629G)) from previously postoperative specimens (No.09085396) (black arrow). (b) A missense mutation (NM 006206.4: c.2525A>T (p.D842V)) in exons 18 of PDGFRA was identified in the last surgical tissues of our hospital (No.1304736) (black arrow).
After that, the patient initiated sorafenib mesylate therapy. However, another mesentery recurrence was detected by CT scan (Figure 6(a–d)). Apparently, the disease progressed again. She began to take regorafenib. During the drug administration, the patient developed grade III Hand-foot Syndrome (HFS), intestine obstruction, ascites, bone marrow suppression (WBC: 2.8x109/L, RBC: 65g/L, PLT: 71x109/L), stomatitis, diarrhea, intestinal fistula, and acute inferior wall myocardial infarction. After a series of symptomatic treatment, the patient’s discomfort improved. The assessment was conducted based on a CT scan after taking the drug for 6 months. The results showed that a new metastasis located in the liver and an increase in volume of the mass of the mesenteric and the patient was evaluated as disease progression (drug resistance) (Figure 6(e–h)). The time from tumor recurrence to death is only 21 months.
Figure 6.
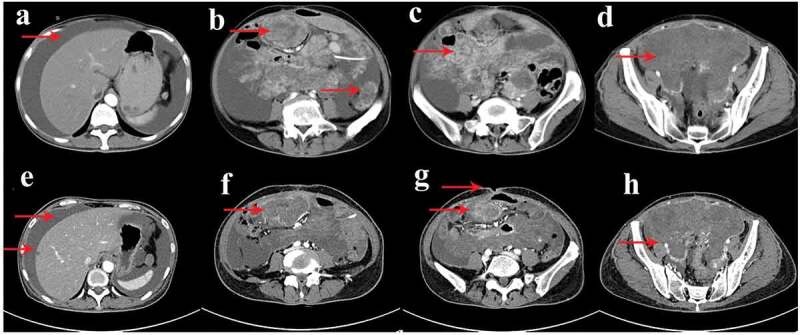
Contrast-enhanced abdominal and pelvic computed tomography (CT) scan after took sorafenib for 3 months (a-d) and took regorafenib for 6 months (e-h).
(a-d) Computed tomography revealed that a mesentery recurrence with large amounts of ascites fill the abdomen and pelvic cavity (red arrow). (e-h) Computed tomography showed a new metastasis was located in the liver and an increase in volume of the mass of the mesenteric; and the patient developed ascites, intestinal fistula (red arrow).
To measure the expression levels of cytoprotective proteins (BCL-2, BCL-XL, and MCL-1) and cytotoxic proteins (BAK and BAX), IHC was performed in tumor materials removed from the patient at all time points. And the expression differences of these proteins were compared between the two postoperative tissues. Representative IHC images of the proteins are shown in Figure 7. IHC staining showed that the expression of BCL-2, MCL-1, BCL-XL and BAK was positive while and BAX was negative in both postoperative specimens. The results also demonstrated that there was no obvious difference between the two postoperative tissues.
Figure 7.
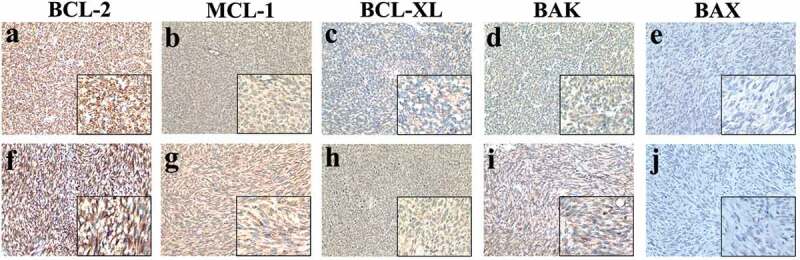
The expression levels of cytoprotective proteins (BCL-2, BCL-XL, and MCL-1) and cytotoxic proteins (BAK and BAX) in the two tumor materials. No.09085396 (a-e) and No.1304736 (f-j).
(a-e) IHC staining showed that the expression of BCL-2 (a), MCL-1 (b), BCL-XL (c) and BAK (d) were positive while BAX (e) was negative in the previously postoperative specimens (No.09085396). (f-j) IHC staining also showed that the expression of BCL-2 (f), MCL-1 (g), BCL-XL (h) and BAK (i) were positive while BAX (j) was negative in postoperative samples from the second operation (No.1304736). (magnifications of all images, ×200, ×400).
Discussion
GISTs are the most well-known mesenchymal tumors in the digestive tract; they may occur wherever of the alimentary tract, from the stomach (60-70%) to the small intestine (20-30%) to the rectum (5-12%) and esophagus (2-5%).9–11 Neoplasms can also arise from the omentum, retroperitoneum, mesentery, or pleura; and GISTs that transpires in these areas are known as EGISTs, which are a very rare clinicopathological entity.12 It is difficult to diagnose the disease correctly so that the diagnosis can be made only organically combined with the clinical manifestations, the imaging features, the origin of tissue, the pathological characteristics, the immunohistochemical phenotypes, and gene analysis results.
Clinical presentations often depend on where the tumor is located. Common manifestations of the EGISTs include abdominal mass, gastrointestinal discomfort (abdominal distension or pain), hemorrhage of digestive tract, intestinal obstruction, and wasting.10,13 Histologically, the tumors are divided into three cell types: spindle cell (60–70%), epithelioid cell type (20%) or mixture of both.14,15 Besides, the mitotic activity must be calculated by counting the number of mitotic cells in 50 HPFs in the region where mitosis is most active. Immunohistochemically, the tumor cells usually behave strong positivity for CD117(95%), CD34(50-100%), and DOG1(92%), with negative expression of SMA, Desmin, and S-100 protein.16
In our patient, the main clinical manifestation was dysuria and abdominal mass, which located in the mesenteric and pelvic regions, and no abnormalities were informed in digestive endoscopy. The resected tissue composed by spindle-shaped cells, and DOG-1 (extensively and strongly) and CD117 (focally and weakly) were positive in the neoplasm cells. Therefore, according to the preoperative examinations, intraoperative investigations, morphological observation and immunohistochemical features, this case (No.1304736) can be diagnosed as EGISTs.17
CD117-positive can support the diagnosis of EGISTs. However, roughly 5% of the cases presented negative immunohistochemistry for it.8 So, we can use the results of DOG-1 and gene mutation report to help diagnose the diseases. The vast majority of GISTs carry activating mutations of the C-KIT or PDGFRA genes (85%-90%).10,18 In GISTs, C-KIT mutations account for 80%, of which more than 80% mutations are in exons 11, approximately 15% are in exons 9, and the mutation rates of exons 13 and 17 are less than 2%; PDGFRA mutations account for 30% of C-KIT mutation-negative GISTs.19 C-KIT and PDGFRA mutations similarly arise in EGISTs. Contrast to GISTs, the frequency of C-KIT mutations was lower in EGISTs, with only 41.4%, and the most common gene variation was also exon 11, and mutations in exons 12 or 18 of the PDGFRA gene are extremely rare in one mutational study.16,20,21 However, mutations in both genes do not occur simultaneously in the same case.22,23 C-KIT and PDGFRA mutations appear to be mutually exclusive oncogenic mechanisms in EGISTs.24,25
A mutation analysis of our present case was performed, and we detected that the tumor cells existed one missense mutation (NM_000222.2:c.1886C>G (p.A629G)) in exons 13 of C-KIT from previously postoperative specimens (No.09085396); and a missense mutation (NM_006206.4:c.2525A>T (p.D842V)) in exons 18 of PDGFRA was identified in the last surgical tissues of our hospital (No.1304736). Meanwhile, we did not find C-KIT mutation involving in our hospital specimens (No.1304736), and no PDGFRA mutation was found in the counterpart of another hospital (No.09085396). The finding of previously postoperative specimens (No.09085396) supported the diagnosis of EGISTs. Therefore, there is beyond all doubt that the masses of mesenteric and pelvic of the last operation (No.1304736) can be described as multiple progression and recurrence after surgery for malignant EGISTs of the mesentery. To date, there is no clinic precedent in the literature where two mutations (C-KIT and PDGFRA) occur in one case of EGISTs. Here, for the first time, we report of it.
It is well known that GISTs/EGISTs are very insensitive to traditional chemotherapy, and the effectiveness of chemotherapy drugs is less than 5%.26 Imatinib, a gene-targeted drug for the C-KIT or PDGFRA mutated GISTs/EGISTs, is a tyrosine kinase inhibitor that was approved by The Food and Drug Administration (FDA) as a first-line treatment for the disease in 2001 and has been confirmed to improve relapse-free survival (RFS) even in patients with advanced GISTs/EGISTs.27 Therefore, imatinib was recommended after our patients were diagnosed with EGISTs. However, the disease progressed within 6 months of treatment, which considered to be the primary resistance existed in tumor cells. Some studies have shown that the response rate of exon 11 of C-KIT mutation on imatinib can reach 90%, while the effect in exon 9 is only 50%. Because exons 13 and 17 mutations are rare, there are insufficient data to confirm the relationship between imatinib efficacy and these types of variations.27,28 In addition, studies have shown that GISTs/EGISTs with PDGFRA mutation have relatively benign biological behavior, clinical prognosis, and a good response to imatinib.23 But, the study of Weisberg E et al.29,30 suggested that patients with D842V-PDGFRA mutation (D842V-PDGFRA-associated GIST patients) could not benefit from imatinib therapy and that D842V-PDGFRA mutation was considered to be the reason of tumor cell unreactive to imatinib. Our patient was found to have a PDGFRA (D842V) mutation, which explained why tumors recurred within a short period.
For patients with widespread progression after standard doses of imatinib, sunitinib was recommended as a second-line treatment by the FDA;31 when the tumor becomes resistant to sunitinib again, sorafenib can be selected for third-line treatment.18 In addition, for imatinib and sunitinib-resistant GISTs/EGISTs, regorafenib has been approved for subsequent treatment in 2013.18 When imatinib was invalid, our patients began using sunitinib for second-line treatment, but the tumor resistance to the drug again. After that, she initiated sorafenib therapy; however, the disease progressed after 3 months of treatment. Subsequently, the patient was treated with regorafenib, but liver metastases and many side effects occurred. Our case may suggest that the presence of both C-KIT and PDGFRA mutations in patients with EGISTs may indicate a very poor prognosis. Meanwhile, it reminds us of the importance of detecting C-KIT and PDGFRA mutations in the diagnosis of GISTs/EGISTs.
Restraining of apoptosis promotes tumor progression. BCL-2, MCL-1, BCL-XL, BAK and BAX are the members of BCL-2 family who play a central role in inducing or inhibition cell apoptosis through a mitochondria-dependent pathway.32 BCL-2, MCL-1 and BCL-XL belong to an anti-apoptotic protein, also known as cytoprotective protein, which can suppress the apoptosis of cancer cells when they are anomalously expressed.32 On the contrary, BAK and BAX belong to pro-apoptosis proteins, also known as cytotoxic proteins, which can direct tumor cells to undergo programmed cell death.32 The balance of anti-apoptotic protein and pro-apoptosis proteins is a well-tuned mechanism dictating the fate of cells. Reported in the previous researches, expression of members of BCL-2 family gene may be helpful in predicting the clinical prognosis,33,34 the survival time of patients,35 and the resistance to chemotherapeutic agents.36 As the two most important members of BCL-2 family, BCL-2 and BAX are a pair of homologous genes that work together to maintain normal cell activities.32 It has been demonstrated that the high BCL-2 expression and low BAX expression are associated with poor prognosis of patients diagnosed with tumors, such as colorectal carcinoma,37 hepatocellular carcinoma,38 bladder cell carcinoma,39 lung adenocarcinoma,39,40 oral squamous cell carcinoma,41 and so on. In our case, the IHC results show that strongly positive BCL-2 expression and negative BAX expression in both postoperative specimens, which may also be a factor for the poor prognosis.
After the first surgery, the patient was misdiagnosed and did not receive anti-tumor treatment until the tumor recurred and metastasized four years later. That maybe the best explanation for no significant difference in IHC results between the two postoperative tissues. Interestingly, BCL-2, MCL-1 and BCL-XL all belong to an anti-apoptotic protein, but the expression of MCL-1 and BCL-XL was lower than the expression of BCL-2 in all postoperative specimens. The result confirms the previous conclusion of MCL-1 and BCL-XL can work independently from BCL-2, put forward by Khodapasand E et al.37 It is difficult to understand why the expressions of BAX and BAK are ambivalent. Paradoxical phenomenon of cancer cell in expressing pro- and anti-oncogene has also been reported in various studies.42 We suspected that the cell signal transduction pathway, an extremely complicated process, is regulated at several different levels, which might be accounted for this result.
In conclusion, in the diagnosis of EGISTs, it is necessary to combine clinical manifestations, imaging features, pathological characteristics, and gene test results. And it is well to be reminded that the sensitivity of DOG-1 is greater than CD117 in the diagnosis of the disease.11,27 Furthermore, genetic mutation testing can be used not only to accurately diagnose diseases, but also to predict patients’ prognosis;19 and the expression level of BCL-2 and BAX could serve as a prognostic factor.43 Till now, this is the first reported case of mesentery EGISTs with C-KIT gene and PDGFRA gene mutations occurred in the same patient at different times.
Funding Statement
This work was supported by the [Natural Science Foundation of Fujian Province] under Grant [No. 2018J01292]; [Fujian Province Health Commission] under Grant [No. 2017-2-32; No. 2017-CXB-10]; and [The Nursery of the Second Affiliated Hospital of Fujian Medical University] under Grant [No. 2016MP20].
Abbreviations
| GISTs | Gastrointestinal stromal tumors |
| GIT | gastrointestinal tract |
| ICC | Interstitial cells of Cajal |
| EGISTs | Extragastrointestinal stromal tumors |
| SIFM | spontaneous isolated fibromatosis of mesentery |
| CT | Computed tomography |
| FDA | The Food and Drug Administration |
| RFS | Relapse-free survival |
| SMA | Smooth muscle actin |
| HPFs | High power fields |
| HFS | Hand-foot Syndrome |
| IHC | Immunohistochemistry |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Ethics statement
The case report was approved by the ethics committee of The Second Affiliated Hospital of Fujian Medical University (Quanzhou, China), and received informed consent from patient, and complied strictly with national ethical guidelines.
References
- 1.Laurini JA, Carter JE.. 2010. Gastrointestinal stromal tumors: a review of the literature. Arch Pathol Lab Med. 134:134–141. doi: 10.1043/2008-0083-RSR2.1. [DOI] [PubMed] [Google Scholar]
- 2.Kang J, Jeon TJ, Yoon SO, Lee KY, Sohn S.. 2014. An extragastrointestinal stromal tumor in the omentum with peritoneal seeding mimicking an appendiceal mucinous cancer with carcinomatosis. Ann Coloproctol. 30:93. doi: 10.3393/ac.2014.30.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franck C, Rosania R, Franke S, Haybaeck J, Canbay A, Venerito M. 2018. The BRAF status may predict response to sorafenib in gastrointestinal stromal tumors resistant to Imatinib, sunitinib, and regorafenib: case series and review of the literature. Digestion. 1–6. doi: 10.1159/000490886. [DOI] [PubMed] [Google Scholar]
- 4.Miyahira CK, Bonfitto M, de Lima Farto JF, de Figueiredo Calili A, Da Silva Sousa NR, de Figueiredo Calili AP. 2018. Extragastrointestinal stromal tumor: a differential diagnosis of compressive upper abdominal tumor. Case Rep Surg. 2018:1–3. doi: 10.1155/2018/1052960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watal P, Bahri N, Brahmbhatt S, Thoriya P. 2014. Retroperitoneal extragastrointestinal stromal tumor: radiologic pathologic correlation. J Clin Imaging Sci. 4:34. doi: 10.4103/2156-7514.135484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monabati A, Safavi M, Solhjoo F. 2016. Extragastrointestinal stromal tumor presenting as omental cyst. J Gastrointest Surg. 20:1275–1277. doi: 10.1007/s11605-016-3098-y. [DOI] [PubMed] [Google Scholar]
- 7.Ko K, Shimanuki K, Sakamoto W, Hara K, Uchida E. 2017. Extragastrointestinal stromal tumor of the inferior vena cava: a case report. Surg Case Rep. 3. doi: 10.1186/s40792-017-0329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph P. 2015. Pancreatic extra-gastrointestinal stromal tumour with documentation of C-Kit Mutation: a case report. J Clin DIAGNOSTIC RES. DOI: 10.7860/JCDR/2015/13018.5821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iqbal N, Sharma A, Iqbal N. 2015. Clinicopathological and treatment analysis of 13 extragastrointestinal stromal tumors of mesentery and retroperitoneum. Ann Gastroenterol. 28:105. doi: 10.1073/pnas.0914878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miettinen M, Lasota J. 2013. Gastrointestinal stromal tumors. Gastroenterol Clin North Am. 42:399–415. doi: 10.1016/j.gtc.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long KB, Butrynski JE, Blank SD, Ebrahim KS, Dressel DM, Heinrich MC, Corless CL, Hornick JL. 2010. Primary extragastrointestinal stromal tumor of the pleura: report of a unique case with genetic confirmation. Am J Surg Pathol. 34:907–912. doi: 10.1097/PAS.0b013e3181d9f18f. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Feng G, Liu Z, Qiao L, Zhang T, Gao C, Xu y. 2014. A young man with primary prostatic extra-gastrointestinal stromal tumor: a rare case report and review of the literature. Int J Clin Exp Pathol. 7:1764. PMID: 25608620. [PMC free article] [PubMed] [Google Scholar]
- 13.Fagkrezos D, Touloumis Z, Giannila M, Penlidis C, Papaparaskeva K, Triantopoulou C. 2012. Extra-gastrointestinal stromal tumor of the omentum: a rare case report and review of the literature. Rare Tumors. 4:e44. doi: 10.4081/rt.2012.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Li J, Li X, Kang Y, Wei Q. 2013. An unexpected but interesting response to a novel therapy for malignant extragastrointestinal stromal tumor of the mesoileum: a case report and review of the literature. World J Surg Oncol. 11:174. doi: 10.1186/1477-7819-11-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto H, Kojima A, Nagata S, Tomita Y, Takahashi S, Oda Y. 2011. KIT-negative gastrointestinal stromal tumor of the abdominal soft tissue: a clinicopathologic and genetic study of 10 cases. Am J Surg Pathol. 35:1287–1295. doi: 10.1097/PAS.0b013e3182206f15. [DOI] [PubMed] [Google Scholar]
- 16.He X, Chen N, Lin L, Wang C, Wang Y. 2017. Extragastrointestinal stromal tumor of the abdominal subcutaneous tissue: report of a very rare case at an unusual site. J Int Med Res. 45:1273–1278. doi: 10.1177/0300060517706577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watal P, Brahmbhatt SG, Thoriya PJ, Bahri NU. 2014. Retroperitoneal extragastrointestinal stromal tumor: radiologic pathologic correlation. J Clin Imaging Sci. 4:34. doi: 10.4103/2156-7514.135484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane JM, et al. 2018. Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 16:536–563. doi: 10.6004/jnccn.2018.0025. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Zhao Y, Xie H, Liu Q, Kong L. 2014. Extragastrointestinal stromal tumor presenting as a recurrent vulvar mass. J Obstetrics Gynaecology Res. 40:1459–1462. doi: 10.1111/jog.12316. [DOI] [PubMed] [Google Scholar]
- 20.Arpaci T, Tokat F, RB Arpaci, Akbas T, Ugurluer G, yavuz S. 2015. Primary pericardial extragastrointestinal stromal tumor: a case report and literature review. Oncol Lett. 9:2726–2728. doi: 10.3892/ol.2015.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terada T. 2008. Primary multiple extragastrointestinal stromal tumors of the omentum with different mutations of c-kit gene. World J Gastroenterol. 14:7256. doi: 10.3748/wjg.14.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liegl-Atzwanger B, Fletcher JA, Fletcher CD. 2010. Gastrointestinal stromal tumors. Virchows Arch. 456:111–127. doi: 10.1007/s00428-010-0891-y. [DOI] [PubMed] [Google Scholar]
- 23.Daniels M, Lurkin I, Pauli R, Erbstosser E, Hildebrandt U, Hellwig K, Zschille U, Lüders P, Krüger G, Knolle J, et al. 2011. Spectrum of KIT/PDGFRA/BRAF mutations and Phosphatidylinositol-3-Kinase pathway gene alterations in gastrointestinal stromal tumors (GIST). Cancer Lett. 312:43–54. doi: 10.1016/j.canlet.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 24.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, et al. 2003. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 25.Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, Kitamura Y. 2003. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology. 125:660–667. doi: 10.1016/s.0016-5085(03)01046-1. [DOI] [PubMed] [Google Scholar]
- 26.Iqbal N, Iqbal N. 2014. Imatinib: a breakthrough of targeted therapy in cancer. Chemother Res Pract. 2014:357027. doi: 10.1155/2014/357027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.HE F, FANG Z, ZHU P, HUANG W, LI L. 2014. Bladder extragastrointestinal stromal tumor in an adolescent patient: a case-based review. Mol Clin Oncol. 2:960–962. doi: 10.3892/mco.2014.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muñoz M, Echeverri C, Ramirez PT, Echeverri L, Pareja LR. 2013. Extragastrointestinal stromal tumor in the rectovaginal septum in an adolescent. Gynecologic Oncol Case Rep. 5:67–69. doi: 10.1016/j.gynor.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serrano C, George S. 2014. Recent advances in the treatment of gastrointestinal stromal tumors. Ther Adv Med Oncol. 6:115–127. doi: 10.1177/1758834014522491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weisberg E, Wright RD, Jiang J, Ray A, Moreno D, Manley PW, Fabbro D, Hall-Meyers E, Catley L, Podar K, et al. 2006. Effects of PKC412, nilotinib, and imatinib against GIST-associated PDGFRA mutants with differential imatinib sensitivity. Gastroenterology. 131:1734–1742. doi: 10.1053/j.gastro.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, et al. 2006. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 32.Ryan JA, Brunelle JK, Letai A. 2010. Heightened mitochondrial priming is the basis for apoptotic hypersensitivity of CD4+ CD8+ thymocytes. Proc Natl Acad Sci U S A. 107:12895–12900. doi: 10.1073/pnas.0914878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jansson A, Sun XF. 2002. Bax expression decreases significantly from primary tumor to metastasis in colorectal cancer. J Clin Oncol. 20:811–816. doi: 10.1200/JCO.2002.20.3.811. [DOI] [PubMed] [Google Scholar]
- 34.Tsamandas AC, Kardamakis D, Petsas T, Zolota V, Vassiliou V, Matatsoris T, Kalofonos H, Vagianos CE, Scopa CD. 2007. Bcl-2, Bax and p53 expression in rectal adenocarcinoma. Correlation with classic pathologic prognostic factors and patients’ outcome. In Vivo. 21:113–118. PMID: 17354623. [PubMed] [Google Scholar]
- 35.Sturm I, Kohne CH, Wolff G, Petrowsky H, Hillebrand T, Hauptmann S, Lorenz M, Dorken B, Daniel PT. 1999. Analysis of the p53/BAX pathway in colorectal cancer: low BAX is a negative prognostic factor in patients with resected liver metastases. J Clin Oncol. 17:1364–1374. doi: 10.1200/JCO.1999.17.5.1364. [DOI] [PubMed] [Google Scholar]
- 36.Katkoori VR, Suarez-Cuervo C, Shanmugam C, Jhala NC, Callens T, Messiaen L, Posey J, Bumpers HL, Meleth S, Grizzle WE, et al. 2010. Bax expression is a candidate prognostic and predictive marker of colorectal cancer. J Gastrointest Oncol. 1:76–89. doi: 10.3978/j.issn.2078-6891.2010.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khodapasand E, Jafarzadeh N, Farrokhi F, Kamalidehghan B, Houshmand M. 2015. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran Biomed J. 19:69–75. doi: 10.6091/ibj.1366.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao N, Sun BC, Zhao XL, Wang Y, Meng J, Che N, Dong X-Y, Gu Q. 2015. Role of Bcl-2 and its associated miRNAs in vasculogenic mimicry of hepatocellular carcinoma. Int J Clin Exp Pathol. 8:15759–15768. PMID: 26884845. [PMC free article] [PubMed] [Google Scholar]
- 39.Golestani EB, Sanati MH, Houshmand M, Ataei M, Akbarian F, Shakhssalim N. 2014. Expression and prognostic significance of bcl-2 and bax in the progression and clinical outcome of transitional bladder cell carcinoma. Cell J. 15:356–363. PMID: 24381861. [PMC free article] [PubMed] [Google Scholar]
- 40.Groeger AM, Esposito V, De Luca A, Cassandro R, Tonini G, Ambrogi V, Baldi F, Goldfarb R, Mineo TC, Baldi A, et al. 2004. Prognostic value of immunohistochemical expression of p53, Bax, Bcl-2 and Bcl-xl in resected non-small-cell lung cancers. Histopathology. 44:54–63. PMID: 14717670. doi: 10.1111/j.1365-2559.2004.01750.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang M, Zhang P, Zhang C, Sun J, Wang L, Li J, Tian Z, Chen W. 2009. Prognostic significance of Bcl-2 and Bax protein expression in the patients with oral squamous cell carcinoma. J Oral Pathol Med. 38:307–313. doi: 10.1111/j.1600-0714.2008.00689.x. [DOI] [PubMed] [Google Scholar]
- 42.Paul-Samojedny M, Kokocinska D, Samojedny A, Mazurek U, Partyka R, Lorenz Z, Wilczok T. 2005. Expression of cell survival/death genes: bcl-2 and Bax at the rate of colon cancer prognosis. Biochim Biophys Acta. 1741:25–29. doi: 10.1016/j.bbadis.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 43.Kulsoom B, Shamsi TS, Afsar NA, Memon Z, Ahmed N, Hasnain SN. 2018. Bax, Bcl-2, and Bax/Bcl-2 as prognostic markers in acute myeloid leukemia: are we ready for Bcl-2-directed therapy? Cancer Manag Res. 10:403–416. doi: 10.2147/CMAR.S154608. [DOI] [PMC free article] [PubMed] [Google Scholar]


