ABSTRACT
The combination of some parameters, including the neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR) and neutrophil to monocyte ratio (NMR), which are associated with patient prognosis, our goal is to find the best indicator to predict the efficacy of neoadjuvant chemotherapy(NAC)in breast cancer patients. A cohort of 808 breast cancer patients treated with NAC and subsequent surgery was analyzed retrospectively. In addition, 2424 people without breast cancer served as the normal group, which included three-fold more individuals compared with the breast cancer group. Receiver operating characteristics (ROC) curves were used to determine the optimal cutoff values of inflammatory markers and compare their predictive capacity. No significant differences in age, PLR, LMR and NMR were noted between the normal group and the patient group. However, the mean value of the NLR was significantly increased in breast cancer patients (2.28) compared with the normal population (2.04) (P < .05). The LMR was significantly associated with age (P = .003), menopausal status (P = .004), cT category (P = .017), cN category (P = .024) and response to NAC (P = .001). The multivariate analysis indicated that among these inflammatory markers, the LMR (6.1 < vs ≥ 6.1) was the only independent predictive factor for the efficacy of NAC (OR = 1.771, 95% CI = 1.273–2.464, P = .001). A low LMR is considered a favorable predicative factor of the efficacy of NAC in breast cancer patients.
KEYWORDS: Breast cancer, inflammatory biomarkers, neoadjuvant chemotherapy, lymphocyte/monocyte ratio, predictive factor
Introduction
Breast cancer is currently the most common cancer in women and is one of the leading causes of cancer-related deaths. Although its incidence has increased with time, its mortality rate has declined in recent decades due to an improvement in early diagnosis and neoadjuvant chemotherapy (NAC).1 The purpose of NAC is to increase the rate of breast-conserving surgery and downsize the risk of postoperative recurrence in patients with resectable breast cancer.2 Therefore, it would be advantageous to have accurate methods for predicting the outcome of NAC to help to identify the direct treatment effects and reduce adverse events caused by inappropriate treatment.3
Some research and clinical trials have demonstrated that magnetic resonance imaging,4 positron emission tomography5 and gene expression profiling6 predict the outcome of NAC in BC patients. However, these methods are costly and cannot be routinely performed. Recently, growing evidence has indicated that the host inflammatory response plays a considerable role in carcinogenesis and disease progression.7,8 The inflammatory response, which is not only standardized and reproducible but also cheap and easy to assess, includes neutrophils, lymphocytes, monocyte and platelets in peripheral blood. Neutrophils and monocytes promote tumor development via different mechanisms.9 In addition, high platelets not only reflect systemic inflammation but also increase metastasis through platelet clumps in neoplastic cells.10 Low lymphocyte counts can lead to an inadequate immune response, which subsequently results in low survival in multiple cancers.11,12 A combination of some parameters, including the neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR) and neutrophil to monocyte ratio (NMR), has been used as a cost-effective and simple metric of systemic inflammation,13 and this combination is associated with prognosis in breast cancer.14–16 However, there is controversy concerning the best indicator to predict the efficacy of neoadjuvant chemotherapy in breast cancer.
Therefore, the purpose of our study was to evaluate the predictive value of the NLR, PLR, LMR and NMR for the efficacy of NAC in breast cancer patients.
Results
Clinicopathological features and inflammatory biomarkers
Pre-NAC clinicopathological features of the patients are summarized in Table 1. Among the 808 patients evaluated, the median age was 50 (range 20–72). In total, 489 (60.52%) of patients were premenopausal, and 319 (39.48%) of patients were postmenopausal. Invasive ductal carcinoma (IDC) was diagnosed in 94.18%, invasive lobular carcinoma was diagnosed in 1.49% and other types of cancer were diagnosed in 4.33% of patients. ER-positive tumors were found in 59.78% of patients, and ER-negative tumors were noted in 40.22% of patients. PR-positive tumors were diagnosed in 44.06% of patients, and PR-negative tumors were noted in 54.44%. HER2 expression was negative in 52.72% of patients, positive in 37.00% patients and unknown in 10.27% patients. In total, 753 (93.19%) patients received at least 3 cycles of treatment with neoadjuvant chemotherapy, and 55 (6.81%) patients received more than 4 cycles of treatment. In total, 57.3% of patients exhibited clinically positive lymph nodes. The mean values of the NLR, PLR, LMR, and NMR were 2.28 ± 1.03, 128.75 ± 48.53, 6.05 ± 5.93 and 12.50 ± 10.68, respectively. As shown in Table 2, we collected 2,424 normal individuals from the physical examination centre using a 3:1 ratio of normal individuals to breast cancer patients. No significant differences in age, PLR, LMR and NMR were noted between the normal group and the patient group. However, the mean value of the NLR was significantly increased in breast cancer patients (2.28) compared with the normal population (2.04) (P < .05). Among the patients, 575 (71.16%) patients were classified into the responder group (pCR+cPR), and 233 (28.84%) patients were classified into the nonresponder group (cPD+cSD).
Table 1.
Patients’ characteristics (n = 808).
| Parameter (pre-NAC) | Number | Percent (%) |
|---|---|---|
| Age | 49.45 ± 9.27 | |
| Menopausal Status | ||
| Postmenopausal | 319 | 39.48 |
| Premenopausal | 489 | 60.52 |
| Subtypes of Cancer | ||
| Ductal | 761 | 94.18 |
| Lobular | 12 | 1.49 |
| Others | 35 | 4.33 |
| Molecular subtype | ||
| Luminal A | 106 | 13.00 |
| Luminal B | 319 | 39.60 |
| HER2 | 179 | 22.15 |
| Triple negative | 121 | 14.98 |
| Unknown | 83 | 10.27 |
| ER status | ||
| Negative | 325 | 40.22 |
| Positive | 483 | 59.78 |
| PR status | ||
| Negative | 439 | 54.44 |
| Positive | 369 | 45.56 |
| Her2 status | ||
| Negative | 426 | 52.72 |
| Positive | 299 | 37.00 |
| Unknown | 83 | 10.27 |
| KI67 | ||
| < 14% | 245 | 30.32 |
| ≥ 14% | 563 | 69.68 |
| Chemotherapy cycles | ||
| ≤ 4 | 753 | 93.19 |
| > 4 | 55 | 6.81 |
| cT category | ||
| T1 | 76 | 9.41 |
| T2 | 565 | 69.92 |
| T3 | 167 | 20.67 |
| cN category | ||
| N0 | 345 | 42.70 |
| N1 | 336 | 41.58 |
| N2 | 127 | 15.72 |
| Response | ||
| Responder | 575 | 71.16 |
| Non-responder | 233 | 28.84 |
| NLR | 2.28 ± 1.03 | |
| PLR | 128.75 ± 48.53 | |
| LMR | 6.05 ± 5.93 | |
| NMR | 12.50 ± 10.68 |
Abbreviations: NAC neoadjuvant chemotherapy, HER2 human epidermal growth factor receptor 2, ER estrogen receptor, PR progesterone receptor, NLR neutrophil to lymphocyte ratio, PLR platelet to lymphocyte ratio, LMR lymphocyte to monocyte ratio, NMR neutrophil to monocyte ratio
Table 2.
Compared with normal group and patient group in inflammatory biomarkers.
| Mean (SD) |
|||
|---|---|---|---|
| Breast Cancer Group |
Control Group |
||
| Characteristics | (n = 808) | (n = 2424) | P value |
| Age, y | 49.45 (9.27) | 49.43 (9.29) | 0.939 |
| NLR | 2.28 (1.03) | 2.04 (0.83) | 0.000 |
| PLR | 128.76 (48.53) | 128.96 (44.2) | 0.914 |
| LMR | 6.05 (5.93) | 6.28 (3.06) | 0.306 |
| NMR | 12.50 (10.68) | 11.88 (5.55) | 0.119 |
Abbreviations: SD standard deviation, NLR neutrophil to lymphocyte ratio, PLR platelet to lymphocyte ratio, LMR lymphocyte to monocyte ratio, NMR neutrophil to monocyte ratio
Optimal cutoff values of inflammatory biomarkers
Receiver operating characteristics (ROC) curves were used to calculate the optimal cutoff values for the inflammatory biomarkers. Our results indicated that the cutoff values of NLR, PLR, LMR and NMR were 3.0 (P = .420, 95% CI 0.473–0.563), 151.3 (P = .103, 95% CI 0.492–0.581), 6.1 (P = .003, 95% CI 0.521–0.611) and 9.7 (P = .537, 95% CI 0.492–0.583), respectively (Figure 2). Then, the patients were divided into groups based on their low or high ratios (NLR < 3.0 and ≥ 3.0; PLR < 151.3 and ≥ 151.3; LMR < 6.1 and ≥ 6.1; NMR < 9.7 and ≥ 9.7) as shown in Table 3.
Figure 2.
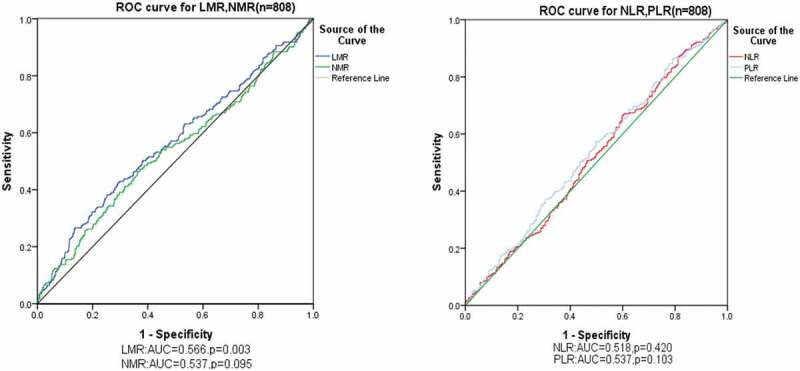
ROC curve analysis for the predictive roles of inflammatory biomarkers for the response to neoadjuvant chemotherapy in breast cancer. The cutoff values of NLR, PLR, LMR and NMR were 3.0 (P = .420, 95% CI 0.473–0.563), 151.3 (P = .103, 95% CI 0.492–0.581), 6.1 (P = .003, 95% CI 0.521–0.611) and 9.7 (P = .537, 95% CI 0.492–0.583), respectively.
Abbreviations: ROC receiver operating characteristics, NLR neutrophil to lymphocyte ratio, PLR platelet to lymphocyte ratio, LMR lymphocyte to monocyte ratio, NMR neutrophil to monocyte ratio, AUC area under curve
Table 3.
Associations of clinicopathological features with NLR, PLR LMR and NMR in breast cancer.
| NLR |
PLR |
LMR |
NMR |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| variable | < 3 | ≥ 3 | P | < 151.3 | ≥ 151.3 | P | < 6.1 | ≥ 6.1 | P | < 9.7 | ≥ 9.7 | P |
| Age(y) | 0.001 | 0.018 | 0.003 | 0.407 | ||||||||
| ≥ 50 | 335 (49.9) | 47 (34.6) | 306 (49.6) | 76 (39.8) | 239 (43.7) | 143 (54.8) | 158 (49.1) | 224 (46.1) | ||||
| < 50 | 337 (50.1) | 89 (65.4) | 311 (50.4) | 115 (60.2) | 308 (56.3) | 118 (45.2) | 164 (50.9) | 262 (53.9) | ||||
| Menopausal Status | 0.001 | 0.005 | 0.004 | 0.192 | ||||||||
| Postmenopausal | 283 (42.1) | 36 (26.5) | 260 (42.1) | 59 (30.9) | 197 (36.0) | 122 (46.7) | 136 (42.2) | 183 (37.7) | ||||
| Premenopausal | 389 (57.9) | 100 (73.5) | 357 (57.9) | 132 (69.1) | 350 (64.0) | 139 (53.3) | 186 (57.8) | 303 (62.3) | ||||
| Subtypes of Cancer | 0.622 | 0.816 | 0.110 | 0.054 | ||||||||
| Ductal | 635 (94.5) | 126 (92.6) | 581 (94.2) | 180 (94.2) | 516 (94.3) | 245 (93.9) | 296 (91.9) | 465 (95.7) | ||||
| Lobular | 10 (1.5) | 2 (1.5) | 10 (1.6) | 2 (1.0) | 5 (0.9) | 7 (2.7) | 8 (2.5) | 4 (0.8) | ||||
| Others | 27 (4.0) | 8 (5.9) | 26 (4.2) | 9 (4.7) | 26 (4.8) | 9 (3.4) | 18 (5.6) | 17 (3.5) | ||||
| Molecular subtype | 0.574 | 0.152 | 0.098 | 0.023 | ||||||||
| Luminal | 355 (52.8) | 70 (51.5) | 331 (53.6) | 94 (49.2) | 286 (52.3) | 139 (53.3) | 171 (53.1) | 254 (52.3) | ||||
| HER2 | 154 (22.9) | 25 (18.4) | 132 (21.4) | 47 (24.6) | 131 (23.9) | 48 (18.4) | 86 (26.7) | 93 (19.1) | ||||
| Triple negative | 96 (14.3) | 25 (18.4) | 85 (13.8) | 36 (18.8) | 82 (15.0) | 39 (14.9) | 41 (12.7) | 80 (16.5) | ||||
| Unknown | 67 (10.0) | 16 (11.8) | 69 (11.2) | 14 (7.3) | 48 (8.8) | 35 (13.4) | 24 (7.5) | 59 (12.1) | ||||
| ER status | 0.527 | 0.059 | 0.284 | 0.422 | ||||||||
| Negative | 267 (39.7) | 58 (42.6) | 237 (38.4) | 88 (46.1) | 227 (41.5) | 98 (37.5) | 135 (41.9) | 190 (39.1) | ||||
| Positive | 405 (60.3) | 78 (57.4) | 380 (61.6) | 103 (53.9) | 320 (58.5) | 163 (62.5) | 187 (58.1) | 296 (60.9) | ||||
| PR status | 0.881 | 0.407 | 0.475 | 0.595 | ||||||||
| Negative | 364 (54.2) | 75 (55.1) | 328 (53.2) | 111 (58.1) | 304 (55.6) | 135 (51.7) | 179 (55.6) | 260 (53.5) | ||||
| Positive | 308 (45.8) | 61 (44.9) | 289 (46.8) | 80 (41.9) | 243 (44.4) | 126 (48.3) | 143 (44.4) | 226 (46.5) | ||||
| Her2 status | 0.641 | 0.240 | 0.077 | 0.017 | ||||||||
| Negative | 352 (52.4) | 74 (54.4) | 326 (52.8) | 100 (11.2) | 287 (52.5) | 139 (53.3) | 163 (50.6) | 263 (54.1) | ||||
| Positive | 253 (37.6) | 46 (33.8) | 222 (36.0) | 77 (87.2) | 212 (38.8) | 87 (33.3) | 135 (41.9) | 164 (33.7) | ||||
| Unknown | 67 (10.0) | 16 (11.8) | 69 (11.2) | 14 (1.6) | 48 (8.8) | 35 (13.4) | 24 (7.5) | 59 (12.1) | ||||
| KI67 | 0.876 | 0.845 | 0.147 | 0.132 | ||||||||
| < 14% | 203 (30.2) | 42 (30.9) | 186 (30.1) | 59 (30.9) | 157 (28.7) | 88 (33.7) | 88 (27.3) | 157 (32.3) | ||||
| ≥ 14% | 469 (69.8) | 94 (69.1) | 431 (69.9) | 132 (69.1) | 390 (71.3) | 173 (66.3) | 234 (72.7) | 329 (67.7) | ||||
| Chemotherapy cycles | 0.077 | 0.511 | 0.598 | 0.161 | ||||||||
| ≤ 4 | 631 (93.9) | 122 (89.7) | 577 (93.5) | 176 (92.1) | 508 (92.9) | 245 (93.9) | 305 (94.7) | 448 (92.2) | ||||
| > 4 | 41 (6.1) | 14 (10.3) | 40 (6.5) | 15 (7.9) | 39 (7.1) | 16 (6.1) | 17 (5.3) | 38 (7.8) | ||||
| cT category | 0.174 | 0.034 | 0.017 | 0.893 | ||||||||
| T1 | 63 (9.4) | 13 (9.6) | 61 (9.9) | 15 (7.9) | 44 (8.0) | 32 (12.3) | 30 (9.3) | 46 (9.5) | ||||
| T2 | 478 (71.1) | 87 (64.0) | 441 (71.5) | 124 (64.9) | 377 (68.9) | 188 (72.0) | 228 (70.8) | 337 (69.3) | ||||
| T3 | 131 (19.5) | 36 (26.5) | 115 (18.6) | 52 (27.2) | 126 (23.0) | 41 (15.7) | 64 (19.9) | 103 (21.2) | ||||
| cN category | 0.443 | 0.601 | 0.024 | 0.509 | ||||||||
| N0 | 293 (43.6) | 52 (38.2) | 263 (42.6) | 82 (42.9) | 230 (42.0) | 115 (44.1) | 144 (44.7) | 201 (41.4) | ||||
| N1 | 277 (41.2) | 59 (43.4) | 261 (42.3) | 75 (39.3) | 218 (39.9) | 118 (45.2) | 126 (39.1) | 210 (43.2) | ||||
| N2 | 102 (15.2) | 25 (18.4) | 93 (15.1) | 34 (17.8) | 99 (18.1) | 28 (10.7) | 52 (16.1) | 75 (15.4) | ||||
| Response to NAC | 0.800 | 0.704 | 0.001 | 0.541 | ||||||||
| Non-responder (cSD and cPD) | 195 (29.0) | 38 (27.9) | 180 (29.2) | 53 (27.7) | 138 (25.2) | 95 (36.5) | 89 (27.6) | 144 (29.6) | ||||
| Responder (pCR and cPR) | 477 (71.0) | 98 (72.1) | 437 (70.8) | 138 (72.3) | 410 (74.8) | 165 (63.5) | 233 (72.4) | 342 (70.4) | ||||
Abbreviations: HER2 human epidermal growth factor receptor 2, ER estrogen receptor, PR progesterone receptor, NLR neutrophil to lymphocyte ratio, PLR platelet to lymphocyte ratio, LMR lymphocyte to monocyte ratio, NMR neutrophil to monocyte ratio, NAC neoadjuvant chemotherapy, cSD clinical stable diseases, cPD clinical progress diseases, pCR pathological complete remission, cPR clinical partial remission
Associations of clinicopathological features with inflammatory biomarkers
The chi-square (χ2) test was used to assess the relationship between inflammatory biomarkers and clinicopathological characteristics in breast cancer patients (Table 3). The results indicated that the NLR was significantly associated with age (P = .001) and menopausal status (P = .001). The PLR was significantly associated with age (P = .018), menopausal status (P = .005) and cT category (P = .034). The LMR was significantly associated with age (P = .003), menopausal status (P = .004), cT category (P = .017), cN category (P = .024) and response to NAC (P = .001). NMR was significantly associated with molecular subtype (P = .023) and HER2 status (P = .017).
Predictive ability for efficacy of neoadjuvant chemotherapy of inflammatory biomarkers
According to univariate analysis, our results indicated that NLR, PLR and NMR were not significantly associated with the response to NAC treatment (all P > .05, Table 4). However, univariate and multivariate analyzes indicated that LMR was the only independent predictive factor among the four biomarkers for the efficacy of neoadjuvant chemotherapy (OR = 1.771, 95% CI = 1.273–2.464, P = .001) (Table 4). Moreover, Table 4 demonstrates that age (OR = 1.379, 95% CI = 1.005–1.891, P = .046), Ki67 (OR = 1.683, 95% CI = 1.207–2.345, P = .002), chemotherapy cycles (OR = 1.821, 95% CI = 1.013–3,273, P = .045), and cN category (P < .000) were independently correlated with the response to NAC.
Table 4.
Univariate and multivariate effective of NAC in patients with breast cancer.
| Variable |
response |
|||
|---|---|---|---|---|
| Univariate HR (95% CI) |
P | Multivariate HR (95% CI) |
P | |
| Age (y) | 0.014 | 0.046 | ||
| ≥ 50 | 1 | 1 | ||
| < 50 | 1.467 (1.081–1.992) | 1.379 (1.005–1.891) | ||
| Menopausal Status | 0.017 | |||
| Postmenopausal | 1 | |||
| Premenopausal | 1.454 (1.068–1.979) | |||
| Subtypes of Cancer | ||||
| Others | 1 | 0.329 | ||
| Ductal | 1.501 (0.743–3.033) | 0.258 | ||
| Lobular | 0.827 (0.217–3.149) | 0.781 | ||
| Molecular subtype | ||||
| Unknown | 1 | 0.051 | ||
| LuminalA | 0.540 (0.292–1.001) | 0.050 | ||
| LuminalB | 1.072 (0.624–1.843) | 0.800 | ||
| HER2 | 1.017 (0.568–1.820) | 0.955 | ||
| Triple negative | 1.022 (.0547–1.910) | 0.945 | ||
| ER status | 0.556 | |||
| Positive | 1 | |||
| Negative | 1.098 (0.804–1.500) | |||
| PR status | 0.304 | |||
| Positive | 1 | |||
| Negative | 1.173 (0.865–1.592) | |||
| Her2 status | ||||
| Unknown | 1 | 0.151 | ||
| Negative | 0.826 (0.490–1.393) | 0.474 | ||
| Positive | 1.145 (0.662–1.979) | 0.628 | ||
| KI67 | 0.000 | 0.002 | ||
| < 14% | 1 | 1 | ||
| ≥ 14% | 1.800 (1.305–2.481) | 1.683 (1.207–2.345) | ||
| Chemotherapy cycles | 0.116 | 0.045 | ||
| > 4 | 1 | 1 | ||
| ≤ 4 | 1,576 (0.894–2.778) | 1.821 (1.013–3.273) | ||
| cT category | ||||
| cT1 | 0.905 (0.496–1.653) | 0.746 | ||
| cT2 | 0.887 (0.602–1.305) | 0.542 | ||
| cT3 | 1 | 0.830 | ||
| cN category | 0.000 | |||
| cN0 | 2.416 (1.560–3.741) | 0.000 | 2.545 (1.621–3.995) | |
| cN1 | 1.436 (0.941–2.191) | 0.093 | 1.596 (1.015–2.426) | |
| cN2 | 1 | 0.000 | 1 | |
| NLR | 0.800 | |||
| ≥ 3 | 1 | |||
| < 3 | 1.054 (0.700–1.589) | |||
| PLR | 0.704 | |||
| ≥ 151.2 | 1 | |||
| < 151.2 | 1.072 (0.747–1.539) | |||
| LMR | 0.001 | 0.001 | ||
| ≥ 6.1 | 1 | 1 | ||
| < 6.1 | 1.741 (1.268–2.392) | 1.771 (1.273–2.464) | ||
| NMR | 0.541 | |||
| ≥ 9.7 | 1 | |||
| < 9.7 | 0.907 (0.664–1.240) | |||
Abbreviations: CI confidence interval, HR hazard ratio, HER2 human epidermal growth factor receptor 2, ER estrogen receptor, PR progesterone receptor, NLR neutrophil to lymphocyte ratio, PLR platelet to lymphocyte ratio, LMR lymphocyte to monocyte ratio, NMR neutrophil to monocyte ratio, NAC neoadjuvant chemotherapy
Discussion
NAC is being increasingly used to treat locally advanced breast cancer patients and operable breast cancer patients to reduce the size of the tumor and increase the likelihood of eligibility for breast-conserving surgery.17 However, readily available and reliable biomarkers for predicting the response to this treatment are currently not available. Features of the inflammatory biomarkers, such as the NLR, PLR, LMR and NMR, have been frequently related to patient prognosis and clinical outcome.14–16,18 However, controversy exists regarding the best indicator to predict the efficacy of neoadjuvant chemotherapy in breast cancer. Therefore, we performed a large-scale cohort study to evaluate the predictive value of NLR, PLR, LMR and NMR for the efficacy of NAC in breast cancer patients.
First, 2,424 normal individuals were collected from the physical examination centre using a 3:1 ratio of normal individuals to breast cancer patients. Then, we compared the inflammatory biomarkers between these groups to determine whether any differences in these inflammatory biomarkers were observed between the two groups. The results indicated no differences in age, PLR, LMR and NMR between the normal group and the patient group. However, NLR is significantly increased in the patient group compared with the normal group (P < .05). The potential explanation is that neutrophils are a major component of white blood cells and induce a variety of pro-cancer factors, including vascular endothelial growth factor (VEGF), neutrophil elastase and matrix metalloprotein 9 (MMP9), which promote angiogenesis and tumor development.19,20 Lymphocytes are an important part of the host immune system and are essential for the elimination of cancer cells, and lymphocyte infiltration of tumors is considered to be an anticancer immune response associated with improved survival.21 Therefore, the NLR may represent a balance between anticancer immune function and a pro-cancer inflammatory reaction.22
In our study, a relationship between the NLR and the response to NAC was not observed. Our data were consistent with previous findings suggesting no relationship between pCR and the NLR value prior to treatment.23,24 However, a growing body of evidence suggests that the NLR is superior to other inflammatory biomarkers as a prognostic biomarker in several different cancers, including breast cancer.9,22,25 In addition, J. Xu et al.16 indicated that the patients with low pretreatment NLR exhibited better treatment response to NAC. Potential reasons for this discrepancy are that the systemic blood count is unlikely to be reflected by local lymphocytic infiltration at the primary tumor site. Inflammation plays a key role in cardiovascular disease,26 which is an independent marker of mortality in patients with bacteremia.27 Therefore, inflammatory cells may be affected by systemic factors. The same reason may partially explain the lack of a significant difference among the PLR, NMR and the response to NAC in our study.
The LMR is associated with multiple cancers, and high ratios have been connected with a better prognosis in breast cancer patients treated with NAC.25,28 However, fewer studies have reported the association of the LMR and tumor response with chemotherapy in breast cancer patients. Hernández CM25 reported that a high LMR (≥ 5.46) was associated with a lower percentage of relapse (P = .048). However, a low LMR (< 5.46) was associated with a better response to neoadjuvant chemotherapy although without statistical significance (P = .067). We found that a low LMR (<6.1) was an independent predictive factor for the efficacy of NAC (OR = 1.771, 95% CI = 1.273–2.464, P = .001). Unfortunately, our patient follow-up has not been completed as of this writing. Accordingly, we are not able to calculate the relationship among these inflammatory biomarkers, disease-free survival (DFS), and overall survival (OS). Therefore, there is limited evidence to clarify the mechanism to explain why a lower LMR represents a worse prognosis in other studies but represents improved chemosensitivity in our study. The immune response of host lymphocytes and monocytes may serve as a possible explanation. T lymphocytes may cause cancer cell death by presenting tumor-associated antigens on immune cells.29,30 Low lymphocyte counts can lead to an inadequate immune response, which results in low survival in multiple cancers.11,12 Additionally, monocytes and macrophages release cytokines and free radicals that are associated with angiogenesis, tumor cell invasion, and metastasis and are related to a worse prognosis.31 Hence, a low LMR may represent better chemosensitivity. The advantage of this index is its ability to combine information from lymphocytes and monocytes and reveal the inflammatory response status in an all-inclusive manner.
In addition, we demonstrated that the LMR value of 6.1 was the optimal cutoff value to predict the response to NAC in breast cancer patients. To the best of our knowledge, there are few studies investigating an appropriate LMR cutoff value to predicate chemosensitivity for breast cancer. Among different breast cancer subtypes, inflammatory cells related to tumors are highly heterogeneous.32,33 LMR cutoff values have been reported by several previous studies; however, different studies used different cutoff values and different methods to calculate these values. Therefore, it is necessary to define a cutoff value for the LMR applied exclusively to predict chemosensitivity.
In conclusion, the findings of this study support the possibility of using the LMR as a predictive factor, and the NLR is potentially increased in breast cancer patients compared with normal individuals. However, this was a retrospective study using a small number of patients from only one centre, and our data were inconsistent with previous findings in breast cancer. Further prospective and multicenter studies are needed to clarify the relationship between the inflammatory biomarkers and the curative effects of neoadjuvant chemotherapy as well as its role in breast cancer prognosis.
Materials and methods
Patients and treatments
842 invasive breast cancer patients who received chemotherapy have been collected at the department of Endocrine Breast Surgery in The First Affiliated Hospital of Chongqing Medical University between January 2013 and July 2017. However, 29 patients who have distant metastasis (stage IV and TNM system) and 1 patient who did not undergo surgery and 3 patients who had bilateral breast cancer and 1 male breast cancer had been excluded. Finally, 808 patients were eligible for analysis (Figure 1). All clinical data, including age, menopausal status, subtypes of cancer, cT category, cN category, cycles of NAC, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) receptor status, Ki67 status and molecular subtype were obtained from the medical records. All of them received at least 3 but up to 8 cycles of treatment with the TEC regimen: cyclophosphamide (500 mg/m2), epirubicin (75 mg/m2), and docetaxel (75 mg/m2) every 21 days. In our study, Herceptin was not used in patients with Her-2 positive status before operation. 2,424 normal individuals were collected from the physical examination centre using a 3:1 ratio of normal individuals to breast cancer patients.
Figure 1.
842 breast cancer patients were collected and the exclusion criteria. Patients with distant metastasis (stage IV of the TNM system) (29) and those who did not undergo sugery (1) or who had bilateral breast cancer (3) or male breast cancer (1) were excluded.
Blood sample analysis
Peripheral blood was collected when a breast cancer diagnosis was made and before NAC. The number of lymphocytes, platelets, monocytes and neutrophils was determined using a hemocytometer. NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. PLR was calculated by dividing the absolute platelet count by the absolute lymphocyte count. LMR was calculated by dividing the absolute lymphocyte count by the absolute monocyte count. NMR was calculated by dividing the absolute neutrophil count by the absolute monocyte count.
Immunohistochemical staining and scoring
All breast cancer specimens were confirmed by core needle biopsy and tested using immunohistochemistry to determine the tumor type. According to the 2011 St. Gallen consensus,34 the cutoffs for ER positivity and PR positivity were both > 0% positive tumor cells with nuclear staining, and the HER2 status was considered to be positive if more greater 10% of the tumor cells exhibited a 3+ score by IHC or a > 2.2-fold increase in fluorescence in situ hybridization (FISH). Regarding Ki67, between 400 and 500 cells were counted to calculate the percentage of positive tumor cell nuclei, including hot spots, and 14% was defined as the optimal cutoff value. The patients were classified according to the following subtypes: luminal, expressed estrogen receptors (ER) and/or progesterone receptors (PR); HER-2, HER-2 receptor overexpression; and triple negative, negative for ER, PR and HER-2. For luminal tumors, Ki67 levels were analyzed and classified as luminal A or B if the level was greater than or less than 14%, respectively.
Evaluation of treatment efficacy
NAC efficacy was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines version 1.135 based on the clinical responses using computed tomography (CT), ultrasonography (US) and magnetic resonance imaging (MRI). Briefly, cPR (clinical partial remission) indicated that the longest diameter of the tumor lesion decreased by 30% or more, cPD (clinical progress diseases) indicated that the longest tumor diameter increased by 20% or more, cSD (clinical stable diseases) indicated that the longest tumor diameter was decreased by less than 30% but increased by less than 20%, pCR (pathological complete remission) indicated that no residual tumor lesion was present in any breast tissues or lymph node. Patients were also classified into the responder group (pCR and cPR) and the nonresponder (cSD and cPD) group.
Statistical methods
Data analyses were conducted using SPSS (version 25.0) software (SPSS Inc., Chicago, IL, USA). Continuous variables are represented as means with standard deviations. Receiver operating characteristic (ROC) curves were used to calculate optimal cutoff values for the inflammatory biomarkers (Figure 2). Frequency distributions of categorical variables between discrimination of inflammatory biomarkers were compared using chi-square tests. The relationship between histologic response to chemotherapy and variables were performed using univariate analyses. Significant factors for histologic response were included in the multivariate analyses using the logistic regression model with a forward LR method. Statistical significance was defined as P-values <0.05.
Funding Statement
This work was supported by the National Natural Science Foundation of China under Grant number [81272265]; the National Natural Science Foundation of China under Grant number [81472658].
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC) under Grant number 81272265 and 81472658. The contributions of all authors are as follows: Conceptualization: Yang Peng, Shengchun Liu; Investigation: Yang Peng, Rui Chen, Fanli Qu, Ying Ye, Yong Fu; Data collect: Zhenrong Tang, Yihua Wang, Beige Zong, Haochen Yu; Writing - original draft: Yang Peng; Writing - review & editing: Yang Peng., Rui Chen Shengchun Liu; Funding acquisition: Feng Luo, Shengchun Liu.
Disclosure of potential conflicts of interest
The authors declare that they have no conflicts of interest. All authors agreed the final version of the manuscript for publication.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A.. 2016. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Mayer EL, Carey LA, Burstein JH.. Commentary Clinical trial update: implications and management of residual disease after neoadjuvant therapy for breast cancer. Breast Cancer Res. 2007;9:110. doi: 10.1186/bcr1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Provenzano E, Vallier AL, Champ R, Walland K, Bowden S, Grier A, Fenwick N, Abraham J, Iddawela M, Caldas C, et al. A central review of histopathology reports after breast cancer neoadjuvant chemotherapy in the neo-tango trial. Br J Cancer. 2013;108:866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marinovich ML, Sardanelli F, Ciatto S, Mamounas E, Brennan M, Macaskill P, Irwig L, von Minckwitz G, Houssami N. Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast. 2012;21:669–677. doi: 10.1016/j.breast.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Coudert B, Pierga JY, Mouret-Reynier MA, Kerrou K, Ferrero JM, Petit T, Kerbrat P, Dupré P-F, Bachelot T, Gabelle P, et al. Use of [(18)F]-FDG PET to predict response to neoadjuvant trastuzumab and docetaxel in patients with HER2-positive breast cancer, and addition of bevacizumab to neoadjuvant trastuzumab and docetaxel in [(18)F]-FDG PET-predicted non-responders (AVATAXHER). Lancet Oncol. 2014;15:1493–1502. doi: 10.1016/S1470-2045(14)70475-9. [DOI] [PubMed] [Google Scholar]
- 6.Fuksa L, Micuda S, Grim J, Ryska A, Hornychova H. Predictive biomarkers in breast cancer: their value in neoadjuvant chemotherapy. Cancer Invest. 2012;30:663. doi: 10.3109/07357907.2012.725441. [DOI] [PubMed] [Google Scholar]
- 7.Proctor MJ, Horgan PG, Talwar D, Fletcher CD, Morrison DS, Mcmillan DC. Optimization of the systemic inflammation-based glasgow prognostic score: a glasgow inflammation outcome study. Cancer. 2013;119:2325–2332. doi: 10.1002/cncr.28018. [DOI] [PubMed] [Google Scholar]
- 8.Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Song Y, Yang Y, Gao P, Chen X, Yu D, Xu Y, Zhao J, Wang Z. The preoperative neutrophil to lymphocyte ratio is a superior indicator of prognosis compared with other inflammatory biomarkers in resectable colorectal cancer. BMC Cancer. 2017;17:744. doi: 10.1186/s12885-017-3752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roxburgh CS, Mcmillan DC. Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer. 2014;110:1409–1412. doi: 10.1038/bjc.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Väyrynen JP, Tuomisto A, Klintrup K, Mäkelä J, Karttunen TJ, Mäkinen MJ. Detailed analysis of inflammatory cell infiltration in colorectal cancer. Br J Cancer. 2013;109:1839–1847. doi: 10.1038/bjc.2013.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van EF, Rouas G, Francis P, Crown JPA, Hitre E, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 13.Noh H, Eomm M, Han A. 2013. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer. 16:55–59. doi: 10.4048/jbc.2013.16.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pistelli M, De Lisa M, Ballatore Z, Caramanti M, Pagliacci A, Battelli N, Ridolfi F, Santoni M, Maccaroni E, Bracci R, et al. 2015. Pre-treatment neutrophil to lymphocyte ratio may be a useful tool in predicting survival in early triple negative breast cancer patients. BMC Cancer. 15:195. doi: 10.1186/s12885-015-1204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marin Hernandez C, Pinero Madrona A, Gil Vazquez PJ, Galindo Fernandez PJ, Ruiz Merino G, Alonso Romero JLParicio PP.. 2017. Usefulness of lymphocyte-to-monocyte, neutrophil-to-monocyte and neutrophil-to-lymphocyte ratios as prognostic markers in breast cancer patients treated with neoadjuvant chemotherapy. Clin Transl Oncol. doi: 10.1007/s12094-017-1732-0. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Ni C, Ma C, Zhang L, Jing X, Li C, Liu Y, Qu X. 2017. Association of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio with ER and PR in breast cancer patients and their changes after neoadjuvant chemotherapy. Clin Transl Oncol. 19:989–996. doi: 10.1007/s12094-017-1630-5. [DOI] [PubMed] [Google Scholar]
- 17.Mamounas EP, Fisher B. Preoperative (neoadjuvant) chemotherapy in patients with breast cancer. Semin Oncol. 2001;28:389–399. doi: 10.1016/S0093-7754(01)90132-0. [DOI] [PubMed] [Google Scholar]
- 18.Yi C, Kai C, Xiao X, Yan N, Qu S, Chang G, Su F, Song E, et al. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer. 2016;16:320. doi: 10.1186/s12885-016-2352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell DR, Huttenlocher A. Neutrophils in the Tumor Microenvironment. Trends Immunol. 2016;37:41–52. doi: 10.1016/j.it.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013;23:141–148. doi: 10.1016/j.semcancer.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 22.Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng J-W, Liu Z-W, Ma L, et al. Prognostic role of neutrophil‐to‐lymphocyte ratio in colorectal cancer: A systematic review and meta‐analysis. Int J Cancer. 2014;134:2403–2413. doi: 10.1002/ijc.v134.10. [DOI] [PubMed] [Google Scholar]
- 23.Eryilmaz MK, Mutlu H, Salim DK, Musri FY, Tural D, Coskun HS. The neutrophil to lymphocyte ratio has a high negative predictive value for pathologic complete response in locally advanced breast cancer patients receiving neoadjuvant chemotherapy. Asian Pac J Cancer Prev. 2014;15:7737–7740. doi: 10.7314/APJCP.2014.15.18.7737. [DOI] [PubMed] [Google Scholar]
- 24.Adachi K, Sakurai K, Suzuki S, Hara Y, Nagashima S, Hirano T, Enomoto K, Amano S. Study of the response rate and neutrophil lymphocyte ratio in breast cancer patients undergoing neoadjuvant chemotherapy. Gan to Kagaku Ryoho Cancer Chemother. 2015;42:1283–1285. [PubMed] [Google Scholar]
- 25.Hernández CM, Madrona AP, Vázquez PJG, Fernández PJG, Merino GR, Romero JLA, Paricio PP.. Usefulness of lymphocyte-to-monocyte, neutrophil-to-monocyte and neutrophil-to-lymphocyte ratios as prognostic markers in breast cancer patients treated with neoadjuvant chemotherapy. Clin Transl Oncol. 2017;20:1–8. [DOI] [PubMed] [Google Scholar]
- 26.Tsai JC, Sheu SH, Chiu HC, Chung FM, Chang DM, Chen MP, Shin S-J, Lee Y-J. Association of peripheral total and differential leukocyte counts with metabolic syndrome and risk of ischemic cardiovascular diseases in patients with type 2 diabetes mellitus. Diabetes/metab Res Rev. 2007;23:111–118. doi: 10.1002/(ISSN)1520-7560. [DOI] [PubMed] [Google Scholar]
- 27.Terradas R, Grau S, Blanch J, Riu M, Saballs P, Castells X, Horcajada JP, Knobel H. Eosinophil count and neutrophil-lymphocyte count ratio as prognostic markers in patients with bacteremia: a retrospective cohort study. PLoS One. 2012;7:e42860. doi: 10.1371/journal.pone.0042860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang GM, Zhu Y, Luo L, Wan FN, Zhu YP, Sun LJ, Ye D-W. Preoperative lymphocyte-monocyte and platelet-lymphocyte ratios as predictors of overall survival in patients with bladder cancer undergoing radical cystectomy. Tumor Biol. 2015;36:1–7. doi: 10.1007/s13277-015-3613-x. [DOI] [PubMed] [Google Scholar]
- 29.Azab B, Shah N, Radbel J, Tan P, Bhatt V, Vonfrolio S, Habeshy A, Picon A, Bloom S. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol. 2013;30:432. doi: 10.1007/s12032-012-0432-4. [DOI] [PubMed] [Google Scholar]
- 30.Dworacki G, Meidenbauer N, Kuss I, Hoffmann TK, Gooding W, Lotze M, Whiteside TL.. Decreased ζ chain expression and apoptosis in CD3+ peripheral blood T lymphocytes of patients with melanoma. Clin Cancer Res. 2001;7:947s–57s. [PubMed] [Google Scholar]
- 31.Tang X. 2013. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett. 332:3–10. doi: 10.1016/j.canlet.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Bianchini G, Qi Y, Alvarez RH, Iwamoto T, Coutant C, Ibrahim NK, Valero V, Cristofanilli M, Green MC, Radvanyi L, et al. Molecular anatomy of breast cancer stroma and its prognostic value in estrogen receptor-positive and -negative cancers. J Clin Oncol. 2010;28:4316–4323. doi: 10.1200/JCO.2009.27.2419. [DOI] [PubMed] [Google Scholar]
- 33.Siker K, Anikó K, Anna D, Pär-Ola B, Kristina L, Frostvik SM, Tobin NP, Lindström L, Bergh J, Einbeigi Z, et al. Contrasting breast cancer molecular subtypes across serial tumor progression stages: biological and prognostic implications. Oncotarget. 2015;6:33306–33318. doi: 10.18632/oncotarget.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, 2011. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dede DS, Gumuskaya B, Guler G, Onat D, Altundag K, Ozisik Y. Evaluation of changes in biologic markers ER, PR, HER 2 and Ki-67 index in breast cancer with administration of neoadjuvant dose dense doxorubicin, cyclophosphamide followed by paclitaxel chemotherapy. J Buon. 2013;18:366. [PubMed] [Google Scholar]


