Abstract
There is growing evidence that oxytocin (OXT), a hypothalamic hormone well recognized for its effects in inducing parturition and lactation, has important metabolic effects in both sexes. The purpose of this review is to summarize the physiologic effects of OXT on metabolism and to explore its therapeutic potential for metabolic disorders. In model systems, OXT promotes weight loss by decreasing energy intake. Pair-feeding studies suggest that OXT-induced weight loss may also be partly due to increased energy expenditure and/or lipolysis. In humans, OXT appears to modulate both homeostatic and reward-driven food intake, although the observed response depends on nutrient milieu (eg, obese vs. nonobese), clinical characteristics (eg, sex), and experimental paradigm. In animal models, OXT is anabolic to muscle and bone, which is consistent with OXT-induced weight loss occurring primarily via fat loss. In some human observational studies, circulating OXT concentrations are also positively associated with lean mass and bone mineral density. The impact of exogenous OXT on human obesity is the focus of ongoing investigation. Future randomized, placebo-controlled clinical trials in humans should include rigorous, standardized, and detailed assessments of adherence, adverse effects, pharmacokinetics/pharmacodynamics, and efficacy in the diverse populations that may benefit from OXT, in particular those in whom hypothalamic OXT signaling may be abnormal or impaired (eg, individuals with Sim1 deficiency, Prader–Willi syndrome, or craniopharyngioma). Future studies will also have the opportunity to investigate the characteristics of new OXT mimetic peptides and the obligation to consider long-term effects, especially when OXT is given to children and adolescents. (Endocrine Reviews XX: XX – XX, 2020)
Keywords: oxytocin, metabolism, body composition, feeding behavior, energy balance
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS.
Oxytocin (OXT) is synthesized in the hypothalamus, specifically in the parvocellular neurons of the paraventricular nucleus (PVN) and in the magnocellular neurons of both the PVN and the supraoptic nucleus.
Apart from its role in parturition and lactation in females, OXT appears to act as a “nutrient status sensor” in both sexes.
In both humans and model systems, OXT may be anorectic, with increased OXT secretion and/or signaling leading to decreased food intake via net effects on multiple different homeostatic and neurobehavioral pathways.
Studies in model systems suggest that weight loss in response to OXT is related to decreased energy intake and also increased energy expenditure; weight loss tends to occur as a result of loss of fat mass, not lean mass.
OXT may be anabolic to muscle and bone.
Additional rigorous clinical trials in humans are needed to evaluate the translational potential of OXT for obesity and other metabolic disorders.
There is growing evidence that oxytocin (OXT), a hypothalamic hormone well known for its role in parturition and lactation in females, also has important metabolic effects. Oxytocin is a 9-amino acid peptide that is structurally similar to vasopressin (Fig. 1), a hormone produced in the same hypothalamic nuclei that is critical for maintaining water balance.
Figure 1.
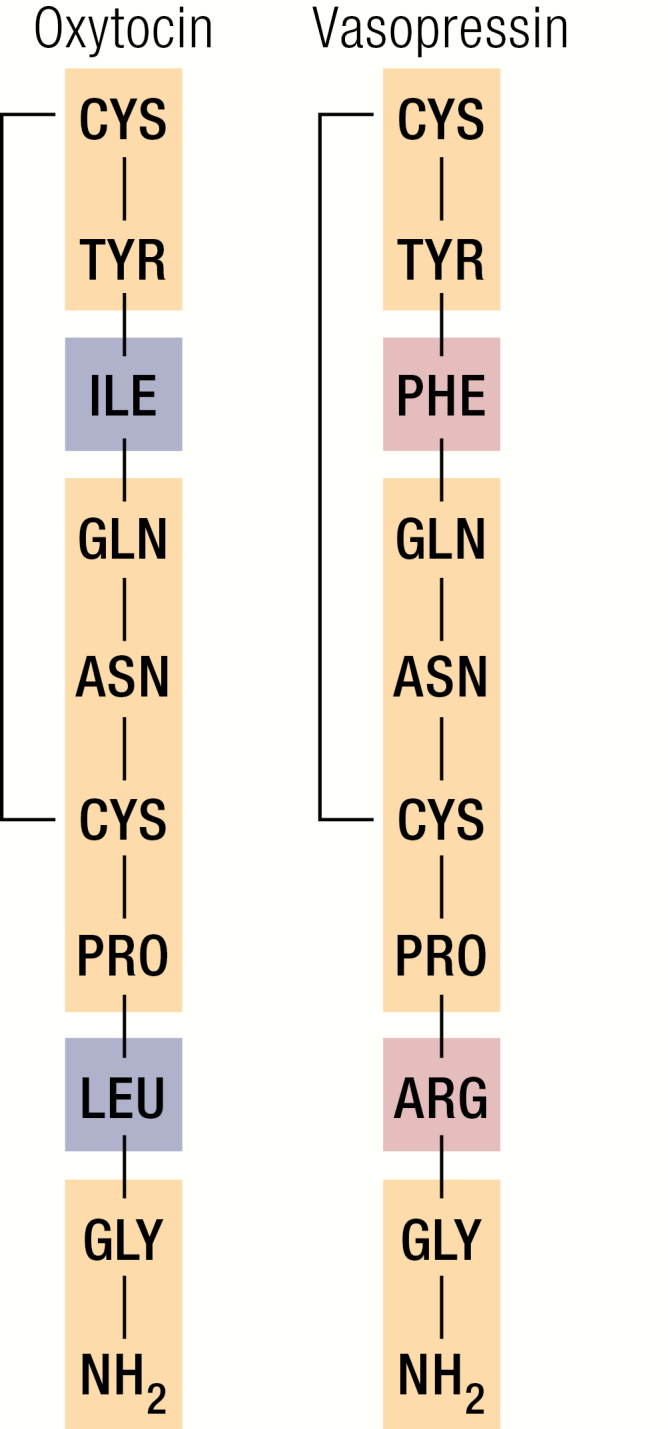
Structural similarity between OXT and vasopressin. Identical amino acids (AAs) are shown in yellow, differing AAs are shown in blue (oxytocin) and red (vasopressin).
OXT also appears to act as a “nutrient status sensor” in both sexes (1). Studies in both model systems and in humans indicate that OXT impacts both energy intake and energy expenditure. The purpose of this review is to summarize what is known about the physiologic effects of OXT on metabolism, including energy intake, energy expenditure, body composition, musculoskeletal health, lipid flux, and glucose homeostasis. We synthesize preclinical findings in model systems and physiologic investigations in humans. We also summarize current evidence regarding the translational potential of OXT for the treatment of endocrine disorders including obesity, diabetes mellitus, and osteoporosis. In the course of reviewing these topics, we include a discussion of potential developmental- and sex-specific effects of OXT. We conclude by outlining important areas for future research, with a focus on clinical trials in humans.
Overview of the OXT System
Anatomic localization of OXT secretion
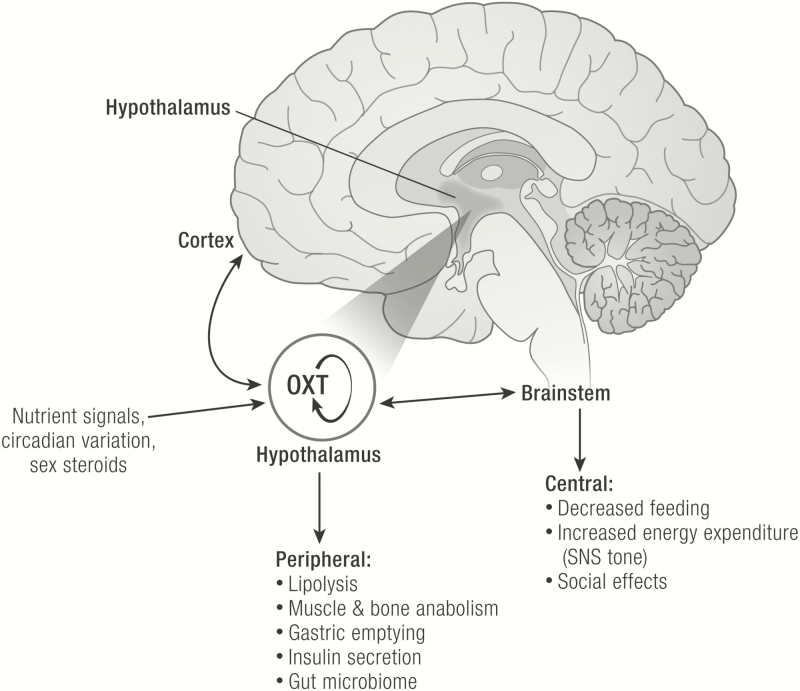
Oxytocin is synthesized in the hypothalamus (2), specifically in the parvocellular neurons of the paraventricular nucleus (PVN) and in the magnocellular neurons of both the PVN and the supraoptic nucleus (SON) (Fig. 2). Oxytocin is also produced in other brain areas outside the hypothalamus, albeit in much smaller quantities (3). There are several ways in which OXT produced in the hypothalamus reaches other hypothalamic regions, for example, the ventromedial nucleus (VMN), as well as other areas of the brain, the spinal cord, and the peripheral circulation (4). First, OXT release occurs locally, from somatodendrites within the PVN and SON. Also, OXT release occurs distally, at synaptic terminals that arise from axons originating from neurons in the PVN and SON. There are magnocelluar PVN and SON projections to the neurohypophysis, from which OXT, like vasopressin, is released into the circulation. In addition, OXT is produced in parvocellular PVN projections to other brain areas that may affect metabolism, including the arcuate nucleus (ARC) in the hypothalamus (5), the nucleus accumbens in the forebrain (6), the ventral tegmental area (VTA) (7) in the midbrain, and nucleus of the solitary tract (NTS) (8) in the brainstem, as well as the spinal cord (9). Finally, there is evidence in support of OXT-producing magnocellular PVN and SON projections to the hypothalamic ARC (5).
Figure 2.
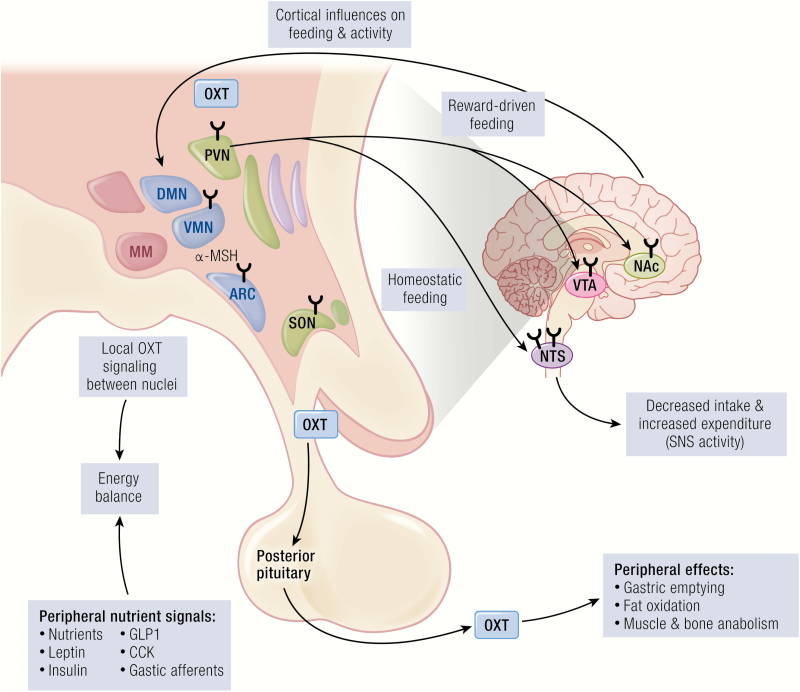
Metabolic effects of OXT. OXT is produced in the paraventricular nucleus (PVN) and supraoptic nucleus (SON), shown in dark text. PVN and SON are sensitive to indicators of nutrient status, including nutrients themselves (eg, glucose, sucrose, leucine, fat-derived satiety factors) as well as other hormones (eg, leptin, insulin, GLP1, CCK, gastric afferents). Nuclei with OXT receptors (indicated by the Y shape) both within the hypothalamus and outside the hypothalamus respond to the presence of OXT and influence energy intake and energy balance. Hypothalamus and brainstem regulate the homeostatic drive to eat, and the brain reward network regulates the hedonic drive to eat. Oxytocin-producing neurons in the PVN also receive regulatory input from the central melanocortinergic signaling pathways that influence appetite. Hypothalamus and brainstem also impact energy expenditure, with the most consistent evidence in support of this occurring via increased sympathetic nervous system tone. The entire OXT signaling network also influences social function, which affects complex behaviors related to metabolism (eg, eating, voluntary physical activity, psychosocial stress). Oxytocin is also secreted into the periphery via the posterior pituitary, where it exerts metabolic effects in multiple organ systems (eg, GI motility, muscle and bone anabolism, lipolysis, pancreatic insulin secretion). Abbreviations: α-MSH, α-melanocyte stimulating hormone; ARC, arcuate nucleus; CCK, cholecystokinin; DMN, dorsomedial nucleus; GI, gastrointestinal; GLP1, glucagon-like peptide 1; MM, mammillary nuclei; NAc, nucleus accumbens; NTS, nucleus tractus solitarius; OXT, oxytocin; PVN, paraventricular nucleus; SNS, sympathetic nervous system; SON, supraoptic nucleus; VMN, ventromedial nucleus; VTA, ventral tegmental area.
Although OXT production is most abundant in the hypothalamus, OXT-producing neurons are also located outside the central nervous system. Specifically, in humans, myenteric and submucous ganglia and nerve fibers throughout the human gastrointestinal tract express OXT (10). In addition, OXT is expressed in bone marrow osteoblasts (11), liver, and subcutaneous adipose tissue (12).
OXT synthesis, secretion, and degradation
Oxytocin is synthesized in the hypothalamus as an inactive precursor along with its carrier protein neurophysin I (13). Labor and infant suckling are 2 well-established stimuli for OXT secretion, consistent with its known physiological roles. Effects of other stimuli including exercise, sexual stimulation, and stress have also been explored, for example (14); however, the full range of physiologic stimuli has not been well-characterized (15). Oxytocin is degraded by multiple aminopeptidases expressed in different cell types, including adipocytes and leukocytes (16). There is some evidence that the degradation of OXT is influenced by metabolic status. For example, in one study of obese Zucker rats, decreased peripheral concentrations of OXT were attributed to increased activity of oxytocinase in both liver and adipose tissue (17).
OXT receptors
Oxytocin receptors (OXTRs) are found in the uterus, consistent with its known role in parturition, and in diverse other tissues that are relevant to its effects on metabolism. In rodents, OXT receptors structurally similar to those found in the uterus were also expressed in brain areas, including the VMN, bed nucleus of the stria terminalis, amygdala, subiculum, hippocampus, olfactory nuclei, NTS, dorsal motor nucleus of the vagus, area postrema, and VTA (18–22). In humans, OXTRs have also been identified in multiple regions of the brain including basal ganglia, hypothalamus, amygdala, hippocampus, and multiple cortical regions, as well as other tissues including breast, aorta, and esophagus (23). Recent expression maps in humans demonstrate that OXT pathway genes (ie, OXT, OXTRs, and the modifier of OXT secretion CD38) are enriched in subcortical and olfactory brain regions, including areas that may influence appetite (24). Particularly relevant to its role in central nervous system (CNS)–gut interactions, OXTRs are also present on the inferior ganglion of the vagus nerve (nodose ganglion) that transmits information from the viscera to the brain, including the NTS (25). Oxytocin receptors are also found on smooth muscle cells in the stomach (26) and on the myenteric plexus, the nerve fibers found between muscle layers in the gastrointestinal (GI) tract (27). Oxytocin receptor expression in these regions may underlie observed OXT-related changes in GI motility (26, 27). Oxytocin receptors are also present on adipocytes, and rodent studies suggest that their distribution may vary with metabolic phenotype (28). In vitro, OXTR expression increases as adipocytes become more differentiated (29). Oxytocin receptors are also expressed in muscle. In rats, OXTRs were expressed mostly in muscle groups composed of glycolytic fibers (eg, quadriceps) and less in muscle groups composed of oxidative fibers (eg, soleus), and expression may be reduced in obese animals (28).
Pulsatility of OXT secretion
Oxytocin has complex secretory patterns. There is substantial evidence in animal models, and accumulating evidence in humans, that OXT secretion is pulsatile, in particular in response to physiologic stimuli. For example, OXT pulses have been detected in both pregnant and nonpregnant sheep (30). In both animal models and humans, the short half-life of OXT in the circulation, on the order of 5 to 7 minutes (31, 32), means that a combination of frequent sampling and deconvolution or other analysis technique (33) is needed to identify and characterize pulses. In lactating women who were evaluated with 2-minute sampling, pulsatile OXT secretion was detected, with approximately 1 to 2 pulses per 20-minute sampling period (34). Most studies of OXT pulsatility in humans have focused on characterizing OXT secretory dynamics in response to physiologic stimuli, and fewer have characterized OXT secretory dynamics at rest. In 1 study, deconvolution analysis of serum OXT concentrations obtained every 5 minutes overnight in 5 healthy men was performed. Pulsatile OXT secretion was demonstrated, with mean interpulse interval of 27 ± 4 minutes (35).
Impact of gonadal steroids on OXT signaling
Estrogen alters OXT signaling by affecting both OXT secretion and OXTR expression, likely related to the known role of OXT in parturition (32). In rodents, there is substantially increased expression of the gene encoding the OXTR in both brain and uterus in response to estrogen (18). In humans, 1 study examined circulating OXT concentrations across the menstrual cycle in healthy women and did not detect a difference between follicular and luteal phases (36). In contrast, a different investigation found that fasting and postprandial serum OXT concentrations were lower in human females in the early to mid-follicular phase, when estradiol levels are low, compared to other phases of the menstrual cycle (37). Other studies in humans have shown that increasing endogenous estrogen concentrations, as occurs in the ovulatory phase of the menstrual cycle, or in response to oral estrogen administration, as with oral contraceptive treatment, leads to increases in circulating OXT concentrations (eg, (36, 38–40)). Estrogen additionally augments OXT effects by increasing the expression of OXTRs. Other steroid hormones impact OXT as well. Progesterone could affect OXTR expression on the cell surface indirectly by altering cholesterol metabolism (41). Testosterone has been posited to affect OXT signaling, either indirectly, via its aromatization to estradiol, and/or directly, via androgen signaling. Specifically, in rats, testosterone administration increased OXT binding to its own receptor, including in the VMN of the hypothalamus (42), a structure with potential relevance for its role in metabolic regulation. In light of the posited effects of sex steroids on OXT production and action, mechanistic and interventional studies should test for the presence of sexually dimorphic effects of OXT on metabolism.
Appetite and Food Intake
OXT responsiveness to nutrient-signaling hormones
Particularly relevant to its role in metabolism, OXT has been shown to participate in the regulation of appetite (43). Oxytocin is anorectic, with increased OXT production leading to decreased food intake. Specifically, OXT-producing neurons of the SON and the PVN can act as nutrient status sensors and then interact with cortical and brainstem structures influencing eating behaviors (Fig. 2) (1). Oxytocin-producing neurons can respond directly to nutrients, as discussed in subsequent sections, and/or to other hormones that reflect adipose stores and overall energy balance. Oxytocin-producing parvocellular PVN neurons, including a subset of those that project to the hindbrain, are sensitive to leptin and appear to decrease meal size by increasing the sensitivity of hindbrain NTS neurons to satiety signals (44, 45). Oxytocin receptors are also highly expressed in key hypothalamic nuclei that affect energy balance, including the VMN (Fig. 2) (46). Moreover, one of the known effects of the neuropeptide α-melanocyte stimulating hormone (α-MSH) signaling via anorectic melanocortin 4 receptor (MC4R) pathways that decrease food intake (4) is increased local dendritic release of OXT (shown in Fig. 2) and decreased systemic circulating OXT. Oxytocin signaling to the NTS in the hindbrain appears to simultaneously reduce food intake and increase sympathetic nervous system (SNS) activity, leading to increased energy expenditure and net negative energy balance (Fig. 2) (29). In addition to OXT signaling to the hindbrain, some studies indicate that OXT signaling to the spinal cord also has effects on energy expenditure. Oxytocin receptors are present in the mouse spinal cord (47), and chemogenetic activation of OXT-expressing PVN neurons that project to the thoracic spinal cord increases energy expenditure in Oxytocin-Ires Cre mice (48). Additionally, there is some evidence that OXT impacts metabolically relevant serotonergic signaling, although the details of its effects are incompletely understood (49).
OXT responsiveness to nutrients
Several additional lines of evidence suggest that OXT responds directly to nutrient sources of energy. Specifically, OXT-producing neurons respond to a variety of nutrient signals, including sucrose (50), the branched-chain amino acid leucine (51), and a fat-derived satiety factor, oleoylethanolamide (52), as well as the act of refeeding itself (53), and its correlates, including gastric distension, the gut hormone cholecystokinin (CCK) (54), signaling via gastric afferents (55), and fibroblast growth factor 21 (56). In addition, in explants of the rat hypothalamo–hypophyseal system, supraoptic neurons release OXT in response to glucose and insulin, concentrations of which are highest in the fed state. OXT release is blocked by administration of a glucokinase inhibitor, which suggests that glucose uptake and metabolism may be coupled to OXT secretion (1). Foundational work by Verbalis et al. (57), demonstrated that in animal models, the increase in endogenous hypothalamic OXT secretion in response to CCK and nausea-inducing aversive agents was even greater than the increase in endogenous hypothalamic OXT secretion in response to food. The investigators concluded that these disparate signals (ie, nausea and satiety) activated a common OXT signaling pathway to decrease energy intake.
Despite this evidence, an anorectic role for OXT may seem inconsistent with its well-established role in promoting lactation, since additional nutrition is required to support lactation. However, the coupling between nutrient status and OXT release is altered in explants from lactating rats. Specifically, glucose alone fails to promote OXT release in these explants, and OXT secretion related to the presence of glucose and insulin loses its dependence on glucokinase (58), thus OXT secretion may be decoupled from nutrient status in the context of lactation. Also, some animal studies suggest that changes in OXTR expression occur in the brain during pregnancy (59). For example, in prairie voles, in ventrum pallidum and PVN, OXTR expression increased in proportion to gestational length, while OXTR expression in other areas of the brain remained unchanged. Other studies have found that pregnancy also leads to increases in the synthesis and release of OXT, for example (60). In summary, the role of OXT in energy sensing may be distinct in the unique hormonal milieu of lactation and/or pregnancy.
In addition, there is evidence in support of developmental variation in the role of OXT for nutrient sensing. For example, mice that are homozygous for deletions of either OXT (61), or the OXTR (62) develop late-onset obesity, suggesting the metabolic role of OXT diverges in younger versus older animals. Also, mice homozygous for deletions in OXT (–/–) are not hyperphagic, contrary to the expectation that hypothalamic OXT decreases food intake in a leptin-dependent manner (61). It is possible that the lack of change in leptin-dependent food intake in OXT (–/–) mice reflects redundancy in the hormonal systems that govern appetite regulation. Alternatively, the appetite of OXT (–/–) mice, reported as “normal,” despite high circulating leptin levels, may actually be excessive relative to the degree of adiposity. In the setting of heightened leptin sensitivity, as occurs in animal models with Soc3 deficiency in the mediobasal hypothalamus, there was an increase in OXT content in the dorsal vagal complex and a corresponding increase in meal-related satiety signals (63). Blocking OXTRs (by administering an OXT receptor antagonist in the fourth ventricle) abrogated the enhanced satiety signals related to Soc3 deficiency in the mediobasal hypothalamus. Similar to the OXT (–/–) mice, total daily food intake was not different in mice homozygous for deletions in the OXTR, despite increases in their body mass and fat (62). However, meal size during the dark, active cycle (but not the light, resting cycle) was increased in OXTR (–/–) mice, and meal size was also less responsive to CCK (64). Also, administration of an OXTR antagonist into the fourth ventricle increased meal size in mice (51). Consistent with these findings, either fourth ventricular administration of an OXTR antagonist (65) or lesions in hindbrain neurons expressing the OXTR (66) attenuated the response to meal related satiety signals like CCK. Taken together, these studies suggest that physiologic OXT modestly reduces meal size and enhances responsiveness of feeding behavior to time of day and to meal-related satiety signals like CCK (67) in ways that appear to leave total daily food intake not appreciably changed. The metabolic phenotype of the OXTR deficient animals (excess adiposity despite similar total food intake) also raises the question of differences in energy expenditure; these are discussed in a subsequent section.
Studies in genetic models of obesity highlight that nutritional status does not uniquely determine peripheral OXT concentrations. For example, in leptin-deficient, obese ob/ob mice, no differences in serum OXT were detected relative to saline-treated C57BL6/J mice (68). In another model, the obese CCK-1 receptor knock-out Otuska Long-Evans Tokushima Fatty (OLETF) rat, serum OXT concentrations were increased relative to lean control Long-Evans Tokushima Otsuka (LETO) rats (69). Chronic pair-feeding of obese OLETF rats to equal lean control LETO rats led to reductions in adiposity, as well as decreases in peripheral OXT concentrations, in the obese OLETF rats (70). In contrast, in different rodent models of obesity and diabetes, including the obese leptin receptor deficient db/db mice (71) and Zucker fatty rats, serum OXT concentrations were decreased relative to lean control animals (17).
Mechanisms of OXT-induced decreases in appetite and food intake via homeostatic pathways
The homeostatic pathways that govern food intake are a set of neural circuits that converge in the hypothalamus and lead an organism to eat in response to energy deficit (72). This is in contrast to eating for pleasure that may occur despite adequate energy availability; the brain’s reward system is often implicated in this “hedonic” pathway, which is discussed later. As described previously and illustrated in Fig. 2, OXT interacts with the central melanocortinergic network that governs energy balance (5). Current evidence indicates that OXT signals via OXTRs on catabolic pro-opiomelanocortin (POMC) neurons on either the ARC and/or the NTS (73). Oxytocin led to increases in cytosolic Ca2+ in POMC neurons in the ARC and NTS, suggesting that OXT has downstream signaling effects. Terminal mapping studies indicated that physiologic OXT produced in the PVN and SON in response to nutrient availability alters feeding behavior by signaling via projections to POMC neurons in the ARC.
Other signaling molecules have also been proposed to impact OXT secretion and effect. For example, an anorectic peptide, nesfatin-1, stimulates OXT secretion (73). Importantly, the anorectic effects of nesfatin-1 are blocked by an OXTR antagonist. This result suggests that the effects of nesfatin-1 depend on its capacity to promote OXT secretion. Hypothalamic-hindbrain connections may also mediate the anorectic effects of OXT in response to noxious stimuli (toxins, salt loading, gastric distension) (43). Studies have also found that exogenous OXT, when administered peripherally, activates vagal afferent neurons that project directly to the NTS, implying that this may be one mechanism by which peripheral OXT acts outside the hypothalamus to decrease feeding, for example (74). A follow-up study found that either sub-diaphragmatic vagotomy, intracerebroventricular injection of an OXTR antagonist, and/or use of OXT KO mice blunted the decrease in food intake produced by intraperitoneal injection of OXT, suggesting that vagal afferents mediate the effect of peripheral OXT on central PVN OXT neurons that impact appetite (75).
Studies in mice have disparate findings with respect to the dispensability of hypothalamic OXT-producing PVN neurons for normal feeding behavior and body weight regulation. For example, 1 study (76) directly manipulated OXT-producing PVN neurons and failed to identify any effects on appetite, feeding behavior, or body weight. These results are unlike those produced by similar disruption paradigms on cells expressing GLP1R or MC4R, which had substantial effects. From these investigations, disruption of PVN cells producing OXT appeared insufficient to impact whole body energy balance. In contrast, in a different study, destruction of PVN OXT-producing neurons via a different technique (diphtheria toxin) led to increased body weight, increased body fat, and reduced energy expenditure in diet-induced male mice only (in contrast, no effect was observed in females on high-fat diet (HFD) or males on normal chow) (77). Destruction of OXT-producing PVN neurons via yet a different technique (inhibition of OXT exocytosis) led to increased food intake (on either HFD or regular chow) and associated weight gain (on either HFD or regular chow) (78). Alternatively, and/or in addition, OXT-producing PVN neurons may exert their effects via the relay of metabolically relevant signals when these are present, with examples including central insulin (79), caffeine (80), and/or nicotine (81). In humans, neuroimaging studies have found that a dose of 24 international units (IU) of intranasal OXT reduces functional magnetic resonance imaging activation in the hypothalamus, a region key for homeostatic control of eating behavior, in response to images of foods (82, 83), supporting preclinical data indicating that OXT reduces food intake in part via homeostatic pathways. However, these studies did not observe consistent correlation between the extent of hypothalamic activation and ad libitum food intake (Table 1).
Table 1.
Interventional studies of OXT in humans with either direct measurement of metabolic outcomes, and/or performed in individuals with metabolic disorders including obesity, Prader–Willi syndrome, and craniopharyngioma
| Title (year) | PMID | Intervention(s) | Main outcome(s) | Key observations |
|---|---|---|---|---|
| Effect of pharmacological doses of oxytocin on insulin response to glucose in normal man (1984)a | 6386646 | Intravenous OXT Dose: 3 IU (1 IU then 2 IU over 1h), 6 IU (2 IU then 4 IU over 1h) Total n doses: 1 Waiting time: 10 minutes (before glucose infusion) | Glucose tolerance Insulin homeostasis Counter-regulatory response | IV glucose was infused (0.33g/kg) on 2 separate days with either (i) no additional intervention (n = 12 healthy men), or (ii) OXT 3 IU (2 IU, then 1 IU over 1 hour, n = 6 of these subjects), or (iii) OXT 6 IU (2 IU, then 4 IU over 1h) A dose of 6 IU (but not 3 IU) increased insulin secretion in response to the intravenous glucose tolerance test (0.33g/kg). No other changes were noted. |
| Pharmacologic doses of oxytocin affect plasma hormone levels modulating glucose homeostasis in normal man (1988)a | 3065208 | Intravenous OXT Dose: 0.2 IU/minute Total n doses: 4 Waiting time: 0 minutes (OXT started at same time as physiologic studies) | Glucose Glucagon Adrenaline Insulin secretion | OXT was infused on 4 separate days during either (i) steady-state (no intervention), (ii) hypoglycemic clamp, (iii) eu-insulinemic/hyperglycemic clamp, or (iv) hyperglycemic clamp in n = 7 fasting, healthy, normal weight men Intravenous OXT led to increases in glucose and glucagon concentrations. Intravenous OXT (with somatostatin and glucagon/low-dose insulin replacement) produced increases in glucose, but not at higher doses of insulin. OXT potentiated glucose-stimulated insulin secretion during hyperglycemic clamp. |
| A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research (2011)a | 21429671 | Intranasal OXT Dose: 18–40 IU Schedule: single dose or qd–qid Total n doses: 1–182 Waiting time: 0–30 minutes (20%), 45–60 minutes (72%), Other/NA (8%) | Adverse events | Systematic review of 38 RCTs (1990–2010) in N = 1529 (79% male) of intranasal OXT. Most studies were single doses, up to 182 administrations. No consistent differences between cases/controls were found, no consistent associated adverse effects were found. Two SAEs (hyponatremia) due to obligate fluid intake during intranasal OXT treatment for lactation in women were reported. A 55-year-old man with OCD also developed hyponatremia (126 mmol/L) after 4 weeks of 8.4–16.8 IU in total daily (divided tid). Overall, AEs were not well documented in studies. |
| Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models (2013)a | 23700406 | Intranasal OXT Dose: 24 IU Schedule: qid (before meals and bedtime) Total n doses: 224 Waiting time: 20 minutes | Weight Waist circumference Hip circumference Lipid profile 2h-glucose/insulin (OGTT) Adverse events | Randomized, placebo-controlled trial of intranasal OXT (N = 24 randomized; n = 12 OXT with 9 complete; n = 12 placebo with 11 complete) in obese, nondiabetic adult men and women for 8 weeks After 8 weeks, weight loss was 8.9 ± 5.4 kg (SD) after OXT treatment with corresponding changes in waist and hip circumference. No statistically significant weight loss was reported after placebo. Total cholesterol and LDL decreased with OXT. No change in glycemic parameters were noted. No differences in AEs; no SAEs. |
| Oxytocin reduces reward-driven food intake in humans (2013)a | 23835346 | Intranasal OXT Dose: 24 IU Schedule: single dose Total n doses: 1 Waiting time: 45 minutes (fasting), 175 minutes (postmeal) | Caloric intake (after overnight fast) Caloric intake (post-meal) REE HPA axis (ACTH, cortisol, NE ) Appetite Glycemic excursion Appetite-regulating hormones | Randomized, double-blind, placebo-controlled crossover study of intranasal OXT (N = 20 healthy, fasting men, normal weight), single dose during each treatment block Caloric intake post fast was unchanged, but postmeal was decreased by ~100 kcal with OXT. REE was not changed. ACTH, cortisol, and NE were decreased with OXT. Fifteen-minute postmeal glucose was decreased with OXT. No other changes were noted. |
| A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome (2014)a | 24980612 | Intranasal OXT Dose: 18–40 IU Schedule: bid Total n doses: 112 Waiting time: NA (outcomes are cumulative) | PWS symptoms (behaviors, hyperphagia, social function, sleepiness) Body weight Adverse events | Randomized, double-blind, placebo-controlled crossover study of intranasal OXT (N = 30 individuals randomized with PWS, male and female, aged 12–30 years), each treatment block 8 weeks with minimum of 2-week washout No impact of OXT was observed in any measure except temper outbursts, scored from 0 (not a problem today) to 3 (major problem today). Means were displayed for these scores. Placebo condition: 1–1.5; OXT (all): 2–2.5; OXT (18–24 IU): 1–1.5; OXT (32–40 IU): 2.5–3. Changes were statistically different from placebo for OXT (when all doses combined were tested together vs. placebo) and OXT (when highest doses, 32–40 IU, were tested vs. placebo). |
| Oxytocin reduces caloric intake in men (2015)a | 25865924 | Intranasal OXT Dose: 24 IU Schedule: single dose Total n doses: 1 Waiting time: 60 minutes (breakfast) | Caloric intake (after overnight fast) Appetite Appetite-modulating hormones REE Fat oxidation HOMA-IR Adverse events | Randomized, double-blind, placebo-controlled crossover study of intranasal OXT (N = 25 healthy, fasting men, 13 normal weight, 12 overweight/obese), single dose during each treatment block Over all participants, test meal caloric intake decreased by 122 ± 51 kcal (SEM), fat intake decreased by 8.7 ± 3.8 g, RQ decreased by 0.03 ± 0.01, CCK increased by 10.9 ± 3.1 pg/mL, and HOMA-IR decreased by 0.4 ± 0.2. No change in appetite was observed. No differences in AEs; no SAEs. |
| Oxytocin’s inhibitory effect on food intake is stronger in obese than normal weight men (2016)a | 27553712 | Intranasal OXT: Dose: 24 IU Schedule: single dose Total n doses: 1 Waiting time: 45 minutes (ad libitum test meal), 170 minutes (postmeal snack) | Caloric intake (after overnight fast) Caloric intake (post-prandial snack) REE Glucose/counterregulatory hormones | Randomized, double-blind, placebo-controlled study of a single dose of intranasal OXT (n = 18 obese men, n = 20 normal weight men) OXT decreased post–fast-food intake in obese men only and decreased postprandial snack food intake in both obese and normal weight men. No change in REE was observed. In both groups, OXT decreased post-meal glucose excursion and HPA activity. |
| Promising effects of oxytocin on social and food-related behavior in young children with Prader-Willi syndrome: a randomized, double-blind, controlled, crossover trial (2016)a | 27486141 | Intranasal OXT Dose: 12–24 IU Schedule: bid Total n doses: 56 Waiting time: NA | Social behavior Food-related behavior | Randomized, double-blind, placebo-controlled crossover study of intranasal OXT (N = 25 children with PWS aged 6–14 years) for 4 weeks No significant effects of OXT were observed in the overall group. When the group was stratified by age, improvements in behavior and hyperphagia were seen in those younger than 11 years old, and worsening behaviors were observed in those 11 years and older. No differences in AEs; no SAEs. |
| Oxytocin enhances cognitive control of food craving in women (2016)a | 27381253 | Intranasal OXT Dose: 24 IU Schedule: single dose Total n doses: 1 Waiting time: 30 minutes (fMRI start) | Brain response to palatable food | Randomized, double-blind, placebo-controlled crossover study of intranasal OXT (N = 31 fasting, healthy, non-obese women), single dose during each treatment block OXT increased activity in top–down cognitive control and self-referential processing. |
| Oxytocin improves beta-cell responsivity and glucose tolerance in healthy men (2017)a | 27554476 | Intranasal OXT Dose: 24 IU Schedule: single dose Total n doses: 1 Waiting time: 60 minutes (OGTT start) | Glucose tolerance Insulin homeostasis REE Adverse events | Randomized, double-blind, placebo-controlled crossover study of intranasal OXT (N = 29 fasting, healthy normal weight men), single dose during each treatment block OXT produced a blunted glucose excursion and a more rapid increase in insulin in response to glucose, manifest by >2-fold increase in disposition index, 258 ± 84 (SEM) with OXT vs. 111 ± 34 with placebo. REE was not changed. Systolic BP at 180 min was 129 mm Hg with OXT vs. 122 mg Hg with placebo; otherwise, no difference in AEs; no SAEs. |
| The use of oxytocin to improve feeding and social skills in infants with Prader–Willi syndrome (2017) | 28100688 | Intranasal OXT Dose: 4 IU Schedule: qod, qd, or bid Total n doses: 4–14 | Tolerance Sucking Social skills Ghrelin Brain connectivity | Phase 2 escalating dose study (7 days) of open label intranasal OXT (N = 18 infants with PWS under 6 months, n = 3 in each dose group) No AEs or dose effects were found. Improvements in sucking, swallowing, clinical global impression, social behavior, and mother-infant interactions were noted. Increases in acylated ghrelin and right superior orbitofrontal brain network activation were associated with changes in sucking and behavior. |
| Oxytocin treatment in children with Prader-Willi syndrome: a double-blind, placebo-controlled, cross-over study (2017)a | 28371242 | Intranasal OXT Dose: 16 IU Schedule: qd Total n doses: 5 Waiting time: 60 minutes (safety assessment with heart rate and BP) | Safety parameters Aberrant behavior Social responsiveness Hyperphagia Clinical global impressions | Randomized, double-blind, placebo-controlled crossover study of intranasal OXT (N = 24 children with PWS aged 11–15 years), assessing primarily safety No significant differences in heart rate, BP, sodium concentration, glucose/insulin, body weight were noted between OXT and placebo. With respect to adverse events, nasal irritation (n = 2 instances) and increased irritability (n = 2 instances) all occurred during OXT and were self-resolved. No statistically significant differences in secondary endpoints were found, though nominal improvements were reported related to OXT. |
| Oxytocin and naltrexone successfully treat hypothalamic obesity in a boy post-craniopharyngioma resection (2017) | 29220529 | Intranasal OXT Dose: 6 IU (titrated up from every 3 days to qd, with brief interval at 9 IU qd) Schedule: qd Total n doses: 2660 Waiting time: NA | BMI Z-score Parent-reported observations | A 13-year-old boy with pan-hypopituitarism s/p transcranial resection of craniopharyngioma (8 years before) receiving pituitary replacement (since diagnosis), low-CHO diet (x1y) and dextroamphetamine was started on intranasal OXT × 10 weeks, then naltrexone was added × 38wks. BMI Z-score decreased from 1.77 to 0.82 over the entire OXT treatment time, no AEs were noted except worsening of food-seeking behaviors on 9 IU OXT daily. |
| Oxytocin administration suppresses hypothalamic activation in response to visual food cues (2017)a | 28655900 | Intranasal OXT Dose: 24 IU Schedule: single dose Total n doses: 2 Waiting time: 45 minutes (fMRI start) or 45 minutes (breakfast start) | Brain activation to high calorie foods Ad libitum food intake | Randomized, double-blind, placebo-controlled crossover study of intranasal OXT (N = 24 fasting, healthy, normal weight men and women), single dose during each treatment block (4 visits in total) Decreased activation in response to high-calorie foods was observed in hypothalamus, with no change activation in reward areas, and no change in food intake. |
| First experiences with neuropsychological effects of oxytocin administration in childhood-onset craniopharyngioma (2017) | 28213803 | Intranasal OXT Dose: 24 IU Schedule: single dose Total n doses: 1 Waiting time: 45 minutes (mood questionnaire, then experimental paradigm) | Emotional identification Correct identification of emotions OXT concentrations in saliva & urine | Intranasal OXT was given to N = 10 adults s/p surgical treatment for craniopharyngioma, BMI SDS 1.3–13.4 Increases in emotional identifications (correct assignment of negative emotion categories) were noted in those with anterior hypothalamic defects only. |
| Intranasal oxytocin fails to acutely improve glucose metabolism in obese men (2018)a | 30203535 | Intranasal OXT Dose: 24 IU Schedule: single dose Total n doses: 1 Waiting time: 60 minutes (OGTT start) | Glucose tolerance Insulin homeostasis REE | Randomized, double-blind, placebo-controlled crossover study of intranasal OXT (N = 15 fasting, otherwise healthy, obese men), single dose during each treatment block No differences in outcomes were noted, except a modestly increased insulin concentration at the 30-minute time point only after OXT. |
| Rapid-onset anorectic effects of intranasal oxytocin in young men (2018)a | 30081055 | Intranasal OXT Dose: 24 IU Schedule: single dose Total n doses: 1 Waiting time: 15 minutes (lunch administered), 45 minutes (snack administered) | Caloric intake (snacks) Appetite Mood | Randomized, double-blind, placebo-controlled crossover study of intranasal OXT (N = 20 fasting, otherwise healthy, normal weight, overweight, and obese men), single dose during each treatment block There was no difference in caloric intake at lunch. There was a decrease in both sweet and salty snack intake with OXT. Anxiety did not differ between conditions. |
| Effects of intranasal oxytocin on the blood oxygenation level-dependent signal in food motivation and cognitive control pathways in overweight and obese men (2018)a | 28930284 | Intranasal OXT Dose: 24 IU Schedule: single dose Total n doses: 1 Waiting time: 60 minutes (fMRI start) | Brain activation to high calorie foods | Randomized, double-blind, placebo-controlled crossover study of intranasal OXT (N = 10 fasting, otherwise healthy, obese men), single dose during each treatment block Decreased activation was observed in response to high-calorie foods in hedonic regions (eg, VTA), and hypothalamus; and increased activation in cognitive control areas (anterior cingulate, frontopolar cortex). |
| Oxytocin curbs calorie intake via food-specific increases in the activity of brain areas that process reward and establish cognitive control (2018)a | 29426874 | Intranasal OXT Dose: 24 IU Schedule: single dose Total n doses: 1 Waiting time: 35 minutes (fMRI start—fasted), 75–105 minutes (breakfast), 155 minutes (fMRI start—fed), 195 minutes (snack) | Brain activation to high calorie foods Brain activation to monetary incentive task | Randomized, double-blind, placebo-controlled crossover study of intranasal OXT (N = 15 fasting, healthy, normal weight men), single dose during each treatment block In response to high-calorie foods, OXT treatment was associated with increased activation in cognitive control and reward areas; OXT also increased activation in anticipation of generalized rewards. Effects were confined to fasted state. Caloric intake at breakfast was decreased by ~150 kcal, but at snack was unchanged. |
| Intranasal carbetocin reduces hyperphagia in individuals with Prader-Willi syndrome (2018)a | 29925684 | Intranasal carbetocin (OXT analog) Dose: 9.6 mg Schedule: tid Total n doses: 42 Waiting time: “prior to meals” | Hyperphagia questionnaire (HPWSQ-R) Obsessive/compulsive symptoms (CY-BOCS) Clinical Global Impression | Randomized, double-blind, placebo controlled trial (N = 37; carbetocin, n = 17; placebo, n = 20 children/adolescents with PWS aged 10–18 years) Decreases in hyperphagia and obsessive/compulsive symptoms were noted, greater with carbetocin than placebo. |
“Wait time” refers to time between OXT administration and assessment of primary outcome, where relevant.
Abbreviations: ACTH, adrenocorticotropin; AE, adverse events; bid, two times a day; BMI, body mass index; BP, blood pressure; CCK, cholecystokinin; CHO, cholesterol; CY-BOCS, Children's Yale–Brown Obsessive Compulsive Scale; fMRI, functional magnetic resonance imaging; HOMA-IR, homeostatic model assessment–insulin resistance; HPA, hypothalamic–pituitary–adrenal; HPWSQ-R, Hyperphagia in PWS Questionnaire–Responsiveness; IU, international units; IV, intravenous; LDL, low-density lipoprotein; NE, norepinephrine; OCD, obsessive-compulsive disorder; OGTT, oral glucose tolerance test; OXT, oxytocin; PWS, Prader–Willi syndrome; qd, once a day; qid, 4 times a day; qod, every other day; RCT, randomized controlled trial; REE, resting energy expenditure; SAE, serious adverse event; SD, standard deviation; SDS, standard deviation score; SEM, standard error of the mean; s/p, status-post; tid, 3 times a day; VTA, ventral tegmental area.
aIndicates a controlled study and/or a meta-analysis of controlled studies.
Mechanisms of OXT-induced decreases in appetite and food intake via hedonic pathways
Dopaminergic neurons in the VTA on the floor of the midbrain form part of the brain’s mesolimbic reward system. Palatable food stimuli produce brain activation in the VTA as well as other brain structures in the brain’s reward network, including the insula, nucleus accumbens, hippocampus, and orbitofrontal cortex (84). The specific brain areas engaged by palatable food stimuli and the degree of activation varies according to nutritional status (ie, fed vs. fasted state). Persistent engagement of reward circuits by palatable foods may also explain the drive to eat the absence of hunger. Cells in the VTA express the OXTR (85) and receive projections from OXT-producing neurons in the SON and PVN (86); thus, their role in modulating the effect of OXT on reward-driven eating has been investigated in both model systems and in humans. Direct injection of OXT into the VTA of rats in a social setting decreased subsequent sucrose ingestion that was reversed by pre-administration of an OXTR-blocking agent (87). Also in rats, this time in a nonsocial setting, administration of OXT to the nucleus accumbens, downstream from the VTA, decreased sucrose intake. In the latter experiment, the effect of OXT was reversed by either concurrent administration of an OXTR blocker and/or return to a social setting (88). This finding presents the intriguing possibility that social stimulation alters responsiveness of feeding behaviors to OXT. In humans, in overweight or obese men, a single dose of 24 IU of intranasal OXT reduced activation in the VTA and other brain areas involved in regulation of “hedonic” or reward-driven food intake (orbitofrontal cortex, insula, globus pallidus, putamen, hippocampus, and amygdala) in response to images of high-calorie foods (83). However, separate studies of normal weight individuals using the same dose of intranasal OXT found either no effects on reward circuits in a study including both men and women (82) or increased activation of reward circuits in a study including only men (89). Thus, the acute effects of OXT on reward-related eating in humans may depend on experimental paradigm and/or metabolic/hormonal milieu.
Circadian variation in OXT and effects on energy intake
Oxytocin secretion and response are related to food intake, a behavior that has clear circadian variation. As a result, the potential interactions between circadian and/or ultradian rhythms and OXT signaling have been studied. Findings in wild-type, chow-fed mice are consistent with a role for OXT in the circadian regulation of eating behavior (90). That is, there was a daytime rise in peripheral circulating OXT (corresponding to when mice are resting and not eating) and a nighttime fall in peripheral circulating OXT (corresponding to when mice are awake and eating). In contrast, in mice fed HFD, there was a loss in this circadian secretion of OXT. Specifically, OXT production was decreased during the usual rest phase in mice fed a HFD, which may explain why animals ate more during this time. Further supporting a causal role for OXT in these eating patterns, daytime injection of OXT into the third ventricle restored physiologic circadian variation in eating and led to an adaptive increase in energy expenditure in the context of a HFD, with a reduction in associated weight gain. In these experiments, OXT administered in the early part of the light cycle (vs. in the early part of the dark cycle) produced more robust increases in energy expenditure and decreases in body weight and fat mass (38). Peripheral administration of exogenous OXT also decreased weight gain in this model. It is also possible that food ingestion is a circadian cue that itself leads to OXT secretion. For example, in rats, food ingestion led to increases in OXT, as did exogenous administration of the entero-endocrine hormone CCK (57).
In humans, we might expect that if circadian variation in circulating OXT contributes to diurnal eating patterns analogously to mice and rats, then peripheral OXT concentrations would be expected to be lowest during the day and highest at night. In contrast to this expectation, in a study of 6 humans with indwelling cerebrospinal fluid (CSF) catheters (inserted for the clinical indication of CSF rhinorrhea), with sample collection every 6 hours for 30 hours, the highest OXT concentrations were found at 12 pm (91). A corresponding peak at 12 pm was not identified in peripheral blood samples from a subset of these individuals, nor was a 12 pm peak found in peripheral blood samples from healthy volunteers (n = 6 female, n = 6 male). At least 1 study has tested for evidence of circadian variation by examining within-participant differences in 12 am versus 7 am measurements in women (92). No time-of-day differences were found in any of the subgroups studied. Assessments may be complicated in humans by evidence that OXT is secreted in a pulsatile manner, such that high-frequency sampling (~every 5 minutes) may be required to fully appreciate these patterns (93). In addition, results of OXT measurements in peripheral blood may diverge from those made in tissues potentially more relevant to OXT signaling (eg, CNS).
OXT and macronutrient preference
In addition to impacting the timing of eating, OXT can also affect the choice of food that is consumed, that is, relative preferences for fat, carbohydrate, and protein. Oxytocin suppresses food intake in a variety of animal models, including rats, mice, and rhesus monkeys, when they are fed diets containing standard chow, which usually has a substantial proportion of calories from carbohydrates (29, 78, 94–97). In these same models, OXT has been found to reduce intake of sucrose (88, 98, 99), glucose (100), fructose-sweetened beverages (101), and HFDs sweetened with sucrose (78, 90, 94, 95, 102–105). Oxytocin has also been shown to suppress energy intake in animals fed HFDs without sucrose (67). Oxytocin appears also to have different effects on macronutrient preference depending on whether it is administered in the fed or fasted state. For example, in 1 study, CNS administration of OXT to rats that had been fasted for 20 hours led to a reduction in the subsequent consumption of glucose, but this effect was not observed in fed rats (100). In a follow-up study, chronic consumption of sugar by rats led to an overall decrease in hypothalamic OXT production, leading the authors to speculate that individuals who chronically consume high amounts of sugar might have diminished OXT-based satiety signaling. Humans with central melanocortin signaling defects offer another interesting case. Given the relationship between melanocortin signaling and OXT, individuals with central melanocortin signaling defects might also be expected to have decreased α-MSH induced stimulation of hypothalamic OXT secretion. These individuals have a strong preference for high-fat foods, but a reduced preference for sucrose (106). The direct effects of exogenous OXT on macronutrient preference in humans are discussed in more detail in a subsequent section, when we consider the therapeutic potential of OXT for obesity.
Energy Expenditure and Body Composition
Whole body energy expenditure
In addition to affecting energy intake, OXT has an important role in modulating energy expenditure and body composition. Multiple studies have found that exogenous OXT produced more weight loss than would be expected based on its impact on food intake alone (68), suggesting that OXT may also increase energy expenditure. For example, pair-feeding experiments in genetically obese leptin-deficient ob/ob mice indicated that decreased food intake was insufficient to explain weight loss related to exogenous OXT treatment (68). In a different study of diet-induced obese rats, OXT treatment also produced weight loss even when food intake was not altered (104). These findings were recapitulated in diet-induced obese rats (95, 104). Multiple studies in animals have also indicated that OXT-mediated weight loss is maintained even after its effects on food intake have waned (103). In rodents, OXT administration into the VMN (107) or third ventricle (78, 90) was sufficient to increase energy expenditure measured directly. In male rhesus monkeys, twice daily subcutaneous OXT also increased energy expenditure (101). Exogenous OXT administration has not been shown to increase locomotor activity (67, 94, 95), thus additional studies have focused on other potential factors contributing to increased energy expenditure at rest, including SNS activity, fat oxidation, and body temperature.
Sympathetic nervous system activity, lipolysis, and temperature regulation
Relevant to its potential effects on energy expenditure, OXT can promote lipolysis by increasing SNS activity and/or acting directly at the level of adipocytes (67). Specifically, OXT appears to drive SNS activity, leading to thermogenesis in interscapular brown adipose tissue and lipolysis in white adipose tissue. Alternatively, and/or in addition, OXT has been found to produce lipolytic effects by interacting directly with OXTRs present on target adipocytes (104). Anatomic evidence also supports the existence of a regulatory connection between OXT-producing neurons in the PVN and interscapular brown adipose tissue (108) or white adipose tissue (109, 110). Circulating levels of adrenaline are also lower in mice homozygous for deletions of OXT, suggesting that decreased SNS tone may contribute to their late-onset obesity phenotype (61).
While OXT appears to increase resting energy expenditure, as described previously, OXT signaling does not appear to directly affect spontaneous physical activity. For example, mice homozygous for deletions of the OXTR do not demonstrate appreciable changes in voluntary activity (62). Further, exogenous OXT administration has not been shown to increase locomotor activity (67). Importantly, mice lacking in OXT or its receptor also have deficits in cold-induced thermogenesis (62, 111), consistent with an inability to appropriately increase sympathetic tone and burn energy in response to cold temperatures. In mice lacking the OXTR, brown adipose tissue also contained large lipid droplets, suggesting reduced fatty acid oxidative capacity (62). Ablation of OXT-producing PVN neurons leads to a reduction in temperature in both adipose tissue as well as overall body temperature in response to cold (112). In contrast, OXT administration has been shown to increase brown fat temperature as well as overall core temperature (29, 113).
Effects of OXT on fat mass
Interventional studies have also indicated that OXT-induced increases in lipolysis lead to decreases in fat mass. In particular, studies in animals have shown that OXT treatment reduces fat mass while either preserving or increasing lean mass (29, 67, 71, 90, 104, 114). In addition, animal studies suggest that specific fat depots (ie, visceral vs. subcutaneous) may be more affected than others by treatment with OXT. Although there are limitations in the translatability of studies between species, some of white adipose tissue depots in mice may reflect metabolically relevant visceral fat depots in humans (115). The fat-burning, muscle-sparing effects of OXT have been replicated in multiple different animal models of obesity, both nutritional and genetic. For example, in mice fed a HFD, 10 days of chronic infusion of subcutaneous OXT led to a reduction of both visceral and subcutaneous fat pads in male mice, but a reduction in only subcutaneous fat in female mice (103). In a genetic model of obesity (the ob/ob mouse), 14 days of chronic infusion of subcutaneous OXT led to a reduction in visceral fat and preservation of lean mass. In contrast, ablation of the OXT-producing PVN neurons led to decreased energy expenditure and increased fat mass without any change in lean mass; effects were specific to male mice in this study (77). Mice lacking OXT also have larger abdominal fat pads (61) and mice lacking the OXTR have increased volume of multiple visceral adipose depots, including perirenal, epididymal, and mesenteric fat pads (94). Fat loss in response to exogenous OXT may also be confined to specific adipose depots; for example, in rodents, 13 to 14 days of subcutaneous OXT reduced adipocyte size (116) and area (94) of visceral depots. In another study, 12 weeks of subcutaneous OXT reduced adipocyte size in subcutaneous as well as multiple visceral depots, including perirenal and epicardial fat pads (71). Taken together, these studies suggest that the decreased body weight in response to OXT occurs as a result of loss of fat mass, not lean mass, and the loss of fat mass may come disproportionately from the metabolically harmful visceral fat. Although sexually dimorphic effects have been reported, a more recent study in diet-induced obese mice reported equal decreases in both visceral and subcutaneous fat in males and females (103). The translational relevance of OXT for use in human obesity is addressed in a separate section.
OXT effects on muscle and bone
Oxytocin receptors are present on mesenchymal stem cells (117, 118), myoblasts (119), osteoblasts (120), osteoclasts (121), and, as might be expected, OXT has direct effects on muscle and bone (122, 123). In mice, genetic lack of OXT results in premature sarcopenia and bone loss, both of which are reversible with OXT administration (124). Although OXT treatment has been shown to promote the loss of fat mass while sparing lean mass in animals, less is known about the direct effects of OXT on muscle. In animal models that lack OXT, development of muscle appears unchanged, but with aging, there is a loss of muscle stem cell regenerative capacity via mitogen-activated protein kinase/extracellularly regulated kinase-mediated muscle stem cell proliferation (124).
The effects of OXT on bone may also derive from its effects on cell lineage. In particular, OXT appears to shunt common mesenchymal stem cell precursors toward osteoblast instead of adipocyte lineage (122). Oxytocin also has been shown to stimulate proliferation of osteoblasts (125). Oxytocin restores lost bone mass and reduces fat gain in female ovariectomized mice, but does not improve bone parameters in male orchidectomized mice. One possible explanation for these findings is that the anabolic effects of estrogen on bone could require OXT signaling (126). Estrogen status is established as a primary determinant of bone health, even in men (127). Osteoblasts typically produce OXT in response to estrogen (11). Then, in a “feed-forward” response, OXT binds to its own receptor and further enhances OXT production (this phenomenon has been observed in cells from both male and female mice) (11). It is also possible that the “feed-forward” interaction between estrogen and OXT plays a role in the recovery of depleted bone mineral density following pregnancy. In women in later phases of lactation, estrogen concentrations begin to rise and the local OXT-mediated anabolic response would be expected to promote increases in bone mineral density. Finally, OXT and vasopressin have been proposed to have opposing effects on bone (ie, OXT is anabolic while vasopressin is catabolic), and the 2 signaling systems may interact to influence bone mass regulation (128). More investigation is needed to understand the potential interactions between these closely related hormones on bone.
Evidence in humans on the interaction between OXT and bone extends these findings from model systems. In humans, there is evidence of positive associations between peripheral circulating OXT concentrations and bone mass, particularly in females (129). Indeed, most of the studies on OXT and bone have focused on women. One study that did include men did not detect an association between OXT and bone density, but did find a weak negative association between peripheral OXT concentrations and fracture risk (130). There is also evidence that OXT mediates the effects of nutritional status on bone in both men and women (92, 130, 131). For example, lower nocturnal OXT levels in female athletes with low percentage of body fat were associated with worse bone microarchitecture, in particular at the distal radius (91, 132). Positive associations have also been observed between OXT concentrations and muscle mass in females (129). In obese individuals, preservation of OXT production may be associated with anabolic benefits for both muscle and bone; in contrast, when OXT signaling declines, as may occur in metabolic decompensation (eg, type 2 diabetes), lack of OXT may lead to adverse effects on muscle and bone health (133). The many complex relationships between OXT, sex steroids, metabolism, and bone remain to be elucidated (122, 134).
Glucose and Lipid Homeostasis
Rationale for investigation of effects of OXT on carbohydrate and lipid metabolism
The demonstrated effects of OXT on energy balance suggest that OXT may also affect fuel utilization and nutrient homeostasis. Insights regarding the effects of OXT on glucose and lipid fluxes can be gleaned from studies in both model organisms and humans. As might be expected in the setting of excess weight gain, mice lacking OXT demonstrate decreased insulin sensitivity and increased glucose excursions (61) and mice lacking the OXTR have increased abdominal fat pads and increased triglycerides (62). The constellation of abnormalities that occurs in the absence of OXT signaling is evocative of human metabolic syndrome. In addition, OXT deficient mice have larger abdominal fat pads (61), recapitulating the increased waist circumference that is observed in human metabolic syndrome. As a result, OXT has been considered as a potential therapy for obesity-related comorbidities including diabetes mellitus and dyslipidemia, and its effects have been studied in both animal models and humans.
Insights from model systems: glucose flux
In a mouse model of prediabetes (C57BL/6 mice fed a HFD for 2 months), 2 doses of exogenous OXT (separated by 12 hours) administered via injection into the brain’s third ventricle, modified glucose homeostasis without altering body weight (135). Specifically, in this model, fasting insulin levels were decreased without a change in fasting glucose, consistent with improved insulin-induced suppression of hepatic gluconeogenesis. In addition, glucose excursion following an enteral glucose load was decreased. In a model of pancreatic beta-cell deficiency (C57BL/6 mice fed normal chow, receiving streptozocin injections), 7 days of centrally administered OXT produced a modest decrease in body weight; insulin levels were increased while fasting and postprandially, leading to reduced glucose excursion on oral glucose tolerance test (135). However, in a different study, OXT treatment of ob/ob mice led to worse glucose tolerance despite loss of fat mass; worsening glycemia was attributed to increased production of corticosterone and increased hepatic gluconeogenesis (68). Oxytocin analogs (generated by either an amino acid substitution, lipid conjugation, or both) have been developed with longer half-life and increased specificity for the OXTR and have been shown to decrease body weight and improve glucose tolerance (136) while avoiding some of the increase in blood pressure that is attributed to off-target binding to the vasopressin receptor.
Insights from model systems: lipid flux
Other studies have also examined how the effects of OXT on insulin sensitivity may be mediated by changes in lipid flux. In a rat model of diet-induced obesity, exogenous OXT increased expression of stearoyl-coenzyme A desaturase 1 and tissue content of oleoyl-phosphatidylethanolamine, which is the precursor of oleoylethanolamide, a known activator of peroxisome proliferator-activated receptor-α (PPAR-α) (104). PPAR-α is a key regulator of mitochondrial fatty acid oxidation. In animals deficient in PPAR-α, OXT failed to induce weight loss and lipolysis, suggesting that the effects of OXT may be dependent on PPAR-α. In this study where OXT was administered centrally, increased peripheral levels of OXT were also detected, thus OXT may have been acting directly via OXTRs present on adipocytes. Peripherally administered OXT also decreased liver fat and visceral fat mass and improved glucose homeostasis in a mouse model of diet-induced obesity; weight loss occurred in this model at least in part via decreased food intake (94). Evidence of increased fat oxidation (decreased respiratory quotient at rest) was also observed. Oxytocin analogs have also been found to increase lipid oxidation in mice (136).
Endogenous OXT concentrations in humans
Some observational studies have tested for associations between circulating concentrations of OXT and metabolic status in humans, and a clear consensus has not yet emerged. For example, in one study, peripheral OXT levels were ~4-fold higher in obese individuals relative to normal-weight controls and fell after gastric banding (40). Other studies have also found a positive association between peripheral OXT concentration and adiposity status in women, as reflected by body mass index (BMI) and/or total, visceral, or subcutaneous fat (129). In this work, peripheral concentrations of OXT were highest in obese women, intermediate in normal weight women, and lowest in women with anorexia nervosa (129). Similarly, a different observational study in adult men reported higher OXT levels in individuals with metabolic syndrome (137). However, in other studies, associations in the opposite direction have been reported. For example, independent of obesity, decreased levels of OXT have been reported in adults with type 2 diabetes mellitus (T2D) (138) and children with metabolic syndrome (139). Another study in African-American men found that individuals with T2D had lower levels of urinary OXT, assessed by ELISA (140). In these studies, it may be that the development of T2D is associated with decreased OXT production. In the same cohort, investigators also reported an association between OXT and gut microbiome speciation (141).
Oxytocin status has also been investigated in individuals who have had brain tumors affecting the hypothalamus and pituitary, most notably craniopharyngioma, because altered OXT secretion could plausibly contribute to the substantial obesity that can occur after surgical treatment and hypothalamic damage in many of those affected (142, 143). In individuals with craniopharyngioma, the change in salivary OXT in response to meals (144) and exercise (145) appears to be related to BMI and eating behaviors (146). In particular, a larger decrease in OXT after meals occurred in individuals with craniopharyngioma and higher BMI. In the preceding study, no association was detected between change in salivary OXT in response to meals and BMI in healthy controls. However, in a different study of healthy girls and women (aged 10–45 years), greater postprandial decreases in peripheral OXT were associated with higher postmeal ratings of hunger and lower postmeal ratings of fullness (36). With respect to fasting measurements, no differences between individuals with craniopharyngioma and controls were appreciated in fasting salivary OXT (144).
The complex findings in humans recapitulate some of the studies in animal models. Whereas 1 study found lower concentrations of serum OXT in mice with diet-induced obesity relative to mice consuming regular chow (135), another study did not detect a difference in OXT concentrations using a similar nutritional approach (95), and a third study detected no difference between rats fed with low-fat versus HFD (103). The duration of exposure to HFD varied across these approaches and could explain observed differences.
Overall, differences in cohorts, sample collection, and OXT measurement technique could contribute to heterogeneity in these reports. Accounting for pulsatility of OXT secretion (35) might also be important and could explain differing findings across various investigations. Some investigators have posited that increased circulating levels of OXT could reflect a degree of unresponsiveness, that is, “resistance” to OXT and/or the presence of abnormal/less functional forms OXT in some conditions (eg, Prader–Willi syndrome [PWS]) (147). In addition, OXTR capacity for recycling (148) may influence its sensitivity and responsiveness to therapy. In one study in rats, although HFD had no effect on OXT or vasopressin 1a receptor binding in forebrain or hindbrain, chronic OXT treatment decreased OXT and vasopressin 1a receptor binding in the forebrain, while hindbrain OXTR binding was unchanged (149). The possibility that decreased peripheral OXT concentrations may actually reflect increased central OXT concentrations in appetite circuits has also been raised, for example (147, 150). Discordant concentrations of central (vs. peripheral) OXT may occur when α-MSH signaling via anorectic MC4R pathways that decrease food intake (4) leads to increased local dendritic release of OXT (shown in Fig. 2) and decreased systemic circulating OXT.
To overcome the limitations in sensitivity of random and/or fasting measurements of OXT concentrations, provocative testing using the response to insulin-induced hypoglycemia has also been performed to test OXT status in humans. For example, in 1 study investigators found an attenuated increase of OXT to insulin-induced hypoglycemia in obese individuals (as compared to lean individuals) that was restored following weight loss (151). As a follow-up to this study, the opioid antagonist drug naloxone was also found to generate an ITT-induced increase in OXT in obese participants that approached the increase observed in nonobese participants (152).
Interventional studies in humans
In humans, there have also been several studies investigating the effects of acute OXT administration on glucose homeostasis. In one study of healthy adults (n = 6 per group), intravenous administration of 6 IU (but not 3 IU) OXT increased insulin secretion in response to an intravenous glucose challenge (153) but did not lead to changes in circulating counterregulatory hormones. In another study of healthy normal weight men (n = 29), 24 IU of intranasal OXT increased the pancreatic β-cell responsiveness to oral glucose, manifesting as increased acute insulin secretion and modestly decreased peak glucose levels (154). On the basis of these types of results, the potential of OXT as an antidiabetic therapy has been proposed. However, a follow-up study using a similar paradigm in obese men did not demonstrate effects on insulin or glucose excursions, pancreatic β-cell responsiveness to oral glucose or insulin sensitivity (155). In exploratory analyses, the investigators did find that endogenous OXT concentrations were positively associated with homeostatic model assessment–insulin resistance in obese men (but not normal weight men). Insulin concentrations were also slightly higher after OXT at the 30-minute time point only. The investigators found that postdose OXT concentrations were lower in obese men as compared to normal weight men, raising the additional possibility of differences in exogenous and/or endogenous OXT metabolism in the setting of obesity. Also, they situate their finding in the ongoing discussions regarding the relationship between peripheral OXT concentrations with adiposity status; while some studies suggest a positive association between peripheral OXT and body weight (eg, (129)), others suggest peripheral OXT concentrations may be lower in individuals with abnormal glucose homeostasis (eg, (138)). Oxytocin also appears to exert both central and peripheral actions, and its integrated effects may depend on baseline nutritional status. In a previous investigation in humans, OXT was found to potentiate glucose-stimulated insulin secretion as well as glycogenolysis and glucagon release, such that the glycemic excursion would depend on the net effects in a given individual (156). In the previously referenced study of obese men, OXT was not found to affect other potential mediators of metabolic effects, including hypothalamic–pituitary–adrenal axis activity, glucagon, nonesterified fatty acid concentrations, energy expenditure, hemodynamic measures, or psychological parameters (155). In addition, an 8-week clinical trial of intranasal OXT in obese adults did not produce detectable effects of OXT treatment on glucose homeostasis despite OXT-related weight loss (135). This complexity highlights the need for additional investigation before the translational potential of OXT as an antidiabetes therapy is determined.
Oxytocin and the gut microbiome
Gut microbes have been proposed to influence human metabolism in multiple ways, including by participating in the digestion of ingested nutrients and by producing molecules that cross the gut–blood barrier and have roles in metabolic signaling. Several studies in animals have suggested that dietary supplementation with feeding with Lactobacillus reuteri and/or dietary supplementation with the sterile lysate of this microbe leads to increased circulating OXT concentrations and a corresponding increase in the number of OXT-producing cells in the caudal PVN (157).
In humans, an association has been reported between high Dialister abundance and higher circulating OXT concentrations in nondiabetic African-American men (141). Interventional studies in humans have not yet demonstrated a direct causal relationship between changes in gut microbiome ecology and OXT production; thus, this is an area for potential future research.
A Focus on Therapy
OXT in models of diet-induced obesity: central administration
Animal models have yielded useful insights into the translational potential of OXT for obesity treatment. In rats with diet-induced obesity, centrally administered OXT led to decreased body weight, increased lipolysis and fatty acid oxidation, and increased insulin sensitivity and glucose tolerance (104). Notably, centrally administered OXT also typically produces increased peripheral concentrations of OXT, suggesting at least some of the observed metabolic effects may be, at least in part, peripherally mediated.
The metabolic effects of centrally administered OXT may be dissociable depending on the neuroanatomical circuits that are engaged. For example, when OXT was administered in rodents only in the fourth cerebral ventricle, which signals to OXTRs in the hindbrain, weight loss occurred related to decreased energy intake, as well as a possible contribution from increased energy expenditure. Relevant to the latter possibility, OXT-related weight loss coincided with increases in brown fat temperature; thus, increased energy expenditure may have occurred from nonshivering brown adipose tissue thermogenesis (29). However, when animals lacking the OXTR only in the hindbrain/NTS were given OXT, their core temperature increased, but they did not demonstrate the same OXT-mediated inhibition of food intake transmitted by GI satiation signals, suggesting that the NTS may be required for OXT-related inhibition of food intake but not to increase energy expenditure via changes in brown adipose tissue thermogenesis (96, 113). In interpreting data using different routes of OXT administration, it is important to consider that central exogenous OXT administration may also lead to an increase in endogenous OXT secretion and subsequent release of OXT into the periphery via a “feed-forward” loop (29).
OXT in models of hypothalamic obesity: central administration
Exogenous OXT is expected to be most helpful in forms of obesity where OXT deficiency contributes to the pathophysiology of excess adiposity. Mice with haploinsufficiency of the gene single-minded 1 (Sim1+/–), which exhibit abnormal development of the hypothalamic PVN, 1 site of OXT-producing neurons (Fig. 2), and a reduction in hypothalamic OXT production by around 80%, offer an illustrative example (158). Sim1+/– mice develop hyperphagia and obesity, and treatment with an intracerebroventricular OXT analog has been shown to reduce food intake and decrease weight gain in these mice (158). Thus, centrally acting OXT has exciting translational potential in this particular mouse model of human obesity driven by hypothalamic dysfunction. This result highlights the potential utility of centrally administered OXT in forms of obesity due to hypothalamic dysfunction, including related to brain tumors such as craniopharyngioma (159, 160), PWS (161, 162), and PWS-like syndrome related to Sim1 deficiency (163).
OXT in models of diet-induced obesity: peripheral administration
Given potential feasibility challenges posed by central administration of OXT, the effects of peripheral administration have also been explored. In rats with diet-induced obesity, peripherally administered OXT produced a dose-dependent decrease in food intake and body weight (95). These metabolic benefits were also demonstrated in obese Koletsky (fa(k)/fa(k)) rats with deficiencies of the leptin receptor, indicating that OXT does not require intact leptin signaling to produce weight loss in these models. In addition, peripheral OXT produced an increase in neuronal c-Fos induction, suggestive of neuronal activation, in both the NTS and area postrema, 2 hindbrain areas that may be responsible for decreased food intake and preserved energy expenditure in response to OXT. The effects of exogenous OXT, whether administered centrally or peripherally, has also been found to vary according to adiposity status (lean vs. obese) and nutrient milieu (diet composition). In one study, the effects of exogenous OXT administered into the fourth ventricle were more apparent in diet-induced obese animals fed a HFD as compared to lean control animals receiving a low-fat diet. Other studies have also found that OXT is more effective at decreasing energy intake, attenuating weight gain, and/or promoting weight loss in HFD-induced obese mice (67, 78, 94, 103) or rats (67, 104), as compared to control animals fed normal chow. In a separate study in mice, peripheral administration of OXT decreased food intake to a similar extent in animals fed normal chow versus a HFD, although the effects of a single dose persisted longer in those receiving a HFD (94). Oxytocin administered peripherally also reduced liver fat and improved glucose homeostasis in animals fed a HFD (94). Peripheral administration of OXT both in rats (116) and mice (94) also preferentially reduced the size of visceral adipose tissue depots. Oxytocin analogs have been generated by either an amino acid substitution, lipid conjugation, or both, with the rationale that these will have increased specificity for the OXTR, thus avoid unfavorable effects of binding to the vasopressin receptor (eg, increased blood pressure), and have a longer half-life. Treatment with OXT analogs in mice produce decreases in body weight and improvements in lipid profile and glucose tolerance (136).
OXT administration in nonhuman primates
Results of studies in rodent models may not directly transfer to primates, therefore, another key study tested the capacity of OXT to produce weight loss in nonhuman primates. In these monkeys, 4 weeks of subcutaneous OXT administered twice daily produced weight loss as a result of decreased food intake and increased energy expenditure (101). Oxytocin-treated monkeys also had higher concentrations of circulating free fatty acids and glycerol and decreased concentrations of triglycerides suggestive of higher rates of fat breakdown.
Summary of studies in model systems
In summary, studies in animals have shown that OXT can reduce fat mass while either preserving or increasing lean mass (71, 90, 104, 114). The route of administration of OXT is an important consideration in relation to potential translation to humans. In rats, central administration of OXT leads to an increase in detectable OXT concentrations in the periphery (104). In mice, intranasal and intraperitoneal administration of OXT also leads to increased concentrations of OXT in peripheral blood, and it is not clear whether these reflect that exogenous OXT has reached the central nervous system. Alternatively and/or in addition, increased peripheral OXT concentrations in response to exogenous OXT could reflect OXT-stimulated central endogenous OXT release (90). Indeed, several studies have proposed that exogenous OXT potentiates endogenous OXT release, although at least 1 study in nonhuman primates did not detect this phenomenon (164). As a result, it is challenging to discern which specific anatomic and/or functional site(s) mediate the effects of exogenous OXT action. Also, there may be neuroanatomic site-specific effects of OXT action. For example, effects on nutrient intake and thermogenesis may be produced by distinct signaling mechanisms (113).
Studies performed in specific gene knock-out animal models suggest that at least some of OXT effect(s) may require intact PPAR-α signaling (104), but may not require intact leptin signaling (95). Oxytocin has been shown to produce weight loss even in the setting of abnormal leptin signaling, as can occur in animal models of diet-induced obesity (78, 94, 95, 103, 104) and animal models of leptin deficiency or leptin receptor mutations (165). These insights regarding the effect of OXT in the setting of abnormal leptin signaling are relevant to the therapeutic potential of OXT in humans with diet-induced obesity and high circulating leptin concentrations. Finally, OXT effects may also depend on adiposity status, usual diet, sex, and other factors that as of yet are undiscovered.
Rationale for investigation of OXT in humans
Promising results in model systems have led to interest in the therapeutic potential of OXT for humans with metabolic disorders. In observational studies in humans, genetic variation in OXT signaling has been proposed as a risk factor for the development of obesity. For example, in 1 study, genetic copy-number variants in the OXTR were associated with severe, early-onset obesity (166). Also, the potential prosocial effects of OXT have been investigated in other neurodevelopmental and psychiatric conditions (167). This considerable body of research is beyond the scope of this review, but it is plausible that the social effects of OXT could be related to its metabolic effects, for example, by influencing eating patterns, response to stress, and physical activity, all complex behaviors with social dimensions. These insights, along with the foundational studies of OXT physiology in model systems and in humans, have led to excitement about the possible use of OXT as a therapy for human obesity and obesity-related comorbidities. A summary of trials performed to date is presented in Table 1 and details are discussed later.
There are several areas of uncertainty with respect to OXT therapeutics in humans that are important to bear in mind in critically evaluating the results of initial human studies. First, pharmacologic parameters (eg, pharmacokinetics and pharmacodynamics of various doses and routes of administration) (168–170) and tolerability (171, 172) of OXT are the focus of continuing investigation and will be discussed in subsequent sections. In addition, in animal models, OXTR downregulation in the forebrain, but not hindbrain, has been demonstrated in response to chronic OXT administration that may impact outcomes in response to prolonged OXT therapy (149). Thus, the issue of desensitization/tachyphylaxis in chronic administration is also potentially relevant. Weight loss studies have shown effect of OXT to ~6 weeks in mice (eg, (90)) and ~8 weeks in humans (eg, (135)); thus, it remains to be determined the extent to which neuron-specific OXTR expression changes modulate metabolic effects of chronic OXT treatment. Finally, the optimization and standardization of techniques for measurement of OXT concentrations, both endogenous and exogeneous, in peripheral blood (173, 174) will allow between-study comparisons to be made more readily.
OXT administration in humans: route of administration
The practical aspects of OXT administration in humans have implications for translatability of studies in model systems. Pharmacologic OXT is more challenging to administer enterally because it is readily broken down in the gut, likely by chymotrypsin and/or other proteases (175). Pharmacologic OXT can also be given intravenously and/or intramuscularly, as for induction of labor (168, 169). In some parts of the world, OXT administered intranasally is used to promote lactation (in doses of 4 IU); intranasal OXT was previously Food and Drug Administration–approved in the United States for lactation but was voluntarily withdrawn, not related to any safety signals (171). Intranasal OXT has been posited to produce both central and peripheral effects in humans; however, there at least 2 important considerations related to this hypothesis (176). First, an increase in OXT concentration detected in the CSF does not necessarily reflect that intranasal OXT has reached potentially responsive areas of brain parenchyma and in physiologically relevant quantities. Second, this route of delivery may lead to supraphysiologic concentrations of OXT in the periphery whose effects are important to consider. In humans, studies have measured peripheral concentrations of OXT following intranasal OXT administration, for example (160), and/or sought evidence of central effects of intranasal OXT using functional magnetic resonance imaging of the brain, for example (82, 83). In one study in humans, mean CSF concentrations of intranasally administered vasopressin, which is structurally similar to OXT, began to rise within 10 minutes of intranasal administration and lasted for up to 80 minutes or longer (177). Delayed clearance of OXT from CSF has been proposed as one potential mechanism for persistence of increased CSF OXT after ~70 min (178). The site of CSF sampling may also be relevant, depending on how intranasal OXT either travels to CSF and/or induces endogenous OXT secretion. In a study of rhesus macaques (n = 4, all female), OXT was administered at doses of 0.1 IU/kg, 1 IU/kg, and 5 IU/kg via intravenous and intranasal routes in a cross-over design, and concurrent measurements of CSF and plasma OXT were made throughout. For intravenous administration, there was a dose-dependent increase in plasma OXT (peak at 5 minutes, return to baseline by 120 minutes); at the 5 U/kg dose only, CSF OXT peaked at 15 minutes and returned to baseline by 120 minutes. For intranasal administration, there was no change in plasma OXT; at the 5 U/kg dose only, an increase in CSF OXT was detected at 15 to 30 minutes (179). Adding to this complexity, it has also been suggested that aerosolization increases exogenous OXT delivery to the CSF relative to intranasal OXT in nonhuman primates, and variability in permeability of the blood–brain barrier in different brain regions could also lead to variability in exogenous OXT passing from the periphery to central areas (180).
OXT administration in humans: safety considerations
Safety is another important consideration for studies in humans. The safety of intranasal OXT in adults has been reviewed (171). This review represents a collection of studies in 1529 subjects (72% male). The authors identified and summarized 3 case reports of hyponatremia related to OXT. In 2 out of these 3, hyponatremia was demonstrably related to excess fluid intake while continuing OXT (in one case, an individual received intravenous fluids for viral gastroenteritis, and in a second case was taking amounts of OXT more than as prescribed and was also consuming approximately 5 L fluids daily). Otherwise, no reproducible side effects have been observed in mostly short-term studies of doses of 18–40 IU per day. In at least 1 longer-term adult study (n = 59), participants received 40 IU 2 times per day for 13 weeks, representing a larger net exposure (181). In a weight loss study in adults, nine were administered 24 IU of OXT 4 times per day for 8 weeks and reported no serious adverse effects (AEs) (135). The safety of intranasal OXT in children across a range of indications has also been reviewed (172). No significant side effects were reported in any of the 6 pediatric studies included, in which OXT was administered at variable doses and durations, ranging from a single dose of 12 units to 24 IU twice daily for 8 weeks. In another review of 11 studies, including 8 randomized controlled clinical trials, again with variable doses/durations of up to 40 IU bid for 8 weeks, no clear association could be identified between OXT and any specific adverse event, although documentation of both drug adherence and AEs were noted to be inconsistent in these studies (182). The authors note the lack of consistent reporting of AEs, including their potential relationship to study drug, is a significant limitation of the current literature and make recommendations for improved reporting in future studies. Their recommendations relate not only to standardized collection of AEs, but also to making multi-modal assessments of adherence, and gathering more information regarding the implications of variations in formulation and delivery of intranasal OXT. One particularly important recommendation is to consider use of a safety monitoring uniform report form that systematically gathers information regarding potential adverse events via a brief general inquiry, a drug-specific inquiry, and a body system review (183).
As per the package insert of intranasal OXT (Syntocinon, Novartis), side effects of intranasal OXT may include nasal irritation, runny nose, or tearing of the eyes, as well as an allergic reaction. Also listed are rare side effects, reported in single cases, and of unknown relationship with medication, including bleeding, convulsions, nausea, and drowsiness. Headache and mood changes are also listed as rare side effects, however in contrast there are also studies proposing benefits of OXT with respect to migraine headache (184), mood (185), and social functioning (186). Uterine contractions could occur in women, related to the known role of OXT in parturition, and would be expected to be more likely in pregnant women, especially with the approach of parturition. Oxytocin receptor expression may be modulated by the substantial rise in estrogen that occurs during pregnancy (187). The time course of the change in OXTR concentration reflects the important role of OXT in parturition; myometrial OXTR concentrations are substantially higher at 37–41 weeks of gestation (vs. 13–17 weeks), and increase even more with the onset of labor (188). In animal models, OXTRs also seem to be more highly expressed on the vasculature at the time of parturition (189). Thus, late pregnancy may generate a unique uterine responsiveness to OXT in women. When considering the therapeutic potential of OXT, late pregnancy is also a stage at which women are aware of their pregnancies and treatment decisions can readily be made in context. Oxytocin may have other effects when administered pharmacologically that are related to its known physiologic properties. In individuals who may also be receiving anti-diuretic therapy for diabetes insipidus, for example, those with hypopituitarism and hypothalamic obesity related to craniopharyngioma, iatrogenic hyponatremia is a potential risk. Cardiovascular side effects, including blood pressure and heart rate changes and potential to prolong the QTc interval have been noted when the parenteral form of OXT is administered to women to promote parturition; in these cases, women in the latest stages of pregnancy may also be receiving other pharmacological therapies that also affect the cardiovascular system, making it challenging to isolate OXT-specific effects. Finally, the unknown potential effects of OXT on physical and psychosocial development will be important to assess in pediatric studies.
OXT for adults with obesity
Initial studies have begun to investigate the potential effects of OXT on food intake and energy expenditure in adults. In healthy adult men who were normal weight or overweight/obese (n = 25), a single 24 IU dose of intranasal OXT modestly decreased food intake at a test meal (190). In a separate study, a single 24 IU dose of intranasal OXT decreased hedonic eating, ie, reward-driven snacking, in both normal weight and overweight/obese men, however a decrease in homeostatic, hunger-driven food intake occurred only in overweight/obese men (191). Functional brain imaging studies have evaluated the effects of OXT on brain regions involved in regulation of eating behaviors, and a subset of studies also included direct testing of eating behaviors in the same participants. Oxytocin was found to decrease activation in the hypothalamus in response to high-calorie foods vs. low calorie foods (82) and high-calorie foods vs. non-food objects (83). These results may reflect a decrease in the homeostatic drive to seek food. These studies also examined other neurobiological pathways involved in eating behavior. One of these studies reported that OXT produced a decrease in activation of reward-related food motivation areas (eg, VTA) in response to high-calorie foods vs. non-food objects in overweight and obese individuals (83). A second study found a directionally consistent but not statistically significant decrease in activation of one brain area involved in reward-related food motivation, the parabranchial nucleus, in normal-weight participants (82). A different study in normal-weight participants reported an OXT-related increase in activation of the brain’s reward circuits in anticipation of desired items (high-calorie food, monetary reward) (89). Finally, OXT also increased activation in the brain’s cognitive control regions (eg, anterior cingulate cortex) and could thereby reduce impulsive eating (83, 89, 192). Although few studies have been adequately powered to examine sex-specific responses, increased activation in areas of cognitive control in response to OXT has been found in men (83) as well as women (192).
The brain imaging studies that also included assessments of food intake in response to OXT found that brain activation patterns did not always correlate with eating behavior. For example, one study in lean male and female volunteers found that while intranasal OXT decreased hypothalamic activation in response to images high-calorie (versus low-calorie) food, ad libitum food intake was not altered (82). It may be that obese individuals demonstrate more sensitivity to OXT in decreasing food intake, and/or there may have been methodological differences in assessment of ad libitum food intake across studies that included normal weight vs. obese individuals. Alternatively, OXT effects may differ in men vs. women and/or depend on the reasons for which eating occurred (ie, for pleasure or for hunger), as in (191).
Longer-term studies have also been pursued and/or proposed to investigate the effects of OXT on energy balance. Obese adults (n = 24, 12 randomized to placebo and 12 to OXT, with 9/12 treated individuals with complete data) were treated for 8 weeks with 24 IU of intranasal OXT or placebo 4 times daily (before meals and bedtime) (135). Over the course of this study, OXT treatment produced weight loss of around 8.9 kg, and there were no significant AEs reported. Of the 9 treated individuals with complete data, 6 of these appeared to lose a substantial amount of weight. Of note, these were the heaviest individuals included in the study (>80 kg in body weight). Viewed according to BMI, little or no weight loss was observed in individuals with BMI 25–30 kg/m2, and more substantial weight loss was observed in those individuals with higher BMI. To our knowledge, at the time of this writing, this is the only available published study of chronic intranasal OXT therapy to achieve weight loss. A randomized controlled study of intranasal OXT on body weight in obese men and women is ongoing (NCT03043053).
OXT for children with Prader–Willi syndrome
In children, there is a growing literature in particular with respect to the chronic treatment of autism and related disorders of social-emotional functioning (172), but much less in the way of metabolic studies. To our knowledge, no pediatric studies have looked specifically at weight loss as a primary endpoint. Relevant to the topic of this review, a randomized controlled cross-over trial (8 weeks each of OXT or placebo, in random order, separated by a minimum of 2-week wash-out) was performed in children and young adults with PWS (18–32 IU twice a day [bid] for ages 13–15 years; 24–40 IU bid for ages 16–30 years) who often develop significant hyperphagia as well as decreased activity that can contribute to obesity (193). The trial was designed to examine behavioral endpoints, including a hyperphagia scale. The investigators did not detect an effect of OXT on any of the measures tested, and no weight changes were observed. They did find an increase in temper tantrums for the older participants at the higher dose tested (aged 16–30 years, 40 IU bid). A different cross-over study in PWS tested the effect of 16 IU daily for 5 days of intranasal OXT versus placebo; its primary focus was safety, and with careful review, no difference in safety parameters could be detected between OXT and placebo (161). No single behavioral subscale tested demonstrated a statistically significant improvement, but the nominal direction of effect favored OXT in many subscales, in particular with respect to decreased anxiety. The investigators concluded that OXT holds promise in this condition and that longer-term and dose-finding studies should be performed before clinicians prescribe OXT in PWS (161). A separate randomized controlled trial of intranasal carbetocin, an OXT analog, in 37 children aged 10 to 17 years with PWS (n = 17 receiving carbetocin) found that 14 days of carbetocin decreased parent-reported hyperphagia and obsessive-compulsive symptoms more than placebo (162). A follow-up phase 3 study in PWS of intranasal carbetocin is ongoing (NCT03649477).
OXT for children with hypothalamic obesity
OXT has also been considered for use in other patients with recalcitrant forms of obesity, most notably, hypothalamic obesity following treatment of hypothalamic/pituitary brain tumors, such as craniopharyngioma. In addition to hypothalamic obesity, these patients frequently have hypopituitarism, including diabetes insipidus (deficiency of vasopressin). Given the co-localization of production, storage, and secretory pathways for vasopressin and OXT, a state of OXT deficiency has been hypothesized in patients with diabetes insipidus. Consistent with this concept, low OXT levels and increased relevant psychopathology (eg, socioemotional functioning difficulties) has recently been identified in men with hypopituitarism and diabetes insipidus (194). Notably, in the 20 participants with diabetes insipidus in this study, there was a nominal association between higher BMI and lower OXT concentrations, suggesting a potential role for OXT in modulating weight in these patients. In a case report of a child with hypopituitarism, diabetes insipidus and obesity following therapy for a craniopharyngioma, compounded intranasal OXT was used along with naltrexone and carbohydrate restriction to achieve sustained weight loss (159). The authors of this report call for additional research into OXT for use in hypothalamic obesity syndrome, in particular in light of the profound needs in this population and the dearth of effective treatments. Indeed, one such follow-up study tested a single dose of 24 IU of intranasal OXT in 10 individuals with a history of childhood-onset craniopharyngioma. This study focused on OXT effects on emotional perception, and suggested that OXT-related responses were most discernible in those whose tumor-associated injuries were limited to the anterior hypothalamus (vs. also in mammillary bodies and posterior hypothalamus) (160). A study of intranasal OXT for obesity related to hypothalamic/pituitary tumors in children and young adults is ongoing (NCT02849743).
Conclusions
In summary, studies in animal models and in humans demonstrate that OXT, a hormone characterized initially for its role in parturition, has effects on energy balance. Oxytocin may also have the capacity to promote weight loss without leading to excess loss of muscle and bone. Future work in model systems will help to characterize these effects more fully, for example, testing the association of OXT with immune function, inflammation, and the gut microbiome (195). Much remains to be learned from mechanism-focused translational studies in humans that leverage important insights from ongoing studies in model systems. Future randomized, placebo-controlled clinical trials in humans should include rigorous, standardized, and detailed assessment of adherence, adverse effects, pharmacokinetics/pharmacodynamics, and efficacy in the diverse populations that may benefit from OXT (182). Future studies will have the opportunity to investigate the characteristics of new OXT mimetic peptides (175) and the obligation to consider long-term, multisystemic effects (196), in particular when OXT is given to children and adolescents.
Acknowledgments
The authors appreciate the support of Anna Dedio and Zahra Tara in preparation of the summary table and figure.
Financial Support: This work was supported by the Doris Duke Charitable Foundation (Doris Duke Clinical Scientist Development Award, SEM), Catherine and Roger Chiang (CHOP Neuroendocrine Center, SEM); NIH grants R01DK109932 (EAL), K24MH120568 (EAL), P30DK040561 (EAL), R01DK115976 (JEB), Merit Review Award BX004102, Office of Research and Development, Medical Research Service, United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service (JEB).
Glossary
Abbreviations
- α-MSH
α -melanocyte stimulating hormone
- ARC
arcuate nucleus
- BMI
body mass index
- CCK
cholecystokinin
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DMN
dorsomedial nucleus
- GLP1
glucagon-like peptide 1
- HFD
high-fat diet
- Hyp
hypothalamus
- IU
international units
- MC4R
melanocortin 4 receptor
- MM
mammary nuclei
- NAc
nucleus accumbens
- NTS
nucleus of the solitary tract
- OXT
oxytocin
- OXTR
oxytocin receptor
- POMC
pro-opiomelanocortin
- PPAR-α
peroxisome proliferator-activated receptor-α
- PVN
paraventricular nucleus
- PWS
Prader–Willi syndrome
- SON
supraoptic nucleus
- SNS
sympathetic nervous system
- VMN
ventromedial nucleus
- VTA
ventral tegmental area.
Additional Information
Disclosure Summary: EAL and JEB have a financial interest in OXT Therapeutics, Inc., a company developing an intranasal oxytocin and long-acting analogs of oxytocin to treat obesity and metabolic disease. SEM has served as a consultant for Rhythm Pharmaceuticals, a company whose activities include development of therapies for rare genetic disorders of obesity. The authors’ interests were reviewed and are managed by their local institutions in accordance with their conflict of interest policies. The contents do not represent the views of the US Department of Veterans Affairs or the US government.
Data Availability: (The Genotype-Tissue Expression [GTEx] Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by National Cancer Institute, National Human Genome Research Institute, National Heart, Lung, and Blood Institute, National Institute on Drug Abuse, National Institute of Mental Health, and National Institute of Neurological Disorders and Stroke. The data used for the analyses described in this manuscript were obtained from the GTEx Portal (https://www.gtexportal.org/home/gene/OXT) on May 29, 2018.
References
- 1. Song Z, Levin BE, Stevens W, Sladek CD. Supraoptic oxytocin and vasopressin neurons function as glucose and metabolic sensors. Am J Physiol Regul Integr Comp Physiol. 2014;306(7):R447–R456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. George JM. Immunoreactive vasopressin and oxytocin: concentration in individual human hypothalamic nuclei. Science. 1978;200(4339):342–343. [DOI] [PubMed] [Google Scholar]
- 3. Rosen GJ, de Vries GJ, Goldman SL, Goldman BD, Forger NG. Distribution of oxytocin in the brain of a eusocial rodent. Neuroscience. 2008;155(3):809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sabatier N, Caquineau C, Dayanithi G, et al. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci. 2003;23(32):10351–10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maejima Y, Sakuma K, Santoso P, et al. Oxytocinergic circuit from paraventricular and supraoptic nuclei to arcuate POMC neurons in hypothalamus. FEBS Lett. 2014;588(23):4404–4412. [DOI] [PubMed] [Google Scholar]
- 6. Ross HE, Cole CD, Smith Y, et al. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162(4):892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151(5):2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rinaman L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. J Comp Neurol. 1998;399(1):101–109. [DOI] [PubMed] [Google Scholar]
- 9. Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205(3):260–272. [DOI] [PubMed] [Google Scholar]
- 10. Ohlsson B, Truedsson M, Djerf P, Sundler F. Oxytocin is expressed throughout the human gastrointestinal tract. Regul Pept. 2006;135(1-2):7–11. [DOI] [PubMed] [Google Scholar]
- 11. Colaianni G, Sun L, Di Benedetto A, et al. Bone marrow oxytocin mediates the anabolic action of estrogen on the skeleton. J Biol Chem. 2012;287(34):29159–29167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gene expression for OXT (ENSG00000101405.3). GTEx Portal.https://www.gtexportal.org/home/gene/OXT. Accessed May 29, 2018.
- 13. Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980;207(4429):373–378. [DOI] [PubMed] [Google Scholar]
- 14. Jong TR, Menon R, Bludau A, et al. Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: The Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology. 2015;62:381–388. [DOI] [PubMed] [Google Scholar]
- 15. Leng G, Sabatier N. Measuring oxytocin and vasopressin: bioassays, immunoassays and random numbers. J Neuroendocrinol. 2016;28(10). doi: 10.1111/jne.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsujimoto M, Hattori A. The oxytocinase subfamily of M1 aminopeptidases. Biochim Biophys Acta. 2005;1751(1):9–18. [DOI] [PubMed] [Google Scholar]
- 17. Gajdosechova L, Krskova K, Segarra AB, et al. Hypooxytocinaemia in obese Zucker rats relates to oxytocin degradation in liver and adipose tissue. J Endocrinol. 2014;220(3):333–343. [DOI] [PubMed] [Google Scholar]
- 18. Breton C, Zingg HH. Expression and region-specific regulation of the oxytocin receptor gene in rat brain. Endocrinology. 1997;138(5):1857–1862. [DOI] [PubMed] [Google Scholar]
- 19. Gould BR, Zingg HH. Mapping oxytocin receptor gene expression in the mouse brain and mammary gland using an oxytocin receptor-LacZ reporter mouse. Neuroscience. 2003;122(1):155–167. [DOI] [PubMed] [Google Scholar]
- 20. Yoshida M, Hasselmo ME. Persistent firing supported by an intrinsic cellular mechanism in a component of the head direction system. J Neurosci. 2009;29(15):4945–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verbalis JG. The brain oxytocin receptor(s)? Front Neuroendocrinol. 1999;20(2):146–156. [DOI] [PubMed] [Google Scholar]
- 22. Hidema S, Fukuda T, Hiraoka Y, et al. Generation of Oxtr cDNA(HA)-Ires-Cre mice for gene expression in an oxytocin receptor specific manner. J Cell Biochem. 2016;117(5):1099–1111. [DOI] [PubMed] [Google Scholar]
- 23.Gene expression for OXTR (ENSG00000180914.10). GTEx Portal. https://www.gtexportal.org/home/gene/OXTR. Accessed May 29, 2018.
- 24. Quintana DS, Rokicki J, van der Meer D, et al. Oxytocin pathway gene networks in the human brain. Nat Commun. 2019;10(1):668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Welch MG, Tamir H, Gross KJ, Chen J, Anwar M, Gershon MD. Expression and developmental regulation of oxytocin (OT) and oxytocin receptors (OTR) in the enteric nervous system (ENS) and intestinal epithelium. J Comp Neurol. 2009;512(2):256–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qin J, Feng M, Wang C, Ye Y, Wang PS, Liu C. Oxytocin receptor expressed on the smooth muscle mediates the excitatory effect of oxytocin on gastric motility in rats. Neurogastroenterol Motil. 2009;21(4):430–438. [DOI] [PubMed] [Google Scholar]
- 27. Yang XX, Lin JM, Xu KJ, et al. Hepatic actinomycosis: report of one case and analysis of 32 previously reported cases. World J Gastroenterol. 2014;20(43):16372–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gajdosechova L, Krskova K, Olszanecki R, Zorad S. Differential regulation of oxytocin receptor in various adipose tissue depots and skeletal muscle types in obese Zucker rats. Horm Metab Res. 2015;47(8):600–604. [DOI] [PubMed] [Google Scholar]
- 29. Roberts ZS, Wolden-Hanson T, Matsen ME, et al. Chronic hindbrain administration of oxytocin is sufficient to elicit weight loss in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol. 2017;313(4):R357–R371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mitchell MD, Kraemer DL, Brennecke SP, Webb R. Pulsatile release of oxytocin during the estrous cycle, pregnancy and parturition in sheep. Biol Reprod. 1982;27(5):1169–1173. [DOI] [PubMed] [Google Scholar]
- 31. Fabian M, Forsling ML, Jones JJ, Pryor JS. The clearance and antidiuretic potency of neurohypophysial hormones in man, and their plasma binding and stability. J Physiol. 1969;204(3):653–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chard T, Boyd NR, Forsling ML, McNeilly AS, Landon J. The development of a radioimmunoassay for oxytocin: the extraction of oxytocin from plasma, and its measurement during parturition in human and goat blood. J Endocrinol. 1970;48(2):223–234. [DOI] [PubMed] [Google Scholar]
- 33. Johnson ML, Suratt P. Quantifying asynchronous breathing. Methods Enzymol. 2004;384:130–138. [DOI] [PubMed] [Google Scholar]
- 34. Ueda T, Yokoyama Y, Irahara M, Aono T. Influence of psychological stress on suckling-induced pulsatile oxytocin release. Obstet Gynecol. 1994;84(2):259–262. [PubMed] [Google Scholar]
- 35. Baskaran C, Plessow F, Silva L, et al. Oxytocin secretion is pulsatile in men and is related to social-emotional functioning. Psychoneuroendocrinology. 2017;85:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aulinas A, Plessow F, Pulumo RL, et al. Disrupted oxytocin-appetite signaling in females with anorexia nervosa. J Clin Endocrinol Metab. 2019;104(10):4931–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kostoglou-Athanassiou I, Athanassiou P, Treacher DF, Wheeler MJ, Forsling ML. Neurohypophysial hormone and melatonin secretion over the natural and suppressed menstrual cycle in premenopausal women. Clin Endocrinol (Oxf). 1998;49(2):209–216. [DOI] [PubMed] [Google Scholar]
- 38. Amico JA, Seif SM, Robinson AG. Oxytocin in human plasma: correlation with neurophysin and stimulation with estrogen. J Clin Endocrinol Metab. 1981;52(5):988–993. [DOI] [PubMed] [Google Scholar]
- 39. Fuchs AR, Romero R, Keefe D, Parra M, Oyarzun E, Behnke E. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. Am J Obstet Gynecol. 1991;165(5 Pt 1):1515–1523. [DOI] [PubMed] [Google Scholar]
- 40. Stock S, Granström L, Backman L, Matthiesen AS, Uvnäs-Moberg K. Elevated plasma levels of oxytocin in obese subjects before and after gastric banding. Int J Obes. 1989;13(2):213–222. [PubMed] [Google Scholar]
- 41. Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81(2):629–683. [DOI] [PubMed] [Google Scholar]
- 42. Tribollet E, Audigier S, Dubois-Dauphin M, Dreifuss JJ. Gonadal steroids regulate oxytocin receptors but not vasopressin receptors in the brain of male and female rats. An autoradiographical study. Brain Res. 1990;511(1):129–140. [DOI] [PubMed] [Google Scholar]
- 43. Klockars A, Levine AS, Olszewski PK. Central oxytocin and food intake: focus on macronutrient-driven reward. Front Endocrinol (Lausanne). 2015;6:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287(1):R87–R96. [DOI] [PubMed] [Google Scholar]
- 45. Perello M, Raingo J. Leptin activates oxytocin neurons of the hypothalamic paraventricular nucleus in both control and diet-induced obese rodents. PLoS One. 2013;8(3):e59625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van der Klaauw AA, Farooqi IS. The hunger genes: pathways to obesity. Cell. 2015;161(1):119–132. [DOI] [PubMed] [Google Scholar]
- 47. Wrobel L, Schorscher-Petcu A, Dupré A, Yoshida M, Nishimori K, Tribollet E. Distribution and identity of neurons expressing the oxytocin receptor in the mouse spinal cord. Neurosci Lett. 2011;495(1):49–54. [DOI] [PubMed] [Google Scholar]
- 48. Sutton AK, Pei H, Burnett KH, Myers MG Jr, Rhodes CJ, Olson DP. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J Neurosci. 2014;34(46):15306–15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mottolese R, Redouté J, Costes N, Le Bars D, Sirigu A. Switching brain serotonin with oxytocin. Proc Natl Acad Sci U S A. 2014;111(23):8637–8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Olszewski PK, Klockars A, Olszewska AM, Fredriksson R, Schiöth HB, Levine AS. Molecular, immunohistochemical, and pharmacological evidence of oxytocin’s role as inhibitor of carbohydrate but not fat intake. Endocrinology. 2010;151(10):4736–4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Blouet C, Jo YH, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci. 2009;29(26):8302–8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gaetani S, Fu J, Cassano T, et al. The fat-induced satiety factor oleoylethanolamide suppresses feeding through central release of oxytocin. J Neurosci. 2010;30(24):8096–8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Johnstone LE, Fong TM, Leng G. Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab. 2006;4(4):313–321. [DOI] [PubMed] [Google Scholar]
- 54. Renaud LP, Tang M, McCann MJ, Stricker EM, Verbalis JG. Cholecystokinin and gastric distension activate oxytocinergic cells in rat hypothalamus. Am J Physiol. 1987;253(4 Pt 2):R661–R665. [DOI] [PubMed] [Google Scholar]
- 55. Ueta Y, Kannan H, Higuchi T, Negoro H, Yamaguchi K, Yamashita H. Activation of gastric afferents increases noradrenaline release in the paraventricular nucleus and plasma oxytocin level. J Auton Nerv Syst. 2000;78(2-3):69–76. [DOI] [PubMed] [Google Scholar]
- 56. Matsui S, Sasaki T, Kohno D, et al. Neuronal SIRT1 regulates macronutrient-based diet selection through FGF21 and oxytocin signalling in mice. Nat Commun. 2018;9(1):4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Verbalis JG, McCann MJ, McHale CM, Stricker EM. Oxytocin secretion in response to cholecystokinin and food: differentiation of nausea from satiety. Science. 1986;232(4756):1417–1419. [DOI] [PubMed] [Google Scholar]
- 58. Sladek CD, Stevens W, Song Z, Johnson GC, MacLean PS. The “metabolic sensor” function of rat supraoptic oxytocin and vasopressin neurons is attenuated during lactation but not in diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2016;310(4):R337–R345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Young LJ, Muns S, Wang Z, Insel TR. Changes in oxytocin receptor mRNA in rat brain during pregnancy and the effects of estrogen and interleukin-6. J Neuroendocrinol. 1997;9(11):859–865. [DOI] [PubMed] [Google Scholar]
- 60. Ophir AG, Sorochman G, Evans BL, Prounis GS. Stability and dynamics of forebrain vasopressin receptor and oxytocin receptor during pregnancy in prairie voles. J Neuroendocrinol. 2013;25(8):719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Camerino C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity (Silver Spring). 2009;17(5):980–984. [DOI] [PubMed] [Google Scholar]
- 62. Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. 2008;19(9):951–955. [DOI] [PubMed] [Google Scholar]
- 63. Matarazzo V, Schaller F, Nédélec E, et al. Inactivation of Socs3 in the hypothalamus enhances the hindbrain response to endogenous satiety signals via oxytocin signaling. J Neurosci. 2012;32(48):17097–17107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yamashita M, Takayanagi Y, Yoshida M, Nishimori K, Kusama M, Onaka T. Involvement of prolactin-releasing peptide in the activation of oxytocin neurones in response to food intake. J Neuroendocrinol. 2013;25(5):455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res. 2003;993(1-2):30–41. [DOI] [PubMed] [Google Scholar]
- 66. Baskin DG, Kim F, Gelling RW, et al. A new oxytocin-saporin cytotoxin for lesioning oxytocin-receptive neurons in the rat hindbrain. Endocrinology. 2010;151(9):4207–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Blevins JE, Thompson BW, Anekonda VT, et al. Chronic CNS oxytocin signaling preferentially induces fat loss in high-fat diet-fed rats by enhancing satiety responses and increasing lipid utilization. Am J Physiol Regul Integr Comp Physiol. 2016;310(7):R640–R658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Altirriba J, Poher AL, Caillon A, et al. Divergent effects of oxytocin treatment of obese diabetic mice on adiposity and diabetes. Endocrinology. 2014;155(11):4189–4201. [DOI] [PubMed] [Google Scholar]
- 69. Schroeder M, Zagoory-Sharon O, Shbiro L, et al. Development of obesity in the Otsuka Long-Evans Tokushima Fatty rat. Am J Physiol Regul Integr Comp Physiol. 2009;297(6):R1749–R1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schroeder M, Gelber V, Moran TH, Weller A. Long-term obesity levels in female OLETF rats following time-specific post-weaning food restriction. Horm Behav. 2010;58(5):844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Plante E, Menaouar A, Danalache BA, et al. Oxytocin treatment prevents the cardiomyopathy observed in obese diabetic male db/db mice. Endocrinology. 2015;156(4):1416–1428. [DOI] [PubMed] [Google Scholar]
- 72. Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr. 2009;139(3):629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Maejima Y, Sedbazar U, Suyama S, et al. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab. 2009;10(5):355–365. [DOI] [PubMed] [Google Scholar]
- 74. Iwasaki Y, Maejima Y, Suyama S, et al. Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: a route for ameliorating hyperphagia and obesity. Am J Physiol Regul Integr Comp Physiol. 2015;308(5):R360–R369. [DOI] [PubMed] [Google Scholar]
- 75. Iwasaki Y, Kumari P, Wang L, Hidema S, Nishimori K, Yada T. Relay of peripheral oxytocin to central oxytocin neurons via vagal afferents for regulating feeding. Biochem Biophys Res Commun. 2019;519(3):553–558. [DOI] [PubMed] [Google Scholar]
- 76. Li C, Navarrete J, Liang-Guallpa J, et al. Defined paraventricular hypothalamic populations exhibit differential responses to food contingent on caloric state. Cell Metab. 2019;29(3):681–694.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wu Z, Lou Y, Jin W, Liu Y, Lu L, Lu G. The Pro12Ala polymorphism in the peroxisome proliferator-activated receptor gamma-2 gene (PPARγ2) is associated with increased risk of coronary artery disease: a meta-analysis. PLoS One. 2012;7(12):e53105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang G, Bai H, Zhang H, et al. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron. 2011;69(3):523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang B, Nakata M, Nakae J, Ogawa W, Yada T. Central insulin action induces activation of paraventricular oxytocin neurons to release oxytocin into circulation. Sci Rep. 2018;8(1):10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wu L, Meng J, Shen Q, et al. Caffeine inhibits hypothalamic A1R to excite oxytocin neuron and ameliorate dietary obesity in mice. Nat Commun. 2017;8:15904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang KM, Zhao GY, Zhang BB, Xu Q, Chu CP, Jin H, Qiu DL. Nicotine enhances GABAergic inhibition of oxytocin mRNA-expressing neuron in the hypothalamic paraventricular nucleus in vitro in rats. Neurosci Lett. 2017;638:5–11. [DOI] [PubMed] [Google Scholar]
- 82. van der Klaauw AA, Ziauddeen H, Keogh JM, et al. Oxytocin administration suppresses hypothalamic activation in response to visual food cues. Sci Rep. 2017;7(1):4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Plessow F, Marengi DA, Perry SK, et al. Effects of intranasal oxytocin on the blood oxygenation level-dependent signal in food motivation and cognitive control pathways in overweight and obese men. Neuropsychopharmacology. 2018;43(3):638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115(2):493–500. [DOI] [PubMed] [Google Scholar]
- 85. Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555(2):220–232. [DOI] [PubMed] [Google Scholar]
- 86. Sofroniew MV. Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog Brain Res. 1983;60:101–114. [DOI] [PubMed] [Google Scholar]
- 87. Mullis K, Kay K, Williams DL. Oxytocin action in the ventral tegmental area affects sucrose intake. Brain Res. 2013;1513:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Herisson FM, Waas JR, Fredriksson R, Schioth HB, Levine AS, Olszewski PK. Oxytocin acting in the nucleus accumbens core decreases food intake. J Neuroendocrinol. 2016;28(4). doi: 10.1111/jne.12381. [DOI] [PubMed] [Google Scholar]
- 89. Spetter MS, Feld GB, Thienel M, Preissl H, Hege MA, Hallschmid M. Oxytocin curbs calorie intake via food-specific increases in the activity of brain areas that process reward and establish cognitive control. Sci Rep. 2018;8(1):2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhang G, Cai D. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. Am J Physiol Endocrinol Metab. 2011;301(5):E1004–E1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Amico JA, Tenicela R, Johnston J, Robinson AG. A time-dependent peak of oxytocin exists in cerebrospinal fluid but not in plasma of humans. J Clin Endocrinol Metab. 1983;57(5):947–951. [DOI] [PubMed] [Google Scholar]
- 92. Lawson EA, Ackerman KE, Estella NM, et al. Nocturnal oxytocin secretion is lower in amenorrheic athletes than nonathletes and associated with bone microarchitecture and finite element analysis parameters. Eur J Endocrinol. 2013;168(3):457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Baskaran C, Sluss PM, Misra M, Lawson EA. Oxytocin Secretion in Healthy Men. Boston, MA: Endocrine Society Annual Meeting; 2016. [Google Scholar]
- 94. Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany NY). 2011;3(12):1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Morton GJ, Thatcher BS, Reidelberger RD, et al. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am J Physiol Endocrinol Metab. 2012;302(1):E134–E144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ong ZY, Alhadeff AL, Grill HJ. Medial nucleus tractus solitarius oxytocin receptor signaling and food intake control: the role of gastrointestinal satiation signal processing. Am J Physiol Regul Integr Comp Physiol. 2015;308(9):R800–R806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Klockars OA, Klockars A, Levine AS, Olszewski PK. Oxytocin administration in the basolateral and central nuclei of amygdala moderately suppresses food intake. Neuroreport. 2018;29(6):504–510. [DOI] [PubMed] [Google Scholar]
- 98. Mullis K, Kay K, Williams DL. Oxytocin action in the ventral tegmental area affects sucrose intake. Brain Res. 2013;1513:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Thienel M, Fritsche A, Heinrichs M, et al. Oxytocin’s inhibitory effect on food intake is stronger in obese than normal-weight men. Int J Obes (Lond). 2016;40(11):1707–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lokrantz CM, Uvnäs-Moberg K, Kaplan JM. Effects of central oxytocin administration on intraoral intake of glucose in deprived and nondeprived rats. Physiol Behav. 1997;62(2):347–352. [DOI] [PubMed] [Google Scholar]
- 101. Blevins JE, Graham JL, Morton GJ, et al. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2015;308(5):R431–R438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Blevins JE, Thompson BW, Anekonda VT, et al. Chronic CNS oxytocin signaling preferentially induces fat loss in high-fat diet-fed rats by enhancing satiety responses and increasing lipid utilization. Am J Physiol Regul Integr Comp Physiol. 2016;310(7):R640–R658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Maejima Y, Aoyama M, Sakamoto K, et al. Impact of sex, fat distribution and initial body weight on oxytocin’s body weight regulation. Sci Rep. 2017;7(1):8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Deblon N, Veyrat-Durebex C, Bourgoin L, et al. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS One. 2011;6(9):e25565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Roberts ZS, Wolden-Hanson TH, Matsen ME, et al. Chronic hindbrain administration of oxytocin is sufficient to elicit weight loss in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol. 2017;313(4):R357–R371. doi: 10.1152/ajpregu.00169.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. van der Klaauw AA, Keogh JM, Henning E, et al. Divergent effects of central melanocortin signalling on fat and sucrose preference in humans. Nat Commun. 2016;7:13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Noble EE, Billington CJ, Kotz CM, Wang C. Oxytocin in the ventromedial hypothalamic nucleus reduces feeding and acutely increases energy expenditure. Am J Physiol Regul Integr Comp Physiol. 2014;307(6):R737–R745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110(3):515–526. [DOI] [PubMed] [Google Scholar]
- 109. Stanley SM. Relation of Phanerozoic stable isotope excursions to climate, bacterial metabolism, and major extinctions. Proc Natl Acad Sci U S A. 2010;107(45):19185–19189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yi KJ, So KH, Hata Y, et al. The regulation of oxytocin receptor gene expression during adipogenesis. J Neuroendocrinol. 2015;27(5):335–342. [DOI] [PubMed] [Google Scholar]
- 111. Kasahara Y, Takayanagi Y, Kawada T, Itoi K, Nishimori K. Impaired thermoregulatory ability of oxytocin-deficient mice during cold-exposure. Biosci Biotechnol Biochem. 2007;71(12):3122–3126. [DOI] [PubMed] [Google Scholar]
- 112. Xi D, Long C, Lai M, et al. Ablation of oxytocin neurons causes a deficit in cold stress response. J Endocr Soc. 2017;1(8):1041–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ong ZY, Bongiorno DM, Hernando MA, Grill HJ. Effects of endogenous oxytocin receptor signaling in nucleus tractus solitarius on satiation-mediated feeding and thermogenic control in male rats. Endocrinology. 2017;158(9):2826–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Blevins JE, Baskin DG. Translational and therapeutic potential of oxytocin as an anti-obesity strategy: insights from rodents, nonhuman primates and humans. Physiol Behav. 2015;152(Pt B):438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chusyd DE, Wang D, Huffman DM, Nagy TR. Relationships between rodent white adipose fat pads and human white adipose fat depots. Front Nutr. 2016;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Eckertova M, Ondrejcakova M, Krskova K, Zorad S, Jezova D. Subchronic treatment of rats with oxytocin results in improved adipocyte differentiation and increased gene expression of factors involved in adipogenesis. Br J Pharmacol. 2011;162(2):452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Elabd C, Basillais A, Beaupied H, et al. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells. 2008;26(9):2399–2407. [DOI] [PubMed] [Google Scholar]
- 118. Santos LF, Singulani MP, Stringhetta-Garcia CT, Oliveira SHP, Chaves-Neto AH, Dornelles RCM. Oxytocin effects on osteoblastic differentiation of bone marrow mesenchymal stem cells from adult and aging female Wistar rats. Exp Gerontol. 2018;113:58–63. [DOI] [PubMed] [Google Scholar]
- 119. Breton C, Haenggeli C, Barberis C, et al. Presence of functional oxytocin receptors in cultured human myoblasts. J Clin Endocrinol Metab. 2002;87(3):1415–1418. [DOI] [PubMed] [Google Scholar]
- 120. Copland JA, Ives KL, Simmons DJ, Soloff MS. Functional oxytocin receptors discovered in human osteoblasts. Endocrinology. 1999;140(9):4371–4374. [DOI] [PubMed] [Google Scholar]
- 121. Colucci S, Colaianni G, Mori G, Grano M, Zallone A. Human osteoclasts express oxytocin receptor. Biochem Biophys Res Commun. 2002;297(3):442–445. [DOI] [PubMed] [Google Scholar]
- 122. Amri EZ, Pisani DF. Control of bone and fat mass by oxytocin. Horm Mol Biol Clin Investig. 2016;28(2):95–104. [DOI] [PubMed] [Google Scholar]
- 123. Zaidi M, New MI, Blair HC, et al. Actions of pituitary hormones beyond traditional targets. J Endocrinol. 2018;237(3):R83–R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Elabd C, Cousin W, Upadhyayula P, et al. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun. 2014;5:4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Petersson M, Lagumdzija A, Stark A, Bucht E. Oxytocin stimulates proliferation of human osteoblast-like cells. Peptides. 2002;23(6):1121–1126. [DOI] [PubMed] [Google Scholar]
- 126. Beranger GE, Djedaini M, Battaglia S, et al. Oxytocin reverses osteoporosis in a sex-dependent manner. Front Endocrinol (Lausanne). 2015;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Weber TJ. Battle of the sex steroids in the male skeleton: and the winner is. J Clin Invest. 2016;126(3):829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sun L, Tamma R, Yuen T, et al. Functions of vasopressin and oxytocin in bone mass regulation. Proc Natl Acad Sci U S A. 2016;113(1):164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Schorr M, Marengi DA, Pulumo RL, et al. Oxytocin and its relationship to body composition, bone mineral density, and hip geometry across the weight spectrum. J Clin Endocrinol Metab. 2017;102(8):2814–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Breuil V, Fontas E, Chapurlat R, et al. Oxytocin and bone status in men: analysis of the MINOS cohort. Osteoporos Int. 2015;26(12):2877–2882. [DOI] [PubMed] [Google Scholar]
- 131. Breuil V, Panaia-Ferrari P, Fontas E, et al. Oxytocin, a new determinant of bone mineral density in post-menopausal women: analysis of the OPUS cohort. J Clin Endocrinol Metab. 2014;99(4):E634–E641. [DOI] [PubMed] [Google Scholar]
- 132. Lawson EA, Ackerman KE, Slattery M, Marengi DA, Clarke H, Misra M. Oxytocin secretion is related to measures of energy homeostasis in young amenorrheic athletes. J Clin Endocrinol Metab. 2014;99(5):E881–E885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Elabd S, Sabry I. Two birds with one stone: possible dual-role of oxytocin in the treatment of diabetes and osteoporosis. Front Endocrinol (Lausanne). 2015;6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Colaianni G, Sun L, Zaidi M, Zallone A. The “love hormone” oxytocin regulates the loss and gain of the fat-bone relationship. Front Endocrinol (Lausanne). 2015;6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zhang H, Wu C, Chen Q, et al. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS One. 2013;8(5):e61477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Snider B, Geiser A, Yu XP, et al. Long-acting and selective oxytocin peptide analogs show antidiabetic and antiobesity effects in male mice. J Endocr Soc. 2019;3(7):1423–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Szulc P, Amri EZ, Varennes A, et al. High serum oxytocin is associated with metabolic syndrome in older men - The MINOS study. Diabetes Res Clin Pract. 2016;122:17–27. [DOI] [PubMed] [Google Scholar]
- 138. Qian W, Zhu T, Tang B, et al. Decreased circulating levels of oxytocin in obesity and newly diagnosed type 2 diabetic patients. J Clin Endocrinol Metab. 2014;99(12):4683–4689. [DOI] [PubMed] [Google Scholar]
- 139. Binay Ç, Paketçi C, Güzel S, Samancı N. Serum irisin and oxytocin levels as predictors of metabolic parameters in Obese children. J Clin Res Pediatr Endocrinol. 2017;9(2):124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Eisenberg Y, Dugas LR, Akbar A, Reddivari B, Layden BT, Barengolts E. Oxytocin is lower in African American men with diabetes and associates with psycho-social and metabolic health factors. PLoS One. 2018;13(1):e0190301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Barengolts E, Green SJ, Eisenberg Y, et al. Gut microbiota varies by opioid use, circulating leptin and oxytocin in African American men with diabetes and high burden of chronic disease. PLoS One. 2018;13(3):e0194171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Karavitaki N, Brufani C, Warner JT, et al. Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol (Oxf). 2005;62(4):397–409. [DOI] [PubMed] [Google Scholar]
- 143. Roth CL, Eslamy H, Werny D, et al. Semiquantitative analysis of hypothalamic damage on MRI predicts risk for hypothalamic obesity. Obesity (Silver Spring). 2015;23(6):1226–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Daubenbüchel AM, Hoffmann A, Eveslage M, et al. Oxytocin in survivors of childhood-onset craniopharyngioma. Endocrine. 2016;54(2):524–531. [DOI] [PubMed] [Google Scholar]
- 145. Gebert D, Auer MK, Stieg MR, et al. De-masking oxytocin-deficiency in craniopharyngioma and assessing its link with affective function. Psychoneuroendocrinology. 2018;88:61–69. [DOI] [PubMed] [Google Scholar]
- 146. Daubenbuchel AM, Ozyurt J, Boekhoff S, Warmuth-Metz M, Eveslage M, Muller HL. Eating behaviour and oxytocin in patients with childhood-onset craniopharyngioma and different grades of hypothalamic involvement. Pediatric Obesity. 2019; e12527. doi: 10.1111/ijpo.12527. [DOI] [PubMed] [Google Scholar]
- 147. Johnson L, Manzardo AM, Miller JL, Driscoll DJ, Butler MG. Elevated plasma oxytocin levels in children with Prader–Willi syndrome compared with healthy unrelated siblings. Am J Med Genet A. 2016;170(3):594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Conti F, Sertic S, Reversi A, Chini B. Intracellular trafficking of the human oxytocin receptor: evidence of receptor recycling via a Rab4/Rab5 “short cycle”. Am J Physiol Endocrinol Metab. 2009;296(3):E532–E542. [DOI] [PubMed] [Google Scholar]
- 149. Freeman SM, Ngo J, Singh B, Masnaghetti M, Bales KL, Blevins JE. Effects of chronic oxytocin administration and diet composition on oxytocin and vasopressin 1a receptor binding in the rat brain. Neuroscience. 2018;392:241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Lawson EA, Holsen LM, Santin M, et al. Oxytocin secretion is associated with severity of disordered eating psychopathology and insular cortex hypoactivation in anorexia nervosa. J Clin Endocrinol Metab. 2012;97(10):E1898–E1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Coiro V, Passeri M, Davoli C, et al. Oxytocin response to insulin-induced hypoglycemia in obese subjects before and after weight loss. J Endocrinol Invest. 1988;11(2):125–128. [DOI] [PubMed] [Google Scholar]
- 152. Coiro V, Capretti L, Speroni G, et al. Increase by naloxone of arginine vasopressin and oxytocin responses to insulin-induced hypoglycemia in obese men. J Endocrinol Invest. 1990;13(9):757–763. [DOI] [PubMed] [Google Scholar]
- 153. Chiodera P, Coiro V, Camellini L, et al. Effect of pharmacological doses of oxytocin on insulin response to glucose in normal man. Horm Res. 1984;20(2):150–154. [DOI] [PubMed] [Google Scholar]
- 154. Klement J, Ott V, Rapp K, et al. Oxytocin improves β-cell responsivity and glucose tolerance in healthy men. Diabetes. 2017;66(2):264–271. [DOI] [PubMed] [Google Scholar]
- 155. Brede S, Fehr S, Dalla-Man C, et al. Intranasal oxytocin fails to acutely improve glucose metabolism in obese men. Diabetes Obes Metab. 2019;21(2):424–428. [DOI] [PubMed] [Google Scholar]
- 156. Paolisso G, Sgambato S, Passariello N, et al. Pharmacological doses of oxytocin affect plasma hormone levels modulating glucose homeostasis in normal man. Horm Res. 1988;30(1):10–16. [DOI] [PubMed] [Google Scholar]
- 157. Varian BJ, Poutahidis T, DiBenedictis BT, et al. Microbial lysate upregulates host oxytocin. Brain Behav Immun. 2017;61:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol. 2008;22(7):1723–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hsu EA, Miller JL, Perez FA, Roth CL. Oxytocin and Naltrexone successfully treat hypothalamic obesity in a boy post-craniopharyngioma resection. J Clin Endocrinol Metabol. 2017. doi: 10.1210/jc.2017-02080. [DOI] [PubMed] [Google Scholar]
- 160. Hoffmann A, Özyurt J, Lohle K, Reichel J, Thiel CM, Müller HL. First experiences with neuropsychological effects of oxytocin administration in childhood-onset craniopharyngioma. Endocrine. 2017;56(1):175–185. [DOI] [PubMed] [Google Scholar]
- 161. Miller JL, Tamura R, Butler MG, et al. Oxytocin treatment in children with Prader–Willi syndrome: a double-blind, placebo-controlled, crossover study. Am J Med Genet A. 2017;173(5):1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Dykens EM, Miller J, Angulo M, Roof et al. Intranasal carbetocin reduces hyperphagia in individuals with Prader–Willi syndrome. JCI Insight. 2018;3(12):e98333. doi: 10.1172/jci.insight.98333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ramachandrappa S, Raimondo A, Cali AM, et al. Rare variants in single-minded 1 (SIM1) are associated with severe obesity. J Clin Invest. 2013;123(7):3042–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Lee MR, Scheidweiler KB, Diao XX, et al. Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Mol Psychiatry. 2018;23(1):115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Altirriba J, Poher AL, Rohner-Jeanrenaud F. Chronic oxytocin administration as a treatment against impaired leptin signaling or leptin resistance in obesity. Front Endocrinol (Lausanne). 2015;6:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wheeler E, Huang N, Bochukova EG, et al. Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nat Genet. 2013;45(5):513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Fineberg SK, Ross DA. Oxytocin and the social brain. Biol Psychiatry. 2017;81(3):e19–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Kruse J. Oxytocin: pharmacology and clinical application. J Fam Pract. 1986;23(5):473–479. [PubMed] [Google Scholar]
- 169. Arias F. Pharmacology of oxytocin and prostaglandins. Clin Obstet Gynecol. 2000;43(3):455–468. [DOI] [PubMed] [Google Scholar]
- 170. Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38(10):1985–1993. [DOI] [PubMed] [Google Scholar]
- 171. MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36(8):1114–1126. [DOI] [PubMed] [Google Scholar]
- 172. Taylor AE, Lee HE, Buisman-Pijlman FT. Oxytocin treatment in pediatric populations. Front Behav Neurosci. 2014;8:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. McCullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci Biobehav Rev. 2013;37(8):1485–1492. [DOI] [PubMed] [Google Scholar]
- 174. Johnsen E, Leknes S, Wilson SR, Lundanes E. Liquid chromatography-mass spectrometry platform for both small neurotransmitters and neuropeptides in blood, with automatic and robust solid phase extraction. Sci Rep. 2015;5:9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ferrie JJ, Gruskos JJ, Goldwaser AL, Decker ME, Guarracino DA. A comparative protease stability study of synthetic macrocyclic peptides that mimic two endocrine hormones. Bioorg Med Chem Lett. 2013;23(4):989–995. [DOI] [PubMed] [Google Scholar]
- 176. Leng G, Ludwig M. Intranasal Oxytocin: Myths and Delusions. Biol Psychiatry. 2016;79(3):243–250. [DOI] [PubMed] [Google Scholar]
- 177. Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5(6):514–516. [DOI] [PubMed] [Google Scholar]
- 178. Striepens N, Kendrick KM, Hanking V, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 2013;3:3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Freeman SM, Samineni S, Allen PC, et al. Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques. Psychoneuroendocrinology. 2016;66:185–194. [DOI] [PubMed] [Google Scholar]
- 180. Modi ME, Connor-Stroud F, Landgraf R, Young LJ, Parr LA. Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques. Psychoneuroendocrinology. 2014;45:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Ohlsson B, Truedsson M, Bengtsson M, et al. Effects of long-term treatment with oxytocin in chronic constipation; a double blind, placebo-controlled pilot trial. Neurogastroenterol Motil. 2005;17(5):697–704. [DOI] [PubMed] [Google Scholar]
- 182. DeMayo MM, Song YJC, Hickie IB, Guastella AJ. A review of the safety, efficacy and mechanisms of delivery of nasal oxytocin in children: therapeutic potential for autism and prader-willi syndrome, and recommendations for future research. Paediatr Drugs. 2017;19(5):391–410. [DOI] [PubMed] [Google Scholar]
- 183. Greenhill LL, Vitiello B, Fisher P, et al. Comparison of increasingly detailed elicitation methods for the assessment of adverse events in pediatric psychopharmacology. J Am Acad Child Adolesc Psychiatry. 2004;43(12):1488–1496. [DOI] [PubMed] [Google Scholar]
- 184. Tzabazis A, Kori S, Mechanic J, et al. Oxytocin and migraine headache. Headache. 2017;57(Suppl 2):64–75. [DOI] [PubMed] [Google Scholar]
- 185. Scantamburlo G, Hansenne M, Geenen V, Legros JJ, Ansseau M. Additional intranasal oxytocin to escitalopram improves depressive symptoms in resistant depression: an open trial. Eur Psychiatry. 2015;30(1):65–68. [DOI] [PubMed] [Google Scholar]
- 186. Parker KJ, Oztan O, Libove RA, et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc Natl Acad Sci U S A. 2017;114(30):8119–8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Fuchs AR, Fuchs F. Endocrinology of human parturition: a review. Br J Obstet Gynaecol. 1984;91(10):948–967. [DOI] [PubMed] [Google Scholar]
- 188. Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150(6):734–741. [DOI] [PubMed] [Google Scholar]
- 189. Habashi JP, MacFarlane EG, Bagirzadeh R, et al. Oxytocin antagonism prevents pregnancy-associated aortic dissection in a mouse model of Marfan syndrome. Sci Transl Med. 2019;11(490). doi: 10.1126/scitranslmed.aat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Lawson EA, Marengi DA, DeSanti RL, Holmes TM, Schoenfeld DA, Tolley CJ. Oxytocin reduces caloric intake in men. Obesity (Silver Spring). 2015;23(5):950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Ott V, Finlayson G, Lehnert H, et al. Oxytocin reduces reward-driven food intake in humans. Diabetes. 2013;62(10):3418–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Striepens N, Schröter F, Stoffel-Wagner B, Maier W, Hurlemann R, Scheele D. Oxytocin enhances cognitive control of food craving in women. Hum Brain Mapp. 2016;37(12):4276–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Einfeld SL, Smith E, McGregor IS, et al. A double-blind randomized controlled trial of oxytocin nasal spray in Prader–Willi syndrome. Am J Med Genet A. 2014;164A(9):2232–2239. [DOI] [PubMed] [Google Scholar]
- 194. Aulinas A, Plessow F, Asanza E, et al. low plasma oxytocin levels and increased psychopathology in hypopituitary men with diabetes insipidus. J Clin Endocrinol Metabol. 2019;104(8):3181–3191 doi: 10.1210/jc.2018-02608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Poutahidis T, Kearney SM, Levkovich T, et al. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS One. 2013;8(10):e78898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Bales KL, Solomon M, Jacob S, et al. Long-term exposure to intranasal oxytocin in a mouse autism model. Transl Psychiatry. 2014;4:e480. [DOI] [PMC free article] [PubMed] [Google Scholar]