Abstract
Background:
Preclinical studies suggest that the non-selective phosphodiesterase inhibitor, Ibudilast (IBUD) may contribute to the treatment of METH use disorder through the attenuation of METH-induced inflammatory markers such as adhesion molecules, sICAM-1 and sVCAM-1, and cytokines, IL-6 and TNF-α.
Objective:
The present study aimed to test whether treatment with IBUD can attenuate peripheral markers of inflammation during a METH challenge in an inpatient clinical trial of 11 patients.
Methods:
This trial followed a randomized, within-subjects crossover design where participants received a METH challenge, during which five participants were treated with placebo then with IBUD, while the remaining six participants were treated with IBUD prior to placebo. Mixed effects regression modeled changes in peripheral markers of inflammation—sICAM-1, sVCAM-1, TNF-α, IL-6, MIF, and cathepsin D—by treatment condition, with measurements at baseline, 60 minutes post-METH infusion, and 360 minutes post-METH infusion.
Results:
While on placebo, sICAM-1, sVCAM-1, and cathepsin D significantly increased by 60 minutes post-METH infusion, while IL-6 significantly increased 360 minutes post-METH infusion. Treatment with IBUD significantly reduced METH-induced levels of sICAM-1, sVCAM-1, and cathepsin D at 60 minutes post-METH infusion.
Conclusions:
Our findings demonstrate that IBUD attenuated acute pro-inflammatory effects of METH administration, which may have implications for treatment of METH use disorder.
Keywords: phosphodiesterase inhibitor, Ibudilast, methamphetamine, inflammation, cytokine, anti-inflammatory
1. Background
Methamphetamine (METH) use disorder (MUD) poses risk of serious medical and psychiatric problems, yet in the U.S., there are currently no approved medications with demonstrated efficacy for the treatment of MUD (Ballester et al., 2017). However, accumulating evidence from both preclinical and human subjects research suggest that targeting factors involved in METH-induced neuroinflammation may be a strategy for developing a potential therapy for MUD (Beardsley et al., 2010; Birath et al., 2017; DeYoung et al., 2016; Loftis and Janowsky, 2014; Snider et al., 2013; Worley et al., 2016).
METH-induced inflammatory signaling involving cytokines (Loftis et al., 2011; Loftis and Janowsky, 2014; Snider et al., 2013) and adhesion molecules (Loftis et al., 2011; Loftis and Janowsky, 2014), as well as changes in neuroplasticity due to dysregulation of cell death factors (Kanthasamy et al., 2006) may be among the factors driving METH neurotoxicity. Acute inflammation vital to protecting the central nervous system from acute tissue damage or infection. For example, acute inflammatory responses to lipopolysaccharide and METH have been shown to protect dopaminergic neurons and play a role in reestablishing homoeostasis following METH-induced vascular and neuronal injury (Czeh et al., 2011; Shaerzadeh et al., 2018). However, based on experimental research, chronic inflammation has recently been considered an underlying risk factor in the development of depression, anxiety, and impaired neurocognition (Bollen et al., 2017; Michopoulos et al., 2017; Miller and Raison, 2016), problems often associated with MUD (Zweben et al., 2004). In response to persistent or high doses of METH use, chronic inflammation is associated with impaired memory loss, depression, anxiety, and other psychiatric problems, and may contribute to MUD (Huckans et al., 2015; Loftis et al., 2011; Loftis and Janowsky, 2014; McAfoose and Baune, 2009). In preclinical models, high METH doses and repeated METH exposure have been shown to be associated with activation of microglia, and in turn, increased expression of cytokines (e.g., IL-6, TNF-α, and MIF), adhesion molecules (e.g., ICAM-1), and the lysosomal protease (e.g., cathepsin D) (Kanthasamy et al., 2006; Loftis et al., 2011; Loftis and Janowsky, 2014; McAfoose and Baune, 2009; Snider et al., 2013). Although the mechanisms by which chronic METH-induced inflammation may impair cognitive functioning is not fully understood, it has been suggested that that these effects may be explained by prolonged, inflammatory-mediated damage to the dopaminergic, serotonergic, and vascular systems, as well as to the blood–brain barrier (Blaker et al., 2016; Ghanbari et al., 2019; Loftis et al., 2011). For these reasons, examining inflammatory marker expression in response to METH challenges in humans may help to evaluate new pharmacological treatments for MUD, particularly those that target neuroinflammation (Beardsley et al., 2010; Worley et al., 2016).
Ibudilast (IBUD) is a non-selective phosphodiesterase (PDE) inhibitor and glial activation inhibitor that has shown potential to reduce METH self-administration and reinstatement in rodents (Brensilver et al., 2013; Worley et al., 2016). IBUD potently inhibits a range of phosphodiesterase families, primarily PDE4, as well as PDEs 3, 10, and PDE11 with IC50s ranging from approximately 1 – 10 μM (Gibson et al., 2006; Huang et al., 2006; Rolan et al., 2009). The inhibition of PDEs is considered to be the principle mode of action by which IBUD bestows its anti-inflammatory effects in both the peripheral immune system and central nervous system (Huang et al., 2006; Kishi et al., 2001; Mizuno et al., 2004; Rolan et al., 2009). As such, IBUD has been tested for its clinical utility in attenuating inflammatory-related diseases such as neurodegenerative disorders, asthma, allergies, and neuropathic pain (Barkhof et al., 2010; Fox et al., 2018; Kishi et al., 2001; Mizuno et al., 2004; Rolan et al., 2009). Given these pharmacologic properties, IBUD has recently been studied for its potential to reduce the symptoms of substance use disorders and substance use withdrawals—such as with alcohol, opioids, and METH. In preclinical research. IBUD has been shown to suppress expression of adhesion molecules and cytokines in rat brains, suggesting that IBUD may have the potential to inhibit the inflammatory response induced by METH use (Kitazato et al., 2010; Lee et al., 2012). Preliminary research has indicated that treatment with IBUD during METH infusion was associated with reduced subjective effects of METH (e.g., discomfort, sadness, nervousness) (DeYoung et al., 2016; Worley et al., 2016) and improved sustained attention during early abstinence from METH (Birath et al., 2017). However, further research is needed to determine whether IBUD can attenuate inflammatory responses to METH use (Beardsley et al., 2010; Worley et al., 2016).
The purpose of this study is to determine whether IBUD can attenuate METH-induced production of peripheral markers of inflammation, including soluble cellular adhesion molecules (sICAM-1 and sVCAM-1), cytokines (TNF-α, IL-6, and MIF), and the lysosomal aspartyl protease cathepsin D.
2. Methods
2.1. Participants
Participants were 11 people with METH use disorder (MUD) who completed a placebo-controlled, planned crossover trial of IBUD treatment during METH infusion (Birath et al., 2017; DeYoung et al., 2016; Worley et al., 2016). The mean age of the sample was 42 years (SD = 6.7), 82% (n = 9) were male, and 63.6% (n = 7) identified as white. Participants were eligible for the study if they were aged 18–55, met the DSM-IV-TR criteria for METH use disorder (MUD) as verified by the Structured Clinical Interview for DSM-IV Disorders (First and Gibbon, 2004), were not seeking MUD treatment, urine-tested positive for METH at least once prior to admission, and had normal vital signs for cardiovascular, hepatic, and renal functioning. Participants were excluded if they had current alcohol use or other substance use disorder, seizure disorder, history of head trauma, current psychotropic treatment, recent suicide attempt, or major medical or psychiatric illness, including infections such as HIV and Hepatitis C that could induce inflammatory marker expression. The institutional review boards of LA Biomed and UCLA oversaw all study procedures.
2.2. Study design
The present study tests an a priori stated hypothesis using secondary data analysis of a within-subjects, planned crossover trial with repeated measurements within each treatment period. The 11 participants were randomized to two treatment sequences where five participants received placebo followed by steady state of 50 mg IBUD BID, while the remaining six participants received 50 mg IBUD BID followed by placebo. Participants received each treatment for seven consecutive days with a five-day washout period between each treatment. After five days at each dose (steady state), participants received a 30 mg METH intravenous (IV) challenge delivered over two minutes. Immunoassays were performed on blood samples taken during both 50 mg IBUD treatment and placebo at the following time points: baseline prior to METH infusion, 60 minutes post-METH-infusion, and 360 minutes post-METH-infusion. As such, analyses encompass six data points across 11 participants, for a total of 66 participant-observations. More information on the clinical trial’s methods and primary findings are described in prior publication (DeYoung et al., 2016).
2.3. Inflammatory Marker Assay and Analysis
Whole blood was collected in EDTA vacutainers and plasma was extracted, aliquotted, and stored at −80°C until analyzed. Peripheral levels of cytokines, chemokines, and cellular adhesion molecules were measured in duplicate by commercial assay services (Eve Technologies, Calgary AB, Canada) using multiplex immunoassays analyzed with a BioPlex 200. Mixed effects linear regression was used to model within-subject effects of METH-infusion (30 mg) on inflammatory marker levels across three time points within treatment period (baseline, 60 minutes post-METH, and 360 minutes post-METH), as well as within-subject effects of IBUD (50 mg BID) compared to placebo (0 mg BID). Use of mixed effects linear regression allowed us to model treatment (IBUD vs. placebo) as varying within-participant (as all participants received both IBUD and placebo), and this approach accounts for random intercepts between-participants and constant correlation within-participant. Therefore, all participants served as their own controls. The fixed effects parameters included time (to estimate temporal effects of METH infusion), IBUD (to estimate baseline differences by treatment), and time x IBUD interaction term (to test whether IBUD attenuates temporal effects of METH). Treatment sequence was included as a covariate to adjust for possible carryover effects among the six participants who received placebo after completing both IBUD dose phases of the design, though carryover effects are minimized with the five-day washout period prior to blood draw. Based on the fitted models, we computed post hoc predicted means of these inflammatory markers for both IBUD and placebo across time, and then tested for significant changes in these predicted means within each treatment condition. Random intercepts adjusted for within-participant correlations. Power analysis indicated that the 66 participant-observations would meet the minimum threshold of 60 observations for this mixed effects regression, with an anticipated medium effect size of 0.15 and a power of 0.80.
3. Results
Table 1 displays the regression estimates for the mixed models of IBUD on peripheral inflammatory markers sICAM-1, sVCAM-1, cathepsin D, IL-6, TNF-α, and MIF over 360 minutes following METH infusion. Post hoc predicted means of these inflammatory markers are reported over time for both IBUD and placebo at the bottom of Table 1, along with p-values corresponding to changes from the prior time point. Figure 1 illustrates these trajectories for sICAM-1, sVCAM-1, cathepsin D, and IL-6 over the 360-minute period, by treatment condition (note that the illustrated confidence intervals correspond to individual point estimates).
Table 1.
Mixed effects regression estimates (B) and post-hoc predicted means (M) of peripheral inflammatory markers by IBUD (vs. placebo) after METH infusion
| sICAM-1 (ng) | sVCAM-1 (ng) | cathepsin D (ng) | IL-6 (pg) | TNF-α (pg) | MIF (ng) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regression estimates | B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | B (95% CI) | p |
| IBUD | 0.16 (−13.30, 13.63) |
0.981 | −40.36 (−108.09, 27.37) |
0.243 | −5.86 (−31.62, 19.89) |
0.655 | 5.25 (0.61, 9.89) |
0.027 | 11.55 (−12.93, 36.03) |
0.355 | 2.08 (−0.18, 4.35) |
0.071 |
| Time | ||||||||||||
| 60 min. | 17.80 (6.49, 29.11) |
0.002 | 93.70 (13.06, 174.34) |
0.023 | 27.93 (7.19, 48.66) |
0.008 | 6.07 (−1.75, 13.89) |
0.128 | −8.89 (−32.78 14.99) |
0.466 | 0.08 (−0.25, 0.42) |
0.626 |
| 360 min. | 3.09 (−15.43, 21.61) |
0.744 | 41.56 (−61.25, 144.37) |
0.428 | 4.89 (−17.62, 27.40) |
0.670 | 24.50 (3.24, 45.75) |
0.024 | 41.76 (−41.75, 125.27) |
0.327 | 0.93 (−0.31, 2.17) |
0.143 |
| IBUD x time | ||||||||||||
| 60 min. | −17.29 (−34.50, −0.07) |
0.049 | −113.21 (−224.00, −2.42) |
0.045 | −30.32 (−60.36, −0.29) |
0.048 | −6.08 (−13.69, 1.54) |
0.118 | 11.79 (−15.13, 38.70) |
0.391 | −1.93 (−4.11, 0.25) |
0.082 |
| 360 min. | −10.04 (−24.77, 4.70) |
0.182 | −55.94 (−163.53, 51.65) |
0.308 | −7.38 (−43.15, 28.39) |
0.686 | −16.39 (−34.53, 1.76) |
0.077 | −17.50 | 0.383 | −1.38 (−4.68, 1.93) |
0.414 |
| (−56.83, 21.83) | ||||||||||||
| Sequence | 18.15 (−18.61, 54.91) |
0.333 | −8.76 (−207.11, 189.59) |
0.931 | 50.24 (−24.62, 125.10) |
0.188 | 4.36 (−10.67, 19.39) |
0.144 | 53.94 (−49.10, 156.98) |
0.305 | 0.18 (−1.35, 1.71) |
0.820 |
| Predicted means | M (95% CI) | p† | M (95% CI) | p† | M (95% CI) | p† | M (95% CI) | p† | M (95% CI) | p† | M (95% CI) | p† |
| Placebo | ||||||||||||
| 0 min. | 116.39 (90.47, 142.30) |
614.50 (498.84, 730.15) |
163.91 (122.74, 205.07) |
6.94 (4.06, 9.81) |
28.23 (−0.44 56.90) |
0.57 (0.18, 0.97) |
||||||
| 60 min. | 134.19 (110.38, 157.99) |
0.002 | 708.19 (564.11, 852.27) |
0.023 | 191.83 (138.08, 245.59) |
0.008 | 13.00 (6.26, 19.75) |
0.128 | 19.34 (2.46, 36.22) |
0.466 | 0.66 (0.37, 0.94) |
0.626 |
| 360 min. | 119.47 (103.64, 135.31) |
0.076 | 656.06 (538.02, 774.09) |
0.193 | 168.79 (131.06, 206.53) |
0.069 | 31.43 (8.15, 54.72) |
0.106 | 69.99 (−38.43, 178.41) |
0.354 | 1.50 (0.30, 2.70) |
0.105 |
| IBUD | ||||||||||||
| 0 min. | 116.55 (93.07, 140.03) |
574.14 (471.13, 677.15) |
158.04 (117.19, 198.90) |
12.18 (5.35, 19.02) |
39.78 (−11.09, 90.65) |
2.66 (0.44, 4.87) |
||||||
| 60 min. | 117.06 (95.38, 138.74) |
0.911 | 554.63 (446.04, 663.22) |
0.420 | 155.65 (110.92, 200.37) |
0.828 | 12.18 (5.71, 18.65) |
0.997 | 42.67 (−10.99, 96.33) |
0.218 | 0.81 (0.30, 1.32) |
0.069 |
| 360 min. | 109.60 (90.92, 128.28) |
0.169 | 559.76 (453.48, 666.03) |
0.873 | 155.55 (116.79, 194.31) |
0.987 | 20.29 (5.52, 35.06) |
0.152 | 64.04 (−29.79, 157.87) |
0.310 | 2.21 (0.93, 3.49) |
0.066 |
p-value corresponds to change in the predicted mean since the prior time point (e.g., 360 min. compared to 60 min.)
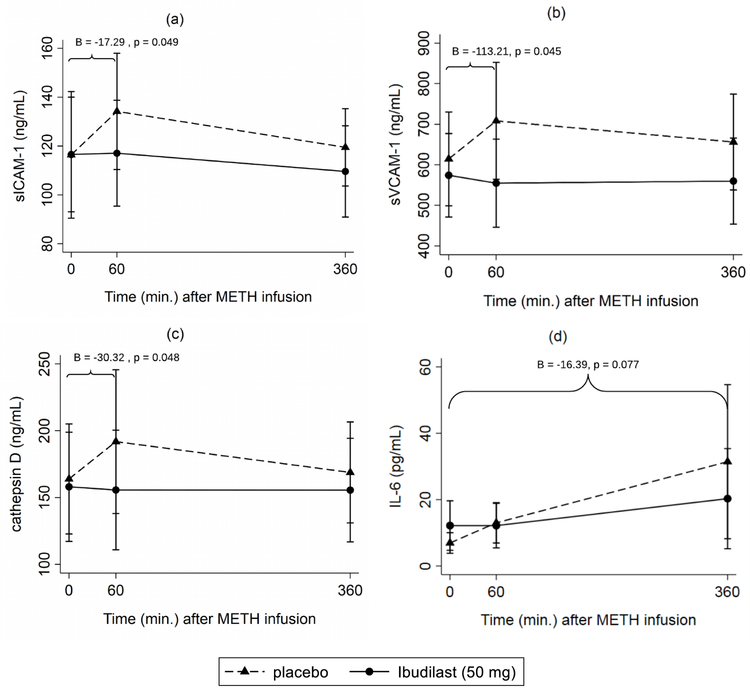
Figure 1.
Predicted means of (a) sICAM-1, (b) sVCAM-1, (c) cathepsin D, and (d) IL-6 by treatment condition (50 mg IBUD vs. placebo) over time. Regression estimates (B) correspond to time intervals in which METH-induced biomarker changes (slopes) are significantly lower with IBUD compared to placebo.
Note: Illustrated confidence intervals correspond to individual point estimates rather than regression estimates of treatment effects.
3.1. Inflammatory marker expression following methamphetamine infusion
In the absence of IBUD, 60 minutes following METH infusion sICAM-1 increased by 17.80 ng/mL (95 % CI [6.49, 29.11], p=.002), sVCAM-1 increased by 93.70 ng/mL (95% CI [13.06, 174.34], p = .023), and cathepsin D increased by 27.93 ng/mL (95% CI [7.19, 48.66], p = .008) (Table 1). After this increase, levels of sICAM-1, sVCAM-1, and cathepsin D levels gradually decreased from 60 to 360 minutes in the absence of IBUD, albeit not significantly (see predicted means in Table 1 and Figure 1). IL-6, TNF-α, and MIF did not significantly increase 60 minutes post-METH. At 360 minutes post-METH infusion, IL-6 significantly increased compared to baseline by 24.50 pg/mL (95% CI [3.24, 45.75], p = .024). There were no statistically significant increases in other inflammatory markers following METH-infusion.
3.2. IBUD treatment and inflammatory marker expression following methamphetamine infusion
From baseline to 60 minutes post-METH infusion, treatment with IBUD attenuated METH-induced pro-inflammatory response of sICAM-1 by −17.29 ng/mL (95% CI [−34.50, −0.07] p = .049, sVCAM-1 by −113 ng/mL (95% CI [−224.00, −2.42], p = 0.045) and cathepsin D by −30.32 ng/mL (95% CI [−60.36, −0.29], p = 0.048) compared to the placebo condition (Table 1). With IBUD, levels of sICAM-1, sVCAM-1, and cathepsin D remained steady during the entire 360-minute period following MA infusion, and did not significantly change from baseline levels (see predicted means in Table 1 and Figure 1). By 360 minutes post-METH, treatment with IBUD attenuated the METH-induced response of IL-6 by −16.39 pg/mL but did not meet the threshold for significance (95% CI [−34.53, 1.76], p = .077). IBUD was not associated with reduced METH-related changes in other pro-inflammatory markers at 60 or 360 minutes.
4. Discussion
Within-subjects analysis of this randomized, escalation trial of steady state 50 mg IBUD BID versus placebo show significant reductions in specific peripheral inflammatory markers observed at 60 minutes following 30 mg METH infusion. As predicted, in the absence of IBUD, METH administration increased several peripheral markers of inflammation, specifically adhesion molecules sVCAM-1 and sICAM-1, and the protease cathepsin D, which then decreased gradually from 60 to 360 minutes post-METH infusion. These inflammatory patterns are consistent with the pharmacokinetics of METH, where METH levels peak at one hour following intravenous administration before gradually decreasing via metabolism (Cook et al., 1993). Treatment with IBUD reduced the acute effects of METH on sICAM-1, sVCAM-1, and cathepsin D levels 60 minutes after METH infusion. It is also worth noting that with IBUD, sICAM-1, sVCAM-1, and cathepsin D levels remained steady during the entire 360-minute observation period as shown in the predicted means in Table 1 and Figure 1. Therefore, it also is possible that IBUD played a role in sustaining these lower levels even after 60 minutes. Together, these findings suggest that IBUD may have protective utility against the acute inflammatory responses that would otherwise occur soon after METH administration.
Our findings are consistent with prior experimental research showing that administration of METH in both humans and mice induces expression of sICAM-1 and sVCAM-1 (Gonçalves et al., 2017; Loftis et al., 2011). IBUD’s reductions of METH-induced sICAM-1 and sVCAM-1 may have important implications for METH treatment. In multiple studies, elevated ICAM-1 and VCAM-1 has shown to mediate anxiety, depression, and memory problems in adults with METH use disorder (MUD) (Huckans et al., 2015; Loftis et al., 2011), psychiatric problems commonly comorbid with chronic METH use (Loftis and Janowsky, 2014; Vearrier et al., 2012) and recently theorized to be rooted in inflammation (Michopoulos et al., 2017; Miller and Raison, 2016). Furthermore, prior IBUD research has shown that IBUD buffered the subjective effects of METH and improved attention in people with MUD (Birath et al., 2017; Worley et al., 2016). The protective effects of IBUD against inflammation, as demonstrated in this study, may provide a biological explanation for these cognitive and self-reported improvements.
Limited inferences can be made about IBUD’s direct impact on METH-induced inflammation in the brain because this study solely measured peripheral levels of inflammatory markers. However, multiple studies have shown that peripheral markers of inflammation coincide with neuroinflammation (McColl et al., 2014), especially in the presence of METH, which increases blood brain barrier permeability (Northrop and Yamamoto, 2012, 2015). Therefore, it is quite possible that the effects of IBUD on peripheral inflammation are linked with reductions in neuroinflammation. Although our findings show a direct effect of dampening inflammatory marker expression relevant to active use of methamphetamine in humans with METH addiction, it is difficult to ascertain the degree to which these differences in inflammation by IBUD are clinically meaningful without longitudinally assessing these inflammatory markers alongside long-term health outcomes among human subjects using METH. Furthermore, this pilot analysis of peripheral inflammatory markers was limited to 360 minutes after infusion of 30 mg METH. It is uncertain whether IBUD would continue to maintain baseline levels of inflammatory markers beyond 360 minutes after METH administration, or by extension, during an extended period of abstinence. Lastly, due to the small sample size of our pilot analysis, our study may not be fully powered to detect other anti-inflammatory effects.
Our findings provide insight into possible protective effects of IBUD against inflammation in METH use, which may be an important component of METH treatment and management of inflammation-related symptomatology. Future research in larger studies is needed to conduct tests of mediation to determine whether reductions in inflammation mediate the association between IBUD treatment and improved subjective effects, as well as whether these effects might have bearing on the use of IBUD in the context of METH abstinence.
Highlights.
Without Ibudilast, 30 mg methamphetamine infusion elevated sICAM-1, sVCAM-1, and cathepsin D in 60 minutes.
Without Ibudilast, 30 mg methamphetamine infusion elevated IL-6 in 360 minutes.
Treatment with Ibudilast decreased methamphetamine-induced responses of sICAM-1, sVCAM-1, and cathepsin D compared to the placebo condition.
Acknowledgements
This study was supported by the National Institute on Drug Abuse [R01DA029804, S. Shoptaw; and R01DA035054, K. Heinzerling], the National Institute of Mental Health (Center for HIV Identification, Prevention, and Treatment Services [P30MH058107; S. Shoptaw], and the University of California, Los Angeles Postdoctoral Fellowship Training Program in Global HIV Prevention Research [T32MH080634; Judith Currier]).
Role of Funding Source
No role declared.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No conflict declared.
Conflict of Interest
The authors declare no conflict of interest with this manuscript.
References
- Ballester J, Valentine G, Sofuoglu M, 2017. Pharmacological treatments for methamphetamine addiction: current status and future directions. Expert Review of Clinical Pharmacology 10(3), 305–314. [DOI] [PubMed] [Google Scholar]
- Barkhof F, Hulst HE, Drulović J, Uitdehaag BMJ, Matsuda K, Landin R, 2010. Ibudilast in relapsing-remitting multiple sclerosis: a neuroprotectant? Neurology 74(13), 1033–1040. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Shelton KL, Hendrick E, Johnson KW, 2010. The glial cell modulator and phosphodiesterase inhibitor, AV411 (Ibudilast), attenuates prime- and stress-induced methamphetamine relapse. Eur. J. Pharmacol 637(1), 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birath JB, Briones M, Amaya S, Shoptaw S, Swanson A-N, Tsuang J, Furst B, Heinzerling K, Obermeit L, Maes L, McKay C, Wright MJ, 2017. Ibudilast may improve attention during early abstinence from methamphetamine. Drug Alcohol Depend 178, 386–390. [DOI] [PubMed] [Google Scholar]
- Blaker AL, Northrop NA, Yamamoto BK, 2016. Chapter 30 - Peripheral Influences of Methamphetamine Neurotoxicity, in: Preedy VR (Ed.) Neuropathology of Drug Addictions and Substance Misuse Academic Press, San Diego, pp. 309–319. [Google Scholar]
- Bollen J, Trick L, Llewellyn D, Dickens C, 2017. The effects of acute inflammation on cognitive functioning and emotional processing in humans: A systematic review of experimental studies. J. Psychosom. Res 94, 47–55. [DOI] [PubMed] [Google Scholar]
- Brensilver M, Heinzerling KG, Shoptaw S, 2013. Pharmacotherapy of amphetamine-type stimulant dependence: An update. Drug and Alcohol Review 32(5), 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CE, Jeffcoat AR, Hill JM, Pugh DE, Patetta PK, Sadler BM, White WR, Perez-Reyes M, 1993. Pharmacokinetics of methamphetamine self-administered to human subjects by smoking S-(+)-methamphetamine hydrochloride. Drug Metab. Dispos 21(4), 717–723. [PubMed] [Google Scholar]
- Czeh M, Gressens P, Kaindl AM, 2011. The Yin and Yang of Microglia. Dev. Neurosci 33(3–4), 199–209. [DOI] [PubMed] [Google Scholar]
- DeYoung DZ, Heinzerling KG, Swanson A-N, Tsuang J, Furst BA, Yi Y, Wu YN, Moody DE, Andrenyak DM, Shoptaw SJ, 2016. Safety of Intravenous Methamphetamine Administration During Ibudilast Treatment. J. Clin. Psychopharmacol 36(4), 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, 2004. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II)
- Fox RJ, Coffey CS, Conwit R, Cudkowicz ME, Gleason T, Goodman A, Klawiter EC, Matsuda K, McGovern M, Naismith RT, Ashokkumar A, Barnes J, Ecklund D, Klingner E, Koepp M, Long JD, Natarajan S, Thornell B, Yankey J, Bermel RA, Debbins JP, Huang X, Jagodnik P, Lowe MJ, Nakamura K, Narayanan S, Sakaie KE, Thoomukuntla B, Zhou X, Krieger S, Alvarez E, Apperson M, Bashir K, Cohen BA, Coyle PK, Delgado S, Dewitt LD, Flores A, Giesser BS, Goldman MD, Jubelt B, Lava N, Lynch SG, Moses H, Ontaneda D, Perumal JS, Racke M, Repovic P, Riley CS, Severson C, Shinnar S, Suski V, Weinstock-Guttman B, Yadav V, Zabeti A, 2018. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. N. Engl. J. Med 379(9), 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanbari F, Khaksari M, Vaezi G, Hojati V, Shiravi A, 2019. Hydrogen Sulfide Protects Hippocampal Neurons Against Methamphetamine Neurotoxicity Via Inhibition of Apoptosis and Neuroinflammation. J. Mol. Neurosci 67(1), 133–141. [DOI] [PubMed] [Google Scholar]
- Gibson LCD, Hastings SF, McPhee I, Clayton RA, Darroch CE, Mackenzie A, MacKenzie FL, Nagasawa M, Stevens PA, MacKenzie SJ, 2006. The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. Eur. J. Pharmacol 538(1–3), 39–42. [DOI] [PubMed] [Google Scholar]
- Gonçalves J, Leitão RA, Higuera-Matas A, Assis MA, Coria SM, Fontes-Ribeiro C, Ambrosio E, Silva AP, 2017. Extended-access methamphetamine self-administration elicits neuroinflammatory response along with blood-brain barrier breakdown. Brain. Behav. Immun 62, 306–317. [DOI] [PubMed] [Google Scholar]
- Huang Z, Liu S, Zhang L, Salem M, Greig GM, Chi Chung C, Natsumeda Y, Noguchi K, 2006. Preferential inhibition of human phosphodiesterase 4 by ibudilast. Life Sci 78(23), 2663–2668. [DOI] [PubMed] [Google Scholar]
- Huckans M, Fuller BE, Chalker ALN, Adams M, Loftis JM, 2015. Plasma Inflammatory Factors Are Associated with Anxiety, Depression, and Cognitive Problems in Adults with and without Methamphetamine Dependence: An Exploratory Protein Array Study. Frontiers in Psychiatry 6(178). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanthasamy A, Anantharam V, Ali SF, Kanthasamy AG, 2006. Methamphetamine induces autophagy and apoptosis in a mesencephalic dopaminergic neuronal culture model: role of cathepsin D in methamphetamine-induced apoptotic cell death. Ann. N. Y. Acad. Sci 1074(1), 234–244. [DOI] [PubMed] [Google Scholar]
- Kishi Y, Ohta S, Kasuya N, Sakita S, Ashikaga T, Isobe M, 2001. Ibudilast: A Non-selective PDE Inhibitor with Multiple Actions on Blood Cells and the Vascular Wall. Cardiovascular Drug Reviews 19(3), 215–225. [DOI] [PubMed] [Google Scholar]
- Kitazato KT, Tamura T, Tada Y, Yagi K, Satomi J, Nagahiro S, 2010. Ibudilast Inhibits Cerebral Aneurysms by Down-Regulating Inflammation-Related Molecules in the Vascular Wall of Rats. Neurosurgery 66(3), 551–559. [DOI] [PubMed] [Google Scholar]
- Lee J-Y, Cho E, Ko YE, Kim I, Lee KJ, Kwon SU, Kang D-W, Kim JS, 2012. Ibudilast, a phosphodiesterase inhibitor with anti-inflammatory activity, protects against ischemic brain injury in rats. Brain Res 1431, 97–106. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Choi D, Hoffman W, Huckans MS, 2011. Methamphetamine Causes Persistent Immune Dysregulation: A Cross-Species, Translational Report. Neurotoxicity Res 20(1), 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A, 2014. Neuroimmune basis of methamphetamine toxicity. Int. Rev. Neurobiol 118, 165–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfoose J, Baune BT, 2009. Evidence for a cytokine model of cognitive function. Neurosci. Biobehav. Rev 33(3), 355–366. [DOI] [PubMed] [Google Scholar]
- McColl A, Thomson C, Graham G, Cavanagh J, 2014. Peripheral inflammation affects the central nervous system and biology of the brain. J. Neuroimmunol 275(1), 163. [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T, 2017. Inflammation in fear-and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 42(1), 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature reviews immunology 16(1), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Kurotani T, Komatsu Y, Kawanokuchi J, Kato H, Mitsuma N, Suzumura A, 2004. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology 46(3), 404–411. [DOI] [PubMed] [Google Scholar]
- Northrop NA, Yamamoto BK, 2012. Persistent neuroinflammatory effects of serial exposure to stress and methamphetamine on the blood-brain barrier. J. Neuroimmune Pharmacol 7(4), 951–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop NA, Yamamoto BK, 2015. Methamphetamine effects on blood-brain barrier structure and function. Front. Neurosci 9, 69–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolan P, Hutchinson MR, Johnson KW, 2009. Ibudilast: a review of its pharmacology, efficacy and safety in respiratory and neurological disease. Expert Opin. Pharmacother 10(17), 2897–2904. [DOI] [PubMed] [Google Scholar]
- Shaerzadeh F, Streit WJ, Heysieattalab S, Khoshbouei H, 2018. Methamphetamine neurotoxicity, microglia, and neuroinflammation. J. Neuroinflammation 15(1), 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, Hendrick ES, Beardsley PM, 2013. Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur. J. Pharmacol 701(1–3), 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vearrier D, Greenberg MI, Miller SN, Okaneku JT, Haggerty DA, 2012. Methamphetamine: history, pathophysiology, adverse health effects, current trends, and hazards associated with the clandestine manufacture of methamphetamine. Disease-a-Month 58(2), 38–89. [DOI] [PubMed] [Google Scholar]
- Worley MJ, Heinzerling KG, Roche DJO, Shoptaw S, 2016. Ibudilast attenuates subjective effects of methamphetamine in a placebo-controlled inpatient study. Drug Alcohol Depend. 162, 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi M, Parent D, Iguchi M, Methamphetamine Treatment, P., 2004. Psychiatric Symptoms in Methamphetamine Users. The American Journal on Addictions 13(2), 181–190. [DOI] [PubMed] [Google Scholar]