ABSTRACT
Brassinosteroids (BRs) are known to be endogenous regulators of ethylene production, suggesting that some BR activity in plant growth and development is associated with ethylene. Here, we demonstrated that ethylene production in Arabidopsis thaliana roots is increased by BR signaling via the ethylene biosynthetic gene for ACC oxidase 1 (ACO1). Electrophoretic mobility shift and chromatin immune-precipitation assays showed that the BR transcription factor BES1 directly binds to two E-box sequences located in the intergenic region of ACO1. GUS expression using site mutations of the E-box sequences verified that ACO1 is normally expressed only when BES1 binds to the E-boxes in the putative promoter of ACO1, indicating that this binding is essential for ACO1 expression and the subsequent production of ethylene in A. thaliana roots. BR exogenously applied to A. thaliana roots enhanced the gravitropic response. Additionally, bes1-D exhibited a greater gravitropic response than did the wild-type specimens, proving that BR is a positive regulator of the gravitropic response in A. thaliana roots. The knock-down mutant aco1-1 showed a slightly lower gravitropic response than did the wild-type specimens, while bes1-D X aco1-1 exhibited a lower gravitropic response than did bes1-D. Therefore, ACO1 is a direct downstream target for BR transcription factor BES1, which controls ethylene production for gravitropism in A. thaliana roots.
KEYWORDS: ACO1, BES1, brassinosteroids, ethylene, root gravitropism
Introduction
Brassinosteroids (BRs) are steroidal plant hormones that are involved in a wide range of growth-regulating activities associated with germination, cell expansion, vascular differentiation, photomorphorgenesis, pathogen resistance, senescence, and the stress response in the vegetative and reproductive growth of plants.1,2–9,10 BRs are known to have their own signal transduction systems to modify the expression of target genes and promote hormonal activity. BR signals are perceived by the heterodimeric membrane-localized receptor kinases brassinosteroid insensitive 1 (BRI1) and BRI1-associated receptor kinase 1 (BAK1).11,12 The signal from BRI1/BAK1 is sequentially transferred to BR-signaling kinases (BSKs) and BRI1 suppressor 1 (BSU1), which suppresses the kinase activity of brassinosteroid insensitive 2 (BIN2), a negative regulator in BR signaling.13–16 The inactivation of BIN2 via BR signaling leads to the nuclear accumulation of BR transcription factors such as brassinazole resistant 1 (BZR1) and BRI1 EMS suppressor 1 (BES1), which regulate the transcription of BR-responsive genes.11,17,18 To date, a number of BR-responsive genes for BZR1 and BES1 have been identified, but the molecular mechanisms underlying the regulation of the expression of target genes by BZR1 and BES1 in plant growth and development remain poorly understood.
Ethylene is a gaseous plant hormone that controls a diverse range of processes across the plant life cycle, from seed germination to organ senescence and fruit ripening.19–23 In plants, ethylene is biosynthesized from S-adenosyl-L-methionine (SAM), with 1-aminocyclopropane-1-carboxylic acid (ACC) as an intermediate. ACC synthases (ACSs) and ACC oxidases (ACOs) are known to catalyze the conversion of SAM to ACC and from ACC to ethylene, respectively.24 The conversion of SAM to ACC catalyzed by ACSs is regarded as rate-limiting.25–27 For this reason, the function and regulation of ACSs in ethylene biosynthesis have been extensively studied in plants.28–34,35 The conversion of ACC to ethylene by ACOs is the final regulatory step in ethylene biosynthesis in plants. Nevertheless, compared to ACSs, the biochemical and physiological function of ACOs remain largely unknown.
Exogenously applied BRs have been shown to increase both ethylene production and endogenous levels of ACC in Arabidopsis thaliana. The expression of several ACSs, such as ACS4, ACS5, and ACS6, is also enhanced by BR application, suggesting that BRs regulate ethylene production via ACSs in A. thaliana.36–39 Recent transcriptome analysis in A. thaliana has revealed that BRs alter the transcript levels of not only ACSs but also ACOs, suggesting that the biosynthetic conversion of ACC to ethylene by ACOs may also be regulated by BRs in this species.40 However, how BRs regulate ethylene production via ACSs and/or ACOs has not yet been elucidated.
We recently demonstrated that ACO1 is dominantly expressed in A. thaliana roots, especially in the root tips.41 An ACO1 knock-down mutant, aco1, exhibited lower ethylene production and increased lateral roots compared to wild-type specimens, indicating that ACO1 is a functional ACO that regulates ethylene production in the growth and development of A. thaliana roots. We also found that ACO1 promoter activity is enhanced by exogenously applied BRs, which suggests that BRs may control the expression of ACO1 in A. thaliana roots. This prompted us to investigate the binding of BR transcription factors to the ACO1 promoter, the change of ACO1 expression in BR-dominant mutants, and the phenotypic variation in related mutants in the present study. The results can thus help to clarify the molecular regulatory mechanisms underlying BR-induced ethylene production in plant tissue.
Materials and methods
Plant materials, growth conditions, and root length and angle measurements
Arabidopsis thaliana ecotypes Col-0 and En-2 were used as wild-type specimens in this study, and the aco1-1 (SALK_127963) line was obtained from SALK. The seeds were surface-sterilized with EtOH-H2O (70:30, v/v) for 5 min and washed with distilled H2O and stratified at 4°C for three days. The surface-sterilized seeds were planted on 0.8% agar (Phytagel, Sigma) containing 0.5X Murashige and Skoog (MS-)salt medium and 1% (w/v) sucrose and grown in the light (120 μmol m−2 s−1) at 22 ± 1°C for 16 h and in the dark at 20 ± 1°C for 8 h in an environmental growth chamber (Sanyo, Osaka). To measure the root length, seedlings were incubated for two days in the presence of 0.01 μM BL, 2 ppm ethylene, 0.01 μM BL with 2 ppm ethylene, 1 μM ACC, 0.01 μM BL with 1 μM ACC, 1 μM cobalt ions, 0.01 μM BL with 1 μM cobalt ions, or without these compounds. Prior to incubation, the seedlings were grown for seven days on MS plates. For gravitational stimulation, seven-day-old seedlings were transferred to fresh MS plates, leading to the straightening of the primary roots. After the transfer, a gravitational stimulus was applied by rotating the plates 90 degrees, followed by incubation under light at 22 ± 1°C. The seedlings were photographed in order to measure the length and angle of the roots. For etiolated conditions, the plates were wrapped with aluminum foil after exposure to white light for one day. Two-week-old seedlings were transferred from agar plates to pots to obtain adult plants.
Total RNA isolation and quantitative RT-PCR analysis
Total RNA was extracted from the wild-type, mutant, and BL-treated seedlings using TRI reagent (Sigma) according to the manufacturer’s instructions. For BL treatment, seven-day-old seedlings were transferred to 1X liquid MS medium with or without 0.01 μM BL and incubated for 6 h. For quantitative RT-PCR, first-strand cDNA was synthesized using 5 μg of total RNA and M-MLV reverse transcriptase (Promega). PCR was performed in a reaction containing 1 μL of cDNA, 0.25 μL of RealTaq (RBC, Taiwan), 2.5 μL of 2.5 mM dNTP mixture, 2.5 μL of 10 X buffer (TaKaRa, Japan), and 1 μl of 10 pmol of each primer in a 25 μL reaction. The gene-specific primers for the ACO genes are listed in Supplementary Table S1. UBQ forward (5′-GACCATAACCCTTGAGGTTGAATC-3′) and reverse (5′-AGAGAGAAAGAGAAGGATCGATC-3′) primers were used for the loading control. The NOS terminator reverse primer (5′-TTATCCTAGTTTGCGCGCTA-3′) was used for transgene amplification. From a total of 25 μL of PCR products, 10 μL was loaded onto 0.8 or 1% agarose gel and stained with EtBr. Stained bands in the gel were scanned and densitometrically analyzed using the Quantity One program (BIO-RAD).
Histochemical GUS staining and GUS activity measurement
Histochemical and quantitative analyzes of the transgenic lines expressing the GUS gene were performed as described previously.42 For histochemical GUS staining, samples were incubated in a staining solution containing 2 mM 5-bromo-4chloro-3-indolyl-β-D-glucuronic acid (Duchefa) in a 50 mM Na2HPO4 buffer (pH 7.2), 2 mM potassium ferrocyanide, 2 mM potassium ferricyanide, and 0.2% Triton X-100 at 37°C overnight after infiltration under a vacuum on ice for 20 min. The samples were cleared using 70% ethanol and observed via dissection (Olympus SZ-PT) and with a light microscope (Olympus CX21). To measure GUS activity, plant tissue was ground in liquid nitrogen. Crude plant extracts were resolved in a GUS extraction buffer (50 mM Na2HPO4 buffer [pH 7.0], 10 mM EDTA, pH 8.0, 0.1% SDS, 0.1% Triton X-100, 10 mM β-mercaptoethanol, and 25 μg mL−1 PMSF). The crude extracts were mixed with a reaction buffer (1 mM 4-methylumbelliferyl-β-D-glucuronide [Duchefa] in the GUS extraction buffer). After exactly 10 and 20 min, the reactions were stopped using Na2CO3. The fluorescence was measured with a spectrofluorometer.
Electrophoretic mobility shift assays (EMSAs)
EMSAs were performed using the following process. First, the N-terminal of full-length BES1 was fused to maltose-binding protein (MBP) using a pMALc2X vector (NEB). The recombinant protein was then expressed and affinity-purified from E. Coli (BL21-CodonPlus [DE3]-RIL) using amylose resin. MBP2 protein, which was used as a negative control in the assays, was purchased from NEB. The ACO1 promoter fragments (145–164 bp) used for the EMSAs were amplified with PCR using the following primer combinations: Probe 1 forward (5′- GAAAAATTCAAAAACTTGTGAT-CACATGG-3′) and reverse (5′-CTCATTGAATTTGAAGCATCATTACG-3′), Probe 2 forward (5′-CGATACTGTAGCTTTAGGCCAAGTAG-3′) and reverse (5′-GCGTCATTGGCGTACTTGTAA-AATGTG-3′), Probe 3 forward (5′-CTTTCGCTTAATTCC-GCTTAGACCC-3′) and reverse (5′-CTACTTGGCCTAAAGCTACAGTATCG-3′), and Probe 4 forward (5′-CCGTGTTAACGATAAA-CCATCATTTCG-3′) and reverse (5′-GGGCCAAGACC-TAATCTACAAAGAC-3′). The PCR products were gel-purified, and 100 ng of the probes was then labeled with α-32P-dCTP using a Rediprime II random prime labeling system (Amersham Biosciences, UK). The ACO1 promoter fragments (34–39 bp) used for the EMSAs were obtained from the annealing of synthetic oligonucleotide pairs (Supplement Table S2). The oligonucleotide pairs were labeled with α-32P-dCTP using Klenow Fragment (TaKaRa, Japan).
The binding reactions were carried out in 2 μL of 5X binding buffer (50 mM Tris-HCl [pH 7.5], 250 mM NaCl, 2.5mM EDTA, 2.5 mM DTT, 5mM MgCl2, and 20% glycerol), 4 μL of recombinant BES1 proteins, 1 μL of sonicated salmon sperm DNA, and 2 μL of distilled H2O with ~1 μL of a radioisotope-labeled probe. After 30 min of incubation at RT, the reactions were resolved using 4% native polyacrylamide gels with a 1X TBE buffer (10.78 g/L Tris, ~5.5 g/L boric acid, and 0.744 g/L EDTA, pH 8.0) and exposed to a phosphorimaging screen.
Chromatin immunoprecipitation (ChIP)
A transgenic plant expressing the BES1-YFP fusion protein in an sgs3-11 mutant background was used for ChIP assays following a procedure described previously.43 Briefly, a chromatin-protein complex was isolated from about 3 g of two-week-old seedlings, resuspended in 2x lysis buffer (100 mM Tris-HCl [pH 8.0], 20 mM EDTA, 400 mM NaCl, 1% Triton X-100, and 2 mM PMSF) and incubated with anti-GFP antibodies (Abcam) at 4°C for 4 h. The chromatin-protein-antibody complex was precipitated with salmon sperm DNA/protein-A agarose beads (Millipore). The precipitated DNA was purified using a DNA elution kit (QIAGEN) and amplified with 32 to 34 cycles of PCR using a ProACO1:GUS 536 For and ProACO1:GUS 1 Rev primer pair (Supplementary Table S2) for the ACO1 promoter. Aliquots of the samples before immunoprecipitation were used as inputs and amplified with primers for the ACO1 promoter.
Results
BRs regulate the conversion of ACC to ethylene in A. thaliana roots
The application of brassinolide (BL), the most active BR, reduced the growth of the roots in A. thaliana (Figure 1a). Similarly, the application of ethylene greatly reduced the growth of A. thaliana roots, which was enhanced in the present of BL. This suggests that BRs and ethylene function interactively in the growth of A. thaliana roots. The application of ACC, a direct biosynthetic precursor of ethylene, also reduced the growth of A. thaliana roots (Figure 1b). Compared to ACC treatment only, the application of ACC with BL enhanced the inhibitory growth of A. thaliana roots. The application of cobalt ions, known as a biosynthetic inhibitor of the conversion of ACC to ethylene, increased the growth of the roots without BL treatment, but this growth increase was reduced in the presence of BL (Figure 1c). Therefore, the inhibitory activity of BRs in the growth of A. thaliana roots appears to be mediated by the regulation of the conversion of ACC to ethylene.
Figure 1.

Effect of BL (10−8 M), ethylene (2 ppm), ACC (10−6 M), and cobalt ions (10−6 M) on primary root growth in Arabidopsis thaliana. (a-c) Data from at least two independent experiments are presented as the mean ± standard error. (n > 120). The asterisks indicate significant difference between control and chemical-treated at *P < .05 and **P < .01 using student’s t-test.
BRs activate the expression of ACO1 in the roots of A. thaliana
In A. thaliana, ACOs are a multigene family. Of the 13 putative ACOs, designated as ACO1 to ACO13 by their AGI number (Supplement Table S1), the expression levels of 11 of these were examined in the roots of A. thaliana. As shown in Figure 2a, the expression of only ACO1 and ACO2 was significantly affected by BL treatment. ACO2 expression was inhibited by BL, and BR-activated ethylene production appears to mainly occur via ACO1 in A. thaliana roots. Previously, we reported that ACO1 is normally expressed when E-box sequences known to be cis-regulatory elements for BES1 binding sites are present in the intergenic region of ACO1.41 As expected, the expression of ACO1 was enhanced in the BES1-dominant mutant (bes1-D) compared to the wild-type specimens (Figure 2b), indicating that ACO1 expression is upregulated by BES1-mediated BR signaling. To verify this, a construct was prepared in which a 1843-bp 5′-intergenic region from the start codon of ACO1 to the 3′-untranslated region of the adjacent gene, which is considered a putative promoter, was transcriptionally fused to β-glucuronidase (GUS). This was transferred to A. thaliana to create ProACO1:GUS 1843 transgenic specimens. When ProACO1:GUS 1843 was transferred into bes1-D (ProACO1:GUS 1843/bes1-D), the tissue-specific expression of ACO1 was unaffected, but the gene expression in the apical regions of the roots was clearly higher compared to the wild-type specimens (Figure 2c and 2d). These findings indicate that BES1 positively controls the transcription of ACO1 in A. thaliana roots, especially in the root tips.
Figure 2.

Expression pattern of ACO genes and GUS expression under the control of the 1843-bp ACO1 promoter. (a) Transcript levels of ACO genes in the roots of BL-untreated (-BL) and BL-treated (+BL) A. thaliana seedlings. (b) Transcript levels of ACO1 in the roots of wild-type and bes1-D seedlings. UBQ5 was used as a loading control. (c) GUS expression in wild-type, and (d) bes1-D seedlings. Five-day-old seedlings were subjected to GUS staining.
BES1 directly binds to the ACO1 promoter in A. thaliana
The presence of E-box sequences (CANNTG) for BES1 binding in the intergenic region of ACO1 was investigated in the A. thaliana genome. As shown in Figure 3a, four E-boxes designated as E-boxes A to D ordered by their distance from the ACO1 start codon were identified. To investigate the interaction between the ACO1 promoter and BES1 protein, radioisotope-labeled probes (approximately 160 bp each) for the DNA fragments containing E-boxes A, B, C, and D were prepared. Maltose-binding protein (MBP)-BES1 was obtained from E. coli using a construct in which MBP was fused to the N-terminal of full-length BES1, and EMSAs were conducted. As shown in Figure 3b, BES1 protein was bound to two probes containing E-boxes B and D. To clarify the binding of BES1 to the E-box sequences, approximately 40-bp DNA probes for the four E-boxes were prepared, and EMSAs were re-conducted. The binding of BES1 to E-boxes B and D was also detected with these shorter probes in a concentration-dependent manner (Figure 3c). The E-box sequence CANNTG was then mutated to TCTGAA and EMSAs carried out. The binding of BES1 to E-boxes B and D was reduced by the mutation (Figure 3b), which confirms that BES1 directly binds to E-boxes B and D in the ACO1 promoter. These findings indicate that the ACO1 promoter contains at least two important regulatory sequences, E-box B and E-box D, which act as the binding site for BES1 in ACO1 expression.
Figure 3.

Location of E-boxes in the ACO1 promoter and the binding of BES1 to the promoter. (a) The names and loci of E-boxes in the 5′-intergenic region of ACO1 are indicated. Minus numeric denotes a base pair from the start codon of ACO1 gene. The orange boxes indicate E-box elements. (b) and (c) Electrophoretic mobility shift assays (EMSAs) with BES1 protein and the ACO1 promoter. (b) Probes containing the E-boxes indicated in (a) were incubated with (+) or without (-) 0.5 μg of recombinant BES1 protein. (c) Approximately 0.5, 1, and 2 μg of recombinant BES1 protein or 1 μg of MBP2 protein were incubated with probes containing E-box B or E-box D. The triangles represent increasing protein concentration. (d) Chromatin immunoprecipitation (ChIP) assays with wild-type and BES1-YFP transgenic plants using an anti-GFP antibody. The SAUR-AC1 promoter was used as a positive control.44 Five biological repeats were performed and showed similar results.
Next, to confirm BES1 binding to the ACO1 promoter in vivo, ChIP assays were performed with transgenic plant specimens in which the BES1-YFP fusion protein was expressed. As shown in Figure 3d, the in vivo binding of BES1 to the ACO1 promoter was identified. Taken together, the results indicate that BES1 is a BR transcription factor that directly binds to the ACO1 promoter to regulate the expression of ACO1 in A. thaliana roots.
BR-induced gravitropic response is mediated by ACO1 in A. thaliana roots
As shown in Figure 2c, the expression of ACO1 was concentrated in A. thaliana root tips. This expression was significantly higher in the root tips of bes1-D (Figure 2d), suggesting that both ACO1 and BES1 are important to the physiology of A. thaliana root tips. Exogenously applied ACC and ethylene increased the gravitropic response in A. thaliana root tips (Figure 4a), while exogenously applied BL also enhanced the gravitropic response in the tips, indicating that both BRs and ethylene are positive regulators that control gravitropism in A. thaliana roots.
Figure 4.
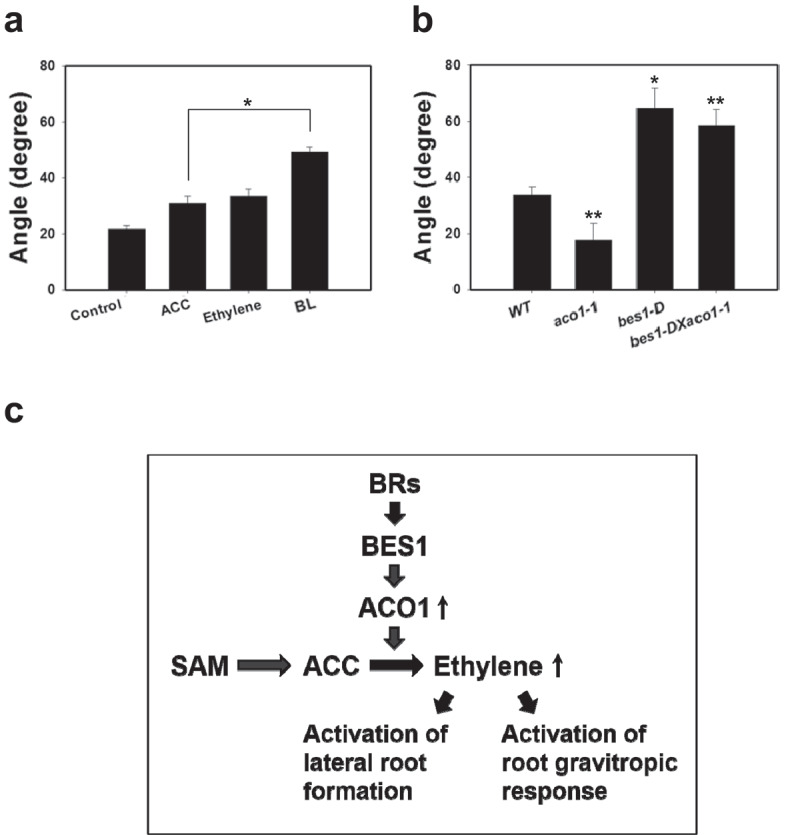
Co-ordination of BR and ethylene via ACO1 in growth and development of Arabidopsis roots. (a) Effect of ACC, ethylene, and BL on root gravitropic response. (b) Gravitropic response in wild-type, aco1-1, bes1-D, and bes1-D X aco1-1 seedlings. (a) and (b) Data from at three independent experiments are presented as the mean ± standard error. (n > 50). The asterisks indicate significant difference between control and chemical-treated at *P < .05 and **P < .01 using student’s t-test. (c) Proposed scheme for ACO1-mediated BR signaling in growth and development of roots in A. thaliana. BES1-mediated BR signals activate ACO1 expression to increase ethylene production which activate formation of lateral roots and gravitropic response in A. thaliana root tips.
As shown in Figure 4b, aco1-1 lowered the gravitropic response of roots slightly compared to the wild-type specimens, suggesting that ACO1 has a role in the gravitropic response of the roots. In contrast, bes1-D, for which ACO expression and ethylene production were higher compared to wild-type specimens, showed an enhanced gravitropic response in its roots compared to wild-type specimens (Figure 4b), indicating that the BR-induced gravitropic response is mediated by ethylene production via ACO1 in A. thaliana roots. The enhanced gravitropic response of the roots exhibited by bes1-D was significantly reduced in bes1-D X aco1-1, which confirms that the BR-induced gravitropic response of the roots is associated with ACO1-mediated ethylene production in A. thaliana.
Discussion
In Arabidopsis and rice, BRs alter the expression of GA metabolic genes such as GA20 oxidase/GA3 oxidase for activation and GA2 oxidase for inactivation by binding BES1/BZR1 to cis-elements such as E-box/G-box, BRRE, or non-E-box motifs in the promoter regions of the genes, which results in the regulation of endogenous levels of active GAs in plants.45 In this study, we found that BES1 directly binds to E-box sequences in the promoter region of ACO1, which increases ethylene production in A. thaliana roots. In a recent study, we also found that BES1 can bind to E-box sequences in the promoter region of ABA oxidase 2, which catalyzes the conversion of xanthoxin to ABA aldehyde in ABA biosynthesis, resulting in a reduction in the endogenous ABA levels in A. thaliana (data will be published elsewhere). These findings suggest that BRs are likely master regulators for the homeostasis of other plant hormones, such as GA, ethylene, and ABA in A. thaliana.
Sun et al. reported that BES1 can bind to not only E-box sequences but also BRRE sequences in the promoter region of BR target genes.46 In opposite, BRZ1 can bind to E-box sequences and BRRE sequences in the promoter region of BR target genes. The 1843-bp intergenic region of ACO1 contains four E-box sequences but no BRRE sequences. EMSAs using the protein MBP-BZR1 revealed that BZR1 does not bind to any E-box sequences in the intergenic region (data not shown). Although there is a possibility that unidentified BR transcription factors bind to the ACO1 promoter, the regulation of BR signals for ethylene production via ACO1 is likely to be specific for the BES transcription factor in A. thaliana roots.
In the presence of auxin, BRs enhance ethylene production in plants, indicating that ethylene production is coordinated by BRs and auxin in plants.47,26,29,48 It has been found that exogenously applied auxin affects the expression of several ACS genes, such as ACS2, 4, 5, 6, 8, and 11 in A. thaliana.49 Of these auxin-regulated genes, the expression of ACS4, 5, and 6 are also affected by the application of BRs.36,38,50 All three genes contain E-box and BRRE sequences in their putative promoter regions, allowing for the possibility of the binding of BES1 and/or BZR1 to the gene promoters. In the promoter regions of ACS4, 5, and 6, auxin-responsive elements (AuxREs) containing the consensus sequence TGTCTC have also been found, suggesting that auxin transcription factors (ARFs) can also bind to AuxREs in the promoter region of the ACS genes. The possible binding of both transcription factors for BR and auxin to the promoter regions of ACS4, 5, and 6 suggests that both hormones interact via the transcriptional regulation of these ACS genes to control ethylene production in A. thaliana.
Arabidopsis thaliana contains three functional ACO genes (ACO1, 2, and 4) and 13 ACO homologs. In A. thaliana roots, the application of BL greatly increased the expression of only ACO1, indicating that the increase in ethylene production due to BL treatment occurs primarily via ACO1. The ACO1 promoter contains two core AuxREs (1,154 and 769 bp from the ACO1 start codon), and the ACO1 expression patterns were altered with IAA treatment.41,51 Therefore, these two core AuxREs in the transcriptional regulatory region might also regulate promoter activity for ACO1. Taken together, tissue-specific ACO1 expression in A. thaliana roots may be mediated by the combinatorial activation of transcription factors for BR and auxin that coordinates multiple signals.
Exogenously applied ACC and ethylene inhibited the growth of primary roots in A. thaliana. To test whether ACO1 expression in roots contributed to ethylene-mediated root growth inhibition, we measured the primary root length of aco1-1 and wild-type plants, but no statistically significant differences were found (data not shown), indicating that ACO1 may not play a major role in ethylene-regulated primary root growth.
In Arabidopsis roots, ACO1 was strongly expressed in the lateral root primordia and root tips, suggesting that ACO1 might be required for ethylene biosynthesis in the regulation of root development, especially in the lateral root formation and the physiological responses in the root tips. In aco1-1, the number of lateral roots was higher than that in the wild-type specimens, confirming that the expression of ACO1 is necessary for lateral root formation in A. thaliana.41 We found that ACC and ethylene increase the gravitropic response in A. thaliana roots. Additionally, the gravitropic response in aco1-1 was slightly reduced compared to that in the wild-type specimens, demonstrating that the conversion of ACC to ethylene by ACO1 is involved in the gravitropism of A. thaliana roots. In the gravitropic response, aco1-1 and bes1-D exhibited contrasting activity. To determine the order of action for ACO1 and BES1, bes1-D X aco1-1 mutants were developed, and their gravitropic response was compared to those of the single mutants aco1-1 and bes1-D. The enhanced gravitropic response in bes1-D was weaker in bes1-D X aco1-1, which is similar to the inhibitory patterns exhibited by aco1-1 in comparison to wild-type plants (Figure 4b), proving that ACO1 is epistatic to BES1. Based on these results, Figure 4c presents a summary of the proposed regulatory action mechanisms underlying BR signaling via BES1 and ACO1 in the growth and development of A. thaliana roots.
Funding Statement
This work was supported by a grant from the Next-Generation BioGreen 21 Program (SSAC, grant#: PJ01320801), Rural Development Administration, Republic of Korea, and by the Chung-Ang University Graduate Research Scholarship in 2018.
Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Sasse JM. Physiological actions of brassinosteroids: an update. J Plant Growth Regul. 2003;22:1–8. doi: 10.1007/s00344-003-0062-3. [DOI] [PubMed] [Google Scholar]
- 2.Mandava NB. Plant growth-promoting brassinosteroids. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:23–52. doi: 10.1146/annurev.pp.39.060188.000323. [DOI] [Google Scholar]
- 3.Clouse SD, Zurek D. 1991. ‘Molecular analysis of brassinolide action in plant growth and development.’ in (ACS Publications).
- 4.Zhu J-Y, Sae-Seaw J, Wang Z-Y. Brassinosteroid signalling. Development. 2013;140:1615–1620. doi: 10.1242/dev.060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell JW, Mandava N, Worley JF, Plimmer JR, Smith MV. Brassins—a new family of plant hormones from rape pollen. Nature. 1970;225:1065–1066. doi: 10.1038/2251065a0. [DOI] [PubMed] [Google Scholar]
- 7.Fujioka S, Yokota T. Biosynthesis and metabolism of brassinosteroids. Annu Rev Plant Biol. 2003;54:137–164. doi: 10.1146/annurev.arplant.54.031902.134921. [DOI] [PubMed] [Google Scholar]
- 8.Grove MD, Spencer GF, Rohwedder WK, Mandava N, Worley JF, David Warthen J, Steffens GL, Flippen-Anderson JL, Cook JC. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature. 1979;281:216–217. doi: 10.1038/281216a0. [DOI] [Google Scholar]
- 9.Domagalska MA, Sarnowska E, Nagy F, Davis SJ. Genetic analyses of interactions among gibberellin, abscisic acid, and brassinosteroids in the control of flowering time in Arabidopsis thaliana. PLoS One. 2010;5:e14012. doi: 10.1371/journal.pone.0014012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zurek DM, Rayle DL, McMorris TC, Clouse SD. Investigation of gene expression, growth kinetics, and wall extensibility during brassinosteroid-regulated stem elongation. Plant Physiol. 1994;104:505–513. doi: 10.1104/pp.104.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z-Y, Nakano T, Gendron J, Junxian H, Chen M, Vafeados D, Yang Y, Fujioka S, Yoshida S, Asami T. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell. 2002;2:505–513. doi: 10.1016/S1534-5807(02)00153-3. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997;90:929–938. doi: 10.1016/S0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- 13.Kim T-W, Guan S, Burlingame AL, Wang Z-Y. ‘The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2ʹ. Mol Cell. 2011;43:561–571. doi: 10.1016/j.molcel.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mora-García S, Vert G, Yin Y, Caño-Delgado A, Cheong H, Chory J. Nuclear protein phosphatases with Kelch-repeat domains modulate the response to brassinosteroids in Arabidopsis. Genes Dev. 2004;18:448–460. doi: 10.1101/gad.1174204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim T-W, Guan S, Sun Y, Deng Z, Tang W, Shang J-X, Sun Y, Burlingame AL, Wang Z-Y. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat Cell Biol. 2009;11:1254. doi: 10.1038/ncb1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nam KH, Li. J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/S0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- 17.Yin Y, Wang Z-Y, Mora-Garcia S, Jianming L, Yoshida S, Asami T, Chory J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell. 2002;109:181–191. doi: 10.1016/S0092-8674(02)00721-3. [DOI] [PubMed] [Google Scholar]
- 18.Tang W, Kim T-W, Oses-Prieto JA, Sun Y, Deng Z, Zhu S, Wang R, Burlingame AL, Wang Z-Y. BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science. 2008;321:557–560. doi: 10.1126/science.1156973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picton S, Barton SL, Bouzayen M, Hamilton AJ, Grierson D. Altered fruit ripening and leaf senescence in tomatoes expressing an antisense ethylene‐forming enzyme transgene. Plant J. 1993;3:469–481. doi: 10.1111/j.1365-313X.1993.tb00167.x. [DOI] [Google Scholar]
- 20.Maunders MJ, Holdsworth MJ, Slater A, Knapp JE, Bird CR, Schuch W, Grierson D. Ethylene stimulates the accumulation of ripening‐related mRNAs in tomatoes. Plant Cell Environ. 1987;10:177–184. [Google Scholar]
- 21.Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, Peter M. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell. 2000;12:1117–1126. doi: 10.1105/tpc.12.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Peng J, Wen X, Guo H. Ethylene-insensitive3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell. 2013;25:3311–3328. doi: 10.1105/tpc.113.113340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaudoin N, Serizet C, Gosti F, Giraudat J. Interactions between abscisic acid and ethylene signaling cascades. Plant Cell. 2000;12:1103–1115. doi: 10.1105/tpc.12.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. doi: 10.1146/annurev.pp.35.060184.001103. [DOI] [Google Scholar]
- 25.Kende H. Ethylene biosynthesis. Annu Rev Plant Biol. 1993;44:283–307. doi: 10.1146/annurev.pp.44.060193.001435. [DOI] [Google Scholar]
- 26.Wang KL-C, Li H, Ecker JR. Ethylene biosynthesis and signaling networks. Plant Cell. 2002;14:S131–S51. doi: 10.1105/tpc.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams DO, Yang SF. Ethylene biosynthesis: identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc National Acad Sci. 1979;76:170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barry CS, Llop-Tous MI, Grierson D. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol. 2000;123:979–986. doi: 10.1104/pp.123.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi HC, Joo S, Nam KH, Lee JS, Kang BG, Kim WT. Auxin and brassinosteroid differentially regulate the expression of three members of the 1-aminocyclopropane-1-carboxylate synthase gene family in mung bean (Vigna radiata L.). Plant Mol Biol. 1999;41:443–454. doi: 10.1023/A:1006372612574. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Zhang S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell. 2004;16:3386–3399. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye L, Lin L, Wang L, Wang S, Sen L, Juan D, Zhang S, Shou H. MPK3/MPK6 are involved in iron deficiency-induced ethylene production in Arabidopsis. Front Plant Sci. 2015;6:953. doi: 10.3389/fpls.2015.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skottke KR, Yoon GM, Kieber JJ, Alison D. Protein phosphatase 2A controls ethylene biosynthesis by differentially regulating the turnover of ACC synthase isoforms. PLoS Genet. 2011;7:e1001370. doi: 10.1371/journal.pgen.1001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thain SC, Vandenbussche F, Laarhoven LJJ, Dowson-Day MJ, Wang Z-Y, Tobin EM, Harren FJM, Millar AJ, Van Der Straeten D. Circadian rhythms of ethylene emission in Arabidopsis. Plant Physiol. 2004;136:3751–3761. doi: 10.1104/pp.104.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chae HS, Faure F, Kieber JJ. The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell. 2003;15:545–559. doi: 10.1105/tpc.006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon GM, Kieber JJ. 14-3-3 regulates 1-aminocyclopropane-1-carboxylate synthase protein turnover in Arabidopsis. Plant Cell. 2013;25:1016–1028. doi: 10.1105/tpc.113.110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemhauser JL, Hong F, Chory J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell. 2006;126:467–475. doi: 10.1016/j.cell.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 37.Arteca RN, Bachman JM. Light inhibition of brassinosteroid-induced ethylene production. J Plant Physiol. 1987;129:13–18. doi: 10.1016/S0176-1617(87)80097-4. [DOI] [Google Scholar]
- 38.Hansen M, Chae HS, Kieber JJ. Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J. 2009;57:606–614. doi: 10.1111/j.1365-313X.2008.03711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joo S, Seo YS, Kim SM, Hong DK, Young Park KY, Kim WT. Brassinosteroid induction of AtACS4 encoding an auxin‐responsive 1‐aminocyclopropane‐1‐carboxylate synthase 4 in Arabidopsis seedlings. Physiol Plant. 2006;126:592–604. [Google Scholar]
- 40.Müssig C, Shin G-H, Altmann T. Brassinosteroids promote root growth in Arabidopsis. Plant Physiol. 2003;133:1261–1271. doi: 10.1104/pp.103.028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park CH, Roh J, Youn J-H, Son S-H, Park JH, Kim SY, Kim T-W, Kim S-K. Arabidopsis ACC oxidase 1 coordinated by multiple signals mediates ethylene biosynthesis and is involved in root development. Mol Cells. 2018;41:923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weigel D, Glazebrook J. 2002. Arabidopsis: a laboratory manual (CSHL Press).
- 43.He J-X, Gendron JM, Sun Y, Gampala SSL, Gendron N, Sun CQ, Wang Z-Y. BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science. 2005;307:1634–1638. doi: 10.1126/science.1107580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin Y, Vafeados D, Tao Y, Yoshida S, Asami T, Chory J. A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell. 2005;120:249–259. doi: 10.1016/j.cell.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 45.Unterholzner SJ, Rozhon W, Papacek M, Ciomas J, Lange T, Kugler KG, Mayer KF, Sieberer T, Poppenberger B. Brassinosteroids are master regulators of gibberellin biosynthesis in Arabidopsis. Plant Cell. 2015;27:2261–2272. doi: 10.1105/tpc.15.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Y, Fan X-Y, Cao D-M, Tang W, Kun H, Zhu J-Y, Jun-Xian H, Bai M-Y, Zhu S, Eunkyoo O. Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev Cell. 2010;19:765–777. doi: 10.1016/j.devcel.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clouse SD, Sasse JM. Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Biol. 1998;49:427–451. doi: 10.1146/annurev.arplant.49.1.427. [DOI] [PubMed] [Google Scholar]
- 48.Hardtke CS, Dorcey E, Osmont KS, Sibout R. Phytohormone collaboration: zooming in on auxin–brassinosteroid interactions. Trends Cell Biol. 2007;17:485–492. doi: 10.1016/j.tcb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Tsuchisaka A, Theologis A. Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol. 2004;136:2982–3000. doi: 10.1104/pp.104.049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lv B, Tian H, Zhang F, Liu J, Songchong L, Bai M, Chuanyou L, Ding Z. Brassinosteroids regulate root growth by controlling reactive oxygen species homeostasis and dual effect on ethylene synthesis in Arabidopsis. PLoS Genet. 2018;14:e1007144. doi: 10.1371/journal.pgen.1007144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ulmasov T, Hagen G, Guilfoyle TJ. Dimerization and DNA binding of auxin response factors. Plant J. 1999;19:309–319. doi: 10.1046/j.1365-313X.1999.00538.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


