ABSTRACT
Despite improvements in surgical resection and adjuvant chemotherapy, the prognosis and outcomes of patients with osteosarcoma remains poor due to the occurrence of metastasis or relapse. Monocyte chemoattractant protein-1-induced protein-1 (MCPIP1), a zinc-finger RNA-binding protein, is known to regulate inflammatory responses and repress breast cancer growth. However, the regulation of MCPIP1 by microRNAs has not been clearly elucidated in osteosarcoma. In this study, we found that miR-421 expression was upregulated and MCPIP1 expression was downregulated in the osteosarcoma specimens from patients. Moreover, MCPIP1 expression was inversely correlated with miR-421 expression in the clinical samples. Furthermore, the upregulation of miR-421 and downregulation of MCPIP1 resulted in poor overall survival and severe disease progression, respectively, in the patients with osteosarcoma. Bioinformatics analysis and luciferase reporter gene assays confirmed that miR-421 specifically targets and binds to the 3ʹ-UTR of MCPIP1. The overexpression of miR-421 induced cell proliferation, invasion, and migration, and the release of pro-inflammatory IL-6 in cultured human osteosarcoma cells. Additionally, the administration of miR-421 to tumor-bearing mice facilitated osteosarcoma growth by downregulating MCPIP1 expression. Taken together, these findings indicate that miR-421 is able to promote the development of osteosarcoma by regulating MCPIP1 expression, and can be a potential therapeutic target for osteosarcoma.
KEYWORDS: Mir-421, MCPIP1, osteosarcoma, growth, migration
Introduction
Osteosarcoma is the most common primary malignancy of the bone and usually affects children and adolescents1 Surgical resection with adjuvant or neoadjuvant chemotherapy using doxorubicin, cisplatin, methotrexate, or ifosfamide) has effectively cured nearly 70% of patients. However, the overall five-year survival of osteosarcoma patients with metastasis or relapse has remained unaltered at approximately 20% over the past 30 years.1–3 Therefore, detailed studies of the molecular mechanism of osteosarcoma are necessary to identify the therapeutic targets for prevention and intervention of osteosarcoma.
MicroRNAs (miRNAs) are emerging as important biomarkers to predict the occurrence, development, and prognosis of osteosarcoma. Studies on miRNA have revived the identification of therapeutic targets of osteosarcoma. Of note, miR-421-targeted programmed cell death protein 4 (PDCD4) regulates breast cancer cell proliferation.4 Additionally, miR-421 expression is associated with poor prognosis in non-small cell lung cancer.5 However, it remains unknown whether miR-421 plays a role in regulating osteosarcoma progression.
Monocyte chemoattractant protein-1-induced protein-1 (MCPIP1) is a transcription factor that contains the CCCH zinc finger binding motif, and has been shown to activate glial fibrillary acid protein (GFAP) to promote cell differentiation and regulate cell morphology.6–8 Moreover, MCPIP1 functions as a ubiquitin-proteasome substrate, and regulates protein degradation and cell metabolism, such as growth, development, and signal transduction.9,10 MCPIP1 has been shown to suppress breast cancer cell growth by downregulating the expression of anti-apoptotic genes.
The purpose of this present study was to investigate whether the crosstalk between miR-421 and MCPIP1 contributes to osteosarcoma progression. We initially detected the expression of miR-421 and MCPIP1 in human osteosarcoma tissues, and found that high expression of miR-421 and low expression of MCPIP1 were correlated with short overall survival and severe clinical stage of osteosarcoma, respectively. Subsequently, we examined the ability of miR-421 to target MCPIP1, and determined the effect of miR-421 on tumorigenesis both in vitro and in vivo using human osteosarcoma cell lines and xenograft models, respectively. Our results demonstrated that miR-421 could accelerate tumor growth, migration, and inflammatory imbalance in osteosarcoma by targeting MCPIP1.
Results
Mir-421 expression is inversely correlated with MCPIP1 expression in human osteosarcoma tissues
To investigate the potential clinical relevance of miR-421, we quantified miR-421 expression in the tumor tissues and adjacent non-tumor bone tissues from 30 patients with primary osteosarcoma (Figure 1(a)). Real-time PCR analysis showed that miR-421 expression in the tumor tissues was significantly higher than that in the non-tumor tissues (Figure 1(b)). Kaplan-Meier analysis also revealed that patients with high expression of miR-421 had a shorter overall survival than those with low expression of miR-421 (χ2 = 3.842; P = .0299) (Figure 1(c)). Moreover, high expression of miR-421 significantly correlated with the severe clinical stage of osteosarcoma (Table 2).
Figure 1.
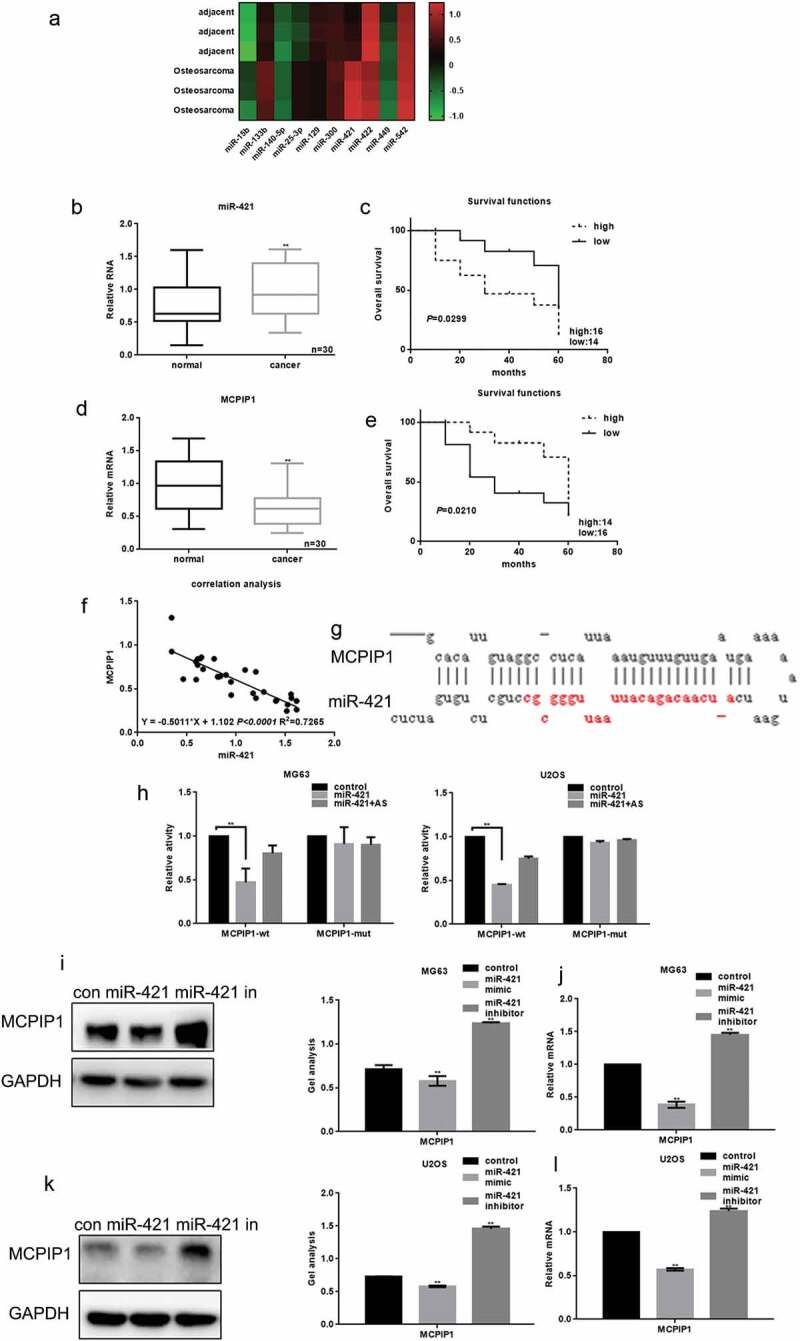
The relationship between miR-421 and MCPIP1 in osteosarcoma (a) The expressions of miR-421 and other microRNAs in 30 samples of osteosarcoma tissues and adjacent tissues were detected by real-time PCR. Data are shown as mean ± SEM. ** P < .05 vs. adjacent tissues group (b) MiR-421 expression was markedly elevated in osteosarcoma tissues (c)The relationship between the expression of miR-421 and the survival time of osteosarcoma patients. (d) The levels of MCPIP1 in 30 samples of osteosarcoma tissues and adjacent tissues were detected by real-time PCR. The levels of MCPIP1 were strikingly decreased in osteosarcoma tissues. Data are shown as mean ± SEM. ** P < .05 vs. adjacent tissues group (e) The relationship between the expression of MCPIP1 and the survival time of osteosarcoma patients. (f) The correlation between the expression of miR-421 and MCPIP1 (g) miRDB predicted that miR-421 could target MCPIP1 (h) The interaction between miR-421 and the MCPIP1 was tested by luciferase reporter assays in MG63 and U2OS cells. Data are shown as mean ± SEM. ** P < .05 vs. TK-MCPIP1 group (i-l) After transfecting miR-421 mimic/inhibitor in MG63 and U2OS cells, the levels of MCPIP1 were detected by western blot and real-time PCR. Data are shown as mean ± SEM. ** P < .05.
Table 2.
The correlations between the expression of miR-421 and clinic pathologic features in osteosarcoma.
| miR-421 expression |
||||||
|---|---|---|---|---|---|---|
| Parameters | Description | No. of patient | High low | χ2 | P value | |
| Gender | Male | 19 | 8 | 11 | 2.625 | 0.105 |
| Female | 11 | 8 | 3 | |||
| Age(years) | <18 | 16 | 9 | 7 | 0.536 | 0.464 |
| ≥18 | 14 | 6 | 8 | |||
| TNM stage | I | 14 | 10 | 4 | 6.735 | 0.034* |
| II | 10 | 2 | 8 | |||
| III | 6 | 4 | 2 | |||
However, MCPIP1 expression in the osteosarcoma tissues from 30 patients was considerably lower than that in the adjacent non-tumor skeletal tissues (Figure 1(d)). Kaplan-Meier analysis showed that the patients with high mRNA levels of MCPIP1 had a longer overall survival than those with low mRNA levels of MCPIP1 (χ2 = 5.840; P = .0210) (Figure 1(e)). Additionally, low mRNA levels of MCPIP1 were significantly correlated with the severe clinical stage of osteosarcoma (Table 3).
Table 3.
The correlations between the expression of MCPIP1 and clinic pathologic features in osteosarcoma.
| MCPIP1 expression |
||||||
|---|---|---|---|---|---|---|
| Parameters | Description | No. of patient | High low | χ2 | Pvalue | |
| Gender | Male | 19 | 9 | 10 | 0.741 | 0.398 |
| Female | 11 | 7 | 4 | |||
| Age(years) | <18 | 16 | 6 | 10 | 3.453 | 0.063 |
| ≥18 | 14 | 10 | 4 | |||
| TNM stage | I | 14 | 5 | 9 | 7.309 | 0.026* |
| II | 10 | 8 | 2 | |||
| III | 6 | 1 | 5 | |||
We further explored the crosstalk between miR-421 and MCPIP1 expression in human osteosarcoma tissues. Remarkably, the Spearman’s correlation analysis revealed an inverse correlation between miR-421 expression and MCPIP1 expression (Figure 1(f)). We also used bioinformatics tools to predict a target sequence in the 3ʹ-UTR of MCPIP1 for miR-421 (Figure 1(g)). We then performed the luciferase reporter gene assay to demonstrate that miR-421 can directly bind to the 3ʹ-UTR of MCPIP1 (Figure 1(h)). Western blotting and real-time PCR analysis showed that miR-421 can significantly downregulate MCPIP1 expression in MG63 and U2OS cells (Figure 1(i–l)).
Mir-421 promotes osteosarcoma cell proliferation
The effect of miR-421 on the proliferation of human osteosarcoma cell lines was assessed using MTT assays. MG63 cells and U2OS cells were transfected with miR-421 mimic and miR-421 inhibitor, respectively. The overexpression of miR-421 promoted the proliferation of both MG63 and U2OS cells, whereas inhibition of miR-421 expression attenuated proliferation (Figure 2(a)).
Figure 2.
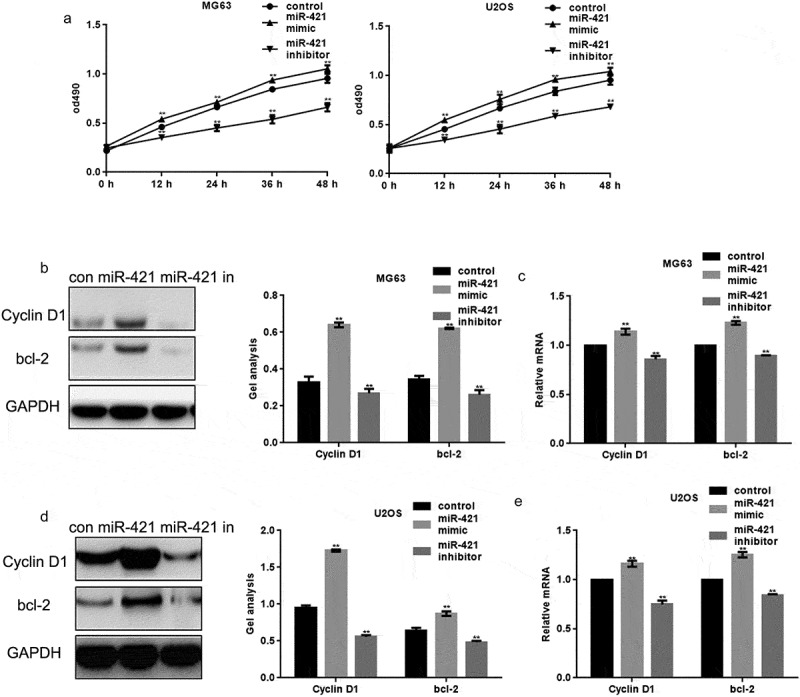
MiR-421 promotes the growth of osteosarcoma cells (a) After transfecting with miR-421 mimic/inhibitor, the proliferations of MG63 and U2OS cells were detected by MTT assay. Data are shown as mean ± SEM. ** P < .05(b-e) After transfecting miR-421 mimic/inhibitor in MG63 and U2OS cells, the levels of Cyclin D1 and bcl-2 were detected by western blot and real-time PCR. Data are shown as mean ± SEM. ** P < .05.
Moreover, the protein and mRNA levels of cyclin D1 and Bcl-2 in MG63 and U2OS cells were assessed. The protein and mRNA levels of cyclin D1 and Bcl-2 were increased upon the overexpression of miR-421 and decreased upon inhibition of miR-421 expression in MG63 and U2OS cells (Figure 2(b–e)).
Mir-421 enhances osteosarcoma cell migration
The miR-421 mimic or inhibitor was transfected into MG63 and U2OS cells using the transwell assay to assess whether miR-421 mediates osteosarcoma cell migration (Figure 3(a,b)). The migration and invasion of both MG63 and U2OS cells were remarkably increased upon the overexpression of miR-421, compared to the control cells (Figure 3(a,b)). Conversely, the migration and invasion of these cells were significantly decreased upon inhibition of miR-421 expression (Figure 3(a,b)).
Figure 3.
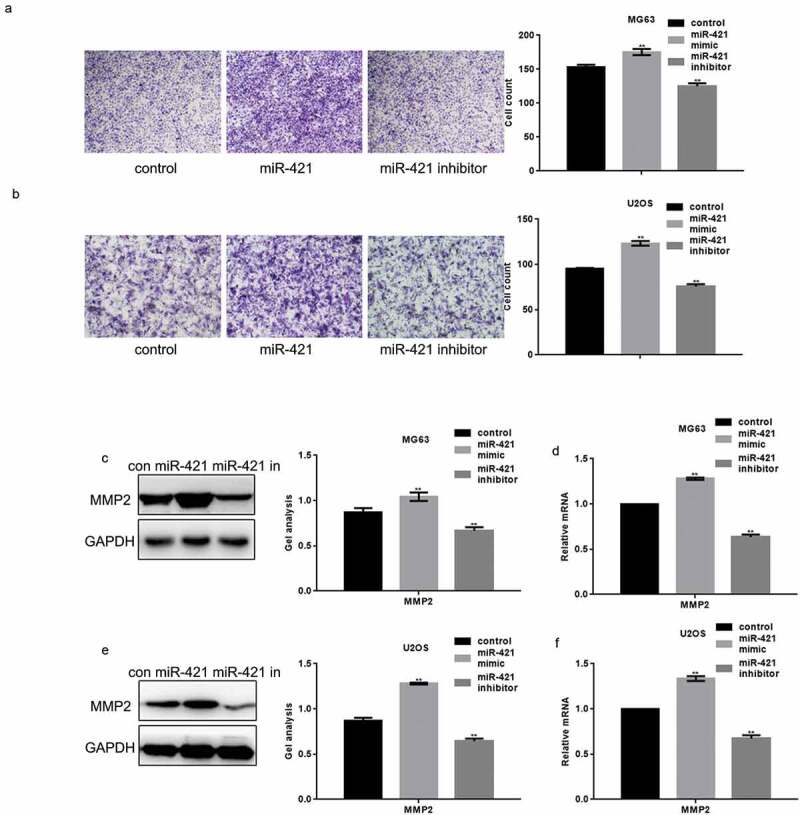
MiR-421 promotes the migration of osteosarcoma cells (a) After transfecting miR-421 mimic or inhibitor in MG63 cells, transwell assays were performed. Cells were counted and results represent the mean±SD for three experiments. ** P < .05 (b) After transfecting miR-421 mimic/inhibitor in U2OS cells, transwell assays were performed. Cells were counted and results represent the mean±SD for three experiments. ** P < .05 (c-f) After transfecting miR-421 mimic/inhibitor in MG63 and U2OS cells, the levels of MMP2 were detected by western blot and real-time PCR. Data are shown as mean ± SEM. ** P < .05.
Western blotting and real-time PCR analysis showed that the protein and mRNA levels of matrix metalloproteinase-2 (MMP2) was increased upon the overexpression of miR-421 and decreased upon inhibition of miR-421 expression in both MG63 and U2OS cells (Figure 3(c–f)).
Mir-421 promotes IL-6 secretion from osteosarcoma cells
MCPIP1 can regulate immune responses and induce IL-6 expression to promote tumor growth and metastasis in human osteosarcoma. Hence, we determined the ability of miR-421 to regulate IL-6 expression in osteosarcoma cell lines. The miR-421 mimic or miR-421 inhibitor was transfected into MG63 and U2OS cells. ELISA analysis revealed that IL-6 expression is increased upon the overexpression of miR-421 and decreased upon its inhibition (Figure 4(a,b)). Western blotting and real-time PCR assays further confirmed these results (Figure 4(c–f)).
Figure 4.
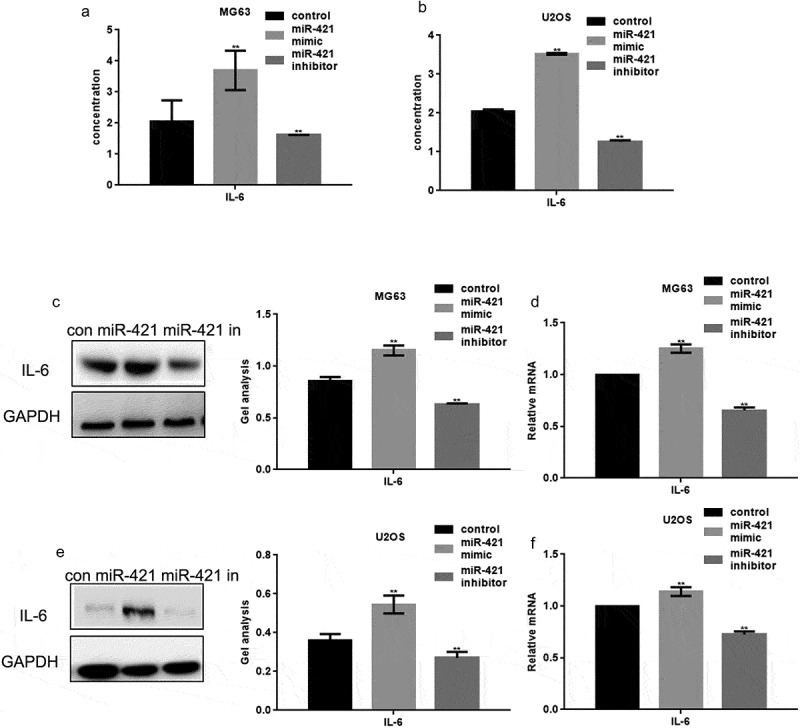
MiR-421 promotes the release of IL-6 in osteosarcoma cells (a, b) After transfecting miR-421 mimic/inhibitor in MG63 and U2OS cells, the expression of IL-6 was detected by ELISA. (c-f) After transfecting miR-421 mimic/inhibitor in MG63 and U2OS cells, the levels of IL-6 were detected by western blot and real-time PCR. Data are shown as mean ± SEM. ** P < .05.
Tumorigenic effect of mir-421 in vivo
The tumorigenic effect of miR-421 on MG63 cells was illustrated in a nude mouse xenograft. Mice treated with the miR-421 agomir exhibited significantly increased (17.61%) tumor volume compared to the control mice (Figure 5(a)). Additionally, treatment with the miR-421 agomir decreased the protein and mRNA levels of MCPIP1, and increased those of cyclin D1, Bcl-2, MMP2, and IL-6 (Figure 5(b,c)). Conversely, mice treated with the miR-421 antagomir exhibited significantly decreased (43.69%) tumor volume compared to the control mice (Figure 5(d)). Furthermore, treatment with the miR-421 antagomir increased the protein and mRNA levels of MCPIP1, and decreased those of cyclin D1, Bcl-2, MMP2, and IL-6 (Figure 5(e,f)).
Figure 5.
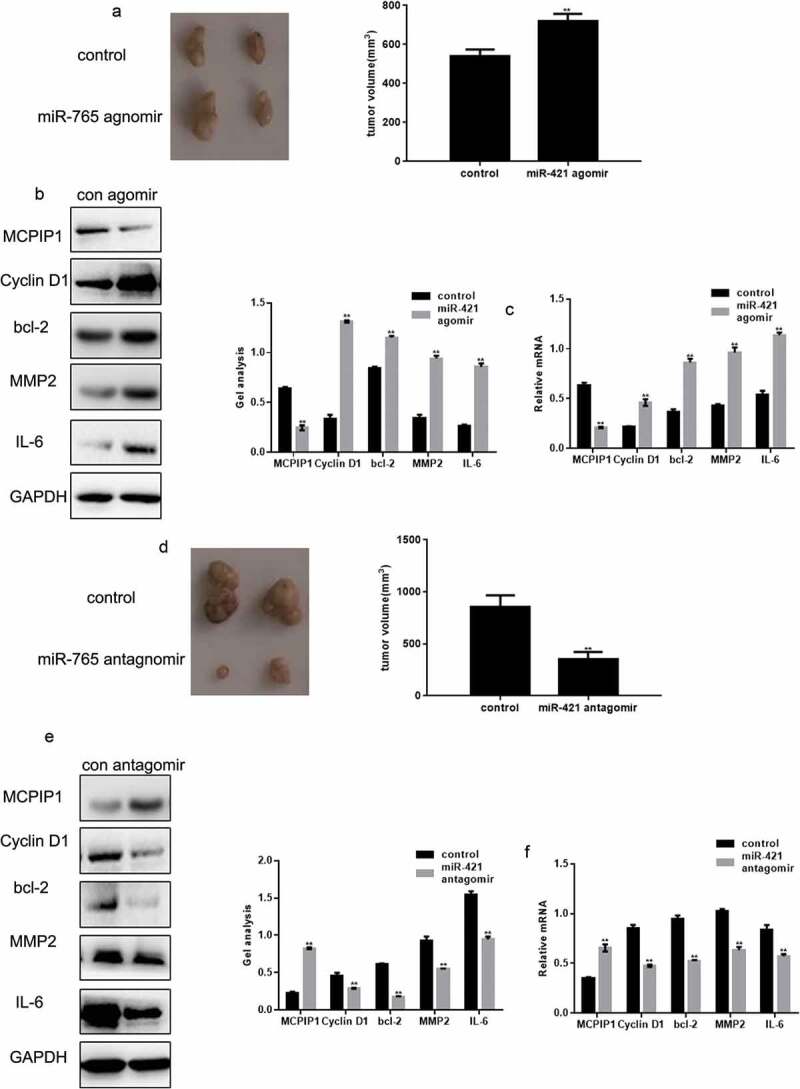
The tumorigenic effect of miR-421 in vivo (a) Photographic illustration of tumors from controls and miR-421 agomir-treated nude mice. The tumor volumes were showed in the graph. (b, c) The levels of MCPIP1, Cyclin D1, bcl-2, MMP2 and IL-6 in tumors from controls and miR-421 agomir-treated nude mice were detected by western blot and real-time PCR. Data are shown as mean ± SEM. ** P < .05 (d) Photographic illustration of tumors from controls and miR-421 antagomir-treated nude mice. The tumor volumes were showed in the graph. (e, f) The levels of MCPIP1, Cyclin D1, bcl-2, MMP2 and IL-6 in tumors from controls and miR-421 antagomir-treated nude mice were detected by western blot and real-time PCR. Data are shown as mean ± SEM. ** P < .05.
Mir-421 targets MCPIP1 to promote osteosarcoma cell growth and migration
We performed MTT and transwell assays to demonstrate that MCPIP1 can promote cell growth and migration upon the overexpression of miR-421 (Figure 6(a,b)). In addition, MCPIP1 also reduced the increased mRNA and protein levels of cyclin D1, Bcl-2, MMP2, and IL-6 induced by the overexpression of miR-421 (Figure 6(c,d)).
Figure 6.
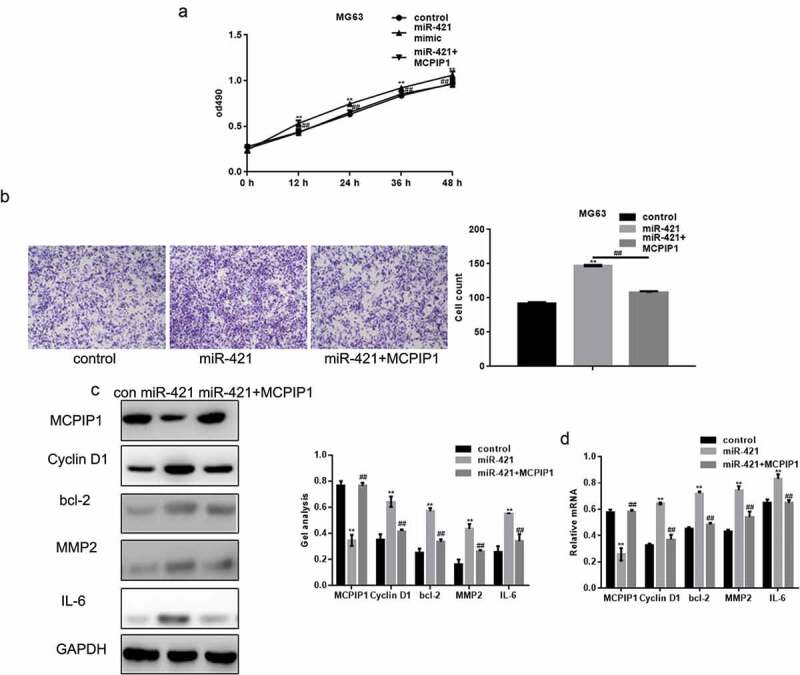
MiR-421 promotes the growth and migration of osteosarcoma cells by targeting MCPIP1 (a) Cells were divided into three groups and transfected with control, miR-421 and miR-421 with MCPIP1 respectively. The proliferation of MG63 cells was detected by MTT assay. Data are shown as mean ± SEM. ** P < .05 control vs miR-421, ##P < .05 miR-421 vs miR-421+ MCPIP1 (b) The migration of MG63 cells was detected by transwell assay. Cells were counted and results represent the mean±SD for three experiments. ** P < .05 control vs miR-421, ## P < .05 miR-421 vs miR-421+ MCPIP1 (c, d) The levels of MCPIP1, Cyclin D1, bcl-2, MMP2 and IL-6 in MG63 cells were detected by western blot and real-time PCR. Data are shown as mean ± SEM. ** P < .05 control vs miR-421, ## P < .05 miR-421 vs miR-421+ MCPIP1.
Discussion
Osteosarcoma is the most common primary malignancy of the bone with its peak incidence in the elderly, and primarily affects children and adolescents. It frequently occurs in the metaphysis of the long bone, including the distal femur, proximal tibia, and proximal humerus. The application of surgical resection and chemotherapy has increased the five-year survival rate of primary and non-metastatic patients from 55% to 80% over the past few years. However, the five-year overall survival of primary osteosarcoma patients with metastasis or relapse has remained unchanged at approximately 20% over several decades.
miRNAs represent a key class of epigenetic and genetic regulators, and play an important role in osteosarcoma pathogenesis. Dysregulated miRNA expression can result in the aberrant expression of osteosarcoma genes. miRNAs perform important functions such as tumor suppression, cell proliferation, apoptosis, cell cycle arrest, pro-metastasis, migration, and chemotherapeutic resistance.11 Several miRNAs, such as miR-34, miR-133, miR-143, miR-16, miR-199, miR-335, and miR-340, are downregulated in human osteosarcoma cells or tissues.12–14 However, several other miRNAs, such as miR-140, miR-20a, miR-29a, and miR-181, are upregulated in osteosarcoma cells.15–18
The identification of miRNAs or proteins that correlate with osteosarcoma progression in patients is important to establish prognostic markers to improve therapeutic efficiency and increase overall survival.15 In our study, we found that the expression of miR-421 and MCPIP1 in human osteosarcoma tissues was higher and lower, respectively, than that in the adjacent non-tumor tissues. Remarkably, the high expression of miR-421 and low expression of MCPIP1 correlated with short overall survival and severe clinical stage of osteosarcoma, respectively, resulting in poor prognosis and aggressive progression. Considering the potential application of miR-421 as a biomarker, it would be interesting to investigate the expression patterns of miR-421 and MCPIP1 in larger cohorts of patients with osteosarcoma at different clinical stages. Furthermore, it might be useful to determine the expression of miR-421 and MCPIP1 in patients with osteosarcoma during the metastatic stage and in response to chemotherapy.
MCPIP1 is encoded by the zc3h12a gene and was recently discovered as a suppressor protein in breast cancer. MCPIP1 can regulate the expression of anti-apoptotic genes.19 Lu et al.6 have demonstrated that MCPIP1 expression in the breast cancer cells is higher than that in the normal epithelial cells. Furthermore, the expression of MCPIP1 in the breast tumor tissues from patients was higher than that in the surrounding normal tissues, and positively correlated with prognosis in breast cancer patients. MCPIP1 also promotes the toxicity of MG-132 in HeLa cells, and the inhibition of proteasome by MG-132 markedly increases MCPIP1 expression.20 In this study, we used biological software analysis to demonstrate that miR-421 has specific binding sites in the 3ʹ-UTR of MCPIP1. Using the luciferase reporter assay, we further confirmed that miR-421 significantly inhibits the relative activity of MCPIP1 and represses MCPIP1 expression, indicating that miR-421 directly targets MCPIP1.
We also overexpressed or inhibited miR-421 expression in human osteosarcoma cell lines using a miR-421 mimic or miR-421 inhibitor to determine the effect of miR-421 on osteosarcoma cells. The proliferation, invasion, and migration of osteosarcoma cells was increased under the overexpression of miR-421 and decreased under inhibition of miR-421 expression. Cyclin D1 is not only an anti-apoptotic and proliferation-associated protein, but also involved in the inflammatory response of keratinocytes by regulating MCPIP1 expression.21 Cyclin D1 expression was quantified to assess whether the effect of miR-421 on cell proliferation and apoptosis was influenced by the effect of MCPIP1 on cyclin D1 expression. As MMP2 plays an important role in osteosarcoma metastasis by promoting the degradation of the extracellular membrane,22,23 we first determined MMP2 expression levels and subsequently confirmed that miR-421 promotes the metastasis of osteosarcoma cells by affecting the expression of MMP2, which is presumed to be downstream of MCPIP1. Using a murine model of osteosarcoma induced by an intramuscular injection of osteosarcoma cells close to tibia, we demonstrated that the overexpression of miR-421 promotes primary tumor growth in vivo, a process associated with the downregulation of MCPIP1 expression in the osteosarcoma tissues of mice. However, the inhibition of miR-421 expression reduced primary tumor growth and increased MCPIP1 expression. These results suggest that miR-421 can promote osteosarcoma by regulating MCPIP1 expression both in vitro and in vivo. In addition to its anti-tumor effect, MCPIP1 can downregulate the expression of proinflammatory cytokines, such as IL-1, IL-6, and IL-12 by promoting mRNA degradation. In particular, IL-6 contributes to tumorigenesis and metastasis of osteosarcoma by directly promoting cell migration and angiogenesis, and indirectly affecting cell seeding and the tumor-stroma microenvironment. Finally, we demonstrated that miR-421 promotes IL-6 secretion from the osteosarcoma cells by targeting MCPIP1.
Our findings suggest that miR-421 promotes oncogenesis and progression of osteosarcoma in human specimens, cultured cells, and the murine model by regulating MCPIP1 expression. miR-421 and MCPIP1 can be potentially used as a biomarker for disease prognosis and progression in the patients with osteosarcoma. As a potential therapeutic biomarker, miR-421 could be worth exploring in a clinical setting of drug resistance in combination with surgical resection and chemotherapy.
Materials and methods
Tissue samples
Tumor tissues and adjacent non-tumor bone tissues were obtained between June 2011 and July 2013 from 30 patients with osteosarcoma treated at the Shengjing Hospital of China Medical University. Patient consent was obtained in writing according to institutional regulations. The experimental protocols were approved by the Ethics Committee of Shengjing Hospital of China Medical University.
Real-time PCR
Total RNA was extracted from tissues or cells using TRIzol reagent (Invitrogen) following the manufacturer’s protocol. Real-time PCR was performed using the following cycling conditions: 94 °C for 5 min, and followed by 30 cycles of 94 °C for 30 s, and 58–61 °C for 30 s.24 The synthesized primer sequences are shown in Table 1 The expression of miR-421 was assessed using the Stem-Loop RT-PCR assay.25–27 All experiments were performed in triplicate.
Table 1.
Primer sequences for detection of RNA expression.
| Name | Forward primer(5ʹ->3ʹ) | Reverse primer(5ʹ->3ʹ) |
|---|---|---|
| miR-421 | GTCGCGCGGGUUAAUGCCTC | GGACATUAGUUGUCUGUAAATAG |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| MCPIP1 | CAACAAGGCTTGCCATCGG | TGAGTGGCTTCTTACGCAGG |
| Cyclin D | CCGAGGAGCTGCTGCAAATGG | GAAATCGTGCGGGGTCATTGCG |
| bcl-2 | GGTGAACTGGGGGAGGATTG | GGCAGGCATGTTGACTTCAC |
| MMP2 | TGATCTTGACCAGAATACCATCG | GGCTTGCGAGGGAAGAAGTT |
| IL-6 GAPDH |
ATGAACTCCTTCTCCACAAGC AACGACCCCTTCATTGAC |
CTACATTTGCCGAAGAGCCCTC TCCACGACATACTCAGGGACAAC |
Western blotting
The cells were lysed with RIPA lysis buffer (Sigma). To determine the expression of different proteins, a total of 30 μg protein/lane was resolved by 10% SDS-PAGE and transferred onto polyvinylidene fluoride membranes (Corning). The membranes were incubated overnight at 4°C with the primary antibody, and subsequently with an appropriate horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. Target proteins were probed with specific antibodies: MCPIP1 (sc-515275), IL-6 (sc-130326), cyclin D1 (sc-4074), Bax (sc-4239), Bcl-2 (sc-56015), MMP2 (sc-13594), and GAPDH (sc-365062) (Santa Cruz Biotechnology, Inc.).
Dual luciferase reporter assay
Dual luciferase activity assays were performed as described previously.28 The 3′-UTR of MCPIP1was PCR-amplified and cloned into the pMIR-REPORT™ vector (Ambion). The following primers were used: MCPIP1-wt, F: 5′- CATCACAGATAGCGGTCCCC-3′, R: 5′- GAGGATTCCCTTGCCTGCTT-3′, MCPIP1-del, F: 5′- CAACACGGTGCTAGGGGAAT-3′, R: 5′- CGAAGGAAGTTGTCCAGGCT-3′. Control, miR-421, and miR-421 antisense (AS; Guangzhou RiboBio Co. Ltd.) were simultaneously transfected. Luciferase activity was measured using the Dual-Luciferase Reporter Assay System (BioTek Instruments, Inc., Winooski, VT, USA).
Cell culture
MG63 and U2OS cells were purchased from the Chinese Academy of Medical Sciences. Cells were cultured in DMEM (Gibco) supplemented with 10% FBS (Gibco) under 5% CO2 at 37°C.
Cell transfection
Cells were plated in 6-well plates and transfected with 100 nM miR-421 mimic/control (Guangzhou RiboBio Co. Ltd.) or miR-421 inhibitor/control (Guangzhou RiboBio Co. Ltd.) after 24 h using the HiGene transfection reagent (Beyotime Institute of Biotechnology) according to the manufacturer’s protocols. MCPIP1 and MCPIP1mut were transfected into cells using Lipo3000.
MTT assay
3-(4,5-dimethylthiazolyl)2,5diphenyltetrazolium bromide (MTT) assay was used to analyze cell proliferation. Cells were seeded at a density of 1 × 103 per well in 96-well plates. After 24 h, cells were transfected with different miRNAs. The optical density of samples was measured at 490 nm on a microplate reader (BioRad, USA).
Tumor xenografts in nude mice
Six-week-old male Balb/c nude mice were purchased from the Laboratory Animal Center of Shenyang, China. MG63 cells (1 × 107) were suspended in 100 μl PBS and subcutaneously injected into mice. The mice were intravenously injected with miR-421agomir/control (Ruibo) or miR-421 antagomir/control (Ruibo) once daily (days 0–16), according to the approved protocol. The mice were monitored for 16 d after tumor inoculation. All experimental procedures were approved by the Institutional Animal Research Ethics Committee with reference to the Chinese Community guidelines for the use of experimental animals (No.201309). All experimental procedures involving animals were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication no. 80–23, revised 1996) and were performed according to the institutional ethical guidelines for animal experiments.
Transwell assay
Migration assays were performed using modified Boyden chambers (Corning), according to the manufacturer’s protocols. The transwell chambers were coated with 20 µl Matrigel (1:4, BD Bioscience). Following 24 h after transfection, cells were seeded in the chambers in serum-free media; the lower wells were filled with media containing 20% FBS. After 8 h, cells were stained with crystal violet for 10 min and counted under a microscope.
Enzyme-linked immunosorbent assay (ELISA)
Using human IL-6 detection kit, the ELISA method was used to determine the concentration of IL-6 in cells samples. The operation method is strictly in accordance with the specification.
Statistical analysis
All data were analyzed with PRISM 6.0 (GraphPad, Inc.). Data are presented as the mean ± standard deviation. Two groups were compared using Student’s t test (two-tailed) and one-way ANOVA. Multiple comparisons were made using one-way ANOVA and the LSD-t method. Statistical significance was defined as P < .05. All experiments were repeated three times.
Funding Statement
This study was supported by Grants from the National Natural Science Foundation of China (No. 81370981; H0726) and the Outstanding Scientific Fund of Shengjing Hospital of China Medical University (MD31). Zhaozhou Ren’s study was supported by an excellent PhD research fund from Shengjing Hospital of China Medical University (201410263); National Natural Science Foundation of China [81370981]; National Natural Science Foundation of China [81370981]; the Outstanding Scientific Fund of Shengjing Hospital of China Medical University [MD31]; excellent PhD research fund from Shengjing Hospital of China Medical University [201410263].
Acknowledgments
All personnel who have contributed to this article are in the list of authors.
Authors’ Contributions
Z Ren and Q Fu conceived and designed the experiments.
T Shen, K Wang and Q Meng collected clinical specimens and analysed the correlation.
Z Ren, K Wang, L Zhou, Y Han and S Liu performed the experiments.
K Wang, L Zhou, C Ji and X Chen analysed the data.
Z Ren, Kejia Wang, X Chen, and Q Fu wrote the article.
Availability of data and material
No restriction on data or material availability.
Conflict of interest
The authors declare no conflict of interest.
Consent for publication
Written informed consent for the publication of all manuscript details was obtained from Shengjing Hospital of China Medical University.
Ethics approval and consent to participate
Research involving human subjects, human material, or human data, was performed in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committee of Shengjing Hospital of China Medical University (R20151242).
References
- 1.Dong J, Liu Y, Liao W, Liu R, Shi P, Wang L.. 2016. miRNA-223 is a potential diagnostic and prognostic marker for osteosarcoma. J Bone Oncol. 5(2):74–79. Doi: 10.1016/j.jbo.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Fiore R, Drago-Ferrante R, Pentimalli F, Di Marzo D, Forte IM, Carlisi D, De Blasio A, Tesoriere G, Giordano A, Vento R. 2016. Let-7d miRNA shows both antioncogenic and oncogenic functions in osteosarcoma-derived 3AB-OS cancer stem cells. J Cell Physiol. 231(8):1832–1841. doi: 10.1002/jcp.25291. [DOI] [PubMed] [Google Scholar]
- 3.Wang M, Xie R, Si H, Shen B. 2017. Integrated bioinformatics analysis of miRNA expression in osteosarcoma. Artif Cells Nanomed Biotechnol. 45(5):936–943. doi: 10.1080/21691401.2016.1196456. [DOI] [PubMed] [Google Scholar]
- 4.Farooqi AA, Qureshi MZ, Coskunpinar E, Naqvi SK, Yaylim I, Ismail M. 2014. MiR-421, miR-155 and miR-650: emerging trends of regulation of cancer and apoptosis. Asian Pac J Cancer Prev. 15(5):1909–1912. doi: 10.7314/apjcp.2014.15.5.1909. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Cui X, Li Y, Zhang T, Li S. 2018. Upregulated expression of miR-421 is associated with poor prognosis in non-small-cell lung cancer. Cancer Manag Res. 10:2627–2633. DOI: 10.2147/CMAR.S167432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu W, Ning H, Gu L, Peng H, Wang Q, Hou R, Fu M, Hoft DF, Liu J. 2016. MCPIP1 selectively destabilizes transcripts associated with an antiapoptotic gene expression program in breast cancer cells that can elicit complete tumor regression. Cancer Res. 76(6):1429–1440. doi: 10.1158/0008-5472.CAN-15-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boratyn E, Nowak I, Horwacik I, Durbas M, Mistarz A, Kukla M, Kaczówka P, Łastowska M, Jura J, Rokita H. 2016. Monocyte chemoattractant protein-induced protein 1 overexpression modulates transcriptome, including microRNA, in human neuroblastoma cells. J Cell Biochem. 117(3):694–707. doi: 10.1002/jcb.25354. [DOI] [PubMed] [Google Scholar]
- 8.Labedz-Maslowska A, Lipert B, Berdecka D, Kedracka-Krok S, Jankowska U, Kamycka E, Sekula M, Madeja Z, Dawn B, Jura J, et al. 2015. Monocyte Chemoattractant Protein-Induced Protein 1 (MCPIP1) enhances angiogenic and cardiomyogenic potential of murine bone marrow-derived mesenchymal stem cells. PLoS One. 10(7):e0133746. DOI: 10.1371/journal.pone.0133746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipert B, Wegrzyn P, Sell H, Eckel J, Winiarski M, Budzynski A, Matlok M, Kotlinowski J, Ramage L, Malecki M, et al. 2014. Monocyte chemoattractant protein-induced protein 1 impairs adipogenesis in 3T3-L1 cells. Biochim Biophys Acta. 1843(4):780–788. DOI: 10.1016/j.bbamcr.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Makki MS, Haseeb A, Haqqi TM. 2015. MicroRNA-9 promotion of interleukin-6 expression by inhibiting monocyte chemoattractant protein-induced protein 1 expression in interleukin-1β-stimulated human chondrocytes. Arthritis Rheumatol. 67(8):2117–2128. doi: 10.1002/art.39173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrow JJ, Khanna C. Osteosarcoma genetics and epigenetics: emerging biology and candidate therapies. Crit Rev Oncog. 2015;20(3–4):173–197. doi: 10.1615/CritRevOncog.v20.i3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li JP, Liu LH, Li J, Chen Y, Jiang XW, Ouyang YR, Liu YQ, Zhong H, Li H, Xiao T. 2013. Microarray expression profile of long noncoding RNAs in human osteosarcoma. Biochem Biophys Res Commun. 433(2):200–206. doi: 10.1016/j.bbrc.2013.02.083. [DOI] [PubMed] [Google Scholar]
- 13.Zhou X, Wei M, Wang W. 2013. MicroRNA-340 suppresses osteosarcoma tumor growth and metastasis by directly targeting ROCK1. Biochem Biophys Res Commun. 437(4):653–658. doi: 10.1016/j.bbrc.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 14.He C, Xiong J, Xu X, Lu W, Liu L, Xiao D, Wang D. 2009. Functional elucidation of MiR-34 in osteosarcoma cells and primary tumor samples. Biochem Biophys Res Commun. 388(1):35–40. doi: 10.1016/j.bbrc.2009.07.101. [DOI] [PubMed] [Google Scholar]
- 15.Jones KB, Salah Z, Del Mare S, Galasso M, Gaudio E, Nuovo GJ, Lovat F, LeBlanc K, Palatini J, Randall RL, et al. 2012. MiRNA signatures associate with pathogenesis and progression of osteosarcoma. Cancer Res. 72(7):1865–1877. DOI: 10.1158/0008-5472.CAN-11-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Namløs HM, Meza-Zepeda LA, Barøy T, Østensen IH, Kresse SH, Kuijjer ML, Serra M, Bürger H, Cleton-Jansen AM, Myklebost O. 2012. Modulation of the osteosarcoma expression phenotype by microRNAs. PLoS One. 7(10):e48086. doi: 10.1371/journal.pone.0048086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang G, Nishimoto K, Zhou Z, Hughes D, Kleinerman ES. 2012. MiR-20a encoded by the miR-17-92 cluster increases the metastatic potential of osteosarcoma cells by regulating Fas expression. Cancer Res. 72(4):908–916. doi: 10.1158/0008-5472.CAN-11-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song B, Wang Y, Xi Y, Kudo K, Bruheim S, Botchkina GI, Gavin E, Wan Y, Formentini A, Kornmann M, et al. 2009. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene. 28(46):4065–4074. DOI: 10.1038/onc.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyu JH, Park DW, Huang B, Kang SH, Lee SJ, Lee C, Bae YS, Lee JG, Baek SH. 2015. RGS2 suppresses breast cancer cell growth via a MCPIP1-dependent pathway. J Cell Biochem. 116(2):260–267. doi: 10.1002/jcb.24964. [DOI] [PubMed] [Google Scholar]
- 20.Skalniak L, Dziendziel M, Jura J. 2014. MCPIP1 contributes to the toxicity of proteasome inhibitor MG-132 in HeLa cells by the inhibition of NF-κB. Mol Cell Biochem. 395(1–2):253–263. doi: 10.1007/s11010-014-2134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang T, Li S, Li J, Yin F, Hua Y, Wang Z, Lin B, Wang H, Zou D, Zhou Z, et al. 2016. Natural product pectolinarigenin inhibits osteosarcoma growth and metastasis via SHP-1-mediated STAT3 signaling inhibition. Cell Death Dis. 7(10):e2421. DOI: 10.1038/cddis.2016.305. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Roomi MW, Kalinovsky T, Rath M, Niedzwiecki A. 2014. In vitro modulation of MMP-2 and MMP-9 in pediatric human sarcoma cell lines by cytokines, inducers and inhibitors. Int J Oncol. 44(1):27–34. doi: 10.3892/ijo.2013.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ory B, Baud’huin M, Verrecchia F, Royer BB, Quillard T, Amiaud J, Battaglia S, Heymann D, Redini F, Lamoureux F. 2016. Blocking HSP90 addiction inhibits tumor cell proliferation, metastasis development, and synergistically acts with zoledronic acid to delay osteosarcoma progression. Clin Cancer Res. 22(10):2520–2533. doi: 10.1158/1078-0432.CCR-15-1925. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Sápi Z, Papp G, Szendrői M, Pápai Z, Plótár V, Krausz T, Fletcher CD. 2016. Epigenetic regulation of SMARCB1 By miR-206, −381 and −671-5p is evident in a variety of SMARCB1 immunonegative soft tissue sarcomas, while miR-765 appears specific for epithelioid sarcoma. A miRNA study of 223 soft tissue sarcomas. Genes Chromosomes Cancer. 55(10):786–802. doi: 10.1002/gcc.22379. [DOI] [PubMed] [Google Scholar]
- 26.Xie BH, He X, Hua RX, Zhang B, Tan GS, Xiong SQ, Liu LS, Chen W, Yang JY, Wang XN, et al. 2016. Mir-765 promotes cell proliferation by downregulating INPP4B expression in human hepatocellular carcinoma. Cancer Biomark. 16(3):405–413. DOI: 10.3233/CBM-160579. [DOI] [PubMed] [Google Scholar]
- 27.Ali Sheikh MS, Xia K, Li F, Deng X, Salma U, Deng H, Wei Wei L, Yang TL, Peng J. 2015. Circulating miR-765 and miR-149: potential noninvasive diagnostic biomarkers for geriatric coronary artery disease patients. Biomed Res Int. 2015:740301. DOI: 10.1155/2015/740301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang TS, Yang XH, Wang XD, Wang YL, Zhou B, Song ZS. 2013. MiR-214 regulate gastric cancer cell proliferation, migration and invasion by targeting PTEN. Cancer Cell Int. 13(1):68. doi: 10.1186/1475-2867-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No restriction on data or material availability.


