Abstract
Background
Urinary stone disease is a common condition characterised by increasing prevalence and high rates of recurrence. Observational studies have reported that increased water intake played a role in the prevention of urinary stone formation but with limited strength of evidence.
Objectives
To compare the effects of increased water intake with standard water intake for the prevention of urinary stone formation in participants with or without a history of urinary stones.
Search methods
We performed a systematic search of PubMed (MEDLINE), EMBASE (Ovid) and the Cochrane Library to 15 October 2019. We handsearched review articles, clinical trial registries, and reference lists of retrieved articles. We did not apply any restrictions to publication language or publication status.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs looking at the benefits and harms of increased water intake versus standard water intake for the prevention of urinary stone formation in participants with or without a history of urinary stones.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two review authors independently extracted data and assessed the risk of bias of included studies. We pooled dichotomous outcomes (e.g. incidence/recurrence rate of urinary stones; adverse events) using risk ratios (RRs) with 95% confidence intervals (CIs). We calculated hazard ratio (HRs) and corresponding 95% CIs to assess the intervention effect for time‐to‐event outcomes. We assessed the certainty of the evidence by using the GRADE criteria.
Main results
Our search identified no RCTs investigating the role of increased water intake for the prevention of urinary stone formation in participants with no history of urinary stones (primary prevention). We found one RCT assessing the effects of increased water intake versus standard water intake for the prevention of urinary stone formation in people with a history of urinary stones (secondary prevention). This trial randomised 220 participants (110 participants in the intervention group with increased water intake and 110 in the control group with standard water intake). Increased water intake was defined as achieving a urine volume of at least 2.0 L per day by drinking water.
Based on this study, increased water intake may decrease stone recurrences (RR 0.45, 95% CI 0.24 to 0.84; 199 participants; low‐certainty evidence); this corresponds to 149 fewer (43 fewer to 205 fewer) stone recurrences per 1000 participants with 270 stone recurrence per 1000 participants over five years in the control group.
Increased water intake may also prolong the time to urinary stone recurrence compared to standard water intake (HR 0.40, 95% CI 0.20 to 0.79; 199 participants; low‐certainty evidence); based on a stone recurrence rate of 270 per 1000 participants over five years, this corresponds to 152 fewer (209 fewer to 50 fewer) recurrences per 1000 participants.
For both outcomes we downgraded the certainty of evidence for study limitations and imprecision. We found no evidence for the outcome of adverse events
Authors' conclusions
We found no RCT evidence on the role of increased water intake for primary prevention of urinary stones. For secondary prevention, increased water intake achieving a urine volume of at least 2.0 L/day may reduce urinary stone recurrence and prolong time to recurrence for people with a history of urinary stone disease. However, our confidence in these findings is limited. We did not find evidence for adverse events.
Plain language summary
Increased water intake for preventing urinary stones
Review question
We performed this review to find out whether drinking more water prevents people from getting kidney stones.
Background
Kidney stones are common in people. Drinking more water may prevent people who have never had stones to get stones and/or help people who have had stones in the past to not get them again. We are uncertain how well this works and whether drinking more water has unwanted effects.
Study characteristics
We examined research published up to October 2019. We included studies which by chance decided whether people were asked to drink more water (to produce at least 2 litres of urine) or were given no special instructions. We found no studies of people who had never had kidney stones. We found one study, performed in 220 people who had calcium‐containing stones in the past, but were stone‐free when they started the study. The average age was approximately 41 years, and two‐thirds of participants were men.
Key results
We found that drinking more water may reduce the risk of stones coming back. It may also prolong the time it takes for stones to come back. We found no evidence of unwanted effects.
Certainty of evidence
The certainty of evidence for both outcomes for which we found evidence was low. This means that the true results may be quite different.
Summary of findings
Summary of findings for the main comparison. Increased water intake compared to standard water intake for secondary prevention of urinary stones.
| Increased water intake compared to standard water intake for secondary prevention of urinary stones | |||||
| Patient or population: participants with a prior history of nephrolithiasis Setting: community care, outpatient Intervention: increased water intake (≥ 2 L/day) Comparison: standard water intake | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with standard water intake | Risk difference with increased water intake | ||||
| Stone recurrence (assessed as symptomatic renal colic/stone expulsion episodes and imaging) |
199 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | RR 0.45 (0.24 to 0.84) | Study population | |
| 270 per 1000 | 149 fewer per 1000 (205 fewer to 43 fewer) | ||||
| Time to recurrence (absolute event rate based on 5‐year follow‐up; assessed as symptomatic renal colic/stone expulsion episodes and imaging) |
199 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | HR 0.40 (0.20 to 0.79) | Study population | |
| 270 per 1000 | 152 fewer per 1000 (209 fewer to 50 fewer) | ||||
| Adverse events | (0 studies) | N/A | not estimable | Study population | |
| 0 per 1000 | 0 fewer per 1000 (0 fewer to 0 fewer) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded for study limitations mainly for unclear risk of bias for selection bias and unclear or high risk of performance, detection bias, attrition and selective reporting bias. bDowngraded for wide confidence interval that crossed threshold for a clinically relevant effect size (absolute risk reduction of 5%).
Background
Description of the condition
Urinary stones are common in the general population and the prevalence varies regionally (Medina‐Escobedo 2002; Pinduli 2006; Ramello 2000). A higher incidence rate is observed in certain parts of the world, like Southern England, Asia, the Middle East (Pak 1980), and South‐East USA (Brikowski 2008). According to a more recent cross‐sectional analysis of the National Health and Nutrition Examination Survey (NHANES), the overall prevalence of urinary stones in 2007 to 2010 was 8.8% in the USA, representing a 70% increase over the last 15 years (Scales 2012). Similar trends for increased stone prevalence were also observed in some other countries, like England and Japan (Rukin 2017; Yasui 2008).
Urinary stones can originate from the upper (kidney and ureter) and lower (bladder and urethra) urinary tract, with the majority occurring in the kidneys initially. Treatment options mainly include conservative treatment (observation), medical expulsive treatments and surgical procedures, based on stone size, location and with or without associated symptoms (Assimos 2016; Türk 2018). The application of minimal invasive technologies by using shock wave lithotripsy (SWL), as well as other endourologic procedures in recent years, has greatly improved efficiency in the managing process (Pradere 2018). However, the high probability of recurrence should not be ignored. The symptomatic recurrence rates at 10 and 15 years were reported to be 31% and 39%, respectively (Rule 2014). And a higher risk of recurrence was observed for those patients without sufficient preventive procedures (Kirkali 2015).
The increasing prevalence and high recurrence rate of urinary stone disease pose a significant healthcare burden. In the USA, emergency department visit rates for urinary stones has nearly doubled from 1992 to 2009, with recurrent episodes accounting for about 10% of emergency care visits (Fwu 2013). Furthermore, the annual direct medical cost of urinary stone disease was estimated to be USD 10 billion, making it one of the most expensive urologic conditions (Litwin 2012). Therefore, there is an urgency to improve prevention strategies for urinary stones.
Description of the intervention
The prevention of urinary stones aims at modifying concentrations of lithogenic factors in the urine. A variety of pharmacologic interventions and dietary changes may help (Qaseem 2014). For example, preventive medications might work for specific patients under risk. However, the financial burden, potential side effects as well as poor patient compliance limits their routine use in clinical practice (Escribano 2009). Dietary changes mainly refer to reducing intake of oxalate, animal protein and other purines (Taylor 2006). However, these modifications were usually combined as a multi‐component diet. Their independent role remained unclear and could not be easily assessed separately (Dussol 2008; Muldowney 2002).
Increasing water intake is an effective and economical way to increase urine volume and decrease concentration of calcium, oxalate and other salts in the urine, which may help slow down the stone formation process. The potential role of water intake in preventing urinary stones was firstly introduced by Frank et al in the 1960s. They reported that people living in a tropical climate without adequate fluid intake harboured higher incidence of urinary stones (Frank 1966). More direct evidence of its prevention effect came from two large observational studies. The National Health Professionals cohort recruited 51,529 participants without a history of nephrolithiasis. During a follow‐up of 14 years, they found that participants drinking more than 2.5 L water/day had decreased stone formation risk compared with those drinking less than 1.2 L/day (Curhan 1997a; Taylor 2004). Another study covered more than 90,000 women (Nurses' Health Study I) with no history of kidney stones. The study reported that the daily water intake of more than 2.5 L resulted in a lower incidence of urinary stones compared with that of less than 1.4 L (116 cases/100,000 person‐years versus 232 cases/100,000 person‐years) (Curhan 1997b). The association was strengthened by revealing an inverse relationship between the volume of water intake and the risk of stone formation in the Nurses' Health Study II in 2004 (Curhan 2004).
On the other hand, researchers have also reported the role of water intake in the secondary prevention of urinary stones in their studies with sample size ranging from 108 to 256 participants and follow‐up time ranging from 3.0 to 5.2 years (Daudon 2005; Embon 1990; Hosking 1983; Strauss 1982). Participants with a history of urinary stones in these studies were encouraged to increase daily water intake (ranging from 1.9 L/day to 3.0 L/day). Their results indicated that participants who suffered from stone recurrence tended to have less daily urine volume compared with those who remained stone‐free.
Taking into account all the evidence reported, increased water intake may potentially play a role for the prevention of stone formation in people with or without a history of urinary stones.
How the intervention might work
One prevailing hypothesis is that increasing water intake leads to an increased urine volume and helps increase the threshold level at which calcium oxalate crystallises. Eventually, the formation potential of urinary stones decreases. The rationale is that the prerequisite of stone formation is considered to be the result of supersaturation of stone‐forming compounds including calcium, oxalate, phosphorus and uric acid. Increasing urine volume may help reduce the saturation of calcium oxalate, calcium phosphate and monosodium urate, and theoretically benefit all people predisposed to the disease.
Why it is important to do this review
Urinary stone disease is common, affecting almost one out of every 11 people, and poses a significant healthcare burden worldwide. Given the increasing prevalence and high recurrence rate of urinary stone disease, there is an urgency to improve prevention strategies. Increasing water intake is an effective and economical way to increase urine volume and reduce saturation of stone‐forming compounds, which may help slow down the stone formation process. Observational studies have reported that increasing water intake played a role in the prevention of urinary stone formation. And our previous work revealed potential effects of increased water intake in preventing recurrence of urinary stones on the basis of prospective intervention trials (Bao 2012). We have updated our previous work based on the latest evidence and rated the certainty of evidence using GRADE.
Objectives
To compare the effects of increased water intake with standard water intake for the prevention of urinary stone formation in participants with or without a history of urinary stones.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled studies (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at the benefits and harms of increased water intake for the prevention of urinary stone disease. We included the first period of randomised cross‐over studies.
Types of participants
Inclusion criteria
-
Participants without a history of urinary stones (primary prevention)
aged more than 18 years old
with no history of urinary stones
-
Participants with a history of urinary stones (secondary prevention)
aged more than 18 years old
with a history of urinary stones, as documented on imaging by ultrasound; kidney, ureter and bladder (KUB) radiography; or computer tomography (CT) scan
the primary stone episode was resolved by spontaneous expulsion of the calculus, medical expulsive treatment, shock wave lithotripsy (SWL), percutaneous nephrolithotomy or other procedures (primary procedures)
Exclusion criteria
Studies recruiting participants with genetically defined metabolic stone diseases (e.g. cystinuria, primary hyperoxaluria etc.)
Studies recruiting participants on medications predisposing to urinary stones, or participants taking urinary stone preventive medications (e.g. thiazide diuretics, potassium salts, allopurinol, phosphate etc.)
Types of interventions
We investigated the following comparisons of experimental versus comparator interventions.
Experimental interventions
Increased water intake
Participants were put into an enforced fluid intake programme (e.g. plain drinking water, beverages, mineral water, and carbonated water etc.) to achieve a daily urine volume of at least 2 litres and with a minimum duration of intervention for 12 months.
Comparator interventions
Standard water intake
No specific diet changes apart from close follow‐up.
Comparisons
Increased water intake versus standard water intake for the primary/secondary prevention of urinary stones
If we included a study with more than two intervention arms, we only included experimental and comparator intervention groups that met the eligibility criteria of the review.
Types of outcome measures
Proportion of participants with new urinary stones
Time to urinary stone formation, as measured from the date of randomisation to the date of new urinary stone formation (symptomatic stones in case of colic or expulsion of calculi during follow‐up, or asymptomatic stones detected by imaging studies)
Proportion of participants with adverse events
Main outcomes for 'Summary of findings' table
We presented all outcomes of this updated review in a GRADE 'Summary of findings' table.
Search methods for identification of studies
This is an updated review originally published in 2004 and updated in 2011. We performed a systematic and updated search (from January 2011 to October 2019) with no restriction on publication status or languages.
Electronic searches
We searched the following databases using search terms relevant to this review (See Appendix 1).
-
The Cochrane Library
Cochrane Database of Systematic Reviews (CDSR)
Cochrane Central Register of Controlled Trials (CENTRAL)
Database of Abstracts of Reviews of Effects (DARE)
Health Technology Assessment Database (HTA)
PubMed (MEDLINE)
EMBASE (Journals@Ovid, EMBASE)
We also searched the following trial registries.
ClinicalTrials.gov (www.clinicaltrials.gov/).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/).
Searching other resources
We identified other potentially eligible trials or ancillary publications by screening reference lists of retrieved included trials, reviews, meta‐analyses and health technology assessment reports (from January 2011 to October 2019). We contacted study authors of included trials to identify any further studies that we might have missed.
We also searched abstract proceedings of major relevant meetings (American Urological Association and European Association of Urology) for the last three years (2017 to 2019) to identify unpublished studies.
Data collection and analysis
Selection of studies
After removing duplicate records, two review authors (Bao Y, Tu X) independently screened abstracts and titles of the remaining records to determine which studies should be assessed further. We then investigated all potentially relevant records as full‐text and classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies (Higgins 2011a). We documented reasons for exclusion of studies. We resolved discrepancies through consensus or recourse to a third review author (Wei Q). We presented the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram showing the process of study selection (Liberati 2009).
Data extraction and management
We developed a dedicated data extraction form. For studies that fulfilled inclusion criteria, two review authors (Bao Y, Tu X) independently extracted the following information.
Study design
Study dates
Study settings and country
Participant inclusion and exclusion criteria
Participant details, baseline demographics
The number of participants by study and by study arm
Details of relevant experimental and comparator interventions, such as fluid type and volume
Definitions of relevant outcomes, and method and timing of outcome measurement as well as any relevant subgroups
Study funding sources
Declarations of interest by primary investigators
We extracted outcome data relevant to this Cochrane Review as needed for calculation of summary statistics and measures of variance. For dichotomous outcomes, we obtained numbers of events and totals for population of a 2 x 2 table, as well as summary statistics with corresponding measures of variance. For continuous outcomes, we obtained means and standard deviations (SDs) or data necessary to calculate this information. We resolved any disagreements by discussion, or, if required, by consultation with a third review author (Wei Q). We provided information, including trial identifier, about potentially relevant ongoing studies in the table 'Characteristics of ongoing studies'. We attempted to contact authors of included studies to obtain key missing data as needed.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we sought to maximise the yield of information by mapping all publications to unique studies and collating all available data. We used the most complete data set aggregated across all known publications. In case of doubt, we had planned to give priority to the publication reporting the longest follow‐up associated with our primary outcomes.
Assessment of risk of bias in included studies
Two review authors (Bao Y, Tu X) assessed the risk of bias for each included study independently. We resolved disagreements by consensus, or by consultation with a third review author (Wei Q). We assessed the risk of bias using Cochrane's 'Risk of bias' assessment tool which included the following domains (Higgins 2011b).
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other sources of bias
We judged the domains as 'low risk', 'high risk' or 'unclear risk' as well as evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions(Higgins 2011b, see Appendix 2). We presented a 'Risk of bias' summary figure to illustrate these findings.
We assessed 'Risk of bias' on a per outcome basis. Since the two outcomes for which we found data had identical risk of bias ratings, we collapsed their risk of bias presentation.
Measures of treatment effect
We pooled dichotomous outcomes (e.g. incidence/recurrence rate of urinary stones; adverse events) using risk ratio (RRs) with 95% confidence intervals (CIs). We calculated hazard ratio (HRs) and corresponding 95% CIs to assess the intervention effect for time‐to‐event outcomes. Estimated HRs were to be calculated on the basis of Tierney's relevant equations in case of the absence of individual participant data (Tierney 2007). When data aggregation was not feasible, results were presented in a descriptive analysis.
Unit of analysis issues
The unit of analysis was the individual participant. We handled trials with more than two intervention groups for inclusion in the review in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Dealing with missing data
Any further information required from the original trial author was requested by contacting the authors (email to corresponding author) and any relevant information obtained was included in the review. We performed intention‐to‐treat (ITT) analyses if data were available. Otherwise we performed available case analyses.
Assessment of heterogeneity
We planned to identify heterogeneity (inconsistency) through visual inspection of the forest plots to assess the amount of overlap of CIs, and the I2 statistic, which quantified inconsistency across studies to assess the impact of heterogeneity in the meta‐analysis (Higgins 2002; Higgins 2003); We interpreted the I2 statistic as follows (Deeks 2011):
0% to 40%: may not be important;
30% to 60%: may indicate moderate heterogeneity;
50% to 90%: may indicate substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Since we only found a single study, we did not assess inconsistency.
Assessment of reporting biases
We planned to assess publication bias using funnel plot analysis if we had included 10 studies or more investigating a particular outcome (Higgins 2011a).
Data synthesis
We performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). For dichotomous outcomes, we used the Mantel‐Haenszel method; for continuous outcomes and time‐to‐event outcomes, we used the inverse variance method. The summary estimates were generated using a fixed‐effect model considering that only one RCT was included. A random‐effect model was to be performed in case of the presence of heterogeneity when we included more than one study. We used Review Manager 5 to perform analysis (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
The increased water intake may help prevent stone formation by increasing urine volume and reducing the concentration of calcium, oxalate and other salts in the urine. Different volumes of water intake may have different impacts on the concentration of urinary stones; this may produce heterogeneity. And the adverse events (e.g. water intoxication) may be related to water volume. Besides, different types of water may be used to increase urine volume which may also have different effects on the outcomes. And participants with different types of primary stones may have different reactions to the water therapy. With sufficient studies, we planned to perform the following subgroup analyses to determine whether the effects of the intervention vary and whether these subgroups introduce heterogeneity.
Different volumes of water intake (to determine the threshold level for water intake).
Different types of water (e.g. plain drinking water, beverages, mineral water, and carbonated water etc.)
Different types of urinary stones (e.g. calcium, uric acid).
We were unable to conduct any of the preplanned subgroup analyses.
Sensitivity analysis
We planned to perform sensitivity analyses by excluding studies judged to be at 'high risk' or 'unclear risk' of bias for the particular outcome but were unable to do so.
'Summary of findings' table
We presented the overall certainty of the evidence for each outcome according to the GRADE approach, which considers five criteria related to internal validity (risk of bias, inconsistency, imprecision, publication bias) and external validity (directness of results) (Guyatt 2008). For each comparison, two review authors (Bao Y and Tu X) independently rated the certainty of evidence for each outcome as ’high,’ ’moderate,’ ’low’ or ’very low’ using GRADEpro GDT (GRADEpro GDT 2015). We resolved any discrepancies by consensus, or, if needed, by arbitration with a third review author (Wei Q). For each comparison, we presented a summary of the evidence for the main outcomes in a 'Summary of findings' table, which provided key information about the best estimate of the magnitude of the effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of the overall confidence in effect estimates for each outcome (Guyatt 2011; Schünemann 2011).
Results
Description of studies
Results of the search
Through an updated search of PubMed (MEDLINE), EMBASE (Ovid) and the Cochrane Library, we identified 522 records from January 2011 to October 2019 (See Figure 1). We identified 25 additional records by searching trial registries and manually searching citing and reference lists. We screened 15 full‐text studies and excluded studies that were reviews, wrong comparator or non‐randomised controlled trial (RCTs) (Characteristics of excluded studies). We also excluded one RCT (Sarica 2006; see below) which randomised participants with renal stone disease prior to shock wave lithotripsy (SWL). Ultimately, only one RCT was eligible for quantitative analysis (Borghi 1996).
1.
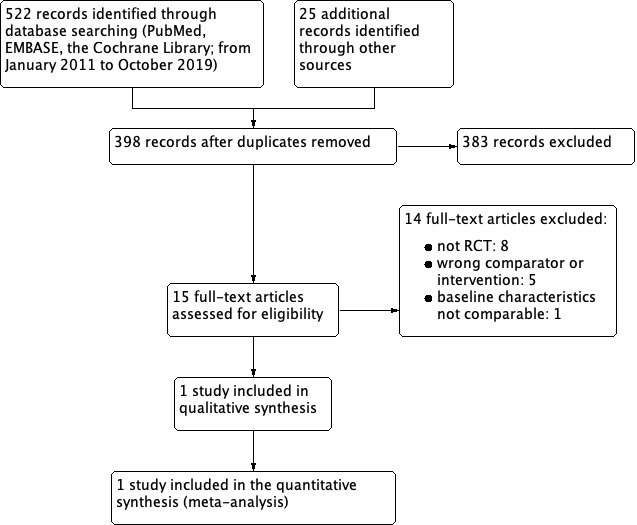
Study flow diagram.
Included studies
We found no studies that investigated the role of increased water intake for the primary prevention of urinary stone formation.
For patients with a history of urinary stones, we included one study with 220 participants which evaluated the preventive effects of increased water intake for stone recurrence (Characteristics of included studies; Table 2; Table 3). The study was conducted in Parma, Italy and enrolled patients with one prior episode of idiopathic calcium nephrolithiasis (with chemical analysis showing a calcium oxalate stone with or without traces of calcium phosphate) that had resolved by spontaneous stone passage or had been treated with SWL, percutaneous technique or other procedures (Borghi 1996). It randomised 220 participants to either increase water intake reaching a urine volume ≥ 2 L/day (intervention group) or to drink water as usual (control group). With a follow‐up of five years, 199 participants completed the study (intervention group: 99; control group: 100).
1. Description of the interventions.
| Intervention(s) (route, frequency, total dose/day) | Intervention(s) appropriate as applied in a clinical practice settinga (description) | Comparator(s) (route, frequency, total dose/day) | Comparator(s) appropriate as applied in a clinical practice settinga (description) | |
| Borghi 1996 | High water intake (give a urine volume ≥ 2 L/day) | N/CPS | No high water treatment or any special procedures | N/CPS |
|
aThe term 'clinical practice setting' refers to the specification of the intervention/comparator as used in the course of a standard medical treatment (such as dose, dose escalation, dosing scheme, provision for contraindications, and other important features). N/CPS: no specification of clinical practice setting possible | ||||
2. Baseline characteristics.
| Intervention(s) and comparator(s) | Duration of intervention (duration of follow‐up) (days, months, years) | Description of participants | Trial period (year to year) | Country | Setting | Ethnic groups (%) | Duration of disease (mean/range years (SD), or as reported) | |
| Borghi 1996 | I1: high water intake (≥ 2 L/day for urine volume) | 5 years | Participants had first episode of idiopathic calcium nephrolithiasis (calculus found at the chemical examination to be composed of pure calcium oxalate or mixed with traces of calcium phosphate) without other retained calculi (renal echography and intravenous pyelography) or arterial hypertension or other metabolic pathology that required regular dietary measures or drug therapy | 1986 to 1991 | Italy | Group 1 (I1, n =110) A high water intake which would give a urine volume ≥ 2 L/day Group 2 (C1, n = 110) No treatment |
‐ | ‐ |
| C1: no treatment | ‐ | ‐ | ||||||
| ‐ denotes not reported C: comparator; I: intervention. | ||||||||
Excluded studies
We identified 14 studies which we fully screened and subsequently excluded (see Characteristics of excluded studies). One ongoing study previously identified was not yet recruiting participants (NCT01100580; Characteristics of ongoing studies).
Of note, we identified one RCT which recruited 70 consecutive patients with urinary stones in the renal pelvis (Sarica 2006). Participants were randomised prior to SWL into three different groups (medication group: 25; increased water intake group: 25; control group: 20). Participants in the increased water intake group (intervention group) were put into an enforced fluid intake programme (to achieve urine volume of more than 2.5 L/day) and those in the control group received no intervention. Only a subset of 21 participants who achieved stone freedom after SWL (intervention group: 12; control group: 9) were then followed up for stone recurrence for three years. Given that these participants were randomised prior to SWL, we cannot assume that the subset of participants that become stone‐free and were then followed represented two groups that were comparable at baseline; thus, we excluded this study.
Risk of bias in included studies
We assessed risk of bias according to the Cochrane 'Risk of bias' tool (Higgins 2011b; Figure 2; Characteristics of included studies).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The included study provided no sufficient information for random sequence generation or allocation concealment. The risk of bias was unclear.
Blinding
Performance bias
Blinding was not performed for participants (not feasible). We assessed the risk of performance bias as high.
Detection bias
No sufficient available data reported blinding outcome assessment in the included study. We rated the risk of bias as unclear.
Incomplete outcome data
The missing outcome data balanced in numbers across intervention (11 out of 110 participants) and control group (10 out of 110 participants) in the included study (Borghi 1996). Given the attrition rate of 10% we judged the risk of bias as unclear.
Selective reporting
There was no protocol available for the included study; therefore we rated the risk of bias as unclear.
Other potential sources of bias
We detected no other potential threats to validity; therefore we rated the risk of bias as low.
Effects of interventions
See: Table 1
Increased water intake versus standard water intake for primary prevention
No available studies investigated the effects of increased water intake for the prevention of urinary stone formation in participants with no history of urinary stones.
Increased water intake versus standard water intake for secondary prevention
One study with 220 participants evaluated the preventive effects of increased water intake for participants with a history of urinary stones and reported the time to recurrence for both groups (Borghi 1996). See Table 1.
Primary outcomes
Stone recurrence
Increased water intake may reduce the recurrence rate of urinary stones (risk ratio (RR) 0.45, 95% confidence interval (CI) 0.24 to 0.84; 1 study, 199 participants; Analysis 1.1). This would correspond to 149 fewer (43 fewer to 205 fewer) stone recurrences per 1000 patients. We rated the certainty of evidence to low due to study limitations and imprecision.
1.1. Analysis.

Comparison 1 Increased water intake versus standard water intake for secondary prevention, Outcome 1 Stone recurrence.
Time to recurrence
Increased water intake prolonged the time to urinary stone recurrence compared with standard water intake (hazard ratio (HR) 0.40, 95% CI 0.20 to 0.79; 1 study, 199 participants; Analysis 1.2). The duration of follow‐up was five years. This would correspond to 152 fewer (50 fewer to 209 fewer) stone recurrences per 1000 patients. We downgraded the certainty of evidence to low due to study limitations and imprecision.
1.2. Analysis.
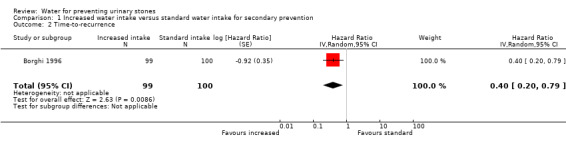
Comparison 1 Increased water intake versus standard water intake for secondary prevention, Outcome 2 Time‐to‐recurrence.
Secondary outcomes
Adverse events
The included study reported similar withdrawal rates (9.5%) in participants in the increased water intake group versus standard water intake group, but without explicitly reporting details on adverse events (Borghi 1996). Therefore, no data were available for this outcome.
Subgroup and sensitivity analysis
We were unable to perform any of the planned subgroup/sensitivity analyses due to paucity of included studies or lack of relevant data in the included studies.
Discussion
Summary of main results
We found no randomised controlled trials (RCTs) investigating the role of increased water intake for the prevention of urinary stone formation in participants without a history of urinary stones.
For secondary prevention, we identified one study suggesting that increased water intake may reduce the risk of recurrent urinary stones and prolong the time to stone recurrence. However, our confidence in both findings is limited. We did not find evidence for adverse events.
Overall completeness and applicability of evidence
The only study included in this review focused on the role of secondary stone prevention for calcium oxalate and phosphate stone formers with a single prior stone episode (Borghi 1996); it does not address primary prevention or the impact of increased fluid intake in individuals who have had multiple stones or other types of stones.
The study predated modern imaging technology for stone disease using computer tomography (CT) and relied entirely on ultrasound and plain x‐rays in identifying recurrent stone formers.
It is unclear from the reported study what percentage of patients presented with symptoms of renal colic versus asymptomatic stone disease and in what location of the kidney.
The study also does not provide information about participants' consumption of other non‐water beverages; this matters as certain types of beverages, such as coffee, teas, juices and soda may have potentially different effects on urinary stone risk (Ticinesi 2017).
Our review did not find evidence on adverse events related to increased water intake. However, we expect the risk to be low in healthy individuals without cardiovascular disease. Pollakisuria and nocturia have been reported to be the most common complications for patients with increased water intake (Wang 2013).
Quality of the evidence
We downgraded the certainty of evidence due to study limitations and imprecision (risk of bias and small sample size). For study limitations, the included study did not provide sufficient information about random sequence generation, allocation concealment as well as blinding process, rasing concerns about selection bias and detection bias. We further downgraded one level further for imprecision given the small sample size, low event rate and resulting wide confidence intervals. As a result, we judged the certainty of evidence for recurrence rate and time to recurrence to be low. Our confidence in these effect size estimates is therefore limited.
Potential biases in the review process
To reduce publication bias in our review, we followed the Cochrane guidelines and conducted an extensive literature search without restriction of publication language or publication status. In addition, we searched trial registries for unpublished or ongoing studies and identified other potentially eligible trials by screening reference lists of retrieved studies. It is possible that additional studies have been published but not yet identified, or conducted but not published. Should any such studies be identified, we would include them in the updates of this review. Moreover, two review authors performed study selection, assessment of the risk of bias, and data extraction which may help reduce the risk of error and bias.
We considered only RCTs or quasi‐RCTs for inclusion in our review. Evidence suggested that there may not be significant differences in the risk estimates of events between RCTs and observational studies (Golder 2011). Of note, for the comprehensive assessment of some rare or serious adverse events, studies of other designs (such as cohort studies, case‐control studies, case series and other observational studies) may offer information, nevertheless, which was beyond the scope of this review.
Agreements and disagreements with other studies or reviews
The Health Professionals Follow‐Up Study (Curhan 1997a; Taylor 2004), and the Nurses' Health Study I (Curhan 1997b) and II (Curhan 2004) were large observational studies that supported an important role for increased fluid intake to reduce the risk of stone recurrence (see Background).
We identified several reviews which assessed the effects of increased water intake for the prevention of urinary stones. However, no review has applied the same rigorous Cochrane methodology to this topic that includes rating the certainty of evidence using GRADE. Prasetyo 2013 included five studies: the RCT which we included in our review (Borghi 1996), three prospective cohort studies, as well as one case‐control study. Results of the three cohorts separately demonstrated increased water intake of more than 2.5 L/day for participants with no history of urinary stones had a positive effect to decrease the risk of stone formation (Curhan 1996; Curhan 1998; Taylor 2004). The result of the case‐control study confirmed its protective role (fluid intake volume of more than 2.0 L/day versus fluid intake volume of less than 500 mLs/day) (odds ratio (OR) 0.57, 95% CI 0.36 to 0.88; Dai 2013). Two other reviews reported that increased water intake may help lower the risk of recurrence for participants with a history of urinary stones (Cheungpasitporn 2016a; Fink 2013). Cheungpasitporn 2016a did not perform a pooled analysis. Fink 2013 differs in that it included Sarica 2006 which addressed a different question (effect of increased water intake in patients undergoing shock wave lithotripsy (SWL)), which is different than the question addressed by this review.
Authors' conclusions
Implications for practice.
Based on this review, there is evidence of low certainty that increasing water intake to achieve a urine volume of at least 2.0 L reduces stone recurrence and extends time to recurrence for secondary prevention. Given these findings and indirect evidence (although not formally assessed in this review) that there are no associated serious adverse effects of this intervention, increasing water intake to achieve at least 2.0 L urine daily is also the basis of current evidence‐based practice guidelines of the American Urological Association (Pearle 2014), the American College of Physicians (Qaseem 2015), and the European Association of Urology (Skolarikos 2015).
Implications for research.
There are no prospective randomised controlled trials (RCTs) available to assess the effects of increased water intake for the primary prevention of urinary stones. Given the increasing incidence of urinary stone disease and its economic burden on society, such trials appear important, especially in geographic areas with a high incidence of urinary stone disease.
We identified only one study which defined increased water intake as achieving a daily urine volume of more than 2.0 L. Further studies should further assess the association of fluid volume and stone risk to confirm these findings.
Further studies should further study the effects of increased water intake for prevention of different types of stone formers including non‐calcium stones.
Future studies should provide more detailed information on potential adverse events of increased water intake, especially for those patients with a high probability of urinary stone recurrence and volume‐sensitive diseases (cirrhosis, heart failure and moderate to severe chronic kidney disease).
What's new
| Date | Event | Description |
|---|---|---|
| 25 March 2020 | Amended | Added "not" to second sentence of Background section. No other changes. |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 20 November 2019 | New search has been performed | Updated electronic search strategies (up to October 2019); GRADE assessment and 'Summary of findings' tables using GRADEpro GDT software were reported; 'time to recurrence' analysed as 'time‐to‐event' outcome; review updated in accordance with current guidance provided in the MECIR standards and the MECIR standards for Plain Language Summaries (PLEACS standards). |
| 20 November 2019 | New citation required but conclusions have not changed | Conclusions not changed. |
| 18 April 2012 | New citation required but conclusions have not changed | One ongoing study identified |
| 18 April 2012 | New search has been performed | Updated electronic search strategies; 'Risk of bias' assessment tool used; PRISMA flowchart included. |
| 15 October 2008 | Amended | Converted to new review format |
Acknowledgements
The authors would like to thank Zhang Ke who contributed to the design, quality assessment, data collection, entry, analysis and interpretation of the initial version of this review (Ke 2004). The authors thank RDT Rajaguru for reviewing and editing the English text of a draft of this manuscript.
The authors are grateful for the help and support from Alea J Miller, Robert Lane, Kourosh Afshar, Philipp Dahm, and the Information Specialist and all relevant personnel from Cochrane Urology.
Appendices
Appendix 1. Electronic search strategies
| DATABASE | Search terms |
| PubMed (MEDLINE) | 1. water[mh] OR water[tiab] 2. drinking water[mh] OR drinking water[tiab] 3. fluid therapy[mh] OR fluid therapy[tiab] 4. beverages[mh] OR beverages[tiab] 5. mineral water[mh] OR mineral water[tiab] 6. carbonated water[mh] OR carbonated water[tiab] 7. water* OR fluid* OR drink* 8. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 9. urinary calculi[mh] OR urinary calculi[tiab] 10. urolithiasis[mh] OR urolithiasis[tiab] 11. urin*stone* OR uin* calcul* 12. 9 OR 10 OR 11 13. 8 AND 12 14. randomized controlled trial[pt] 15. controlled clinical trial[pt] 16. randomized[tiab] 17. placebo[tiab] 18. clinical trials as topic[mesh:noexp] 19. randomly[tiab] 20. trial[ti] 21. animals[mh] NOT human[mh] 22. 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 23. 22 NOT 21 24. 13 AND 23 |
| EMBASE & Ovid | #1 exp Urinary Calculi/ or exp Urolithiasis/ #2 urolithiasis or urin* ston* or urin* calcul* .af. #3 (#1 or #2) #4 exp Water/ OR exp Drinking water/ OR exp Fluid Therapy/ OR exp Body Water/ OR exp Beverages/ OR exp Mineral Water/ OR exp Carbonated Water/ #5 water* or fluid* or drink* .af. #7 (#4 or #5) #8 (#3 and #7) #9 exp crossover‐procedure/ or exp double‐blind procedure/ or exp randomized controlled trial/ or exp single‐blind procedure/ #10 (random* or factorial* or crossover* or placebo*) .af. #11 (#9 or #10) #12 (#8 and #11) |
| Cochrane Library | #1 MeSH descriptor: [Urinary Calculi] explode all trees #2 MeSH descriptor: [Urolithiasis] explode all trees #3 urolithiasis or urin* ston* or urin* calcul* #4 (#1 or #2 or #3) #5 MeSH descriptor: [Water] explode all trees #6 MeSH descriptor: [Drinking Water] explode all trees #7 MeSH descriptor: [Fluid Therapy] explode all trees #8 MeSH descriptor: [Body Water] explode all trees #9 MeSH descriptor: [Beverages] explode all trees #10 MeSH descriptor: [Mineral Water] explode all trees #11 MeSH descriptor: [Carbonated Water] explode all trees #12 water* or fluid* or drink* #13 (#5 or #6 or #7 or #8 or #9 or #10 or #11 or #12) #14 (#4 and #13) |
Appendix 2. 'Risk of bias' assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: insufficient information to permit judgement. | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors |
Low risk of bias: no blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: insufficient information to permit judgement. | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data |
Low risk of bias: no missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; 'as‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: insufficient information to permit judgement. | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: the study protocol is available and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). |
| High risk of bias: not all of the study's prespecified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified; one or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: insufficient information to permit judgement. | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: the study appears to be free of other sources of bias. |
| High risk of bias: had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Increased water intake versus standard water intake for secondary prevention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stone recurrence | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.24, 0.84] |
| 2 Time‐to‐recurrence | 1 | 199 | Hazard Ratio (Random, 95% CI) | 0.40 [0.20, 0.79] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Borghi 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group 1 (n = 99) (intervention group)
Group 2 (n = 100) (control group)
|
|
| Outcomes |
|
|
| Funding Sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes | Baseline of the intervention group, including urine volume, creatine, urea etc. was equal to that of the control group. During the study period, urine volume of the intervention group was significantly greater than that of the control group (2621 ± 443 mL/24h versus 1014 ± 195 mL/24 h, P < 0.0001). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "... patients were randomly placed in 2 different follow‐up programs..." Comment: the study did not provide information about the sequence generation process. We judged the risk of bias as unclear. |
| Allocation concealment (selection bias) | Unclear risk | Quote from publication: N/A Comment: method of concealment was not described. We judged the risk of bias as unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from publication: N/A Comment: participants in group 1 were instructed to tailor their fluid intake to a urinary output of ≥ 2 L/day; participants in group 2 were given no such instructions. We therefore judged participants and personnel to have been unblinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote from publication: N/A Comment: no information was provided as to whether outcome assessors were masked to the group assignment; we judged the risk of bias as unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from publication: "... 11 dropouts during follow‐up in group 1 and 10 in group 2 ..." Comment: 99/110 participants in the intervention group and 100/110 in the control group were included in the analyses of outcomes. Given the attrition rate of 10% we judged the risk of bias as unclear. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available to assess the risk of bias from selective reporting. |
| Other bias | Low risk | We detected no other potential threats to validity. |
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cheungpasitporn 2016a | Not RCT |
| Cheungpasitporn 2016b | Wrong comparator |
| de La Guéronnière 2011 | Intervention given for only 6 days |
| Ferraro 2017 | Not RCT |
| Fink 2013 | Not RCT |
| Khan 2015 | Wrong comparator |
| Lotan 2013 | Not RCT |
| Lotan 2016 | Not RCT |
| Maughan 2016 | Wrong comparator |
| Prasetyo 2013 | Not RCT |
| Sarica 2006 | Participants were randomised prior to SWL; only a subset of participants who were determined stone‐free were then followed, thereby addressing a different question. Participants followed may not have been comparable at baseline. |
| Ticinesi 2015 | Not RCT |
| Tsang 2012 | Wrong comparator |
| Wang 2013 | Not RCT |
RCT: randomised controlled trial SWL: shock wave lithotripsy
Characteristics of ongoing studies [ordered by study ID]
NCT01100580.
| Trial name or title | The links between water and salt intake, body weight, hypertension and kidney stones: a difficult puzzle |
| Methods | Prospective, randomised, open‐label, interventional study |
| Participants | For stone formers main inclusion criteria
|
| Interventions | Dietary supplement: low salt diet + water therapy |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | May 2010 |
| Contact information | Prof Loris Borghi +390521703375 loris.borghi@unipr.it |
| Notes | Latest information: Study not yet recruiting (1 December 2018) |
BMI: body mass index
Differences between protocol and review
The review is based on a published protocol (Wei 2003), with any differences described below.
The review was updated in accordance with current guidance provided in the MECIR standards, the MECIR standards for Plain Language Summaries (PLEACS standards), and the latest online version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We reported GRADE assessments in 'Summary of findings' tables using GRADEpro GDT software in the updated review (GRADEpro GDT 2015).
We found no studies that reported incidence rate/patient/year and recurrence rate/patient/year. We therefore chose to report proportion of participants (no history of urinary stones/stone‐free after primary procedures) who developed a new urinary stone after initiation of intervention as primary outcomes instead. We calculated hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) to assess the intervention effect for time‐to‐event outcomes. Estimated HRs were to be calculated on the basis of Tierney's relevant equations in the case of the absence of individual participant data (Tierney 2007).
Contributions of authors
BY: updating the review, selection of studies, 'Risk of bias' assessment, data extraction, data analysis
TX: updating the review, selection of studies, 'Risk of bias' assessment, data extraction, data analysis
WQ: conception and study design, provision of clinical and methodological advice on the updated review, drafting of the review, and final approval
Sources of support
Internal sources
Chinese Cochrane Centre, Chinese Centre of Evidence‐based Medicine, West China Hospital of Sichuan University, China.
Department of Urology, Institute of Urology, West China Hospital of Sichuan University, China.
External sources
-
National Natural Science Foundation of China, China.
Grant number: 81500522
Declarations of interest
BY: none known TX: none known WQ: none known
"These authors contributed equally to this work."
"These authors contributed equally to this work."
Edited (no change to conclusions)
References
References to studies included in this review
Borghi 1996 {published data only}
- Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5‐year randomized prospective study. Journal of Urology 1996;155(3):839‐43. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Cheungpasitporn 2016a {published data only}
- Cheungpasitporn W, Rossetti S, Friend K, Erickson SB, Lieske JC. Treatment effect, adherence, and safety of high fluid intake for the prevention of incident and recurrent kidney stones: a systematic review and meta‐analysis. Journal of Nephrology 2016;29(2):211‐9. [PMID: 26022722] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheungpasitporn 2016b {published data only}
- Cheungpasitporn W, Erickson SB, Rule AD, Enders F, Lieske JC. Short term tolvaptan increases water intake and effectively decreases urinary calcium oxalate, calcium phosphate, and uric acid supersaturations. Journal of Urology 2016;195(5):1476‐81. [PMID: 26598423] [DOI] [PMC free article] [PubMed] [Google Scholar]
de La Guéronnière 2011 {published data only}
- Guéronnière V, Bellego L, Jimenez IB, Dohein O, Tack I, Daudon M. Increasing water intake by 2 liters reduces crystallization risk indexes in healthy subjects. Archivio Italiano di Urologia, Andrologia 2011; Vol. 83, issue 1:43‐50. [MEDLINE: ] [PubMed]
Ferraro 2017 {published data only}
- Ferraro PM, Curhan GC, D'Addessi A, Gambaro G. Risk of recurrence of idiopathic calcium kidney stones: analysis of data from the literature. Journal of Nephrology 2017;30(2):227‐33. [PMID: 26969574] [DOI] [PubMed] [Google Scholar]
Fink 2013 {published data only}
- Fink HA, Wilt TJ, Eidman KE, Garimella PS, Macdonald R, Rutks IR, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Annals of Internal Medicine 2013;158(7):535‐43. [PMID: 23546565] [DOI] [PubMed] [Google Scholar]
Khan 2015 {published data only}
- Khan MM, Khan MW, Qadir I, Ch Z Ali, Liaquat H. A study regarding Citrus juices effect (lemonade & orange juices only) in urolithiasis when compared to plain drinking water. Pakistan Journal of Medical & Health Sciences 2015;9(1):239‐42. [Google Scholar]
Lotan 2013 {published data only}
- Lotan Y, Buendia Jiménez I, Lenoirwijnkoop I, Daudon M, Molinier L, Tack I, et al. Increased water intake as a prevention strategy for recurrent urolithiasis: major impact of compliance on cost‐effectiveness. Journal of Urology 2013;189(3):935‐9. [PMID: 23017509] [DOI] [PubMed] [Google Scholar]
Lotan 2016 {published data only}
- Lotan Y, Antonelli J, Jiménez IB, Gharbi H, Herring R, Beaver A, et al. The kidney stone and increased water intake trial in steel workers: results from a pilot study. Urolithiasis 2016;45(2):177‐83. [DOI: 10.1007/s00240-016-0892-7] [DOI] [PubMed] [Google Scholar]
Maughan 2016 {published data only}
- Maughan RJ, Watson P, Cordery PA, Walsh NP, Oliver SJ, Dolci A, et al. A randomized trial to assess the potential of different beverages to affect hydration status: development of a beverage hydration index. American Journal of Clinical Nutrition 2016;103(3):717‐23. [PMID: 26702122] [DOI] [PubMed] [Google Scholar]
Prasetyo 2013 {published data only}
- Prasetyo T, Birowo P, Rasyid N. The influence of increased fluid intake in the prevention of urinary stone formation: a systematic review. Acta Medica Indonesiana 2013;45(4):253‐8. [PMID: 24448328] [PubMed] [Google Scholar]
Sarica 2006 {published data only}
- Sarica K, Inal Y, Erturhan S, Yağci F. The effect of calcium channel blockers on stone regrowth and recurrence after shock wave lithotripsy. Urological Research 2006;34(3):184‐9. [PMID: 16463053] [DOI] [PubMed] [Google Scholar]
Ticinesi 2015 {published data only}
- Ticinesi A, Nouvenne A, Borghi L, Meschi T. Water and other fluids in nephrolithiasis: State of the art and future challenges. Critical Reviews in Food Science and Nutrition 2017;57(5):963‐74. [PMID: 25975220] [DOI] [PubMed] [Google Scholar]
Tsang 2012 {published data only}
- Tsang C, Smail NF, Almoosawi S, Davidson I, Al‐Dujaili EA. Intake of polyphenol‐rich pomegranate pure juice influences urinary glucocorticoids, blood pressure and homeostasis model assessment of insulin resistance in human volunteers. Journal of Nutritonal Science 2012;1:e9. [DOI: 10.1017/jns.2012.10] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2013 {published data only}
- Wang CJ, Grantham JJ, Wetmore JB. The medicinal use of water in renal disease. Kidney International 2013;84(1):45‐53. [DOI: 10.1038/ki.2013.23] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01100580 {published data only}
- NCT01100580. The links between water and salt intake, body weight, hypertension and kidney stones: a difficult puzzle. clinicaltrials.gov/ct2/show/NCT01100580 (accessed 18 April 2012).
Additional references
Assimos 2016
- Assimos D, Krambeck A, Miller NL, Monga M, Murad MH, Nelson CP, et al. Surgical management of stones: American Urological Association/Endourological Society Guideline, PART I. The Journal of Urology 2016;196(4):1153‐60. [DOI: 10.1016/j.juro.2016.05.090; PMID: 27238616] [DOI] [PubMed] [Google Scholar]
Brikowski 2008
- Brikowski TH, Lotan Y, Pearle MS. Climate‐related increase in the prevalence of urolithiasis in the United States. Proceedings of the National Academy of Sciences of the United States of America 2008;105(28):9841‐6. [DOI: 10.1073/pnas.0709652105; PMID: 18626008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Curhan 1996
- Curhan GC, Willett WC, Rimm EB, Spiegelman D, Stampfer MJ. Prospective study of beverage use and the risk of kidney stones. American Journal of Epidemiology 1996;143(3):240‐7. [PMID: 8561157] [DOI] [PubMed] [Google Scholar]
Curhan 1997a
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. Family history and risk of kidney stones. Journal of the American Society of Nephrology: JASN 1997;8(10):1568‐73. [PMID: 9335385] [DOI] [PubMed] [Google Scholar]
Curhan 1997b
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Annals of Internal Medicine 1997;126(7):497‐504. [PMID: 9092314] [DOI] [PubMed] [Google Scholar]
Curhan 1998
- Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Beverage use and risk for kidney stones in women. Annals of Internal Medicine 1998;128(7):534‐40. [PMID: 9518397] [DOI] [PubMed] [Google Scholar]
Curhan 2004
- Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses' Health Study II. Journal of Urology 2004;164(8):885‐91. [DOI: 10.1001/archinte.164.8.885; PMID: 15111375] [DOI] [PubMed] [Google Scholar]
Dai 2013
- Dai M, Zhao A, Liu A, You L, Wang P. Dietary factors and risk of kidney stone: a case‐control study in southern China. Journal of Renal Nutrition: the Official Journal of the Council on Renal Nutrition of the National Kidney Foundation 2013;23(2):e21‐8. [DOI: 10.1053/j.jrn.2012.04.003; PMID: 22658934] [DOI] [PubMed] [Google Scholar]
Daudon 2005
- Daudon M, Hennequin C, Boujelben G, Lacour B, Jungers P. Serial crystalluria determination and the risk of recurrence in calcium stone formers. Kidney International 2005;67(5):1934‐43. [DOI: 10.1111/j.1523-1755.2005.00292.x; PMID: 15840041] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Dussol 2008
- Dussol B, Iovanna C, Rotily M, Morange S, Leonetti F, Dupuy P, et al. A randomized trial of low‐animal‐protein or high‐fiber diets for secondary prevention of calcium nephrolithiasis. Nephron Clinical Practice 2008;110(3):c185‐94. [DOI: 10.1159/000167271; PMID: 18957869] [DOI] [PubMed] [Google Scholar]
Embon 1990
- Embon OM, Rose GA, Rosenbaum T. Chronic dehydration stone disease. BJU International 1990;66(4):357‐62. [PMID: 2224429] [DOI] [PubMed] [Google Scholar]
Escribano 2009
- Escribano J, Balaguer A, Pagone F, Feliu A, Roqué I Figuls M. Pharmacological interventions for preventing complications in idiopathic hypercalciuria. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004754.pub2; PMID: 19160242] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frank 1966
- Frank M, Vries A. Prevention of urolithiasis. Education to adequate fluid intake in a new town situated in the Judean Desert Mountains. Archives of Environmental Health 1966;13(5):625‐30. [PMID: 5925636] [DOI] [PubMed] [Google Scholar]
Fwu 2013
- Fwu CW, Eggers PW, Kimmel PL, Kusek JW, Kirkali Z. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney International 2013;83(3):479‐86. [DOI: ; PMID: 23283137] [DOI] [PMC free article] [PubMed] [Google Scholar]
Golder 2011
- Golder S, Loke YK, Bland M. Meta‐analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. Plos Medicine 2011;8(5):e1001026. [DOI: 10.1371/journal.pmed.1001026; PMID: 21559325] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed prior to 20 January 2020.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Schünemann HJ, et al. What is 'quality of evidence' and why is it important to clinicians?. BMJ (Clinical Research Ed.) 2008;336(7651):995‐8. [DOI: 10.1136/bmj.39490.551019.BE; PMID: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, BrozekJ, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI: 10.1016/j.jclinepi.2010.04.026; PMID: 21195583] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Higgins 2011c
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Hosking 1983
- Hosking DH, Erickson SB, Berg CJ, Wilson DM, Smith LH. The stone clinic effect in patients with idiopathic calcium urolithiasis. Journal of Urology 1983;130(6):1115‐8. [PMID: 6644890] [DOI] [PubMed] [Google Scholar]
Kirkali 2015
- Kirkali Z, Rasooly R, Star RA, Rodgers GP. Urinary stone disease: progress, status, and needs. Urology 2015;86(4):651‐3. [DOI: 10.1016/j.urology.2015.07.006; PMID: 26190090] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical Research Ed.) 2009;339:b2700. [DOI: 10.1136/bmj.b2700; PMID: 19622552] [DOI] [PMC free article] [PubMed] [Google Scholar]
Litwin 2012
- Litwin MS, Saigal CS, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Public Health Service, US Department of Health and Human Services. Table 14‐47: Economic impact of urologic disease. Urologic Diseases in America. Vol. 486, Washington, DC: NIH publication, 2012:12‐7865. [Google Scholar]
Medina‐Escobedo 2002
- Medina‐Escobedo M, Zaidi M, Real‐de León E, Orozco‐Rivadeneyra S. Urolithiasis prevalence and risk factors in Yucatan, Mexico. Salud Pública De Mexico 2002;44(6):541‐5. [PMID: 20383456] [PubMed] [Google Scholar]
Muldowney 2002
- Muldowney FP. Prevention of recurrent stones in idiopathic hypercalciuria. New England Journal of Medicine 2002;346(21):1667‐9. [PMID: 12030262] [PubMed] [Google Scholar]
Pak 1980
- Pak CY, Sakhaee K, Crowther C, Brinkley L. Evidence justifying a high fluid intake in treatment of nephrolithiasis. Annals of Internal Medicine 1980;93(1):36‐9. [PMID: 7396311] [DOI] [PubMed] [Google Scholar]
Pearle 2014
- Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu‐Ciocca CJ, Matlaga BR, et al. Medical management of kidney stones: AUA guideline. Journal of Urology 2014;192(2):316‐24. [DOI] [PubMed] [Google Scholar]
Pinduli 2006
- Pinduli I, Spivacow R, Valle E, Vidal S, Negri AL, Previgliano H, et al. Prevalence of urolithiasis in the autonomous city of Buenos Aires, Argentina. Urological Research 2006;34(1):8‐11. [DOI: 10.1007/s00240-005-0003-7; PMID: 16425020 ] [DOI] [PubMed] [Google Scholar]
Pradere 2018
- Pradere B, Doizi S, Proietti S, Brachlow J, Traxer O. Evaluation of guidelines for surgical management of urolithiasis. Journal of Urology 2018;199(5):1267‐71. [DOI: 10.1016/j.juro.2017.11.111; PMID: 29221932] [DOI] [PubMed] [Google Scholar]
Qaseem 2014
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD. Dietary and pharmacologic management to prevent recurrent nephrolithiasis in adults: a clinical practice guideline from the American College of Physicians. Annals of Internal Medicine 2014;161(9):659‐67. [DOI: 10.7326/M13-2908; PMID: 25364887] [DOI] [PubMed] [Google Scholar]
Qaseem 2015
- Qaseem A, Fink HA, Denberg TD. Prevention of recurrent nephrolithiasis in adults. Annals of Internal Medicine 2015;162(7):529. [DOI] [PubMed] [Google Scholar]
Ramello 2000
- Ramello A, Vitale C, Marangella M. Epidemiology of nephrolithiasis. Journal of Nephrology 2000;13 Suppl 3:S45‐50. [DOI: 10.1159/000046295] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rukin 2017
- Rukin NJ, Siddiqui ZA, Chedgy EC, Somani BK. Trends in upper tract stone disease in England: evidence from the Hospital Episodes Statistics Database. Urologia Internationalis 2017;98(4):391‐6. [DOI: 10.1159/000449510; PMID: 27694759] [DOI] [PubMed] [Google Scholar]
Rule 2014
- Rule AD, Lieske JC, Li X, Melton LJ 3rd, Krambeck AE, Bergstralh EJ. The ROKS nomogram for predicting a second symptomatic stone episode. Journal of the American Society of Nephrology: JASN 2014;25(12):2878‐86. [DOI: 10.1681/ASN.2013091011; PMID: 25104803 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scales 2012
- Scales CD Jr, Smith AC, Hanley JM, Saigal CS, Urologic Diseases in America Project. Prevalence of kidney stones in the United States. European Urology 2012;62(1):160‐5. [DOI: 10.1016/j.eururo.2012.03.052; PMID: 22498635 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Skolarikos 2015
- Skolarikos A, Straub M, Knoll T, Sarica K, Seitz C, Petrik A, et al. Metabolic evaluation and recurrence prevention for urinary stone patients: EAU guidelines. European Urology 2015;67(4):750‐63. [DOI] [PubMed] [Google Scholar]
Strauss 1982
- Strauss AL, Coe FL, Deutsch L, Parks JH. Factors that predict relapse of calcium nephrolithiasis during treatment: a prospective study. American Journal of Medicine 1982;72(1):17‐24. [PMID: 7058820] [DOI] [PubMed] [Google Scholar]
Taylor 2004
- Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow‐up. Journal of the American Society of Nephrology 2004;15(12):3225‐32. [DOI: 10.1097/01.ASN.0000146012.44570.20; PMID: 15579526] [DOI] [PubMed] [Google Scholar]
Taylor 2006
- Taylor EN, Curhan GC. Diet and fluid prescription in stone disease. Kidney International 2006;70(5):835‐9. [DOI: 10.1038/sj.ki.5001656; PMID: 16837923] [DOI] [PubMed] [Google Scholar]
Ticinesi 2017
- Ticinesi A, Nouvenne A, Borghi L, Meschi T. Water and other fluids in nephrolithiasis: State of the art and future challenges. Critical Reviews in Food Science and Nutrition 2017;57(5):963‐74. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Türk 2018
- Türk C, Neisius A, Petřík A, Seitz C, Thomas K, Skolarikos A. EAU Guidelines on Urolithiasis 2018. www.uroweb.org/guideline/urolithiasis (accessed 2 January 2019). [ISBN: 978‐94‐92671‐01‐1]
Yasui 2008
- Yasui T, Igucho M, Suzuki S, Kohri K. Prevalence and epidemiological characteristics of urolithiasis in Japan: national trends between 1965 and 2005. Urology 2008;71(2):209‐13. [DOI: 10.1016/j.urology.2007.09.034; PMID: 18308085] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bao 2012
- Bao Y, Wei Q. Water for preventing urinary stones. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD004292.pub3] [DOI] [PubMed] [Google Scholar]
Ke 2004
- Ke Z, Wei Q. Water for preventing urinary calculi. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004292.pub2] [DOI] [PubMed] [Google Scholar]
Wei 2003
- Wei Q, Zhang K. Water for preventing urinary calculi. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD004292] [DOI] [Google Scholar]


