ABSTRACT
This systematic literature review was conducted to better understand the epidemiology and burden of varicella across the Middle East, gain insight into the evidence to support using universal varicella vaccination (UVV), and identify potential data gaps. Both epidemiology and economic data on the burden of varicella were limited and varied significantly. Most of the data focussed on varicella burden in the absence of a UVV program. In the absence of UVV, varicella incidence is increasing across this region with varicella infection associated with substantial morbidity. Although limited, data on the impact of vaccination at a population level indicated UVV programs reduce varicella incidence and hospitalizations, in line with global experience. Further research and action are needed to better understand varicella epidemiology in the Middle East, increase awareness and understanding in the region, and provide local data to support national public-health decisions regarding the implementation of UVV programs.
KEYWORDS: Middle East, seroprevalence, systematic literature review, varicella, vaccination
Introduction
Varicella is a common, highly contagious illness caused by the varicella-zoster virus.1 The disease is characterized by an itchy, vesicular rash that usually starts on the scalp and face and is accompanied by fever and malaise. Typically, the disease is mild, with the vesicular rash disappearing over 7–10 days.2 However, severe complications such as bacterial infections and encephalitis can arise.2 Varicella can be transmitted through direct contact with individuals with rash, through aerosol droplets from an infected person sneezing or coughing, or by an individual coming in contact with the vesicular fluid of skin lesions. The global annual varicella disease burden is estimated to include 140 million cases, of which 4.2 million have severe complications that can lead to hospitalization and death; neonates and the immunocompromised are at particularly high risk.1
Vaccination can provide long-term protection against varicella. Varicella vaccines are based on live-attenuated varicella; most varicella vaccines available globally are based on the OKA virus strain, although some are based on the less well-studied MAV virus strain. Two different presentations are available: a single-component vaccine (either OKA or MAV) and a tetravalent vaccine that also protects against measles, mumps, and rubella (OKA only). Recommended vaccination schedules typically involve 1 (median prevention of 83%) or 2 (median prevention of 95%) doses.3
The World Health Organization recommends that routine childhood immunization is considered in countries where varicella has an important health impact.1 Over the last decade, a number of countries in the Middle East have introduced varicella vaccination into their national immunization programs, and over 50% of the Middle Eastern population now lives in countries offering universal varicella vaccination (UVV).4 However, this still leaves a substantial proportion of the region’s population with only private and/or limited access to the vaccine.
Understanding the true local burden of disease and the potential impact of a UVV program is central to support varicella vaccination decision-making in the Middle East. In 2012 a working group on pediatric vaccine-preventable disease in low- to middle-income countries, which included experts from the Middle East, acknowledged that data on varicella epidemiology (specifically morbidity and mortality) and the use of varicella vaccine (as a single component or multicomponent vaccine) are lacking for these countries.5 There are numerous global and regional reviews that discuss the epidemiology of varicella and the impact of vaccination programs on incidence, herd immunity, morbidity, and mortality.6–8 However, very little has been published for the Middle East; a recent review by Al-Turab et al. provides a general overview of varicella infection in the Middle East with a specific focus on seroprevalence.9 This review highlighted that, as in most countries, varicella is a childhood disease with seropositivity to varicella increasing with age.
To the best of the authors’ knowledge, this is the first systematic literature review (SLR) to assess the clinical and economic burden of varicella and the impact of varicella vaccination in the Middle East, addressing the need for a comprehensive review of varicella in the region. To gain a better understanding of burden of disease in the Middle East and to identify data gaps to be addressed in future research, this SLR was conducted to: (1) assess the epidemiology (incidence/prevalence) of varicella disease in the Middle East; (2) provide data on the status of vaccination programs, including coverage rates; and (3) examine the economic burden of varicella, including healthcare resource utilization.
Results/findings
The SLR identified a total of 210 studies of which 35 were from the Middle East (Figure 1) specifically Iran, Iraq, the State of Palestine, Saudi Arabia, Turkey, and the United Arab Emirates (UAE). Of the studies identified, 34 reported epidemiology data; 13 were cross-sectional studies, 10 were retrospective studies, 10 were prospective studies, and 1 was a database/registry study. Studies varied greatly in terms of size, with samples ranging from 102 to 20,788 patients. Data were collected mainly through interviews and questionnaires/surveys. Seroprevalence was the most commonly reported outcome (21 studies), followed by incidence (9), complication (7), and mortality (3)
Figure 1.
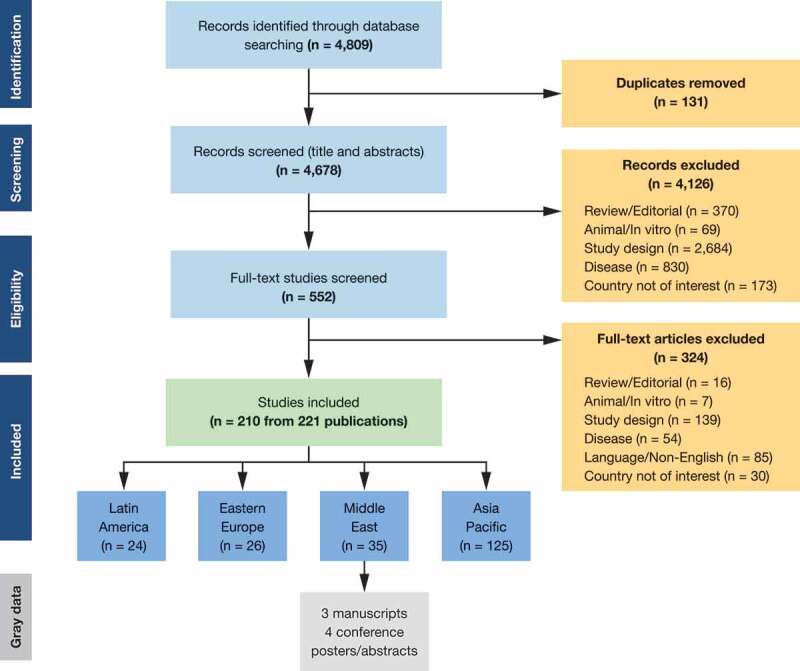
PRISMA flow chart for the global search with regional-level outcomes.
Studies identified in the SLR were supplemented with six pieces of gray data: four conference posters (one multinational, three in Turkey) and two publications (both Iran).
Vaccination program and coverage
The SLR identified five studies that provided information on the inclusion of the varicella vaccine in national immunization programs (NIPs) in the Middle East; four in Turkey and one in Saudi Arabia. A single-dose universal schedule was mandated and government-funded in Turkey (2013), while a two-dose program was implemented (and supported by the government) in Saudi Arabia (2008) (Table 1).10,11 Review of gray data also found that a single-dose schedule universal government-funded vaccination program has been implemented in Oman in 2010, and two-dose programs in the UAE (2009), Bahrain (2015), Kuwait (2017), and Qatar (2010).4,12,13 Those countries in the Middle East where the vaccine is available privately only – particularly Lebanon and Egypt – tend to follow the US Centers for Disease Control and Prevention recommendations for target age and dosing.
Table 1.
Universal varicella vaccination programs in the Middle East.
| Country | Dose | Schedule | Implementation date/approval date |
|---|---|---|---|
| Bahrain*4 | 2 doses | 12 months; 3 y | 2015 |
| Kuwait*4 | 2 doses | 12 and 24 months | 2017 |
| Oman*4 | 1 dose | 12 months | 2010 |
| Qatar*4 | 2 doses | 12 months; 4–6 y | 2010 |
| Turkey14,15 | 1 dose | 12 months | 2013 |
| Saudi Arabia11 | 2 doses | 12 months; 4–6 y | Childhood varicella vaccine introduced in 1998; made mandatory in 2008 |
| UAE16 | 2 doses | 12 months; 5–6 y | 2009 |
UAE, the United Arab Emirates.
*Identified via gray data review.
Very limited data on vaccine coverage were identified in the SLR – only three studies reporting coverage data were found, all in Turkey. Before the introduction of the vaccination program in Turkey in 2013, varicella vaccine coverage varied substantially in the private sector. A 2002 survey of 3,405 subjects (aged 2–59 months) from the Umraniye Health District in Istanbul reported a varicella vaccination coverage rate of 2.7%.17 In comparison, an evaluation of 144 children attending a nursery school during a varicella epidemic in 2006 reported a vaccination coverage of 50.6%, while a cross-sectional study of 2,802 children <2 y, conducted 2008–2009, reported a 60.1% vaccine coverage rate.14,18 Since this SLR was conducted, data from Dubai in the UAE suggest that during the period of 2013–2018, following the introduction of the vaccination program, the varicella vaccine coverage rate was 94 %. Based on the limited and varied coverage data found, this is an area that requires further research.
Surveillance and incidence reporting
A total of 10 studies provided evidence on the incidence of varicella in the Middle East (Table 2).
Table 2.
Incidence of varicella across the Middle East.
| Incidence |
|||||
|---|---|---|---|---|---|
| Country | Study | Design and vaccine availability status | Age group | Minimum | Maximum |
| Iraq | Khaleel 201319 | Retrospective study investigating trend of registered clinical cases 2007–2011 (pre-UVV era) | All ages | 73.4 per 100,000 population in 2007 | 222.6 per 100,000 population in 2011 |
| Jordan | World Health Association20 | Surveillance report. Pre-vaccine incidence rate, 1991 (no UVV program) |
All ages | 458 per 100,000 population | |
| Lebanon | World Health Association20 | Surveillance report. Pre-vaccine incidence rate, 1991 (no UVV program) |
All ages | 454 per 100,000 population | |
| Saudi Arabia | Almuneef 200621 | Retrospective study in an outpatient setting 2001–2003 (pre-UVV era) | All ages | 4,121 patients reported with varicella, 60% of cases in age group 5–14 y | |
| Jahan 200722 | Retrospective study, 1999 and 2003 (pre-UVV era) | All ages | 207 per 100,000 population in 1999 | 759 per 100,000 population in 2003 | |
| Al-Tawfiq 201311 | Retrospective study of 19,577 patients 1994–2011 | All ages | 88.1 per 100,000 population in 2011 (post-UVV era) | 739.8 per 100,000 population in 1994 (pre-vaccine availability) | |
| Saleh 201423 | Retrospective review of surveillance records and book registries from the Armed Forces Hospital of the Southern Region of Saudi Arabia 2007–2012. | All ages | 227 cases in 2012 (post-UVV era) |
754 cases in 2007 (pre-UVV era) |
|
| Syria | World Health Association*20 | Surveillance report. Pre-vaccine incidence rate, 1991 (no UVV program) |
All ages | 1,435 per 100,000 population | |
| Turkey | Dinleyici 2012, 201510,15 | Retrospective study of 824 patients hospitalized due to varicella between October 2008 and September 2010 (pre-UVV era) | Ages < 1–15 y | Incidence of varicella-related hospitalization: 5.3 per 100,000 children (overall) 3.9 per 100,000 children aged 5–10 y |
Incidence of varicella-related hospitalization: 6.9 per 100,000 children (overall) 13.8 per 100,000 children aged < 5 y |
| Kurugol 201114 | Cross-sectional study of 2,802 children from April 2008 to March 2009 (pre-UVV era) | Ages 2–15.5 y | 4,550 per 100,000 person-years for vaccinated (breakthrough) children | 12,020 per 100,000 person-year for unvaccinated (wild-type varicella) children | |
| UAE | Uduman 200924 | Retrospective study conducted between 2000–2004 (pre-UVV era) | All ages | 373 per 100,000 population | 790 per 100,000 population |
UVV, universal varicella vaccine.
*No gray data on incidence rates were identified.
There is substantial variation in reported varicella incidence in the Middle East. In the absence of the UVV in NIPs (pre-UVV) the reported annual incidence per 100,000 population ranged from 73.4 in Iraq (2007) to 759 in Saudi Arabia (2003).19,22 Since varicella was not a notifiable disease in most of the countries where it was reported, very little is known about the exact case definitions use, or the reporting efficiency of these surveillance systems, which may partially explain the variation. Differences in climate may have contributed to the variation observed as incidence varied with the seasons, peaking in the spring; incidence was highest in April and May in Iraq, in March in Saudi Arabia, and in March and May in the UAE.19,23 Differences in study design, in particular, study population, may also have contributed to the observed variation: in the UAE and Saudi Arabia the incidence of varicella varied according to gender (higher in men compared with women), and in Iraq and the UAE incidence varied with age (higher in patients aged <15 y compared with older patients).19,21,24 As indicated by a quarterly summary report of communicable diseases, the annual incidence rate of varicella in Abu Dhabi was 486 per 100,000 population in 2011. Following the introduction of government-funded universal varicella vaccination in 2012, there was a decrease in the rates of varicella that ranged between 147 and 168 per 100,000 population starting from 2013.25
However, despite the variation in data, in the absence of a UVV program (pre-UVV era), an increase in varicella incidence over time was observed across the two studies that published longitudinal data (Table 2). In Iraq, a threefold increase in incidence was observed from 2007 to 2011.19 Similarly, in Saudi Arabia incidence increased from 207/100,000 population in 1999 to 759/100,000 in 2003.22
Post-UVV incidence data were only found for Saudi Arabia, where a UVV was introduced in 2008. A study by Al-Tawfiq et al. in patients with varicella infection reported a 2011 post-UVV incidence of 88.1 per 100,000 population, which had fallen substantially from 739.8 per 100,000 population in 1994, prior to vaccine availability.11 This finding was supported by Saleh et al., who reported a decline in the number of cases recorded at an armed forces hospital from 754 in 2007 (pre-UVV era) to 227 in 2012 (post-UVV era).23
Comparison of varicella incidence data for vaccinated (breakthrough) and unvaccinated cases provides further insight into the potential impact of a UVV program. One study conducted in children (aged 2.0–15.5 y) in Turkey reported that the incidence of varicella was lower in vaccinated compared with unvaccinated children (4,550 per 100,000 patient-years vs 12,020 per 100,000 patient-years, respectively).14 Similar findings were reported in a study of a varicella outbreak in preschool children (n = 124) in Turkey that was identified as part of the gray data.26 Among vaccinated children (who had received one dose of vaccine before the outbreak), 23.4% had varicella compared with 34% among unvaccinated children.26 It was also noted that the risk of disease increased with time since vaccination; the risk was 3.5 times higher in children vaccinated ≥5 y versus those vaccinated more recently.26
Seroprevalence
A total of 35 studies from the SLR provided evidence on the seroprevalence of the varicella-zoster virus in the Middle East; most of the data were from Iran (12 studies) (Table 3). Based on the available SLR data, the seroprevalence of varicella in different age groups mentioned in Table 3 was shown to range from 27.6% to 94.6% in Iran, 74.4% to 88.5% in Saudi Arabia, 22.3% to 98.2% in Turkey, 91.0% in Syria, 80.6% to 88.0% in the UAE, and 92.2% in Qatar.28,29,35,36,39–45,48,49,53,54,56–60 The large ranges in overall seroprevalence for Iran and Turkey are likely due to differences in the overall age of the study population as many studies from the Middle East suggest an increase in seroprevalence with increasing age (Iran, Turkey, and the UAE) (Figure 2). Based on gender distribution, a comparable seroprevalence was observed in men and women in Saudi Arabia and Turkey.40,47
Table 3.
Seroprevalence data.
| Country/Study | Population | Seroprevalence |
|---|---|---|
| Iran | ||
| Allami 201427 | Overall, n = 270 Aged 18–49 y |
74.5% |
| Bayani 201328 | Overall n = 459 Mean age 32.2 ± 1.1 y |
94.6% |
| History of varicella | 95.5% | |
| Ardakani 201329 | Overall population of patients aged Aged 1–15 y, n = 558 |
27.6% |
| Talebi-Taher 201030 | Pregnant women, according to age, Aged 16–43 y n = 400; min–max values presented |
77.8% at ≤ 20 y 94.7% at ≥ 30 y |
| Vojgani 201331 | Children, by age, n = not available Aged 7 months to 6 y Min–max values presented |
6.6% at 7 months 21.3% at 6 y |
| Hoseini 201632 | Patients aged 10–18 y, n = 2,753 | 87.4% |
| Motamedifar 200633 | Children aged 0–10 y, n = 270 | 35.2% |
| Pourahmad 201034 | Women aged 11–45 y, n = 334 | 72.7% |
| Pourakbari 201235 | Individuals aged between 10–25 y, n = 412 | 65.3% |
| Majidy 201636 | Young women aged 13–40 y for pre-marital medical checkup, n = 250 | 71.2% |
| Sharif 200537 | Overall, n = 635, aged 1–60 y | 83.6% |
| Amjadi 2017*38 | Overall (meta-analysis), n = 7,867, aged ≤7–88 y | 78.5% |
| Iraq | ||
| Yassien 201239 | Overall, children aged <1–15 y, n = 92 | 53.3% |
| Saudi Arabia | ||
| Almuneef 200640 | Overall, adults, n = 4,006 | 86.0% |
| Hossain 198941 | Children aged 1–15 y, n = 224 | 68.0% |
| Adults – healthy male blood donors and pregnant women, n = 452 | 90.0% | |
| Memish 200142 | Saudi Arabian National Guard soldiers, aged 15–40+ y, n = 1,350 | 88.5% |
| Ghazi 200243 | Pregnant Saudi Arabian women, ages unspecified, n = 926 | 74.4% |
| Almuneef 200344 | Healthcare workers, ages unspecified, n = 2,047 | 64.0% |
| Abbas 200745 | Healthcare workers, ages unspecified | 68.4% |
| Turkey | ||
| Alp 200546 | Subjects aged < 30 y, n = 568 | 78.0% |
| Alp 201247 | Healthcare-worker population (pre-vaccination era), aged 19–60 y, n = 1,255 | 98.0% |
| Celikbas 200648 | Healthcare-worker population, mean age 29 y, n = 363 | 98% |
| Gürgöze 200649 | Unvaccinated children aged 1–16 y, n = 803 | 26.8% for 1–4 y 90.3% for 13–16 y |
| Karasahin 201450 | Healthcare-worker population, ages unspecified, n = 811 | 95.2% |
| Kanra 200251 | Subjects aged < 30 y, n = 4,387 | 77.8% |
| Kose 201352 | Subjects aged > 15 y, n = 2,136 | 94.3% |
| Aypak 201253 | Healthcare workers, mean age 33.5 ± 11 y, n = 284 | 98.2% |
| Ozkan 200554 | Children aged 9–60 months, n = 292 | 22.3% |
| Kurugol 200755 | Overall (ages 1–30 y), n = 600 | 84.1% |
| Savas 200456 | Children aged 0–15 y, n = 885 | 41.2% for 4–5 y 80% for 10–11 y 85% for 13–15 y |
| Syria | ||
| Barah 201257 | Females of childbearing age, n = 316 | 91.0% |
| UAE | ||
| Sheek-Hussein 201258 | Medical students, aged 16–33 y, post-vaccination era, n = 261 |
88.0% |
| Uduman 200159 | Overall, aged <10–>41 y, n = 648 | 80.6% |
| Qatar | ||
| Guanche Garcell, 201660 | Healthcare workers, 84% aged 30–49 y, n = 705 | 92.2% |
UAE, the United Arab Emirates.
*Denotes identified gray data.
Figure 2.
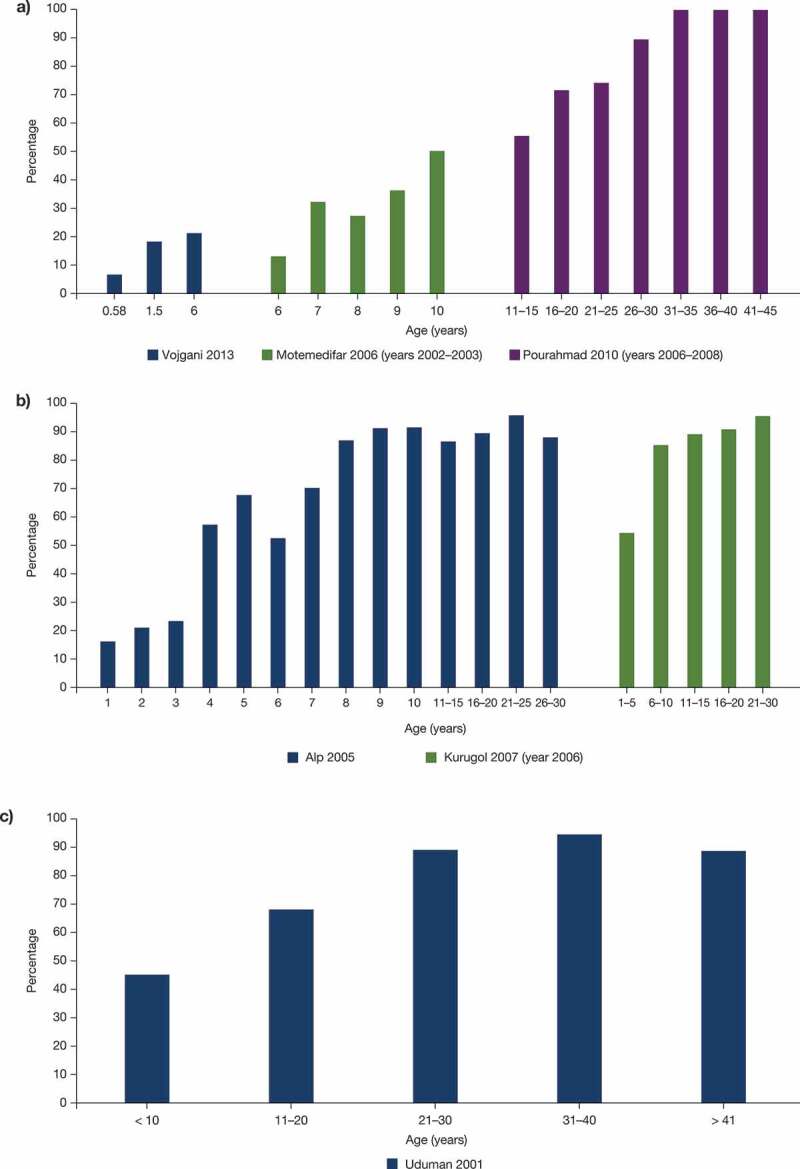
Seroprevalence by age in Iran (a), Turkey (b), and the UAE (c). References: Alp 2005,46 Kurugol 2007,14 Motemedifar 2006,33 Pourahmad 2010,34 Uduman 2001,59 Vojgani 201331 UAE, the United Arab Emirates.
A recent meta-analysis of 22 publications provides further information on seroprevalence. The overall seroprevalence of varicella-zoster infection in Iran was 78.5%.38 When studies of high-risk populations (such as pregnant women and hemodialysis patients) were excluded, the overall seroprevalence rate of varicella-zoster virus in the general population was 67%. In agreement with other studies, seroprevalence in this analysis increased with age. In Lebanon, in adolescents and young adults aged 15–18 y, attending school and at high-risk, the rate of seroprevalence was 96.6%.61
Mortality
A limited number of publications (three studies) reported varicella-related fatality in the Middle East. In Saudi Arabia during 2001–2003 (pre-UVV era), there were two deaths in a cohort of 3,802 patients (0.05%); one adult died of pneumonia and one child died due to Group A beta-hemolytic Streptococcus septic shock.21 A study of 102 hospitalized patients in the UAE from March 2005 to February 2008 (pre-UVV era) reported five deaths (4.9%) due to severe varicella pneumonia.62 In a study of 36 children in Turkey, hospitalized with breakthrough varicella, one patient with hematological-oncological malignancy died due to varicella-related complications such as secondary bacterial infections and sepsis.10
Complications
A total of seven studies (eight publications) provided data for the complications associated with varicella in the Middle East. Commonly reported varicella-associated complications in Saudi Arabia, Turkey and the UAE included bacterial infections, skin, and soft tissue infections, followed by neurologic complications and respiratory complications.10,15,18,21,24,62–64 Two studies highlighted that bacterial infections often manifested as skin and soft tissue infections.10,24 Bacterial infections have been long established as common complications associated with varicella.65
In Saudi Arabia, the incidence of varicella complications in a general cohort of 3,802 patients was 1.50% prior to the introduction of the UVV; the most commonly reported complications were skin and soft tissue infections (0.50%), pneumonia (0.42%), bacteremia (0.16%), encephalitis and cerebellitis (0.11%), and myositis and necrotizing fasciitis (0.11%).21
Four publications reported complications in patients hospitalized with varicella in Turkey: the most commonly reported were skin/soft tissue infection, pneumonia/pulmonary symptoms, central nervous system complications, hematological complications, and bacterial infection (Figure 3). In a study of varicella outbreak in children in Turkey (non-hospitalized), none of the vaccinated children experienced varicella-associated complications; however, in the unvaccinated group, 20.3% had secondary skin infections, 18.6% had vomiting, 11.8% diarrhea, 6.7% vertigo, and 5% pneumonia.18 However, other studies have reported cases of hospitalization due to complications of breakthrough varicella, mainly neurological complications, fever and dehydration, respiratory complications, secondary bacterial infections, and hematological complications.10
Figure 3.
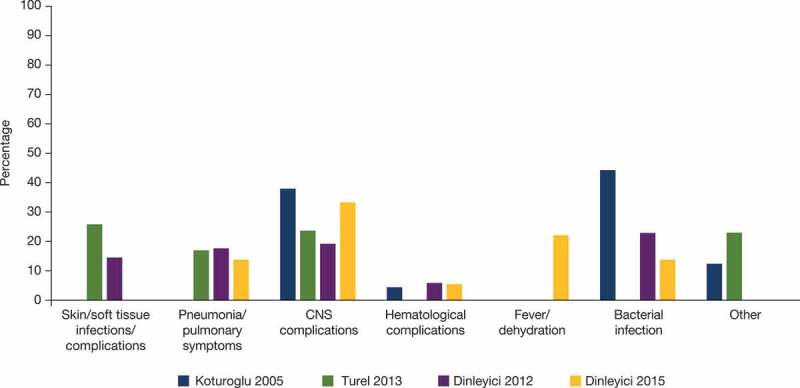
Common complications of varicella reported in hospitalized patients in Turkey. References: Koturoglu 2005,63 Turel 2013,64 Dinleyici 2012,15 Dinleyici 201510.
Two studies reported varicella-associated complications in the UAE, both prior to the introduction of the UVV. Between 2000 and 2004, the most frequent varicella-associated complications in a group of 187 hospitalized children were bacterial infections (affecting 50.3% of cases; 25.1% of patients had secondary bacterial infection of the skin or soft tissue and 24.1% had respiratory tract infection including clinical pneumonia), fever (associated with respiratory tract infection in 20.3%, superficial skin infection in 19.3%, and dehydration in 15.5%), and cerebellar ataxia 12.3%.24 In the other study, with 102 hospitalized patients, the most frequent were an increase of alanine aminotransferase (51.9%), thrombocytopenia (42.1%), varicella pneumonia (28.4%), leukocytosis (26.4%), and skin infection (25.4%).62
Resource use and health economic aspects
Eight studies identified in the SLR evaluated the economic burden of varicella in the Middle East: five in Turkey, two in the UAE, and one in Saudi Arabia (five retrospective studies, one prospective study, one cross-sectional study, and one study that assessed economic evaluations). Sample size ranged from 102 to 137,310 patients.62,66 In addition, four gray data sources were identified: two conference proceedings and one publication presenting data on the impact of varicella vaccination in Turkey and one publication about the cost-effectiveness of varicella vaccination in Iran.67,68 The majority of studies (11) provided evidence on resource use or hospitalization rates (Table 4). As seen for the epidemiology parameters, hospitalization data were predominantly prior to the introduction of the UVV into the NIP (pre-UVV era) and varied greatly between studies.
Table 4.
Hospitalization rate and duration of stay due to varicella.
| Study | Study description/Population | Hospitalization rate | Average duration of stay |
|---|---|---|---|
| Turkey | |||
| Dinleyici 201215 | VARICOMP: Children between age 0–15 y hospitalized due to varicella, October 2008 to September 2010 | Overall estimated incidence of varicella-related hospitalization: 5.3–6.9 per 100,000 children per year |
6 d (median) 11 d in ICU (median) |
| Dinleyici 201510 | VARICOMP: Children between age 0–15 y hospitalized due to breakthrough varicella, October 2008 to September 2013 | NR | 8 d (median) |
| Dinleyici 2016*67 | VARICOMP: Varicella-related hospitalization among children aged 1–5 y Pre-UVV era Post-UVV era |
6.1–9.1 per 100,000 children per year 3.1–4.3 per 100,000 children per year |
NR |
| Turel 201364 | Study of 186 patients hospitalized with varicella, November 2006 to June 2011 | 74.2% of patients were aged < 3 y, of which 64.2% were ≤ 1 y | 5 d (median) |
| Kurugol 201114 | Study of 466 cases of breakthrough varicella in vaccinated children, and 723 cases of varicella in unvaccinated children | 0.2% of children in the vaccinated group and 0.6% in the unvaccinated group | NR |
| Koturoglu 200563 | Study of 178 cases in children between 2007 and 2011 | NR | 6.2 d (mean) |
| UAE | |||
| Uduman24 | 187 hospitalized during 2000–2004 (pre-UVV era) | NR | 7.5 d (mean) |
| Abro 200962 | Study period March 2005 to February 2008 (pre-UVV era) | NR | 7.45 d (mean) |
| Saudi Arabia | |||
| Almuneef 200621 | Data collection period June 1, 2001, to December 30, 2003 (Pre-UVV era) | Hospitalization rate due to varicella: 2,050 per 100,000 reported cases |
NR |
ICU, intensive care unit; NR, not reported; UVV, universal varicella vaccine.
*Denotes gray data.
A study from Saudi Arabia conducted prior to introduction of the UVV, indicated that approximately 2% of varicella cases required hospitalization.21 Only the VARICOMP study in Turkey provided population-level data on varicella-hospitalization rates. The overall estimated annual incidence of varicella-related hospitalization per 100,000 children (aged 0–15 y) was 5.3–6.9 in Turkey in the pre-UVV era.15 Following the inclusion of varicella vaccine into the NIP in 2013, hospitalization in children aged 1–5 y fell significantly from 6.1 to 9.1 per 100,000 children per year (pre-UVV era) to 3.1–4.3 per 100,000 children per year (post-UVV era).67 However, herd protection for other age groups was not observed. The VARICOMP study also indicated that in the post-UVV era, the mean age of hospitalized children was slightly older (52.8 vs 48.6 months p < .005) and the hospitalization rate of children with underlying conditions was lower compared with the pre-UVV era.69 Over a 7-y period from 2008 to 2015, neurological complications occurred in 17.7% of children hospitalized with varicella.70 Authors highlighted that the proportion of children hospitalized with varicella and neurological complications was not significantly lower in the post-vaccine versus the pre-vaccine program era; however, the percentage of children with seizures (including febrile seizures) was significantly reduced (the VARICOMP study).70
In Turkey, most patients hospitalized were aged <3 y, with the majority being ≤1 y.64 The majority of hospitalized cases were reported in the spring and early summer months, and hospitalization peaked in May in Turkey, reflecting the seasonal incidence of varicella infection.
Hospital stays due to varicella ranged from 5–8 d (median) in Turkey to 7.5 d (mean) in the UAE. In Turkey, patients with central nervous system complications had the longest duration of hospital stay (9 d) (Table 4).15 Other complications associated with long hospital stays were cerebellitis (8 d), hematologic complications (8 d), and respiratory complications (7 d). The most commonly reported treatments among hospitalized patients with varicella were acyclovir, antibiotics (intravenous and oral), and intravenous immunoglobulin.15
There are limited published data on the economic burden of varicella in the Middle East. The SLR identified two studies, conducted in Turkey, that provided evidence on economic burden prior to the implementation of the UVV in 2013. In a retrospective study, the estimated total cost to treat 824 patients hospitalized with varicella between October 2008 and September 2009 in Turkey was US$ 422,102 (equivalent to US$ 453,011 in 2017), with median cost of hospitalization of US$ 338 per patient (equivalent to US$ 363 in 2017).15 In the second study of 186 patients hospitalized with varicella between November 2005 and June 2011, median cost of hospitalization was US$ 283 per patient (US$ 315 in 2017), and the annual cost for varicella hospitalizations in Turkey was estimated at US$ 396,200 (equivalent to US$ 440,369 in 2017).64
One study, conducted in Turkey, provided economic evaluations on screening and vaccination. The study concluded that the cost of vaccination without screening was significantly more expensive versus vaccination with screening (US$ 43,566 [equivalent to US$ 47,475 in 2017] and US$ 10,159 [equivalent to US$ 11,071 in 2017], respectively, for a cost difference of US$ 33,407 [equivalent to US$ 36,411 in 2017]).47
In a study published in 2017 in Iran, a decision-tree model was used to evaluate the cost-effectiveness of the varicella vaccination program in a cohort of 12-month-old children. The incremental cost-effectiveness ratios per disability-adjusted life years averted were US$ 17,280 and US$ 41,531 for one-dose and two-dose regimens, respectively.68 Based on the assumptions used in the model, the authors concluded that neither the one-dose nor the two-dose vaccination program would be cost-effective in Iran, although based on the sensitivity analysis, the one-dose vaccination may be cost-effective in a scenario of greater epidemiological burden.68
Rationale supporting varicella vaccination decision-making in the Middle East
Currently, a substantial proportion of the population in the Middle East does not have access to varicella vaccination via UVV programs incorporated into NIPs.4 Understanding the importance of varicella from a public health and economic perspective is critical in supporting UVV decision-making. To help address the data need, this review provides an insight into what is currently known about the clinical and economic burden of varicella in the Middle East.
Data identified in this review indicate that in the absence of a UVV program, the incidence of varicella is increasing over time across the Middle East; based on data from Iraq and Saudi Arabia, incidence increased at a rate of 3–3.7-fold over a 5-y period in the pre-UVV era.19,22 Seroprevalence identified in the SLR showed a rapid increase in seropositivity in early childhood, tailing off in adolescence, and plateauing in adulthood, indicating that infection rates are highest in early childhood in the Middle East, consistent with the general understanding of varicella epidemiology.
Varicella is often perceived as a mild childhood disease of low severity; however, morbidity can be high, particularly in infants, adults, and immunocompromised people who are at risk of more severe disease and have a higher incidence of complications.71,72 Consistent with this, the SLR identified studies reporting substantial varicella-related morbidity across the Middle East. Data from Turkey indicated that in the absence of a UVV program 5.3–6.9 children per 100,000 per year were hospitalized due to varicella.15 Furthermore, general incidence data from Saudi Arabia indicated that 1.5% of patients with varicella experienced complications and approximately 2% of varicella cases required hospitalization.21 Morbidity in hospitalized patients in the Middle East was high, with a substantial proportion of patients reporting soft tissue/skin infections, bacterial infections, and central nervous system and respiratory complications.10,15,18,24,62,63 Fatality rates as high as 4.9% were also reported in varicella-hospitalized patients.62
In addition to clinical burden, varicella infection also carried a resource and economic burden in the Middle East. Patients hospitalized due to varicella had a median stay of duration of 5–8 d; those patients requiring intensive care had a median stay of 11 d.10,15,64 In turn, hospitalization due to varicella carried a substantial economic burden – the annual cost of varicella hospitalization between 2005 and 2011 in Turkey was estimated at US$ 396,200 (equivalent to US$ 440,368.97 in 2017).64 This substantial economic burden is not unique to countries in the Middle East and has been observed globally. Recent studies report a significant clinical, resource, and economic burden associated with varicella in countries where UVV programs have not been implemented, such as Poland, Argentina, and Hungary.73–75 Occurrence of complications and the need for hospitalizations placed demand on health-care resources (e.g., need for medications, tests/diagnoses, health-professional consultations) as well as having an indirect economic impact (e.g., time taken off work to look after children).73–75
Although data on the impact of varicella vaccination in the Middle East are limited, the data that are available indicate that the UVV has the potential to substantially reduce the clinical burden of the disease. A Turkish study found that incidence rates were over 2.5-fold higher in unvaccinated compared with vaccinated populations.14 Data from Saudi Arabia suggested that varicella incidence fell substantially, by approximately 88%, following the introduction of a two-dose UVV program.11 This fall in incidence observed in the Saudi Arabia study is in a similar range to that reported with other two-dose regimens.6
Owing to a lack of published data from the Middle East in the post-UVV era, it is difficult to gain insight into the impact of UVV programs on hard outcomes such as mortality, complication rates, and hospitalization rates in this area. In Turkey, the proportion of children hospitalized with varicella was higher in unvaccinated compared with vaccinated children (0.6% vs 0.2%).14 Data from Turkey (the VARICOMP study) suggested an approximate twofold reduction in varicella-related hospitalizations in children aged 1–5 y following the introduction of the UVV program.67 The beneficial impact of vaccination on outcomes is also illustrated by data from other countries, highlighted by a reduction in varicella-hospitalization rate and death.6 Collectively these data support the introduction of UVV programs from a clinical perspective.
The review only identified one study looking at the cost-effectiveness of a UVV program. This study concluded that neither a one- nor two-dose vaccination program in 12-month-old children would be cost-effective in Iran, based on the assumptions (including epidemiology assumptions) used in the model. However, the authors emphasized that the one-dose schedule may be cost-effective if there was a greater epidemiological burden than that captured in the primary model; this emphasizes the need for accurate epidemiological data to enable realistic cost-effectiveness analyses and support varicella vaccination decision-making.68
Based on results from the SLR, published data on varicella epidemiology and economic burden are relatively sparse in the Middle East. Furthermore, most of the data focussed on pre-UVV data, therefore understanding of the potential impact of inclusion of the varicella vaccine into the NIP is limited in this region. This lack of data may be partially responsible for inhibiting or delaying the introduction of the UVV in the Middle East.
Those countries that do not have the resources to obtain local epidemiology and economic burden data may rely on data from neighboring countries. However, where available, the data are markedly different across the Middle East. For example, incidence in the general population in the pre-UVV era ranged from 73.4 per 100,000 population (Iran, 2007) to 7,590 per 100,000 population (Saudi Arabia, 2003).19,22 This may in part be due to differences in study design (e.g. age of the study population) as well as variation in surveillance systems and reporting across the region. For example, in some countries such as Iran, Lebanon, and Turkey, varicella is not a reportable or notifiable disease,56,76,77 therefore incidence rates associated with morbidity/mortality, and consequently burden of disease, may be underestimated. Furthermore, differences in climate and urbanization, which influence infection rates, may also contribute to the observed variation.
This variation in epidemiology is not unique to this review or to the Middle East. Standardized incidence rates for overall populations of 300 to 1,291 per 100,000 per year have been reported in Europe, which lies within the range observed in this review of epidemiology in the Middle East.6 However, this variation in data, due to a variety of potential factors, makes it difficult to compare data from different countries and to extrapolate data between countries.
As demonstrated by the Iran cost-effectiveness analysis that highlights the need for accurate epidemiology data, lack of local-data makes varicella vaccination decision-making very challenging. A further potential challenge to the implementation of UVV programs is that varicella is currently perceived as being less important than other infectious diseases, which in part may be due to an underestimation of the disease burden, lack of awareness, and/or poor reporting. Therefore, progress on improving surveillance and reporting in the region should be sought if resources allow. Taken together, such improvements are likely to lead to a better understanding of the burden of disease, which will initially mean that an increase in cases will be noted. This is likely to be a positive outcome and lead to increased awareness and acceptance of varicella as a common, important, vaccine-preventable disease.
There are a number of limitations in our review relating to the interpretation of the burden of varicella in the Middle East: the limited-published data in the region, a high likelihood that the data available is an underestimate of the burden and costs of varicella, and the fact that many countries in the Middle East are not represented in the manuscript, as no data have been collected or analyzed.
In summary, the limited data identified for the Middle East indicate that in the absence of a UVV program the incidence of varicella infection is increasing across the region, which in turn will increase the already substantial varicella-associated morbidity, hospitalizations, and economic burden. Due to the large degree of variation in epidemiology data and the need for accurate data to determine the true cost-effectiveness of immunization programs, varicella needs to be better understood in the Middle East. In addition, there is a need for improved surveillance and reporting (where possible), changes in care-seeking behaviors, and increased public awareness. Hopefully, with such changes, decision-makers will recognize the importance of varicella and the need for inclusion of varicella vaccine in their NIPs.
Methodology and data sources
A global SLR on varicella was conducted and publications with data from Middle East countries (including Bahrain, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Oman, Qatar, Saudi Arabia, Syria, Turkey, the UAE, and Yemen) were identified and reviewed for this article.
In brief, key biomedical electronic literature databases were searched using a comprehensive search strategy to identify studies that provided relevant data on the epidemiology, economic burden, and coverage of vaccination programs associated with varicella. Databases used were: The Excerpta Medica Database (Embase®), Medical Literature Analysis and Retrieval System Online (MEDLINE®), Index Medicus for the WHO Eastern Mediterranean Region (IMEMR), Latin American and Caribbean Health Sciences Literature (LILACS), and Health Literature Libraries and Information Services (HELLIS). Studies that published data from database inception (pre-1966) up to the search date, February 1, 2016, were captured. The study was carried out in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.78 Further details are given in the Supplemental Online Material.
Abstracts and subsequently full texts were screened based on the predefined eligibility criteria specified in the protocol. Studies evaluating individuals (of any age) with primary and/or breakthrough varicella infection were included as part of the review. The SLR was restricted to papers published in the English language.
Searches were also conducted on country-specific/global websites to address any data gaps. Gray data, such as country-specific health bulletins and locally published manuscripts, were also sourced.
Funding Statement
The study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. The authors take full responsibility for the scope, direction, and content of the manuscript, and have approved the submitted manuscript.
Acknowledgments
Medical writing assistance was provided by Anil Dandu, PhD, and Aruna Jeans, PhD, of Parexel and was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ USA. Project Management assistance was provided by Tracey Weiss of Merck & Co., Inc., Kenilworth, NJ, USA.
Disclosure of potential conflicts of interest
N Al Kaabi, M Al-Qaseer, and FMAS Al-Olama have nothing to disclose. EC Dinleyici reports grants from GlaxoSmithKline Vaccines and Pfizer Vaccines outside the submitted work. WA Hayajneh has nothing to disclose. M Loulou, T Ndao, and LJ Wolfson are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, who may own stock and/or hold stock options in Merck & Co., Inc., Kenilworth, NJ, USA.
Author contributions
N Al-Kaabi, M Al-Qaseer, and LJ Wolfson have contributed to the conception, design or planning of the study. N Al-Kaabi, FMAS Al-Olama, EC Dinleyici, M Loulou, LJ Wolfson, and AR Bizri were involved in data acquisition. LJ Wolfson, EC Dinleyici and WA Hayajneh were responsible for analysis of the data. M Al-Qaseer, LJ Wolfson, and EC Dinleyici were involved in interpretation of the data. FMAS Al-Olama, EC Dinleyici, and WA Hayajneh contributed to writing the manuscript. All authors had full access to the data, were involved in critically reviewing or revising the manuscript and gave final approval before submission.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.World Health Organization . Varicella and herpes zoster vaccination position paper. [accessed 2019. January 10]. https://apps.who.int/iris/bitstream/handle/10665/242227/WER8925_265-287.PDF.
- 2.World Health Organization . Immunization, vaccines and biologicals: varicella. [accessed 2018. December 7]. https://www.who.int/immunization/diseases/varicella/en/.
- 3.World Health Organization . Weekly epidemiological record Relevé épidémiologique hebdomadair. [accessed 2018. December 7]. https://www.who.int/wer/2014/wer8925.pdf?ua=1.
- 4.LouLou M, Siddiqui K, Mangat GS, Weiss TJ. A review of the health and economic burden of varicella in the Middle East. World Congress of the World Soceity for Paediatric Infections Disease; 2017; Shenzen (China). [Google Scholar]
- 5.Dbaibo G, Tatochenko V, Wutzler P.. Issues in pediatric vaccine-preventable diseases in low- to middle-income countries. Hum Vaccin Immunother. 2016;12(9):2365–77. doi: 10.1080/21645515.2016.1181243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wutzler P, Bonanni P, Burgess M, Gershon A, Safadi MA, Casabona G.. Varicella vaccination - the global experience. Expert Rev Vaccines. 2017;16(8):833–43. doi: 10.1080/14760584.2017.1343669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang HK, Huang MY. Burden of varicella in Asia-Pacific countries: A systematic review and critical analysis. Value Health. 2014;17(7):A803–04. doi: 10.1016/j.jval.2014.08.508. [DOI] [PubMed] [Google Scholar]
- 8.Hussey H, Abdullahi L, Collins J, Muloiwa R, Hussey G, Kagina B. Varicella zoster virus-associated morbidity and mortality in Africa - a systematic review. BMC Infect Dis. 2017;17(1):717. doi: 10.1186/s12879-017-2815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Turab M, Chehadeh W. Varicella infection in the Middle East: prevalence, complications, and vaccination. J Res Med Sci. 2018;23(4):19. doi: 10.4103/jrms.JRMS_979_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinleyici EC, Kurugol Z, Kara A, Tezer H, Tas MA, Guler E, Yasa O, Devrim I, Ciftci E, Ozdemir H, et al. Children with breakthrough varicella infection requiring hospitalization in Turkey (VARICOMP Study 2008–2013). Vaccine. 2015;33(32):3983–87. doi: 10.1016/j.vaccine.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 11.Al-Tawfiq JA, AbuKhamsin A, Memish ZA. Epidemiology and impact of varicella vaccination: A longitudinal study 1994–2011. Travel Med Infect Dis. 2013;11(5):310–14. doi: 10.1016/j.tmaid.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Department of Health – Abu Dhabi. Varicella vaccine added to school immunization schedule for 2012–2013. [accessed 2018. October 26]. https://www.haad.ae/haad/tabid/58/Mid/417/ItemID/311/ctl/Details/Default.aspx.
- 13.Dubai Health Authority . Immunization guidelines. [accessed 2019. January 10]. https://www.dha.gov.ae/Documents/HRD/Immunization%20Guidelines.pdf.
- 14.Kurugol Z, Halicioglu O, Koc F, Koturoglu G, Aksit S. Varicella rates among unvaccinated and one-dose vaccinated healthy children in Izmir, Turkey. Int J Infect Dis. 2011;15(7):e475–80. doi: 10.1016/j.ijid.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Dinleyici EC, Kurugol Z, Turel O, Hatipoglu N, Devrim I, Agin H, Gunay I, Yasa O, Erguven M, Bayram N, et al. The epidemiology and economic impact of varicella-related hospitalizations in Turkey from 2008 to 2010: A nationwide survey during the pre-vaccine era (VARICOMP study). Eur J Pediatr. 2012;171(5):817–25. doi: 10.1007/s00431-011-1650-z. [DOI] [PubMed] [Google Scholar]
- 16.Al-Mekaini LA, Kamal SM, Al-Jabri O, Soliman M, Alshamsi H, Narchi H, Souid AK, Alsuwaidi AR. Seroprevalence of vaccine-preventable diseases among young children in the United Arab Emirates. Int J Infect Dis. 2016;50:67–71. doi: 10.1016/j.ijid.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Topuzoglu A, Ozaydin GA, Cali S, Cebeci D, Kalaca S, Harmanci H. Assessment of sociodemographic factors and socio-economic status affecting the coverage of compulsory and private immunization services in Istanbul, Turkey. Public Health. 2005;119(10):862–69. doi: 10.1016/j.puhe.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Kilic A, Unuvar E, Yilmaz C, Yildiz I, Oguz F, Sidal M. The effectiveness of varicella vaccination during an outbreak in a children’s day-care center. Vaccine. 2008;26(27–28):3371–72. doi: 10.1016/j.vaccine.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Khaleel HA, Abdelhussein HM. Clinical epidemiology of chickenpox in Iraq from 2007–2011. Glob J Health Sci. 2012;5(1):180–86. doi: 10.5539/gjhs.v5n1p180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization . Health conditions of the Arab population in the occupied Arab territories, including Palestine. World Health Organization, 45th World Health Assembly; 1992.
- 21.Almuneef M, Memish ZA, Balkhy HH, Alotaibi B, Helmy M. Chickenpox complications in Saudi Arabia: is it time for routine varicella vaccination? Int J Infect Dis. 2006;10(2):156–61. doi: 10.1016/j.ijid.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Jahan S, Al-Saigul AM, Hamed SA. Five-year surveillance of chickenpox in Qassim, Central Saudi Arabia. Saudi Med J. 2007;28(5):808–10. [PubMed] [Google Scholar]
- 23.Saleh N, Al Moghazy B. Seasonal variation and trend of chicken pox in the southern region of Saudi Arabia (2007–2012). J Egypt Public Health Assoc. 2014;89(3):143–47. doi: 10.1097/01.EPX.0000456619.36915.df. [DOI] [PubMed] [Google Scholar]
- 24.Uduman SA, Sheek-Hussein M, Bakir M, Trad O, Al-Hussani M, Uduman J, Sheikhs F. Pattern of varicella and associated complications in children in United Arab Emirates: 5-year descriptive study. East Mediterr Health J. 2009;15(4):800–06. doi: 10.26719/2009.15.4.800. [DOI] [PubMed] [Google Scholar]
- 25.Department of Health – Abu Dhabi. Communicable Diseases Bulletin Quarterly Summary Report. 2017;8(1). [accessed 2018 Dec 7]. https://www.haad.ae/HAAD/LinkClick.aspx?fileticket=lZtbRD2akIU%3D&tabid=1177.
- 26.Kurugol Z, Gokce S. Outbreak of varicella in preschool children despite one-dose vaccination. Turk J Pediatr. 2018;60(1):56–62. doi: 10.24953/turkjped.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Allami A, Mohammadi N, Najar A. Seroepidemiology of varicella and value of self-reported history of varicella infection in Iranian medical students. Int J Occup Med Environ Health. 2014;27(2):304–13. doi: 10.2478/s13382-014-0265-9. [DOI] [PubMed] [Google Scholar]
- 28.Bayani M, Hasanjani-Roushan MR, Siadati S, Javanian M, Sadeghi-Haddad-Zavareh M, Shokri M, Mohammadpour M, Zarghami A, Asghari S. Seroepidemiology of varicella zoster virus in healthcare workers in Babol, Northern Iran. Caspian J Intern Med. 2013;4(3):686–91. [PMC free article] [PubMed] [Google Scholar]
- 29.Ardakani AT, Soltani B, Sehat M, Namjoo S. Seroprevalence and risk factors of varicella-zoster among children of Kashan-center of Iran. Jundishapur J Microbiol. 2013;6(5). doi: 10.5812/jjm.8388. [DOI] [Google Scholar]
- 30.Talebi-Taher M, Kashanian M, Khalili K. Seroprevalence of varicella-zoster virus among pregnant women in two teaching hospitals, Tehran, Iran. Iran J Microbiol. 2014;6(1):37–40. [PMC free article] [PubMed] [Google Scholar]
- 31.Vojgani Y, Tavangar B, Zarie S, Jedi Tehrani M. Assessment of anti-varicella antibody levels in sera of Iranian children. Iran J Allergy, Asthma Immunol. 2013;12(1):S49. [Google Scholar]
- 32.Hoseini SG, Kelishadi R, Kasaeian A, Ataei B, Yaran M, Motlagh ME, Heshmat R, Ardalan G, Safari O, Qorbani M, et al. Seroprevalence and risk factors of varicella zoster infection in Iranian adolescents: A multilevel analysis. The CASPIAN-III study. PLoS One. 2016;11(6):e0158398. doi: 10.1371/journal.pone.0158398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motamedifar M. Seroprevalence of varicella-zoster virus in children from Shiraz-Iran. Iran J Immunol. 2006;3(1):43–46. [Google Scholar]
- 34.Pourahmad M, Davami MH, Jahromi AR. Evaluation of anti-varicella antibody in young women before their marriage: A sero-epidemiologic study in Iran. J Clin Virol. 2010;48(4):260–63. doi: 10.1016/j.jcv.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 35.Pourakbari B, Shahbaznezhad L, Parvaneh N, Nikkhah S, Mahmoudi S, Teymuri M, Alyari A, Mamishi S. Seroepidemiology of varicella zoster virus among children, adolescents and medical students in a referral children medical center, Tehran, Iran. Iran J Microbiol. 2012;4(3):136–38. [PMC free article] [PubMed] [Google Scholar]
- 36.Majidy P, Khodabandehloo M, Azadi NA. Seroprevalence of varicella zoster virus antibody among young women before marriage in Sanandaj, Iran. Iran J Microbiol. 2016;8(2):147–52. [PMC free article] [PubMed] [Google Scholar]
- 37.Sharifi Z, Emadi Ghanjin S. The seroepidemiology of varicella zoster virus (VZV) in different age groups in Tehran, Iran. Iran J Allergy Asthma Immunol. 2005;4(2):95–98. [PubMed] [Google Scholar]
- 38.Amjadi O, Rafiei A, Haghshenas M, Navaei RA, Valadan R, Hosseini-Khah Z, Omran AH, Arabi M, Shakib RJ, Mousavi T, et al. A systematic review and meta-analysis of seroprevalence of varicella zoster virus: A nationwide population-based study. J Clin Virol. 2017;87:49–59. doi: 10.1016/j.jcv.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Yassien WT, Hasony HJ. Immunological responses to varicella-zoster virus (vzv) in Basrah with special emphasis on the pattern of exposure. Med J Basrah Univ. 2012;30(2):106–14. doi: 10.33762/mjbu.2012.75807. [DOI] [Google Scholar]
- 40.Almuneef MA, Memish ZA, Balkhy HH, Otaibi B, Helmi M. Seroprevalence survey of varicella, measles, rubella, and hepatitis A and B viruses in a multinational healthcare workforce in Saudi Arabia. Infect Control Hosp Epidemiol. 2006;27(11):1178–83. doi: 10.1017/S0195941700074932. [DOI] [PubMed] [Google Scholar]
- 41.Hossain A. Herpes simplex virus type 1 (HSV-1) and varicella-zoster virus (VZV) infections in Saudi Arabia. J Trop Pediatr. 1989;35(4):171–74. doi: 10.1093/tropej/35.4.171. [DOI] [PubMed] [Google Scholar]
- 42.Memish ZA, Oni GA, Bannatyne RM, Qasem L. The cost-saving potential of prevaccination antibody tests when implementing a mass immunization program. Mil Med. 2001;166(1):11–13. doi: 10.1093/milmed/166.1.11. [DOI] [PubMed] [Google Scholar]
- 43.Ghazi HO, Telmesani AM, Mahomed MF. TORCH agents in pregnant Saudi women. Med Princ Pract. 2002;11(4):180–2. doi: 10.1159/000065813. [DOI] [PubMed] [Google Scholar]
- 44.Almuneef M, Dillon J, Abbas MF, Memish Z. Varicella zoster virus immunity in multinational health care workers of a Saudi Arabian hospital. Am J Infect Control. 2003;31(6):375–81. doi: 10.1016/S0196-6553(02)48204-1. [DOI] [PubMed] [Google Scholar]
- 45.Abbas M, Atwa M, Emara A. Seroprevalence of measles, mumps, rubella and varicella among staff of a hospital in Riyadh, Saudi Arabia. J Egypt Public Health Assoc. 2007;82:283–97. [PubMed] [Google Scholar]
- 46.Alp H, Altinkaynak S, Ertekin V, Kilicaslan B, Giiraksin A. Seroepidemiology of varicella-zoster virus infection in a cosmopolitan city (Erzurum) in the eastern Turkey. Health Policy. 2005;72(1):119–24. doi: 10.1016/j.healthpol.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Alp E, Cevahir F, Gokahmetoglu S, Demiraslan H, Doganay M. Prevaccination screening of health-care workers for immunity to measles, rubella, mumps, and varicella in a developing country: what do we save? J Infect Public Health. 2012;5(2):127–32. doi: 10.1016/j.jiph.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Celikbas A, Ergonul O, Aksaray S, Tuygun N, Esener H, Tanir G, Eren S, Baykam N, Guvener E, Dokuzoguz B. Measles, rubella, mumps, and varicella seroprevalence among health care workers in Turkey: is prevaccination screening cost-effective? Am J Infect Control. 2006;34(9):583–87. doi: 10.1016/j.ajic.2006.04.213. [DOI] [PubMed] [Google Scholar]
- 49.Gurgoze MK, Yilmaz E, Godekmerdan A, Akca Z, Dogan Y, Akarsu S, Aygun AD. Seroprevalence of mumps, varicella and rubella antibodies in children 1–16 years of age in eastern Turkey. Turk J Pediatr. 2006;48:185–88. [PubMed] [Google Scholar]
- 50.Karasahin O, Civil F, Ozger S, Measles-mumps-rubeola HK. (MMR), varicella, and hepatitis A (HAV) seroprevalances among healthcare workers and their compliance to vaccination. Int J Infect Dis. 2014;21(S1):252–53. doi: 10.1016/j.ijid.2014.03.945. [DOI] [Google Scholar]
- 51.Kanra G, Tezcan S, Badur S. Turkish National Study Team. Varicella seroprevalence in a random sample of the Turkish population. Vaccine. 2002;20(9–10):1425–28. doi: 10.1016/S0264-410X(01)00459-5. [DOI] [PubMed] [Google Scholar]
- 52.Kose S, Mandiracioglu A, Senger SS, Ulu Y, Cavdar G, Gol B, Gurbuz I, Sariavci S, Nohutcu N. Seroprevalence of varicella-zoster virus in the prevaccine era: A population-based study in Izmir, Turkey. J Infect Public Health. 2013;6(2):115–19. doi: 10.1016/j.jiph.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Aypak C, Bayram Y, Eren H, Altunsoy A, Berktaş M. Susceptibility to measles, rubella, mumps, and varicella-zoster viruses among healthcare workers. J Nippon Med Sch. 2012;79(6):453–58. doi: 10.1272/jnms.79.453. [DOI] [PubMed] [Google Scholar]
- 54.Ozkan S, Maral I, Ilhan F, Aycan S, Cirak MY, Beyazova U, Aygun R. Varicella zoster seroprevalence in children less than 5 years old. J Trop Pediatr. 2005;51(3):141–44. doi: 10.1093/tropej/fmh102. [DOI] [PubMed] [Google Scholar]
- 55.Kurugol Z, Koturoglu G, Aksit S, Ozacar T. Varicella seroprevalence in Turkish population in Cyprus. Acta Paediatr. 2007;96(6):861–63. doi: 10.1111/j.1651-2227.2007.00289.x. [DOI] [PubMed] [Google Scholar]
- 56.Savas S, Dallar Y, Arikan I, Onde U. Varicella-zoster virus seroprevalence in children between 0–15 years old. Mikrobiyol Bul. 2004;38:69–75. [PubMed] [Google Scholar]
- 57.Barah F. Prevalence of herpes simplex types 1 and 2, varicella zoster virus, cytomegalovirus, immunoglobulin G antibodies among female university students in Syria. Saudi Med J. 2012;33(9):990–94. [PubMed] [Google Scholar]
- 58.Sheek-Hussein M, Hashmey R, Alsuwaidi AR, Al Maskari F, Amiri L, Souid AK. Seroprevalence of measles, mumps, rubella, varicella-zoster and hepatitis A-C in Emirati medical students. BMC Public Health. 2012;12:1047. doi: 10.1186/1471-2458-12-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uduman SA, Tahira AM, Al-Wash R, Usmani MA, Bener A. Varicella susceptibility among children and healthy adults in the United Arab Emirates. East Mediterr Health J. 2001;7(4-5):604–08. [PubMed] [Google Scholar]
- 60.Guanche Garcell H, Villanueva Arias A, Guilarte Garcia E, Alfonso Serrano RN. Seroprotection against vaccine-preventable diseases amongst health care workers in a community hospital, Qatar. Int J Occup Environ Med. 2016;7(4):234–40. doi: 10.15171/ijoem.2016.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Musharrafieh UM, Nuwayhid IA, Hamadeh GN, Steitieh SW, Bizri AR. Immunity to chickenpox among school adolescents in Lebanon and options for vaccination. Epidemiol Infect. 2002;129(3):607–15. doi: 10.1017/S0950268802007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abro AH, Ustadi AM, Das K, Abdou AM, Hussaini HS, Chandra FS. Chickenpox: presentation and complications in adults. J Pak Med Assoc. 2009;59(12):828–31. [PubMed] [Google Scholar]
- 63.Koturoglu G, Kurugol Z, Cetin N, Hizarcioglu M, Vardar F, Helvaci M, Capar Z, Ozkinay F, Ozkinay C. Complications of varicella in healthy children in Izmir, Turkey. Pediatr Int. 2005;47(3):296–99. doi: 10.1111/j.1442-200x.2005.02054.x. [DOI] [PubMed] [Google Scholar]
- 64.Turel O, Bakir M, Gonen I, Hatipoglu N, Aydogmus C, Hosaf E, Siraneci R. Children hospitalized for varicella: complications and cost burden. Value Health Reg Issues. 2013;2(2):226–30. doi: 10.1016/j.vhri.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Aebi C, Ahmed A, Ramilo O. Bacterial complications of primary varicella in children. Clin Infect Dis. 1996;23(4):698–705. doi: 10.1093/clinids/23.4.698. [DOI] [PubMed] [Google Scholar]
- 66.Ginsberg GM, Somekh E. Cost containment analysis of childhood vaccination against varicella in Israel. J Infect. 2004;48(2):119–33. doi: 10.1016/S0163-4453(03)00079-3. [DOI] [PubMed] [Google Scholar]
- 67.Dinleyici EC, Kurugol Z, Bayram N, Devrim I, Tezer H, Yasa O, Kuzdan C, Siraneci R, Alhan E, Dalgic N, et al. Early impact of universal single dose varicella vaccine on hospitalization in Turkey (VARICOMP study 2008–2015). 34th Annual Meeting of European Society for Pediatric Infectious Disease (ESPID); 2016. May 10–14; Birghton, United Kingdom. ESP16–0091. https://espid2016.kenes.com/Documents/ESPID16_Abstracts.pdf. [Google Scholar]
- 68.Esmaeeli S, Yaghoubi M, Nojomi M. Cost-effectiveness of varicella vaccination program in Iran. Int J Prev Med. 2017;8:103. doi: 10.4103/ijpvm.IJPVM_295_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dinleyici EC, Kurugol Z, Bayram N, Devrim I, Tezer H, Yasa O, Kuzdan C, Siraneci R, Alhan E, Dalgic N, et al. Vaccine immunogenicity, efficacy, effectiveness of universal single dose varicella vaccine on cause of hospitalizations in Turkey(VARICOMP study 2008–2015). 34th Annual Meeting of European Society for Pediatric Infectious Disease (ESPID); 2016. May 10–14; Birghton, United Kingdom. ESP16–0145. https://espid2016.kenes.com/Documents/ESPID16_Abstracts.pdf. [Google Scholar]
- 70.Dinleyici EC, Kurugol Z, Carman KB. OC68: neurologic causes of varicella related hospitalizations in Turkey (VARICOMP study 2008–2015). Eur J Paediatr Neurol. 2017;21:e91. doi: 10.1016/j.ejpn.2017.04.670. [DOI] [Google Scholar]
- 71.van Lier A, Tostmann A, Harmsen IA, de Melker HE, Hautvast JL, Ruijs WL. Negative attitude and low intention to vaccinate universally against varicella among public health professionals and parents in the Netherlands: two internet surveys. BMC Infect Dis. 2016;16:127. doi: 10.1186/s12879-016-1442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Centers for Disease Control and Prevention . Chickenpox (varicella) complications. [accessed 2018 Dec 7]. https://www.cdc.gov/chickenpox/about/complications.html.
- 73.Wysocki J, Malecka I, Stryczynska-Kazubska J, Rampakakis E, Kuter B, Wolfson LJ. Varicella in Poland: economic burden in children 1–12 years of age in Poland, 2010–2015. BMC Public Health. 2018;18(1):410. doi: 10.1186/s12889-018-5298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meszner Z, Molnar Z, Rampakakis E, Yang HK, Kuter BJ, Wolfson LJ. Economic burden of varicella in children 1–12 years of age in Hungary, 2011–2015. BMC Infect Dis. 2017;17(1):495. doi: 10.1186/s12879-017-2575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giglio N, Monsanto H, Rampakakis E, Yang HK, Kuter BJ, Wolfson LJ. Economic burden of varicella in children 1–12 years of age in Argentina, 2009–2014. J Med Econ. 2018;21(4):416–24. doi: 10.1080/13696998.2018.1431919. [DOI] [PubMed] [Google Scholar]
- 76.Nader F, Askarian M. How do Iranian physicians report notifiable diseases? The first report from Iran. Am J Infect Control. 2009;37(6):500–04. doi: 10.1016/j.ajic.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 77.Republic of Lebanon Ministry of Public Health . Epidemiological surveillance. [accessed 2018 Dec 7]. https://moph.gov.lb/en/Pages/2/193/esu.
- 78.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- World Health Organization . Varicella and herpes zoster vaccination position paper. [accessed 2019. January 10]. https://apps.who.int/iris/bitstream/handle/10665/242227/WER8925_265-287.PDF.
- World Health Organization . Immunization, vaccines and biologicals: varicella. [accessed 2018. December 7]. https://www.who.int/immunization/diseases/varicella/en/.


