ABSTRACT
Introduction: RTS,S/AS01 is currently the most advanced malaria vaccine but provides incomplete, short-term protection. It was developed for use within the expanded program on immunizations (EPI) for African children. Another use could be adding mass RTS,S/AS01 vaccination to the integrated malaria elimination strategy in the Greater Mekong Subregion (GMS), where multidrug-resistant P.falciparum strains have emerged and spread. Prior to evaluating RTS,S/AS01 in large-scale trials we assessed whether the vaccine, administered with and without antimalarial drugs, is safe and immunogenic in Asian populations.
Methods: An open-label, randomized, controlled phase 2 trial was conducted in healthy, adult Thai volunteers. Seven vaccine regimens with and without antimalarial drugs (dihydroartemisinin-piperaquine plus a single low dose primaquine) were assessed. Antibody titres against the PfCSP full-length (NANP) 6, PfCSP anti-C–term, PfCSP full-length (N + C-Terminal) were measured by standard enzyme-linked immunosorbent assays. Liquid chromatography was used to measure piperaquine, primaquine and carboxy-primaquine concentrations.
Results: 193 volunteers were enrolled and 186 study participants completed the 6 months follow-up period. One month after the last vaccination all study participants had seroconverted to the PfCSP (NANP)6, and the PfCSP Full Length (N + C-Terminal). More than 90% had seroconverted to the Pfanti-C-Term CSP. There was no indication that drug concentrations were influenced by vaccine regimens or the antibody levels by the drug regimens. Adverse events were similarly distributed between the seven treatment groups. No serious adverse events attributable to the study interventions were detected.
Conclusion: This study found that RTS,S/AS01 with and without dihydroartemisinin-piperaquine plus a single low dose primaquine was safe and immunogenic in a healthy, adult Asian population.
KEYWORDS: Malaria, vaccine, RTS, S/AS01, P. falciparum, phase 2, ELISA pharmacokinetics, dihydroartemisinin, piperaquine, primaquine
Introduction
The most advanced malaria vaccine, RTS,S/AS01 has been developed to be used in conjunction with the expanded program on immunizations (EPI) in African children. It provides incomplete protection against uncomplicated and severe malaria over a limited time span. Phase 3 evaluation of RTS,S/AS01 showed a vaccine efficacy (VE) against malaria of 55.1% (95% confidence interval [CI], 50.5–59.3%) over 12 months after vaccination when delivered on a 0, 1, and 2-month schedule in children aged 5–17 months at first vaccination.1,2 While this efficacy profile has the potential to provide significant health benefits to vaccinated children it is unlikely, on its own, to make a major impact on malaria transmission when implemented in this context. In the aforementioned Phase 3 trial, the vaccine was more protective in children living in lower transmission settings (presumably more aligned with an elimination setting) than in higher transmission settings, perhaps due to lower forces of infection.3 Furthermore, it has recently been shown that vaccine efficacy against controlled human malaria infection (CHMI) can be improved using alternative immunization schedules.4 These observations have stimulated additional interest in the potential role of RTS,S/AS01 in accelerating P. falciparum parasite elimination in Asia. Mathematical models suggest that adding a temporarily effective vaccine to other basic malaria control measures could interrupt parasite transmission permanently if implemented at scale.5
To accelerate progress towards this ambitious goal, mass drug administrations (MDA) have been piloted in malaria hot-spots in addition to the conventional malaria control strategies; widespread use of long-lasting insecticide-treated bed-nets and early diagnosis and effective treatment.6 As with previous studies, the impact of a recent series of MDA conducted in the GMS with dihydroartemisinin-piperaquine (DP) waned over time.7 The rebound in malaria months after MDA is a predictable consequence of the importation of malarial infections from surrounding areas where no intervention took place and also from residual infections in residents who did not participate in the MDA. A longer lasting or even permanent interruption of transmission could potentially be achieved by combined mass vaccination and drug administration campaigns.5 The emergence and spread of multidrug-resistant P.falciparum strains in the Greater Mekong Subregion (GMS) have increased the urgency for regional elimination of malaria.8,9
While some studies have been conducted in African10 and American adults4,11 the vaccine has not been tested in Asia. As a necessary prelude to the evaluation of RTS,S/AS01 in large-scale trials in the GMS, we assessed in an open-label, randomized, controlled trial whether different dosing regimens of the vaccine, administered with and without the antimalarial drugs used for MDA, are safe and immunogenic in an Asian, adult population.
Results
Two hundred and sixty-seven participants were screened of whom 74 (28%) did not meet the inclusion criteria and were excluded. One hundred and ninety-three volunteers (including 3 replacements) were randomized into one of seven treatment groups (Table 1). Two participants were lost to follow up and one participant withdrew before completing the vaccination schedule (Figure 1).
Table 1.
Treatment groups.
| Group | Vaccine* | Dose Month 0 |
Dose Month 1 |
Dose Month 2 |
Antimalarial‡ | Group name† |
Participants |
|---|---|---|---|---|---|---|---|
| 1 | RTS,S/AS01B | Single standard dose (0.5mL) | Single standard dose (0.5mL) | Fractional dose (0.1mL) | B-SSF | 21 | |
| 2 | RTS,S/AS01E | Double standard dose (1.0mL) | Double standard dose (1.0mL) | Double Fx dose (0.2mL) | EE-SSF | 21 | |
| 3 | RTS,S/AS01E | Single standard dose (0.5mL) | Single standard dose (0.5mL) | Single standard dose (0.5mL) | E-SSS | 30 | |
| 4 | RTS,S/AS01E | Single standard dose (0.5mL) | Single standard dose (0.5mL) | Single standard dose (0.5mL) | DHA-PIP+PQ | E-SSS+D | 30 |
| 5 | RTS,S/AS01E | Single standard dose (0.5mL) | Single standard dose (0.5mL) | Fractional dose (0.1mL) | E-SSF | 30 | |
| 6 | RTS,S/AS01E | Single standard dose (0.5mL) | Single standard dose (0.5mL) | Fractional dose (0.1mL) | DHA-PIP+PQ | E-SSF+D | 30 |
| 7 | RTS,S/AS01E | Single standard dose (0.5mL) | Fractional dose (0.1mL) | DHA-PIP+PQ | E-SF+D | 31 |
* RTS,S/AS01B = adult dose, RTS,S/AS01E = paediatric dose‡DHA-PIP: Dihydroartemisinin-piperaquine; PQ: primaquine † B: RTS,S/AS01B; E: RTS,S/AS01E; EE: double dose of RTS,S/AS01E; S: standard dose; F: fractional dose; +D: DHA-PIP+PQ
Figure 1.
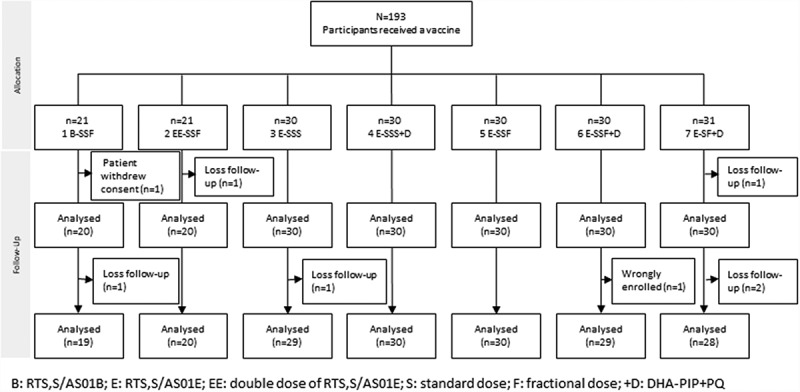
Study participant assembly (Consort chart).
B: RTS,S/AS01B; E: RTS,S/AS01E; EE: double dose of RTS,S/AS01E; S: standard dose; F: fractional dose; +D: DHA-PIP+PQ
Baseline characteristics
The baseline characteristics were well balanced across the groups (Table 2). Forty-five percent of the enrolled participants were male. The median age was 33.8 years, IQR (27.3–40.0) years and the median weight was 61.2 kg (range 53.2–71.2 kg).
Table 2.
Baseline characteristics (M0 day 0 measurements) of the participants by vaccine group.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
|---|---|---|---|---|---|---|---|---|
| Group* | B-SSF | EE-SSF | E-SSS | E-SSS+D | E-SSF | E-SSF+D | E-SF+D | Total |
| n | 20 | 20 | 30 | 30 | 30 | 30 | 30 | 190 |
| Gender male n (%) | 8 (40) | 9 (45) | 14 (46) | 13 (43) | 13 (43) | 14 (46) | 14 (46) | 87 (45) |
| Age (years) median (IQR) | 33.2 (24.8, 41.3) | 30.9 (24.5, 41.9) | 34.1 (28.7, 39.9) | 36.3 (30.7, 42.0) | 37.1 (32.1, 42.8) | 32.4 (29, 39.5) | 29.2 (26.8, 34.5) | 33.8 (27.3, 40.0) |
| Weight (kg) median (IQR) | 57.2 (46.2, 68.5) | 60.9 (54.5, 69.0) | 61.7 (52.5, 70.5) | 61.0 (54.5, 70.3) | 60.0 (53.2, 73.5) | 64.2 (58.1, 75.6) | 59.5 (54.0, 72.3) | 61.2 (53.2, 71.2) |
| Number with fever n | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hb mean (sd) | 13.4 (12.8, 14.5) | 13.4 (13.3, 14.6) | 13.4 (12.7, 14.8) | 14 (12.4, 14.5) | 13.6 (12.2, 14.5) | 13.3 (12.5, 14.7) | 13.1 (12.5, 13.4) | 13.4(12.5, 14.5) |
| WBC median (IQR) | 7.1 (5.9, 7.5) | 7.0 (6.3, 9.1) | 7.0 (6.4, 8.7) | 6.6 (6.0, 7.7) | 7.0 (5.6, 7.4) | 7.0 (6.3, 8.2) | 6.8 (6.0, 8.0) | 6.9 (6.1, 8.0) |
| Platelets median (IQR) | 312 (273, 345) | 297 (263, 329) | 268 (243, 322) | 260 (226, 309) | 284 (233, 354) | 285 (250, 323) | 267 (224, 309) | 280 (243, 323) |
| Creatinine median (IQR) | 0.7 (0.7, 0.9) | 0.9 (0.7, 1.0) | 0.8 (0.7, 1.0) | 0.9 (0.7, 1.0) | 0.8 (0.7, 1.0) | 0.8 (0.7, 1.0) | 0.8 (0.7, 1.0) | 0.8 (0.7, 1.0) |
| AST median (IQR) | 17 (16, 18) | 18 (14.5, 20) | 18 (16, 20) | 23 (19, 28) | 19 (17, 21) | 20 (18, 26) | 21 (19, 28) | 19 (17, 23) |
| ALT median (IQR) | 14 (12, 17) | 14.5 (11, 20) | 14.5 (11, 20) | 18 (13, 26) | 14 (10, 18) | 16 (13, 24) | 20 (16, 34) | 16 (12, 21) |
| Temperature mean (sd) | 36.1 (0.3) | 36.2 (0.5) | 36.1 (0.3) | 36.1 (0.5) | 36.0 (0.4) | 36.0 (0.6) | 36.1 (0.5) | 36.1 (0.4) |
* B: RTS,S/AS01B; E: RTS,S/AS01E; EE: double dose of RTS,S/AS01E; S: standard dose; F: fractional dose; +D: DHA-PIP+PQ
Immunogenicity
One month after the last vaccination all study participants had seroconverted to the PfCSP (NANP)6 repeat region, and the PfCSP full length (N + C-Terminal). More than 90% had seroconverted to the PfCSP C-Term (Supplementary Table S1, S2, S3). Antibody levels against each of the three antigens peaked at month 2 (PfCSP full-length; N + C-Terminal) or month 3 (PfCSP NANP 6 repeat region, PfCSP C – term) and then fell to a lower level by month 6 at which time participants in treatment groups 3 and 4 (3x standard dose with or without antimalarial drugs) had the highest antibody levels and participants in group 7E-SF+D, two doses only, had the lowest antibody levels (Figure 2, Supplementary figures S1, S2). Vaccine regimens which included high vaccine doses (group 1B-SSF and 2EE-SSF) or regimens including fractional third doses (group 1B-SSF, 2EE-SSF, 5E-SSF, 6E-SSF+D) did not result in higher antibody levels than the standard pediatric three-dose regimen. Antibody avidity (PfCSP C-Term and PfCSP full length (N + C-Terminal)) reached over 50% by month 3 in all treatment groups and remained above 50% by month 6 (Figure 3 and Supplementary Figures S3, S4,). Avidity of antibodies against PfCSP (NANP)6 repeat region was below 50% in treatment groups 5E-SSF and 7E-SF+D at month 3 and fell below 40% in groups 3E-SSS and 7E-SF+D by month 6.
Figure 2.
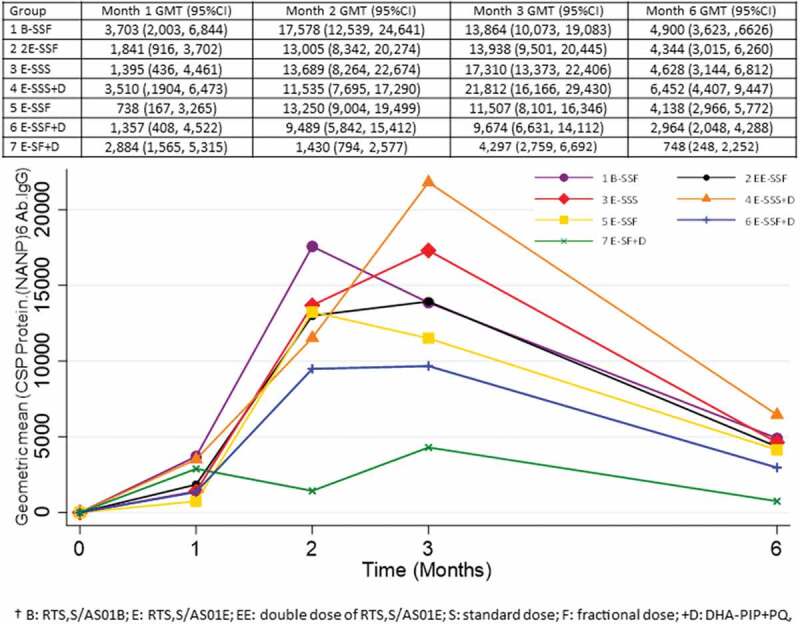
Changes in Pf CSP NANP6 IgG levels over time.
Figure 3.
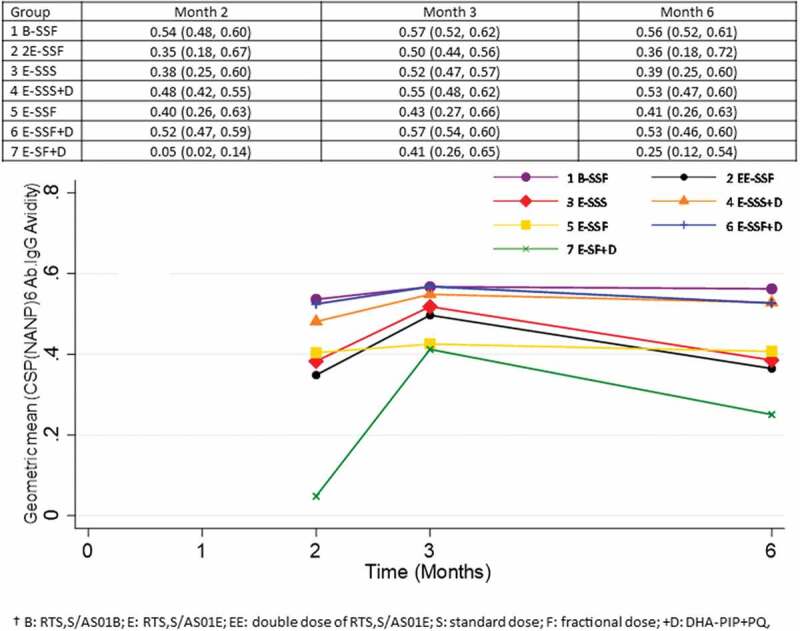
Changes in Pf CSP NANP6 antibody avidity over time (%).
Pharmacokinetics
The median (range) of day 7 piperaquine concentrations during the first month was 32.8 ng/ml (11.4–115 ng/ml). The median trough concentration before the start of the second month of treatment was 6.1 ng/mL which accumulated by 48% to 9.0 ng/mL before the start of the third month of treatment. There were no significant differences between the piperaquine, primaquine or carboxy-primaquine concentrations at each sampling time point in study participants who received the standard or fractional vaccine doses (Figure 4).
Figure 4.
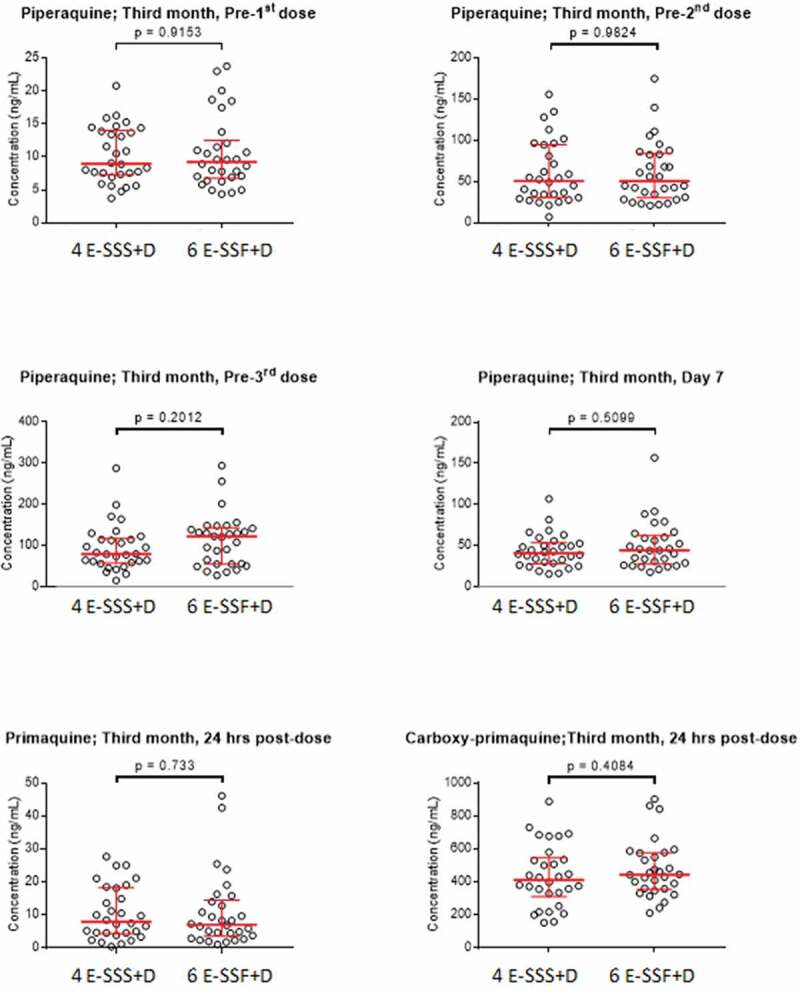
Comparison of concentration measurements when dihydroartemisinin-piperaquine and primaquine were given together with a normal RTS,S/AS01E vaccine dose (4 E-SSS+D) and when given with a fractional dose (6 E-SSF+D).
The population pharmacokinetic model for piperaquine described the data well and suggested a relatively lower bioavailability of piperaquine compared to the prior study, i.e. a scaling factor of 0.327. The vaccine dose (i.e. full and fractional doses) did not have a significant impact on the pharmacokinetic parameters of piperaquine. Simulation-based diagnostics and standard goodness-of-fit plots showed an excellent predictive and descriptive performance of the model (Figure S5 and S6). Final pharmacokinetic parameter estimates are presented in Table S4.
Tolerability and safety
Ninety-nine percent study participants 190/193 reported one or more adverse events following vaccination. Seventy-five percent (1,219/1,630) of the adverse events were mild in character (Figure 5(a)). The most frequent complaint following vaccination was pain around the injection site (24%; 397/1,630) followed by fatigue 282/1630 (17%; Figure 5(c)). Individual study participants reported a median of five adverse events (Figure 5(b)). The number of vaccine-attributable mild, moderate and severe adverse events did not vary significantly between treatment groups (Table S6, S7, S8). The hematology and biochemistry test results stayed within the normal limits except for one study participant who was found to have elevated liver transaminases (ALT 263 U/l, AST 100 U/l) at month 3 which normalized spontaneously by month 6. One participant had thrombocytopenia (84,000/uL) on day 7 following the first round of vaccinations. The participant was diagnosed with dengue fever and recovered without further treatment. Of the 193 volunteers recruited in the study, three participants had a serious adverse event. One participant was diagnosed with acute pharyngitis, one with vestibular migraine and depression, and one with acute sinusitis. None of the serious adverse events were considered to be related to the study vaccine or drugs.
Figure 5.
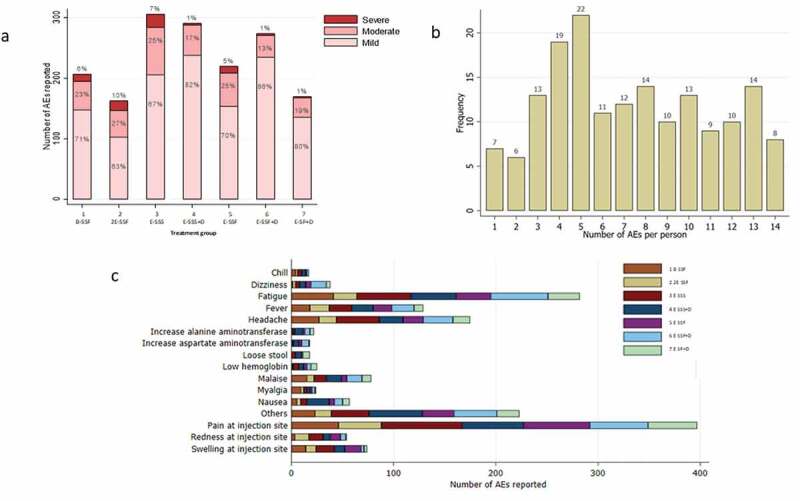
Safety and tolerability of RTS,S/AS01 with and without antimalarial drug administrations a Percentage of mild, moderate, severe adverse (AEs) in each treatment group b The number of AEs per person and the frequency of participants with a specific number of AE c Summary of AEs by frequency.
Discussion
This study found that the RTS,S/AS01 was safe and immunogenic in healthy Asian volunteers as has been reported in Caucasian and African populations.12-15 Comparing the treatment groups receiving vaccine with and without antimalarial drugs suggests that the antibody titers following vaccination with RTS,S/AS01 were not reduced by the co-administration of antimalarial drugs. This could have been an important operational concern when the vaccine is used in addition to MDA for the acceleration of malaria elimination. It has been observed that the immunogenicity of live, cell-culture rabies vaccines is reduced by co-administration with the antimalarial drug chloroquine which is structurally related to piperaquine and could have immunomodulatory effects that will negatively impact immunological responses to the vaccine.16,17
To explore which vaccine dosing schedule would be optimal we compared the immunogenicity of two vaccine doses and formulations, the pediatric dose, the adult dose, and a double pediatric dose. The three antigen and adjuvant doses resulted in comparable antibody titers. Importantly using a higher dose did not result in increased antibody titers or avidity. This finding suggests that the pediatric dose of RTS,S/AS01E can be used for immunization of populations irrespective of age in Asia and perhaps even globally. This finding is of importance for vaccine production which is currently geared to produce pediatric vaccine doses for a large pilot introduction in areas of sub-Saharan Africa.18 Switching production to a different dose for adults would be a logistical challenge which the findings from the current study suggest is not necessary.
Recent work by Regules and co-workers in human malaria challenge studies found vaccine regimens consisting of two standard vaccine doses with a fractional third dose resulted in better protection against controlled human malaria infection compared with a regimen consisting of three standard doses despite the slightly lower ant-CS response.4 In the current study in healthy volunteers, five regimens with a fractional third dose were compared with two regimens using a standard vaccine dose. The fractional dose regimens did not result in higher antibody titers than the three standard dose regimens. The antibody titers measured in Thai volunteers were in the same range as observed in North American volunteers in the earlier study.4
It was hoped that the use of less resources requiring two-dose vaccine schedule would result in a similar immunogenicity as a three-dose regimen. By month 6 the antibody titers against all three antigens evaluated were lowest in the two-dose regimen. Based on antibody titers alone which may not be sufficient for recommendations in the absence of clinical efficacy data, further exploration of such a 2-dose regimen may not be warranted. Protection against P.falciparum infection in challenge studies indicated no discernible difference between groups of participants receiving two doses compared to three doses of RTS,S albeit with a slightly different adjuvant, AS02.19
Compared to the reference study20 the absorption of piperaquine in the current study was lower resulting in a relative reduction in bioavailability by 67.3%. However, vaccine dosing (i.e. full and fractional vaccine doses) had no impact on the pharmacokinetic parameters of piperaquine. The co-administration of the vaccine with the antimalarial drugs is therefore unlikely to be the cause of the reduced bioavailability. The most likely explanation is the absence of fat-containing foods with the drug administration.21 In the reference study on which the model was built a light meal was administered at the time of antimalarial dosing, while food intake was not monitored in the present study.20 Intake of a high-fat meal has been seen to enhance the absorption of piperaquine while smaller amounts of fat have been shown not to have an effect.22,23 The observed piperaquine levels were well above the threshold required to kill Plasmodium species in earlier studies, and the observed piperaquine concentrations are similar to what is seen in patients during acute treatment of uncomplicated malaria.21
Nearly all participants reported one or more adverse events. Some participants reported 14 and more AEs suggesting a highly variable threshold for reporting AEs is in this population. The most frequently observed attributable adverse events were pain at the injection site and fatigue. Three serious adverse events were observed but none of them was considered to be related to the vaccinations. Hematological and biochemistry parameters were monitored with no attributable abnormalities. While the vaccine was found to be safe in this study, the perception of discomfort could be a deterrent for vaccine uptake in combined mass vaccination and drug administration campaigns. To assure adequate coverage of mass drug and vaccinations campaigns it will be essential to provide credible information to target communities regarding the relative risks and benefits of the campaigns.
The study had an excellent follow-up, 98% of enrolled participants completed the follow-up period suggesting that the reported discomfort did not lead to discontinuation in study participation. One hundred and ninety participants were evaluated, hence adverse events which occur at a frequency much smaller than 1/190 were likely to be missed. The absence of a control group receiving placebo prevents the attribution of adverse events to the vaccine or drugs is a limitation of the study. Inclusion of a control group receiving a placebo version of the vaccine and drugs so as to maintain blinding was considered not feasible with the available resources. The study was conducted in unexposed adult Thai volunteers, which leaves the possibility that safety and immunogenicity studies could be different in other Asian populations.
A sequence of interventions, universal access to early diagnosis and adequate treatment, vector control for example in the form of improved access to insecticide-impregnated bed nets, three-monthly rounds of combined mass vaccinations and drug administrations (MVDA), followed by screening and treatment of imported cases, could eliminate falciparum malaria from low transmission areas in the SE Asia over a period of 3 years.5 The optimal schedule for MDA, three rounds of three doses of antimalarials matches the current schedule of three-monthly RTS,S/AS01 injections. Booster doses of RTS,S/AS01 and additional MDA rounds are probably necessary for the permanent interruption of falciparum malaria transmission, but their safety and effectiveness will need to be studied. The impact of MVDA will depend on the uptake of the intervention. In a recent series of MDA in Asia intense community engagement helped achieve MDA coverage above 80% which is thought to be the threshold for the interruption of malaria transmission.24 The benefit of MVDA needs to be carefully communicated to target communities in order to reach adequate coverage. There is little reason to think that an intramuscular injection will reduce the uptake of the intervention substantially, on the contrary, it is possible that communities perceive injections more “powerful” than orally administered drugs and hence a desirable adjunct to drug administrations. The clinical development pathway of MVDA will require proof of individual protection in a randomized phase 3 trial in low transmission settings. Such a trial will require thousands of participants even when the planned endpoint is PCR-detected P.falciparum infection and not clinical malaria as was the case in earlier malaria vaccine trials. Once individual protection has been demonstrated in the target community, community-randomized trials will be needed to assess the impact of this approach on malaria transmission in residents of all ages in high-risk areas. Assuming community-randomised trials demonstrate permanent interruption of transmission on a village level a wider, regional approach will be rolled out which in low-transmission settings is likely to be restricted to communities with evidence of ongoing malaria transmission. In conclusion, MVDA has the potential to interrupt malaria transmission permanently and could play a critical role in the speedy elimination of malaria from low transmission regions.
Methods
This was a phase 2, open-label, computer-randomized, controlled study in healthy, adult Thai volunteers conducted in the Healthy Volunteer Unit of Mahidol University in Bangkok, Thailand between 6 June 2017 and 20 February 2018. The following research questions were addressed.
Is RTS,S/AS01 safe and immunogenic in a malaria-naïve, adult Asian population?
Does the co-administration of RTS,S/AS01 with the antimalarial drugs used in MDA, dihydroartemisinin-piperaquine (DP) and single low dose primaquine (PQ) interfere either with the immunogenicity of the vaccine or the pharmacokinetics of the antimalarial drugs?
Can currently manufactured dosages be deployed? RTS,S/AS01 has been manufactured in a pediatric dose (RTS,S/AS01E) which contains half the antigen and adjuvant system components of the adult dose (RTS,S/AS01B). Using this pediatric dose in adults would simplify vaccine procurement for mass vaccination campaigns but the immunogenicity of the pediatric and adult dose has not been compared previously.
Could a fractional third dose be used? A vaccine regimen in which two standard doses are followed by a fractional dose consisting of a fifth of a standard dose may be more efficacious than a regimen with three standard doses.4
Would a two-dose regimen suffice?
To answer these questions a drug – vaccine interaction study was conducted. In addition, we compared the immunogenicity of vaccine regimens with a fifth of the standard third dose with regimens including standard doses only. We compared the tolerability, safety, and immunogenicity of two and three-dose regimens.
Study vaccines and antimalarials
The vaccines were manufactured by GlaxoSmithKline (Rixensart, Belgium). After reconstitution, 1 dose (0.5 ml) of the paediatric dose RTS,S/AS01E contains 25 micrograms (µg) of RTS,S and 25 µg of each of the components of the AS01E adjuvant system composed of Quillaja saponaria Molina, fraction 21 (QS-21) and 3-Odesacyl-4ʹ- monophosphoryl lipid A (MPL). In the adult dose RTS,S/AS01B each component of the vaccine is doubled. After reconstitution, 1 dose (0.5 ml) contains 50 µg of each RTS,S, QS-21 and MPL. Dihydroartemisinin/piperaquine (DP) tablets (Sigma-Tau Industrie Farmaceutiche Riunite s.p.a., Pomezia, Italy) for adult patients contain 40 mg dihydroartemisinin and 320 mg piperaquine with a therapeutic dose range between 2 and 10 mg/kg/day dihydroartemisinin and 16–26 mg/kg/dose piperaquine. Participants in treatment groups 4 and 6 received the standard DP treatment on Days 0, 1, 2, on Month 0, 1, 2 and in group 7 on Month 0, 2 (Figure S7). In addition, each participant in groups 4, 6, and 7 received a single low dose of primaquine (0.25 mg base/kg; Thai Government Pharmaceutical Organisation, Bangkok, Thailand) on the first day of each vaccination (Day 0).
Sample size calculations
The sample size calculations were based on the primary objective of comparing the serologic response to RTS,S/AS01E vaccine co-administered with DP-PQ versus RTS,S/AS01E or RTS,S/AS01B vaccine given alone in Thai adults. A sample size of 30 participants per group gave 80% power at the 5% significance level to detect a difference in serologic response of 30% for groups 3 to 7. A higher than 30% in the difference in serologic response was anticipated in groups 1 and 2 compared to the other groups. For groups 1 and 2 (in which larger differences were anticipated) a sample size of 20 participants was thought to be sufficient to document immunogenicity. Whenever possible an attempt was made to replace study participants when a participant was withdrawn or chose to withdraw.
Study procedures
After a full explanation of study procedures healthy male or non-pregnant female volunteers, aged 18 to 55 years (inclusive), of Thai origin, without a history of malaria, who provided informed consent and were willing to adhere to the study requirements were recruited. Volunteers were screened before enrolment for hematological or biochemical abnormalities, and for malaria and viral infections (HBsAg, HCV, HIV; Table S5).
Tolerability and safety assessments
Local injection site and general solicited adverse events (AEs) were monitored on days 2, 3, and 7 post-vaccination and graded as mild, moderate, or severe (grades 1, 2, or 3, respectively). All other AEs (unsolicited) were recorded over a 30-day period after each vaccination. Serious AEs (SAEs) were captured throughout the study period. All injection site AEs were considered causally related to vaccination; the causality of all other AEs was assessed by the investigator and these decisions were reviewed by the DSMB and the medical monitor. Hematological and biochemical tests for safety assessment were conducted at screening, on day 0 and 7 of the first vaccination (M0) and Month 3. Abnormal test results were followed until they resolved.
Immunogenicity assessments
The immunology assessments used in this trial were described in detail recently.4 Briefly, antibody levels against PfCSP were measured by standard enzyme-linked immunosorbent assays (ELISAs), using PfCSP (NANP)6 repeat region, PfCSP C – term, and PfCSP full-length (N + C-Terminal).4,25 By using 4M urea as a chaotropic reagent, ELISA-based avidity assays were conducted to assess antibody binding to PfCSP (NANP)6 repeat region, PfCSP C – term, and PfCSP full-length (N + C-Terminal).4 All antibody assays were processed at WRAIR in Silver Spring, Maryland, USA.
Pharmacokinetics
Piperaquine concentrations (groups 4 and 6) were assessed during each vaccination round (Month 0, 1, and 2) on Day 0, 1, 2, and 7. Primaquine concentrations were assessed during each round 24 h after drug administration. The bioanalytical measurements were performed at the Department of Clinical Pharmacology, Mahidol Oxford Research Unit, Bangkok, Thailand using liquid chromatography (LC) coupled with tandem mass spectrometry (MS/MS) detection. All assays were developed and validated according to US FDA regulations. The impact of the vaccine dose on the observed drug concentrations of piperaquine, primaquine, and carboxyprimaquine were investigated by comparing drug concentrations in group 4E-SSS+D (standard vaccine dose) and group 6E-SSF+D (one-fifth vaccine dose at month 3) during the third month of treatment. The pharmacokinetic comparison was made at each time point (pre-1st dose, pre-2nd dose, pre-3rd dose, and 7 days post-dose) using an unpaired Mann-Whitney U-test implemented in Graphpad Prism v7.02 (GraphPad Software, La Jolla California USA). Piperaquine concentration time data were analyzed using a population pharmacokinetic approach as implemented in the software NONMEM v 7.3 (Icon Development Solutions, Ellicott City, Maryland, USA).26 To facilitate the modeling process and to diagnose developed models, Pirana v2.9.2, Perl-Speaks-NONMEM 4.6.0, and R v3.4.3 (the R Foundation for Statistical Computing, Vienna, Austria) with the package Xpose v4.6.1 were used to facilitate the modeling process and model diagnostics.27-30 Due to the sparse nature of the data, a frequentist prior model, based on a healthy volunteers study were implemented (supplementary materials).31 Relative standard error was calculated using the Sampling Importance Resampling function in Perl-Speaks-NONMEM.32
Statistical analysis
All analyses were conducted according to a predefined Statistical Analysis Plan. The main strategy of analysis for the primary outcome analyses (immunogenicity) was carried out using the intention to treat (ITT) population including every participant who was randomized. A per-protocol (PP) analysis including only the study participants who completed the vaccinations and the follow-up schedule was also performed to assess the impact of protocol deviations. The safety outcomes were analyzed using the ITT approach. All participants who received at least one dose of vaccine were included in the safety analyses. Participants lost to follow-up before the completion of the follow-up period assessments were censored on the last day seen. Linear regression was performed on the log values of the protein titers and also on avidity (natural scale). Due to the large number of possible comparisons between vaccine regimens geometric mean titers (GMT) with 95% confidence intervals (95%CI) were reported. P-values less than 0.05 were considered statistically significant in this study. Statistical analyses were conducted using Stata 15 software (StataCorp LLC, College Station, Texas, USA).
Funding Statement
Funding for the project was provided by PATH’s Malaria Vaccine Initiative. The vaccine was provided free of cost by GlaxoSmithKline Biologicals SA. Part of the pharmacokinetic analysis was supported by a grant from the Bill & Melinda Gates Foundation. The Mahidol-Oxford Tropical Medicine Research Unit is supported by the Wellcome Trust of Great Britain.
Ethics review
The protocol was approved by the Mahidol Faculty Tropical Medicine (TMEC 16-097) and Oxford University’s ethics review board (OxTREC ref 54-16) and the Western Institutional Review Board (WIRB # 20162633). The trial was undertaken in accordance with International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines and good clinical practice (ICH GCP). Written informed consent was obtained from each participant before study procedures were initiated (Clinical Trials.gov identifier: NCT02992119)
Data Availability
The data that support the findings of this study can be made available following application to the MORU data sharing committee http://www.tropmedres.ac/data-sharing.
Acknowledgments
We thank the volunteers who kindly agreed to participate in this study. The study was made possible by the dedicated support from the study nurses Somrutai Aurboonkasem, Wasana Saohinkong, Pawinee Khannamkam, and Pawanrat Leungsinsiri. We thank the members of the data safety monitoring board for their support: Prof. Philip Bejon (Chair, Kilifi, Kenya), Prof. Roland Gosling (San Francisco, USA), Prof. Ric Price (Casuarina, Australia) and the medical monitor, Prof. Elizabeth Ashley (Yangon, Myanmar). We thank Marc Lievens (GSK, Rixensart, Belgium) for his support and advice during all stages of the trial. GlaxoSmithKline Biologicals SA was provided the opportunity to review a preliminary version of this manuscript for factual accuracy, but the authors are solely responsible for final content and interpretation. We thank Johan Vekemans (WHO, Geneva, Switzerland) for advice and review of the manuscript.
Authorship contributions
Contribution: LvS, AMD, NJW, SP conceived the study; BH, PJ, SP provided care for participant and sample collection; PoPo coordinated the study activities, KC coordinated the laboratory activities, JT, RMH, MW designed and performed the pharmacokinetic assessment; MM, PiPe performed data analysis and made the figures, PaS managed the database, ZD supervised the regulatory compliance of study activities, CFO, KI, CL, AJB, DCK reviewed and revised the protocol and operating procedures, CFO provided advice for the analysis, KI supported compliance of study activities with regulatory and institutional requirements, PrS, NPJD supported the implementation of the study, all authors read and approved the final manuscript.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.RTSS._Clinical_Trials_Partnership, Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmüller B, Issifou S, Kremsner PG, et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367:2284–95. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.RTSS._Clinical_Trials_Partnership, Agnandji ST, Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BGNO, Doucka Y, Flamen A, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863–75. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 3.RTSS_Clinical_Trials_Partnership . Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regules JA, Cicatelli SB, Bennett JW, Paolino KM, Twomey PS, Moon JE, Kathcart AK, Hauns KD, Komisar JL, Qabar AN, et al. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J Infect Dis. 2016;214:762–71. doi: 10.1093/infdis/jiw237. [DOI] [PubMed] [Google Scholar]
- 5.Tun STT, von Seidlein L, Pongvongsa T, Mayxay M, Saralamba S, Kyaw SS, Chanthavilay P, Celhay O, Nguyen TD, Tran TN-A, et al. Towards malaria elimination in Savannakhet, Lao PDR: mathematical modelling driven strategy design. Malar J. 2017;16:483. doi: 10.1186/s12936-017-2130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Seidlein L, Dondorp A.. Fighting fire with fire: mass antimalarial drug administrations in an era of antimalarial resistance. Expert Rev Anti Infect Ther. 2015;13:1–16. [DOI] [PubMed] [Google Scholar]
- 7.Poirot E, Skarbinski J, Sinclair D, Kachur SP, Slutsker L, Hwang J.. Mass drug administration for malaria. Cochrane Database Syst Rev. 2013;12:CD008846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–23. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tun KM, Imwong M, Lwin KM, Win AA, Hlaing TM, Hlaing T, Lin K, Kyaw MP, Plewes K, Faiz MA, et al. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet Infect Dis. 2015;15:415–21. doi: 10.1016/S1473-3099(15)70032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bojang KA, Milligan PJ, Pinder M, Vigneron L, Alloueche A, Kester KE, Ballou WR, Conway DJ, Reece WH, Gothard P, et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in the Gambia: a randomised trial. Lancet. 2001;358:1927–34. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- 11.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, Wellde BT, Garçon N, Krzych U, Marchand M. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S malaria vaccine evaluation group. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 12.Doherty JF, Pinder M, Tornieporth N, Carton C, Vigneron L, Milligan P, Ballou WR, Holland CA, Kester KE, Voss G, et al. A phase I safety and immunogenicity trial with the candidate malaria vaccine RTS,S/SBAS2 in semi-immune adults in the Gambia. Am J Trop Med Hyg. 1999;61:865–68. doi: 10.4269/ajtmh.1999.61.865. [DOI] [PubMed] [Google Scholar]
- 13.Aponte JJ, Aide P, Renom M, Mandomando I, Bassat Q, Sacarlal J, Manaca MN, Lafuente S, Barbosa A, Leach A, et al. Safety of the RTS,S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. Lancet. 2007;370:1543–51. doi: 10.1016/S0140-6736(07)61542-6. [DOI] [PubMed] [Google Scholar]
- 14.Abdulla S, Oberholzer R, Juma O, Kubhoja S, Machera F, Membi C, Omari S, Urassa A, Mshinda H, Jumanne A, et al. Safety and immunogenicity of RTS,S/AS02D malaria vaccine in infants. N Engl J Med. 2008;359:2533–44. doi: 10.1056/NEJMoa0807773. [DOI] [PubMed] [Google Scholar]
- 15.Gordon DM, McGovern TW, Krzych U, Cohen JC, Schneider I, LaChance R, Heppner DG, Yuan G, Hollingdale M, Slaoui M. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J Infect Dis. 1995;171:1576–85. doi: 10.1093/infdis/171.6.1576. [DOI] [PubMed] [Google Scholar]
- 16.Pappaioanou M, Fishbein DB, Dreesen DW, Schwartz IK, Campbell GH, Sumner JW, Patchen LC, Brown WJ. Antibody response to preexposure human diploid-cell rabies vaccine given concurrently with chloroquine. N Engl J Med. 1986;314:280–84. doi: 10.1056/NEJM198601303140504. [DOI] [PubMed] [Google Scholar]
- 17.Taylor DN, Wasi C, Bernard K. Chloroquine prophylaxis associated with a poor antibody response to human diploid cell rabies vaccine. Lancet. 1984;1:1405. doi: 10.1016/s0140-6736(84)90090-4. [DOI] [PubMed] [Google Scholar]
- 18.WHO . Malaria vaccine implementation programme (MVIP). [Accessed July 30, 2019] http://wwwwhoint/immunization/diseases/malaria/malaria_vaccine_implementation_programme/en/2018.
- 19.Kester KE, McKinney DA, Tornieporth N, Ockenhouse CF, Heppner DG, Hall T, Krzych U, Delchambre M, Voss G, Dowler MG, et al. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J Infect Dis. 2001;183:640–47. doi: 10.1086/318534. [DOI] [PubMed] [Google Scholar]
- 20.Hoglund RM, Adam I, Hanpithakpong W, Ashton M, Lindegardh N, Day NP, White NJ, Nosten F, and Tarning J. A population pharmacokinetic model of piperaquine in pregnant and non-pregnant women with uncomplicated Plasmodium falciparum malaria in Sudan. Malar J. 2012;11:398. doi: 10.1186/1475-2875-11-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoglund RM, Workman L, Edstein MD, Thanh NX, Quang NN, Zongo I, Ouedraogo JB, Borrmann S, Mwai L, Nsanzabana C, et al. Population pharmacokinetic properties of piperaquine in falciparum malaria: an individual participant data meta-analysis. PLoS Med. 2017;14:e1002212. doi: 10.1371/journal.pmed.1002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sim IK, Davis TM, Ilett KF. Effects of a high-fat meal on the relative oral bioavailability of piperaquine. Antimicrob Agents Chemother. 2005;49:2407–11. doi: 10.1128/AAC.49.6.2407-2411.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Annerberg A, Lwin KM, Lindegardh N, Khrutsawadchai S, Ashley E, Day NP, Singhasivanon P, Tarning J, White NJ, Nosten F. A small amount of fat does not affect piperaquine exposure in patients with malaria. Antimicrob Agents Chemother. 2011;55:3971–76. doi: 10.1128/AAC.00279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Seidlein L, Peto TJ, Landier J, Nguyen TN, Tripura R, Phommasone K, Pongvongsa T, Lwin KM, Keereecharoen L, Kajeechiwa L, et al. The impact of targeted malaria elimination with mass drug administrations on falciparum malaria in Southeast Asia: a cluster randomised trial. PLoS Med. 2019;16:e1002745. doi: 10.1371/journal.pmed.1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clement F, Dewar V, Van Braeckel E, Desombere I, Dewerchin M, Swysen C, Demoitié MA, Jongert E, Cohen J, Leroux-Roels G, et al. Validation of an enzyme-linked immunosorbent assay for the quantification of human IgG directed against the repeat region of the circumsporozoite protein of the parasite Plasmodium falciparum. Malar J. 2012;11:384. doi: 10.1186/1475-2875-11-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boeckman A, Sheiner L, Beal S. NONMEM proj group;NONMEM users guide. Univ California. 2010;1–61. [Google Scholar]
- 27.Keizer RJ, van Benten M, Beijnen JH, Schellens JH, Huitema AD. Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Programs Biomed. 2011;101:72–79. doi: 10.1016/j.cmpb.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN) – a Perl module for NONMEM related programming. Comput Methods Programs Biomed. 2004;75:85–94. doi: 10.1016/j.cmpb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Lindbom L, Pihlgren P, Jonsson EN. PsN-toolkit – a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241–57. doi: 10.1016/j.cmpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Jonsson EN, Karlsson MO. Xpose – an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58:51–64. [DOI] [PubMed] [Google Scholar]
- 31.Chotsiri P, Wattanakul T, Hoglund RM, Hanboonkunupakarn B, Pukrittayakamee S, Blessborn D, Jittamala P, White NJ, Day NPJ, Tarning J. Population pharmacokinetics and electrocardiographic effects of dihydroartemisinin-piperaquine in healthy volunteers. Br J Clin Pharmacol. 2017;83:2752–66. doi: 10.1111/bcp.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dosne AG, Bergstrand M, Harling K, Karlsson MO. Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J Pharmacokinet Pharmacodyn. 2016;43:583–96. doi: 10.1007/s10928-016-9487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- WHO . Malaria vaccine implementation programme (MVIP). [Accessed July 30, 2019] http://wwwwhoint/immunization/diseases/malaria/malaria_vaccine_implementation_programme/en/2018.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study can be made available following application to the MORU data sharing committee http://www.tropmedres.ac/data-sharing.


