Abstract
Skin regeneration is a vexing problem in the field of regenerative medicine. A bioactive molecule-based strategy has been frequently used in skin wound healing in recent years. Bioactive molecules are practical tools for regulating cellular processes and have been applied to control cellular differentiation, dedifferentiation, and reprogramming. In this review, we focus on recent progress in the use of bioactive molecules in skin regenerative medicine, by which desired cell types can be generated in vitro for cell therapy and conventional therapeutics can be developed to repair and regenerate skin in vivo through activation of the endogenous repairing potential. We further prospect that the bioactive molecule-base method might be one of the promising strategies to achieve in situ skin regeneration in the future.
1. Introduction
The skin is the largest organ in the body and plays a crucial role in protecting the body from various injuries, such as trauma, heat, chemicals, UV radiation, and microbial infection [1]. However, when the skin is injured, its protective function is lost, and the defects regrow new skin through a wound healing process. This process can be divided into three individual but overlapping phases: inflammation, reepithelialization, and tissue remodeling. It is a well-coordinated process involving a variety of cell types, mainly including immune cells, keratinocytes, fibroblasts, endothelial cells, and hair follicle stem cells [2]. Keratinocytes migrate to the wound site through proliferation and differentiation until the wound is entirely sealed [3]. Fibroblasts are the predominant cell type during the early stages of the wound healing process. A large number of the native fibroblasts transform into myofibroblasts, which are responsible for wound contraction and extracellular matrix (ECM) deposition [4, 5]. In addition, the reconstruction of an injured skin vascular network through the migration and proliferation of endothelial cells is necessary for successful wound healing [6]. The skin includes a large number of appendages, such as hair follicles and sweat glands. Hair follicle stem cells (HFSCs) are currently thought to be essential for hair follicle regeneration and skin repair, including differentiation into epidermal cells, sebaceous gland cells, and different types of hair follicle epithelial cells [7]. Moreover, sweat gland cells are responsible for the regulation of body temperature and contribute significantly to skin repair, presenting a substantial turnover both in wound healing and in homeostasis [8]. More importantly, these cells cooperate to repair/regenerate the injured skin, and abnormal function or an insufficient number of repairing cells frequently lead to scar healing or chronic wound.
Realizing skin regeneration is a worldwide problem. We propose to focus on two pivotal aspects: first is replenishing the sufficient number of repairing cells and second is activating the endogenous repair potential. Therefore, cell transplantation, skin grafts, and tissue-engineered skins are commonly used for skin wound healing. For example, one study illustrated the use of keratinocytes and fibroblasts suspended in the platelet-rich plasma-enriched medium which could promote the full-thickness skin wound healing [9]. Another in vivo study showed that bacterial cellulose/acrylic acid hydrogel loaded with human epidermal keratinocytes and dermal fibroblasts leads to the higher acceleration of burn wound healing, compared with treatment with hydrogel alone [10]. A recent study reported a compound biomaterial which is constructed with nanofibrous collagen, polycaprolactone, and bioactive glass nanoparticles which promoted the proliferation, migration, and vascularization of endothelial progenitor cells through upregulation of the hypoxia-inducible factor-1α/vascular endothelial growth factor/stromal cell-derived factor-1α (HIF-1α/VEGF/SDF-1α) signaling pathway [11]. All of these studies illustrated the importance of supplemental necessary repairing cells for wound repair. Still, it is worth noting that the lack of seed cells is an outstanding limitation for the widespread adaptation of the strategy. Recently, stem cells, mainly induced pluripotent stem cells (iPSCs), have received much attention for their tissue repair and regeneration properties. They can provide unlimited required cells and are theoretically able to differentiate into all cell types in the body, which are considered an ideal cell source. More importantly, iPSCs have the advantage in autotransplantation, which can avoid immune rejection. Nevertheless, many studies have demonstrated that genetic approaches are the conventional methods for cell reprogramming or transdifferentiation, which is low efficiency and has the integration risk. Therefore, it cannot be used as a conventional strategy for getting target cells for cell therapy [12]. Thus, compared to genetic manipulation, bioactive molecule-based reprogramming with high efficiency and improved quality of the reprogrammed cells is one of the critical approaches for obtaining cells of interest.
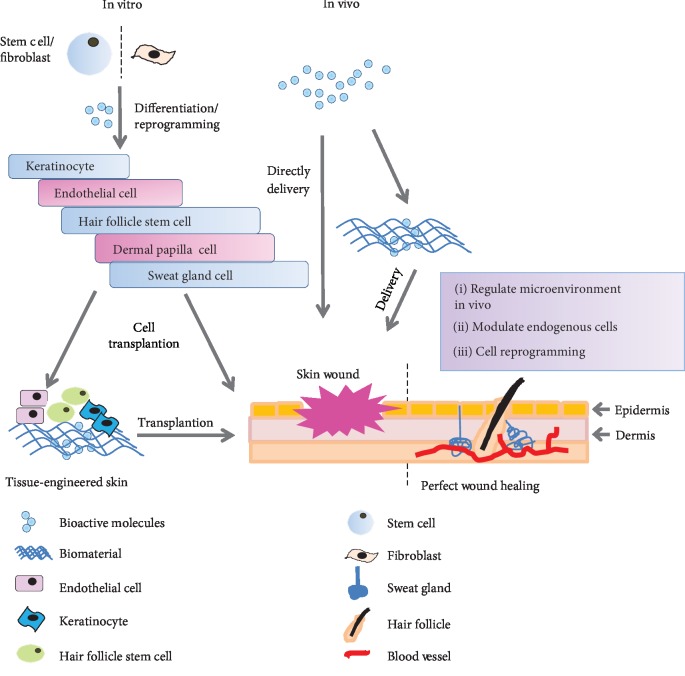
The bioactive molecules mentioned here include large natural molecules, such as proteins, cytokines, and lipids, as well as small molecules that have been demonstrated to regulate specific signaling pathways and contribute to cell reprogramming and tissue repair. Bioactive molecules are mostly cell-permeable and nonimmunogenic and can selectively modulate intracellular processes in a reversible way with their cellular targets [13, 14]. Therefore, bioactive molecules with therapeutic potentials may represent the next generation of regenerative medicine. More importantly, bioactive molecules have been used for cell differentiation or reprogramming to acquire the target cells with biological activity in vitro [15–17]. For example, human pluripotent stem cells (hPSCs) generated mesodermal cells after treatment with CHIR99021 and bone morphogenetic protein4 (BMP4). Mesodermal progenitors differentiated into vascular endothelial cells in the exposure to vascular endothelial growth factor A (VEGF-A) and the small molecule forskolin in vitro. And the hPSC-derived endothelial cells could be further cultured and expand in medium supplemented with serum, VEGF, and SB431542 [18]. Additionally, not only can bioactive molecules promote reprogramming and differentiation efficiency, but they can also activate the local repair potential. The C-X-C chemokine receptor type 4-stromal cell-derived factor 1 (CXCR4-SDF1) axis plays a vital role in the stem or progenitor cell mobilization and home, which can be modulated through bioactive molecules. For example, antagonizing CXCR4 with Plerixafor/Mozobil induced stem cell or progenitor cell mobilization, while increasing CXCR4 expression with prostaglandin E2 or enhancing SDF1 stability with Diprotin A promoted stem cell or progenitor cell homing [19]. Therefore, it is conceivable that bioactive molecules can be developed as a treatment, which can be delivered in vivo directly to repair injured tissues and regenerate damaged or lost cells. This review will focus on the recent developments of bioactive molecules that contribute to skin wound healing. We emphasize on the repairing cells reprogrammed from other cells through bioactive molecules' induction and the endogenous repairing cells recruited from local and distant tissue by bioactive molecules' stimulation (Figure 1).
Figure 1.
The strategies of skin regeneration using bioactive molecules. The repairing of cells induced from stem cells or somatic cells by using bioactive molecules in vitro for skin repair or stimulating skin endogenous cells to regenerate skin in vivo by using bioactive molecules as a conventional therapeutics.
2. Skin-Repairing Cells That Are Induced by Bioactive Molecules
2.1. Keratinocytes Derived by Bioactive Molecule Induction
Keratinocytes make up the first barrier of the skin. They play a critical role in the reepithelialization process which is mediated by keratinocyte proliferation and migration. If this reepithelialization process failed, its barrier function is lost, which might cause dehydration, infection, or even death [20, 21]. Rapid reepithelialization is indispensable for restoring the skin barrier. Keratinocytes can be used as grafts or as a component of other complex matrices to cover the injured sites [22]. Nevertheless, keratinocyte sources are limited. It is necessary to develop new strategies to obtain sufficient keratinocytes for skin wound transplantation.
Employing bioactive molecules to modulate signaling pathways of keratinocyte development and to restrict initial cell to follow the specific differentiation path presents an alternative strategy for obtaining transplantable keratinocytes. Previous studies showed that the transgene method was commonly used for cell reprogramming, while the risk of insertional mutagenesis of viral vectors and spontaneous transgene reactivation limit its application in the clinic [23]. Reprogramming and transdifferentiation through bioactive molecules have been explored and tested in regenerative medicine in recent years. Primarily, iPSCs were initially generated by viral vector-mediated overexpression of pluripotency factors (Oct4, Sox2, Klf4, and c-Myc (OSKM)) from fibroblasts. Then the pluripotency factors were substituted by a combination of bioactive molecules [24]. A large number of studies show promise in obtaining keratinocytes by bioactive molecules' strategy from embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). And in this process, the bioactive molecule selection is a vital prerequisite that depends on an understanding of keratinocyte development and regulatory mechanism, as well as further screening the suitable bioactive molecules to guide initial cell reprogramming into keratinocytes.
In the skin development, the epidermis is derived from the primitive ectoderm, which expressed K8/K18, and then these cells committed to a keratinocyte fate which was marked by the K5/K14 expression replaced by K8/K18 expression. The K5/K14-positive basal keratinocytes initiated epidermal stratification and eventually underwent terminal differentiation to form the mature adult epidermis marked K1/K10 [25], while the primitive ectoderm also gives rise to the nervous system and its choice to epidermal lineage development is governed by coordination of Wnt, bone morphogenetic protein (BMP), and fibroblast growth factor (FGF) signaling. Among them, Wnt signaling was active in early epidermal progenitors and promoted ectodermal cells to differentiate into epidermal [26, 27]. With Wnt signals, the ectodermal cells responded to BMP signaling while inhibiting FGF signaling, which resulted in an epidermal fate. Without Wnt signals, the ectodermal cells responded to FGF signals instead of BMP signaling activity and thus developed towards a neural fate [28, 29]. Also, p63, a transcription factor, which is required for initiating a basal layer of keratinocytes, was expressed throughout the epidermal differentiation [17]. BMP signaling also has been suggested to control p63 expression during ectodermal development. ΔNp63 is an ectoderm-specific direct transcriptional target of BMP signaling, which is essential for the dorsoventral patterning of the ectoderm and skin development. The early expression of ΔNp63 in the ectoderm is directly activated by Smad4/5-mediated BMP signaling and is sufficient to block neural specification while promoting the early steps of the epidermal specification [30]. Moreover, ΔNp63 also can directly induce K14 expression [31].
According to the roles of crucial regulators and signaling pathways during embryonic epidermal development, pluripotent stem cells (PSCs), including ESCs and iPSCs, can differentiate into keratinocytes by sequentially activating the above signaling pathways. Based on previous studies, BMP4 and retinoic acid (RA) have been used as well-known inducers for epidermal differentiation from ESCs in vitro [32, 33]. In vivo, BMP4 is a potent epidermal inducer and a neural inhibitor along with ectodermal fate [34]. During the differentiation of ESC into epidermal cells in vitro, BMP4 activates the expression of ΔNp63, further promoting expression of basal keratinocyte maker K5/14 and the terminal differentiation marker K1/10 expression [26]. BMP4 also could promote epidermal commitment by downregulating Smad6 and induce the apoptosis of Sox1+ neural progenitors [35]. Besides, BMP4 upregulated ectoderm protein AP2γ (a transcription factor activator protein- (AP-) 2 family member) through binding of Smad1 to its promoter, which could restrict neural expansion and initiate epidermal differentiation during the early stages of ectodermal patterning [36]. RA was also discovered as an inducer of keratinocyte differentiation from iPSCs or ESCs. RA was a stage-dependent agent for differentiation which directed pluripotent stem cells differentiating into ectodermal cells and upregulated K8/18 and p63 expression [37]. Therefore, RA and BMP4 play distinct but synergistic roles in promoting iPSC or ESC differentiation along the keratinocyte lineage and inhibit neural differentiation [32, 38]. Additionally, studies showed that BMP4 and RA promoted the differentiation of pluripotent stem cells into the epidermal through inhibition of the canonical Wnt signaling pathway. RA could induce the localization of β-catenin to the cell membrane and diminished the amount of nuclear β-catenin, which downregulated the canonical Wnt signaling and activated the noncanonical Wnt signaling pathway [39]. Therefore, the downregulation of canonical Wnt signaling might be able to induce the epidermal differentiation of pluripotent stem cells. SU6656, a Src family kinase inhibitor, has been shown to modulate β-catenin translocation to the cell membrane and directly phosphorylate β-catenin; this phosphorylation inhibits the binding of β-catenin to E-cadherin, which suppresses the expression of the canonical Wnt-dependent genes. SU6656 induced the differentiation of hPSCs into epithelial cells with the repression of pluripotency gene Oct4 and upregulation of ectoderm marker K18/K8. These hPSC-derived keratinocytes possessed the capacity to terminally differentiate, which were capable of forming coherent epithelial sheets or could be used for skin tissue engineering applications [40, 41]. These keratinocytes provide an abundant source for cellular transplantation applications and regenerative medicine [17, 42]. Also, keratinocytes can be derived from the two-step differentiation process of iPSCs. Recombinant noggin, SB431542, and CHIR99021 created human skin-derived precursor cells from human-induced pluripotent stem cells (hiPSCs) that could differentiate into keratinocyte-like cells on a collagen-chitosan scaffold, which contributes to the regeneration of the epidermal and dermal layers [43]. As mentioned in this section, the iPSC-derived keratinocytes that were induced by the above bioactive molecules have similar biological functions as the keratinocytes in the physiological state [44].
It is worth noting that according to the above differentiation pathway, we need to obtain PSCs first and then further induce the differentiation of PSCs into physiological keratinocytes. Fortunately, the “two-step differentiation process” controlled by bioactive molecules is now available. However, such a reprogramming path for getting keratinocytes will elevate the risk of the accumulation of cellular damage, even tumor formation. Therefore, how to directly reprogram adult stem cells, even the reprogramming of other somatic cells into keratinocytes, is worth exploring.
Direct cellular reprogramming is often dependent on the substitution of key transcription factors. One study has shown the direct conversion of mouse embryonic fibroblasts into functional keratinocytes, in which the fibroblasts transfected with Sox2, Oct4, and Klf4 to initiate part dedifferentiation and then are treated with RA and BMP4 for their differentiation [45]. Another study has reported that the combination of the transcription factors p63 and Klf4 can convert human fibroblasts to keratinocyte-like cells [46]. Unfortunately, these studies still use genetically modified methods. If bioactive molecules replace these critical transcriptional factors, it will achieve the goal of getting keratinocytes through direct reprogramming by bioactive molecules, which is a promising method to get unlimited keratinocytes for cellular transplantation and tissue-engineered skin. The latest research reported that simvastatin could differentiate HFSCs into keratinocyte. Besides, simvastatin accelerated the wound healing through anti-inflammatory, anti-bacterial, immunomodulatory, and antioxidative effects and the improvement of vascularization [47]. Therefore, bioactive molecules with the dual forces of promoting the orientation-inducing ability and promoting the repairing fact are more favored.
2.2. Endothelial Cells Derived by Bioactive Molecule Induction
Revascularization is an essential stage of the wound healing which establishes blood supply to the newly formed tissue. New blood vessels provide the nutrients and oxygen for the newly formed granulation tissue [48]. Endothelial cells and their progenitors are essential in neovascularization [49, 50]. Moreover, endothelial cells have two primary functions for tissue homeostasis. The first one is to release the tissue-specific angiocrine factors that support tissue homeostasis and regeneration, and the second is to regulate the permeability of proteins, ions, bioactive molecules, and even cells through the vascular wall impacting on a trauma microenvironment [51]. Similar to the strategy of obtaining keratinocytes, endothelial cells can be obtained by PSC differentiation or by direct reprogramming of fibroblasts. These induced endothelial cells and their progenitors may provide the cell source for the construction of tissue-engineered vascularization. To support these applications, an efficient and reliable in vitro differentiation induction system is needed. The differentiation method in vitro should follow the process of endothelial cell development, and the cultural environment in vitro should simulate the microenvironment of the target cells in vivo. Endothelial cells are derived from mesodermal progenitors during embryonic development. Activin/Nodal/TGF-β, BMP, Wnt, and fibroblast growth factor (FGF) signaling pathways play crucial roles in the mesodermal transition during gastrulation and are required for epiblast-to-mesoderm transition. Therefore, Activin A/Nodal, BMP4, FGF2, and Wnt ligands or GSK3 inhibitors (such as CHIR99021) were currently the most used mesoderm-endothelial cell inductive factors in vitro [52–54]. Modulation of the signaling as mentioned above should promote the differentiation of PSCs into functional endothelial cells.
So far, there are three methods to differentiate PSCs into endothelial cells: coculture of PSCs with stromal cell lines, three-dimensional (3D) embryoid body- (EB-) mediated differentiation, and monolayer-directed differentiation. The latter two methods are accomplished by using bioactive molecules to follow the developmental process. PSCs differentiated into mesodermal lineages and then differentiated into endothelial cells under the treatment with Activin A, BMP4, FGF2, VEGF-A, SB431542, and so on [48, 55–57]. And it was reported that hESCs aggregated into EBs, and then sequential exposure to BMP4, Activin A, FGF2, VEGF-A, and SB431542 drives the differentiation of the cells into endothelial cells which were functional in vivo. And the SB431542 was the critical small molecule for enhancing cell differentiation efficiency, up to tenfold [58]. In line with the above study, recent studies also have shown that the inhibition of TGF-β using SB431542 enhances the production of endothelial cells from hPSCs, which may be due to SB431542 resisting the inhibitory effect of TGF-β on endothelial expansion [59, 60]. The latter study showed that activation of canonical Wnt signaling via CHIR99021 without exogenous growth factors was sufficient to generate high yields of endothelial progenitors from hPSCs [61–63]. The other study demonstrated that hPSCs differentiated into endothelial cells in the exposure to CHIR99021, and these cells generated intact microvessels in vitro in PDMS-based microfluidic devices, which could be subjected to shear stress to mimic the in vivo physiological conditions [64]. And the promotion effect of CHIR99021 was by the upregulation of CDX/HOX genes, which are expressed in the posterior mesoderm of embryo development [65]. These in vitro differentiation experiments also confirmed the importance of activation of the Wnt signaling pathway for generating mesodermal progenitor cells from PSCs, and these cells could be further differentiated into vascular endothelial cells or cardiomyocytes. Of course, the proper combination of bioactive molecules further improved the differentiation efficiency. For example, high differentiation efficiency was achieved when CHIR99021 was combined with a lower concentration of VEGF-A and DLL4 (a Notch ligand) in the endothelial cell induction system [66]. DLL4 could enhance endothelial cell-lineage differentiation and inhibit hematopoietic-lineage differentiation [67]. In addition, CHIR99021 in combination with FGF2, VEGF, and BMP4 could synergistically induce early vascular progenitors (VPs) from hiPSCs with high purities. It is established that VEGF and FGF2 activated the MAPK and the PI3K pathways, which were crucial not only for the initial commitment to vascular lineages but also for the differentiation of vascular progenitors to endothelial cells. It most likely through regulation of the ETS family transcription factors, ERG and FLI1 which were important to endothelial cell development [68]. Except for growth factors and small molecules, 3D culture also enhanced differentiation efficiency; the 3D thermoreversible PNIPAAm-PEG hydrogel provided 3D space for cell growth and acted as a physical barrier to prevent the cell from agglomeration and the shear force during the differentiation of endothelial cell [69]. More importantly, iPSC- or ESC-derived endothelial cells are capable of forming capillary-like structures in vitro and integrating into the host vasculature in vivo [18]. These studies have proven the possibility of generating intact, engineered microvessels in vitro that replicate some of the fundamental biological features of native microvessels.
Direct reprogramming is another way to obtain endothelial cells. Endothelial cells are derived by direct reprogramming from fibroblasts, and amniotic cells have also been reported [70, 71]. Human fibroblasts could be transdifferentiated into endothelial progenitor cells using lentiviral ETS transcription factors (ETV2) and hypoxia, the derived endothelial progenitor cells similared to human umbilical endothelial cells in phenotypes and tube formation assay [71, 72]. We have mentioned that the use of lentiviral vectors raises safety issues as it may induce insertional mutagenesis, as well as possible generation of replication-competent lentiviruses and germline transfer. Furthermore, the efficiency of the direct reprogramming of lentiviral vectors is low. Therefore, bioactive molecules that can replace critical transcriptional factors may be a feasible strategy for direct reprogramming. It has been proven that with the sequential addition of 5-aza-2′-deoxycytidine and trichostatin A in a fibroblast culture environment and tideglusib for a specific time, the fibroblasts can convert into functional endothelial progenitors. 5-Aza-2′-deoxycytidine, a DNA methyltransferase inhibitor, and trichostatin A, a histone deacetylase inhibitor, may cause chromatin decondensation and induce a short “dedifferentiation” state. Tideglusib inhibited β-catenin phosphorylation, which resulted in its translocation to the nucleus activation of Wnt signaling, thus causing the differentiation [73]. These results illustrate the feasibility of direct reprogramming for obtaining endothelial cells by bioactive molecule induction, but more achievable paths and the critical signaling pathways for reprogramming need to be further explored.
2.3. Hair Follicle Cells Derived by Bioactive Molecule Induction
Hair follicle (HF) undergoes cyclical physiologic regeneration, and their cellular components exhibit robust regenerative capabilities. Hair follicle stem cells (HFSCs) residing in the bulge represent a unique source of stem cells that are maintained through self-renewal and differentiation during the hair cycle and contribute to hair follicle regeneration and wound healing [74]. Following wounding, skin wound healing started not just from the edges of the wound but also around the follicles. Hair follicle-derived epidermal stem cells (EpSCs) are recruited immediately for wound closure. It has been shown that skin injury in high hair density areas tends to heal more quickly than those lacking hair follicles. And a chronic wound treated with skin grafts with hair follicles showed a faster healing rate than that transplanted with skin grafts without hair follicles [75].
Hair follicle morphogenesis needs epithelial-mesenchymal interaction and involves many signaling pathways, such as Wnt, BMP, Shh, Notch, TGF-β, and platelet-derived growth factor (PDGF) [76–79]. For example, Wnt signaling is necessary for hair follicle development and patterning, and Wnt ligands are expressed in the interfollicular epidermis as well as the hair follicle during all stages of follicle development [80]. In the process of epidermis development, a high level of Wnt signaling is essential for HF induction, while the constitutive attenuation of epidermal Wnt signaling impairs HF formation, but does not impact interfollicular epidermis integrity. Deletion of β-catenin in the skin epidermis or overexpression of Dkk1, a soluble inhibitor of Wnt signaling, results in the absence of HF with no sign of HF or dermal papilla (DP) formation [81]. BMP signaling has also been suggested to regulate hair follicle induction and the patterning of follicles in the skin [79]. In the mature HF, the quiescence and activation of HFSCs are tightly controlled by a balance of BMP and Wnt signals coming from their niche cells. Decreased BMP signaling unleashes Wnt signaling activation promoting HF growth. In early epidermal development, Shh is expressed in the placode of developing HFs. Shh is required for hair follicle morphogenesis during embryogenesis and for regulating follicular growth and cycling in adults. While lacking β-catenin in the epidermis, Shh is not expressed, which indicates that Shh signaling serves as a downstream pathway of Wnt/β-catenin signaling to regulate HF induction. Therefore, the development and circulation of hair follicles need the various signal pathways to interconnect and mutually constrain.
At present, there are a few reports on the stem cell-derived EpSCs. Some results showed that BMP and Wnt are necessary for maintaining EpSC features during HF formation in the embryo and adults. BMP signaling is essential for hair cycling, and Wnt signaling pathway activation is required to stimulate hair growth [82]. One study has shown that hiPSCs differentiate into EpSCs through precise temporal control of the activities of epidermal growth factor, RA, and BMP4. These EpSCs can reconstitute the epithelial components of the hair follicle and interfollicular epidermis [83]. And the latest study produced EpSCs from iPSCs using the same method as mentioned above with minor modifications; iPSC-derived EpSCs seeding on the human acellular amniotic membrane (hAAM) promoted the construction of hair follicles and interfollicular epidermis and restored skin functions after transplantation [84]. IM176OUT05 activated HFSC metabolism and further promoted the expansion of ki67-positive progenitors during the early phases of hair follicle regeneration so that IM176OUT05 could be a candidate drug to improve tissue regeneration with high solubility, greater potency, and bioavailability [85].
Well-orchestrated epithelial-mesenchymal interactions are crucial for hair follicle morphogenesis [86]. In HF development, EpSCs are induced by mesenchymal dermal papilla (DP) cell cues to develop HFs. DP, located at the base of the hair follicle, plays a critical role in directing keratinocytes to form the follicle. The role of the DP in hair formation includes determining the size, shape, color of the hair, and the frequency with its regeneration [87]. Therefore, DP cells are important supporting cells for hair follicle regeneration after injury. Research on bioactive molecule-induced DP cells has also been frequently reported. A study has demonstrated that hiPSCs differentiate into induced mesenchymal cells (iMCs) with a bone marrow stromal cell phenotype and are subsequently induction with RA and DP cell culture medium to acquire DP properties. The resultant DP cells interacted with human keratinocytes to upregulate HF-related genes in keratinocytes and, when cografted with human keratinocytes in vivo, gave rise to a hair cuticle-like coat. However, the study also suggested that human DP cells lose key inductive properties after in vitro resemblance of the hair shaft [88]. This loss of inductivity was partially reversible if the cells are reassembled into three-dimensional (3D) spheroids [89]. Also, bioactive molecules play an essential role in maintaining the biological efficiency of DP cells. It showed that topical treatment of human skin with JAK inhibitor, tofacitinib, increased the growth rate of anagen hair shafts and enhanced the inductivity of human DP spheres [90]. Moreover, the bioactive molecule, 3-deoxysappanchalcone, an herbal medicine similar to the JAK inhibitor tofacitinib, promoted the proliferation of human hair follicle DP cells and hair growth via modulation of Wnt/β-catenin and STAT signaling [91].
In addition to the above critical cells, skin wound healing also requires sweat gland cells (SGCs) to help restore sweat glands (SGs). SGCs are responsible for the regulation of body temperature and are also critical for wound repair [8]. Like all skin appendages, sweat glands are derived from embryonic ectoderm. However, following severe skin injury, SGs do not regenerate because of the destruction of the duct and secretory cell coiler [92]. Up to now, SGCs are mainly obtained by environmental induction and the transgenic method. The previous study reported that human umbilical cord mesenchymal stem cells (hucMSCs) could differentiate into sweat gland-like cells under sweat gland cell-induction medium consisting of nine parts of basic SG medium and one part of sterile supernatants from a conditioned heat-shock SG medium, which might help solve the problem of sweat gland depletion in patients [93]. However, this differentiation rate was very low, and it was challenging to meet the needs of large-area transplantations. A later study showed that epimorphin could improve the rate of differentiation of mesenchymal stem cells into SGCs [94]. A recent research suggested that the human keratinocyte growth factor played a pivotal role in promoting hucMSC differentiation to sweat gland-like cells [95]. The first transgenic report transformed the key factors NF-kb and Lef-1 that were related to sweat gland development and directly reprogrammed fibroblasts into sweat gland-like cells [96]. However, no study reported on how to use bioactive molecules alone to obtain the SGCs, which needed further exploration. It is worth recognizing that the critical cell types required for wound repair can be obtained by bioactive molecule induction through the differentiation or direct reprogramming, which may provide a sufficient cell source for wound cell therapy.
It is not difficult to see that skin repair cells can be obtained using bioactive molecule-induced differentiation of stem cells, which is closely related to developmental science. The molecular mechanism explained from the developmental perspective is the essential information for realizing the differentiation of stem cells into target cells. Nevertheless, efforts still need to be made to more in-depth understanding of the developmental process of the skin to obtain a more effective induction strategy. In addition, we should further evaluate the role of repairing cells in tissue repair and the interaction between various cells, such as the hair follicle development mentioned above, and the microenvironmental signals provided by the supporting cells may be the key to tissue regeneration. Additionally, as far as cell therapy is concerned, the researchers tend to perform single-cell type transplantation. In contrast, the combined transplantation of different cells may achieve better therapeutic effects, which are also worth of attention. Even in the process of stem cell differentiation or direct reprogramming, it is also worth to pay attention to whether it is possible to obtain multiple skin cells in one induction system concurrently, thus promoting the regeneration of the skin by cotransplantation.
2.4. In Situ Skin Regeneration Is Induced by Bioactive Molecules
As we discussed above, it will require a large amount of expansion of target cells and takes a lot of time and money to obtain the functional repair cells for cell therapy. Therefore, is there a better way to promote tissue regeneration? Tissue repair requires sophisticated regulatory networks and specific manipulation of certain signaling pathways, while bioactive molecules may serve the purpose of recruiting stem cells, stem cell differentiation, transdifferentiation, etc. in vivo. So the direct application of bioactive molecules to the injured site is an important focus in tissue regeneration. Tissue regeneration stimulated with bioactive molecules in vivo, which is called in situ regeneration, has just emerged in recent years. For example, Thymosin 4 has been found to promote wound healing by decreasing inflammation, increasing angiogenesis, enhancing keratinocyte and endothelial cell migration, and promoting hair follicle growth [97]. Plerixafor in combination with G-CSF for hematopoietic stem cell (HSC) mobilization has been approved in multiple myeloma patients for autologous transplantation. Moreover, they showed that this HSC mobilization effect could be enhanced by BIO5192, a small molecule VLA-4 inhibitor [98, 99]. Another study showed that the sustained delivery of sphingolipid growth factor FTY720 from poly(lactic-co-glycolic acid) (PLAGA) and local targeting of sphingosine 1-phosphate receptors reduced infiltration of CD45+ inflammatory cell, promoted endogenous recruitment of CD29+CD90+ bone progenitor cells, and improved defective vascularization and bone formation [100]. A recent study demonstrated that a natural bioactive molecule from cruciferous vegetables, DIM (3,3′-diindolylmethane), could promote the stemness of hucMSCs, which provided a novel strategy for improving the therapeutic effects of hucMSCs on tissue repair [101]. Recently, in vivo, Tazarotene enhanced the growth of mature and functional microvessels in wound healing models, thereby accelerating wound neovascularization [102]. Hydroxysafflor yellow A and deferoxamine have been reported to accelerate wound healing through promoting angiogenesis and reducing the inflammatory. And the deferoxamine and hydroxysafflor yellow A hydrogel could accelerate diabetic wound healing via enhanced angiogenesis by upregulation of hypoxia-inducible factor-1 alpha expression [103]. AES16-2M, an extracellular signal-regulated kinase-activating peptide, activated the MAPK signaling pathway and promoted wound healing through accelerating the migration of keratinocytes [104]. Our latest research demonstrated that lithium chloride (LiCl) loaded into chitosan hydrogel in full-thickness loss showed reduced inflammation, improved angiogenesis, accelerated reepithelialization, and regenerated hair follicles [105]. Based on these above studies, the possible roles of bioactive molecules for regenerative medicine in vivo are through regulating the wound microenvironment, like reducing inflammation, increasing angiogenesis, modulating endogenous stem cell or progenitor cell proliferation, differentiation, and homing, stimulating somatic cell proliferation or repairing their function, and promoting somatic cell reprogramming into repairing cells.
Of course, these bioactive molecules can not only promote wound repair but also reduce scar formation and treat skin diseases. Inhibition of Wnt/β-catenin signaling has been proved to reduce fibrosis and promote regeneration of wounds. The application of small molecule, XAV-939, could reduce fibrosis and promote regenerative cutaneous wounds in response to inhibition of Wnt/β-catenin signaling [106]. ICG-001 inhibited the endogenous Wnt/β-catenin signaling and had an inhibitory effect on collagen production in fibroblasts, suggesting its potential application in fibrotic diseases [107]. Poly(ε-caprolactone)/gelatin (PGT) fibrous scaffold doped with the TGF-β1 inhibitor (SB525334) could be used for reducing scars [108]. Bacterial cellulose (BC) decorated with 4,6-diamino-2-pyrimidinethiol- (DAPT-) modified gold nanocomposites inhibited bacterial growth and promoted wound repair when applied as wound dressings [109]. Interfering with the JAK/STAT signaling by a small molecular pharmacological inhibitor (the STAT3 inhibitor STA-21) was currently feasible since it could be applied not only systematically but also topically for the treatment of inflammatory skin diseases [110]. Therefore, the use of bioactive molecules to reduce fibrosis and activate the endogenous repair potential can promote wound repair. However, the regeneration of skin wound appendages through the use of bioactive molecules is currently rarely reported. This may be due to the unclear molecular mechanism of appendage regeneration. With the more in-depth exploration of signaling pathways in regenerative skin biology, it is not difficult to efficiently obtain adequate targeted skin cells by bioactive molecules in vitro, and these bioactive molecules also might be used as drugs for skin repair and regeneration in vivo.
3. Perspectives
In this review, bioactive molecule-based reprogramming represents an attractive and valuable alternative approach to the repair/regeneration of skin wounds. Bioactive molecules induce stem cells to differentiate into keratinocytes, endothelial cells, hair follicle stem cells, DP cells, and so on, which may provide sufficient cell source for cell therapy or tissue engineering (Table 1). Moreover, direct reprogramming of somatic cells into skin cells through bioactive molecules by passing stem or progenitor cell status is also crucial for regenerative medicine, which simplifies the process and avoids the risks associated with using stem cells. Most protocols for generating target cells follow the embryonic development of the target cells through the stepwise of bioactive molecule combination induction. Bioactive molecules are stable, cost-effective, and cell-permeable, and they reduce the safety concerns about genetic manipulation and are becoming a traditional alternative in cell reprogramming and regenerative medicine [111, 112]. Nevertheless, there are still some challenges in using bioactive molecules as traditional treatment in regenerative skin medicine.
Table 1.
Bioactive molecules for skin repair and regeneration.
(a).
| In vitro | |||
|---|---|---|---|
| Initial cell | Target cell | Bioactive molecules | Reference |
| Pluripotent stem cells | Reprogramming to keratinocyte | BMP4, RA | [32, 33] |
| SU6656 | [17, 42] | ||
| Noggin, SB431542, CHIR99021 | [43] | ||
| Pluripotent stem cells | Reprogramming to endothelial cells | BMP4, Activin A, FGF2, VEGF-A, SB431542 | [58, 59] |
| CHIR99021 | [61–64] | ||
| CHIR99021, VEGF-A, DLL4 | [66] | ||
| CHIR99021, FGF2, VEGF, BMP4 | [68] | ||
| Fibroblasts | Reprogramming to endothelial cells | 5-Aza-2′-deoxycytidine, trichostatin A, tideglusib | [73] |
| Pluripotent stem cells | Reprogramming to hair follicle | Epidermal growth factor, RA, BMP4 | [83, 84] |
(b).
| In vivo | ||
|---|---|---|
| Roles of bioactive molecules | Bioactive molecules | Reference |
| Regulation of the wound microenvironment Modulation of endogenous stem cell or progenitor cell Stimulation of somatic cell proliferation or repairing their function |
Thymosin 4 | [96, 97] |
| Plerixafor, G-CSF, BIO5192 | [98, 99] | |
| FTY720 | [100] | |
| 3,3′-Diindolylmethane | [101] | |
| Tazarotene | [102] | |
| Hydroxysafflor yellow A, deferoxamine | [103] | |
| AES16-2M | [104] | |
| Lithium chloride | [105] | |
| Reducing fibrosis and treating skin diseases | XAV-939 | [106] |
| ICG-001 | [107] | |
| SB525334 | [108] | |
| Bacterial cellulose decorated with DAPT-modified gold nanocomposites | [109] | |
| STA-21 | [110] | |
The first challenge is the low efficiency of chemical-based reprogramming or transdifferentiation. A better understanding of the detailed mechanisms during reprogramming or transdifferentiation processes can help to select more specific bioactive molecules to improve the efficiency. It is necessary to explore the molecular mechanisms and regulatory networks of skin development in temporal and spatial changes and further to screen for possible proregenerative bioactive molecule combinations which promote dedifferentiation of differentiated cells or transdifferentiation of one cell into another. These problems may be solved with joint efforts by developmental biologists, pharmacologists, and multidisciplinary experts.
The second challenge is to screen suitable bioactive molecules for skin repair in situ. The identification of bioactive molecules that can promote regeneration is primarily based on the in vitro cell model by high-throughput screening. Promoting skin wound healing through bioactive molecules in vivo represents a new direction. We propose that the functions of bioactive molecules mainly focus on the following four aspects: (1) recruit stem cells near and far from the skin wounds, such as HSCs, MSCs, and adipose stem cells, which have been proved to be useful in wound repair and regeneration [113]; (2) promote the dedifferentiation of keratinocytes, fibroblasts, and endothelial cells in order to increase the number of stem cells in skin; (3) regulate the inflammatory microenvironment of the wound, as it is well-known that excessive inflammation inhibits wound regeneration; and (4) increase the biological function of repair cells, such as to improve the ability of wound vascularization and the migration of repairing cells. For example, the bioactive molecule compounds consisting of alprostadil and trimebutine maleate promoted the self-renewal and proliferation of skin-derived precursor in vitro and enhance skin repair in vivo through the pharmacological activation of endogenous precursor cells [114]. As far as skin repair is concerned, we need to find more effective bioactive molecules that can target the activation, proliferation, differentiation, and survival of endogenous stem cells and further clarify their possible molecular mechanism.
The third challenge is that the in vitro results of bioactive molecules do not reflect the in vivo regenerative effects. How to efficiently deliver transplanted cells into the target tissues, to maintain their survival in vivo, and to engraft into the endogenous tissues still needs further study. Furthermore, verifying the regenerative effect of bioactive molecules in vivo requires many experiments in vitro, and we also need to pay attention to the consistency of the effects of bioactive molecules in vivo and in vitro; after all, the microenvironment in vivo is more complicated.
The fourth challenge is to devise a safe and efficient delivery system for transporting bioactive molecules into the target tissues. Because of the lack of specificity, more efforts should be made to reduce the off-target effects and evaluate the tumorigenic risk while using bioactive molecules to promote regeneration in vivo before clinical applications. Nevertheless, we hope that bioactive molecules together with current biological technologies will enable in situ skin regeneration and accelerate the progress in regenerative medicine. Scaffolds, hydrogel, or other biomaterials have been used as vehicles by providing controlled release of therapeutic agents and tunable release of bioactive molecules of skin engineering [115]. A study has shown that electrospun fibers can be bioactive molecule carriers for wound healing [116]. And a wide variety of bioactive molecules has been incorporated into electrospun fibers and delivered into injured sites. Recently, it has reported that a bifunctional hydrogel loaded with the sphingosine analogs, FTY720 and SDF-1α, enhances the recruitment of anti-inflammatory monocytes and promotes microvascular remodeling, thus promoting of tissue repair. This dual-affinity hydrogel overcomes the challenge of codelivering two physiochemically distinct molecules, and the corelease of FTY720 and SDF-1α yields superior proregenerative biological effects over either factor alone [117].
We propose that bioactive molecule-based tissue regeneration is not only an essential method in the field of skin repair but also an important strategy for the restoration of injured tissues in the future. With a better understanding of regenerative mechanisms in different tissues and the development of technologies for high-throughput screening, future studies in tissue regeneration by bioactive molecules will overcome current hurdles, generate discoveries, and benefit human health.
Acknowledgments
This study was supported in part by the National Key Research and Development Plan (2017YFC1104701 and 2017YFC1103300), the National Nature Science Foundation of China (81830064, 81721092, and 81971841), the Military Logistics Research Key Project (AWS17J005), the National S&T Resource Sharing Service Platform Project of China (YCZYPT[2018]07), and the General Hospital of PLA Medical Big Data R&D Project (MBD2018030).
Contributor Information
Meirong Li, Email: meirong811225@126.com.
Xiaobing Fu, Email: fuxiaobing@vip.sina.com.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- 1.Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. European Journal of Dermatology. 2002;12(4):390–401. [PubMed] [Google Scholar]
- 2.Shi J., Ma X., Su Y., et al. MiR-31 Mediates Inflammatory Signaling to Promote Re-Epithelialization during Skin Wound Healing. The Journal of Investigative Dermatology. 2018;138(10):2253–2263. doi: 10.1016/j.jid.2018.03.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rousselle P., Braye F., Dayan G. Re-epithelialization of adult skin wounds: cellular mechanisms and therapeutic strategies. Advanced Drug Delivery Reviews. 2018;146:344–365. doi: 10.1016/j.addr.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Darby I. A., Laverdet B., Bonte F., Desmouliere A. Fibroblasts and myofibroblasts in wound healing. Clinical, Cosmetic and Investigational Dermatology. 2014;2014:301–311. doi: 10.2147/ccid.s50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thulabandu V., Chen D., Atit R. P. Dermal fibroblast in cutaneous development and healing. Wiley Interdisciplinary Reviews: Developmental Biology. 2018;7(2, article e307) doi: 10.1002/wdev.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsakiroglou P., Vanden Akker N. E., Del Bo C., Riso P., Klimis-Zacas D. Role of berry anthocyanins and phenolic acids on cell migration and angiogenesis: an updated overview. Nutrients. 2019;11(5, article 1075) doi: 10.3390/nu11051075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leiros G. J., Kusinsky A. G., Drago H., et al. Dermal papilla cells improve the wound healing process and generate hair bud-like structures in grafted skin substitutes using hair follicle stem cells. Stem Cells Translational Medicine. 2014;3(10):1209–1219. doi: 10.5966/sctm.2013-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao B., Xie J., Liu N., et al. Identification of a new sweat gland progenitor population in mice and the role of their niche in tissue development. Biochemical and Biophysical Research Communications. 2016;479(4):670–675. doi: 10.1016/j.bbrc.2016.09.155. [DOI] [PubMed] [Google Scholar]
- 9.Law J. X., Chowdhury S. R., Saim A. B., Idrus R. B. H. Platelet-rich plasma with keratinocytes and fibroblasts enhance healing of full-thickness wounds. Journal of Tissue Viability. 2017;26(3):208–215. doi: 10.1016/j.jtv.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Mohamad N., Loh E. Y. X., Fauzi M. B., Ng M. H., Mohd Amin M. C. I. In vivo evaluation of bacterial cellulose/acrylic acid wound dressing hydrogel containing keratinocytes and fibroblasts for burn wounds. Drug Delivery and Translational Research. 2019;9(2):444–452. doi: 10.1007/s13346-017-0475-3. [DOI] [PubMed] [Google Scholar]
- 11.Wang C., Wang Q., Gao W., et al. Highly efficient local delivery of endothelial progenitor cells significantly potentiates angiogenesis and full-thickness wound healing. Acta Biomaterialia. 2018;69:156–169. doi: 10.1016/j.actbio.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Li X., Xu J., Deng H. Small molecule-induced cellular fate reprogramming: promising road leading to Rome. Current Opinion in Genetics & Development. 2018;52:29–35. doi: 10.1016/j.gde.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Xie X., Fu Y., Liu J. Chemical reprogramming and transdifferentiation. Current Opinion in Genetics & Development. 2017;46:104–113. doi: 10.1016/j.gde.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Langle D., Halver J., Rathmer B., Willems E., Schade D. Small molecules targeting in vivo tissue regeneration. ACS Chemical Biology. 2014;9(1):57–71. doi: 10.1021/cb4008277. [DOI] [PubMed] [Google Scholar]
- 15.Li X., Zuo X., Jing J., et al. Small-molecule-driven direct reprogramming of mouse fibroblasts into functional neurons. Cell Stem Cell. 2015;17(2):195–203. doi: 10.1016/j.stem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Cao N., Huang Y., Zheng J., et al. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science. 2016;352(6290):1216–1220. doi: 10.1126/science.aaf1502. [DOI] [PubMed] [Google Scholar]
- 17.Selekman J. A., Lian X., Palecek S. P. Generation of epithelial cell populations from human pluripotent stem cells using a small-molecule inhibitor of Src family kinases. Methods in Molecular Biology. 2016;1307:319–327. doi: 10.1007/7651_2014_70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song W., Kaufman D. S., Shen W. Efficient generation of endothelial cells from human pluripotent stem cells and characterization of their functional properties. Journal of Biomedical Materials Research Part A. 2016;104(3):678–687. doi: 10.1002/jbm.a.35607. [DOI] [PubMed] [Google Scholar]
- 19.Zhu S., Wei W., Ding S. Chemical strategies for stem cell biology and regenerative medicine. Annual Review of Biomedical Engineering. 2011;13:73–90. doi: 10.1146/annurev-bioeng-071910-124715. [DOI] [PubMed] [Google Scholar]
- 20.Santoro M. M., Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Experimental Cell Research. 2005;304(1):274–286. doi: 10.1016/j.yexcr.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 21.Krishnaswamy V. R., Korrapati P. S. Role of dermatopontin in re-epithelialization: implications on keratinocyte migration and proliferation. Scientific Reports. 2014;4, article 7385 doi: 10.1038/srep07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McHeik J. N., Barrault C., Pedretti N., et al. Foreskin-isolated keratinocytes provide successful extemporaneous autologous paediatric skin grafts. Journal of Tissue Engineering and Regenerative Medicine. 2016;10(3):252–260. doi: 10.1002/term.1690. [DOI] [PubMed] [Google Scholar]
- 23.Baranek M., Belter A., Naskret-Barciszewska M. Z., Stobiecki M., Markiewicz W. T., Barciszewski J. Effect of small molecules on cell reprogramming. Molecular BioSystems. 2017;13(2):277–313. doi: 10.1039/c6mb00595k. [DOI] [PubMed] [Google Scholar]
- 24.Hou P., Li Y., Zhang X., et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341(6146):651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 25.Tadeu A. M., Lin S., Hou L., et al. Transcriptional profiling of ectoderm specification to keratinocyte fate in human embryonic stem cells. PLoS One. 2015;10(4, article e0122493) doi: 10.1371/journal.pone.0122493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X. J., Liu Y., Dai Z. M., et al. BMP-FGF signaling axis mediates Wnt-induced epidermal stratification in developing mammalian skin. PLoS Genetics. 2014;10(10, article e1004687) doi: 10.1371/journal.pgen.1004687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houschyar K. S., Momeni A., Pyles M. N., Maan Z. N., Whittam A. J., Siemers F. Wnt signaling induces epithelial differentiation during cutaneous wound healing. Organogenesis. 2015;11(3):95–104. doi: 10.1080/15476278.2015.1086052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bielefeld K. A., Amini-Nik S., Alman B. A. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cellular and Molecular Life Sciences. 2013;70(12):2059–2081. doi: 10.1007/s00018-012-1152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S., Zhang H., Duan E. Epidermal development in mammals: key regulators, signals from beneath, and stem cells. International Journal of Molecular Sciences. 2013;14(6):10869–10895. doi: 10.3390/ijms140610869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakkers J., Hild M., Kramer C., Furutani-Seiki M., Hammerschmidt M. Zebrafish DeltaNp63 is a direct target of Bmp signaling and encodes a transcriptional repressor blocking neural specification in the ventral ectoderm. Developmental Cell. 2002;2(5):617–627. doi: 10.1016/s1534-5807(02)00163-6. [DOI] [PubMed] [Google Scholar]
- 31.Romano R. A., Birkaya B., Sinha S. A functional enhancer of keratin14 is a direct transcriptional target of deltaNp63. The Journal of Investigative Dermatology. 2007;127(5):1175–1186. doi: 10.1038/sj.jid.5700652. [DOI] [PubMed] [Google Scholar]
- 32.Metallo C. M., Ji L., de Pablo J. J., Palecek S. P. Retinoic acid and bone morphogenetic protein signaling synergize to efficiently direct epithelial differentiation of human embryonic stem cells. Stem Cells. 2008;26(2):372–380. doi: 10.1634/stemcells.2007-0501. [DOI] [PubMed] [Google Scholar]
- 33.Metallo C. M., Ji L., de Pablo J. J., Palecek S. P. Directed differentiation of human embryonic stem cells to epidermal progenitors. Methods in Molecular Biology. 2010;585:83–92. doi: 10.1007/978-1-60761-380-0_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinstein D. C., Honore E., Hemmati-Brivanlou A. Epidermal induction and inhibition of neural fate by translation initiation factor 4AIII. Development. 1997;124(21):4235–4242. doi: 10.1242/dev.124.21.4235. [DOI] [PubMed] [Google Scholar]
- 35.Gambaro K., Aberdam E., Virolle T., Aberdam D., Rouleau M. BMP-4 induces a Smad-dependent apoptotic cell death of mouse embryonic stem cell-derived neural precursors. Cell Death and Differentiation. 2006;13(7):1075–1087. doi: 10.1038/sj.cdd.4401799. [DOI] [PubMed] [Google Scholar]
- 36.Qiao Y., Zhu Y., Sheng N., et al. AP2γ regulates neural and epidermal development downstream of the BMP pathway at early stages of ectodermal patterning. Cell Research. 2012;22(11):1546–1561. doi: 10.1038/cr.2012.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H., Zhou H., Fu X., Xiao R. Directed differentiation of human embryonic stem cells into keratinocyte progenitors in vitro: an attempt with promise of clinical use. In Vitro Cellular & Developmental Biology Animal. 2016;52(8):885–893. doi: 10.1007/s11626-016-0024-2. [DOI] [PubMed] [Google Scholar]
- 38.Kim Y., Park N., Rim Y. A., et al. Establishment of a complex skin structure via layered co-culture of keratinocytes and fibroblasts derived from induced pluripotent stem cells. Stem Cell Research & Therapy. 2018;9(1):p. 217. doi: 10.1186/s13287-018-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osei-Sarfo K., Gudas L. J. Retinoic acid suppresses the canonical Wnt signaling pathway in embryonic stem cells and activates the noncanonical Wnt signaling pathway. Stem Cells. 2014;32(8):2061–2071. doi: 10.1002/stem.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selekman J. A., Grundl N. J., Kolz J. M., Palecek S. P. Efficient generation of functional epithelial and epidermal cells from human pluripotent stem cells under defined conditions. Tissue Engineering. Part C, Methods. 2013;19(12):949–960. doi: 10.1089/ten.TEC.2013.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bilousova G., Chen J., Roop D. R. Differentiation of mouse induced pluripotent stem cells into a multipotent keratinocyte lineage. The Journal of Investigative Dermatology. 2011;131(4):857–864. doi: 10.1038/jid.2010.364. [DOI] [PubMed] [Google Scholar]
- 42.Lian X., Selekman J., Bao X., Hsiao C., Zhu K., Palecek S. P. A small molecule inhibitor of SRC family kinases promotes simple epithelial differentiation of human pluripotent stem cells. PLoS One. 2013;8(3, article e60016) doi: 10.1371/journal.pone.0060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugiyama-Nakagiri Y., Fujimura T., Moriwaki S. Induction of skin-derived precursor cells from human induced pluripotent stem cells. PLoS One. 2016;11(12, article e0168451) doi: 10.1371/journal.pone.0168451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bayati V., Abbaspour M. R., Neisi N., Hashemitabar M. Skin-derived precursors possess the ability of differentiation into the epidermal progeny and accelerate burn wound healing. Cell Biology International. 2017;41(2):187–196. doi: 10.1002/cbin.10717. [DOI] [PubMed] [Google Scholar]
- 45.Iacovides D., Rizki G., Lapathitis G., Strati K. Direct conversion of mouse embryonic fibroblasts into functional keratinocytes through transient expression of pluripotency-related genes. Stem Cell Research & Therapy. 2016;7(1):p. 98. doi: 10.1186/s13287-016-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y., Mistry D. S., Sen G. L. Highly rapid and efficient conversion of human fibroblasts to keratinocyte- like cells. The Journal of Investigative Dermatology. 2014;134(2):335–344. doi: 10.1038/jid.2013.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babakhani A., Hashemi P., Mohajer Ansari J., Ramhormozi P., Nobakht M. In vitro differentiation of hair follicle stem cell into keratinocyte by simvastatin. Iranian Biomedical Journal. 2019;23(6):404–411. doi: 10.29252/ibj.23.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marcelo K. L., Goldie L. C., Hirschi K. K. Regulation of endothelial cell differentiation and specification. Circulation Research. 2013;112(9):1272–1287. doi: 10.1161/CIRCRESAHA.113.300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chong M. S., Ng W. K., Chan J. K. Concise review: endothelial progenitor cells in regenerative medicine: applications and challenges. Stem Cells Translational Medicine. 2016;5(4):530–538. doi: 10.5966/sctm.2015-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee T. H., Jung H., Park K. H., Bang M. H., Baek N. I., Kim J. Jaceosidin, a natural flavone, promotes angiogenesis via activation of VEGFR2/FAK/PI3K/AKT/NF-κB signaling pathways in endothelial cells. Experimental Biology and Medicine (Maywood, N.J.) 2014;239(10):1325–1334. doi: 10.1177/1535370214533883. [DOI] [PubMed] [Google Scholar]
- 51.Wilson H. K., Canfield S. G., Shusta E. V., Palecek S. P. Concise review: tissue-specific microvascular endothelial cells derived from human pluripotent stem cells. Stem Cells. 2014;32(12):3037–3045. doi: 10.1002/stem.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu M., He J., Zhang C., Xu J., Wang Y. Strategies for derivation of endothelial lineages from human stem cells. Stem Cell Research & Therapy. 2019;10(1):p. 200. doi: 10.1186/s13287-019-1274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan K. S., Tamura K., Lai M. I., et al. Molecular pathways governing development of vascular endothelial cells from ES/iPS cells. Stem Cell Reviews. 2013;9(5):586–598. doi: 10.1007/s12015-013-9450-7. [DOI] [PubMed] [Google Scholar]
- 54.Klein D. iPSCs-based generation of vascular cells: reprogramming approaches and applications. Cellular and Molecular Life Sciences. 2018;75(8):1411–1433. doi: 10.1007/s00018-017-2730-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Era T., Izumi N., Hayashi M., Tada S., Nishikawa S., Nishikawa S. Multiple mesoderm subsets give rise to endothelial cells, whereas hematopoietic cells are differentiated only from a restricted subset in embryonic stem cell differentiation culture. Stem Cells. 2008;26(2):401–411. doi: 10.1634/stemcells.2006-0809. [DOI] [PubMed] [Google Scholar]
- 56.Kelly M. A., Hirschi K. K. Signaling hierarchy regulating human endothelial cell development. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29(5):718–724. doi: 10.1161/ATVBAHA.109.184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bai H., Gao Y., Arzigian M., Wojchowski D. M., Wu W. S., Wang Z. Z. BMP4 regulates vascular progenitor development in human embryonic stem cells through a Smad-dependent pathway. Journal of Cellular Biochemistry. 2010;109(2):363–374. doi: 10.1002/jcb.22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.James D., Nam H. S., Seandel M., et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nature Biotechnology. 2010;28(2):161–166. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang C., Tang X., Sun X., et al. TGFβ inhibition enhances the generation of hematopoietic progenitors from human ES cell-derived hemogenic endothelial cells using a stepwise strategy. Cell Research. 2012;22(1):194–207. doi: 10.1038/cr.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White M. P., Rufaihah A. J., Liu L., et al. Limited gene expression variation in human embryonic stem cell and induced pluripotent stem cell-derived endothelial cells. Stem Cells. 2013;31(1):92–103. doi: 10.1002/stem.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lian X., Bao X., Al-Ahmad A., et al. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Reports. 2014;3(5):804–816. doi: 10.1016/j.stemcr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bao X., Lian X., Palecek S. P. Directed endothelial progenitor differentiation from human pluripotent stem cells via Wnt activation under defined conditions. Methods in Molecular Biology. 2016;1481:183–196. doi: 10.1007/978-1-4939-6393-5_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bao X., Lian X., Dunn K. K., et al. Chemically-defined albumin-free differentiation of human pluripotent stem cells to endothelial progenitor cells. Stem Cell Research. 2015;15(1):122–129. doi: 10.1016/j.scr.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sivarapatna A., Ghaedi M., Xiao Y., et al. Engineered microvasculature in PDMS networks using endothelial cells derived from human induced pluripotent stem cells. Cell Transplantation. 2017;26(8):1365–1379. doi: 10.1177/0963689717720282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kitajima K., Nakajima M., Kanokoda M., et al. GSK3β inhibition activates the CDX/HOX pathway and promotes hemogenic endothelial progenitor differentiation from human pluripotent stem cells. Experimental Hematology. 2016;44(1):68–74.e10. doi: 10.1016/j.exphem.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee S. J., Sohn Y. D., Andukuri A., et al. Enhanced therapeutic and long-term dynamic vascularization effects of human pluripotent stem cell-derived endothelial cells encapsulated in a nanomatrix gel. Circulation. 2017;136(20):1939–1954. doi: 10.1161/CIRCULATIONAHA.116.026329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee S. J., Kim K. H., Yoon Y. S. Generation of human pluripotent stem cell-derived endothelial cells and their therapeutic utility. Current Cardiology Reports. 2018;20(6):p. 45. doi: 10.1007/s11886-018-0985-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harding A., Cortez-Toledo E., Magner N. L., et al. Highly efficient differentiation of endothelial cells from pluripotent stem cells requires the MAPK and the PI3K pathways. Stem Cells. 2017;35(4):909–919. doi: 10.1002/stem.2577. [DOI] [PubMed] [Google Scholar]
- 69.Lin H., Du Q., Li Q., et al. A scalable and efficient bioprocess for manufacturing human pluripotent stem cell-derived endothelial cells. Stem Cell Reports. 2018;11(2):454–469. doi: 10.1016/j.stemcr.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ginsberg M., James D., Ding B. S., et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFβ suppression. Cell. 2012;151(3):559–575. doi: 10.1016/j.cell.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee S., Park C., Han J. W., et al. Direct reprogramming of human dermal fibroblasts into endothelial cells using ER71/ETV2. Circulation Research. 2017;120(5):848–861. doi: 10.1161/CIRCRESAHA.116.309833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Pham P., Vu N. B., Nguyen H. T., Huynh O. T., Truong M. T. Significant improvement of direct reprogramming efficacy of fibroblasts into progenitor endothelial cells by ETV2 and hypoxia. Stem Cell Research & Therapy. 2016;7(1):p. 104. doi: 10.1186/s13287-016-0368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wary A., Wary N., Baruah J., Mastej V., Wary K. K. Chromatin-modifying agents convert fibroblasts to OCT4+ and VEGFR-2+ capillary tube-forming cells. PLoS One. 2017;12(5, article e0176496) doi: 10.1371/journal.pone.0176496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mistriotis P., Andreadis S. T. Hair follicle: a novel source of multipotent stem cells for tissue engineering and regenerative medicine. Tissue Engineering. Part B, Reviews. 2013;19(4):265–278. doi: 10.1089/ten.TEB.2012.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jimenez F., Poblet E., Izeta A. Reflections on how wound healing-promoting effects of the hair follicle can be translated into clinical practice. Experimental Dermatology. 2015;24(2):91–94. doi: 10.1111/exd.12521. [DOI] [PubMed] [Google Scholar]
- 76.Enshell-Seijffers D., Lindon C., Kashiwagi M., Morgan B. A. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Developmental Cell. 2010;18(4):633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao J., Li H., Zhou R., et al. Foxp1 regulates the proliferation of hair follicle stem cells in response to oxidative stress during hair cycling. PLoS One. 2015;10(7, article e0131674) doi: 10.1371/journal.pone.0131674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rishikaysh P., Dev K., Diaz D., Qureshi W. M., Filip S., Mokry J. Signaling involved in hair follicle morphogenesis and development. International Journal of Molecular Sciences. 2014;15(1):1647–1670. doi: 10.3390/ijms15011647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abe Y., Tanaka N. Roles of the hedgehog signaling pathway in epidermal and hair follicle development, homeostasis, and cancer. Journal of Developmental Biology. 2017;5(4):p. 12. doi: 10.3390/jdb5040012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsai S. Y., Sennett R., Rezza A., et al. Wnt/β-catenin signaling in dermal condensates is required for hair follicle formation. Developmental Biology. 2014;385(2):179–188. doi: 10.1016/j.ydbio.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi Y. S., Zhang Y., Xu M., et al. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell. 2013;13(6):720–733. doi: 10.1016/j.stem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kandyba E., Leung Y., Chen Y. B., Widelitz R., Chuong C. M., Kobielak K. Competitive balance of intrabulge BMP/Wnt signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(4):1351–1356. doi: 10.1073/pnas.1121312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang R., Zheng Y., Burrows M., et al. Generation of folliculogenic human epithelial stem cells from induced pluripotent stem cells. Nature Communications. 2014;5(1, article 3071) doi: 10.1038/ncomms4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou H., Wang L., Zhang C., et al. Feasibility of repairing full-thickness skin defects by iPSC-derived epithelial stem cells seeded on a human acellular amniotic membrane. Stem Cell Research & Therapy. 2019;10(1, article 155) doi: 10.1186/s13287-019-1234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Son M. J., Jeong J. K., Kwon Y., et al. A novel and safe small molecule enhances hair follicle regeneration by facilitating metabolic reprogramming. Experimental & Molecular Medicine. 2018;50(12):p. 160. doi: 10.1038/s12276-018-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fuchs E. Epithelial skin biology: three decades of developmental biology, a hundred questions answered and a thousand new ones to address. Current Topics in Developmental Biology. 2016;116:357–374. doi: 10.1016/bs.ctdb.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morgan B. A. The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harbor Perspectives in Medicine. 2014;4(7):p. a015180. doi: 10.1101/cshperspect.a015180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Veraitch O., Mabuchi Y., Matsuzaki Y., et al. Induction of hair follicle dermal papilla cell properties in human induced pluripotent stem cell-derived multipotent LNGFR(+)THY-1(+) mesenchymal cells. Scientific Reports. 2017;7(1, article 42777) doi: 10.1038/srep42777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoshida Y., Soma T., Matsuzaki T., Kishimoto J. Wnt activator CHIR99021-stimulated human dermal papilla spheroids contribute to hair follicle formation and production of reconstituted follicle-enriched human skin. Biochemical and Biophysical Research Communications. 2019;516(3):599–605. doi: 10.1016/j.bbrc.2019.06.038. [DOI] [PubMed] [Google Scholar]
- 90.Harel S., Higgins C. A., Cerise J. E., et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Science Advances. 2015;1(9, article e1500973) doi: 10.1126/sciadv.1500973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim Y. E., Choi H. C., Lee I. C., Yuk D. Y., Lee H., Choi B. Y. 3-Deoxysappanchalcone promotes proliferation of human hair follicle dermal papilla cells and hair growth in C57BL/6 mice by modulating WNT/β-Catenin and STAT signaling. Biomolecules & Therapeutics. 2016;24(6):572–580. doi: 10.4062/biomolther.2016.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li H., Chen L., Zhang M., Tang S., Fu X. Three-dimensional culture and identification of human eccrine sweat glands in matrigel basement membrane matrix. Cell and Tissue Research. 2013;354(3):897–902. doi: 10.1007/s00441-013-1718-3. [DOI] [PubMed] [Google Scholar]
- 93.Xu Y., Huang S., Ma K., Fu X., Han W., Sheng Z. Promising new potential for mesenchymal stem cells derived from human umbilical cord Wharton's jelly: sweat gland cell-like differentiative capacity. Journal of Tissue Engineering and Regenerative Medicine. 2012;6(8):645–654. doi: 10.1002/term.468. [DOI] [PubMed] [Google Scholar]
- 94.Tao R., Sun T. J., Han Y. Q., Xu G., Liu J., Han Y. F. Epimorphin-induced differentiation of human umbilical cord mesenchymal stem cells into sweat gland cells. European Review for Medical and Pharmacological Sciences. 2014;18(9):1404–1410. [PubMed] [Google Scholar]
- 95.Xu Y., Hong Y., Xu M., et al. Role of keratinocyte growth factor in the differentiation of sweat gland-like cells from human umbilical cord-derived mesenchymal stem cells. Stem Cells Translational Medicine. 2016;5(1):106–116. doi: 10.5966/sctm.2015-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao Z., Xu M., Wu M., et al. Direct reprogramming of human fibroblasts into sweat gland-like cells. Cell Cycle. 2015;14(21):3498–3505. doi: 10.1080/15384101.2015.1093707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Philp D., Goldstein A. L., Kleinman H. K. Thymosin beta4 promotes angiogenesis, wound healing, and hair follicle development. Mechanisms of Ageing and Development. 2004;125(2):113–115. doi: 10.1016/j.mad.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 98.Ramirez P., Rettig M. P., Uy G. L., et al. BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood. 2009;114(7):1340–1343. doi: 10.1182/blood-2008-10-184721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu J., Huang H., Chen H., et al. Plerixafor and granulocyte-colony-stimulating factor for mobilization of hematopoietic stem cells for autologous transplantation in Chinese patients with non-Hodgkin's lymphoma: a randomized phase 3 study. Transfusion. 2018;58(1):81–87. doi: 10.1111/trf.14426. [DOI] [PubMed] [Google Scholar]
- 100.Das A., Barker D. A., Wang T., Lau C. M., Lin Y., Botchwey E. A. Delivery of bioactive lipids from composite microgel-microsphere injectable scaffolds enhances stem cell recruitment and skeletal repair. PLoS One. 2014;9(7, article e101276) doi: 10.1371/journal.pone.0101276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi H., Xu X., Zhang B., et al. 3,3′-Diindolylmethane stimulates exosomal Wnt11 autocrine signaling in human umbilical cord mesenchymal stem cells to enhance wound healing. Theranostics. 2017;7(6):1674–1688. doi: 10.7150/thno.18082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zen A. A. H., Nawrot D. A., Howarth A., et al. The retinoid agonist tazarotene promotes angiogenesis and wound healing. Molecular Therapy. 2016;24(10):1745–1759. doi: 10.1038/mt.2016.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gao S. Q., Chang C., Li J. J., et al. Co-delivery of deferoxamine and hydroxysafflor yellow A to accelerate diabetic wound healing via enhanced angiogenesis. Drug Delivery. 2018;25(1):1779–1789. doi: 10.1080/10717544.2018.1513608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee S., Kim M. S., Jung S. J., Kim D., Park H. J., Cho D. ERK activating peptide, AES16-2M promotes wound healing through accelerating migration of keratinocytes. Scientific Reports. 2018;8(1):p. 14398. doi: 10.1038/s41598-018-32851-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yuan J., Hou Q., Chen D., et al. Chitosan/LiCl composite scaffolds promote skin regeneration in full-thickness loss. Science China Life Sciences. 2019:1–11. doi: 10.1007/s11427-018-9389-6. [DOI] [PubMed] [Google Scholar]
- 106.Bastakoty D., Saraswati S., Cates J., Lee E., Nanney L. B., Young P. P. Inhibition of Wnt/β-catenin pathway promotes regenerative repair of cutaneous and cartilage injury. The FASEB Journal. 2015;29(12):4881–4892. doi: 10.1096/fj.15-275941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim K. I., Jeong D. S., Yoon T. J., Jung E. C., Lee J. H., Kim C. D. Inhibition of collagen production by ICG-001, a small molecule inhibitor for Wnt/β-catenin signaling, in skin fibroblasts. Journal of Dermatological Science. 2017;86(1):76–78. doi: 10.1016/j.jdermsci.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 108.Wang L., Yang J., Ran B., et al. Small molecular TGF-β1-Inhibitor-Loaded electrospun fibrous scaffolds for preventing hypertrophic scars. ACS Applied Materials & Interfaces. 2017;9(38):32545–32553. doi: 10.1021/acsami.7b09796. [DOI] [PubMed] [Google Scholar]
- 109.Li Y., Tian Y., Zheng W., et al. Composites of bacterial cellulose and small molecule-decorated gold nanoparticles for treating gram-negative bacteria-infected wounds. Small. 2017;13(27, article 1700130) doi: 10.1002/smll.201700130. [DOI] [PubMed] [Google Scholar]
- 110.Welsch K., Holstein J., Laurence A., Ghoreschi K. Targeting JAK/STAT signalling in inflammatory skin diseases with small molecule inhibitors. European Journal of Immunology. 2017;47(7):1096–1107. doi: 10.1002/eji.201646680. [DOI] [PubMed] [Google Scholar]
- 111.Ma X., Kong L., Zhu S. Reprogramming cell fates by small molecules. Protein & Cell. 2017;8(5):328–348. doi: 10.1007/s13238-016-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu A., Cheng L. Chemical transdifferentiation: closer to regenerative medicine. Frontiers in Medicine. 2016;10(2):152–165. doi: 10.1007/s11684-016-0445-z. [DOI] [PubMed] [Google Scholar]
- 113.Motegi S. I., Ishikawa O. Mesenchymal stem cells: the roles and functions in cutaneous wound healing and tumor growth. Journal of Dermatological Science. 2017;86(2):83–89. doi: 10.1016/j.jdermsci.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 114.Naska S., Yuzwa S. A., Johnston A. P., et al. Identification of drugs that regulate dermal stem cells and enhance skin repair. Stem Cell Reports. 2016;6(1):74–84. doi: 10.1016/j.stemcr.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Laurencin C. T., Ashe K. M., Henry N., Kan H. M., Lo K. W. Delivery of small molecules for bone regenerative engineering: preclinical studies and potential clinical applications. Drug Discovery Today. 2014;19(6):794–800. doi: 10.1016/j.drudis.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gizaw M., Thompson J., Faglie A., Lee S. Y., Neuenschwander P., Chou S. F. Electrospun fibers as a dressing material for drug and biological agent delivery in wound healing applications. Bioengineering. 2018;5(1):p. 9. doi: 10.3390/bioengineering5010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ogle M. E., Krieger J. R., Tellier L. E., McFaline-Figueroa J., Temenoff J. S., Botchwey E. A. Dual affinity heparin-based hydrogels achieve pro-regenerative immunomodulation and microvascular remodeling. ACS Biomaterials Science & Engineering. 2018;4(4):1241–1250. doi: 10.1021/acsbiomaterials.6b00706. [DOI] [PMC free article] [PubMed] [Google Scholar]