ABSTRACT
Diffuse large B cell lymphoma (DLBCL), a heterogeneous group of invasive disease, is the most common type of B-cell non-Hodgkin’s lymphomas. The mechanism of its development is closely related to the constitutive activation of NF-κB. In this study, we investigated the function and the mechanism of β-TRCP1 in DLBCL. CCK8 and EdU assays showed that β-TRCP1 could promote the growth of DLBCL cells under the stimulation of TNFα. Furthermore, overexpression of β-TRCP1 enhanced NF-κB activation in the presence of TNFα. Moreover, ectopic expression of β-TRCP1 decreased IκB-α expression but increased phospho-p65 expression. In addition, β-TRCP1 promoted cell cycle progression by accelerating G1-S phase transition. We also found that silencing of β-TrCP1 increased mitoxantrone-induced cell growth arrest and apoptosis. Based on these, we proposed that the expression of β-TRCP1 promoted cell proliferation via TNF-dependent NF-κB activation in DLBCL cells.
KEYWORDS: Diffuse large B cell lymphoma (DLBCL), tumor necrosis factor-α (TNF-α), nuclear factor kappa B (NF-κB), β-TRCP1, cell proliferation
1. Introduction
Diffuse large B cell lymphoma (DLBCL), a heterogeneous group of invasive disease, is the most common form of B-cell non-Hodgkin’s lymphomas. Most patients achieve sustained remission after treating with frontline treatment regimens such as R-CHOP, but 30–40% of patients still relapse or not sensitive to first-line treatment.1 DLBCL originates from the malignant transformation of B cells in the germinal centre (GC). According to characteristic gene-expression patterns, DLBCL can be subdivided into at least two subtypes. One type is germinal center B cell-like (GCB) expressing genes characteristic of germinal center B cells, and the other type is activated B cell like (ABC) expressing genes activation of peripheral blood B cells.2 Among the different subtypes, patients with ABC DLBCL have a significantly poor 5-year survival rate compared to patients with GCB DLBCL.3
The constitutive activation of nuclear factor kappa B (NF-κB) is one of the typical characteristics of ABC DLBCL and is also a key element that led to the poor prognosis. It was verified that NF-κB pathway was charged with B-cell activation, cell proliferation and apoptosis resistance2,4. NF-κB activation was categorized into two pathways, namely, the canonical (RelA/p50) and the non-canonical pathway (RelB/p50 or RelB/p52). In unstimulated cells, RelA/p50 dimers is usually present in the cytoplasm by interacting with NF-κB inhibitors (IκBα).5 NF-κB can be activated by a variety of factors, such as tumor necrosis factor-α (TNF-α), and bacteria endotoxin lipopolysaccharide (LPS). Under the stimulation of the above activators, IκBα is phosphorylated by the IκB kinase (IKK) and degraded via the ubiquitin-proteasome system. Then, the RelA/p50 dimers expose and translocate to the nucleus, leading to the transcription of target genes which may be involved in controlling cell cycle distribution and apoptosis occurrence5,6.
β-TrCP1, an E3 ubiquitin ligase, belongs to the F-box protein which containing a F-box motif at the N-terminus and seven WD40 repeats at the C-terminus. The functional characteristics of β-TrCP1 are determined by C-terminus7,8. Transcription process of NF-κB was considered to be regulated by β-TrCP1 through promoting IκBα degradation.8 It has been reported that β-TrCP1 promotes growth of a variety of tumors, including breast cancer, pancreatic carcinoma and glioblastoma9,10. In addition, β-TrCP1 plays a pivotal role in cell cycle progression by degradating of some key cell cycle related fators, including early mitotic inhibitor (EMI)-1/2, cell division cycle- (CDC)-25A/B, and β-Catenin.11
Adhesion of hematologic malignant cells to the bone marrow microenvironmental components protects cells from cytotoxic drugs induced apoptosis which is called cell adhesion-mediated drug resistance (CAM-DR).12 Considerable evidence suggests that CAM-DR is closely associated with cell cycle arrest.13 In addition, studies have shown that activation of the NF-κB signaling pathway is important for adhesion microenvironment to support tumor survival14,15. Thus, whether the clinical drug resistance of DLBCL is related to CAM-DR and the underlying mechanisms need further study.
In this article, we explored the role of β-TrCP1 in DLBCL. We found that β-TrCP1 promoted the proliferation of DLBCL cells by enhancing NF-κB activation under TNFα stimulation. Furthermore, we illuminated that silencing of β-TrCP1 sensitized DLBCL cells to chemotherapeutic drugs when cocultured with HS5 cells. These findings suggest that β-TrCP1 may be a promising small molecule for further therapeutic evaluation in DLBCL.
2. Materials and methods
2.1. Immunohistochemistry (IHC)
A total of 114 DLBCL tissue sections were obtained from the Department of Pathology, Nantong Tumor Hospital. The tissue sections were washed in xylene and rehydrated in 100%, 95%, and 80% graded alcohol concentrations. The sections were subsequently submerged in sodium citrate (pH 6.0) and heated at 121°C for 3 min to retrieve antigenicity. Then, 3% hydrogen peroxide was prepared to block endogenous peroxidase by incubating for 10 min at room temperature. After that, the sections were incubated with β-TrCP antibody (1:1000 dilution; Abcam) for 1 h at room temperature. After washing 3 times with phosphate-buffered saline (PBS, 0.1M, PH7.4), the sections were incubated with secondary antibody (Dako Diagnostics, Carpinteria, CA) for 1 h at room temperature, followed by adding DAB solution (Dako Diagnostics, Carpinteria, CA). The slides were then counterstained and dehydrated following a standard procedure, and then covered with coverslips.
2.2. Immunohistochemical staining evaluation
The immunostained slides were evaluated by two independent experienced pathologists. Staining evaluation was based on the extent and intensity of the staining tumor. Extent was recoded according to a percentage scale of positive tumor ranged from 0% to 100%: 0, no positive tumor; 1, ≤25% positive tumor cells; 2, 26‐50% positive tumor cells; 3, 51‐75% positive tumor cells; and 4, ≥76% positive tumor cells. Staining intensity was scored as follows: 0, no staining; 1, very weak; 2, weak; 3, moderate; or 4, strong. The final score was multiplied by the positive percentage score and the staining intensity score. The final score of 0–4 pointed for low expression and the final score of >4 pointed for high expression.
2.3. Western blot
Antibodies used in western blot were: anti-GAPDH (1:1000 dilution; Santa Cruz biotechnology), anti-β-TrCP (1:1000 dilution; Abcam), anti-IκB-α (1:1000 dilution; Cell Signaling Technology), Flag(1:1000 dilution; proteintech), anti-CyclinD1(1:1000 dilution; Cell Signaling Technology), anti-CyclinE1 (1:1000 dilution; Cell Signaling Technology), anti-P27 (1:5000 dilution; Abcam), m-IgGκBP-HRP (1:1000 dilution; Santa Cruz biotechnology) and mouse anti-rabbit IgG-HRP (1:1000 dilution; Santa Cruz biotechnology). For western blot, cells were centrifuged on a 4 ℃ centrifuge for 5 min at 1000 rpm. After washing three times with PBS, whole cell proteins were extracted with ProtLytic Protein Lysis and Sample Loading (NCM; Lot # 6524081). Protein samples were separated using SDS-PAGE gel and transferred to PVDF membranes. The protein samples membrane was nonspecific blocked in TBST containing 5% skim milk for 1 h. The primary antibody was incubated at 4°C overnight and washed 3 times for 5 min each time with TBST. The secondary antibody was incubated for 1 h at room temperature and washed 3 times for 5 min each time with TBST. Band detection was performed using Bio-Rad gel imaging system.
2.4. Cell culture
Human DLBCL cell lines OCI-Ly10, OCI-Ly3 and human embryonic kidney 293 (HEK-293T) cell were purchased from COBIOER (COBIOER BIOSCIENCES CO., Ltd). Human bone marrow stromal cell HS-5 was purchased from EK-Bioscience (Biotechnology Co., Ltd. Shanghai enzyme research). OCI-Ly3 and OCI-Ly10 were cultured in 1640 medium containing 20% FBS, HEK293T was cultured in DMEM medium containing 10% FBS, and HS5 was cultured in DMEM medium containing 10% FBS. The cells were cultured in an incubator with a condition of 37°C, 5% CO2.
2.5. Plasmid construction and lentivirus-mediated transduction
The plasmid and lentiviral vector of Flag-β-TrCP1 was purchased from Genechem (Shanghai Genechem Co., LTD). Transfection of the Flag-β-TrCP1 plasmid was performed with Lipofectamine 2000 according to the manufacturer’s protocol. For the lentivirus infection of target cells, transduction was carried out in the presence of 40 μL/mL virus infection reagent HitransGP, and the medium was changed 12 h later.
2.6. EdU assay
EdU kit was purchased from RIBOBIO (Guangzhou Ribo Bio Co., Ltd.). Fifty micro liters of cell suspension (1 × 105 cells) were smeared on glass slide after general culture or stimulation, and the air-dried cells were treated according to the manufacturer’s protocol. Observation was performed using a fluorescence microscope (Leica 4500).
2.7. CCK-8 assay
One hundred micro liters of cell suspension was inoculated into 96-well plates (Corning Inc., Corning, NY, USA) at approximately 1 × 105 cells per well and 10 μL of CCK-8 regeats (Dojindo, Ku- mamoto, Japan) was added per well. Absorbance was detected by Microplate reader (CMax Plus) with a measurement wavelength of 450 nm.
2.8. Cell cycle detection
Cells were centrifuged, washed three times with PBS, and taken from each tube, fixed with 70% alcohol at −20°C for 24 h, stained with Propidium iodide (propidium iodide SIGMA-ALORIC, P4170). Cells were detected by flow cytometry within 2 h.
2.9. Apoptosis detection
The stimulated cells were double-stained with APC Annexin V and 7AAD. The kit was purchased from BD PharmingenTM (561012) and cells were assessed by flow cytometry (BD FACSCaliburTM Flow Cytometer). TUNEL: The kit (In Situ Cell Death Detection Kit, TMR red) was purchased from SIGMA-ALORIC (Cat. NO. 12156792910). The experimental steps are based on the manufacturer’s protocol.
2.10. Data analysis
Data were analyzed using SPSS software (version 25.0, SPSS, Inc.). The significance of differences between experimental groups was analyzed using the Student’s two-tailed t-test. P < .05 was considered statistically significant.
3. Results
3.1. Expression and clinicopathological parameters of β-TrCP1 in DLBCL tissue specimens
As shown in Figure 1, β-TrCP1 predominantly existed in the cytoplasm. Of the 114 cases of DLBCL, 61 were determined as β-TrCP1 low-expression, and 53 were determined as β-TrCP1 high-expression. As shown in Table 1, β-TrCP1 expression was closely correlated with Ann Arbor staging, but was not associated with gender, age, Hans algorithm and Ki67 labeling index. The percentage of β-TrCP1 high-expression cases was significantly lower in stage I-II (34.04%, 16/47) compared with that in stage III-IV (55.22%, 37/67) (P= .026).
Figure 1.
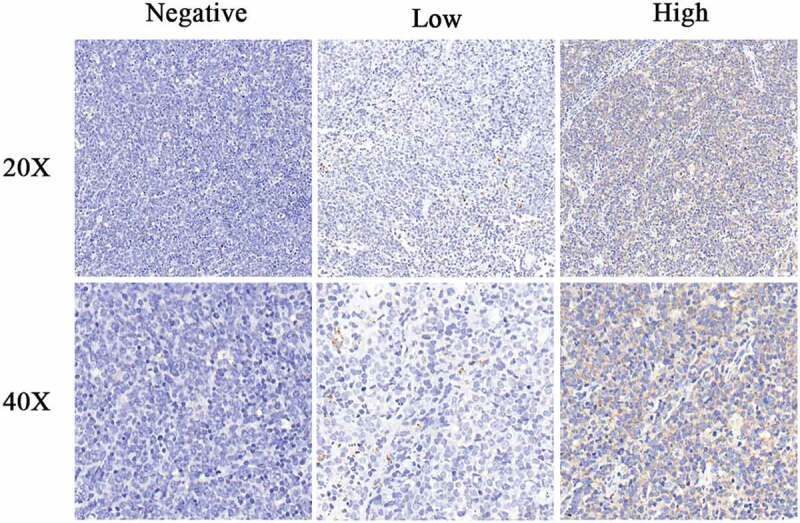
β-TrCP1 expression in DLBCL specimens. Representative images of immunohistochemically staining. Magnification: 200× (up), 400× (down).
Table 1.
Clinicopathological characteristics of DLBCL cases with β-TrCP1 expression.
| β-TrCP1 expression |
||||
|---|---|---|---|---|
| Characteristic | Patients,n | High(n = 53) | Low(n = 61) | P-value |
| Gender | 0.591 | |||
| Male | 55 | 27 | 28 | |
| Female | 59 | 26 | 33 | |
| Age,years | 0.276 | |||
| <60 | 54 | 28 | 26 | |
| ≥60 | 60 | 25 | 35 | |
| Ann Arbor stage | 0.026* | |||
| I-II | 47 | 16 | 31 | |
| III-IV | 67 | 37 | 30 | |
| Phenotype | 0.850 | |||
| GCB | 18 | 8 | 10 | |
| Non-GCB | 96 | 45 | 51 | |
| Ki67# | 0.466 | |||
| <80% | 45 | 23 | 22 | |
| ≥80% | 68 | 30 | 38 | |
Pearson’s χ2 test. *: P value is significant (<0.05). #:One case was not evaluated.
3.2. β-TrCP1 promotes the proliferation of DLBCL cells
EdU and CCK8 assays were applied to evaluate the proliferation and viability of the DLBCL cells. No significant differences of the quantity of EdU-labeled cells were found between control and β-TrCP1-overexpressing (β-TrCP1-OE) group. However, β-TrCP1 observably increased the EdU-labeled cells of OCI-ly10 and OCI-ly3 under the stimulation of TNFα (Figure 2(a–c)). To further verify the role of β-TrCP1 in regulating lymphoma cell growth, we examined the viability of OCI-ly10 and OCI-ly3 cells by CCK8. Cell growth curves showed that the survival cell rate was significantly increased under treatment with TNFα, and was further raised by β-TrCP1 overexpression (Figure 2(d,e)). These results revealed that β-TrCP1 might promote the proliferation of DLBCL cells.
Figure 2.

β-TrCP1 promotes the proliferation of DLBCL cells. (a-b) Immunofluorescence assay showed representative fields of EdU-labeled cells. EdU-incorporated signal were red and nucleus were stained blue with Hoechst in OCI-ly3, OCI-ly10 cells. (c-d) The histograms demonstrated mean with standard deviation of EDU-incorporated ratio from three independent experiments. (e) CCK8 was performed based on cell viability in OCI-ly3, OCI-ly10 cells. Data are shown as mean ± SEM performed with three replicates. Statistical significance was determined by student’s t-test. *, P < .05; #, P < .05.
3.3. β-trcp1 positively regulates NF-κB signaling pathway
It is reported that IκB-α is a classical ubiquitination substrate of the β-TrCP1. To confirm the mechanism underlying β-TrCP1-mediated NF-κB activation in DLBCL cells, β-TrCP1 was overexpressed in HEK-293, OCI-ly10 and OCI-ly3 cells. The luciferase assay revealed that overexpression of β-TrCP1 further promoted the transcriptional activity of the NF-κB under TNFα stimulation, while β-TrCP1 alone showed no significant difference on the activation of NF-κB (Figure 3(a–c)). To further confirm the effect of β-TrCP1 on NF-κB activation, IκBα expression was investigated. We found that overexpression of β-TrCP1 further decreased IκBα protein levels stimulated by TNFα (Figure3(d,e)). In addition, β-TrCP1 overexpression significantly increased phosphorylated p65 (Ser536) expression, but no change in p65 under stimulation of TNFα (Figure3(f,g)). These observations suggest that β-TrCP1 induces the canonical NF-κB signaling pathway in TNFα dependent manner.
Figure 3.

β-TrCP1 positively regulates of NF-κB signaling pathway. (a-c) pNF-κB-luc and pRL-TK were co-transfected in DLBCL cells with or without β-TrCP1 transfection. At 24 h post-transfection, the cells were treated with TNF-α. Then, a luciferase assay was performed. (d-e) IκBα level was decreased. The histograms demonstrated the expression ratio of IκBα relative to GAPDH by densitometry. (f) Enforced expression of β-TrCP1 increased the phosphorylation of p65 in OCI-ly3 and OCI-ly10 cells, while no significant change in p65 under the stimulation of TNF-α. (g) The histograms demonstrated the expression ratio of p-p65 and p65 relative to GAPDH by densitometry. *, P < .05; #, P < .05.
3.4. β-TrCP1 promotes G1-S phase transition
Numerous studies have shown that NF-κB is the key transcription factor of CyclinD1 and CyclinE1, which are critical regulators involved in G1-S phase transition. In human hepatoma cells, β-TrCP1 participates in the proteasomal degradation of CyclinD1.16 To verify whether β-TrCP1 regulates cell cycle progression in DLBCL, we first examined the expression of CyclinD1, CyclinE1 and p27kip in β-TrCP1 overexpressed cells. As shown in Figure 4(a,b), overexpression of β-TrCP1 obviously increased CyclinD1 and CyclinE1 expression, and reduced p27kip level in both OCI-ly10 and OCI-ly3 cells under treatment with TNFα. However, the expression levels of CyclinD1, CyclinE1 and p27kip were not significantly changed in the absence of TNFα. Moreover, flow cytometry analysis showed that TNFα could significantly decrease G0/G1 phase proportion independently, whereas ectopic expression of β-TrCP1 further reduced the percentage of cells in G0/G1 phase in the presence of TNFα compared with cells stimulated by TNFα only (Figure 4(c–e)). These results indicated that β-TrCP1 could promote G1-S phase transition in DLBCL cells.
Figure 4.

β-TrCP1 regulates cell cycle. (a) Expression of cyclinD1, cyclinE1 and P27kip in DLBCL cells. (b) The histograms demonstrated the expression ratio of cyclinD1, cyclinE1 and P27kip relative to GAPDH by densitometry. (c-e) Effect of TNFα and β-TrCP1 on cell cycle distribution in OCI-ly3 and OCI-ly10 cells. *, P < .05; #, P < .05.
3.5. Inhibition of β-TrCP1 attenuates drug resistance of DLBCL cells
Previous studies had shown that adhesion of NHL cells to bone marrow stromal cells or fibronectin could protect tumor cells from chemotherapeutic drugs. We also found that the expression levels of β-TrCP1 were significantly increased in DLBCL cells cocultured with bone marrow stromal cells HS5 (Figure 5(a)). To determine the role of β-TrCP1 in drug-resistance, DLBCL cells were transfected with siRNA-β-TrCP1 (β-TrCP1-KD) or siRNA-control, TUNEL assay showed that silencing of β-TrCP1 significantly increased the number of apoptotic cells (Figure5(b)). Flow cytometry again confirmed that DLBCL cells interfered with β-TrCP1 were more sensitive to Mito compared with control group (Figure5(c)). These findings collectively suggest that adhesion of DLBCL cells to HS5 significantly increased the expression level of β-TrCP1, and silencing of β-TrCP1 may attenuate drug resistance.
Figure 5.

β-TrCP1 promotes CAM-DR. (a) OCI-ly10 and OCI-ly3 were adhered to HS5-coated plates or cultured in suspension for 24 h. Then, the cells were harvested and the expression of β-TrCP1 was detected by western blot. (b) OCI-ly10 and OCI-ly3 cells transfected with β-TrCP1 or vector were added with 1 μM Mito. TUNEL were performed to evaluate the effect of β-TrCP1 on chemosensitivity. (c) OCI-ly10 and OCI-ly3 with or without β-TrCP1 knockdown were adhered to HS5-coated plates or cultured in suspension for 24 h along with adding 1 μM Mito. Flow cytometry assay and TUNEL were performed to evaluate the effect of β-TrCP1 on chemosensitivity. *, P < .05.
4. Discussion
Transcriptional activation of NF-κB is mediated by phosphorylation and degradation of its inhibitor, IκBα. NF-κB p65 forms a heterodimeric complex with p50 in the cytoplasm. IκBα degradation permits translocation of the NF-κB heterodimer p50/p65 to the nucleus, allowing subsequent DNA binding and gene activation17,18. Previously studies have reported that β‐TrCP1 can bind to its substrates IκBα, thereby directly participating in the activation of the NF‐κB.19 Studies have shown that β-TRCP1 can regulate cellular proliferation in many cancers, including breast cancer, myeloma and pancreatic cancer 20,16,21. In triple-negative breast cancers, downregulation of β-TrCP1 remarkably inhibits the proliferation of cancer cells.22 In addition, β-TRCP1 regulates proliferation and migration of breast and prostate cancer cells by degradation of MTSS1 (metastasis suppressor 1).9 A recent study shows that inhibition of β-TRCP1 by PDTC (pyrrolidine dithiocarbamate) significantly inhibits the growth and survival of multiple myeloma cells in mice. Then, we asked whether β-TRCP1 produces proliferative effect on DLBCL cells. EdU and CCK8 assays showed that there was no significant difference between β-TRCP1-overexpressed cells and control group in the absence of TNFα. However, β-TrCP1 could promote the proliferation and growth of DLBCL cells in the presence of TNFα.
Since NF-κB plays a critical role in cancer progression,23 we speculated that β-TrCP1 may promote the proliferation and growth of DLBCL cells through activation of NF-κB pathway. To prove this, dual luciferase reporter assay was performed, and we found that β-TrCP1 could enhance the activation of NF-κB pathway under the stimulation of TNFα in OCI-ly10 and OCI-ly3 cells. Furthermore, we found that overexpression of β-TrCP1 decreased IκBα protein levels stimulated by TNFα. In addition, β-TrCP1 overexpression increased phosphorylated p65 (Ser536) expression, but no change in p65 under stimulation of TNFα As reported, NF-κB is closely related to G1/S phase transition. P65 is an important transcription factor for CyclinD1 and CyclinE124,25. β-TrCP1, a member of the F-box protein family, is involved in G2/M phase transition and ubiquitination of various important cell cycle regulatory genes, including EMI-1/2, CDC-25A/B, and β-Catenin.26 At the same time, β-TrCP1 closely regulates CyclinD1.26 Therefore, we hypothesized that β-TrCP1 could affect cell cycle progression by activating NF-κB. DLBCL cells expressed higher levels of CyclinD1 and CyclinE1 and lower p27kip when NF-κB was activated under treatment with TNFα. β-TrCP1-overexpressing DLBCL cells expressed more CyclinD1 and CyclinE1 and lower p27 in the presence of TNFα.
Adhesion between tumor cells and BMSCs or ECM in hematologic malignancies contributes to drug resistance,27 which is called CAM-DR. In this study, we found that silencing of β-TrCP1 significantly increased the sensitivity of DLBCL cells to chemotherapeutic drug, suggesting that β-TrCP1 has a regulatory effect on CAM-DR.
In this study, β-TrCP1 is shown to be involved in the regulation of NF-κB activation in DLBCL. β-TrCP1 overexpression promotes cell growth and proliferation via accelerating G1/S phase transition. Inhibition of β-TrCP1 attenuates drug resistance of DLBCL cells.
Funding Statement
This work was supported by the Natural Science Foundation of Jiangsu province Grants (No. BK20160060); the Major Research of Natural Science Foundation for Colleges and Universities in Jiangsu Province (16KJ320004), Six Talent Peaks Project in Jiangsu Province (2015-SWYY-021); the National Natural Science Foundation of China Grants (No. 81600158, 81570187, 81600169)
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Dubois S, Jardin F.. Novel molecular classifications of DLBCL. Nat Rev Clin Oncol. 2018;15(8):474–476. doi: 10.1038/s41571-018-0041-z. [DOI] [PubMed] [Google Scholar]
- 2.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 3.Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A, Staudt LM, A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100(17):9991–9996. doi: 10.1073/pnas.1732008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ngo VN, Davis RE, Lamy L, Yu X, Zhao H, Lenz G, Lam LT, Dave S, Yang L, Powell J, et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature. 2006;441(7089):106–110. doi: 10.1038/nature04687. [DOI] [PubMed] [Google Scholar]
- 5.Gasparini C, Celeghini C, Monasta L, Zauli G, NF-kappaB pathways in hematological malignancies. Cell Mol Life Sci. 2014;71(11):2083–2102. doi: 10.1007/s00018-013-1545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Zhou M, Zhang Q-G, Xu J, Lin T, Zhou R-F, Li J, Yang Y-G, Chen B, Ouyang J, et al. Prognostic value of expression of nuclear factor kappa-B/p65 in non-GCB DLBCL patients. Oncotarget. 2017;8(6):9708–9716. doi: 10.18632/oncotarget.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs SY, Spiegelman VS, Kumar KG.. The many faces of beta-TrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene. 2004;23(11):2028–2036. doi: 10.1038/sj.onc.1207389. [DOI] [PubMed] [Google Scholar]
- 8.Hatakeyama S, Kitagawa M, Nakayama K, Shirane M, Matsumoto M, Hattori K, Higashi H, Nakano H, Okumura K, Onoe K, et al. Ubiquitin-dependent degradation of IkappaBalpha is mediated by a ubiquitin ligase Skp1/Cul 1/F-box protein FWD1. Proc Natl Acad Sci U S A. 1999;96(7):3859–3863. doi: 10.1073/pnas.96.7.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong J, Shaik S, Wan L, Tron AE, Wang Z, Sun L, Inuzuka H, Wei W. SCF β-TRCP targets MTSS1 for ubiquitination-mediated destruction to regulate cancer cell proliferation and migration. Oncotarget. 2013;4(12):2339–2353. doi: 10.18632/oncotarget.v4i12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warfel NA, Niederst M, Stevens MW, Brennan PM, Frame MC, Newton AC, Mislocalization of the E3 ligase, beta-transducin repeat-containing protein 1 (beta-TrCP1), in glioblastoma uncouples negative feedback between the pleckstrin homology domain leucine-rich repeat protein phosphatase 1 (PHLPP1) and Akt. J Biol Chem. 2011;286(22):19777–19788. doi: 10.1074/jbc.M111.237081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6(5):369–381. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- 12.Lwin T, Crespo LA, Wu A, Dessureault S, Shu HB, Moscinski LC, Sotomayor E, Dalton WS, Tao J. Lymphoma cell adhesion-induced expression of B cell-activating factor of the TNF family in bone marrow stromal cells protects non-Hodgkin’s B lymphoma cells from apoptosis. Leukemia. 2009;23(1):170–177. doi: 10.1038/leu.2008.266. [DOI] [PubMed] [Google Scholar]
- 13.Hazlehurst LA, Dalton WS. Mechanisms associated with cell adhesion mediated drug resistance (CAM-DR) in hematopoietic malignancies. Cancer Metastasis Rev. 2001;20(1–2):43–50. doi: 10.1023/A:1013156407224. [DOI] [PubMed] [Google Scholar]
- 14.Shankland KR, Armitage JO, Hancock BWJL. Non-Hodgkin lymphoma. Lancet. 2012;380(9844):848–857. doi: 10.1016/S0140-6736(12)60605-9. [DOI] [PubMed] [Google Scholar]
- 15.Lwin T, Hazlehurst LA, Li Z, Dessureault S, Sotomayor E, Moscinski LC, Dalton WS, Tao J. Bone marrow stromal cells prevent apoptosis of lymphoma cells by upregulation of anti-apoptotic proteins associated with activation of NF-kappaB (RelB/p52) in non-Hodgkin’s lymphoma cells. Leukemia. 2007;21(7):1521–1531. doi: 10.1038/sj.leu.2404723. [DOI] [PubMed] [Google Scholar]
- 16.Shi Z, Yang W-M, Chen L-P, Yang D-H, Zhou Q, Zhu J, Chen -J-J, Huang R-C, Chen Z-S, Huang R-P, et al. Enhanced chemosensitization in multidrug-resistant human breast cancer cells by inhibition of IL-6 and IL-8 production. Breast Cancer Res Treat. 2012;135(3):737–747. doi: 10.1007/s10549-012-2196-0. [DOI] [PubMed] [Google Scholar]
- 17.Banerjee S, Zmijewski JW, Lorne E, Liu G, Sha Y, Abraham E, Modulation of SCF beta-TrCP-dependent I kappaB alpha ubiquitination by hydrogen peroxide. J Biol Chem. 2010;285(4):2665–2675. doi: 10.1074/jbc.M109.060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanarek N, Ben-Neriah Y. Regulation of NF-kappaB by ubiquitination and degradation of the IkappaBs. Immunol Rev. 2012;246(1):77–94. doi: 10.1111/j.1600-065X.2012.01098.x. [DOI] [PubMed] [Google Scholar]
- 19.Shi Z, Wu X, Ke Y, Wang L, Hint1 Up-Regulates IkappaBalpha by targeting the beta-TrCP subunit of SCF E3 ligase in human hepatocellular carcinoma cells. Dig Dis Sci. 2016;61(3):785–794. doi: 10.1007/s10620-015-3927-y. [DOI] [PubMed] [Google Scholar]
- 20.Wan M, Huang J, Jhala NC, Tytler EM, Yang L, Vickers SM, Tang Y, Lu C, Wang N, Cao X, et al. SCF(beta-TrCP1) controls Smad4 protein stability in pancreatic cancer cells. Am J Pathol. 2005;166(5):1379–1392. doi: 10.1016/S0002-9440(10)62356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma R, Williams PJ, Gupta A, McCluskey B, Bhaskaran S, Muñoz S, Oyajobi BO, A dominant-negative F-box deleted mutant of E3 ubiquitin ligase, β-TrCP1/FWD1, markedly reduces myeloma cell growth and survival in mice. Oncotarget. 2015;6(25):21589–21602. doi: 10.18632/oncotarget.v6i25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi YW, Kang HJ, Bae EJ, Oh S, Seong Y-S, Bae I, beta-TrCP1 degradation is a novel action mechanism of PI3K/mTOR inhibitors in triple-negative breast cancer cells. Exp Mol Med. 2015;47:e143. doi: 10.1038/emm.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel M, Horgan PG, McMillan DC. NF-kappaB pathways in the development and progression of colorectal cancer. Transl Res. 2018;197:43–56. doi: 10.1016/j.trsl.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Chen JM, Chui SC, Wei TY. The involvement of nuclear factor-kappaappaB in the nuclear targeting and cyclin E1 upregulating activities of hepatoma upregulated protein. Cell Signal. 2015;27(1):26–36. doi: 10.1016/j.cellsig.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Zhou Y, Zheng X, Wu X, Liang Y, Wang S, Zhang Y, Sphk1 promotes breast epithelial cell proliferation via NF-kappaB-p65-mediated cyclin D1 expression. Oncotarget. 2016;7(49):80579–80585. doi: 10.18632/oncotarget.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheon Y, Lee S. CENP-W inhibits CDC25A degradation by destabilizing the SCF(beta-TrCP-1) complex at G2/M. Faseb J. 2018; fj201701358RRR. doi: 10.1096/fj.201701358RRR. [DOI] [PubMed] [Google Scholar]
- 27.Li ZW, Dalton WS. Tumor microenvironment and drug resistance in hematologic malignancies. Blood Rev. 2006;20(6):333–342. doi: 10.1016/j.blre.2005.08.003. [DOI] [PubMed] [Google Scholar]


