Abstract
Introduction
We examined the prevalence of autism spectrum disorders (ASD) in Massachusetts (MA) comparing children born via assisted reproductive technology (ART) and children born to women with indicators of subfertility but no ART [Subfertile], to children born to women with neither ART nor indicators of subfertility [Fertile]. We assessed the direct, indirect, and total effects of ART and subfertility on ASD among singletons.
Methods
This study included 10,147 ART, 8,072 Subfertile and 441,898 Fertile MA resident births from the MA Outcome Study of ART (MOSART) database linked with Early Intervention program participation data. ART included fresh in vitro fertilization (IVF), fresh intracytoplasmic sperm injection (ICSI), and frozen embryo transfer. We estimated the prevalence of ASD by fertility group. We used logistic regression to assess the natural direct (NDE), natural indirect (NIE) through preterm birth, and total effects of each fertility group on ASD.
Results
The NDE indicated that, compared to the Fertile group, the odds of ASD were not statistically higher in the ART (ORNDE: 1.07; 95% CI: 0.88-1.30), Subfertile (ORNDE: 1.11; 95% CI: 0.89-1.38), IVF (ORNDE: 0.91; 95% CI: 0.68-1.22), or ICSI (ORNDE: 1.13; 95% CI: 0.84-1.51) groups, even if the rate of preterm birth was the same across all groups. The total effect (product of NDE and NIE) was not significant for ART (ORTotal Effect: 1.08; 95% CI: 0.89-1.30), Subfertile (ORTotal Effect: 1.11; 95% CI: 0.89-1.38), IVF (ORTotal Effect: 0.92; 95% CI: 0.69-1.23), or ICSI (ORTotal Effect: 1.13; 95% CI: 0.84-1.52).
Conclusion
Compared to children born to Fertile women, children born to ART, ICSI, or IVF, or Subfertile are not at increased risk of receiving an ASD diagnosis.
Keywords: Assisted Reproductive Technology, In vitro fertilization, Intracytoplasmic sperm injection, Autism spectrum disorders, Subfertile, MOSART
Introduction
The use of assisted reproductive technology (ART) procedures has steadily risen over the last decades. The proportion of births attributable to ART in Massachusetts reached 4.8% compared to 1.6% for the national average in 2013 (Sunderam, Kissin et al. 2013). Parallel to this increase, the prevalence of autism spectrum disorders (ASDs) in the US and other developed countries has increased significantly (Newschaffer, Falb et al. 2005; Fombonne, Zakarian et al. 2006; Parner, Schendel et al. 2008; Hvidtjorn, Grove et al. 2011). In Massachusetts, the incidence of ASDs increased from 56 per 10,000 children in 2001 to 93 per 10,000 in 2005. Data from the Massachusetts Department of Public Health (MDPH) Early Intervention (EI) program indicate a 50% increase in ASDs incidence from 94 in 2006 to 141 per 10,000 in 2012, or 1 in 71 births (unpublished data, MDPH).
Previous research showed that children conceived via ART and those born to women with indicators of subfertility but no ART (“Subfertile” in this study, defined from hospital records and birth certificate data indicating prior treatment for conditions related to infertility), are more likely to have adverse perinatal outcomes including multiple birth, preterm birth, low birthweight, and small for gestational age, even among singletons (Wright, Schieve et al. 2003; Schieve, Ferre et al. 2004; Declercq, Luke et al. 2015; Luke, Stern et al. 2016). These same adverse outcomes have been associated with a higher likelihood of ASDs (Manning, Davin et al. 2011). Past research also indicated that regardless of preterm status, singletons born to women who used ART and Subfertile women had higher odds of being enrolled in EI programs, a proxy for developmental delays (Diop, Gopal et al. 2016).
Concerns about the risk of autism following infertility and/or infertility treatment have been suggested by various studies. In a population-based study in the United States, the authors found that ICSI (Intracytoplasmic Sperm Injection) is associated with an increased incidence of autism diagnosis. However, this study was restricted to ART-conceived children and concluded that additional studies are needed to explain the increased risk and safety of ICSI (Kissin, Zhang et al. 2015). A Danish population-based study found that the risk of infantile autism was lower among children born to women who used in vitro fertilization (IVF) and ICSI (Maimburg and Vaeth 2007). A nested case-control study did not find an association between ART and history of infertility and ASD, but suggested an increased risk with ovulation inducing drug and artificial insemination (Lyall, Pauls et al. 2012). A systematic review of seven studies concluded that ART did not appear to be a risk factor for ASDs, but suggested that large and high quality studies are needed to further explain the association between ART and ASDs (Conti, Mazzotti et al. 2013).
While results from past studies on the risk of autism after ART have been mixed, few have examined the risk of autism among children born to parents with a history of infertility. In a case-control study, the authors found that several infertility indicators were associated with an increased risk of ASD among multiples. Infertility indicators were defined as having documented infertility medications from pharmacy records dispensed to women any time in the past or around the time of the index pregnancy or ICD-9-MC (International Classification of Diseases, Ninth Revision, Clinical Modification) codes for female or male infertility. However, they concluded that their findings should be interpreted with caution due to insufficiently detailed data on treatments associated with ART and small study sample (n=21 cases and n=54 controls) (Grether, Qian et al. 2013). Recent research suggests that with few exceptions, the excess morbidity for women and children results from the underlying cause of infertility rather than ART treatment parameters alone (Luke, Stern et al. 2016).
ASDs are serious lifelong neurodevelopmental disorders characterized by impairments in social function, communication, and social behaviors (First 2013) and can be diagnosed as early as 1–2 years of age (Kotsopoulos 2015). The Healthy People 2020 goal is to have children with ASD be evaluated by age 36 months and community-based support and services initiated by age 48 months, for improved developmental and educational outcomes (Rogers, Vismara et al. 2014; Christensen, Baio et al. 2016).
The association of ART, subfertility and ASDs among children aged 0–3 years has not been clearly established in population-based studies in the US. Our objectives were to (1) examine the prevalence of, and characteristics associated with ASD diagnosis comparing children conceived via ART, children born to women with indicators of subfertility but no ART [Subfertile], and children born to women with neither ART nor indicators of subfertility [Fertile]; (2) conduct a sub-analysis of specific types of ART; and (3) assess the direct, indirect (through preterm birth), and total effects of ART and subfertility on early ASD among singletons using a mediation approach. Prior studies have adjusted for preterm birth as confounder, which is “not only unnecessary”, but “it can also be harmful” for estimation of total effects (Ananth and Schisterman 2017). To our knowledge, this is the first population-based study in the US that used a mediation approach to show the degree to which intervening on preterm could better estimate ASD.
Methods
Study Design and Setting
We conducted a longitudinal cohort study of live births in Massachusetts from July 1, 2004 through December 31, 2010 and children who participated in EI programs between July 1, 2004 and December 31, 2013.
Data Sources
This study linked the Society for Assisted Reproductive Technology Clinic Outcome Reporting System (SART CORS), the Massachusetts Pregnancy to Early Life Longitudinal (PELL), and EI program participation data. All ART clinics in Massachusetts during our study period contributed data to SART CORS, including patient demographic characteristics, medical history, infertility diagnoses, specific ART treatments and pregnancy outcomes. SART CORS has been described elsewhere (Luke, Brown et al. 2012).
PELL is a population-based data system that links delivery records to hospital discharge records for both women and children. This core dataset is longitudinally linked probabilistically to non-birth-related inpatient admissions, observational stays, and emergency room visits. More than 99% of Massachusetts deliveries from 1998–2010 have been linked in PELL. PELL has been described elsewhere (Nannini, Lazar et al. 2011). PELL allows for uniquely identifying deliveries and children born to the same woman, and multiple hospitalization records belonging to the same women or children to be classified as such.
EI program participation data are collected by MDPH; EI provides services at no cost to children aged 0–3 years with established developmental delays, social/emotional, and environmental factors. Massachusetts children at risk for ASD are screened using the Modified Checklist for Autism in Toddlers (Snow and Lecavalier 2008) administered by an interdisciplinary team, including a pediatrician, child psychologist, behavioral specialist, child neurologist, and child psychiatrist, or licensed mental health counselor. This interdisciplinary team evaluates young children to establish the diagnosis of ASD before children are enrolled in autism specialty services (Manning, Davin et al. 2011). Once children are determined to be eligible for ASD services, an Individualized Family Service Plan (IFSP) is developed by EI providers and parents. Treatment data for ASDs are obtained through statewide coordinated EI services (Clements, Barfield et al. 2006).
Data Linkage and Study Sample
The Massachusetts Outcomes Study of Assisted Reproductive Technology (MOSART) database was created using a five-phase algorithm to link PELL and SART CORS, via women’s first and last name, father’s last name, and women’s and baby’s dates of birth and yielding an overall linkage rate of 89.7% (Kotelchuck, Hoang et al. 2014). The MOSART database from July 1, 2004 through December 31, 2010 included 474,784 deliveries resulting in 486,075 live births and fetal deaths, linked to 70,086 ART deliveries from SART CORS.
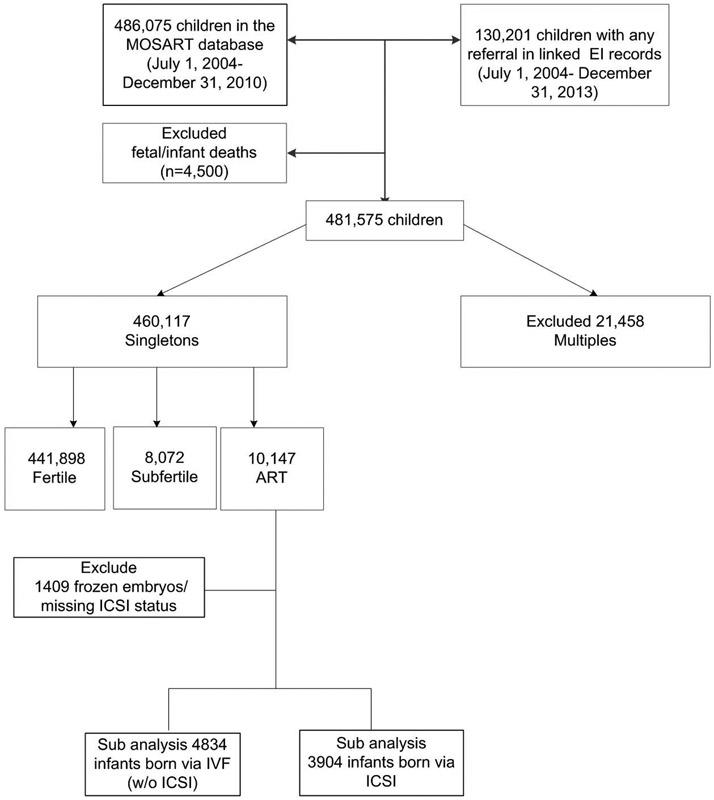
Children enrolled in the EI program are linked to PELL using a unique child ID. A probabilistic algorithm comprised of nine steps was used to match EI program data with PELL using LinkPro software (InfoSoft, Inc, Winnipeg, Manitoba, Canada) and multiple permutations of child’s first and last names, mother’s last name, child’s and mother’s date of birth, child’s sex, and zip code. Overall 86% of EI children linked to a PELL record. Linked PELL-EI data including children born from July 1, 2004 through December 31, 2010 (n=486,075) and children with any referral in EI between July 1, 2004 and December 31, 2013 (n=130,201) were merged with MOSART. After excluding fetal and infant deaths (n=4,500) and multiple births (n=21,458), our final study sample included 460,117 singletons, which were classified according to maternal fertility group (10,147 ART, 8,072 Subfertile and 441,898 Fertile). ART is defined as all treatments in which both eggs and sperm are manipulated in vitro and includes fresh in vitro fertilization (IVF), fresh intracytoplasmic sperm injection (ICSI), and frozen embryo transfer (FET). For FET cycles the method of insemination (IVF versus ICSI) is not recorded in SART CORS. After excluding FET and missing insemination method, we conducted a sub-analysis of IVF (n=4,834) and ICSI (n=3,904). Figure 1 illustrates our study sample.
Fig. 1.
Flow diagram of study smple with inclusion and exclusion criteria
Dependent Variable
Our dependent variable was an ASD diagnosis among children aged 0–3 years, defined as receiving autism-related services in the EI specialty services program with a diagnosis of autistic disorder (ICD-9-CM code 299.00), Asperger’s disorder (299.80), pervasive disorders not otherwise specified (299.90), or autism spectrum disorder or childhood disintegrative disorder (299.10).
Independent Variables and Mediator
Our main independent variable was maternal fertility status (ART, Subfertile, or Fertile group) for the delivery of the index child; our mediator was preterm birth defined as a delivery at <37 weeks gestation. To assess the direct, indirect and total effects of maternal fertility group on ASDs, we conducted separate analyses comparing children born to Fertile women with (1) children born via ART; (2) children born to Subfertile women; (3) children born via IVF; and (4) children born via ICSI.
Our subfertility measure [Subfertile] was derived from the MOSART database and was defined as having (1) at least one of the two fertility-treatment questions [Assisted Reproductive Technology (e.g., artificial insemination, in vitro fertilization) or Fertility Drug (e.g., Clomid, or Pergonal)] on the Massachusetts birth certificate checked as “yes” for the index delivery; (2) a prior hospital encounter for a condition specifically related to infertility (ICD-9-CM codes 628.0, 628.2, 628.3, 628.8, 628.9, V230); or (3) if a woman had an ART cycle or other fertility treatment indicated on the birth certificate in the five years prior to the index delivery but did not have an ART cycle associated with the current index delivery. We removed those cases where a woman received ART for the current index delivery and the remaining deliveries were classified as Subfertile (Declercq, Belanoff et al. 2014).
Covariates
Covariates were selected based on known risk factors associated with ASDs and ART and included parental demographics (maternal and paternal age, race, education, marital status, and nativity), insurance, smoking, prenatal care, parity, gender, method of delivery, gestational and chronic hypertension and diabetes, and breech presentation.
Mediation Approach
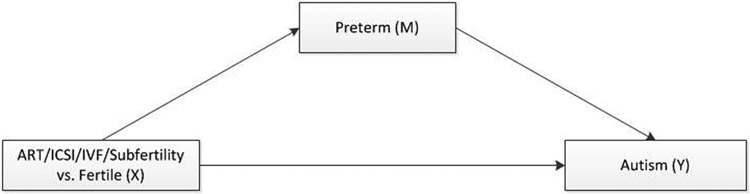
We used a mediation approach which assumes no unmeasured confounding to account for the known associations of ASD and prematurity and prematurity and ART (Ananth and VanderWeele 2011). We assumed that adjusting for the covariates listed above would be satisfactory to control for confounding of (1) exposures—outcome (fertility groups-ASD), (2) mediator—outcome (preterm-ASD), and (3) exposures—mediator (fertility groups-preterm). The mediation approach is used when a variable of interest is on the causal pathway between the exposure and the outcome (MacKinnon, Fairchild et al. 2007; Ananth and VanderWeele 2011). Prior studies have adjusted for preterm birth as confounder, which is “not only unnecessary,” but “it can also be harmful” for estimation of total effects (Ananth and Schisterman 2017). In this study, our mediator, preterm birth, is associated with higher odds of ASD (Manning, Davin et al. 2011; Diop, Gopal et al. 2016), and ART and subfertility are associated with higher odds of preterm birth (Schieve, Ferre et al. 2004; Luke, Stern et al. 2015). The mediation approach allowed us to split the total effect of each of our exposure groups on early ASDs into a natural direct effect (NDE) and a natural indirect effect (NIE) (Valeri and Vanderweele 2013). Our theoretical causal framework for mediation is shown in Figure 2.
Fig. 2.
Mediation framework used to asses whether preterm is a mediator of ART or subfertility in autism spectrum disorders
Statistical analysis
We examined maternal and infant characteristics among singleton ART, Subfertile, Fertile, IVF, and ICSI, and estimated the prevalence of early ASD using χ2 statistics (α=0.05). We used logistic regression to estimate crude and adjusted odds ratios (aORs) and 95% confidence intervals (CIs) to verify the three relationships for determining mediation. Based on our mediation framework, we estimated the NDE and NIE of the ART–ASD relationship by fitting a model for ASD (Y), conditional on ART (X), preterm birth (M), and ART–preterm birth interaction (X × M) and the covariates (C). We then fit a model for preterm birth (M), conditional on ART (X) and the covariates (C). We conducted similar analyses using Subfertile, IVF, and ICSI as exposures. We ruled out the possibility of effect modification of preterm birth on the association of ART, IVF, ICSI and Subfertile and ASD after we examined the interaction terms in our models. The mediation analysis was conducted using a SAS macro (Valeri and Vanderweele 2013). All analyses were conducted using SAS software version 9.3 (SAS Institute Inc., Cary, NC, USA. This study was approved by the Institutional Review Boards of the MDPH and Dartmouth College and the SART Research Committee.
Results
Compared to Fertile, women in the ART, Subfertile, IVF, and ICSI groups were generally older, with a higher proportion giving birth at or after age 35. Compared to Fertile (66.3%), the proportion of White non-Hispanic women was higher in the ART (85.1%), Subfertile (84.4%), IVF (84.4%), and ICSI (85.7%) groups. In addition, women in the ART, Subfertile, IVF, and ICSI groups were more likely to be US born, highly educated, married, on private insurance, with less inadequate prenatal care, deliver via primary cesarean, with higher proportion of pregnancy and chronic hypertension, gestational diabetes and chronic diabetes, preterm birth, and breech delivery (Table 1). Table 2 shows the prevalence of early ASD by maternal, paternal, and infant characteristics. The prevalence of ASD was higher among children born to women and fathers aged 38 years or more, Hispanic and black non-Hispanic women, women with some college or associate degree, hypertension and diabetes (chronic and pregnancy), cesarean delivery, on public insurance, male children, and children born preterm or via breech. Table 3 shows the prevalence of early ASDs by fertility group. Compared to the Fertile group, the prevalence of ASD was 1.2% (p<0.05) in the ART group, 1.1% (p=0.35) in the Subfertile group, 1.0% (p=0.85) in the IVF, and 1.3% (p=0.07) in the ICSI group.
Table 1.
Characteristics of Study Sample, by Fertility Groups, Massachusetts Birth July 1, 2004–December 31, 2010
| Demographic Characteristics |
Fertile | Subfertile | ART | ICSI | IVF | ||||
|---|---|---|---|---|---|---|---|---|---|
| N |
441,898 |
8,072 |
10,147 |
3,904 |
4,834 |
||||
| % | % | p- value |
% | p- value |
% | p- value |
% | p- value |
|
| Mother's Age | |||||||||
| <=30 | 55.0 | 16.4 | <0.0001 | 11.9 | <0.0001 | 10.9 | <0.0001 | 13.7 | <0.0001 |
| 31-34 | 24.2 | 28.9 | 27.2 | 26.8 | 27.9 | ||||
| 35-37 | 12.5 | 26.2 | 24.8 | 25.1 | 24.1 | ||||
| 38-40 | 6.1 | 18.3 | 20.4 | 21.3 | 20.2 | ||||
| 41-42 | 1.5 | 6.7 | 8.7 | 9.6 | 8.4 | ||||
| 43+ | 0.6 | 3.5 | 6.9 | 6.2 | 5.8 | ||||
| Race/Ethnicity | |||||||||
| Hispanic | 14.9 | 4.5 | <0.0001 | 3.4 | <0.0001 | 3.1 | <0.0001 | 3.8 | <0.0001 |
| Non-Hispanic White | 66.3 | 84.4 | 85.1 | 85.7 | 84.4 | ||||
| Non-Hispanic Black | 8.9 | 3.1 | 3.1 | 2.6 | 3.4 | ||||
| Asian/Pacific Islands | 7.7 | 6.9 | 7.4 | 7.7 | 7.3 | ||||
| American Indians & Others | 2.2 | 1.1 | 1.0 | 0.9 | 1.1 | ||||
| Education | |||||||||
| <High school or HS/GED | 37.8 | 11.8 | <0.0001 | 9.4 | <0.0001 | 8.6 | <0.0001 | 10.4 | <0.0001 |
| Some college/Associate | 21.5 | 17.7 | 15.4 | 14.9 | 16.3 | ||||
| BA degree or Post-graduate | 40.7 | 70.5 | 75.2 | 76.5 | 73.3 | ||||
| Marital Status | |||||||||
| Married | 65.1 | 94.3 | <0.0001 | 95.6 | <0.0001 | 95.5 | <0.0001 | 95.8 | <0.0001 |
| Not Married | 34.9 | 5.7 | 4.4 | 4.5 | 4.2 | ||||
| Prenatal Care | |||||||||
| Inadequate/Intermediate | 16.8 | 9.0 | <0.0001 | 7.2 | <0.0001 | 7.0 | <0.0001 | 7.0 | <0.0001 |
| Adequate | 45.9 | 43.9 | 43.7 | 44.2 | 42.3 | ||||
| Adequate Plus | 37.3 | 47.1 | 49.2 | 48.7 | 50.7 | ||||
| Parity | |||||||||
| 1 | 45.8 | 37.9 | <0.0001 | 62.4 | <0.0001 | 65.9 | <0.0001 | 65.2 | <0.0001 |
| 2 | 34.1 | 40.2 | 30.0 | 27.5 | 28.9 | ||||
| 3+ | 20.1 | 21.9 | 7.5 | 6.6 | 5.9 | ||||
| Method of delivery | |||||||||
| Vaginal | 67.5 | 53.9 | <0.0001 | 52.9 | <0.0001 | 54.8 | <0.0001 | 53.8 | <0.0001 |
| VBAC | 2.0 | 3.2 | 1.4 | 1.4 | 1.2 | ||||
| Primary C-Section | 18.0 | 20.5 | 32.1 | 32.4 | 32.6 | ||||
| Repeated C-Section | 12.6 | 22.4 | 13.6 | 11.4 | 12.3 | ||||
| Father's Age | |||||||||
| <=30 | 38.9 | 10.3 | <0.0001 | 7.1 | <0.0001 | 6.9 | <0.0001 | 7.8 | <0.0001 |
| 31-34 | 25.0 | 23.2 | 21.9 | 23.2 | 21.1 | ||||
| 35-37 | 15.6 | 24.3 | 23.1 | 23.8 | 22.1 | ||||
| 38-40 | 10.1 | 20.0 | 19.8 | 20.8 | 18.5 | ||||
| 41-42 | 4.0 | 8.5 | 9.4 | 9.5 | 9.3 | ||||
| 43+ | 6.4 | 13.8 | 18.7 | 15.8 | 21.2 | ||||
| Chronic Hypertension | |||||||||
| No | 98.4 | 97.4 | <0.0001 | 96.9 | <0.0001 | 97.0 | <0.0001 | 97.3 | <0.0001 |
| Yes | 1.6 | 2.6 | 3.1 | 3.0 | 2.7 | ||||
| Pregnancy Hypertension | |||||||||
| No | 91.4 | 89.8 | <0.0001 | 87.4 | <0.0001 | 88.5 | <0.0001 | 87.4 | <0.0001 |
| Yes | 8.6 | 10.2 | 12.6 | 11.5 | 12.6 | ||||
| Chronic Diabetes | |||||||||
| No | 98.8 | 98.3 | <0.0001 | 98.0 | <0.0001 | 98.0 | <0.0001 | 98.0 | <0.0001 |
| Yes | 1.2 | 1.7 | 2.0 | 2.0 | 2.0 | ||||
| Gestational Diabetes | |||||||||
| No | 94.4 | 91.5 | <0.0001 | 91.8 | <0.0001 | 92.4 | <0.0001 | 91.4 | <0.0001 |
| Yes | 5.6 | 8.5 | 8.2 | 7.6 | 8.6 | ||||
| Preterm | |||||||||
| No | 93.8 | 92.5 | <0.0001 | 89.9 | <0.0001 | 90.2 | <0.0001 | 90.2 | <0.0001 |
| Yes | 6.2 | 7.5 | 10.1 | 9.8 | 9.8 | ||||
| Breech | |||||||||
| No | 97.0 | 96.3 | <0.01 | 94.2 | <0.0001 | 94.4 | <0.0001 | 94.4 | <0.0001 |
| Yes | 3.0 | 3.7 | 5.8 | 5.6 | 5.6 | ||||
| Mother's nativity | |||||||||
| Non-US | 28.0 | 18.3 | <0.0001 | 18.9 | <0.0001 | 17.7 | <0.0001 | 20.3 | <0.0001 |
| US-born | 72.0 | 81.7 | 81.1 | 82.3 | 79.7 | ||||
| Infant gender | |||||||||
| Male | 51.2 | 51.2 | 0.90 | 51.3 | 0.82 | 53.1 | 0.04 | 49.5 | 0.01 |
| Female | 48.8 | 48.8 | 48.7 | 46.9 | 50.5 | ||||
| Payer | |||||||||
| Private | 57.7 | 91.3 | <0.0001 | 96.7 | <0.0001 | 96.9 | <0.0001 | 96.5 | <0.0001 |
| Public | 42.3 | 8.7 | 3.3 | 3.1 | 3.5 | ||||
HS/GED, high school/general educational development
Table 2.
Prevalence of Autism Spectrum Disorder among Singletons, Massachusetts Birth July 1, 2004–December 31, 2010
| Demographic Characteristics |
Autism (%) |
No Autism (%) |
p-value |
|---|---|---|---|
| Mother's Age | |||
| <=30 | 1.0 | 99.0 | <0.0001 |
| 31-34 | 0.9 | 99.1 | |
| 35-37 | 1.0 | 99.0 | |
| 38-40 | 1.2 | 98.8 | |
| 41-42 | 1.2 | 98.8 | |
| 43+ | 1.3 | 98.7 | |
| Race/Ethnicity | |||
| Hispanic | 1.2 | 98.8 | <0.0001 |
| Non-Hispanic White | 1.0 | 99.0 | |
| Non-Hispanic Black | 1.1 | 98.9 | |
| Asian/Pacific Islands | 0.8 | 99.2 | |
| American Indians & Others | 0.9 | 99.1 | |
| Education | |||
| <High school or HS/GED | 1.1 | 98.9 | <0.0001 |
| Some college or Associate | 1.2 | 98.8 | |
| BA degree or Post-graduate | 0.8 | 99.2 | |
| Marital Status | |||
| Married | 0.9 | 99.1 | <0.0001 |
| Not Married | 1.1 | 98.9 | |
| Prenatal Care | |||
| Inadequate/Intermediate | 1.0 | 99.0 | 0.0001 |
| Adequate | 0.9 | 99.1 | |
| Adequate Plus | 1.1 | 98.9 | |
| Parity | |||
| 1 | 1.1 | 98.9 | <0.0001 |
| 2 | 1.0 | 99.0 | |
| 3+ | 0.8 | 99.2 | |
| Method of delivery | |||
| Vaginal | 0.9 | 99.1 | <0.0001 |
| VBAC | 0.7 | 99.3 | |
| Primary C-Section | 1.3 | 98.7 | |
| Repeated C-Section | 1.0 | 99.0 | |
| Father's Age | |||
| <=30 | 1.0 | 99.0 | <0.0001 |
| 31-34 | 0.9 | 99.1 | |
| 35-37 | 0.9 | 99.1 | |
| 38-40 | 1.1 | 98.9 | |
| 41-42 | 1.2 | 98.8 | |
| 43+ | 1.2 | 98.8 | |
| Chronic Hypertension | |||
| No | 1.0 | 99.0 | <0.01 |
| Yes | 1.3 | 98.7 | |
| Pregnancy Hypertension | |||
| No | 1.0 | 99.0 | <0.0001 |
| Yes | 1.3 | 98.7 | |
| Chronic Diabetes | |||
| No | 1.0 | 99.0 | <0.0001 |
| Yes | 1.5 | 98.5 | |
| Gestational Diabetes | |||
| No | 1.0 | 99.0 | <0.0001 |
| Yes | 1.4 | 98.6 | |
| Preterm | |||
| No | 1.0 | 99.0 | <0.0001 |
| Yes | 1.5 | 98.5 | |
| Breech | |||
| No | 1.0 | 99.0 | 0.01 |
| Yes | 1.2 | 98.8 | |
| Mother's nativity | |||
| Non-US | 0.8 | 99.2 | <0.0001 |
| US-born | 1.1 | 98.9 | |
| Infant gender | |||
| Male | 1.6 | 98.4 | <0.0001 |
| Female | 0.4 | 99.6 | |
| Payer | |||
| Private | 0.9 | 99.1 | <0.0001 |
| Public | 1.1 | 98.9 |
HS/GED, high school/general educational development
Table 3.
Prevalence of Autism Spectrum Disorder among Singletons, by Fertility Groups, Massachusetts Birth July 1, 2004–December 31, 2010
| Demographic Characteristics |
Autism N (%) |
No Autism N (%) |
p-value |
|---|---|---|---|
| Fertility Group | |||
| Fertile | 4,363 (1.0) | 437,535 (99.0) | Ref |
| Subfertile | 88 (1.1) | 7,984 (98.9) | 0.35 |
| ART | 120 (1.2) | 10,027 (98.8) | <0.05 |
| ICSI | 50 (1.3) | 3,854 (98.7) | 0.07 |
| IVF | 49 (1.0) | 4,785 (99.0) | 0.85 |
Crude and aORs used to assess our mediation conditions are presented in Table 4. In the adjusted models, compared to the Fertile group, the relationship between ART and ASD was not significant (aOR: 1.08; 95% CI: 0.89–1.31). Similarly, the relationships between Subfertile, IVF, ICSI, and ASD were not significant. However, ART was significantly associated with preterm birth (aOR: 1.51; 95% CI: 1.40–1.63) and preterm birth was significantly associated with ASD (aOR: 1.31; 95% CI: 1.17–1.47). Likewise, when compared to the Fertile group, Subfertile (aOR: 1.17; 95% CI: 1.06–1.28), IVF (aOR: 1.50; 95% CI: 1.35–1.67), and ICSI (aOR: 1.42; 95% CI: 1.27–1.60) were all significantly associated with preterm birth, and preterm birth was significantly associated with ASD in the Subfertile (aOR: 1.34; 95% CI: 1.20–1.50), IVF (aOR: 1.33; 95% CI: 1.18–1.49), and ICSI (aOR: 1.32; 95% CI: 1.17–1.48) groups.
Table 4.
Unadjusted and Adjusted Odds Rations from Logistic Regression Models for Each of the Criteria for the Mediation Analysis in Singletons, Massachusetts Birth July 1, 2004–December 31, 2010
| Criteria for Mediation | Unadjusted ORa (95% CI) |
P-value | Adjusted ORb (95% CI) |
P-value |
|---|---|---|---|---|
| ART. vs. Fertile (N= 404,604) | ||||
| 1. Relationship between ART and ASD | 1.20 (1.00, 1.45) | 0.05 | 1.08 (0.89, 1.31) | 0.43 |
| 2. Relationship between ART and preterm | 1.80 (1.69, 1.93) | <0.0001 | 1.51 (1.40, 1.63) | <0.0001 |
| 3. Relationship between preterm and ASD | 1.57 (1.41, 1.75) | <0.0001 | 1.31 (1.17, 1.47) | <0.0001 |
| Subfertile vs. Fertile (N=402,511) | ||||
| 1. Relationship between Subfertile and ASD | 1.13 (0.91, 1.40) | 0.26 | 1.11 (0.89, 1.39) | 0.40 |
| 2. Relationship between Subfertile and preterm | 1.28 (1.17, 1.39 | <0.0001 | 1.17 (1.06, 1.28) | <0.01 |
| 3. Relationship between preterm and ASD | 1.61 (1.44, 1.79) | <0.0001 | 1.34 (1.20, 1.50) | <0.0001 |
| ICSI vs. Fertile (N=398,564) | ||||
| 1. Relationship between ICSI and ASD | 1.26 (0.94, 1.68) | 0.12 | 1.13 (0.85, 1.53) | 0.29 |
| 2. Relationship between ICSI and preterm | 1.75 (1.57, 1.95) | <0.0001 | 1.42 (1.27, 1.60) | <0.0001 |
| 3. Relationship between preterm and ASD | 1.59 (1.42, 1.77) | <0.0001 | 1.32 (1.17, 1.48) | <0.0001 |
| IVF vs. Fertile(N=399,421) | ||||
| 1. Relationship between IVF and ASD | 1.05 (0.79, 1.40) | 0.74 | 0.92 (0.69, 1.23) | 0.58 |
| 2. Relationship between IVF and preterm | 1.76 (1.59, 1.94) | <0.0001 | 1.50 (1.35, 1.67) | <0.0001 |
| 3. Relationship between preterm and ASD | 1.59 (1.43, 1.78) | <0.0001 | 1.33 (1.18, 1.49) | <0.0001 |
For the first two assumptions, the unadjusted OR is the crude OR of the association. For the third assumption, the unadjusted OR is the association of ASD (outcome) and preterm (mediator), controlling only for fertility status (exposure)
Models adjusted for maternal demographics (maternal paternal age, race, education, marital status, nativity), insurance, smoking, prenatal care, parity, gender, method of delivery, chronic and pregnancy hypertension, gestational and chronic diabetes, breech.
Data with missing covariates are excluded in all models.
Table 5 shows the results of the mediation analysis. The natural direct effect (NDE) indicated that, compared to the Fertile group, the odds of early ASD were not meaningfully higher in the ART (ORNDE: 1.07; 95% CI: 0.88–1.30), Subfertile (ORNDE: 1.11; 95% CI: 0.89–1.38), IVF (ORNDE: 0.91; 95% CI: 0.68–1.22) and ICSI (ORNDE: 1.13; 95% CI: 0.84–1.51) groups, even if the rate of preterm birth is the same across all groups. The natural indirect effects (NIEs) (odds of early ASD among the exposure groups under their observed preterm rates) are statistically significant; however, the ORs were equal to 1.00 for the Subfertile (ORNIE Subfertile versus Fertile: 1.00; 95% CI: 1.00–1.00) and ICSI (ORNIE ICSI versus Fertile: 1.00; 95% CI: 1.00–1.01) groups, and nearly equal to 1.00 for both ART (ORNIE ART versus Fertile: 1.01; 95% CI: 1.00–1.01) and IVF (ORNIE IVF versus Fertile: 1.01; 95% CI: 1.00–1.01). The total effect which is the product of NDE and NIE was not significant for ART (ORTotal Effect: 1.08; 95% CI: 0.89–1.30), Subfertile (ORTotal Effect: 1.11; 95% CI: 0.89–1.38), ICSI (ORTotal Effect: 1.13; 95% CI: 0.84–1.52), and IVF (ORTotal Effect: 0.92; 95% CI: 0.69–1.23). The total effect was equal to the aORs in Table 4 across all exposure groups.
Table 5.
Estimates of Natural Direct, Natural Indirect and Total Effect of the Association between ART, Subfertility, ICIS, IVF and ASD Mediated through Preterm Birth, in Singletons, Massachusetts Birth July 1, 2004–December 31, 2010 a
| Natural Direct effect | Natural Indirect Effect | Total Effect | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| ART vs. Fertile | 1.07 (0.88, 1.30) | 0.49 | 1.01 (1.00, 1.01) | <0.01 | 1.08 (0.89, 1.30) | 0.46 |
| Subfertile vs. Fertile | 1.11 (0.89, 1.38) | 0.35 | 1.00 (1.00, 1.00) | 0.01 | 1.11 (0.89, 1.38) | 0.34 |
| ICSI vs. Fertile | 1.13 (0.84, 1.51) | 0.43 | 1.00 (1.00, 1.01) | <0.01 | 1.13 (0.84, 1.52) | 0.41 |
| IVF vs. Fertile | 0.91 (0.68, 1.22) | 0.53 | 1.01 (1.00, 1.01) | <0.01 | 0.92 (0.69, 1.23) | 0.56 |
Models adjusted for maternal demographics (maternal paternal age, race, education, marital status, nativity), insurance, smoking, prenatal care, parity, gender, method of delivery, chronic and pregnancy hypertension, gestational and chronic diabetes, breech. Data with missing covariates are excluded in all models.
Discussion
As the proportion of ART infants remains significantly above national averages in Massachusetts, it is important to improve our understanding of the long-term health outcomes of these children. This study examined the likelihood of receiving an ASD diagnosis in the first three years of life among singletons. Compared to children born to Fertile women, the odds of early ASD were not higher among children born to ART, Subfertile, IVF, and ICSI groups through a direct mechanism (i.e., the NDE effects were not significant). Our findings are consistent with other case-control and prospective studies in the US and Sweden (Lyall, Pauls et al. 2012; Conti, Mazzotti et al. 2013; Grether, Qian et al. 2013; Rogers, Vismara et al. 2014).
A Danish population-based study found that the risk of infantile autism was lower among children born to women who used in vitro fertilization (IVF) and ICSI; the authors speculate that women who use assisted conception were more likely to interact with the health care system, and may have better behaviors and experiences early on during pregnancy. While this study raised an interesting question, it was limited by the small study sample as there were only 10 cases and 23 controls (Maimburg and Vaeth 2007).
A large population-based retrospective study in California found that the incidence of autism diagnosis was higher among children born to women who conceived via ICSI when compared to those who utilized traditional IVF. The study also found that compared to other types of infertility, there is reduced association of autism diagnosis among singletons born to parents with unexplained infertility and among multiples born to parents with tubal factors. While this study had a large sample size (n=42,383), it was restricted to ART-conceived children and did not compare autism diagnosis among ART to non-ART conceived children and to children born to women with indicators of subfertility (Kissin, Zhang et al. 2015).
Our study confirmed that singletons born to ART, Subfertile, IVF, ICSI groups were associated with a greater risk of preterm birth, which is consistent with prior studies (Perri, Chen et al. 2001; Pandey, Shetty et al. 2012; Diop, Gopal et al. 2016). We also found that compared to the Fertile group, the NIEs (odds of early ASD among children born to ART, Subfertile, IVF, and ICSI groups under their observed preterm birth rate) were significant, however, the ORs were close to 1, indicating that risk of ASD increased by only 1% or less through preterm birth. The total effect which is the product of NDE and NIE was not significant for ART, Subfertile, IVF, or ICSI.
Our study has limitations. The study population was limited to Massachusetts and may not be generalizable to other states or countries. Children born in Massachusetts who received EI services outside of Massachusetts were not captured. While the American Academy of Pediatrics (AAP) recommends routine developmental screening to identify young children at risk of developmental delays (Duby, Lipkin et al. 2006), prior research has shown that ASD is often identified later in life (Maenner, Schieve et al. 2013). In our study, ASD diagnosis was only captured among children 0–3 years of age; the median age at diagnosis was 19 months. Screening at 18 months is essential, as symptoms of ASD often materialize by this age and can be “reliably” diagnosed, thus enabling early intervention; rescreening at 24 months is also critical for children who retrogress or could be missed at earlier ages (Dai, Miller et al. 2019).
Additionally, children with early ASD not enrolled in EI were not captured in this study since identification of ASD cases was based on participation and receipt of autism-related services in the EI specialty services dataset. However, the EI program in Massachusetts is very active and most children with ASD are believed to be in the program. Finally, we have likely underestimated the number of women in the Subfertile group, since it is based on a complex multi-factor algorithm, in which we were deliberatively conservative in our definitions. Given that women with fertility problems can be treated in outpatient settings, it is possible that we may have misclassified the Subfertile group, and the Fertile group may contain a small proportion of women who were actually Subfertile. However, since the Fertile group is much larger than the Subfertile group, it is unlikely this small number is sufficient to affect our findings.
Our study also has several strengths. MOSART is a large, population-based database which includes detailed ART treatment, post birth hospital utilization, and EI program participation data. Most ART studies in the US have relied on the national database and clinic-based data, which do not allow investigators to follow children post birth. To identify women with a history of infertility but no ART, we used a previously described algorithm that examined hospital discharge records available through our longitudinally linked MOSART database, rather than reports of infertility diagnosis by women. In addition, categorization of ART, IVF, and ICSI groups were based on SART CORS, the gold standard for ART treatment cycles obtained directly from all ART clinics in Massachusetts, and subsequently linked to in-state delivery records for resident women. About 95% of eligible ART treatment records were linked to a delivery record suggesting that the vast majority of ART births in Massachusetts were correctly classified.
Finally, our study used the mediation approach to disentangle the direct and indirect (through preterm birth) effects of ART, Subfertile, IVF, and ICSI on ASD, while prior studies have adjusted for preterm birth as confounder, which is detrimental for estimation of total effects (Ananth and Schisterman 2017). Our study showed that independent of preterm birth there was no direct effect of ART, Subfertile, IVF, and ICSI on ASD diagnosis. Our study improves upon the previous literature because it allowed us to show the degree to which intervening on preterm could better estimate ASD among children born to women in the ART, Subfertile, IVF, and ICSI groups. The natural indirect effects (NIEs) (odds of early ASD among the exposure groups under their observed preterm rates) are statistically significant.
As the prevalence of ASD has continued to increase, the search for its causes has continued to evolve; from the strong genetic origins (Muhle, Trentacoste et al. 2004), to environmental exposures which may have teratogenic effect on the central nervous system early in pregnancy and play an important role in gene expression without DNA modification (Johnson and Myers 2007). Given that older parental age has been found to be associated with ASD due to alteration in genetic imprinting (Croen, Najjar et al. 2007; Reichenberg, Gross et al. 2010); and that the use ART, IVF, ICSI, and subfertility are more prevalent among older women who have elevated adverse birth outcomes, there is a legitimate concern about the potential risk of ASD among children born to these women.
Conclusion
We have found that children born to women in the ART, Subfertile, IVF, and ICSI groups are not at increased risk of receiving an early ASD diagnosis when compared to children born to Fertile women. Although there was no causal association between ART/subfertility and ASD we confirmed an association between preterm birth and ASD and between ART and preterm birth. Long term studies are needed to further elucidate the causes of ASD, while research on other areas of potential underlying child health sequelae of infertility and its treatment (beyond ASD) must continue.
Significance.
What is already known about the subject?
Increased risk of autism following infertility and/or infertility treatment has been suggested by various studies; however, the effect of assisted reproductive technology (ART) and maternal subfertility on autism spectrum disorders (ASD) among children aged 0-3 years has not been clearly established in population-based studies in the US.
What this study adds?
Compared to singleton children born to Fertile women, the odds of ASD were not higher among children born via ART and children born to women with subfertility. This study shows the degree to which intervening on preterm birth using a mediation model could better estimate ASD.
Acknowledgements
The authors would like to thank SART members for providing clinical information to the SART CORS database for use by patients and researchers. Without the efforts of SART members, this research would not have been possible. The authors also want to thank Gabriela Kernan from the Massachusetts Department of Public Health for providing technical support around EI data acquisition. This work was supported by R01HD064595 and R01HD067270. The views expressed in this article are those of the authors and do not necessarily represent the official view of the National Institutes of Health or the Massachusetts Department of Public Health.
References
- Ananth CV and Schisterman EF (2017). “Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics.” Am J Obstet Gynecol 217(2): 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananth CV and VanderWeele TJ (2011). “Placental abruption and perinatal mortality with preterm delivery as a mediator: disentangling direct and indirect effects.” Am J Epidemiol 174(1): 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Baio J, et al. (2016). “Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years--Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012.” MMWR Surveill Summ 65(3): 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements KM, Barfield WD, et al. (2006). “Birth characteristics associated with early intervention referral, evaluation for eligibility, and program eligibility in the first year of life.” Matern Child Health J 10(5): 433–441. [DOI] [PubMed] [Google Scholar]
- Conti E, Mazzotti S, et al. (2013). “Are children born after assisted reproductive technology at increased risk of autism spectrum disorders? A systematic review.” Hum Reprod 28(12): 3316–3327. [DOI] [PubMed] [Google Scholar]
- Croen LA, Najjar DV, et al. (2007). “Maternal and paternal age and risk of autism spectrum disorders.” Arch Pediatr Adolesc Med 161(4): 334–340. [DOI] [PubMed] [Google Scholar]
- Dai YG, Miller LE, et al. (2019). “Incremental Utility of 24-Month Autism Spectrum Disorder Screening After Negative 18-Month Screening.” J Autism Dev Disord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declercq E, Luke B, et al. (2015). “Perinatal outcomes associated with assisted reproductive technology: the Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART).” Fertil Steril 103(4): 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declercq ER, Belanoff C, et al. (2014). “Identifying women with indicators of subfertility in a statewide population database: operationalizing the missing link in assisted reproductive technology research.” Fertil Steril 101(2): 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop H, Gopal D, et al. (2016). “Assisted Reproductive Technology and Early Intervention Program Enrollment.” Pediatrics 137(3): e20152007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duby JC, Lipkin PH, et al. (2006). “Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening.” Pediatrics 118(1): 405–420. [DOI] [PubMed] [Google Scholar]
- First MB (2013). “Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility.” J Nerv Ment Dis 201(9): 727–729. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Zakarian R, et al. (2006). “Pervasive developmental disorders in Montreal, Quebec, Canada: prevalence and links with immunizations.” Pediatrics 118(1): e139–150. [DOI] [PubMed] [Google Scholar]
- Grether JK, Qian Y, et al. (2013). “Is infertility associated with childhood autism?” J Autism Dev Disord 43(3): 663–672. [DOI] [PubMed] [Google Scholar]
- Hvidtjorn D, Grove J, et al. (2011). “Risk of autism spectrum disorders in children born after assisted conception: a population-based follow-up study.” J Epidemiol Community Health 65(6): 497–502. [DOI] [PubMed] [Google Scholar]
- Johnson CP and Myers SM (2007). “Identification and evaluation of children with autism spectrum disorders.” Pediatrics 120(5): 1183–1215. [DOI] [PubMed] [Google Scholar]
- Kissin DM, Zhang Y, et al. (2015). “Association of assisted reproductive technology (ART) treatment and parental infertility diagnosis with autism in ART-conceived children.” Hum Reprod 30(2): 454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelchuck M, Hoang L, et al. (2014). “The MOSART database: linking the SART CORS clinical database to the population-based Massachusetts PELL reproductive public health data system.” Matern Child Health J 18(9): 2167–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsopoulos S (2015). “[Early diagnosis of autism: Phenotype-endophenotype].” Psychiatriki 25(4): 273–281. [PubMed] [Google Scholar]
- Luke B, Brown MB, et al. (2012). “Cumulative birth rates with linked assisted reproductive technology cycles.” N Engl J Med 366(26): 2483–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke B, Stern JE, et al. (2016). “Is the wrong question being asked in infertility research?” J Assist Reprod Genet 33(1): 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke B, Stern JE, et al. (2016). “Birth Outcomes by Infertility Treatment: Analyses of the Population-Based Cohort: Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART).” J Reprod Med 61(3–4): 114–127. [PMC free article] [PubMed] [Google Scholar]
- Luke B, Stern JE, et al. (2015). “Birth Outcomes by Infertility Diagnosis Analyses of the Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART).” J Reprod Med 60(11–12): 480–490. [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Pauls DL, et al. (2012). “Fertility therapies, infertility and autism spectrum disorders in the Nurses’ Health Study II.” Paediatr Perinat Epidemiol 26(4): 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, et al. (2007). “Mediation analysis.” Annu Rev Psychol 58: 593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner MJ, Schieve LA, et al. (2013). “Frequency and pattern of documented diagnostic features and the age of autism identification.” J Am Acad Child Adolesc Psychiatry 52(4): 401–413 e408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimburg RD and Vaeth M (2007). “Do children born after assisted conception have less risk of developing infantile autism?” Hum Reprod 22(7): 1841–1843. [DOI] [PubMed] [Google Scholar]
- Manning SE, Davin CA, et al. (2011). “Early diagnoses of autism spectrum disorders in Massachusetts birth cohorts, 2001–2005.” Pediatrics 127(6): 1043–1051. [DOI] [PubMed] [Google Scholar]
- Muhle R, Trentacoste SV, et al. (2004). “The genetics of autism.” Pediatrics 113(5): e472–486. [DOI] [PubMed] [Google Scholar]
- Nannini A, Lazar J, et al. (2011). “Rates of hospital visits for assault during pregnancy and the year postpartum: timing matters.” Public Health Rep 126(5): 664–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newschaffer CJ, Falb MD, et al. (2005). “National autism prevalence trends from United States special education data.” Pediatrics 115(3): e277–282. [DOI] [PubMed] [Google Scholar]
- Pandey S, Shetty A, et al. (2012). “Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis.” Hum Reprod Update 18(5): 485–503. [DOI] [PubMed] [Google Scholar]
- Parner ET, Schendel DE, et al. (2008). “Autism prevalence trends over time in Denmark: changes in prevalence and age at diagnosis.” Arch Pediatr Adolesc Med 162(12): 1150–1156. [DOI] [PubMed] [Google Scholar]
- Perri T, Chen R, et al. (2001). “Are singleton assisted reproductive technology pregnancies at risk of prematurity?” J Assist Reprod Genet 18(5): 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Gross R, et al. (2010). “Advancing paternal and maternal age are both important for autism risk.” Am J Public Health 100(5): 772–773; author reply 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Vismara L, et al. (2014). “Autism treatment in the first year of life: a pilot study of infant start, a parent-implemented intervention for symptomatic infants.” J Autism Dev Disord 44(12): 2981–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieve LA, Ferre C, et al. (2004). “Perinatal outcome among singleton infants conceived through assisted reproductive technology in the United States.” Obstet Gynecol 103(6): 1144–1153. [DOI] [PubMed] [Google Scholar]
- Snow AV and Lecavalier L (2008). “Sensitivity and specificity of the Modified Checklist for Autism in Toddlers and the Social Communication Questionnaire in preschoolers suspected of having pervasive developmental disorders.” Autism 12(6): 627–644. [DOI] [PubMed] [Google Scholar]
- Sunderam S, Kissin DM, et al. (2013). “Assisted reproductive technology surveillance -- United States, 2010.” MMWR Surveill Summ 62(9): 1–24. [PubMed] [Google Scholar]
- Valeri L and Vanderweele TJ (2013). “Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros.” Psychol Methods 18(2): 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright VC, Schieve LA, et al. (2003). “Assisted reproductive technology surveillance--United States, 2000.” MMWR Surveill Summ 52(9): 1–16. [PubMed] [Google Scholar]