Abstract
Due to the critical role of cochlear ion channels for hearing, the focus of the present study was to examine age-related changes of Na, K-ATPase (NKA) subunits in the lateral wall of mouse cochlea. We combined qRT-PCR, western blot and immunocytochemistry methodologies in order to determine gene and protein expression levels in the lateral wall of young and aged CBA/CaJ mice. Of the seven NKA subunits, only the mRNA expressions of α1, β1 and β2 subunit isoforms were detected in the lateral wall of CBA/CaJ mice. Aging was accompanied by dys-regulation of gene and protein expression of all three subunits detected. Hematoxylin and eosin (H&E) staining revealed atrophy of the cochlear stria vascularis (SV). The SV atrophy rate (20%) was much less than the ~80% decline in expression of all three NKA isoforms, indicating lateral wall atrophy and NKA dys-regulation are independent factors and that there is a combination of changes involving the morphology of SV and NKA expression in the aging cochlea which may concomitantly affect cochlear function. Immunoprecipitation assays showed that the α1-β1 heterodimer is the selective preferential heterodimer over the α1-β2 heterodimer in cochlea lateral wall. Interestingly, in vitro pathway experiments utilizing cultured mouse cochlear marginal cells from the SV (SV-K1 cells) indicated that decreased mRNA and protein expressions of α1, β1 and β2 subunit isoforms are not associated with reduction of NKA activity following in vitro application of ouabain, but ouabain did disrupt the α1-β1 heterodimer interaction. Lastly, the association between the α1 and β1 subunit isoforms was present in the cochlear lateral wall of young adult mice, but this interaction could not be detected in old mice. Taken together, these data suggest that in the young adult mouse there is a specific, functional selection and assembly of NKA subunit isoforms in the SV lateral wall, which is disrupted and dys-regulated with age. Interventions for this age-linked ion channel disruption may have the potential to help diagnose, prevent, or treat age-related hearing loss.
Keywords: Age-related hearing loss, Stria vascularis, Na, K-ATPase, Ouabain
1. Introduction
The Na, K-ATPase (NKA) ion channel is an integral membrane protein located in cellular plasma membranes, including the cochlear lateral wall, and particularly in the stria vascularis (SV). Under normal conditions, cells maintain relatively high intracellular concentrations of K+ but low concentrations of Na+. NKA, an adenosine triphosphate (ATP) dependent protein (Kaplan, 2002), plays a key homeostatic role in moving Na+ and K+ ions in opposite directions across the plasma membrane. X-ray crystallography analysis has shown that NKA is an oligomeric enzyme consisting of two essential subunits, the catalytic α-subunit (α), and a regulatory β-subunit (β), which are required for the translation, stability, and membrane insertion of the α-subunit (Geering, 2008; Shinoda et al., 2009; Lingrel et al., 1988). For normal ion transport, NKA must contain at least one α and one β subunit. There are four isoforms of the α subunits and three isoforms of the β subunits. In vivo transfection studies have demonstrated that each a subunit isoform can partner with a β subunit to form a functional antiporter enzyme. Selective co-immunoprecipitation of α and β subunits suggests there is a preferred binding partner for the co-assembly of these subunits, and this preferential binding may be related to the tissue type and differences in the functional roles of the NKA isoforms, such as K+ affinity and isoform stability (Colonna et al., 1997a,b; Schmalzing et al., 1997; Tokhtaeva et al., 2012a,b).
NKA is expressed ubiquitously in most cells, and theoretically, each of the four α subunit isoforms can interact with each of the three β subunit isoforms to form an active unit. However, not all isoforms are expressed simultaneously in a single cell or tissue type, and their configurations depend on their functional roles. The α and β heterodimer configurations can have different tissue-specific forms. For example, α1-β1 NKA is a monomer combination present in most cell types (Tokhtaeva et al., 2012a, b), whereas the α2 isoforms are found primarily in muscle and nervous system tissues (Sweadner, 1989; Kaplan, 2002; Lingrel, 2010; Blanco and Mercer, 1998; Blanco, 2005); α3 isoforms are expressed mainly in neurons (McLean et al., 2009; Dobretsov and Stimers, 2005), whereas the α4 isoforms reside only in the testis (Woo et al., 2000; Jimenez et al., 2011). The β2 isoforms are expressed predominantly in brain and muscle (Lingrel, 2010; Blanco, 2005), while the β3 isoforms are located in lung, testis, skeletal muscle and liver (Malik et al., 1996; Arystarkhova and Sweadner, 1997, 1996). NKA carries out many cellular functions, such as modulating a cell’s resting potential, promoting ion transport, controlling cell volume, and functioning as a signal transducer (Hall and Guyton, 2006; Yuan et al., 2005; Li et al., 2009). The enzyme also regulates contractility of cardiac, smooth, and skeletal muscle, modulates blood pressure under stress, and affects intercellular adhesion in epithelia (Blaustein et al., 2009; Rindler et al., 2011; Tokhtaeva et al., 2012a,b). Moreover, a third subunit, small membrane proteins FXYD, have been shown to be associated with the αβ complex of NKA. FXYD proteins are a small group of seven transmembrane proteins, which are localized in tissues and organs involved in solute and fluid transport, where they are proposed to act as regulators of ion transport. All members of this family are known to associate with NKA and modulate its properties in a tissue- and isoform-specific way. In rat, FXYD6 was found to be co-localized with NKA in the stria vascularis and to be expressed in rat in various epithelial cells bordering the endolymphatic space and in the auditory neurons (Delprat et al., 2007).
Most relevant to the present study is the unique functional role of NKA in the lateral wall of the inner ear, as it is one of key factors which plays an important role in the maintenance of the endocochlear potential (EP). Like its counterpart, such as sodium/potassium/chloride cotransporter (NKCC1), this is accomplished by pumping cations out of the SV against a voltage and concentration gradient, thereby maintaining a high K+ concentration and positive voltage potential in the endolymphatic space of scala media (Nishiyama et al., 1994; Furukawa et al., 1996; Nin et al., 2008; Lang et al., 2007). In this study we focus on the changes of NKA in the aged cochlea compared to the young adult cochlea. Previous studies have found a slow but progressive loss of the EP with age, in both gerbils and several strains of mice, including CBA/CaJs (Jimenez et al., 1999; Johnson et al., 2000; Schulte and Schmiedt, 1992). This loss of the EP has also been associated with atrophy of the SV, and reduced NKA activity (Gratton et al., 1996; Ichimiya et al., 2000; Schmiedt, 1996). In addition, cardiac glycosides, such as ouabain, if applied to the lateral wall in vivo, can decrease the EP and also lead to down-regulation of NKA activity followed by degeneration of SV (Kusakari et al., 1978; Rybak and Morizono, 1982; Shugyo et al., 1990; Wangemann, 2002; Schulte and Schmiedt, 1992; Gratton et al., 1996, 1997; Spicer et al., 1997).
In a previous immunohistochemistry study, α1, β1 and β2 subunits were found in the young adult mouse cochlea, especially in SV marginal cells (Schulte and Steel, 1994). Another report demonstrated that the dominant isoforms in young adult rat cochlea have α1 and β1 subunits (ten Cate et al., 1994). However, inconsistency in the literature is present. For example, McGuirt and colleagues reported that the distribution of α and β subunits in the young gerbil cochlea are different from mouse and rat (McGuirt and Schulte, 1994). In addition, the subunits detected in the rat cochlea using in situ mRNA hybridization (Ryan et al., 1991) are not identical to the subunits detected using immunohistochemistry (ten Cate et al., 1994). Finally in another study, using RT-PCR with primers specific for the various isoforms of NKA α and β-subunits (Pierre et al., 2001), the results contradict the immunocytochemistry findings (Mayol et al., 1998). Such inconsistencies raise the question about the possibility of the lack of specificity of available antibodies and DNA probes directed against the different NKA isoforms, as well as the physiological relevance of the diversity of the NKA isoforms. In order to overcome these inconsistencies we explored both the mRNA and protein expression of NKA isoforms using semi-quantitative and real-time RT-PCR combined with western blots to gain a better understanding of their subunit expression patterns in the mouse SV. Furthermore, we used immunochemical methods and quantitative image analysis to map the anatomical location of NKA isoforms in mouse SV. Specifically, we selected primers designed to span key introns and bridge an exon-to-exon junction, which excluded the possibility of amplification from genomic DNA (Arystarkhova and Sweadner, 1997). In addition, we only used antibodies for which specificity was confirmed by reputable vendors, i.e., there was no cross detection (reactivity) between the various isoforms. Since very little is known about the particular α-β heterodimers responsible for the functional role in aging processes of the cochlea, we investigated whether diversity of the α-β NKA heterodimers is determined not only by cell-specific co-expression of particular isoforms, but also by selective association of the α and β subunit isoforms.
2. Materials and methods
2.1. Animal
Breeding pairs of CBA/CaJ mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The offspring were housed and maintained in approved animal husbandry facilities. All procedures were compliant with NIH policies and approved by the University of South Florida IACUC and Vivarium.
2.2. Tissue preparation
2.2.1. Molecular biology
Young adult (N = 6, 3 mon) and old aged (N = 6, 30 mon) male CBA/CaJ mice were sacrificed by decapitation. The cochleae were quickly removed from the temporal bone, and transferred into ice-cold Dulbecco’s phosphate-buffered saline (DPBS, HyClone Lab Inc., Logan, Utah, #84321). The soft tissues of the cochlear lateral wall were dissected under a Zeiss stereomicroscope. Then the samples were transferred into an Eppendorf tube on dry ice, and finally stored at a −80 °C freezer for later RT-PCR and western blots. Care was taken to isolate the cochlear lateral wall so that only stria vascularis lateral wall was dissected.
2.2.2. Histology and immunohistochemistry
The freshly removed cochleae were placed into glass vials with 20 ml fresh 4% paraformaldehyde (Thermo scientific # 28,908, Rockford, IL) in phosphate buffered saline (PBS, 0.1 M, pH 7.6) overnight at 4 °C. After fixation, the cochleae were washed for 3 × 10 min in PBS, then decalcified in 20 ml 10% EDTA (Ethyl-enediamine Tetra-acetic Acid, Fisher Scientific, Fair Lawn, NJ) in PBS at 4 °C. The cochleae samples were checked daily until decalcification was complete (approximately 1 week). Then the cochleae were washed 3 × 10 min in PBS, cryoprotected in 10% and 20% sucrose (Acros, NJ) in PBS for 2 h each, followed by 30% sucrose in PBS overnight at 4 °C, until the cochleae sank. Next, cochleae were embedded in degassed OCT (Tissue-Tek #4583, Torrance, CA) overnight at 4 °C, orientated into cryomolds (Tissue-Tek # 4565) with OCT, and degassed for 1h, then frozen at −80 °C. Cryosectioning was performed at 5 μm per section.
2.3. Cultured cells
An epithelial cell line, SV-K1 cloned from SV of an immortomouse (H2kbtsA58) was utilized (provided courtesy of Dr. Federico Kalinec, House Ear Institute, Los Angeles, CA; Kalinec et al., 2003; Belyantseva et al., 2002; Gratton et al., 2002). Following a 1 week initiation of the cultures in the permissive condition [33 °C, 10% CO2, Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS)], cells were transferred to grow in the non-permissive condition (39 °C, 5% CO2, DMEM with 5% FCS) and the cells were split onto 100 mm plates (Falcon). Experiments were performed at 70–80% confluence. All treatment started after the switch from permissive to non-permissive condition. Then adherent cells were scraped off the dish using a cold plastic cell scraper, then gently transferred to the cell suspension into a precooled microcentrifuge tube. Centrifuge in a microcentrifuge at 4 °C for 20 min at 12,000 rpm was performed. Then the tubes were gently removed from the centrifuge and placed on ice, then the supernatant was aspirated and placed in a fresh tube kept on ice, and the pellet discarded.
2.4. Immunohistochemistry and H&E staining
Cochlear lateral wall tissue was obtained from young male adult (N = 4, 3 mon) and old aged mice (N = 4, 30–33 mon). Rosenthal’s canal was divided into three regions (apical, middle, and basal), and the three regions were used for evaluation of cochlear histology. We used 3 mice per group for SV thickness measurements. Antibodies for NKA α1 and β2 subunits were purchased from Abcam (Cambridge, MA; ab7671, ab110730), and the β1 subunit antibody was purchased from Santa Cruz (Santa Cruz, CA; sc-25709). The slides were washed 2 × 5 min in TBS-T (0.001% Tween 20, Fisher scientific #BP337) in Tris-buffer saline with gentle agitation. Sections were then incubated with 1% Triton X-100 (Alfa Aesar, Ward Hill, MA) in PBS at room temperature for 30 min. Sections were then transferred to 10% goat serum (Sigma G9023) with 1% BSA (Bovine Serum Albumin, Fisher Scientific, #BP1600) in TBS for 2 h at room temperature. Slides were drained and blotted with tissue paper. Antibody diluted 1:50 in TBS with 1% BSA was applied to the slides and incubated overnight. Then, slides were rinsed with TBS-T 2 × 5 min, incubated in 0.3% H2O2 in TBS for 15 min, and washed 2 × 5 min with TBS-T. Next, 1 to 3 drops of SignalStain detection reagent with the secondary antibody (HRP, mouse, Cell Signaling Tech, #8125) were applied to the slides and incubated 1h at room temperature. Next, the slides were rinsed 3 × 5 min in TBS-T, and stained with DAB to visualize the staining. The slides were dehydrated, cleared and mounted with coverslips. The slides without the primary antibody incubation were used as negative controls. To determine the NKA expression intensity, ImageJ Region of Interest (ROI) was used with freehand tool software. These ROI can be drawn directly by placing marks of different colors onto positive and negative areas. ImageJ then automatically generates the immunohistochemistry intensity index.
Adjacent serial sections were stained H&E to identify histopathological details. The sections were displayed on the monitor using a digital camera connected to a personal computer. The calibrated image was obtained at a magnification of 20X. Measurements of SV thickness from the portions of the basal, middle and apical turns were performed in each age group using a singleblinded design with three investigators. We followed the Nguyen method to measure the intensity of ROI (Nguyen et al., 2013). The measurement was made by using a cursor to draw a line from the margin of the ROI. Measurements were made at the basal, middle, and apical regions of the cochlea for each mouse, and averages of each region were calculated for each section, using background staining as a scaling factor for the dynamic range of the DAB. Specifically, we subtracted the background activity from the intensity of the DAB signal of interest. This method scaled all of the images to the same intensity range then 10 sections of the apical, middle, and basal turns were evaluated in one cochlea per mouse.
2.5. Western blot analysis
Cell (scraping) or tissue (homogenized) lysates were prepared in radioimmunoprecipitation assay buffer (RIPA, Pierce #89901, Thermo Scientific, Waltham MA) with protease inhibitor cocktail (#78430, Thermo Scientific, Rockford, IL). Cell and tissue samples were homogenized in buffer, followed by centrifugation at 2000 X for 10 min at 4 °C. Supernatants were subjected to western blot analysis by loading 20 μg protein per lane, after the protein concentrations were determined by the Bradford protein assay. Proteins were fractionated by SDS-PAGE gel electrophoresis and transferred to a PVDF blotting membrane. The blot was incubated with primary antibodies and subsequently with the secondary antibody. First antibody dilution was 1:1000 and the second antibody was 1: 2000. Our pre-experiments demonstrated that target protein expression was proportional to the expression of β-actin. In addition, the pre-experiments showed that target protein expression was proportional to housekeeping gene expression levels (β-actin).
2.6. Relative quantitative RT-PCR
Semi-quantitative and real-time RT-PCR analysis was performed as previously described (Marone et al., 2001; Ding et al., 2013). In brief, total RNA was extracted using the RNAeasy Mini Kit (Qiagen, Valencia, CA). Samples were vortexed for 1 min to shear genomic DNA before loading onto the RNeasy mini columns, and then eluted in a minimum volume of 30 ml and a maximum volume of 2 × 50 ml RNAse-free water. RNA obtained with this procedure was essentially free of genomic DNA. 10 ng of RNA was reverse transcribed and complementary DNA was subjected to PCR amplification. Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) was performed using the Enhanced Avian HS RT-PCR-100 Kit (HSRT20, Sigma, St. Louis, MO). The reverse transcript (RT) reaction took place at 45 °C for 50 min. The competition between primer sets was excluded by adjusting the reaction condition. Then the RT products went to PCR amplification directly. A first cycle of 10 min at 95 °C, 45 s at 65 °C and 1 min at 72 °C was followed by 45 s at 95 °C, 45 s at 65 °C and 1 min at 72 °C for 25 cycles. The conditions were chosen so that none of the RNAs analyzed reached a plateau at the end of the amplification protocol, i.e. they were in the exponential phase of amplification. Each set of reactions always included a no-sample negative control. We also performed a negative control containing RNA instead of cDNA to rule out genomic DNA contamination. The PCR products were analyzed on agarose gels stained with Gel Red Nucleic Acid Stain (Biotium, Hayward, CA).
The quantitative real-time RT-PCR reaction mixture was prepared using the SYBR-Green PCR Master Mix. Thermal cycling conditions were the same as in the semi-quantitative method. Amplification specificity was checked using melting curves. Both negative and positive controls were included in each PCR reaction. All assays were carried out three times as independent PCR runs for each cDNA sample. Gene expression was referenced to the expression of β-actin as the housekeeping gene. Each gene expression level was normalized with respect to β-actin mRNA content. Calculations of expression were performed with the 2∆∆CT method (Bustin et al., 2005). The primers are given in Table 1.
Table 1.
Oligonucleotide primers used to detect Na, K-ATPase isoforms.
| Isoform | Primer | Tm (°C) | Size (bp) |
|---|---|---|---|
| α1 | CCACTACTCCCGAATGGGTG(F) | 59.82 | 141 |
| ACAGATCATCGTTTGGCGGT (R) | 60.04 | ||
| α2 | GACAGATTGGCATGATCCAG(F) | 59.04 | 166 |
| TGCTCGTAGGTCCACTCTTG(R) | 59.03 | ||
| α3 | TATGGGCAGCAGTGGACTTA (F) | 59.3 | 177 |
| CTCAAACAAGCCGAAGATCA (R) | 59.0 | ||
| α4 | CCTGGTGAGCTGAATCAGAA (F) | 58.96 | 182 |
| AGACCCTTGGTCAAGTCCAC (R) | 59.0 | ||
| β1 | TCCCAGTGAACCCAAGGAAC (F) | 59.52 | 166 |
| CCAACACTCGGTTGAGCTTG (R) | 59.41 | ||
| β2 | TGAATCGGGTCATCAACTTC (F) | 58.49 | 276 |
| GCTCATCGTCTGTGGCAATA (R) | 59.83 | ||
| β3 | TTTCCAGTCTCCTTGCTTGA (F) | 58.72 | 242 |
| CGATATCCAACATGCCGTT (R) | 57.85 | ||
| β-actin | GCTCTGGCTCCTAGCACCAT (F) | 56.45 | 112 |
| ACATCTGCTGGAAGGTGGACAGT (R) | 57.69 |
2.7. Na, K-ATPase activity assay
Enzyme activity was determined by measuring the amount of inorganic phosphate (Pi) liberated from ATP conversion using a high throughout colorimetric ATPase assay kit from Innova Biosciences (601–0120) and follows the protocol of Fishburn et al. (2015). In brief, 40-μL reactions contained 10 mM Hepes (pH 7.6), 100 mM potassium glutamate, 10 mM magnesium acetate, 3.5% glycerol, 1 mM DTT, 4 μg BSA, 10 ml lysate (cell or tissue standardized as 10 μg/ml). After 40 min at room temperature, purified ATP was added to 0.5 mM, and reactions were incubated 1–20 min at 26 °C. Reactions were stopped by the addition of 10 μl gold mix and, after 4 min, 4 μl stabilizer 2. After 30 min at room temperature, absorbance was measured at 600 nm and plotted against protein concentration. A standard curve was established for every experiment using the phosphate standard.
2.8. Immunoprecipitation
SV-K1 cell or cochlea lateral wall tissue extracts were prepared in a modified radioimmunoprecipitation assay 1 (RIPA) buffer: 50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1 mM EDTA; 1% Nonidet P-40; 0.1% sodium dodecyl sulfate (SDS); 1 mM dithiothreitol; 1:200-diluted protease inhibitor cocktail (Sigma); 1 mM PMSF; 10 mM NEM; and 0.1 mM iodoacetamide. 200 μl of protein was incubated with 30 ml of protein A-agarose suspension (Santa Cruz, Huston, TX) and primary antibody at 4 °C with continuous rotation for overnight. After microcentrifuging, for 30 s at 4 °C, the pellet was resuspended with 20 μl 3X SDS sample buffer. Following vortexing, microcentrifuging for 30 s was done. Then the sample was heated to 95 °C for 5 min and microcentrifuged for 1 min at 18,000 × g. It was then loaded (30 μl) on an SDS-PAGE gel (12%). Bound proteins were released in 1 × SDS sample buffer, resolved by SDS-polyacrylamide gel electrophoresis, transferred onto a PVDC membrane (GE Healthcare, Piscataway, NJ), the next step for antibody incubation was same like we described in western blot analysis and visualized by using enhanced chemiluminescence detection reagents (Pears, Shelton CT) according to the manufacturer’s instructions.
2.9. Densitometric analysis
Scanning was performed using a film or gel with a desktop scanner using a grey scale mode at a DPI of 300. The file was then saved in a TIFF format and processed using the Process > Subtract Background command to reduce background noise. The “rectangle” tool from ImageJ was used to frame the largest band for each row. For each protein band across the lanes, we defined each single region of interest using the same frame as the largest band. With the same frame as the protein (row) we took a background measurement. This procedure was done for the other rows or loading controls and the measurements for the bands and their backgrounds were exported to Excel. The pixel density for all data (bands/controls + their backgrounds) were inverted and put in new columns. The inverted values are expressed as 255 – X, where X is the value recorded by ImageJ. For the protein bands and loading controls, the net values were expressed by deducting the inverted background from the inverted band value. When the net bands and loading controls were calculated as the final step, a ratio of a net band value over the net loading control of that lane was calculated. The final relative quantification values are the ratio of the net band to the net loading control.
2.10. Statistics
Images from films and Gels were imported into Adobe Photoshop (v 5.0), and analyzed using Adobe Photoshop CS and Meta-Morph Imaging Software (Molecular Devices, Center Valley, PA) for the densitometry analysis. Data are reported as mean ± SD as indicated. Statistical analysis was performed with GraphPad Prism 5.0 (GraphPad, La Jolla, CA). Differences were analyzed with a 1-way or a 2-way repeated measure analysis of variance (ANOVA) as appropriate, or a two-way ANOVA followed by Bonferroni post hoc t-tests, that were corrected for multiple comparisons. Values of p < 0.05 were considered significant.
3. Results
3.1. Determination of the mRNA expression of Na, K-ATPase subunits in mouse cochlear samples
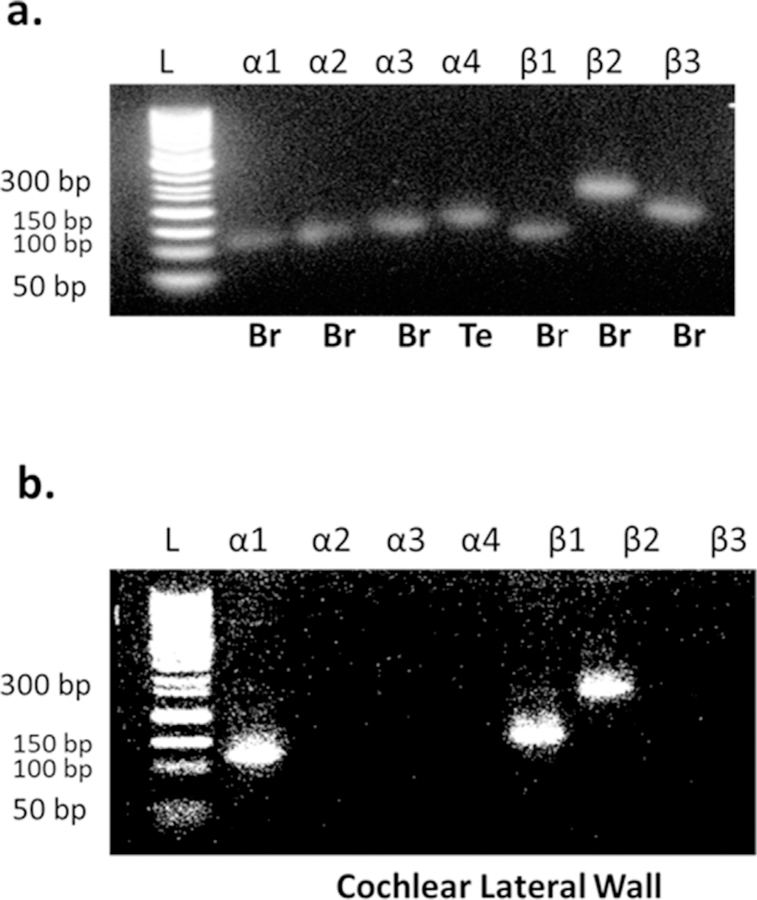
One of the primary goals of the present study was to detect which isoforms of NKA are expressed in the cochlear SV. Fig. 1 provides the results of RT-PCR products from α1, α2, α3, α4, β1, β2 and β3 specific probes for the amplification of mRNA signals in total cochlear RNA. Negative controls were incorporated by performing RT-PCR without the addition of reverse transcriptase. All primers are listed in Table 1. Total RNA from isolated brains of young CBA/CaJ mice were used as positive controls for α1, α2, α3, β1, β2 and β3 subunits except the α4 subunit. We were not able to detect α4 signal which has been reported as present in some reproductive tissues (Peng et al., 1997; Hlivko et al., 2006; Jimenez et al., 2011; Woo et al., 2000), so total RNA from the testis of young CBA/CaJ mice were used for the α4 positive control (Fig. 1a). No mRNA expression was shown in the negative control, but gene expressions of NKA subunits were seen in all positive controls. The RT-PCR method only detected the α1, β1 and β2 mRNA expressions in the cochlear lateral wall of young adult mice, and no mRNA signals representing α2, α3, α4 and β3 were observed (Fig. 1b). No evidence of cross hybridization fragments was noted in any experiments, which suggests that genomic DNA was not biasing the results.
Fig. 1.
Selective NKA subunit mRNA expression in cochlear lateral wall of young adult CBA/CaJ mice (n = 6). Total RNA was isolated from testis, brain and lateral wall of cochlea (CBA/CaJ) and subjected to RT-PCR with primers representing the subunits of NKA isoforms (Sigma, SHRT20), using 10 ng of the diluted RNA. a. mRNA expressions from mouse brain and testis were used to confirm the specific detection for all subunit isoforms of NKA (α1, α2, α3, α4, β1, β2 and β3) without any cross hybridization fragment(s) noted in any experiments, (−) negative control without primers added. Lane marked L shows 50 bp DNA ladder. Br: Brain; Te: testis. b. The positive gene expressions of NKA subunit isoforms were detected by RT-PCR in the cochlea lateral wall of young adult mice (3 mon). The primer sequences and fragment sizes are given in Table 1.
3.2. Gene and protein expression of Na, K-ATPase subunits in aged mouse SV
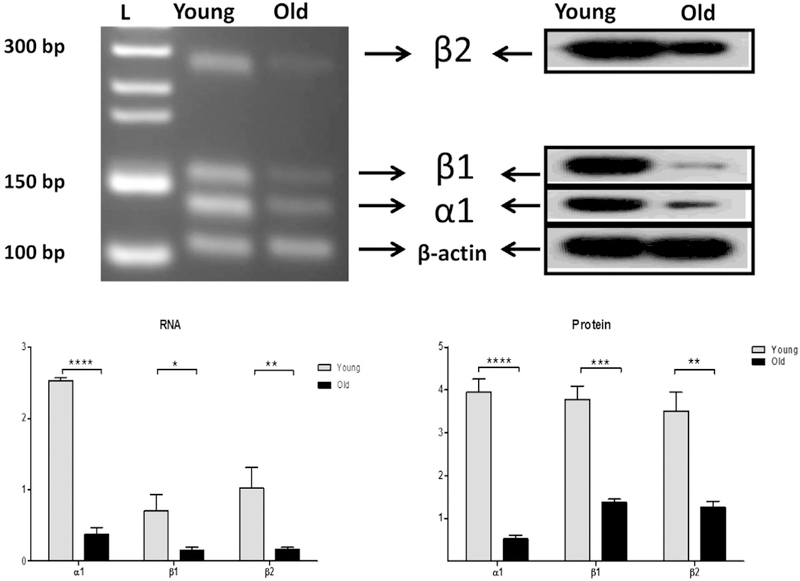
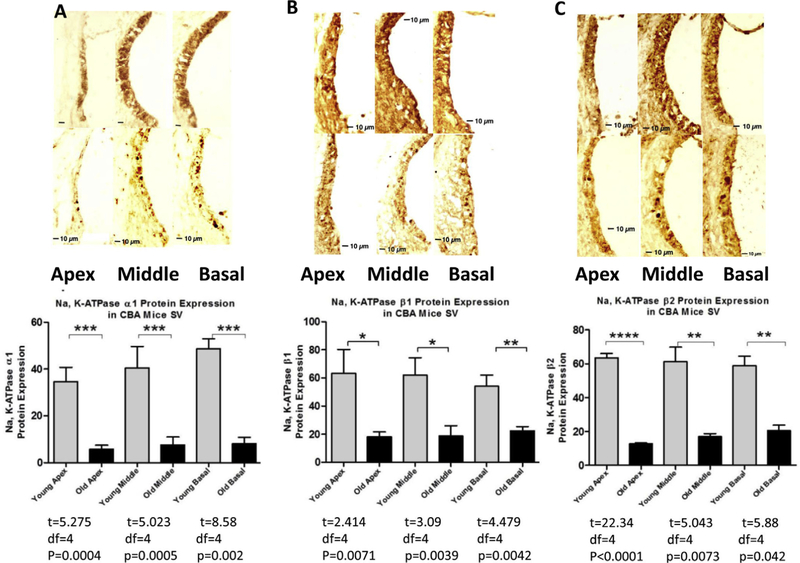
One shortcoming of immunohistochemistry studies is that an antibody may not discriminate between the spliced forms of the protein and the antibody may lack specificity in some circumstances. This may lead to some conflicting results in the studies of NKA distribution (Emanuel et al., 1987; Herrera et al., 1987; Orlowski and Lingrel, 1988; Young and Lingrel, 1987; Mayol et al., 1998; Pierre et al., 2001). To avoid this obstacle, we utilized proteomic (western blot) analysis combined with RT-PCR, especially we selected RT-PCR primers with sequence spanning key introns and bridging an exon-to-exon junction to exclude the possibility of detecting signals from genomic DNA. Semi-quantitative RT-PCR analysis revealed significant declines in mRNA signals α1, β1 and β2 subunit isoforms in old cochlea lateral wall (30 mon) compared to young adult (3 mon) (Fig. 2, a). Real-time RT-PCR presented identical results in mRNA expression of NKA subunits in cochlea lateral wall samples (Fig. 2b). Next, we examined the protein levels of NKA subunit isoforms in samples from same subjects and found that there are identical dys-regulation changes of NKA protein levels (Fig. 2, c) in aged mice (30 mon) compared to young adult (3 mon). These data suggest that the age-related change in NKA subunit protein expressions in cochlea is due to the age –related decline of transcriptional activity. Since the ion channel protein expression is always related with its activity, we measured the NKA activities in young and old cochlea lateral wall samples (Supplement Fig 1, 3 mon vs 30 mon, n = 3 for each group) to determine whether there is a concomitant change between protein expression and activity. The mean level of NKA activity in the cochlear lateral wall of young and old mice was 12.95 ± 1.4 and 3.66 ± 0.7 μmol Pi/mg protein/h. NKA activity was statistically significant different between young and old cochlea with declined activity in old lateral wall samples compared to young samples. To determine the distribution and localization of specific isoforms in the SV lateral wall, whole cochlea sections were used in histology analysis (Fig. 3a,b, and c). NKA is a ubiquitous molecule found in nearly all cell types, such as spiral ganglion, lateral wall fibrocytes, spiral ligament, and hair cells. The dys-regulation of α1, β1 and β2 subunits in the lateral wall of old mice is compatible with the observation in protein expression detected by western blot. However, the decrease in rate is not identical between blot and immunohistochemistry (80% vs. 85%), suggesting there may be an additional factor involving the aging process in regulating NKA’s expression in the lateral wall of SV.
Fig. 2.
Age-related decreases in Na, K-ATPase subunit gene and protein expressions from cochlear lateral walls from young adult (3 mon) and old (30 mon) mice. The protein lysates and mRNA extractions were subjected to RT-PCR analysis and western blot analysis respectively. a. Original RT-PCR result was shown in an agarose gel image. For the α1 subunit, the expected product of 141 bp was observed; b1 subunit, 166 bp; and β2 subunit, 276 βp. Lane L is 50 bp DNA ladder. b. Quantitative real-time RT-PCR is summarized in a bar graph. The differentiation of expressions are shown as arbitrary units. c. Left panel: NKA subunit protein expressions were shown in western blot. Lane L is the protein molecular weight marker. Right panel: The relative expression levels are summarized by the histograms from 3 independent experiments, using densitometry quantification (NIH, Image J). *p < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001. Bar graph results are means ±SD from 3 independent experiments.
Fig. 3.
Cross sections from cochlea samples with immunohistochemistry staining revealed that the NKA subunits of SV declined significantly with age in all three cochlear turns. The three measurement points apex, middle and base were determined by dividing the entire lateral wall as equal parts and measuring the middle points in each part. Immunostaining for the NKA α1, β1 and β2 subunits in the cochlea of young adult CBA/CaJ mice (3 mon, upper row) show stronger staining in the SV compared to old mice (30 mon, lower row). Section-thickness is 5 μm, Magnification: 40X. Histology (lower row) of representative cochlear cross-section of an animal at 30 mon reveals significant decreases of α1 (A), β1(B) and b2(C) subunits in SV of all turns. The relative expression levels, as measured by densitometry performed on the anatomical sections, are summarized by the histograms from 3 independent experiments (lower panel). *p < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001.
3.3. Atrophy of the lateral wall with age
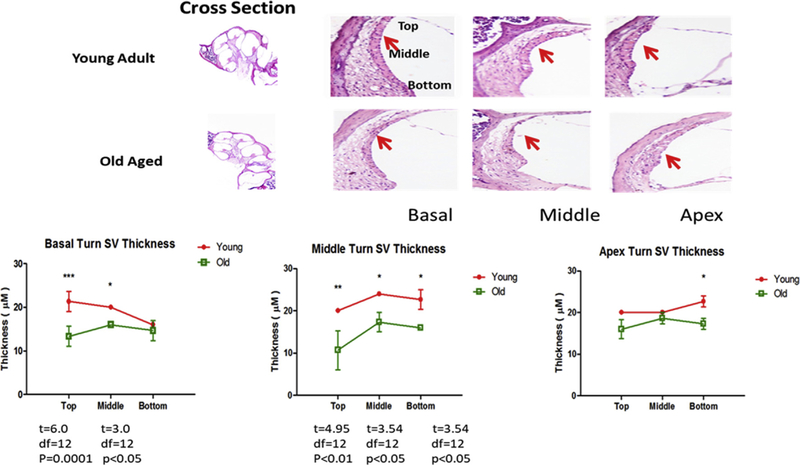
The age-linked morphological abnormalities of the lateral wall, including SV, have been noted in several prior investigations (Wu and Marcus, 2003; Hellier et al., 2002; Johnson et al., 2000; Schulte and Schmiedt, 1992; Spicer et al., 1997; Gratton et al., 1995, 1997). Therefore, we sought to answer the question: are there morphological changes in the lateral wall related to the declines in gene and protein expression? H&E staining was used in the sections from samples from the same subjects studied for immunohistochemistry. It revealed significant changes in the SV in all three turns of the cochlea (Fig. 4). For quantitative analyses, 3 regions on each anatomical section were evaluated: apex, middle and basal turns (anatomy shown in Fig. 4, cross section). Basic atrophy of the lateral wall is defined as the difference in thickness between young adult and old mice. The decrease in lateral wall thickness between old and young mice is up to 20% in all three turns, but the decrease in expression of NKA mRNA and protein is greater than 80% (Fig. 3, using β-actin as the loading control). The difference in the age-related changes in the size of the lateral wall compared to the much greater decline in subunit expression levels with age suggests that lateral wall atrophy is another factor for which NKA activity declines with age, but it may be an independent factor.
Fig. 4.
Young adult and old aged CBA/CaJ cochlear cross sections stained with H&E showed atrophy of SV with age in all three turns. Section-thickness is 5 μm, Magnification: 2.5 × 1.6. All cochlear turns are distinguishable as: apical, middle and basal. Thickness of the SV was measured in the basal, middle and apical turns as shown in the top of figure and the average of thickness is shown in the histogram in lower panel. The difference in SV thickness of young adult and old mice at same locations were calculated, and the statistical significance was represented as * p < 0.05; **p < 0.01; ***p < 0.005. Bar graph results are means ±SD from 3 independent experiments.
3.4. Subunit composition and the selective association of Na, K-ATPase α-β heterodimers
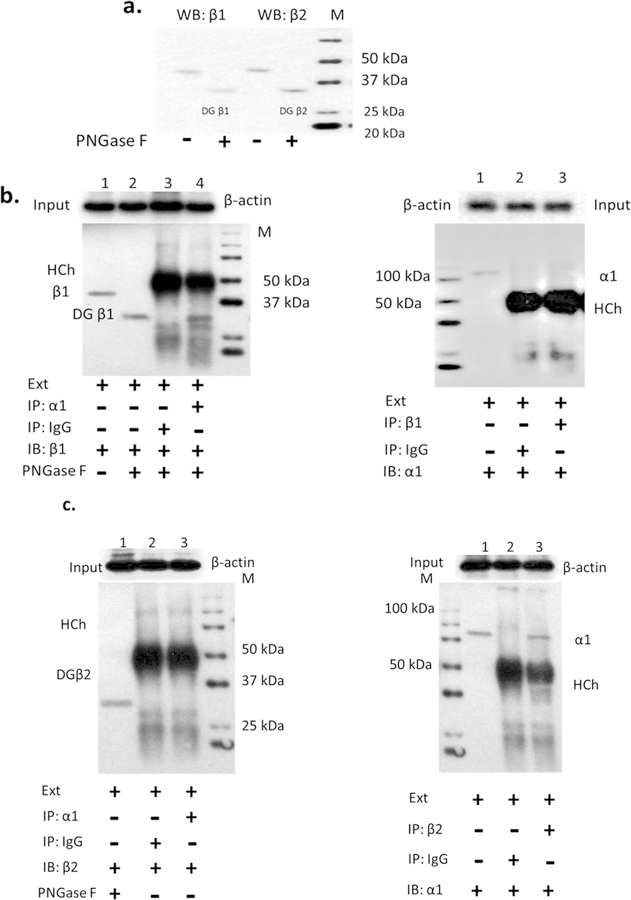
To be an intact catalyzing unit, the NKA channel must form a functional unit containing at least one α and one β subunit (Tokhtaeva et al., 2012a, 2012b, Sweadner et al., 2000, Geering, 2008). Theoretically each of the four α subunit isoforms can assemble with each of the three β subunit isoforms to form an active enzyme, but Tokhtaeva et al. (2012) reported that a selective assembly of NKA α-β heterodimers may be tissue- or regionspecific in mouse brain (Tokhtaeva et al., 2012). Based on this information, we examined the possibility of selective association between α and β subunit isoforms in the cochlear lateral wall using immunoprecipitate technique. The normal molecular weight of NKA β subunits is 38–45 kDa, which approximates the molecular weight of an IgG heavy chain (HCh). If the products of immunoprecipitation are followed by a western blot, the signals from immunoglobulin IgG heavy chain and NKA β subunits will merge due the similarity in molecular weight. In order to avoid this we used PNGase F to cleave N-glycans in the β subunit, which resulted in an increase in electrophoretic mobility and acted to separate NKA β subunits from immunoglobulin heavy chains. We found that a band at 33–35 kDa consists of deglycosylated β subunits, with molecular weights lower than heavy chain bands (50–60 KDa). To validate the antibodies used, western blots were employed to detect β subunit isoforms, with the immunoblot incubated with or without PNGase F (Fig. 5A). The differences of mobility for the conditions with and without PNGase F cleavage revealed that immunoprecipitation of the α1 subunit from the mouse cochlear cell line SV-K1 extracts led to co-precipitation of the β1 isoform (Fig. 5 b left panel, Line 2 vs. Line 4), or vice versa (Fig. 5b right panel line 1 vs. line 3). Co-precipitation of the β2 subunit by immunoprecipitation of α1 or co-precipitation of the α1 subunit by immunoprecipitation β2 did not occur (Fig. 5c both panels line 1 vs. line 3). These data suggest that the interaction between the α1 and β2 subunits in the mouse cell line from the cochlear lateral wall is not as strong as the binding between α1 and β1 subunits.
Fig. 5.
The Na, K-ATPase α1-β1 subunits were co-immunoprecipitated from SV-K1 cells. Proteins were collected from mouse SV-K1 homogenate in RIPE buffer with proteinase cocktail. Antibodies against NKA isoforms subunits were validated by western blot (line 1 in a, b and c). The PNGase F cleaves N-glycans from β isoforms subunit, resulting in elevated mobility leading to separation from precipitated IgG heavy chain (HCh, IgG and NKA β subunits have similar molecular weight around 50–60 KDa). a. The β1 and β2 subunits have different mobility with or without PNGase F pre-incubation: cleaved β1 and β2 subunits (PNGase F) have quicker mobility than non-cleaved ones, it leads to expression signals lower than the normal positions (around 50–60 KDa) and uncovered by the expression signal of IgG. b. Analysis of the immunoprecipitated (IP) α1 or β1 subunit isoforms with immunoblot (IB) of β1 subunit or α1 subunit, shows that the α1 subunit is preferentially co-precipitated with the β1 subunit (left panel, line 2 vs line 4; right panel line 1 vs. line 3). c. β2 antibody was co-immunoprecipitated with the α1 subunit (left panel) or Vis versa (right panel) did not interact with α1 and β2 subunit isoforms (in both panels, line 1 vs line 3). IgG used for co-immunoprecipitation with cell lysate extraction is a negative control. Ext: cell lysate extraction. Input: cell lysate without IP.
3.5. Expression of α1, β1 and β2 subunits
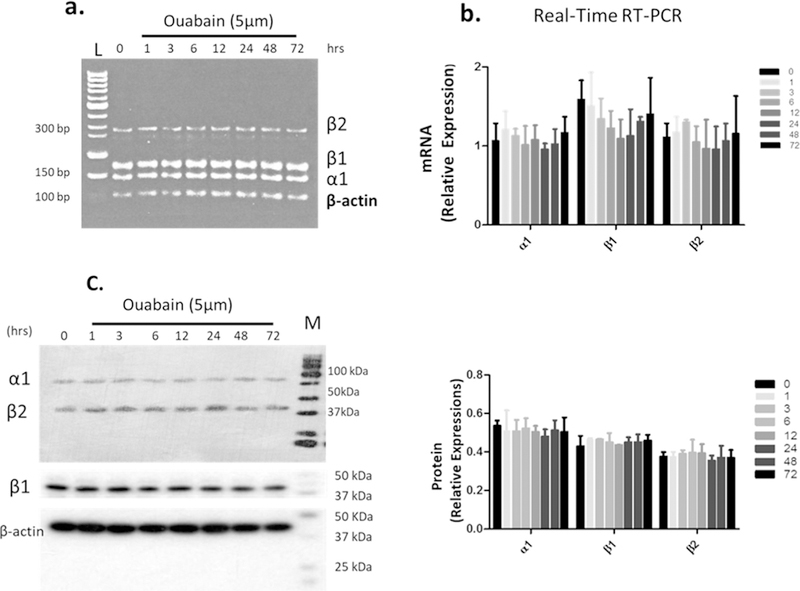
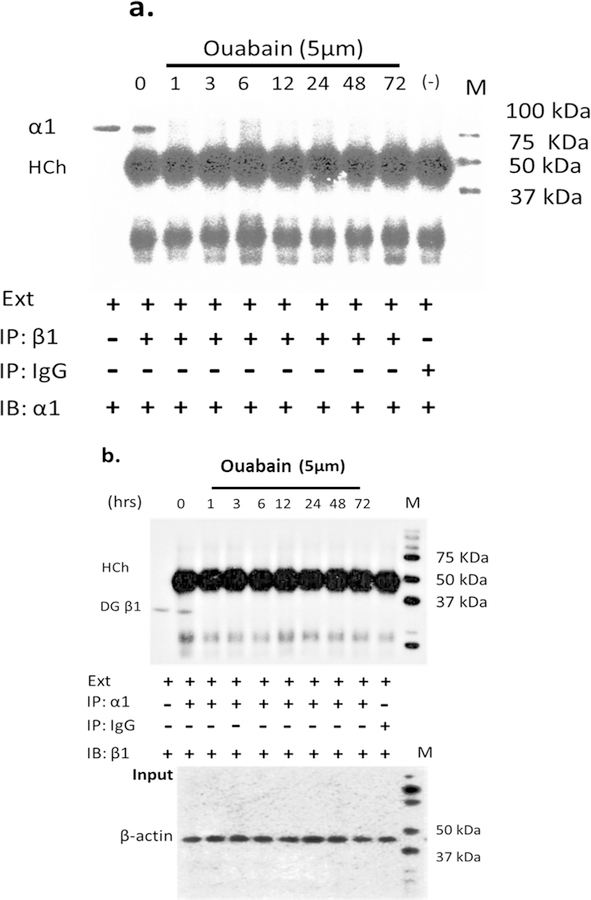
Research on Mongolian gerbils with metabolic presbycusis (i.e., involving SV) has demonstrated a change in NKA activity with dys-regulation in the aging cochlea (Gratton et al., 1995, 1996,1997; Sakaganchi, 1998), suggesting that aging has an effect on blocking NKA activity. But whether or not inhibition of NKA activity will change its heterodimer combination is unknown. Ouabain is a cardiac glycoside whose mechanism of action lies in its binding to and inhibition of NKA activity (Gao et al., 2002). Several reports revealed that ouabain can modulate NKA subunit composition in certain non-proliferating cells, such as neonatal cardiomyocytes and neurons (Yamamoto et al., 1993; Huang et al., 1997; Kometiani et al., 2000; Tian et al., 2009). Therefore, we used ouabain to test our hypothesis. Since the dys-regulation of activity in NKA was observed in the lateral wall of old animals, we simulated aging effect in vitro by administering ouabain to SV cell line-SV-K1, an epithelial cell line cloned from the SV of an immortomouse (H2kbtsA58, House Ear Institute., Los Angeles, CA) and originates in the mouse cochlear lateral wall with non-proliferative characteristics under non-permissive conditions. We used 5 μM ouabain to treat cells based on preliminary results from dose-response experiments. 5 μM ouabain led to an activity blockage rate similar to the activity inhibition rate between the young adult and old cochlea (Supplement Fig. 2); and a time course study showed that this treatment could reach a stable inhibition after 6 h of ouabain application (Supplement Fig 3). There were no significant changes in mRNA and protein expressions of NKA isoforms α1, β1 and β2 for up to 72 h of ouabain administration (Fig. 6). But the co-precipitation of the β1 subunit by immunoprecipitation of α1, or co-precipitation of α1 subunits by the immunoprecipitation of β1 subunits did not occur in ouabain treated cells. Simply put, ouabain prevented the interaction between the α1 and β1 subunits (Figs. 7 and 8, ouabain treatment from 1 to 72 h showed no signals of α1 or β1 after co-precipitation). However, the interaction (binding) between α1 and β1 subunits was observed for the non-treated control cells (Fig. 7a and b, 0 time point for ouabain). Therefore, it is likely that the interactions between the α1-β1 heterodimer are associated with the activity levels of NKA. Our data suggest that gene and protein expression of α1, β1 and β2 subunits is not associated with the inhibition of NKA activity, but the inhibitory action in NKA regulates the association between α1 and β1 subunits.
Fig. 6.
Inhibition of NKA activity by ouabain (5 μM) does not change the mRNA and protein expressions of α1, β1 and β2 subunits. SV-K1 cells (marginal cell line of SV) that were treated with 5 μM ouabain for the time periods (hr) as indicated. a. The mRNA levels were detected by RT-PCR and shown in agarose gel image, Lane L is 50 bp DNA ladder. b. The values of quantitative real time RT-PCR was summarized in a bar graph. c. The protein expression was measured by western blot (left panel) and histogram of relative α1, β1 and β2 subunits protein expressions was obtained with densitometry (NIH, Image J) and shown in right panel. Results indicate that there is no change in NKA mRNA levels and protein expressions due to ouabain treatment (inhibitor of NKA activity). Data shown in bar graph are means ±SD from 3 independent experiments.
Fig. 7.
Ouabain blocks the interaction between α1 and β1 subunits. Cells were treated with 5 μM ouabain and activity was measured up to 72 hrs. Cell lysates were immunoprecipitated (IP) with anti-β1 antibody (a) or anti-α1 antibody (b) and immunoblotted (IB) with anti-α1 or anti-β1 antibody. The signals of α1 and β1 subunits disappeared due to the ouabain treatment compared to non-treatment. IgG used for co-immunoprecipitation with cell lysate extraction is a negative control. Ext: cell lysate extraction. Input: cell lysate without IP.
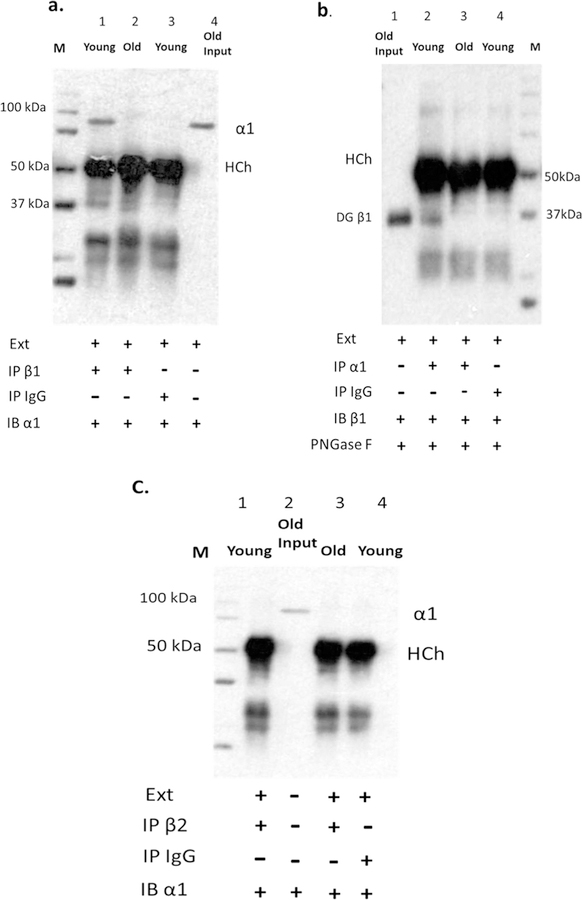
Fig. 8.
The association between α1 and β1 in cochlea of young adult and old CBA/CaJ mice. Cochlear lysates were immunoprecipitated (IP) with anti-β1 antibody (a, line 1 and 2) or anti-α1 antibody (b, line 2 and 3) or anti-β2 antibody (C, line 1 and 3), an equal amount of rabbit IgG was used as negative control (a, line 3; b, line 4; c, line4), and western blots (IB) were performed with anti- α1 (a), anti- β 1 (b), or anti- β2 (c) antibodies. Old Input: cell lysate from cochlear lateral wall of old mouse without IP (as positive control; a, line 4; b, line 1 and c, line 2). IgG used for co-immunoprecipitation with cell lysate extraction is a negative control (a, line 3; b, line 4; c, line4). Ext: cell lysate extraction.
3.6. Age-related disassociation between α1 and β1 subunit isoforms in the mouse cochlea
Decreases in EP and NKA activity levels have been observed in the lateral wall of aged gerbils and old mice (Schulte and Schmiedt, 1992; Gratton et al., 1995, 1997). Consistent with this, when young adult mice were treated with ouabain, the EP was observed to decrease (Higashiyama K. 2009 ORL). Tokhtaeva et al. recently reported that the diversity of the α-β NKA heterodimers in vivo is determined not only by cell-specific co-expression of particular isoforms, but also by selective association of the α and β subunit isoforms (Tokhtaeva et al., 2012a). The ouabain experiments in the present investigation suggest that there may be an alteration of association between α1-β1 heterodimers in the aging cochlea underlying a “decrease” in NKA functionality with age. To test this hypothesis, we compared the immunoprecipitation of the NKA α1 subunit for co-precipitation of β1 for young adult and old cochlear lysates. The α1 subunit was detected in immunoprecipitated fractions from young adult cochlear lysates, but the α1 subunit was not detected in cochlear immunoprecipitated fractions from old mice (Fig. 8 a, line 1 vs line 2). We reversed the immunoprecipitation order by using the β1 subunit, resulting in co-precipitation of the α1 subunit for young adult mice but not for aged mice (Fig. 8b, line 2 vs. line 3), suggesting that aging plays a role in the de-regulation of interactions between α1 and β1 subunit isoforms in the cochlear lateral wall. The interaction between α1 and β2 subunits was further investigated using co-precipitation of the α1 subunit with β2 subunit antibody in cochlear lateral wall lysates from young adult and old mice, and immunoblotting with α1 subunit antibody. There was no α1 subunit signal in western blot analysis from the cochlear lateral wall lysates of young and old mice (Fig. 8 c, line 1 vs. line 3), suggesting that the preferred heterodimer of NKA in lateral wall of SV is α1-β1 format.
4. Discussion
Our study provides insights into the possible selectivity of the various heterodimers of NKA subunit isoforms and the roles they may play in the progression of degenerative age-related changes in SV of the mouse cochlear lateral wall. We observed significant atrophy of SV in all three cochlear turns, and NKA subunit isoform expression decline with age in CBA/CaJ model, similar to the earlier findings in aged gerbils (Schuknecht et al., 1974; Schulte and Schmiedt, 1992., Schmiedt, 1996), and mice (Ohlemiller, 2009, 2010). Age-related atrophy of SV is characterized by a sensorineural hearing loss with flat or mildly sloping audiometric patterns and relatively normal speech discrimination in quiet which Schuknecht describes as “metabolic presbycusis” (Schuknecht et al., 1974; Pauler et al., 1988).
The extent of strial atrophy and loss of immunoreactive NKA in SV lateral wall are of different magnitudes, 20% vs. 80%, respectively. Therefore, we hypothesize that the decreases in expression and activity of NKA with age in the cochlear SV and morphological changes of SV lateral wall are co-contributors in the regulation of function across the lifespan in SV. The decrease in NKA expression would decrease the expression of other proteins interacting with it, even proteins that are involved in cell structure and morphology.
Knowledge of which isoforms of NKA are expressed in SV is important for gaining insights into underlying mechanisms of ARHL, which will have translational pharmacological implications. For example, ouabain is an antagonist of NKA belonging to the cardiac glycoside family. Its therapeutic profile is different from that of digitalis derivatives (Fuerstenwerth, 2014). Both ouabain and digitalis are inhibitors of NKA, but only ouabain affects hearing, likely due to the down-regulation of NKA activity specifically in the cochlea (Higashiyama et al., 2009; Hibino et al., 1997; Kusakari et al., 1978; Konishi and Mendelsohn, 1970). It has been noted that the cardiac glycosides show variable affinity for different NKA α isoforms. In particular, binding affinities of the digitalis glycosides, digoxin, β-methyl digoxin, and digitoxin show moderate but highly significant selectivity for α2/α3 over α1. In contrast, ouabain shows moderate selectivity for α1 over α2. Binding affinities for the three isoforms of: digoxigenin, digitoxigenin, and all other glycones (digitalis family) tested, are the same (Katz et al., 2010). Based on these findings, it is likely that the α1 subunit is more susceptible to the effects of ouabain (selectivity). Similar to the results reported previously (Schulte and Steel, 1994), we found that α1 is the only a subunit among the four α isoforms that are expressed in the cochlea, whereas in the heart and brain the other digitalis isoforms probably interact with α2 or α3 subunits (Fina and Ryan, 1994; Tokhtaeva et al., 2012).
As reported here for the first time, the selective assembly form of the heterodimer is α1 and β1 in the cochlea, but not α1 and β2 isoforms, which may have specific functional implications. However, the molecular mechanisms for the assembly processes of the α and β subunits are still poorly understood (Beggah et al., 1993; Geering et al., 1993; Eakle et al., 1994; Hamrick et al., 1993; Jaunin et al., 1993; Lemas et al., 1994), therefore, it is difficult to draw general conclusions. NKA is an integral membrane protein and a heterodimer comprised of a 100-kDa α-subunit that spans the plasma membrane 10 times, and a 40–60-kDa glycoprotein β-subunit that has a short cytoplasmic N-terminal domain, a single transmembrane domain, and a large extracellular domain (Thomas and Brugge, 1997; Geering, 2008; Kaplan, 2002). It is likely that the interactions between α and β subunits have functional significance for the physiological activity of NKA. For example, reduction of a disulfide bond existing between Cys 158 and Cys 175 of the β-subunit results in loss of enzymatic activity of the purified enzyme and binding ability to α (Beggah et al., 1997). Even though under specific experimental conditions such as oocyte injection of α and β subunit isoforms, there does not seem to be a preference of a given α subunit for a particular β (Geering et al., 1993; Hasler et al., 2001). In contrast, preferential interactions between α and β subunit isoforms were observed in a yeast two-hybrid system (Colonna et al., 1997) and specific tissues such as brain (Tokhtaeva et al., 2012). For example, α2 can be selectively immunoprecipitated by anti-β2 antibodies in the brain tissue. In addition, the interactions between α and β isoforms are tightly associated with the stability and ion affinity of NKA (Schmalzing et al., 1997; Mishra et al., 2011). It is a unique phenomenal as we reported in cochlea that the preferential assembly of NKA involves α1-β1 heterodimer, whereas the unassembled β2 with α1 subunits are also present. The selective interaction between α1 and β1 subunits in mouse cochlea may be a natural selection for the benefit of cochlear physiological function, as it is known that α2 and β1 are less stable that α1 and β1 (Tokhtaeva et al., 2012). Specifically, phosphatidylserine stabilizes NKA and the α2 subunit has weaker interaction ability with phosphatidylserine. In other words, the α-β complex with α2 is less stable compared to other complex combinations such as α1-β1 or α3-β1 (Kapri-Pardes et al., 2011). The presence of the stronger α-β complex, α1-β1 in the cochlea, is no doubt a significant benefit for the maintenance of high K+ concentrations in endolymph and the resulting EP (Blanco and Mercer, 1998; Crambert et al., 2000) based on following findings: a family of small, single-span membrane proteins (the FXYD family) has been defined, based on their sequence and structural homology, as tissue-specific regulators of NKA (Crambert et al., 2002), although the functional characteristics of the FXYD family have not yet been fully studied, most studies suggested that various FXYD proteins can act as tissue-specific regulatory subunit for NKA and preferentially associate with the NKA α 1- β isozymes (Teriete et al., 2007). Specifically, it was found that FXYD6 interacts preferentially with α1, β1, and β2, but not other subunits, for the regulation of K+ affinity in cochlear tissue. It has also been shown that the activity of NKA is dependent on the concentration of cytoplasmic Na+ and extracellular levels of K+ (Therien and Blostein, 2000; Nakao and Gadsby, 1989). Compared with the α1 isoform, the α2 and α3 isoforms have lower affinities for Na+ (Horisberger and Kharoubi-Hess, 2002; Crambert et al., 2000) and may be more suited for physiological systems that create or regulate Na+ concentrations. Therefore we consider that one possible explanation for the absence of other α isoforms except β1 in the cochlear lateral wall including the SV marginal cells is that low concentrations of Na+ may not a preferential condition for the presence of the α2 and α3 subunits. On the other hand, FXYD6 associates with NKA α1-β1 and α1-β2 isozymes, which are preferentially expressed in different regions of the inner ear and can be co-immunoprecipitated with NKA. In addition, based on the functional roles in the cochlea, the K+ and Na+ affinities for the α1-β1 and α1-β2 isozymes are different (Delprat et al., 2007; Nin et al., 2008). The association between FXYD6 and NKA α1-β1 isozymes slightly decreases their apparent K+/Na+ affinities, which may be necessary for the efficient extrusion of Na+ during neuronal activity in cochlea or may be involved in other regulatory mechanisms of the expression or function of NKA isozymes in cochlea cells. The association between FXYD6 and α1-β2 increases their apparent K+/Na+ affinities. Commensurately, FXYD6 is strongly expressed in the SV marginal cells and possibly in the intermediate cells, which mainly express α1-β2 NKA isozymes (Peters et al., 2001). α1-β2 NKA complexes associated with FXYD6 with a high apparent affinity for Na + might be necessary to maintain the low intracellular Na + concentrations in cochlear marginal cells that have been reported by Ikeda et al. since the SV contains a very high K+ concentration (Ikeda and Morizono, 1989; Delprat et al., 2007). Based on our data, it is possible that heterodimer transformations from the EP-2 confirmation to the EP-1 confirmation are related to NKA activity changes and stability, since we obtained similar results for the subunit interactions and immunoprecipitation experiments for in vivo (comparison between young adult and old cochlear samples) and in vitro (comparison between ouabain and non-treated samples). As reported recently by Habeck and colleagues (Habeck et al., 2017), two independent binding sites have been identified that implicate mutual interactions between FXYD and EP-2 confirmation changes for the EP-1. Site A is formed by αTM8–10 and the FXYD transmembrane helix and harbors one molecule of 18:0/18:1 PS and one cholesterol molecule, which together stabilize but do not per se affect NKA activity. At a second site, B, located between αM2, 4, 6, and 9, 18:0/20:4 or 18:0/22:6 PC/PE bind and stimulate activity by accelerating E1P–E2P conformational transitions, but do not affect stability. The different effects of lipid classes at the separate sites are independent, and modulate distinct properties of NKA, so further investigation is needed to understand the mechanisms, including potential associations between FXYD and NKA in the regulation of SV function across the lifespan.
We observed the dys-regulation of NKA activity in lateral wall of SV in old CBA/CaJ mice compared with young SV lateral wall. Interestingly using ouabain to decrease activity in SV-K1 cell lines showed that the interaction between α1 and β1 subunit isoforms disappeared with inhibition of activity of NKA. However, there is no change in the levels of mRNA and protein expression of NKA subunit isoforms. In addition, the dissociation of the α1-β1 complex was observed in the aged cochlea. Taken together, our data suggest that the dys-regulation of NKA activity may be connected to age-related regulation of binding between the α1 and β1 subunits. Our observation that the affinity of purified, native, α1 subunits from the mouse cochlea are primarily associated with β1 but not at all with β2 is compatible with some views about the functions of NKA in the cochlea found by other groups. A unique characteristic of the β1 subunit is the presence of three disulfide bridges in its structure, with locations between Cys125—Cys148, Cys158—Cys174, and Cys212—Cys275 (Beggah et al., 1997). The glycosylation and disulfide bond formation in the β1 secondary structure can alter folding, tertiary and quaternary protein structure, and functional expression of NKA. The removal of just one of the disulfide bonds by site-directed mutagenesis of the involved cysteines has been shown to be sufficient to abolish proper assembly of the α-β-subunits. (Beggah et al., 1997). This type of change could possibly be responsible for the dys-regulation of NKA activity with age, and aging process may weak bonds between the α1 and β1 subunits. However, the mechanism is not fully understand so far, further research is needed to investigate whether there is an alteration in these sulfhydryl bridges in the aged cochlea compared to the young adult cochlea.
For β2 subunits, the story may be different. It has been known that β2 must carry N-glycans to combine with α1, but N-glycans are not a pre-requisite to combine with α2 (Schmalzing et al., 1997). Protein glycosylation is acknowledged as one of the major post-translational modifications for neurodegenerative conditions involved in aging. However the role glycosylation between α1 and β1, or N-glycan, within the cochlear is poorly understood. One possibility, as Tokhtaeva et al., 2012a, b) described, may due to the fact that the β2 subunit persistently binds calnexin (chaperone protein, characterized by assisting protein folding and quality control) in the endoplasmic reticulum and undergoes repeated calnexin-assisted folding prior to its assembly with the α subunit [Tokhtaeva et al., 2010(1); 2010 (2); 2012a,b]. Persistent calnexin binding to glycoproteins is dependent on repeated cycles of de- and re-glycosylation of glycoprotein N-glycans by the ER glucosidase and UGGT1, a folding sensing enzyme (Molinari et al., 2005). It is possible that the β2 subunit is not completely folded by the time calnexin is dissociated from the deglycosylated β2 subunit, and thus is recognized by UGGT1 (Ritter et al., 2005). UGGT1 reglycosylates the β2 subunit’s N-glycans, which results in repeated calnexin binding but not α subunit interaction. Also we agree with Schmalzing and colleagues that the formation of a particular Na, K-pump isozyme is governed by preferential formation of the most stable α-β isozymesdi.e., α1-β1, α2-β2, and presumably α2-β1dperhaps in combination with regulated gene expression, and is tissue specific (Schmalzing et al., 1997).
However, despite the importance of NKA in regulating EP function and its potential key role in ARHL, until the present study, it has not been known which NKA α-β heterodimers are responsible for the cochlear EP. So, these important associations between cochlear NKA heterodimers and the EP are need of further investigation as strial presbycusis, defined by EP reduction, does not appear equally in all strains, and likely has a genetic component (Recanzone et al., 1993). CBA/CaJs are a moderate-to-high-risk strain, with correlations between NKA levels and EP magnitudes (Ohlemiller et al., 2010). Specifically the reduction in the EP occurs more prominently in females than males and the onset of EP reduction seems to track estropause in females (Ohlemiller et al., 2010). So for CBA/CaJs, the magnitude of EP reduction varies across animals, so that epigenetic, stochastic, or environmental factors, are likely involved (Ohlemiller, 2009).
5. Summary and conclusions
Major new findings of the present report are, i) the preferential heterodimer in the mouse cochlear lateral wall is α1-β1, not α1-β2, ii) all NKA subunit isoforms are dys-regulated with age, at gene expression and protein levels, and this dys-regulation is not associated with inhibition of NKA activity, but rather a dissociation of the interactions between α1 and β1 subunit isoforms and iii) interactions between the α1 and β1 subunit isoforms in the young adult CBA/CaJ cochlea cannot be detected in aged mice. Teasing apart these age-related changes in a key homeostatic mechanism in the cochlear lateral wall has the potential to lead to the development of new biotherapeutic or pharmacological interventions to ameliorate or prevent the progression of ARHL.
Supplementary Material
Acknowledgements
This work was supported by NIH Grant P01 AG009524. We thank Dr. Shannon Salvog for project support and Dr. James Willott for critically reading and improving the manuscript. We also thank Dr. Yoshihisa Sakei (Otolaryngology, University of South Florida) for the design of NKA primers; and Drs. Tanika Williamson and Parveen Bazard for procedural support.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heares.2018.07.006.
References
- Arystarkhova E, Sweadner KJ, 1996. Isoform-specific monoclonal antibodies to Na, K-ATPase alpha subunits. Evidence for a tissue-specific post-translational modification of the alpha subunit. J. Biol. Chem 271, 23407–23417. [DOI] [PubMed] [Google Scholar]
- Arystarkhova E, Sweadner KJ, 1997. Hormonal and neurogenic control of Na-K-ATPase and myosin isoforms in neonatal rat cardiac myocytes. Am. J. Physiol 273 (2 Pt 1), C489–C499. [DOI] [PubMed] [Google Scholar]
- Beggah AT, Beguin P, Jaunin P, Peitsch MC, Geering K, 1993. Hydrophobic C-terminal amino acids in the beta-subunit are involved in assembly with the alpha-subunit of Na, K-ATPase. Biochemistry 32, 14117–14124. [DOI] [PubMed] [Google Scholar]
- Beggah AT, Jaunin P, Geering K, 1997. Role of glycosylation and disulfide bond formation in the beta subunit in the folding and functional expression of Na, K-ATPase. J. Biol. Chem 11 (15), 10318–10326, 272. [DOI] [PubMed] [Google Scholar]
- Belyantseva IA, Adler HJ, Curi R, Frolenkov GI, Kachar B, 2002. Expression and localization of prestin and the sugar transporter GLUT-5 during development of electromotility in cochlear outer hair cells. J. Neurosci 20 RC116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco G, 2005. Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin. Nephrol 25 (5), 292–303. [DOI] [PubMed] [Google Scholar]
- Blanco G, Mercer RW, 1998. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol 275 (5 Pt 2), F633–F650. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Zhang J, Chen L, Song H, Raina H, Kinsey SP, Izuka M, Iwamoto T, Kotlikoff MI, Lingrel JB, Philipson KD, Wier WG, Hamlyn JM, 2009. The pump, the exchanger, and endogenous ouabain: signaling mechanisms that link salt retention to hypertension. Hypertension 53 (2), 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Nolan T, Pfaffl MW, 2005. Quantitative real-time RT-PCRea perspective. J. Mol. Endocrinol 34, 597–601. [DOI] [PubMed] [Google Scholar]
- Colonna T, Huynh L, Fambrough DM, 1997a. Subunit interactions in the Na,K-ATPase explored with the yeast two-hybrid system. J. Biol. Chem 272 (19), 12366–12372. Issue of May 9. [DOI] [PubMed] [Google Scholar]
- Colonna T, Kostich M, Hamrick M, Hwang B, Rawn JD, Fambrough DM, 1997b. Subunit interactions in the sodium pump. Ann. N. Y. Acad. Sci 834, 498–513. [DOI] [PubMed] [Google Scholar]
- Crambert G, Hasler U, Beggah AT, Yu C, Modyanov NN, Horisberger JD, Leliévre L, Geering K, 2000. Transport and pharmacological properties of nine different human Na, K-ATPase isozymes. J. Biol. Chem 275 (3), 1976–1986. [DOI] [PubMed] [Google Scholar]
- Crambert G, Fuzesi M, Garty H, Karlish S, Geering K, 2002. Phospholemman (FXYD1) associates with Na,K-ATPase and regulates its transport properties. Proc. Natl. Acad. Sci. U.S.A 20 (17), 11476–11481, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delprat B, Schaer D, Roy S, Wang J, Puel JL, Geering K, 2007. FXYD6 is a novel regulator of Na,K-ATPase expressed in the inner ear. J. Biol. Chem 282 (10), 7450–7456. [DOI] [PubMed] [Google Scholar]
- Ding B, Frisina RD, Zhu X, Sakai Y, Sokolowski B, Walton JP, 2013. Direct control of Na+-K+−2Cl- co-transport protein (NKCC1) expression with aldosterone: biotherapeutic implications. Am. J. Physiol (in press). [DOI] [PMC free article] [PubMed]
- Dobretsov M, Stimers JR, 2005. Neuronal function and alpha 3 isoform of the Na/K-ATPase. Front. Biosci 10, 2373–2396. [DOI] [PubMed] [Google Scholar]
- Eakle KA, Kabalin MA, Wang S-G, Farley RA, 1994. The influence of beta subunit structure on the stability of Na+/K(+)-ATPase complexes and interaction with K+. J. Biol. Chem 269, 6550–6557. [PubMed] [Google Scholar]
- Emanuel JR, Garetz S, Stone L, Levenson R, 1987. Differential expression of Na+,K+-ATPase alpha- and beta-subunit mRNAs in rat tissues and cell lines. Proc. Natl. Acad. Sci. U.S.A 84 (24), 9030–9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fina M, Ryan A, 1994. Expression of mRNAs encoding alpha and beta subunit isoforms of Na,K-ATPase in the vestibular labyrinth and endolymphatic sac of the rat. Mol. Cell. Neurosci 5 (6), 604–613. [DOI] [PubMed] [Google Scholar]
- Fishburn J, Tomko E, Galburt E, Hahn S, 2015. Double-stranded DNA translocase activity of transcription factor TFIIH and the mechanism of RNA polymerase II open complex formation. Proc. Natl. Acad. Sci. U.S.A 31 (13), 3961–3966, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerstenwerth H, 2014. On the differences between ouabain and digitalis glycosides. Am. J. Therapeut 21 (1), 35–42. [DOI] [PubMed] [Google Scholar]
- Furukawa M, Ikeda K, Takeuchi S, Oshima T, Kikuchi T, Takasaka T, 1996. August 9 Na+,K(+)-ATPase activity in the cochlear lateral wall of the gerbil. Neurosci. Lett 213 (3), 165–168. [DOI] [PubMed] [Google Scholar]
- Gao JRS, Wymore Y, Wang GR, Gaudette IB, Krukenkamp IB, Cohen IS, Mathias RT, 2002. Isoform specific stimulation of cardiac Na/K pumps by nM concentrations of glycosides. J. Gen. Physiol 119, 297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering K, 2008. Functional roles of Na,K-ATPase subunits. Curr. Opin. Nephrol. Hypertens 17 (5), 526–532. [DOI] [PubMed] [Google Scholar]
- Geering K, Jaunin P, Jaisser F, Mérillat AM, Horisberger JD, Mathews PM, Lemas V, Fambrough DM, Rossier BC, 1993. Mutation of a conserved proline residue in the beta-subunit ectodomain prevents Na(+)-K(+)-ATPase oligomerization. Am. J. Physiol 265 (4 Pt1), C1169–C1174. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Meehan DT, Smyth BJ, Cosgrove D, 2002. Strial marginal cells play a role in basement membrane homeostasis: in vitro and in vivo evidence. Hear Res 163 (1–2), 27–36. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Smyth BJ, Schulte BA, Vincent DA Jr., 1995. Na,K-ATPase activity decreases in the cochlear lateral wall of quiet-aged gerbils. Hear. Res 83 (1–2), 43–50. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Schmiedt RA, Schulte BA, 1996. Age-related decreases in endocochlear potential are associated with vascular abnormalities in the stria vascularis. Hear. Res 102 (1–2), 181–190. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Smyth BJ, Lam CF, Boettcher FA, Schmiedt RA, 1997. Decline in the endocochlear potential corresponds to decreased Na,K-ATPase activity in the lateral wall of quiet-aged gerbils. Hear. Res 108 (1–2), 9–16. [DOI] [PubMed] [Google Scholar]
- Habeck M, Kapri-Pardes E, Sharon M, Karlish S, 2017. Specific phospholipid binding to Na,K-ATPase at two distinct sites. Proc. Natl. Acad. Sci. Unit. States Am 114 (11), 2904–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall John E., Guyton Arthur C., 2006. Textbook of Medical Physiology St. Louis, Mo: Elsevier Saunders. [Google Scholar]
- Hamrick M, Renaud KJ, Fambrough DM, 1993. Assembly of the extracellular domain of the Na,K-ATPase beta subunit with the alpha subunit. Analysis of beta subunit chimeras and carboxyl-terminal deletions. J. Biol. Chem 268, 24367–24373. [PubMed] [Google Scholar]
- Hasler U, Crambert G, Horisberger JD, Geering K, 2001. Structural and functional features of the transmembrane domain of the Na,K-ATPase beta subunit revealed by tryptophan scanning. J. Biol. Chem 2276 (19), 16356–64. [DOI] [PubMed] [Google Scholar]
- Hellier WP, Wagstaff SA, O’Leary SJ, Shepherd RK, 2002. Functional and morphological response of the stria vascularis following a sensorineural hearing loss. Hear. Res 172 (1e2), 127–136. [DOI] [PubMed] [Google Scholar]
- Herrera VL, Emanuel JR, Ruiz-Opazo N, Levenson R, Nadal-Ginard B, 1987. Three differentially expressed Na,K-ATPase alpha subunit isoforms: structural and functional implications. J. Cell Biol 105 (4), 1855–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Horio Y, Inanobe A, Doi K, Ito M, Yamada M, Gotow T, Uchiyama Y, Kawamura M, Kubo T, Kurachi Y, 1997. An ATP-dependent inwardly rectifying potassium channel, KAB-2 (Kir4.1), in cochlear stria vascularis of inner ear: its specific subcellular localization and correlation with the formation of endocochlear potential. J. Neurosci 17, 4711–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama K, Takeuchi S, Azuma H, Sawada S, Kakigi A, Takeda T, 2009. Ouabain-induced vacuolar formation in marginal cells in the stria vascularis is dependent on perilymphatic Na(+). ORL J. Otorhinolaryngol. Relat. Spec 71 (1), 57–66. 10.1159/000265125. [DOI] [PubMed] [Google Scholar]
- Hlivko JT 1, Chakraborty S, Hlivko TJ, Sengupta A, James PF, 2006. The human Na,K-ATPase alpha 4 isoform is a ouabain-sensitive alpha isoform that is expressed in sperm. Mol. Reprod. Dev 73 (1), 101–115. [DOI] [PubMed] [Google Scholar]
- Horisberger JD 1, Kharoubi-Hess S, 2002. Functional differences between alpha subunit isoforms of the rat Na,K-ATPase expressed in Xenopus oocytes. J. Physiol 15 (Pt 3), 669–680, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Kometiani P, Xie Z, 1997. Differential regulation of Na/K-ATPase alpha-subunit isoform gene expressions in cardiac myocytes by ouabain and other hypertrophic stimuli. J. Mol. Cell. Cardiol 29 (11), 3157–3167. [DOI] [PubMed] [Google Scholar]
- Ichimiya I, Suzuki M, Mogi G, 2000. Age-related changes in the murine cochlear lateral wall. Hear. Res 139 (1–2), 116–122. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Morizono T, 1989. Electrochemical profiles for monovalent ions in the stria vascularis: cellular model of ion transport mechanisms. Hear. Res 39 (3), 279–286. [DOI] [PubMed] [Google Scholar]
- Jaunin P, Jaisser F, Beggah AT, Takeyasu K, Mangeat P, Rossier BC, Horisberger JD, Geering K, 1993. Role of the transmembrane and extracytoplasmic domain of beta subunits in subunit assembly, intracellular transport, and functional expression of Na,K-pumps. J. Cell Biol 123, 1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez AM, Stagner BB, Martin GK, Lonsbury-Martin, 1999. Age-related hearing loss of distortion product otoacoustic emission in four mouse strains. Hear. Res 138, 91–105. [DOI] [PubMed] [Google Scholar]
- Jimenez T, McDermott JP, Sánchez G, Blanco G, 2011. Na,K-ATPase alpha 4 isoform is essential for sperm fertility. Proc. Natl. Acad. Sci. U.S.A 108 (2), 644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Erway LC, 2000. A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics 70 (2), 171–180. [DOI] [PubMed] [Google Scholar]
- Kalinec GM, Webster P, Lim DJ, Kalinec F, 2003. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol. Neuro. Otol 8, 177–189. [DOI] [PubMed] [Google Scholar]
- Kaplan JH, 2002. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem 71, 511–535. [DOI] [PubMed] [Google Scholar]
- Kapri-Pardes E, Katz A, Haviv H, Mahmmoud Y, Ilan M, Khalfin-Penigel I, Carmeli S, Yarden O, Karlish SJ, 2011. Stabilization of the α2 isoform of Na,K-ATPase by mutations in a phospholipid binding pocket. J. Biol. Chem 286 (50), 42888–42899, 2011 Dec 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A, Lifshitz Y, Bab-Dinitz E, Kapri-Pardes E, Goldshleger R, Tal DM, Karlish SJ, 2010. Selectivity of digitalis glycosides for isoforms of human Na,K-ATPase. J. Biol. Chem 18 (25), 19582–19592, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kometiani P, Tian J, Li J, Nabih Z, Gick G, Xie Z, 2000. Regulation of Na/K-ATPase beta1-subunit gene expression by ouabain and other hypertrophic stimuli in neonatal rat cardiac myocytes. Mol. Cell. Biochem 215 (1–2), 65–72. [DOI] [PubMed] [Google Scholar]
- Konishi T, Mendelsohn M, 1970. Effect of ouabain on cochlear potentials and endolymph composition in Guinea pigs. Acta Otolaryngol 69 (3), 192–199. [DOI] [PubMed] [Google Scholar]
- Kusakari J, Kambayashi J, Ise I, Kawamoto K, 1978. Reduction of the endocochlear potential by the new “loop” diuretic, bumetanide. Acta Otolaryngol 86 (5–6), 336–341. [DOI] [PubMed] [Google Scholar]
- Lang F, Vallon V, Knipper M, Wangemann P, 2007. Functional significance of channels and transporters expressed in the inner ear and kidney. Am. J. Physiol. Cell Physiol 293 (4), C1187–C1208. [DOI] [PubMed] [Google Scholar]
- Lemas MV, Hamrick M, Takeyasu K, Fambrough DM, 1994. 26 Amnino acids of an extracellular domain of the Na,K-ATPase a-subunit are sufficient for assembly with the Na,K-ATPase b-subunit. J. Biol. Chem 269, 8255–8259. [PubMed] [Google Scholar]
- Li Z, Cai T, Tian J, Xie JX, Zhao X, Liu L, Shapiro JI, Xie Z, 2009. NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J. Biol. Chem 284 (31), 21066–21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingrel JB, 2010. The physiological significance of the cardiotonic steroid/ouabainbinding site of the Na,K-ATPase. Annu. Rev. Physiol 72, 395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingrel JB, Young RM, Shull MM, 1988. Multiple forms of the Na,K-ATPase: their genes and tissue specific expression. Prog. Clin. Biol. Res 105–112. [PubMed]
- Malik N, Canfield VA, Beckers MC, Gros P, Levenson R, 1996. Identification of the mammalian Na,K-ATPase 3 subunit. J. Biol. Chem 271 (37), 22754–22758. [DOI] [PubMed] [Google Scholar]
- Marone M, Mozzetti S, De Ritis D, Pierelli L, Scambia G, 2001. Semiquantitative RT-PCR analysis to assess the expression levels of multiple transcripts from the same sample. Biol. Proced Online 3, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayol V, Dignat-George F, Gerbi A, Martin-Vasallo P, Lesaule G, Sampol J, Maixent JM, 1998. Evidence that human endothelial cells express different isoforms of Na,K-ATPase. J. Hypertens 16 (2), 145–150. [DOI] [PubMed] [Google Scholar]
- McGuirt JP, Schulte BA, 1994. Distribution of immunoreactive alpha- and beta-subunit isoforms of Na,K-ATPase in the gerbil inner ear. J. Histochem. Cytochem 42 (7), 843–853. [DOI] [PubMed] [Google Scholar]
- McLean WJ, Smith KA, Glowatzki E, Pyott SJ, 2009. Distribution of the Na,K-ATPase alpha subunit in the rat spiral ganglion and organ of corti. J. Assoc. Res. Otolaryngol 10 (1), 37–49. 10.1007/s10162-008-0152-9. Epub 2008 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra NK, Peleg Y, Cirri E, Belogus T, Lifshitz Y, Voelker DR, Apell HJ, Garty H, Karlish SJ, 2011. FXYD proteins stabilize Na,K-ATPase: amplification of specific phosphatidylserine-protein interactions. J Biol Chem 286 (11), 9699–9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M, Galli C, Vanoni O, Arnold SM, Kaufman RJ, 2005. Persistent glycoprotein misfolding activates the glucosidase II/UGT1-driven calnexin cycle to delay aggregation and loss of folding competence. Mol. Cell 20 (4), 503–512. [DOI] [PubMed] [Google Scholar]
- Nakao M, Gadsby DC, 1989. [Na] and [K] dependence of the Na/KPump current-voltage relationship in Guinea pig ventricular myocytes. J. Gen. Physiol 94, 539–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DH, Zhou T, Shu J, Mao JH, 2013. Quantifying chromogen intensity in immunohistochemistry via reciprocal intensity. Cancer InCytes 2 (1) (e). [Google Scholar]
- Nin F, Hibino H, Doi K, Suzuki T, Hisa Y, Kurachi Y, 2008. The endocochlear potential depends on two K+ diffusion potentials and an electrical barrier in the stria vascularis of the inner ear. Proc. Natl. Acad. Sci. U.S.A 105 (5), 1751–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama S, Okada T, Kobayashi T, Garcia del Saz E, Seguchi H, 1994. Na-K-ATPase activity in the Guinea pig stria vascularis in experimentally-induced endo-lymphatic hydrops. Histol. Histopathol 9 (No. 2), 205–209. [PubMed] [Google Scholar]
- Ohlemiller KK, 2009. Mechanisms and genes in human strial presbycusis from animal models. Brain Res 1277, 70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Dahl AR, Gagnon PM, 2010. Divergent aging characteristics in CBA/J and CBA/CaJ mouse cochleae. J. Assoc. Res. Otolaryngol 11 (4), 605–623, 2010 Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski J, Lingrel JB, 1988. Tissue-specific and developmental regulation of rat Na,K-ATPase catalytic alpha isoform and beta subunit mRNAs. J. Biol. Chem 263 (21), 10436–10442. [PubMed] [Google Scholar]
- Pauler M 1, Schuknecht HF, White JA, 1988. July Atrophy of the stria vascularis as a cause of sensorineural hearing loss. Laryngoscope 98 (7), 754–759. [DOI] [PubMed] [Google Scholar]
- Peng L, Martin-Vasallo P, Sweadner KJ, 1997. Isoforms of Na,K-ATPase alpha and beta subunits in the rat cerebellum and in granule cell cultures. J. Neurosci 17 (10), 3488–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters TA, Tonnaer EL, Kuijpers W, Cremers CW, Curfs JH, 2001. Developmental aspects of the rat endolymphatic sac and functional implications. Acta Otolaryngol 121 (2), 125–129. [DOI] [PubMed] [Google Scholar]
- Pierre S, Compe E, Grillasca JP, Plannells R, Sampol J, Pressley TA, Maixent JM, 2001. RT-PCR detection of Na,K-ATPase subunit isoforms in human umbilical vein endothelial cells (HUVEC): evidence for the presence of alpha 1 and beta 3. Cell. Mol. Biol 47 (2), 319–324. [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM, 1993. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J. Neurosci 13 (1), 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindler TN, Dostanic I, Lasko VM, Nieman ML, Neumann JC, Lorenz JN, Lingrel JB, 2011. Knockout of the Na,K-ATPase α₂-isoform in the cardiovascular system does not alter basal blood pressure but prevents ACTH-induced hypertension. Am. J. Physiol. Heart Circ. Physiol 301 (4), H1396–H1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter C, Quirin K, Kowarik M, Helenius A, 2005. Minor folding defects trigger local modification of glycoproteins by the ER folding sensor GT. EMBO J 24 (9), 1730–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AF, Watts AG, Simmons DM, 1991. Preservation of mRNA during in situ hybridization in the cochlea. Hear. Res 56 (1–2), 148–152. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Morizono T, 1982. Effects of topical sodium potassium ATPase inhibitors upon endocochlear potential in chinchilla. Otolaryngol. Head Neck Surg 90 (6), 808–813. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N, Crouch JJ, Lytle C, Schulte BA, 1998. Na-K-Cl cotransporter expression in the developing and senescent gerbil cochlea. Hear Res 118 (1–2), 114–122. [DOI] [PubMed] [Google Scholar]
- Schmalzing G, Ruhl K, Gloor SM, 1997. Isoform-specific interactions of Na,K-ATPase subunits are mediated via extracellular domains and carbohydrates. Proc. Natl. Acad. Sci. U.S.A 94 (4), 1136–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA, 1996. Effects of aging on potassium homeostasis and the endocochlear potential in the gerbil cochlea. Hear. Res 102 (1–2), 125–132. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Watanuki K, Takahashi T, Belal AA Jr., Kimura RS, Jones DD, Ota CY, 1974. Atrophy of the stria vascularis, a common cause for hearing loss. Laryngoscope 84 (10), 1777–1821. [DOI] [PubMed] [Google Scholar]
- Schulte BA, Steel KP, 1994. Steel Expression of α and β subunit isoforms of Na,K-ATPase in the mouse inner ear and changes with mutations at the WV or Sld loci. Hear. Res 78, 65–76. [DOI] [PubMed] [Google Scholar]
- Schulte BA, Schmiedt RA, 1992. Lateral wall Na,K-ATPase and endocochlear potentials decline with age in quiet-reared gerbils. Hear. Res 61 (1–2), 35–46. [DOI] [PubMed] [Google Scholar]
- Schulte BA, Steel KP, 1994. Expression of alpha and beta subunit isoforms of Na,K-ATPase in the mouse inner ear and changes with mutations at the Wv or Sld loci. Hear. Res 78 (1), 65–76. [DOI] [PubMed] [Google Scholar]
- Shinoda T, Ogawa H, Cornelius F, Toyoshima C, 2009. Crystal structure of the sodium-potassium pump at 2.4 A resolution. Nature 459 (7245), 446–450. [DOI] [PubMed] [Google Scholar]
- Shugyo A, Mori N, Matsunaga T, 1990. A comparison of the reduction in the K+ activity of the scala media produced by furosemide and ouabain. Eur. Arch. Oto-Rhino-Laryngol 248 (2), 79–81. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Gratton MA, Schulte BA, 1997. Expression patterns of ion transport enzymes in spiral ligament fibrocytes change in relation to strial atrophy in the aged gerbil cochlea. Hear. Res 111 (1–2), 93–102. [DOI] [PubMed] [Google Scholar]
- Sweadner KJ, 1989. Isozymes of the Na+/K+-ATPase. Biochim. Biophys. Acta 988 (2), 185–220. [DOI] [PubMed] [Google Scholar]
- Sweadner KJ, Wetzel RK, Arystarkhova E, 2000. Genomic organization of the human FXYD2 gene encoding the gamma subunit of the Na,K-ATPase. Biochem. Biophys. Res. Commun 279 (1), 196–201. [DOI] [PubMed] [Google Scholar]
- Ten Cate WJ, Curtis LM, Rarey KE, 1994. Na,K-ATPase subunit isoform expression in the Guinea pig endolymphatic sac. ORL J. Otorhinolaryngol Relat Spec 56 (5), 257–262. [DOI] [PubMed] [Google Scholar]
- Teriete P, Franzin CM, Choi J, Marassi FM, 2007. Structure of the Na,K-ATPase regulatory protein FXYD1 in micelles. Biochemistry 46 (23), 6774–6783. Epub 2007 May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therien AG, Blostein R, 2000. Mechanisms of sodium pump regulation. Am. J. Physiol. Cell Physiol 279 (3), C541–C566. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS, 1997. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol 13, 513–609. [DOI] [PubMed] [Google Scholar]
- Tian J, Li X, Liang M, Liu L, Xie JX, Ye Q, Kometiani P, Tillekeratne M, Jin R, Xie Z, 2009. Changes in sodium pump expression dictate the effects of ouabain on cell growth. J. Biol. Chem 29 (22), 14921–14929, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokhtaeva E, Sachs G, Vagin O, 2010. Diverse pathways for maturation of the Na,K-ATPase β1 and β2 subunits in the endoplasmic reticulum of Madin-Darby canine kidney cells. J. Biol. Chem 285 (50), 39289–39302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokhtaeva E, Clifford RJ, Kaplan JH, Sachs G, Vagin O, 2012a. Subunit isoform selectivity in assembly of Na,K-ATPase α-β heterodimers. J. Biol. Chem 287 (31), 26115–26125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokhtaeva E, Munson K, Sachs G, Vagin O, 2012b. N-glycan-dependent quality control of the Na,K-ATPase beta(2) subunit. Biochemistry 49 (14), 3116–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann PK, 2002. K+ cycling and the endocochlear potential. Hear. Res 165 (1–2), 1–9. Review. [DOI] [PubMed] [Google Scholar]
- Woo AL, James PF, Lingrel JB, 2000. Sperm motility is dependent on a unique isoform of the Na,K-ATPase. J. Biol. Chem 275 (27), 20693–20699. [DOI] [PubMed] [Google Scholar]
- Wu T, Marcus DC, 2003. Age-related changes in cochlear endolymphatic potassium and potential in CD-1 and CBA/CaJ mice. J. Assoc. Res. Otolaryngol 4 (3), 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Ikeda U, Seino Y, Tsuruya Y, Oguchi A, Okada K, Ishikawa S, Saito T, Kawakami K, Hara Y, 1993. Regulation of Na,K-adenosine triphosphatase gene expression by sodium ions in cultured neonatal rat cardiocytes. J. Clin. Invest 92, 1889–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RM, Lingrel JB, 1987. Tissue distribution of mRNAs encoding the alpha isoforms and beta subunit of rat Na+,K+-ATPase. Biochem. Biophys. Res. Commun 145 (1), 52–58. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie Z, 2005. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol. Biol. Cell 16 (9), 4034–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.