ABSTRACT
Abiotic stress is a major threat to plant growth and development, resulting in extensive crop loss worldwide. Plants react to abiotic stresses through physiological, biochemical, molecular, and genetic adaptations that promote survival. Exploring the molecular mechanisms involved in abiotic stress responses across various plant species is essential for improving crop yields in unfavorable environments. Halophytes are characterized as plants that survive to reproduce in soils containing high salt concentrations, and thus act as an ideal model to comprehend complicated genetic and physiological mechanisms of salinity stress tolerance. Plant ecologists classify halophytes into three main groups: euhalophytes, recretohalophytes, and pseudo-halophytes. Recent genetic and molecular research has showed complicated regulatory networks by which halophytes coordinate stress adaptation and tolerance. Furthermore, investigation of natural variations in these stress responses has supplied new perspectives on the evolution of mechanisms that regulate tolerance and adaptation. This review discusses the current understanding of the genetic mechanisms that contribute to salt-stress tolerance among different classes of halophytes.
KEYWORDS: Halophytes, salt stress, salt tolerance, adaptation
Introduction
Abiotic stress is characterized as the adverse effects of non-living factors on living organisms.1,2 Plants must survive under ever-changing environmental conditions characterized by variations in a lot of abiotic stresses, such as salt,3,4 cold,5–7 flooding,8 and drought.9–11 In some environmental niches, plants must respond to multiple abiotic stresses.12–14 As a result, they have evolved mechanisms to respond to abiotic stress, including changes in growth and development resulting from altered metabolism, as well as modifications in morphology (Figure 1). Among abiotic stress, soil salinity has become a serious problem in many parts of the word, especially in arid and semi-arid areas.15,16 As reported, over 800 million ha of land worldwide is salt-affected, which accounts for about 6% of the world’s total land area.17,18 Soil salinity has an enormous effect on the agricultural yield all over the world, for it affects the establishment, development, and growth of plants, which finally lead to crop yield loss.19–21
Figure 1.
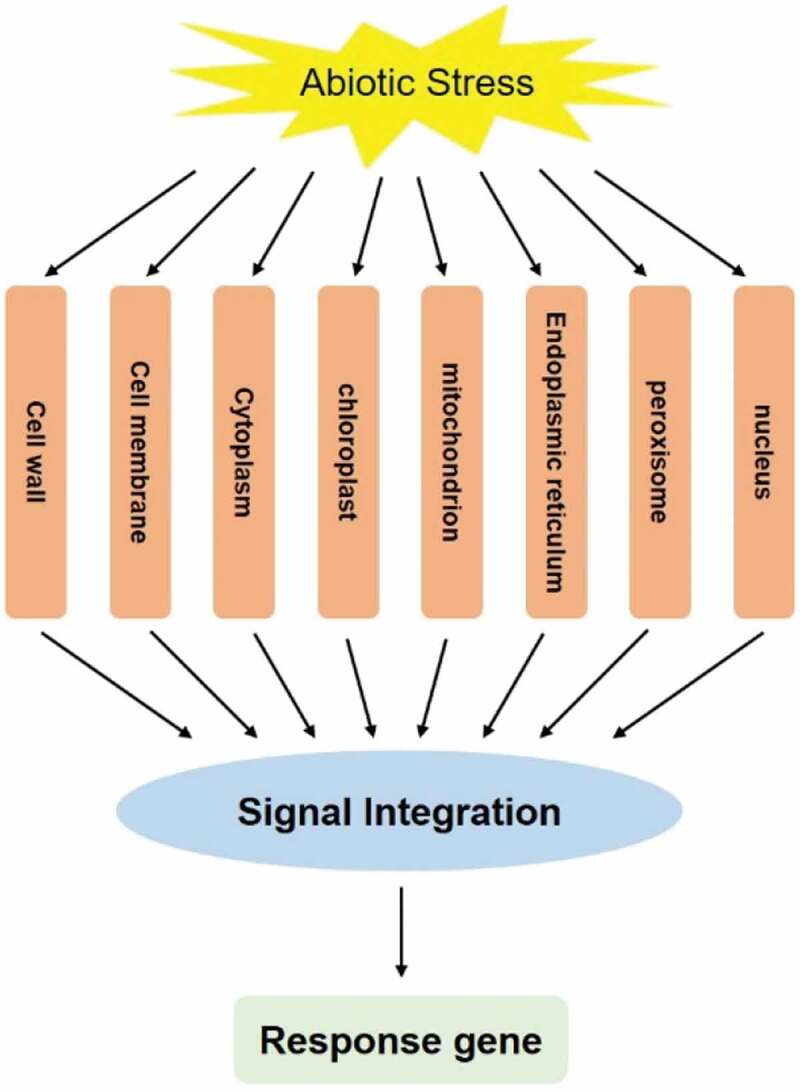
Model of dispersed stress sensing by organelles.
Salt stress decreases the yield of a number of crops, including wheat,22–24 maize,25–27 and rice,28,29 presenting a challenge for feeding the increasing global population.30 To elucidate mechanisms that contribute to stress adaptation and tolerance, it becomes more and more useful to characterize the physiological and genomic characteristics of individual crop plants that thrive in unfavorable environments.31,32 Although many researchers have performed a number of relevant studies in Arabidopsis and other crop model systems,33,34 halophytes could offer valuable resources to help us uncover the mystery of salt-related signaling pathway.35,36 The study of how halophytes tolerate salt could offer biologists' clues to increase salt tolerance in conventional crops.
Halophytes could not only grow well in environments where the salt concentration is around 200 mM NaCl or more, but survive in other harsh environments, including drought, cold or flooding.37 Therefore, they act as an ideal model to understand complicated genetic and physiological mechanisms of abiotic stress tolerance. Plant ecologists classify halophytes into three main groups: euhalophytes, recretohalophytes, and pseudo-halophytes.38,39 Euhalophytes can dilute salt within their stems or leaves and have a strong ability to tolerate salt.40,41 Recretohalophytes can secrete salt from their leaves, and grows widely around the world, inland saline lands and inhabiting seawater.42,43 Salt glands and bladders are salt-secreting structures, which can directly secrete ions out of the plants. Pseudo-halophytes could not only hold up ions in roots but also minimize its transport to the shoot parts, so as to protect itself from metabolic tissue.44,45
Suaeda salsa and Salicornia europaea are typical euhalophytes, which can dilute salt within their stems or leaves and have a strong ability to tolerate salt. S. salsa could survive well in 200mM NaCl concentration,46,47 and also grow in the environments where salt stress and drought stress coexist. S. europae have much broader salinity tolerance. Researchers have performed a lot of studies in S. salsa and S. europae, and cloned a series of genes involved in abiotic tolerance and tested their functions. These studies have indicated that euhalophytes play an essential role in agricultural production.48,49 Recretohalophytes possess unique salt-secreting structures, namely salt glands and bladders, which can directly secrete these ions out of the plants.50 Therefore, salt bladders and salt glands play essential roles for the recretohalophytes to secrete excess salt.51 Limonium bicolor is a good example of recretohalophyte, which can desalinate saline-alkali soil and preserve high rates of photosynthesis under the treatment of 200–300 mM NaCl.52 Reaumuria trigyna is an endangered recretohalophyte and has salt excretion glands to respond to salt stress.53 Pseudo-halophytes could intercept ions in roots and minimize its transport to the shoot parts. Eutrema salsugineum (previously Thellungiella salsuginea, salt cress) is a representative pseudo-halophyte, which could not only survive well under more than 300 mM NaCl but grow well in cold regions.54,55 Different categories of halophytes have distinct abilities to address high salt concentrations or other stresses, finally finish their life cycle.
Halophytes have evolved diverse responses to environmental variations.56,57 Natural variation studies have provided novel views for the evolutionary processes that shape stress responses, also showed previously undiscovered loci related to these mechanisms.58–61 Studies are performed on natural variation in stress response traits, providing essential sources of genetic variation.62–64 These important sources can help us understand the coordinated regulation of stress responses and play a role in improving agronomic crops.65 In plants, it becomes a complex process to understand and identify the crucial regulatory factors that are related to multiple stress responses. However, it is essential to identify traits that can protect plants from environmental stresses, and genomic studies across a wide variety of species are needed to address this aim.66 Advances in functional genomics will help us identify and understand potential stress-tolerant traits.67–71 Therefore, this review focuses on the role of functional genomics and system biology approaches in discovering the mechanisms that underlie stress-tolerance traits in halophytes.
The mechanism of euhalophytes in salt stress
S. salsa, a typical euhalophyte, is a good example to understand the molecular mechanisms in abiotic stress. S. salsa is an annual herbaceous C-3 euhalophyte that belongs to the Chenopodiaceae family,72,73 a group of plants that produce dimorphic seeds on the same plant under standard conditions.74–76 Recent studies have shown that NaCl concentration plays a key role in seed vitality in S. salsa by regulating the levels of stored compounds, such as proteins, starch, and fatty acids.77,78 S. salsa could tolerate high salt concentration and offers good sources for salt-related genes.79 A series of salt-tolerant genes from S. salsa have been cloned and their functions were tested. One important feature of halophytes is highly efficient vacuolar sequestration of cytotoxic Na+, which requires that Na+ could be pumped into the vacuole against the electrochemical gradient and that Na+ in the vacuole could be prevented from leaking back into the cytosol. Na+/H+ antiporters make a contribution to the vacuolar compartmentalization of Na+.80,81 One study showed that the expression of SsNHX1 was up-regulated in S. salsa leaves under 500 mM NaCl treatment. The product of SsNHX1 might be an Na+/H+ antiporter, which plays essential roles in S. salsa salt tolerance. Treatment of S. salsa with increasing concentrations of NaCl increased the activity of the plasma membrane (PM) H+-ATPase to promote salt tolerance.82 Later study illustrated that this increase in PM H+-ATPase activity attribute to both an increase in PM H+-ATPase protein levels and transcriptional levels. Also, Na+ efflux across the plasma membrane is due to Salt-Overly-Sensitive1(SOS1) Na+/H+ antiporter, and researchers have demonstrated that SsSOS1 may be involved in Na+ efflux both in leaves and in roots. Besides these genes, the expression of SsHKT1 increases under salt stress, suggesting that SsHKT1 is essential for K+ uptake under high salinity.83 Further research confirmed that transgenic Arabidopsis, which SsHKT1 was overexpressed, revealed increased salt tolerance. Ca2+ plays an essential role in maintaining K+ and Na+ homeostasis under salt stress, while SsCAX1 is important to lower cytosolic Ca2+ burst under salt stress.84,85 Overexpression S-adenosylmethionine synthetase gene from S. salsa could promote salt tolerance in tobacco, suggesting that this pathway also contributes to salt tolerance.86
S. salsa could also grow in saline inland soils of arid zone, characterized by both high salinity and drought. Compared to salt stress, drought problem is much more pervasive and negatively affects crop yields seriously. Dehydration-responsive element-binding (DREB) transcription factor (TF) is involved in abiotic stress tolerance in plants. Researchers have cloned DREB from succulent halophyte S. salsa (SsDREB) and tested its functions in tobacco. Transgenic tobacco plants transformed with SsDREB showed improved drought and salt tolerance, compared with wild-type controls.87 Also, researchers have cloned a vacuolar H+-pyrophosphatase gene from the halophyte S. salsa (SsVP) and identified its function in Arabidopsis. Transgenic Arabidopsis plants transformed with SsVP showed higher V-ATPase and the V-PPase activities, and increased drought tolerance in comparison with wild-type plants.88 Another study showed that transgenic Arabidopsis, transformed with ssNHX1, grew well under 200Mm NaCl treatment and drought stress.89 Later study demonstrated that transgenic plants showed higher photosynthesis activity and reduced toxic effects of Na+ accumulation in the cytosol. These studies testified euhalophytes S. salsa have evolved many mechanisms to drought stress.
Almost all the halophytes must face the challenge of osmotic adjustment in reaction to lower external water supply, including organic and inorganic solutes.90 Glycinebetaine and proline are related to osmotic adjustment in certain halophytes. The concentration of glycinebetaine exceeded 25 mM kg−1 of fresh weight in S. salsa under 400 mM NaCl treatment, which was deduced to be important in S. salsa osmotic adjustment. While the contribution rate of proline to osmotic potential was <0.5% in S. salsa under salt stress, therefore the cytoplasmic glycinebetaine might act a more essential role in osmotic adjustment compared with proline.91 Researchers have discovered two genes related to glycinebetaine synthesis, including glycinebetaine aldehyde dehydrogenase (BADH) and choline monooxygenase (CMO), which catalyze glycinebetaine synthesis.92 Researchers have cloned SsCMO and SsBADH from S. salsa and identified their functions. Transgenic tobacco overexpressing SsCMO showed higher tolerance to salt stress. Furthermore, researchers have cloned several important genes involved in this function in S. salsa, of which SsINPS is related to myo-inositol synthesis and SsP5CS is related to proline synthesis. In addition, S. salsa expressed more SsP5CS and SsINPS under salt treatment.
Halophytes are famous for their ability to endure and quench toxic reactive oxygen species (ROS), for they possess powerful antioxidant system.93,94 Researchers have detected Mn-SOD and several isoforms of CuZn-SOD and Fe-SOD in S. salsa leaf extracts, also cloned several genes related to oxidative stress tolerance in S. salsa, including Ss.sAPX, SsCAT2, SsPrxQ, SsGST, SsCAT1, SsAPX, and SsTypA1.95,96 Later study displayed that transgenic Arabidopsis plants overexpressing Ss.sAPX improved the germination rate, cotyledon growth, survival rate, and salt tolerance. In addition, transgenic plants showed higher total chlorophyll content, longer roots, higher total APX activity and less cell membrane damage than wild-type. These results demonstrated that Ss.sAPX may be essential to protect higher plants against salt-induced oxidative stress.97,98
S. europaea is another annual succulent euhalophytes and belongs to Chenopodiaceae family. Under seawater irrigation, S. europaea seeds have 28% oil content and 30.2% protein content, also unsaturated fatty acid account for high level in the seed oil, which makes S. europaea act as a potential competitive oilseed crop.99 Recent studies have shown that salinity treatment increased significantly the contents of proline, reducing saccharide, soluble saccharide and oligosaccharide in S. europaea. And MDA contents increased in S. europaea under high salt concentrations exceeding 300mM. Also, proteins' and polysaccharides' contents reduced under salt stress in S. europaea. In addition, salinity treatment decreased the K+ contents as well as carotenoids and chlorophylls in seedlings of S. europaea. S. europaea could tolerate high salt concentration and offers good sources for salt-related genes.100 Researchers have cloned phytoene synthase from S. europaea (SePSY) and tested its function in Arabidopsis. The study showed that SePSY overexpression enhanced the growth of the transgenic line. Under 100mM NaCl treatment, transgenic Arabidopsis displayed higher photosystem II activity and photosynthesis rate, as well as SOD and POD activity, and showed lower MDA and H2O2 contents in comparison with non-transformed line.101 This study demonstrated that the SePSY gene could enhance salt tolerance in Arabidopsis. Another study showed choline monooxygenase from S. europaea (SeCMO) could increase salt tolerance. CMO catalyzes the synthesis of Glycinebetaine, which is an osmoprotectant accumulated by stresses in plants. SeCMO transgenic tobacco could survive under 300mM NaCl treatment and displayed vigorous conditions in comparison with the wild-type control. Also, transgenic tobacco showed higher betaine accumulation and chlorophyll content, lower electrical conductivity. These studies demonstrate that SeCMO could effectively improve salt-tolerance in tobacco.102 Another study showed that researchers have cloned β-lycopene cyclase gene from S. europaea (SeLCY) and tested its functions. SeLCY transgenic Arabidopsis showed increased oxidative stress tolerance and salt-stress tolerance. Also, transgenic Arabidopsis grew better, displayed higher photosystem activity and less H2O2 accumulation, and retained higher carotenoid contents. All these studies demonstrated that euhalophytes S. europaea have evolved complex mechanism to resist salt stress.
The mechanism of recretohalophytes in salt stress
Recretohalophytes, typical halophytes, can excrete excessive salt to the environment through epidermal salt bladders or salt glands (Figure 2).103,104 Therefore, salt bladders and salt glands play essential roles for the recretohalophytes to secrete excess salt.105 L. bicolor, which possess salt glands that secrete excess salt, is extensively studied to understand the high molecular mechanism of recretohalophytes.106,107 The L. bicolor salt gland is comprised of 16 cells and is regulated by levels of specific cations.108 The divalent cation Ca2+ has been demonstrated to function crucially in plant growth, development, and salt tolerance. And increases in Ca2+ levels not only markedly enhance its development, but also promote salt-secretion rates in L. bicolor leaves.109 In addition, under conditions of elevated salinity, K+ accumulation in salt gland cells may play a part in salt secretion in L. bicolor.110 Despite the important roles of Ca2+ and K+ levels, environmental scanning electron microscopy has shown that the chemical composition of secretions from the secretory pores is primarily composed of NaCl.
Figure 2.
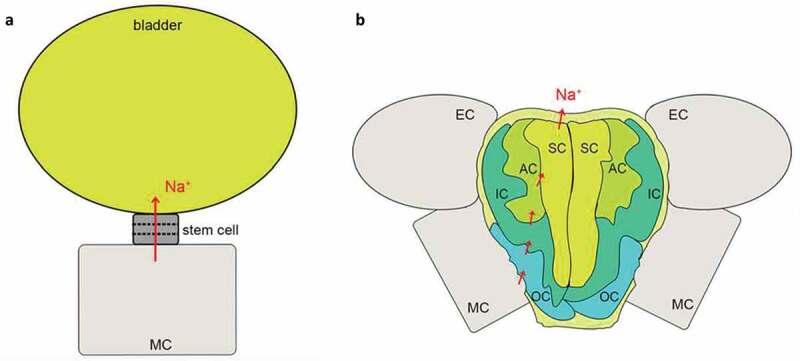
The structure and Na+ secretion pathway of a salt bladder (a) and a salt gland (b)(Yuan et al., 2016).
Many studies are performed to screen candidate genes involved in salt stress in L. bicolor. Researchers used the fluorescence method to screen ~10,000 seedlings in which seeds were gamma-irradiated, and obtained 15 mutants with increased salt gland density and 4 mutants with reduced salt gland density.111 These two group mutants will be helpful to isolate genes related to salt secretion and salt gland development in L. bicolor. Also, high-throughput RNA-sequencing analysis was performed to screen genes related to salt secretion in L. bicolor. This study showed that 2040 genes were differentially expressed among 27311 total genes of database, of which 1260 genes were down-regulated and 744 were up-regulated with the NaCl versus the control treatment. Further analysis showed that 102 of these genes might be related to salt secretion, including NHX genes, vesicle-associated membrane protein (VAMP) and so on.112 This study identifies the candidate genes that are related to salt secretion in the L. bicolor salt glands.113
Apart from salt stress, L. bicolor could also survive in a wide range of harsh environments, implying that it has evolved physiological and molecular systems to adjust to harmful stress conditions. Plant glutathione S-transferases (GSTs) play essential roles in protecting plants against diverse abiotic and biotic stresses. A novel GST gene was cloned from L. bicolor (LbGST1), and its functions were tested in tobacco. Transgenic tobacco plants, which LbGST1 is overexpressed, showed both glutathione peroxidase and GST activities. Furthermore, catalase, peroxidase (POD) and superoxide dismutase showed higher activities in transgenic plants than WT plants, especially under salt treatment.114 Similarly, transgenic plants showed higher levels of proline than WT plants under NaCl treatment. In addition, transgenic plants showed lower Na+ content than WT plants under these stress conditions. Another study showed that transgenic yeast harboring the LbGST1 showed elevated tolerance to drought and freezing compared with the control transformants. These studies suggest that LbGST1 might be involved in many physiological pathways that can improve stress resistance in plants. Also, researchers have cloned a novel dehydration-responsive element-binding (DREB) gene from L. bicolor (LbDREB) and characterized its function in copper stress. The study showed that transgenic tobacco plants transformed with LbDREB revealed higher contents of soluble protein and proline, and higher ratio of K+ to Na+ under the treatment of CuSO4.113 In addition, some genes involved in stress are up-regulated in LbDREB transgenic plants, including late embryogenesis abundant (LEA), PODs, Cu/Zn SOD, and lipid transfer proteins (LTP). This study demonstrates that LbDREB can strengthen copper stress tolerance by up-regulating a series of genes involved in stress, consequently regulating stress tolerance-related physiological processes in plants.
R. trigyna, an endangered dicotyledonous shrub, is another recretohalophyte belonging to Tamaricaceae family. It grows in a salinized desert in Inner Mongolia, China, which is characterized by high salinity, low temperature, and hyper-drought conditions.115 Therefore, exploring the molecular mechanisms employed by R. trigyna will offer us valuable resources in salt-related signaling pathway. Many researches are performed to study the mechanisms in response to salt stress in R. trigyna. Researchers have isolated Na+/H+ antiporter from R. trigyna (RtNHX1) and tested its function in Arabidopsis. Transgenic Arabidopsis, which RtNHX1 was overexpressed, exhibited enhanced seed germination rate, root elongation, biomass accumulation, and chlorophyll content in comparison with control lines. Further study showed that RtNHX1 transgenic Arabidopsis showed increased activities of POD and CAT, RWC, and proline content. Also, the leaves of transgenic Arabidopsis accumulated more K+ and less Na+, and lower ratio of Na+/K+. In addition, RtNHX1 transgenic yeast vacuole showed increased accumulation of Na+ and K+ and decreased Na+/K+ ratio. These studies revealed that RtNHX1 acts as an antiporter which sequesters Na+ and K+ in the vacuole, and could resist salt tolerance.116 Another study showed that a high-affinity potassium transporter gene was isolated from R. trigyna (RtHKT1), which was up-regulated under high Na+ or low K+ treatment in R. trigyna. Transgenic Arabidopsis enhanced the accumulation of K+, prevented the transport of Na+ from roots to shoots, and increased biomass under salt stress in comparison with wild control. This study suggests that RtHKT1could increase salt tolerance by maintaining the homeostasis of Na+/K+.115 Recent research showed that salt stress could activate the flavonoid biosynthesis pathway, while flavanone-3-hydroxylase (F3H) is involved in this process. The study showed that the transcription level of RtF3H1 and RtF3H2 was increased in R. trigyna under salt stress. Also, transgenic RtF3H1 or RtF3H2 Escherichia coli lines showed a higher survival rate than the control lines under salt stress. Another study showed that the Group II WRKY transcription factor was isolated from R. trigyna (RtWRKY23), which was induced by salt treatment. RtWRKY23 transgenic Arabidopsis revealed increased chlorophyll content, fresh weight, and root length. Further study showed that RtWRKY23 transgenic Arabidopsis had a higher content of proline and activity of peroxidase, and lower H2O2 and MDA contents in comparison with wild-type plants under salt stress.117 All these studies demonstrated that recretohalophyte R. trigyna evolved many mechanisms in response to salt stress.
The mechanism of pseudo-halophytes in salt stress
Pseudo-halophytes could not only tolerate strong salinity but escape from it by locating the active part of the root system in less saline soil levels.50,118 E. salsugineum is a representative pseudo-halophyte and could survive well under more than 300 mM NaCl.119 According to high sequence similarity between E. salsugineum and Arabidopsis, researchers have cloned several E. salsugineum genes involved in salt stress and characterized its functions, including TsVP which encodes vacuolar pyrophosphatase, ThCBL9 which encodes a calcineurin-B-like protein, ThHSC70 which encodes a heat-shock protein, ThCYP1 which encodes a cyclophilin, ThZF1which encodes a Cys-2/His-2-type transcription factor, TsnsLTP4 which encodes nonspecific lipid transfer proteins, TsGOLS2 which encodes galactinol synthase, TsLEA1 and so on.120–124 TsVP-transformed transgenic cotton and maize showed improved salt and drought tolerance, which is related to the higher activity of vacuolar H+-PPase. Also, the leaves of TsVP-transformed tobacco accumulated more Na+, owing to efficient vacuolar Na+ compartmentalization. Overexpression of ThCBL9 enhanced its tolerance to salt and osmotic stress in A. thaliana, and overexpression of ThHSC70 in A. thaliana improved tolerance to chilling and high temperature. ThCYP1-transformed fission yeast and tobacco cells showed increased salt tolerance, indicating that ThCYP1 might mediate the correct folding of certain stress-related proteins. Also, ectopic expression of ThZF1 in Arabidopsis mutant azf2 showed that ThZF1 might function similarly as Arabidopsis AZF2 in regulating downstream gene expression and plant development. Overexpression of TsGOLS2, a galactinol synthase, in Arabidopsis thaliana increased its tolerance to high salinity and osmotic stresses.122 In addition, TsLEA1 maintained salt tolerance in yeast as well as in plants, suggesting that TsLEA1 may be involved in protection plant and yeast cells under stress conditions. Apart from salt-related genes, E. salsugineum small RNA libraries were constructed and sequenced, and the study showed that T. salsuginea diverse set of miRNAs could respond to salt stress and act an important role in response to salt stress.125 Clearly, more researches are needed to perform to identify the detailed molecular mechanisms of E. salsugineum in abiotic stress.
E. salsugineum commonly grows in extreme environments, characterized by both high salinity and low temperature.125 Freezing is another major environmental stress, which seriously affects plant growth and development, contributing to the decrease of crop yield and quality.126 The TsFtsH8 gene is related to cold tolerance in E. salsugineum, and TsFtsH8-RNAi lines exhibit severe chlorophyll decomposition and organelle deterioration, also reveal the reductions of the rates of open photosystem II reaction centers.127 Five aquaporin genes involved in cold stress have been identified through RNA-sequencing experiments, including three TIPs, one PIPs, and one NIPs. In addition, in response to low temperatures, expression of DREB1/CBF-cold signaling pathway genes are altered, including COR47, ICE1, and CBF1 (Figure 3). KEGG pathway analysis was performed, showing that cold-regulated genes were also involved in photosynthesis, circadian rhythm, metabolism, and transcriptional regulation.126
Figure 3.
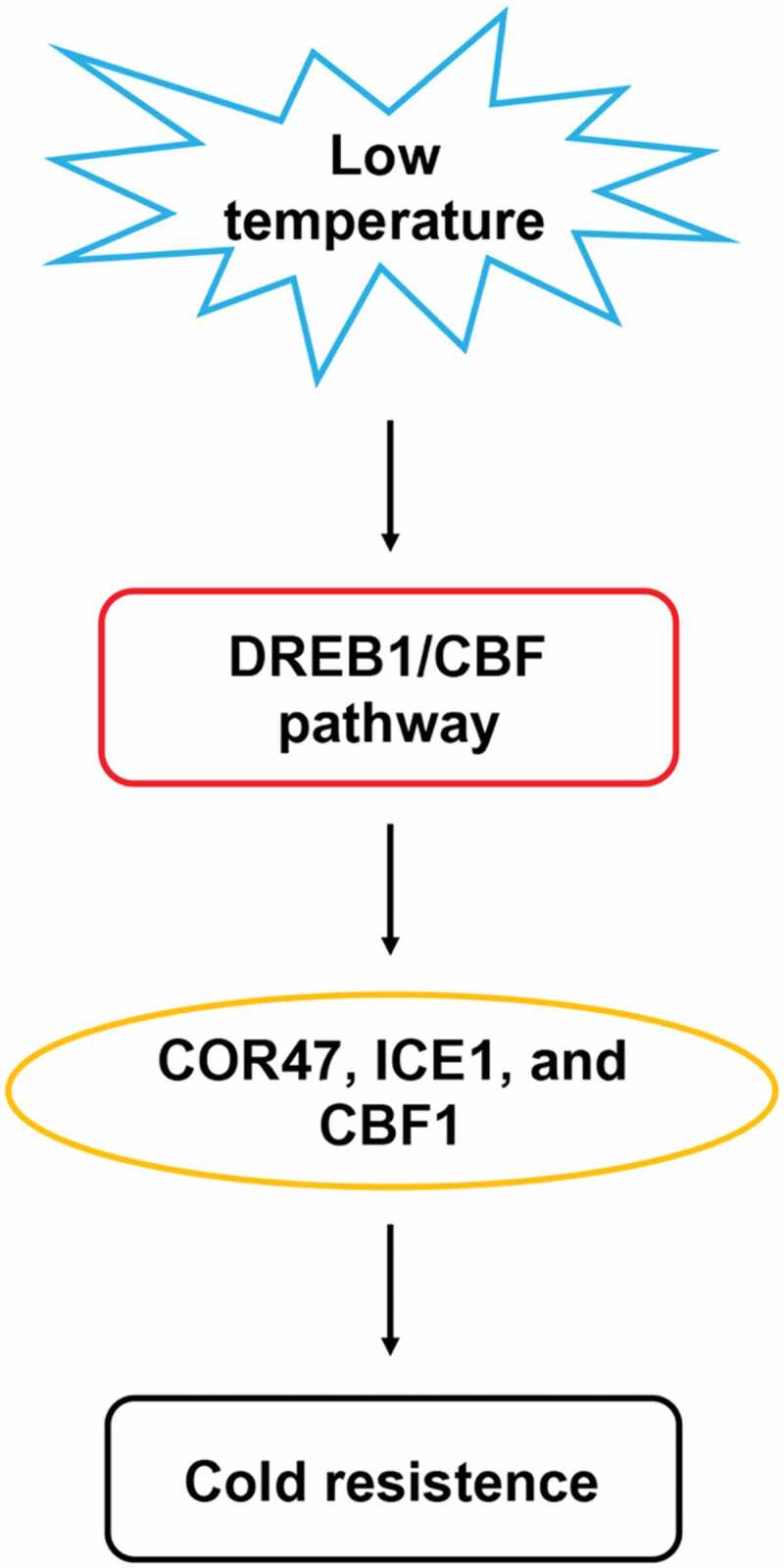
Regulation network model of DREB1/CBF pathway.
Aquaporin (AQP) membrane channels play essential roles in high salt tolerance and drought tolerance in E. salsugineum. Researchers have cloned a tonoplast AQP gene (TsTIP1;2) from the E. salsugineum and identified its functions. TsTIP1;2-transformed Arabidopsis showed strikingly strengthened tolerance to oxidative, drought, and salt stresses. And TsTIP1;2-expressed in Xenopus oocytes revealed water channel activity. Also, TsTIP1;2 could conduct H2O2 molecules into yeast cells under oxidative stress.44 This study showed that TsTIP1;2 played multifunctional roles in the survival of E. salsugineum.
Future perspective
Herein, we have described the complicated regulatory processes that coordinate tolerance and responses to abiotic stresses in halophytes. We anticipate several major future areas of active investigation in plant responses to abiotic stresses. First, emerging sequencing techniques and platforms will help researchers characterize individual regulatory components in detail.128–131 Second, genomic-scale experimental data, coupled with computational biology modeling, will serve as clues to discover stress response regulatory genes.132–135 In future, we will see a much clearer picture of the abiotic stress signaling pathway among various species.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (31900560), China Postdoctoral Science Foundation (2018M632710), and Shandong Provincial Postdoctoral Innovation Project (201901013).
Conflict of interest
Authors state no conflict of interest.
References
- 1.Zhu JK. Abiotic stress signaling and responses in plants. Cell. 2016;167(2):1–10. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao TL, Ren Q.. New records of ochrolechia and placopsis from the Hengduan mountains, China. Mycotaxon. 2012;122:461–466. doi: 10.5248/122.461. [DOI] [Google Scholar]
- 3.Guo J, Dong X, Han G, Wang B. Salt-enhanced reproductive development of Suaeda salsa L. coincided with ion transporter gene upregulation in flowers and increased pollen K(+) Content. Front Plant Sci. 2019;10:333. doi: 10.3389/fpls.2019.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han G, Yuan F, Guo J, Zhang Y, Sui N, Wang B. AtSIZ1 improves salt tolerance by maintaining ionic homeostasis and osmotic balance in Arabidopsis. Plant Sci. 2019;285:55–67. doi: 10.1016/j.plantsci.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Tang GY, Wei LQ, Liu ZJ, Bi YP, Shan L. Ectopic expression of peanut acyl carrier protein in tobacco alters fatty acid composition in the leaf and resistance to cold stress. Biologia Plantarum. 2012;56(3):493–501. doi: 10.1007/s10535-012-0057-7. [DOI] [Google Scholar]
- 6.Liu W, Ji SX, Fang XL, Wang QG, Li Z, Yao FY, Hou L, Dai SJ. Protein kinase LTRPK1 influences cold adaptation and microtubule stability in Rice. J Plant Growth Regul. 2013;32(3):483–490. doi: 10.1007/s00344-012-9314-4. [DOI] [Google Scholar]
- 7.He YA, Li YP, Cui LX, Xie LX, Zheng CK, Zhou GH, Zhou JJ, Xie XZ. Phytochrome B negatively affects cold tolerance by regulating OsDREB1 gene expression through phytochrome interacting factor-like protein OsPIL16 in Rice. Front Plant Sci. 2016;7. doi: 10.3389/fpls.2016.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu L, Xie Y, Fan SJ, Wang ZS, Wang FH, Zhang B, Li HS, Song J, Kong LA. Comparative analysis of root transcriptome profiles between drought-tolerant and susceptible wheat genotypes in response to water stress. Plant Sci. 2018;272:276–293. doi: 10.1016/j.plantsci.2018.03.036. [DOI] [PubMed] [Google Scholar]
- 9.Hou L, Liu W, Li Z, Huang C, Fang XL, Wang Q, Liu X. Identification and expression analysis of genes responsive to drought stress in peanut. Rus J Plant Physio. 2014;61(6):842–852. doi: 10.1134/S1021443714060089. [DOI] [Google Scholar]
- 10.Tang GY, Shao FX, Xu PL, Shan L, Liu ZJ. Overexpression of a peanut NAC gene, AhNAC4, confers enhanced drought tolerance in tobacco. Rus J Plant Physio. 2017;64(4):525–535. doi: 10.1134/S1021443717040161. [DOI] [Google Scholar]
- 11.Guo YY, Tian SS, Liu SS, Wang WQ, Sui N. Energy dissipation and antioxidant enzyme system protect photosystem II of sweet sorghum under drought stress. Photosynthetica. 2018;56(3):861–872. doi: 10.1007/s11099-017-0741-0. [DOI] [Google Scholar]
- 12.Liu J, Zhang F, Zhou JJ, Chen F, Wang BS, Xie XZ. Phytochrome B control of total leaf area and stomatal density affects drought tolerance in rice. Plant Mol Biol. 2012;78(3):289–300. doi: 10.1007/s11103-011-9860-3. [DOI] [PubMed] [Google Scholar]
- 13.Liu SS, Wang WQ, Li M, Wan SB, Sui N. Antioxidants and unsaturated fatty acids are involved in salt tolerance in peanut. Acta Physiologiae Plantarum. 2017;39:9. doi: 10.1007/s11738-017-2501-y. [DOI] [Google Scholar]
- 14.Cui F, Sui N, Duan GY, Liu YY, Han Y, Liu SS, Wan SB, Li GW. Identification of metabolites and transcripts involved in salt stress and recovery in Peanut. Front Plant Sci. 2018;9:16. doi: 10.3389/fpls.2018.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang WQ, Zheng HX, Wang Y, Han GL, Sui N. Overexpression of CCCH zinc finger protein gene delays flowering time and enhances salt tolerance in Arabidopsis by increasing fatty acid unsaturation. Acta Physiologiae Plantarum. 2018;40(11):13. doi: 10.1007/s11738-018-2775-8. [DOI] [Google Scholar]
- 16.Sui N, Wang Y, Liu SS, Yang Z, Wang F, Wan SB. Transcriptomic and physiological evidence for the relationship between unsaturated fatty acid and salt stress in Peanut. Front Plant Sci. 2018;9:12. doi: 10.3389/fpls.2018.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song J, Wang BS. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann Bot. 2015;115(3):541–553. doi: 10.1093/aob/mcu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su T, Li W, Wang P, Ma C. Dynamics of peroxisome homeostasis and its role in stress response and signaling in Plants. Front Plant Sci. 2019;10:705. doi: 10.3389/fpls.2019.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu G, Li S, Li X, Liu Y, Zhao S, Liu B, Zhou H, Lin H. A functional alternative oxidase modulates plant salt tolerance in Physcomitrella patens. Plant Cell Physiol. 2019;60(8):1829–1841. doi: 10.1093/pcp/pcz099. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Li L, Yuan F, Chen M. Exogenous salicylic acid improves the germination of Limonium bicolor seeds under salt stress. Plant Signal Behav. 2019;14(10):e1644595. doi: 10.1080/15592324.2019.1644595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Song J. Analysis of widely targeted metabolites of the euhalophyte Suaeda salsa under saline conditions provides new insights into salt tolerance and nutritional value in halophytic species. BMC Plant Biol. 2019;19(1):388. doi: 10.1186/s12870-019-2006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng YQ, Bao J, Yuan F, Liang X, Feng ZT, Wang BS. Exogenous hydrogen sulfide alleviates salt stress in wheat seedlings by decreasing Na+ content. Plant Growth Regul. 2016;79(3):391–399. doi: 10.1007/s10725-015-0143-x. [DOI] [Google Scholar]
- 23.Xia HY, Xue YF, Liu DY, Kong WL, Xue YH, Tang YY, Li J, Li D, Mei PP. Rational application of fertilizer nitrogen to soil in combination with foliar zn spraying improved zn nutritional quality of wheat grains. Front Plant Sci. 2018;9. doi: 10.3389/fpls.2018.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Li D, Zhang DB, Zhao XG, Cao XM, Dong LL, Liu JX, Chen KL, Zhang HW, Gao CX, et al. Analysis of the functions of TaGW2 homoeologs in wheat grain weight and protein content traits. Plant J. 2018;94(5):857–866. doi: 10.1111/tpj.13903. [DOI] [PubMed] [Google Scholar]
- 25.Li AQ, Li GH, Zhao YH, Meng ZD, Zhao M, Li CS, Zhang Y, Li PC, Ma CL, Xia H, et al. Combined small RNA and gene expression analysis revealed roles of miRNAs in maize response to rice black-streaked dwarf virus infection. Sci Rep. 2018;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang SS, Wang F, Tan SJ, Wang MX, Sui N, Zhang XS. Transcript profiles of maize embryo sacs and preliminary identification of genes involved in the embryo sac-pollen tube interaction. Front Plant Sci. 2014;5. doi: 10.3389/fpls.2014.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun YL, Mu CH, Zheng HX, Lu SP, Zhang H, Zhang XC, Liu X. Exogenous Pi supplementation improved the salt tolerance of maize (Zea mays L.) by promoting Na+ exclusion. Sci Rep. 2018;8:13. doi: 10.1038/s41598-018-34320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JC, Li M, Xie XZ, Han GL, Sui N, Wang BS. DEFICIENCY OF PHYTOCHROME B ALLEVIATES CHILLING-INDUCED PHOTOINHIBITION IN RICE. Am J Bot. 2013;100(9):1860–1870. doi: 10.3732/ajb.1200574. [DOI] [PubMed] [Google Scholar]
- 29.Ruan W, Guo M, Wang X, Guo Z, Xu Z, Xu L, Zhao H, Sun H, Yan C, Yi K. Two RING-Finger Ubiquitin E3 Ligases Regulate the Degradation of SPX4, an internal phosphate sensor, for phosphate homeostasis and signaling in Rice. Mol Plant. 2019;12(8):1060–1074. doi: 10.1016/j.molp.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Kong XQ, Gao XH, Sun W, An J, Zhao YX, Zhang H. Cloning and functional characterization of a cation-chloride cotransporter gene OsCCC1. Plant Mol Biol. 2011;75(6):567–578. doi: 10.1007/s11103-011-9744-6. [DOI] [PubMed] [Google Scholar]
- 31.Liu YJ, Gao SQ, Tang YM, Gong J, Zhang X, Wang YB, Zhang LP, Sun RW, Zhang Q, Chen ZB, et al. Transcriptome analysis of wheat seedling and spike tissues in the hybrid Jingmai 8 uncovered genes involved in heterosis. Planta. 2018;247(6):1307–1321. doi: 10.1007/s00425-018-2848-3. [DOI] [PubMed] [Google Scholar]
- 32.Pan JW, Li Z, Wang QG, Garrell AK, Liu M, Guan YA, Zhou WQ, Liu W. Comparative proteomic investigation of drought responses in foxtail millet. BMC Plant Biol. 2018;18:19. doi: 10.1186/s12870-018-1533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han GL, Wang MJ, Yuan F, Sui N, Song J, Wang BS. The CCCH zinc finger protein gene AtZFP1 improves salt resistance in Arabidopsis thaliana. Plant Mol Biol. 2014;86(3):237–253. doi: 10.1007/s11103-014-0226-5. [DOI] [PubMed] [Google Scholar]
- 34.Jiang CY, Tholen D, Xu JM, Xin CP, Zhang H, Zhu XG, Zhao YX. Increased expression of mitochondria-localized carbonic anhydrase activity resulted in an increased biomass accumulation in Arabidopsis thaliana. J Plant Bio. 2014;57(6):366–374. doi: 10.1007/s12374-014-0330-8. [DOI] [Google Scholar]
- 35.Zhou JC, Fu TT, Sui N, Guo JR, Feng G, Fan JL, Song J. The role of salinity in seed maturation of the euhalophyte Suaeda salsa. Plant Biosyst. 2016;150(1):83–90. doi: 10.1080/11263504.2014.976294. [DOI] [Google Scholar]
- 36.Feng ZT, Sun QJ, Deng YQ, Sun SF, Zhang JG, Wang BS. Study on pathway and characteristics of ion secretion of salt glands of Limonium bicolor. Acta Physiologiae Plantarum. 2014;36(10):2729–2741. doi: 10.1007/s11738-014-1644-3. [DOI] [Google Scholar]
- 37.Leng BY, Yuan F, Dong XX, Wang J, Wang BS. Distribution pattern and salt excretion rate of salt glands in two recretohalophyte species of Limonium (Plumbaginaceae). S Afr J Bot. 2018;115:74–80. doi: 10.1016/j.sajb.2018.01.002. [DOI] [Google Scholar]
- 38.Zhang T, Song J, Fan JL, Feng G. Effects of saline-waterlogging and dryness/moist alternations on seed germination of halophyte and xerophyte. Plant Spec Biol. 2015;30(3):231–236. doi: 10.1111/psbi.2015.30.issue-3. [DOI] [Google Scholar]
- 39.Ma Y, Yang Y, Liu R, Li Q, Song J. Adaptation of euhalophyte Suaeda salsa to nitrogen starvation under salinity. Plant Physiol Biochem. 2019;146:287–293. doi: 10.1016/j.plaphy.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 40.Sui N, Tian SS, Wang WQ, Wang MJ, Fan H. Overexpression of glycerol-3-phosphate acyltransferase from Suaeda salsa improves salt tolerance in Arabidopsis. Front Plant Sci. 2017;8. doi: 10.3389/fpls.2017.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao SZ, Sun HZ, Gao Y, Sui N, Wang BS. Growth regulator-induced betacyanin accumulation and dopa-4,5-dioxygenase (DODA) gene expression in euhalophyte Suaeda salsa calli. In Vitro Cell Develop Biol-Plant. 2011;47(3):391–398. doi: 10.1007/s11627-011-9339-6. [DOI] [Google Scholar]
- 42.Deng YQ, Feng ZT, Yuan F, Guo JR, Suo SS, Wang BS. Identification and functional analysis of the autofluorescent substance in Limonium bicolor salt glands. Plant Physiol Biochem. 2015;97:20–27. doi: 10.1016/j.plaphy.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Zhao C, Zhang M, Yuan F, Chen M. Exogenous melatonin improves seed germination in Limonium bicolor under salt stress. Plant Signal Behav. 2019;14(11):1659705. doi: 10.1080/15592324.2019.1659705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang LL, Chen AP, Zhong NQ, Liu N, Wu XM, Wang F, Yang CL, Romero MF, Xia GX. The Thellungiella salsuginea tonoplast aquaporin TsTIP1;2 functions in protection against multiple abiotic stresses. Plant Cell Physiol. 2014;55(1):148–161. doi: 10.1093/pcp/pct166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Shi SH, Li FL, Zhao CZ, Li AQ, Hou L, Xia H, Wang BS, Baltazar JL, Wang XJ, et al. Global transcriptome analysis provides new insights in Thellungiella salsuginea stress response. Plant Biol (Stuttg). 2019;21(5):796–804. doi: 10.1111/plb.13006. [DOI] [PubMed] [Google Scholar]
- 46.Chen X, Han H, Jiang P, Nie L, Bao H, Fan P, Lv S, Feng J, Li Y. Transformation of beta-lycopene cyclase genes from Salicornia europaea and Arabidopsis conferred salt tolerance in Arabidopsis and tobacco. Plant Cell Physiol. 2011;52(5):909–921. doi: 10.1093/pcp/pcr043. [DOI] [PubMed] [Google Scholar]
- 47.Liu QQ, Liu RR, Ma YC, Song J. Physiological and molecular evidence for Na+ and Cl- exclusion in the roots of two Suaeda salsa populations. Aquatic Botany. 2018;146:1–7. doi: 10.1016/j.aquabot.2018.01.001. [DOI] [Google Scholar]
- 48.Wang FX, Xu YG, Wang S, Shi WW, Liu RR, Feng G, Song J. Salinity affects production and salt tolerance of dimorphic seeds of Suaeda salsa. Plant Physiol Biochem. 2015;95:41–48. doi: 10.1016/j.plaphy.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Wang FX, Yin CH, Song YP, Li Q, Tian CY, Song J. Reproductive allocation and fruit-set pattern in the euhalophyte Suaeda salsa in controlled and field conditions. Plant Biosyst. 2018;152(4):749–758. doi: 10.1080/11263504.2017.1330776. [DOI] [Google Scholar]
- 50.Yuan F, Guo JR, Shabala S, Wang BS. Reproductive Physiology of Halophytes: current Standing. Front Plant Sci. 2019;9:13. doi: 10.3389/fpls.2018.01954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan F, Leng BY, Wang BS. Progress in Studying Salt Secretion from the Salt Glands in Recretohalophytes: how Do Plants Secrete Salt?. Front Plant Sci. 2016;7. doi: 10.3389/fpls.2016.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan F, Leng B, Zhang H, Wang X, Han G, Wang B. A WD40-Repeat Protein From the Recretohalophyte Limonium bicolor Enhances Trichome Formation and Salt Tolerance in Arabidopsis. Front Plant Sci. 2019;10:1456. doi: 10.3389/fpls.2019.01456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dang ZH, Zheng LL, Wang J, Gao Z, Wu SB, Qi Z, Wang YC. Transcriptomic profiling of the salt-stress response in the wild recretohalophyte Reaumuria trigyna. BMC Genomics. 2013;14:29. doi: 10.1186/1471-2164-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo YH, Wang D, Jia WJ, Song J, Yang JC, Wang BS. Effects of seed vernalisation and photoperiod on flowering induction in the halophyte Thellungiella halophila. Aust J Botany. 2012;60(8):743–748. doi: 10.1071/BT12180. [DOI] [Google Scholar]
- 55.Li Y, Sun W, Liu F, Cheng J, Zhang X, Zhang H, Zhao Y. Methods for grafting Arabidopsis thaliana and Eutrema salsugineum. Plant Methods. 2019;15:93. doi: 10.1186/s13007-019-0477-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qi YC, Liu WQ, Qiu LY, Zhang SM, Ma L, Zhang H. Overexpression of glutathione S-transferase gene increases salt tolerance of arabidopsis. Rus J Plant Physio. 2010;57(2):233–240. doi: 10.1134/S102144371002010X. [DOI] [Google Scholar]
- 57.Cheng S, Yang Z, Wang MJ, Song J, Sui N, Fan H. Salinity improves chilling resistance in Suaeda salsa. Acta Physiologiae Plantarum. 2014;36(7):1823–1830. doi: 10.1007/s11738-014-1555-3. [DOI] [Google Scholar]
- 58.Liu RR, Wang L, Tanveer M, Seed Heteromorphism: SJ. An Important Adaptation of Halophytes for Habitat Heterogeneity. Front Plant Sci. 2018;9(10). doi: 10.3389/fpls.2018.01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ren Q. Pertusaria albiglobosa, a new lichen from China. Mycotaxon. 2013;124:349–352. doi: 10.5248/124.349. [DOI] [Google Scholar]
- 60.Ren Q. New species of Pertusaria from China. Telopea. 2014;16:(133–40. doi: 10.7751/telopea20147851. [DOI] [Google Scholar]
- 61.Ren Q, Kou XR. A new species of Pertusaria from China. Lichenologist. 2013;45(3):337–339. doi: 10.1017/S0024282913000066. [DOI] [Google Scholar]
- 62.Wang FR, Zhang CY, Liu GD, Chen Y, Zhang JX, Qiao QH, Yuan ZC, Fan SJ, Zhang J. Phenotypic variation analysis and QTL mapping for cotton (Gossypium hirsutum L.) fiber quality grown in different cotton-producing regions. Euphytica. 2016;211(2):169–183. doi: 10.1007/s10681-016-1728-9. [DOI] [Google Scholar]
- 63.Ren Q, Li SX. New records of crustose lichens from China-1. Mycotaxon. 2013;125:(65–7. doi: 10.5248/125.65. [DOI] [Google Scholar]
- 64.Ren Q, Zhao N. New taxa of the lichen genus Pertusaria from China. Mycotaxon. 2014;127:(221–6. doi: 10.5248/127.221. [DOI] [Google Scholar]
- 65.Li YY, Xu JJ, Ul Haq N, Zhang H, Zhu XG. Was low CO2 a driving force of C-4 evolution: arabidopsis responses to long-term low CO2 stress. J Exp Bot. 2014;65(13):3657–3667. doi: 10.1093/jxb/eru193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kong XQ, Wang T, Li WJ, Tang W, Zhang DM, Dong HZ. Exogenous nitric oxide delays salt-induced leaf senescence in cotton (Gossypium hirsutum L.). Acta Physiologiae Plantarum. 2016;38:3. doi: 10.1007/s11738-016-2079-9. [DOI] [Google Scholar]
- 67.Lin J, Li JP, Yuan F, Yang Z, Wang BS, Chen M. Transcriptome profiling of genes involved in photosynthesis in Elaeagnus angustifolia L. under salt stress. Photosynthetica. 2018;56(4):998–1009. doi: 10.1007/s11099-018-0824-6. [DOI] [Google Scholar]
- 68.Liu F, Yang YJ, Gao JW, Ma CL, Bi YP. A comparative transcriptome analysis of a wild purple potato and its red mutant provides insight into the mechanism of anthocyanin transformation. PLoS One. 2018;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen XY, Wang ZL, Song XF, Xu JJ, Jiang CY, Zhao YX, Ma CL, Zhang H. Transcriptomic profiling revealed an important role of cell wall remodeling and ethylene signaling pathway during salt acclimation in Arabidopsis. Plant Mol Biol. 2014;86(3):303–317. doi: 10.1007/s11103-014-0230-9. [DOI] [PubMed] [Google Scholar]
- 70.Wang PF, Shi SH, Ma JJ, Song H, Zhang Y, Gao C, Zhao CZ, Zhao SZ, Hou L, Lopez-Baltazar J, et al. Global Methylome and gene expression analysis during early Peanut pod development. BMC Plant Biol. 2018;18:13. doi: 10.1186/s12870-018-1546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang PF, Song H, Li CS, Li PC, Li AQ, Guan HS, Hou L, Wang XJ. Genome-Wide Dissection of the Heat Shock Transcription Factor Family Genes in Arachis. Front Plant Sci. 2017;8:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song J, Shi WW, Liu RR, Xu YG, Sui N, Zhou JC, Feng G. The role of the seed coat in adaptation of dimorphic seeds of the euhalophyte Suaeda salsa to salinity. Plant Spec Biol. 2017;32(2):107–114. doi: 10.1111/psbi.2017.32.issue-2. [DOI] [Google Scholar]
- 73.Sui N, Li M, Li K, Song J, Wang BS. Increase in unsaturated fatty acids in membrane lipids of Suaeda salsa L. enhances protection of photosystem II under high salinity. Photosynthetica. 2010;48(4):623–629. doi: 10.1007/s11099-010-0080-x. [DOI] [Google Scholar]
- 74.Duan HM, Ma YC, Liu RR, Li Q, Yang Y, Song J. Effect of combined waterlogging and salinity stresses on euhalophyte Suaeda glauca. Plant Physiol Biochem. 2018;127:231–237. doi: 10.1016/j.plaphy.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 75.Li X, Liu Y, Chen M, Song YP, Song J, Wang BS, Feng G. Relationships between ion and chlorophyll accumulation in seeds and adaptation to saline environments in Suaeda salsa populations. Plant Biosyst. 2012;146:142–149. doi: 10.1080/11263504.2012.727880. [DOI] [Google Scholar]
- 76.Song J, Zhou JC, Zhao WW, Xu HL, Wang FX, Xu YG, Wang L, Tian CY. Effects of salinity and nitrate on production and germination of dimorphic seeds applied both through the mother plant and exogenously during germination in Suaeda salsa. Plant Spec Biol. 2016;31(1):19–28. doi: 10.1111/psbi.2016.31.issue-1. [DOI] [Google Scholar]
- 77.Guo JR, Li YD, Han GL, Song J, Wang BS. NaCl markedly improved the reproductive capacity of the euhalophyte Suaeda salsa. Functional Plant Biology. 2018;45(3):350–361. doi: 10.1071/FP17181. [DOI] [PubMed] [Google Scholar]
- 78.Guo JR, Suo SS, Wang BS. Sodium chloride improves seed vigour of the euhalophyte Suaeda salsa. Seed Sci Res. 2015;25(3):335–344. doi: 10.1017/S0960258515000239. [DOI] [Google Scholar]
- 79.Zhang SR, Song J, Wang H, Feng G. Effect of salinity on seed germination, ion content and photosynthesis of cotyledons in halophytes or xerophyte growing in Central Asia. J Plant Ecolo. 2010;3(4):259–267. doi: 10.1093/jpe/rtq005. [DOI] [Google Scholar]
- 80.Song J, Shi GW, Gao B, Fan H, Wang BS. Waterlogging and salinity effects on two Suaeda salsa populations. Physiol Plant. 2011;141(4):343–351. doi: 10.1111/ppl.2011.141.issue-4. [DOI] [PubMed] [Google Scholar]
- 81.Yang MF, Song J, Wang BS. Organ-Specific Responses of Vacuolar H+-ATPase in the Shoots and Roots of C-3 Halophyte Suaeda salsa to NaCl. J Integr Plant Biol. 2010;52(3):308–314. doi: 10.1111/j.1744-7909.2010.00895.x. [DOI] [PubMed] [Google Scholar]
- 82.Chen M, Song J, Wang BS. NaCl increases the activity of the plasma membrane H+-ATPase in C-3 halophyte Suaeda salsa callus. Acta Physiologiae Plantarum. 2010;32(1):27–36. doi: 10.1007/s11738-009-0371-7. [DOI] [Google Scholar]
- 83.Shao Q, Han N, Ding TL, Zhou F, Wang BS. SsHKT1;1 is a potassium transporter of the C-3 halophyte Suaeda salsa that is involved in salt tolerance. Functional Plant Biology. 2014;41(8):790–802. doi: 10.1071/FP13265. [DOI] [PubMed] [Google Scholar]
- 84.Han N, Lan WJ, He X, Shao Q, Wang BS, Zhao XJ. Expression of a Suaeda salsa Vacuolar H+/Ca2+ Transporter Gene in Arabidopsis Contributes to Physiological Changes in Salinity. Plant Mole Bio Rep. 2012;30(2):470–477. doi: 10.1007/s11105-011-0353-y. [DOI] [Google Scholar]
- 85.Han N, Shao Q, Bao HY, Wang BS. Cloning and characterization of a Ca2+/H+ antiporter from halophyte Suaeda salsa L. Plant Mole Bio Rep. 2011;29(2):449–457. doi: 10.1007/s11105-010-0244-7. [DOI] [Google Scholar]
- 86.Qi YC, Wang FF, Zhang H, Liu WQ. Overexpression of suadea salsa S-adenosylmethionine synthetase gene promotes salt tolerance in transgenic tobacco. Acta Physiologiae Plantarum. 2010;32(2):263–269. doi: 10.1007/s11738-009-0403-3. [DOI] [Google Scholar]
- 87.Zhang X, Liu X, Wu L, Yu G, Wang X, Ma H. The SsDREB Transcription Factor from the Succulent Halophyte Suaeda salsa Enhances Abiotic Stress Tolerance in Transgenic Tobacco. Int J Genomics. 2015;2015:875497. doi: 10.1155/2015/875497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guo S, Yin H, Zhang X, Zhao F, Li P, Chen S, Zhao Y, Zhang H. Molecular cloning and characterization of a vacuolar H+ -pyrophosphatase gene, SsVP, from the halophyte Suaeda salsa and its overexpression increases salt and drought tolerance of Arabidopsis. Plant Mol Biol. 2006;60(1):41–50. doi: 10.1007/s11103-005-2417-6. [DOI] [PubMed] [Google Scholar]
- 89.Zheng Y, Liao CC, Zhao SS, Wang CW, Guo Y. The glycosyltransferase QUA1 regulates chloroplast-associated calcium signaling during salt and drought stress in Arabidopsis. Plant And Cell Physio. 2017;58(2):329–341. doi: 10.1093/pcp/pcw192. [DOI] [PubMed] [Google Scholar]
- 90.Chen TS, Yuan F, Song J, Wang BS. Nitric oxide participates in waterlogging tolerance through enhanced adventitious root formation in the euhalophyte Suaeda salsa. Functional Plant Biology. 2016;43(3):244–253. doi: 10.1071/FP15120. [DOI] [PubMed] [Google Scholar]
- 91.Zhao YQ, Yang Y, Song YP, Li Q, Song J. Analysis of storage compounds and inorganic ions in dimorphic seeds of euhalophyte Suaeda salsa. Plant Physiol Biochem. 2018;130:511–516. doi: 10.1016/j.plaphy.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 92.Su T, Wang PP, Li HJ, Zhao YW, Lu Y, Dai P, Ren TQ, Wang XF, Li XZ, Shao Q, et al. The Arabidopsis catalase triple mutant reveals important roles of catalases and peroxisome-derived signaling in plant development. J Integr Plant Biol. 2018;60(7):591–607. doi: 10.1111/jipb.v60.7. [DOI] [PubMed] [Google Scholar]
- 93.Kong LA, Xie Y, Hu L, Si JS, Wang ZS. Excessive nitrogen application dampens antioxidant capacity and grain filling in wheat as revealed by metabolic and physiological analyses. Sci Rep. 2017;7:43363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun YL, Li F, Su N, Sun XL, Zhao SJ, Meng QW. The increase in unsaturation of fatty acids of phosphatidylglycerol in thylakoid membrane enhanced salt tolerance in tomato. Photosynthetica. 2010;48(3):400–408. doi: 10.1007/s11099-010-0052-1. [DOI] [Google Scholar]
- 95.Li K, Pang CH, Ding F, Sui N, Feng ZT, Wang BS. Overexpression of Suaeda salsa stroma ascorbate peroxidase in Arabidopsis chloroplasts enhances salt tolerance of plants. S Afr J Bot. 2012;78:235–245. doi: 10.1016/j.sajb.2011.09.006. [DOI] [Google Scholar]
- 96.Pang CH, Li K, Wang BS. Overexpression of SsCHLAPXs confers protection against oxidative stress induced by high light in transgenic Arabidopsis thaliana. Physiol Plant. 2011;143(4):355–366. doi: 10.1111/j.1399-3054.2011.01515.x. [DOI] [PubMed] [Google Scholar]
- 97.Qi JS, Song CP, Wang BS, Zhou JM, Kangasjarvi J, Zhu JK, Gong ZZ. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J Integr Plant Biol. 2018;60(9):805–826. doi: 10.1111/jipb.12654. [DOI] [PubMed] [Google Scholar]
- 98.Cao S, Du XH, Li LH, Liu YD, Zhang L, Pan X, Li Y, Li H, Lu H. Overexpression of Populus tomentosa cytosolic ascorbate peroxidase enhances abiotic stress tolerance in tobacco plants. Rus J Plant Physio. 2017;64(2):224–234. doi: 10.1134/S1021443717020029. [DOI] [Google Scholar]
- 99.Zhao KF, Song J, Fan H, Zhou S, Zhao M. Growth response to ionic and osmotic stress of NaCl in salt-tolerant and salt-sensitive maize. J Integr Plant Biol. 2010;52(5):468–475. doi: 10.1111/j.1744-7909.2010.00947.x. [DOI] [PubMed] [Google Scholar]
- 100.Wang X, Fan P, Song H, Chen X, Li X, Li Y. Comparative proteomic analysis of differentially expressed proteins in shoots of Salicornia europaea under different salinity. J Proteome Res. 2009;8(7):3331–3345. doi: 10.1021/pr801083a. [DOI] [PubMed] [Google Scholar]
- 101.Han H, Li Y, Zhou S. Overexpression of phytoene synthase gene from Salicornia europaea alters response to reactive oxygen species under salt stress in transgenic Arabidopsis. Biotechnol Lett. 2008;30(8):1501–1507. doi: 10.1007/s10529-008-9705-6. [DOI] [PubMed] [Google Scholar]
- 102.Wu S, Su Q, An LJ. Isolation of choline monooxygenase (CMO) gene from Salicornia europaea and enhanced salt tolerance of transgenic tobacco with CMO genes. Indian J Biochem Biophys. 2010;47:298–305. [PubMed] [Google Scholar]
- 103.Zhang H, Li YY, Zhu JK. Developing naturally stress-resistant crops for a sustainable agriculture. Nat Plants. 2018;4(12):989–996. doi: 10.1038/s41477-018-0309-4. [DOI] [PubMed] [Google Scholar]
- 104.Zhang JX, Wang FR, Zhang CY, Zhang JH, Chen Y, Liu GD, Zhao YX, Hao FS, Zhang J. A novel VIGS method by agroinoculation of cotton seeds and application for elucidating functions of GhBI-1 in salt-stress response. Plant Cell Rep. 2018;37(8):1091–1100. doi: 10.1007/s00299-018-2294-5. [DOI] [PubMed] [Google Scholar]
- 105.Yuan F, Liang X, Li Y, Yin SS, Wang BS. Methyl jasmonate improves tolerance to high salt stress in the recretohalophyte Limonium bicolor. Functional Plant Biology. 2019;46(1):82–92. doi: 10.1071/FP18120. [DOI] [PubMed] [Google Scholar]
- 106.Yuan F, Lyu MJA, Leng BY, Zheng GY, Feng ZT, Li PH, Zhu XG, Wang BS. Comparative transcriptome analysis of developmental stages of the Limonium bicolor leaf generates insights into salt gland differentiation. Plant Cell And Enviro. 2015;38(8):1637–1657. doi: 10.1111/pce.12514. [DOI] [PubMed] [Google Scholar]
- 107.Yuan F, Chen M, Yang JC, Leng BY, Wang BS. A system for the transformation and regeneration of the recretohalophyte Limonium bicolor. In Vitro Cell Develop Biol-Plant. 2014;50(5):610–617. doi: 10.1007/s11627-014-9611-7. [DOI] [Google Scholar]
- 108.Yuan F, Chen M, Leng BY, Wang BS. An efficient autofluorescence method for screening Limonium bicolor mutants for abnormal salt gland density and salt secretion. S Afr J Bot. 2013;88:110–117. doi: 10.1016/j.sajb.2013.06.007. [DOI] [Google Scholar]
- 109.Ding F, Chen M, Sui N, Wang BS. Ca2+ significantly enhanced development and salt-secretion rate of salt glands of Limonium bicolor under NaCl treatment. S Afr J Bot. 2010;76(1):95–101. doi: 10.1016/j.sajb.2009.09.001. [DOI] [Google Scholar]
- 110.Feng ZT, Deng YQ, Zhang SC, Liang X, Yuan F, Hao JL, Zhang JC, Sun SF, Wang BS. K+ accumulation in the cytoplasm and nucleus of the salt gland cells of Limonium bicolor accompanies increased rates of salt secretion under NaCl treatment using NanoSIMS. Plant Sci. 2015;238:286–296. doi: 10.1016/j.plantsci.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 111.Yuan F, Chen M, Yang JC, Song J, Wang BS. The optimal dosage of Co-60 gamma irradiation for obtaining salt gland mutants of exo-recretohalophyte Limonium bicolor (Bunge) O. Kuntze. Pak J Botany. 2015;47:71–76. [Google Scholar]
- 112.Yuan F, Lyu MJA, Leng BY, Zhu XG, Wang BS. The transcriptome of NaCl-treated Limonium bicolor leaves reveals the genes controlling salt secretion of salt gland. Plant Mol Biol. 2016;91(3):241–256. doi: 10.1007/s11103-016-0460-0. [DOI] [PubMed] [Google Scholar]
- 113.Ban Q, Liu G, Wang Y. A DREB gene from Limonium bicolor mediates molecular and physiological responses to copper stress in transgenic tobacco. J Plant Physiol. 2011;168(5):449–458. doi: 10.1016/j.jplph.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 114.Liu ZH, Yang CP, Qi XT, Xiu LL, Wang YC. Cloning, heterologous expression, and functional characterization of a chitinase gene, Lbchi32, from Limonium bicolor. Biochem Genet. 2010;48(7–8):669–679. doi: 10.1007/s10528-010-9348-x. [DOI] [PubMed] [Google Scholar]
- 115.Li N, Du C, Ma B, Gao Z, Wu Z, Zheng L, Niu Y, Wang Y. Functional analysis of ion transport properties and salt tolerance mechanisms of RtHKT1 from the Recretohalophyte Reaumuria trigyna. Plant Cell Physiol. 2019;60(1):85–106. doi: 10.1093/pcp/pcy187. [DOI] [PubMed] [Google Scholar]
- 116.Li N, Wang X, Ma B, Du C, Zheng L, Wang Y. Expression of a Na(+)/H(+) antiporter RtNHX1 from a recretohalophyte Reaumuria trigyna improved salt tolerance of transgenic Arabidopsis thaliana. J Plant Physiol. 2017;218:109–120. doi: 10.1016/j.jplph.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 117.Du C, Ma B, Wu Z, Li N, Zheng L, Wang Y. Reaumuria trigyna transcription factor RtWRKY23 enhances salt stress tolerance and delays flowering in plants. J Plant Physiol. 2019;239:38–51. doi: 10.1016/j.jplph.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 118.Yang Z, Wang Y, Wei XC, Zhao X, Wang BS, Sui N. Transcription profiles of genes related to hormonal regulations under salt stress in Sweet Sorghum. Plant Mole Bio Rep. 2017;35(6):586–599. doi: 10.1007/s11105-017-1047-x. [DOI] [Google Scholar]
- 119.Sui N, Han GL. Salt-induced photoinhibition of PSII is alleviated in halophyte Thellungiella halophila by increases of unsaturated fatty acids in membrane lipids. Acta Physiologiae Plantarum. 2014;36(4):983–992. doi: 10.1007/s11738-013-1477-5. [DOI] [Google Scholar]
- 120.Guo YH, Jia WJ, Song J, Wang DA, Chen M, Wang BS. Thellungilla halophila is more adaptive to salinity than Arabidopsis thaliana at stages of seed germination and seedling establishment. Acta Physiologiae Plantarum. 2012;34(4):1287–1294. doi: 10.1007/s11738-012-0925-y. [DOI] [Google Scholar]
- 121.Sun W, Li Y, Zhao YX, Zhang H. The TsnsLTP4, a Nonspecific Lipid Transfer Protein Involved in Wax Deposition and Stress Tolerance. Plant Mole Bio Rep. 2015;33(4):962–974. doi: 10.1007/s11105-014-0798-x. [DOI] [Google Scholar]
- 122.Sun ZB, Qi XY, Wang ZL, Li PH, Wu CX, Zhang H, Zhao YX. Overexpression of TsGOLS2, a galactinol synthase, in Arabidopsis thaliana enhances tolerance to high salinity and osmotic stresses. Plant Physiol Biochem. 2013;69:82–89. [DOI] [PubMed] [Google Scholar]
- 123.Zhang JX, Wang C, Yang CY, Wang JY, Chen L, Bao XM, Zhao YX, Zhang H, Liu J. The role of arabidopsis AtFes1A in cytosolic Hsp70 stability and abiotic stress tolerance. Plant J. 2010;62(4):539–548. doi: 10.1111/j.1365-313X.2010.04173.x. [DOI] [PubMed] [Google Scholar]
- 124.Zhang LY, Zhang XJ, Fan SJ. Meta-analysis of salt-related gene expression profiles identifies common signatures of salt stress responses in Arabidopsis. Plant Syst Evol. 2017;303(6):757–774. doi: 10.1007/s00606-017-1407-x. [DOI] [Google Scholar]
- 125.Zhang Q, Zhao CZ, Li M, Sun W, Liu Y, Xia H, Sun MN, Li AQ, Li CS, Zhao SZ, et al. Genome-wide identification of Thellungiella salsuginea microRNAs with putative roles in the salt stress response. BMC Plant Biol. 2013;13. doi: 10.1186/1471-2229-13-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang JS, Zhang Q, Cui F, Hou L, Zhao SZ, Xia H, Qiu JJ, Li TT, Zhang Y, Wang XJ, et al. Genome-wide analysis of gene expression provides new insights into cold responses in Thellungiella salsuginea. Front Plant Sci. 2017;8:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu XX, Fu C, Yang WW, Zhang Q, Fan H, Liu J. The involvement of TsFtsH8 in Thellungiella salsuginea tolerance to cold and high light stresses. Acta Physiologiae Plantarum. 2016;38(3). doi: 10.1007/s11738-016-2080-3. [DOI] [Google Scholar]
- 128.Liang Z, Chen KL, Yan Y, Zhang Y, Gao CX. Genotyping genome-edited mutations in plants using CRISPR ribonucleoprotein complexes. Plant Biotechnol J. 2018;16(12):2053–2062. doi: 10.1111/pbi.2018.16.issue-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.JJ X, YY L, XL M, JF D, Wang K, SS W, Tian Y, Zhang H, XG Z. Whole transcriptome analysis using next-generation sequencing of model species Setaria viridis to support C-4 photosynthesis research. Plant Mol Biol. 2013;83(1–2):77–87. doi: 10.1007/s11103-013-0025-4. [DOI] [PubMed] [Google Scholar]
- 130.Zhao Y, Ma J, Li M, Deng L, Li G, Xia H, Zhao S, Hou L, Li P, Ma C, et al. Whole-genome resequencing-based QTL-seq identified AhTc1 gene encoding a R2R3-MYB transcription factor controlling peanut purple testa colour. In: Plant Biotechnol J. 2019. doi: 10.1111/pbi.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hua K, Zhang J, Botella JR, Ma C, Kong F, Liu B, Zhu JK. Perspectives on the application of genome-editing technologies in crop breeding. Mol Plant. 2019;12(8):1047–1059. doi: 10.1016/j.molp.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 132.Li H, Yuan JY, Wu M, Han ZP, Li LH, Jiang HM, Jia YL, Han X, Liu M, Sun DL, et al. Transcriptome and DNA methylome reveal insights into yield heterosis in the curds of broccoli (Brassica oleracea L var. italic). BMC Plant Biol. 2018;18:17. doi: 10.1186/s12870-018-1384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li YY, Ma XL, Zhao JL, Xu JJ, Shi JF, Zhu XG, Zhao YX, Zhang H. Developmental genetic mechanisms of C-4 syndrome based on transcriptome analysis of C-3 Cotyledons and C-4 assimilating shoots in Haloxylon ammodendron. PLoS One. 2015;10(2):e0117175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jia Y, Tian H, Zhang S, Ding Z, Ma C. GUN1-interacting proteins open the door for retrograde signaling. Trends Plant Sci. 2019;24(10):884–887. doi: 10.1016/j.tplants.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 135.Gu JW, Hou DL, Li YH, Chao HB, Zhang K, Wang H, Xiang J, Raboanatahiry N, Wang BS, Li MT. Integration of proteomic and genomic approaches to dissect seed germination vigor in Brassica napus seeds differing in oil content. BMC Plant Biol. 2019;19:20. doi: 10.1186/s12870-018-1624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


