ABSTRACT
The CDC Advisory Committee on Immunization Practices (ACIP) recommended immunization with the recently licensed 13-valent pneumococcal conjugate vaccine (PCV13) for high-risk (immunocompromised) adults aged ≥19 years in 2012. This was in addition to the 23-valent pneumococcal polysaccharide vaccine (PPSV23). Data on vaccine-specific uptake among these individuals were previously unavailable. This retrospective observational study analyzed PCV13 uptake in immunocompromised patients aged 19–64 years. Data were acquired from insurance claims (N = 267,022) and electronic health records (EHR; N = 572,055) from October 2011–October 2016. Descriptive statistics were provided. Demographics were similar across the two database cohorts: mean age 49.7–51.0 years, 57–62% female, and >70% white. Iatrogenic immunosuppression was the most common high-risk category (33.3–44.2%). PCV13 uptake was 7.3% (95% CI: 7.25–7.45) in insurance claims and 9.9% (95% CI: 9.80–9.96) in EHR. Patients with HIV had the highest rate of PCV13 uptake; patients with multiple risk factors were above the mean in both cohorts. A Kaplan-Meier analysis was conducted to include patients lost to follow-up, with 441,657 and 722,071 patients for insurance claims and EHR, respectively. PCV13 uptake was only slightly higher: 9.3% (95% CI: 9.14–9.47) and 13.1% (95% CI: 12.93–13.19) for insurance claims and EHR, respectively. Four years after the ACIP 2012 recommendation, PCV13 uptake in high-risk adults aged19–64 years was low at <15% in all overall analyses. Clinicians caring for these patients should ensure adherence to the ACIP recommendation to minimize the risk of pneumococcal disease.
KEYWORDS: 13-valent pneumococcal vaccine, pneumococcal conjugate vaccine, PCV13, pneumococcal disease, pneumococcal vaccines, immunization, risk factors
Introduction
Immunocompromising conditions are known to greatly increase the risk of pneumococcal disease in adults.1,2 In patients with severe immunosuppression related to HIV or cancer treatment, for example, the incidence of invasive pneumococcal disease (IPD) has been shown to be 23–48 times greater than in healthy adults.1 As IPD carries a mortality rate of up to 30% in western-world adults,3 preventive measures such as pneumococcal vaccine programs have the potential to greatly diminish this significant public health threat in these high-risk populations.
The US Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) has issued two recent updates to guidelines for pneumococcal vaccination in adults. In 2012, ACIP added the recently licensed 13-valent pneumococcal conjugate vaccine (PCV13 [Prevnar 13®, Pfizer]) to the vaccine protocol for adults aged ≥19 years with immunocompromising conditions.4 The resulting recommendation is for PCV13 to be administered as the first, followed 8 weeks later by the 23-valent pneumococcal polysaccharide vaccine (PPSV23 [Pneumovax 23®, Merck]). Adults with immunocompromising conditions who had already received PPSV23 were also recommended to receive PCV13 one year after PPSV23. In 2014, following the results of an efficacy study of PCV13 against vaccine-type community-acquired pneumonia, ACIP made a similar update to the pneumococcal recommendation for all adults aged ≥65 years, with PCV13 added to the existing recommendation for PPSV23.5,6
Adherence to these guidelines is difficult to assess. In 2016, four years after the 2012 guidance was issued, the National Health Interview Survey (NHIS) indicated 24% pneumococcal vaccine uptake in adults 19–64 years (compared to 67% in adults ≥65 years);7 however, NHIS does not stratify by the specific vaccine administered, nor does it differentiate the high-risk population for which PCV13 is recommended from the remainder of the increased-risk immunocompetent population in whom only PPSV23 is recommended. Consequently, there are very limited data on the adherence to the 2012 ACIP pneumococcal guidelines for PCV13 in high-risk adults aged <65 years.
A comprehensive understanding of PCV13 uptake among high-risk adults may assist in developing interventions to better implement ACIP recommendations in this population. We evaluated electronic health records (EHR) and an administrative insurance claims database over a 4-year period with the objective to assess PCV13 uptake, as a metric for assessment of adherence to the ACIP 2012 guidelines, among immunocompromised and other high-risk adults aged 19–64 years.
Results
Study cohorts
Within the insurance claims database, we identified 267,022 adults aged 19–64 years falling within the risk groups described by the ACIP 2012 guidelines; 572,055 were identified from the EHR database (Figure 1). Due to the known potential for overlap across the two database cohorts, an aggregate population was not evaluated.
Figure 1.
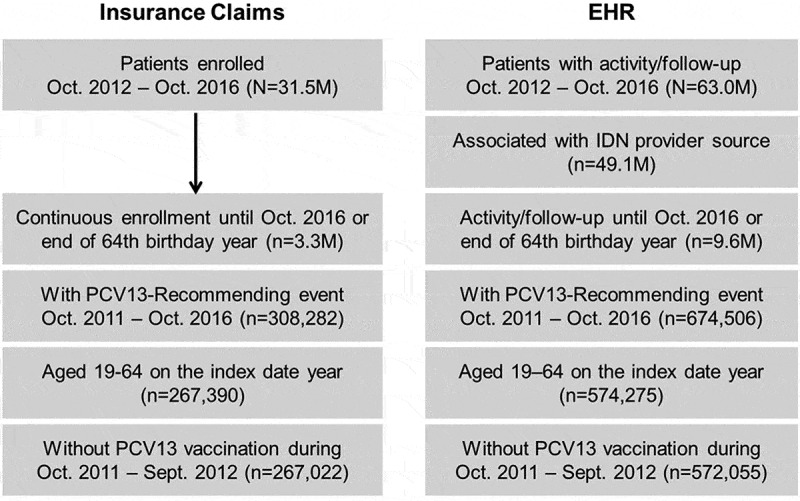
Disposition of eligible patients in cohort databases.
Demographics were generally similar across the two database cohorts (Table 1). Mean ages were 51.0 years and 49.7 years for insurance claims and EHR cohorts, respectively. A majority in both cohorts were female (57% in the insurance claims cohort and 62% in the EHR cohort), and >70% were white. Differences in other characteristics were relatively small, with the exception of household income, which was markedly higher in the insurance claims cohort. Iatrogenic immunosuppression was the most common reason for inclusion in the high-risk category (Figure 2). Cancer or treatment for cancer, renal failure or nephrotic syndrome, and the presence of multiple risk factors were also common reasons for inclusion.
Table 1.
Baseline demographics and characteristics.
| Insurance Claims Cohort (n = 267,022) |
EHR Cohort (n = 572,055) |
|
|---|---|---|
| Age, mean y (SD) | 51.0 (11.2) | 49.7 (11.6) |
| 19–49, y (%) | 99,683 (37.3) | 233,025 (40.7) |
| 50–64, y (%) | 167,339 (62.7) | 339,030 (59.3) |
| Sex, n (%) | ||
| Unknown | 66 (0) | 98 (0) |
| Female | 152,224 (57.0) | 354,846 (62.0) |
| Male | 114,732 (43.0) | 217,111 (38.0) |
| Race, n (%) | ||
| White | 189,999 (71.2) | 444,542 (77.7) |
| Hispanic* | 27,439 (10.3) | |
| Black | 26,678 (10.0) | 82,408 (14.4) |
| Asian | 8663 (3.2) | 8353 (1.5) |
| Missing/Unknown | 14,243 (5.3) | 36,752 (6.4) |
| Ethnicity, n (%) | ||
| Other/Unknown | 26,894 (4.7) | |
| Hispanic* | 31,664 (5.5) | |
| Not Hispanic | 513,497 (89.8) | |
| Annual household income category, n (%) | ||
| Missing/Unknown | 31,638 (11.9) | 13,721 (2.4) |
| <$50k | 49,573 (18.6) | 482,939 (84.4) |
| ≥$50k | 185,811 (69.6) | 75,395 (13.2) |
EHR = electronic health record
*Databases differed in categorizing “Hispanic” as race or ethnicity
Figure 2.
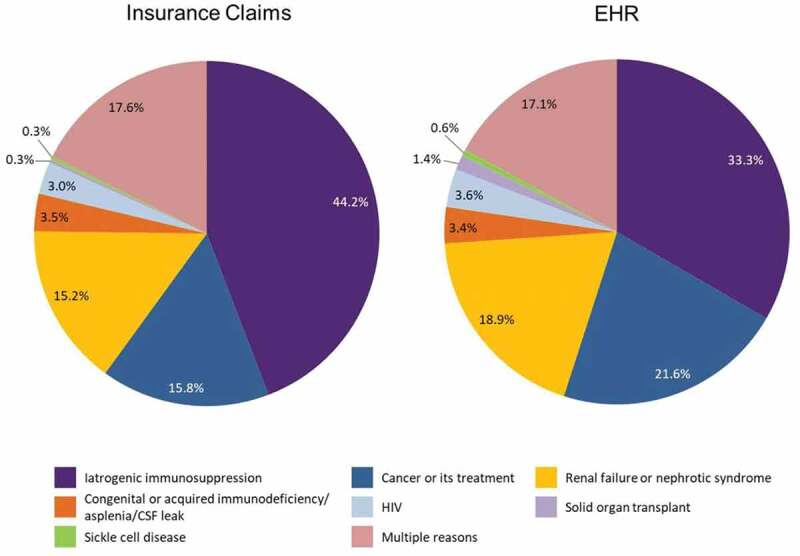
Reason for high-risk ACIP recommendation eligibility by database.
PCV13 uptake per ACIP 2012 guidelines
Overall PCV13 uptake was low in both databases. Within the insurance claims cohort, the unadjusted PCV13 uptake was 7.3% (95% CI: 7.25–7.45); uptake was slightly higher in the EHR cohort at 9.9% (95% CI: 9.80–9.96).
After stratifying PCV13 uptake by demographic characteristics, we found that high-risk adults aged ≥50 years were more likely to be vaccinated than those aged <50 years (Figure 3), and men were more likely to be vaccinated than women. There was no consistent pattern in uptake by race, ethnicity, or income levels.
Figure 3.
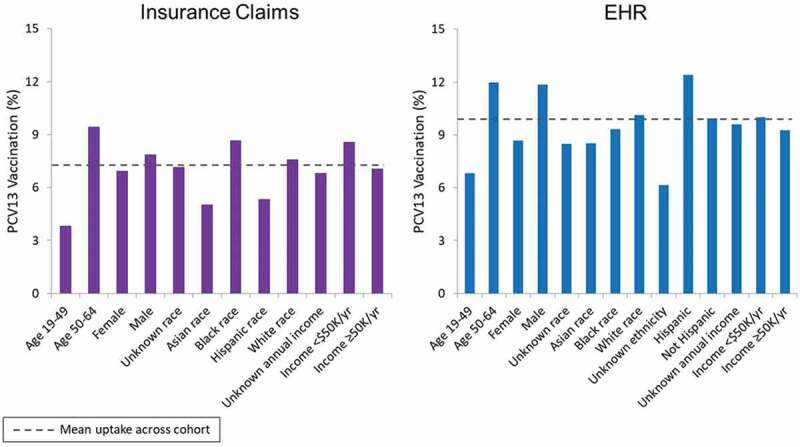
PCV13 uptake among high-risk adults aged 19–64 years according to demographic characteristics.
Stratification by reason for inclusion in the high-risk category indicated that patients with HIV had the highest rate of PCV13 uptake (Figure 4), more than 3 times the overall uptake among the cohorts in each database. Patients with multiple risk factors also showed higher uptake relative to the mean in both database cohorts.
Figure 4.
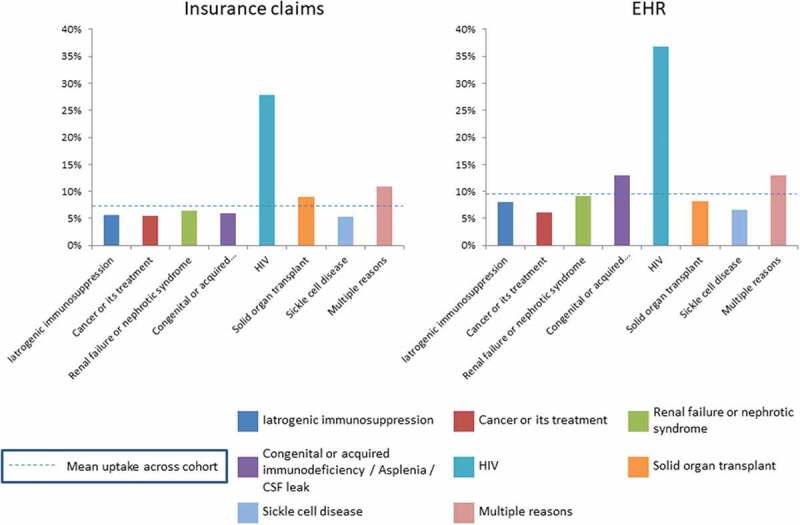
PCV13 uptake according to the reason for high-risk ACIP recommendation eligibility.
Analysis including patients lost to follow-up
The Kaplan-Meier analysis, which included adults who were omitted from the original analysis due to loss to follow-up prior to 2016, enabled assessment of substantially larger populations: 441,657 in the insurance claims cohort and 722,071 in the EHR cohort. Estimates of PCV13 uptake in this sensitivity analysis were slightly higher than that observed among the cohorts with full follow-up, with 9.3% (95% CI: 9.14–9.47) in the insurance claims cohort and 13.1% (95% CI: 12.93–13.19) in the EHR cohort.
This Kaplan-Meier analysis also demonstrated a higher rate of PCV13 uptake in the subset of patients aged 50–64 years vs those aged 19–49 years (Figure 5). In evaluating PCV13 uptake before and after the ACIP 2014 update, which added PCV13 to pneumococcal vaccine guidelines for adults ≥65 years, we found an accelerated PCV13 uptake after the recommendation.
Figure 5.
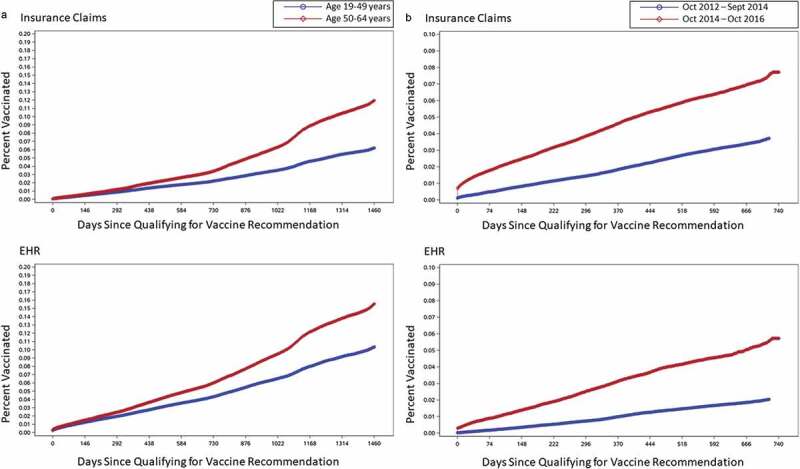
Kaplan-Meier analysis of patients lost to follow-up: (A) uptake across time by age group; (B) uptake across time before and after 2014 ACIP recommendation for PCV13 in adults aged ≥65 years.
Discussion
Pneumococcal vaccines have been associated with a marked decline in IPD,8 due to both direct and indirect (herd) effects of vaccination programs. Considering the effectiveness demonstrated by vaccines in the prevention of pneumococcal disease, it would be prudent to ensure vaccination of high-risk patients. A stated objective of the US Health and Human Services’ Healthy People 2020 initiative is to increase the percentage of high-risk adults aged 18–64 years who are vaccinated against pneumococcal disease from a baseline of 17% in 2008 to a target coverage of 60%.9 It should be noted, however, that his goal for the pneumococcal vaccine in general and does not specify PCV13, PPSV23, or the sequence of both vaccines recommended by ACIP in 2012.4
Previously, the only source of data on pneumococcal vaccine uptake within this patient population was NHIS, and as noted above, NHIS data do not indicate which vaccine (PCV13 vs PPSV23) was administered, thus making it difficult to assess adherence to the ACIP 2012 guidelines. While no single data source will fully capture the adoption of PCV13 uptake, our analysis provides relevant insight on adherence to the ACIP recommendations for adults aged 19–64 years who are at risk for pneumococcal disease.
Our study, including the sensitivity analysis leveraging Kaplan-Meier estimation of uptake, shows PCV13 uptake to be <15% in high-risk adults aged <65 years four years after the implementation of the ACIP 2012 guidelines. This is particularly low when compared with the uptake of 31.5% seen in adults aged ≥65 years, based on insurance claims, only 2 years after ACIP issued its recommendation for US adults aged ≥65 years.5,10 NHIS data for the older group also indicate an upward trend in PCV13 coverage after 2014, increasing from 63.6% in 2015 to 66.9% in 2016.11 Of note, the accelerated uptake we reported in the 19–64 age group that occurred after 2014 (Figure 5) suggests the ACIP age-based recommendation may also have led to greater uptake in this younger age group, despite not being targeted by the 2014 recommendation. The current study did not investigate the reason for increased uptake after the recommendation for older adults; however, potential explanations may include increased awareness of PCV13 and more widespread availability after the broader recommendation was issued.
We noted that the Kaplan-Meier analysis was undertaken to allow the inclusion of patients previously omitted due to loss to follow-up in the databases. Simple change or discontinuation of insurance carriers or providers is a common occurrence and a significant exclusion within this analysis, with more than 90% attrition in the insurance claims cohort and 80% in the EHR cohort due to not meeting the criteria for ≥4 years continuous enrollment or follow-up. A Kaplan-Meier analysis might, therefore, present a more accurate picture of PCV13 uptake, especially if vaccination rates differed between those who retained insurance carriers vs those who changed. However, this analysis showed only slightly higher uptake than in the continuous enrollment/follow-up population analysis.
The stratification of PCV13 uptake by risk category found that patients with HIV, who comprised <4% of each database cohort, had the highest rate of PCV13 uptake; this was markedly higher than patients in other high-risk categories. This may be due to the fact that these patients tend to have higher levels of care coordination, thereby focusing on prevention. The high uptake in patients with HIV may also partially explain the higher uptake in men within our analysis, as HIV is more common in men.12 The category of patients with multiple risk factors was a distant second to HIV in terms of uptake percentage, although these patients had uptake above the mean in both cohorts.
A substantial proportion of patients in our analysis had iatrogenic immunosuppression: 33.3% and 44.2% in the EHR and insurance claims cohorts, respectively. However, in both databases, PCV13 uptake for patients with iatrogenic immunosuppression was lower than in the overall population. This suggests the need for interventions to target prescribers of immunosuppressant therapies for education regarding ACIP guidelines for pneumococcal vaccines.
The low uptake among patients with iatrogenic immunosuppression is particularly concerning, as the current analysis used a narrower definition that did not include individuals who would qualify solely because of long-term corticosteroid use, a treatment regimen that is common for many autoimmune diseases.13 This was due to the complexity of operationalizing the recommendation; i.e., calculating prednisone-equivalent dosages associated with immunosuppression, which would determine a patient’s inclusion in ACIP’s high-risk category. Including these patients would almost certainly increase the number of high-risk patients in the analysis, as well as the proportion of patients at high-risk due to iatrogenic immunosuppression. There is little reason to believe such patients are more likely to be vaccinated than other patients with iatrogenic immunosuppression; therefore, including them would likely have further reduced the overall PCV13 uptake rate.
The current study employed two different databases to evaluate uptake, and some of the findings highlight the relative strengths and weaknesses of each database for this purpose. Interestingly, we found a slightly higher level of PCV13 uptake in high-risk patients using the EHR database as compared to the claims-based cohort, despite the possibility that PCV13 can be obtained at pharmacies which would potentially not be captured in the EHR, thereby theoretically underestimating the vaccination coverage relative to claims. Further, it should be noted that we did not have access to or use the unstructured portions (i.e., HCP notes) of EHR to further capture comorbidities, medications and vaccination status and may have underestimated these measures of interest in the EHR cohort. We also observed higher attrition in the claims cohort with requirement of four years of continuous enrollment (or until the end of the year the individual turned age 64), which raised the possibility that we may have selected a healthier or more stable cohort amongst the high-risk patients; however, sensitivity analysis not requiring the full four years of enrollment or follow up also suggested higher PCV13 uptake in the EHR cohort. In addition, while the potential gaps and challenges with reliability of coding in EHR vs. claims have been explored in the context of risk stratification,14 the prevalence of most high-risk conditions largely defined by presence of International Classification of Diseases, 9th and 10th revision (ICD-9 and ICD-10, respectively) diagnosis codes in our study was similar or higher in the EHR database, the proportion of high-risk patients who were considered to have iatrogenic immunosuppression was higher in claims data. Unlike the other risk factors, iatrogenic immunosuppression risk was primarily determined by a combination of select immunosuppressive medications, with physician specialties that would prescribe or administer those medications (in order to differentiate the use of agents used for cancer as well as other indications). Differences in prevalence may be attributed to potential differences in the availability of provider specialty in the EHR for iatrogenic immunosuppression status classification. There could also be underlying differences in the populations served by the commercial insurance plan which provided claims data and the providers who contribute the EHR data. Including both claims and EHR, as was done in this study, should provide a more representative sample of vaccine uptake in the total population than reliance on either sample alone. Further, future use of a linked EHR (structured and unstructured) and claims database may provide additional insights on the reliability of coding and classification of risk and vaccination status, though potentially at a loss of representativeness.
Limitations
Limitations of the current study include the reliance on databases generated from systems created for billing and clinical practice management. The claims in the current study were primarily from a large national health plan in the US. There may also have been patients whose claims for vaccinations or other qualifying treatments were not processed or paid through an insurance company; however, we expect that few insured patients would pay out-of-pocket for PCV13, given the mandate that insurers cover ACIP-recommended vaccines. Such factors may prevent our results from being generalizable to populations outside of the insured population, although insured populations would be expected to have higher vaccination rates than uninsured patients. The inclusion criterion of continuous enrollment/follow-up over a long period of time, while intended to ensure no records of PCV13 vaccinations were missed, may have resulted in a sample that is not representative of the entire covered population. In addition, it is possible that some patients may have received PCV13 prior to the observation period and were therefore misclassified as not having received the vaccine.
Diagnostic coding (or undercoding) errors may have occurred in some patient records or claims, which may have led to the exclusion of some patients who would have qualified for the study. Likewise, there is room for interpretation in the recommendation, and we chose to exclude some categories of patients who might otherwise be considered part of the high-risk group recommended to receive the vaccine. In particular, patients with metastatic solid tumors that were not coded as generalized malignancies would not have been included in this analysis unless they underwent a treatment putting them at high risk. ACIP recommendations are subject to interpretation by clinicians, and the current analysis represents a conservative interpretation. Other interpretations (such as including the metastatic solid tumor patients mentioned above) are possible and would impact both the total number of patients and the uptake rate.
Finally, it is important to note that these analyses are meant only to describe vaccine uptake; the relationships observed should not be interpreted as causal. Likewise, our study was exploratory and did not test prespecified hypotheses.
Conclusions
Four years after the issuance of the ACIP 2012 guidelines for pneumococcal vaccination of immunocompromised adults aged ≥19 years of age, uptake of PCV13 in this population remained low at <15%. Clinicians caring for high-risk patient populations should ensure adherence to the current pneumococcal vaccine recommendations, including PCV13 and PPSV23 in series, to reduce the incidence of pneumococcal disease in these patients.
Patients and methods
Study design
This was a retrospective observational study of US adults aged 19–64 years between October 2012 and October 2016 who met criteria for the high-risk target population in the ACIP 2012 pneumococcal vaccine guidelines. Reasons for inclusion in the high-risk population were categorized as congenital or acquired immunodeficiency, asplenia, or CSF leak; sickle-cell disease; HIV; renal failure or nephrotic syndrome; cancer or cancer treatment; solid organ transplant; iatrogenic immunosuppression; or a combination of any of these factors using respective ICD-9 and ICD-10 codes. There are several aspects of how the iatrogenic immunosuppression category was operationalized for the study that should be noted. First, iatrogenic immunosuppression is defined in the recommendation as, “Diseases requiring treatment with immunosuppressive drugs, including long-term corticosteroids and radiation therapy.” The current study relied on use of National Drug Code (NDC) for written prescription or fill or Healthcare Common Procedure Coding System (HCPCS) codes for administration of immunosuppressive medication (e.g., methotrexate, adalimumab, rituximab, etc., as recorded in EHR or claims) in addition to the attributable provider specialties rather than diagnosis of a disease requiring such drugs or procedures. Furthermore, the long-term corticosteroid use mentioned in the recommendation was not incorporated into the category due to the complexity of operationalizing, so patients who would qualify as high-risk only because of long-term corticosteroid use were not included in the study. Finally, many therapies and procedures (identified via Current Procedural Terminology [CPT-4] codes) which would cause iatrogenic immunosuppression are oncological, and we chose to include such therapies which are specific to oncology or were prescribed by oncologists as “cancer or cancer treatment”. Eligible individuals were to be identified and followed within the record or claims until the end of the year in which they reached 64 years of age, or October 2016, whichever occurred first.
Cohort selection
Patients included in this analysis were US adults who were aged 19–64 years in the index-event year, the index event being the disease diagnosis, medication prescription, fill or administrative procedure that caused the patient to be considered high-risk. The index event could have occurred anytime between October 12, 2011, and October 11, 2016. Two different types of statistically de-identified, structured, longitudinal real-world databases from the health data and analytics company Optum® were leveraged for this study; one comprised of electronic health records (EHR), and the other of claims. Both databases are compliant with the Health Insurance Portability and Accountability Act (HIPAA). EHR data offers the advantage of being payer agnostic, potentially representing a broader swath of the high-risk populations than the commercially insured and Medicare Advantage Part D lives available in the claims data, and may provide a more comprehensive view of comorbidity, medication and vaccination status than the paid claims for services and filled prescriptions that were covered and reimbursed by health plans. On the other hand, the claims data includes enrollment files that potentially support better follow-up and history across different care settings so long as the services and prescriptions are paid for by the health plan. Lastly, cohort attrition often occurs in claims databases when requiring continuous enrollment for follow-up as it is not uncommon for enrollees to switch health plans, while we expected less loss to follow-up in the EHR database.
Optum’s longitudinal EHR clinical repository is derived from dozens of health-care provider organizations in the United States, which include more than 700 hospitals and 7000 clinics treating more than 94 million patients. Clinical records, claims, and other medical administrative data are obtained from EHRs (both inpatient and outpatient), practice management systems, and numerous other internal systems. Optum’s EHR database is agnostic to EHR platforms and aggregated across various provider sources covering the continuum of care and different formats/structures. Hence, EHR data are processed (i.e., Optum reviews the format, structures the data, and loads it into their databases), normalized (transformed to ensure disparate data can be evaluated across a common denominator, which includes mapping labs, medications, observations, and microbiology values, and use of natural language processing algorithms to retrieve information from text), and standardized (disparate formats in data received from providers are routed into consistent formats) across the continuum of care from both acute inpatient stays and outpatient visits. Optum’s EHR data elements related to this study comprise demographics, including month and year of death from the Death Master File maintained by the Social Security Administration (noting that some states stopped contributing data after 2011), estimated socioeconomic status based on 3-digit zip codes, medications prescribed and administered, and health-care professional-coded diagnoses and procedures.
Optum’s ClinformaticsTM Data Mart comes from a database of administrative health claims for members of a large national managed-care company affiliated with Optum. The database includes approximately 12–14 million covered lives annually, for a total of more than 65 million unique lives over a 19-year period (2000 through 2018). The claims data comprise both commercial and Medicare Advantage health plan data, and the database spans all 50 states. In addition to medical and pharmacy claims, the data include tables with member eligibility and inpatient confinements; the data also include standard pricing for all medical claims, pharmacy claims, and inpatient confinements. The Optum data also include information on additional consumer characteristics (e.g., predefined household income ranges) from an outside vendor, along with month and year of death from the Death Master File maintained by the Social Security Administration (as above, noting that some states stopped contributing data after 2011).
Patients were to be continuously enrolled in the claims database or to have a follow-up in the EHR database, from October 2012 through October 2016 or the end of the year in which the patient turned 64 years of age. Patients in the EHR cohort must have been associated with an integrated delivery network. Patients with PCV13 vaccination between 12 October 2011 and 12 October 2012 (i.e., prior to the publication of the ACIP recommendation) were excluded.
Outcomes of interest
The overall population was to be evaluated for vaccination with PCV13 after the index event. Patients were also stratified by age (19–49 years, 50–64 years), sex, race or ethnicity, household income, and reason for inclusion in this high-risk population.
Statistical analysis
The continuously enrolled population was characterized using descriptive statistics, including means and standard deviations for continuous data, and frequencies and percentages for categorical data, with 95% confidence intervals calculated for vaccine uptake.
Additional sensitivity analyses to assess the immunization rate included application of a Kaplan-Meier approach. The updated population for the Kaplan-Meier analysis included patients lost to follow-up due to disenrollment (or not returning to the provider system captured in EHR) or death prior to the study end date. This analysis excluded patients who died prior to the index event and patients who received PCV13 between the study start date and the index-event date. This population was stratified by age groups and timing of the ACIP 2014 update regarding PCV13 recommendation for adults aged ≥65 years. All analyses were performed using SAS 9.4 software.
Funding Statement
This work was supported by Pfizer Inc.
Acknowledgments
This study was sponsored by Pfizer Inc. Ann L. Davis, MPH, CMPP, an employee of Pfizer Inc., provided writing assistance under the direction of the authors.
Disclosure of potential conflicts of interest
All authors are employees of Pfizer Inc. and may hold stock in the company.
References
- 1.Kyaw MH, Rose CE Jr., Fry AM, Singleton JA, Moore Z, Zell ER, Whitney CG, Active Bacterial Core Surveillance Program of the Emerging Infections Program Network . The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis. 2005;192(3):377–86. doi: 10.1086/431521. [DOI] [PubMed] [Google Scholar]
- 2.Weycker D, Farkouh RA, Strutton DR, Edelsberg J, Shea KM, Pelton SI.. Rates and costs of invasive pneumococcal disease and pneumonia in persons with underlying medical conditions. BMC Health Serv Res. 2016;16:182. doi: 10.1186/s12913-016-1432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drijkoningen JJ, Rohde GG.. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20(Suppl 5):45–51. doi: 10.1111/1469-0691.12461. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention . Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61(40):816–19. [PubMed] [Google Scholar]
- 5.Black CL, Williams WW, Warnock R, Pilishvili T, Kim D, Kelman JA. Pneumococcal vaccination among Medicare beneficiaries occurring after the Advisory Committee on Immunization Practices Recommendation for Routine Use Of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine for Adults Aged >/=65 Years. MMWR Morb Mortal Wkly Rep. 2017;66(27):728–33. doi: 10.15585/mmwr.mm6627a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–25. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention . Vaccination Coverage Among Adults in the United States. National Health Interview Survey. 2016. [accessed 2018 September27]. https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2016.html.
- 8.Centers for Disease Control and Prevention . Pneumococcal Disease Surveillance Reporting and Trends. 2018. August 1 [accessed 2018 Oct 2]. https://www.cdc.gov/pneumococcal/surveillance.html.
- 9.Office of Disease Prevention and Health Promotion . US Department of Health and Human Services. Healthy People 2020. Objective IID-13.2. 2018. October 25 [accessed 2018 Oct 31] https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives.
- 10.Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, Hadler S, Pilishvili T. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged >/=65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822–25. [PMC free article] [PubMed] [Google Scholar]
- 11.Williams WW, Lu PJ, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE, et al. Surveillance of vaccination coverage among adult populations - United States, 2015. MMWR Surveill Summ. 2017;66(11):1–28. doi: 10.15585/mmwr.ss6611a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention . HIV Surveillance Report, 2017, 29, November 2018. [accessed 2018 Dec 7] https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2017-vol-29.pdf. [Google Scholar]
- 13.Zink A, Thiele K, Huscher D, Listing J, Sieper J, Krause A, Gromnica-Ihle E, von Hinueber U, Wassenberg S, Genth E, et al., German Collaborative Arthritis Centres . Healthcare and burden of disease in psoriatic arthritis. A comparison with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2006;33(1):86–90. [PubMed] [Google Scholar]
- 14.Kharrazi H, Chi W, Chang HY, Richards TM, Gallagher JM, Knudson SM, Weiner JP. Comparing population-based risk stratification model performance using demographic, diagnosis, and medication data extracted from outpatient electronic health records versus administrative claims. Med Care. 2017;55(8):789–96. doi: 10.1097/MLR.0000000000000754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Centers for Disease Control and Prevention . Vaccination Coverage Among Adults in the United States. National Health Interview Survey. 2016. [accessed 2018 September27]. https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2016.html.
- Centers for Disease Control and Prevention . Pneumococcal Disease Surveillance Reporting and Trends. 2018. August 1 [accessed 2018 Oct 2]. https://www.cdc.gov/pneumococcal/surveillance.html.


