Telehealth interventions were associated with improvements in obstetric outcomes, perinatal smoking cessation, breastfeeding, early access to medical abortion services, and schedule optimization for high-risk obstetrics.
Abstract
OBJECTIVE:
To systematically review the effectiveness of telehealth interventions for improving obstetric and gynecologic health outcomes.
DATA SOURCES:
We conducted a comprehensive search for primary literature in ClinicalTrials.gov, Cochrane Library, Cochrane Collaboration Registry of Controlled Trials, EMBASE, PubMed, and MEDLINE.
METHODS OF STUDY SELECTION:
Qualifying primary studies had a comparison group, were conducted in countries ranked very high on the United Nations Human Development Index, published in English, and evaluated obstetric and gynecologic health outcomes. Cochrane Collaboration's tool and ROBINS-I tool were used for assessing risk of bias. Summary of evidence tables were created using the United States Preventive Services Task Force Summary of Evidence Table for Evidence Reviews.
TABULATION, INTEGRATION, RESULTS:
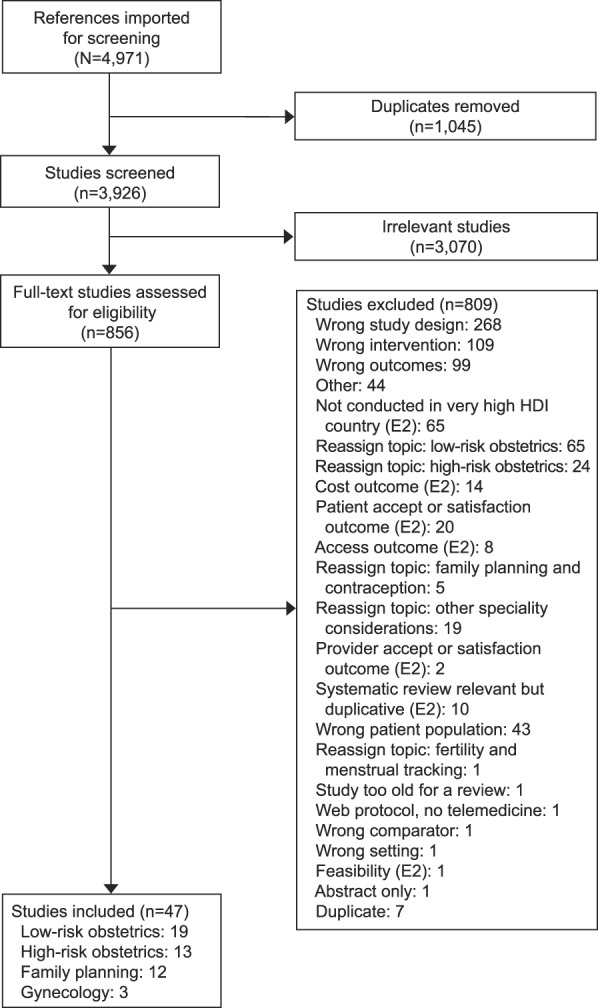
Of the 3,926 published abstracts identified, 47 met criteria for inclusion and included 31,967 participants. Telehealth interventions overall improved obstetric outcomes related to smoking cessation and breastfeeding. Telehealth interventions decreased the need for high-risk obstetric monitoring office visits while maintaining maternal and fetal outcomes. One study found reductions in diagnosed preeclampsia among women with gestational hypertension. Telehealth interventions were effective for continuation of oral and injectable contraception; one text-based study found increased oral contraception rates at 6 months. Telehealth provision of medication abortion services had similar clinical outcomes compared with in-person care and improved access to early abortion. Few studies suggested utility for telehealth to improve notification of sexually transmitted infection test results and app-based intervention to improve urinary incontinence symptoms.
CONCLUSION:
Telehealth interventions were associated with improvements in obstetric outcomes, perinatal smoking cessation, breastfeeding, early access to medical abortion services, and schedule optimization for high-risk obstetrics. Further well-designed studies are needed to examine these interventions and others to generate evidence that can inform decisions about implementation of newer telehealth technologies into obstetrics and gynecology practice.
Technology-enhanced health care delivery has rapidly proliferated with increased use of mobile phone apps, wearable devices, short message service or text messaging, multimedia messaging service and live audio-visual communication. The formulation of evidence-based practices may lag behind technology uptake by patients and physicians. In the absence of comprehensive and rigorous evidence reviews for a clinical area, clinicians may selectively rely on technologies promoted to them or showing early promise in small pilot projects or feasibility studies. Establishing evidence-based practices in this emerging dimension of health care delivery is important to mitigate potential health risks and costs that could be associated with rapid adoption of new technologies that have not been adequately studied, and also for overcoming barriers to adoption of clearly beneficial technologic advances. Robust scientific analysis of existing research in telehealth can both guide clinicians where evidence exists, and highlight areas for future study, so that the benefits of this burgeoning technology can be embraced while minimizing the risks.
Telehealth refers to any health care delivery enhanced by telecommunication. The Telehealth Resource Center, a leading national consortium of telehealth networks, defines telehealth as “a collection of means or methods for enhancing the health care, public health, and health education delivery and support using telecommunications technologies.”1 More specifically, the term telehealth has traditionally been used when referring to clinical diagnosis and monitoring that is augmented by telecommunication. Several recent terms have emerged to describe various aspects of new technology such as apps, wearable devices, and audio-visual communication, including mobile health, connected health, and digital health. The term telehealth is more commonly used to both describe the range of topics, including diagnosis and management, education, and training, and to provide an umbrella term for the numerous emerging technologies used to improve health care.
There is reason to focus specifically on telehealth in obstetrics and gynecology. First, telehealth is increasingly used in nearly every aspect of women's health care. Examples include virtual patient consultation with specialty services, remote observation of ultrasound recordings by maternal–fetal medicine and reproductive endocrinology experts, bladder diary tracking with smartphone apps, postpartum blood pressuring monitoring with Wi-Fi-connected devices, remote provision of medication abortion, and fertility tracking with patient-generated data. Second, in 2014, there were nearly 2,000 obstetric apps alone.2 In 2015, Women's Health & Pregnancy Apps accounted for 7% of all Health Apps.3 Here we present a systematic review of studies using telehealth interventions that report health outcomes in selected areas in low-risk obstetrics, high-risk obstetrics, family planning and gynecologic conditions. Telehealth activities can involve one-way or two-way communication exchanges. For this review, telehealth is defined as the technology-enhanced health care framework that includes novel services such as virtual visits, remote patient monitoring, and mobile apps or text messaging and that uses both synchronous and asynchronous communication.
SOURCES
We developed this review to evaluate evidence on the comparative effectiveness of telehealth as an alternative or adjunct to usual reproductive health care. The review aims to be relevant to medical disciplines involved in gynecologic and obstetric care, both for specialized and primary health services. The specific key questions addressed in this review are listed below.
Does telehealth improve reproductive health outcomes in low-risk obstetrics, high risk obstetrics, family planning, and gynecology?
Key Question 1. Is telehealth an effective adjunct or alternative to standard of care for improving family planning outcomes?
Key Question 2. Is telehealth an effective adjunct or alternative to standard of care for improving low-risk obstetric outcomes?
Key Question 3. Is telehealth an effective adjunct or alternative to standard of care for improving high-risk obstetric outcomes?
Key Question 4. Is telehealth an effective adjunct or alternative to standard of care for improving gynecology outcomes?
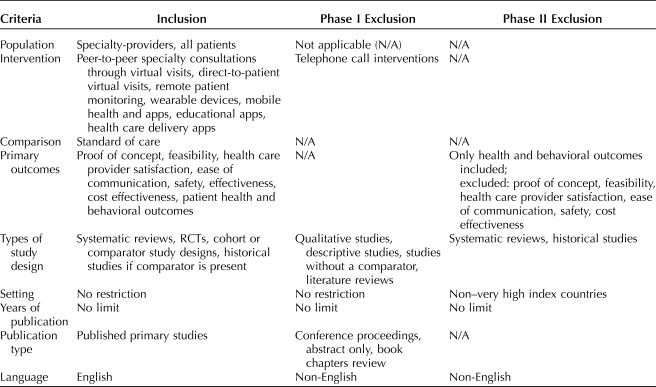
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement4 guided the review. Detailed study inclusion and exclusion criteria for the systematic review are available for review in Table 1. Studies were included and assessed for quality if they satisfied the PICOTS (Population, Intervention, Comparator, Outcomes, Time, Setting) elements.
Table 1.
Study Inclusion and Exclusion Criteria
This systematic review was designed to identify and review existing evidence on the effects of mobile media, remote monitoring and care-delivery, patient-generated data, and virtual visits in women's health care delivery. The synthesis aimed to organize existing evidence into clinically meaningful areas where telehealth has been applied in reproductive health care. The process involved two phases, funneling a comprehensive search into content areas and levels of evidence for the synthesis. The PRISMA method was followed closely, with notable divergences including lack of establishing a priori inclusion and exclusion criteria as well as not publishing our review protocol on a systematic review reporting website.
The systematic review team developed an extensive outline with suggested keywords and PICOTS in collaboration with American College of Obstetricians and Gynecologists Resource Center senior medical librarians. Comprehensive literature searches were performed for primary literature in ClinicalTrials.gov, Cochrane Library, Cochrane Collaboration Registry of Controlled Trials, EMBASE, PubMed, and MEDLINE. Results were uploaded to ProQuest Refworks folders based on level of evidence and topic area. Parameters for the search included: human-only published studies in English, with no date restrictions. Given the broad scope of this manuscript as well as the volume of literature found, we were not resourced to review non–English-language articles. MeSH terms and keywords can be found in Appendix 1, available online at http://links.lww.com/AOG/B689). The Research Manager (J.L.B.), along with the Research Team Chair (N.D.), completed a high-level filtering of the articles. All references were uploaded to an evidence distilling software, Covidence (Covidence systematic review software; available at www.covidence.org), at which point any duplicate articles were removed.
The investigators then initiated a two-step process. In phase I, a research team was established, bringing together clinical and methodologic experts to determine the scope of the inquiry and to design the comprehensive literature review to identify all existing literature on the topic of telehealth in reproductive health care delivery. Investigators met at a two-day in-person meeting to categorize the findings from the initial search into clinical areas and to further specify the original inclusion and exclusion criteria. These criteria were subsequently refined during each phase of the evidence synthesis process as presented in Table 1. During phase I, each pair of authors independently reviewed the title and abstracts for their respective clinical area based on the established inclusion and phase I exclusion criteria. Studies investigating telehealth within a different medical specialty outside of obstetrics and gynecology were excluded. Additionally, studies where the population included men or children were excluded.
Phase II included full-text review whereby authors assessed the studies based on more restrictive inclusion and exclusion criteria. Four workgroups were formed to further refine the scope and carry out the literature processing review activities in four areas: low-risk obstetrics, high-risk obstetrics, family planning, and gynecology. Early in this phase, the team also identified the key outcomes for each clinical area to be abstracted from the included studies. Outcomes were chosen based on ones that were consistently reported amongst the studies and most clinically relevant to the field of obstetrics and gynecology. Additional study designs such as systematic reviews and observational study designs without a comparator were excluded. Phase II also included re-review of full-text articles that had advanced to quality assessment with the additional exclusion criteria (Table 1). Discrepancies in the dual review of study inclusion and quality assessment were resolved through discussion among the investigators. Reference lists from pertinent systematic reviews were reviewed by investigators and any missing studies were added to the first phase of the review and followed the full review process. A targeted literature search was completed on September 26, 2018, to capture any high-impact studies published between initiation and completion of the review.
STUDY SELECTION
Two reviewers were assigned to each of the four different specialized topic groups. The same inclusion and exclusion criteria were used across all groups during review of the articles. Qualifying studies were conducted in countries ranked very high on the United Nations Human Development Index,5 published in English, and included female participants. These restrictions were employed to find literature generalizable to the U.S. health care system and population. Randomized controlled trials (RCTs), comparative observational cohort studies and case–control studies were included to examine associations of telehealth interventions for obstetric and gynecologic care with clinical health and behavioral outcomes. There was no requirement for minimum sample size for inclusion. Studies that only evaluated telehealth in relation to improved access, health care provider acceptability or satisfaction, and patient acceptability or satisfaction were excluded (Fig. 1). Studies focused on evaluating fertility and menstrual tracking telehealth strategies were excluded. Studies involving telephone-only, or website-only interventions were not included as these did not represent novel interventions.
Fig. 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram, overall. HDI, Human Development Index; E2, exclusion 2.
DeNicola. Telehealth to Improve Health Outcomes. Obstet Gynecol 2020.
Two authors independently appraised the internal validity of the eligible studies using the Cochrane Collaboration's tool for assessing the risk of bias for RCTs,6 and the ROBINS-I tool for assessing risk of bias in nonrandomized studies of interventions.7 Biases including selection, performance, detection, attrition, and reporting bias were evaluated. For RCTs, the following six domains were investigated: 1) random sequence generation, 2) allocation concealment, 3) blinding of participants and personnel, 4) blinding of outcome assessment, 5) incomplete outcome data, and 6) selective reporting. For observational studies, the following seven domains were investigated: 1) bias due to confounding, 2) bias in selection of participants into the study, 3) bias in classification of interventions for all outcomes, 4) bias due to departures from intended interventions for all outcomes, 5) bias due to missing data for all outcomes, 6) bias in measurement of outcomes, and 7) bias in selection of the reported result. The quality assessment rubric for both tools followed a low, high, or unclear risk of bias rating per domain. Lastly, trial protocol, registration, and retention rates were examined when making judgments on reporting biases. Differences in study quality assessments were resolved by discussion between the pair of reviewers until consensus was achieved. All studies that underwent quality assessment were included in the summary of evidence tables. The Research Manager (J.L.B.) extracted the study data (study design, sample size, details of interventions, outcomes) into summary of evidence tables. Discussions were held with authors on the presentation of summary tables and relevance of evidence presented. An investigator independently and comprehensively reviewed all data tables for accuracy.
Results were organized into summary of evidence tables and synthesized qualitatively to describe the degree of heterogeneity in study designs, follow-up protocols, outcome measurements, and settings. Owing to clinical heterogeneity amongst the studies and few studies with similar outcomes, quantitative estimates of pooled effects were not generated. Summary tables included study design, study characteristics including intervention, follow-up, study limitations, and the overall assessments of the body of literature. The United States Preventive Services Task Force procedure manual was followed for the summary of evidence tables, specifically Appendix XII, Summary of Evidence Table for Evidence Reviews (United States Preventive Services Task Force procedure manual). This method allowed for narrative presentation of the results while considering the consistency, precision, reporting bias, and applicability of evidence for the body of evidence presented per outcome. Studies were assessed for risk of bias and results are presented in Appendices 2–5, available online at http://links.lww.com/AOG/B689. The risk of bias assessments was incorporated into the limitations section of the summary of evidence tables. Results were organized by key question and sub question, which included relevant intervention and outcome for each group of studies. Within each author group (family planning, low- and high-risk obstetrics, and gynecology) the two authors jointly agreed on subthemes centered around the most common or relevant health outcomes. Family planning outcomes focused on contraception, including: initiation, use, continuation, adherence, and prescription fulfillment as well as telehealth provision of medication abortion and remote follow-up after medication abortion. Low-risk obstetrics outcomes centered on smoking cessation, influenza vaccination, pregnancy wellness, including: weight-loss and physical activity engagement, and breastfeeding. High-risk obstetrics outcomes included diabetes management, visit reduction, asthma control, and preterm labor. The general gynecology outcomes concentrated on sexually transmitted infections and stress urinary incontinence (SUI). Evidence interpretation focused on whether telehealth as a supplement or replacement is equally as effective compared with standard of care for improving reproductive health outcomes.
RESULTS
Two reviewers, KM and NG, independently reviewed 1,248 title and abstracts and 280 full-text articles in low-risk obstetrics (Appendix 6, available online at http://links.lww.com/AOG/B689). Nineteen articles met the inclusion criteria: 17 RCTs, one retrospective cohort and one case–control study for a total of 6,827 participants (Appendix 7, available online at http://links.lww.com/AOG/B689). The 19 studies reviewed telehealth interventions for smoking cessation outcomes, influenza vaccination, pregnancy wellness outcomes, and breastfeeding outcomes. Studies were implemented during the antenatal and postpartum period. Duration of intervention varied among the 17 trials and two studies.
Studies on smoking cessation in pregnancy that met our selection criteria resulted in four RCTs, with a total of 1,889 patients. Participants in the intervention groups received text messages throughout their pregnancies with content on the effects of smoking in pregnancy and the benefits and techniques of abstinence. All of these trials demonstrated a reduction in self-reported smoking at 30 days 15.3% (95% CI 12.08–18.58) in the control compared with 9.6% (95% CI 6.95–12.32) in the treatment group, and up to 3 months in one trial (35.2% of the intervention group and 22.7% of the control group) risk ratio 1.85 (95% CI 1.25–2.75)8–11 (Appendix 7, http://links.lww.com/AOG/B689). However, in the one trial that looked for biochemical validations, this was not confirmed except in older smokers or those who enrolled after the first trimester.8
Text messages encouraging vaccination for influenza in pregnancy did not demonstrate improved vaccination rates. We identified three RCTs, including 1,708 women, evaluating influenza vaccine uptake in women receiving text messages in support of the vaccine's importance in pregnancy12–14 (Appendix 7, http://links.lww.com/AOG/B689). Overall, vaccine uptake was low in all groups (27–62%), which may have contributed to the lack of efficacy of the intervention.
Physical wellness in pregnancy using smartphone apps and text messaging interventions were evaluated in 10 RCTs and one case–control study. The studies evaluated improvements in healthy eating in pregnancy, physical activity in pregnancy, gestational weight gain, and postpartum weight loss (Appendix 7, http://links.lww.com/AOG/B689). No significant improvement was noted in healthy eating using a smartphone app intervention.15 Adherence to the Health and Medicine Division of the National Academies of Sciences, Engineering, and Medicine recommendations for gestational weight gain was improved in one RCT;16 however, gestational weight gain was not significantly improved in two other RCTs that studied this outcome.17,18 Data regarding improved physical activity in pregnancy were mixed.19–21 Postpartum weight retention seemed to improve in the short term (6 months) however no significant difference persists when patients were followed to 12 months.22–25 Notable findings included Gilmore et al,22 which found significant reduction in body weight in five patients with the highest adherence to their platform, and Choi et al,21 which found decreased perceived barrier to being active in the app plus Fitbit intervention group.
Lastly, three studies evaluated the association of text-messaging and web-based interventions with breastfeeding. One RCT showed that a text-based intervention significantly improved continuation of breastfeeding even when controlling for infant age, mother's income, education, and type of delivery.26 Compared with the control group, the intervention group had a significantly higher rate of exclusive breastfeeding at 6 months than the control group, with an adjusted odds ratio (OR) of 2.67 (95% CI 1.45–4.91).26 Ahmed et al27 found a significant difference with 84% of the intervention group compared with 66% of the control group breastfeeding at 3 months. In the case–control study, there was a 6% decrease in exclusive breast feeding in the intervention group, compared with a 14% decrease in the comparison group after adjusting for covariates.28
Two reviewers (CL and YBT) independently reviewed 979 title and abstracts and 310 full-text articles in high-risk obstetrics (Appendix 8, available online at http://links.lww.com/AOG/B689). Appendix 9 (available online at http://links.lww.com/AOG/B689) provides a summary of the evidence for the 13 studies on telehealth interventions for high risk pregnancies. Thirteen articles met the inclusion criteria: 11 randomized RCTs, one non-RCT, and one retrospective cohort study, for a total of 1,514 perinatal women. The 13 studies reviewed focused on telehealth-mediated interventions for diabetes management in pregnancy, interventions for reduction of prenatal visits and optimizing management of asthma during pregnancy. All studies were implemented during the antenatal period. Duration of intervention varied among the 11 trials and one study.
Eight trials (seven RCTs, one nonrandomized controlled experiment) evaluated telehealth interventions in the management of diabetes during pregnancy. Telehealth interventions comprised the use of home internet-based telehealth systems, glucometer-cell phone units with data transmission capabilities, glucometers with integrated electronic log books, and short message service for management of diabetes in pregnancy. Both gestational diabetes mellitus (GDM) and pre-existing diabetes were included. One study29 included patients with either GDM and pre-existing diabetes. Primary outcomes included glycemic control as measured by change in HbA1C; secondary outcomes included a large range of maternal and fetal clinical outcomes, including mode of delivery, gestational age at delivery, neonatal intensive care unit admission, and Apgar scores. All studies were conducted between 1996 and 2018. Six of the eight studies29–34 reported no difference in glycemic control, maternal clinical outcomes, and neonatal outcomes despite higher frequency of data reporting in the telehealth group31 (Appendix 9, http://links.lww.com/AOG/B689). Two studies32,35 reported higher use of insulin therapy in the telehealth group compared with standard care (31% vs 4%). One study29 assessing participants with both GDM and pre-existing diabetes in pregnancy found lower rates of cesarean delivery and macrosomia in the GDM telehealth group, but no difference in quality of life or perceived stress between groups. One study reported higher medication compliance, lower mean blood glucose, lower rates of off-target measurements, and lower rate of pregnancies requiring insulin in the group that received a smartphone-based daily prompt.36 Overall, there was a reasonably consistent trend toward reduction in outpatient clinic visits while maintaining maternal and neonatal clinical outcomes. However, there was inconsistent and unclear blinding and allocation concealment in five of the six studies29–32,35 and selection bias in one study.30 Generalizability to the U.S. population is at best limited to two trials, consisting of 143 participants, as the remaining studies were all based in high middle-income European countries.
Three studies37–39 among 353 pregnant participants evaluated telehealth-mediated reduction in prenatal appointments in Spain and Belgium. The interventions ranged from a web-based clinical decision support tool for monitoring blood sugar in GDM, wireless home monitoring devices including a blood pressure monitor, smart body analyzer, and pulse oximetry for remote monitoring of gestational hypertension and a glucometer linked with a mobile phone–based app for monitoring patients with GDM. Two of the trials were RCTs37,39 and one was a retrospective cohort study.38 All studies reported remote monitoring decreased the number of unscheduled visits. One study38 evaluating clinical outcomes observed less progression to preeclampsia (7/48 [14.58%] vs 43/98 [43.87%]) and less requirement for medical interventions among gestational hypertensives, including induction of labor and maternal and neonatal hospitalizations. Although there was a reasonable consistent trend toward the outcomes observed, outcomes were imprecise, with small sample size and two different clinical parameters (GDM and gestational hypertension). There was a trend toward a high risk of selection bias in two of three studies,38,39 one study38 with an imbalance in number of participants assigned to each group and one study38 reported no difference in clinical outcomes, without reporting data to support this conclusion. Applicability to the U.S. health care system must be viewed with caution, given that all studies were performed outside of the United States.
Two RCTs40,41 among 278 pregnant women assessed telehealth on asthma control outcomes. In a study conducted in Australia, Zairina and colleagues assessed the effectiveness of a mobile app-based monitoring system, with automated feedback messaging for measuring pulmonary function and recording asthma symptoms and weekly asthma medication usage. The primary outcome measured was change in asthma control. Antenatal complications including GDM, hypertensive disorders, postpartum hemorrhage, and neonatal outcomes were also measured. The telehealth intervention improved subjective asthma control as measured by a questionnaire (95% CI −0.66 to −0.07) and asthma-related quality of life (95% CI 0.29 to 1.16) at 6 months follow-up. The telehealth intervention group had a higher proportion of participants with well-controlled asthma than the control group at 6 months (82% vs 58%). No significant difference was found in objective pulmonary function, unscheduled visits, days off work or school, oral corticosteroid use or fetal and maternal clinical outcomes between groups. The study was reasonably precise and with a low risk of bias. The outcomes measured were relevant, complete, and numerical results correlated to statistical analysis. However, the sample size was small, no other similar studies were found, and the setting may not be applicable to the U.S. population. One U.S. randomized control trial evaluated a text-based intervention for monitoring postpartum blood pressure in women with hypertensive disease of pregnancy. Among 206 women in the study, the 103 in the intervention group who remotely monitored blood pressure and communicated results using text-based reporting showed a significant increase in blood pressure measurements obtained in the first 10 days postpartum, and 84% in the text-based surveillance met American College of Obstetricians and Gynecologists' guidelines for blood pressure recording at 3–4 and 7–10 days postpartum.41
Two reviewers, SS and DG, independently reviewed 422 title and abstracts and 67 full-text articles in family planning (Appendix 10, available online at http://links.lww.com/AOG/B689). Twelve articles met the inclusion criteria: seven RCTs, one cluster RCT, and four cohort studies evaluated the effect of text message interventions on emergency contraceptive prescription fulfillment (Wilkinson TA, Berardi MR, Crocker EA, Nordt C, Silverstein M. Feasibility of using text message reminders to increase fulfilment of emergency contraception prescriptions by adolescents [letter]. J Fam Plann Reprod Health Care 2017;43:79–80.), any contraception initiation,42 any contraception use,43–45 and oral contraception adherence46 in 23,221 women (Appendix 11, available online at http://links.lww.com/AOG/B689). Duration of intervention varied among the 12 studies. The types of text message interventions included noninteractive, repetitive texts (Wilkinson et al, Reprod Health Care),42,43,45,46 and interactive bidirectional texts.44 These studies did not show evidence of a beneficial effect of text message intervention on contraceptive initiation, use, or adherence. A pilot study showed a possible temporal effect of text messages on emergency contraception fulfillment, but this study was exploratory in nature (Wilkinson et al, Reprod Health Care).
Two RCTs47–49 assessed the effect of text messaging support on contraceptive continuation. One study of 962 participants evaluated the effect of 47 individual interactive daily educational text messages on oral contraceptive continuation at 6 months. The intervention group had significantly higher continuation rates, and analyses stratified by age, history of oral contraceptive pill use, and race did not alter the effect of the intervention.48 A smaller pilot study of 100 participants assessed the effect on depot medroxyprogesterone acetate continuation of an intervention consisting of daily interactive text appointment reminders and other health messages.47,49 The results for injection cycle 349 and at 20 months47 were published in two reports, and improved depot medroxyprogesterone acetate continuation was noted at 20 months in the intervention group. These studies, taken together, support the beneficial effect of daily interactive text message reminders on oral contraceptive and injectable contraceptive continuation.
Four studies were identified that evaluated interventions related to induced abortion, all of which were related to medication abortion. One RCT explored the utility of remote follow-up after medication abortion, which allowed for text or online completion of a symptom questionnaire, compared with clinic-based follow-up.50 Overall, the proportion of patients receiving follow-up was not different in the two groups.
Three cohort studies evaluated the provision of medication abortion using telehealth in a clinic system in Iowa. One study compared the prevalence of clinically significant adverse events between patients receiving telehealth services compared with those receiving in-person services over a period of 7 years.51 The analysis found that telehealth provision was noninferior to in-person provision in terms of these safety outcomes. Another cohort study found that effectiveness of the medication abortion was similar between patients receiving telehealth services and those receiving in-person services; some measures of acceptability were significantly higher among telehealth patients.52 The third study evaluated changes in the service delivery statistics of the clinic system in the 2 years after telehealth was introduced compared with the 2 years prior.53 In the latter period, patients were significantly more likely to obtain a medication abortion and to obtain a first-trimester abortion. Travel distance to the clinic decreased slightly. Taken together, these findings suggest that telehealth provision of medication improves access to early abortion with similar clinical outcomes compared with in-person provision.
Two reviewers (C.T.W. and N.D.) independently reviewed 1,277 title and abstracts with 199 full-text articles in the gynecology group (Appendix 12, available online at http://links.lww.com/AOG/B689). Appendix 13 (available online at http://links.lww.com/AOG/B689) provides a summary of the evidence for the three studies on telehealth interventions for gynecology. Three articles met the inclusion criteria: two RCTs and one follow-up to RCT, for a total of 704 women. The three studies focused on telehealth interventions for adolescent notification of positive test results for sexually transmitted infections and treatment for SUI. Age of participants and duration of intervention varied among the three studies. No studies that met inclusion criteria were found in reproductive endocrinology or gynecologic oncology subspecialty clinical areas.
One RCT was identified that examined the effectiveness of a telehealth intervention on successful adolescent notification of positive test results for sexually transmitted infections.54 This study used a 2×3 factorial design and randomized 383 women (mean age for women was 17.6 years) and 201 men to one of six combinations including a combination of one of three modes of notification: call alone, text alone, call plus text. The study was judged to have low risk of bias (Appendix 12, http://links.lww.com/AOG/B689). The setting was a pediatric emergency department in a large U.S. city, so applicability to U.S. populations is high. For women, successful notification was greater for call plus text message (OR 3.2; 95% CI 1.4–6.9) as compared with call or text alone and documenting a confidential phone number on the information card (OR 3.6; 95% CI 1.7–7.5) resulted in more successful notification as compared with not (Appendix 13, http://links.lww.com/AOG/B689).
One RCT examined the effectiveness of treatment for SUI delivered using a mobile app focused on pelvic floor muscle training exercises with information describing SUI, the pelvic floor, and lifestyle factors related to incontinence compared with control, which had delayed access to the app.56 A follow-up study to the same RCT examined 62 women who were randomized to the app and compared their outcomes at 2 years postintervention with baseline data.57 The studies had low risk of bias (Appendix 5, http://links.lww.com/AOG/B689) and findings were reasonably precise, with narrow CIs. In the RCT, women in the app group reported improvements in symptom severity as measured by the International Consultation on Incontinence Questionnaire Short Form (mean score reduction: 3.9, 95% CI 3.0–4.7) and condition-specific quality of life using the International Consultation on Incontinence Questionnaire-Lower Urinary Tract Symptom Quality of Life scores (mean score reduction: 4.8, 3.4–6.2), and the groups were significantly different (mean score difference: −3.2, −4.3 to −2.1; mean score difference: −4.6, −7.8 to −1.4). Of women in the app group, 98.4% (60/61) performed pelvic floor muscle training at follow-up, and 41.0% (25/61) performed it daily.56 The women who were followed for 2 years showed a mean decrease of 3.1 (95% CI 2.0–4.2) on the International Consultation on Incontinence Questionnaire Short Form and 4.0 (95% CI 2.1–5.9) on International Consultation on Incontinence Questionnaire-Lower Urinary Tract Symptom Quality of Life scores. Furthermore, 8.7% rated themselves as very much better, 19.6% as much better, and 34.8% as a little better; use of incontinence protection products decreased significantly; and the proportion of women who felt they could contract their pelvic muscles correctly increased from 30.4% (14/46) to 67.4% (31/46).57
DISCUSSION
In the emerging field of telehealth, it was noteworthy that our review screened nearly 3,926 published articles and the final review included 47 articles, which included 31,967 participants (Fig. 1). The review had a broad scope, encompassing low- and high-risk obstetrics, family planning, and general gynecology reflecting the wide reach of telehealth in women's health services. Although there was limited rigorous evidence supporting telehealth interventions in obstetrics and gynecology, we found some promising interventions deserving of clinical uptake and future study.
One theme that emerged from our review is that text messaging may be helpful to reinforce certain health behaviors, such as smoking cessation during pregnancy, breastfeeding, and adherence to contraception. In contrast, text messaging to initiate a behavior or treatment, such as starting a new contraceptive method, was not effective. It may be that patient motivation is a critical prerequisite for the success of telehealth interventions such as text messaging, and more research is needed to identify populations that might be more receptive to interventions aimed at behavior change.
Another theme highlighted in our review is the role that remote monitoring and virtual visits can play in settings where there are barriers to facility-based care. In the case of high-risk obstetrics, we found that patient-generated data transmitted using remote monitoring and mobile phones led to fewer scheduled outpatient visits for the management of diabetes and hypertension. In the case of medication abortion, telehealth improved access to early abortion. In both examples, the safety and effectiveness of the telehealth service was equivalent to in-person care. Future studies should examine the role that these interventions could play in other services that may be difficult for some patients to access, including contraception provision and management.
Future studies are needed to explore these themes with attention to pairing the telehealth modality and the health outcome most likely to benefit from a targeted intervention, some of which are identified here. Additional controlled trials are needed for the more emerging aspects of telehealth, such as remote monitoring and wearable devices, which currently have been studied primarily with pilot or feasibility trials. Also, this review did not examine patient satisfaction, health care provider ratings, or cost analysis, which can also inform future directions of telehealth. Our systematic review has several strengths. First, we included only studies with at least two comparison groups: RCTs, comparative cohort, and case–control studies. Second, the focus on clinical and behavioral outcomes makes the findings relevant to practicing women's health care providers. Finally, the systematic review methodology offers practical signals from the diverse and growing literature on telehealth interventions being tested in obstetrics and gynecology. This review provides an initial framework that can be updated as further evidence is generated. The potential benefits and gaps in evidence highlight the need for further work to understand what interventions are effective and should be implemented in clinical practice.
Limitations of the review include only incorporating peer-reviewed research in our search strategy and excluding gray literature, thus narrowing the available literature and reducing access to negative trials. The review was designed to summarize evidence from RCTs as well as comparative observational and case–control studies. Evidence from observational studies was more cautiously interpreted owing to design-specific threats to internal validity, as were included studies that used self-reporting mechanisms that may have introduced bias. Within the parameters of the evidence synthesis, research was limited to English-only studies conducted in very high United Nations Human Development Index countries.5 As such, this review is less generalizable to developing nations. Additionally, women consented to participate in randomized trials or included in small, focused investigations may have limited generalizability to the general population. Given the variable study follow-up times, we also could not determine whether some effects might be transient or the result of Hawthorne effects. As mentioned in the Methods section, our development process deviated from the PRISMA methodology. Given the large scope of our review, we assessed the quality and content of literature before specifying inclusion and exclusion criteria, and thus did not publicly post our review protocol before study initiation. Nevertheless, we provided details on our process that increase the transparency and replicability of this review, and all stages of the review were managed to ensure consistency across topic groups at all stages of the review. By focusing on the RCT evidence in our synthesis and limiting the review to study designs that compared women exposed and unexposed to telehealth interventions, our synthesis provides a thorough and comprehensive exploration of the effects of telehealth across several domains of women's reproductive health care.
With some notable exceptions, this review highlights the marked gap in knowledge of telehealth-mediated interventions in women's health care. Although the advent of technologic advances has created an opportunity for integration of telehealth into practice, little is known of the potential benefits or possible harms of these interventions. More evidence is needed to help clinicians determine how they might integrate telehealth into practice in ways that clearly improve patient care. Although this systematic review suggests some benefit for specific telehealth interventions, especially text messaging and remote monitoring, further well-designed studies are needed to examine interventions such as wearable devices and virtual visits to encompass the larger integration of telehealth in obstetrics and gynecology.
Footnotes
Research reported in this publication was supported by the University of California, San Francisco and the Susan Thompson Buffett Foundation. The grant number was 10149sc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the University of California, San Francisco.
Financial Disclosure Daniel Grossman has served as a consultant to the Planned Parenthood Federation of America and provided input on the implementation of services providing medication abortion using telehealth. He has received money paid to them by the Planned Parenthood Federation of America and the Center for Reproductive Rights. He is a member of the editorial board for Contraception. He has served as a Senior Advisor with Ibis Reproductive Health. Jillian Henderson is a member of the editorial board for Contraception. Kathryn Marko serves as an unpaid content advisor for Babyscripts. Nihar Ganju serves as an unpaid content advisor for Babyscripts. Sarita Sonalkar received money paid to her institution from the National Institutes of Health. She received money paid as part of her PCORI board membership. Yvonne Butler Tobah serves as one of the lead research physicians for the Mayo Clinic OB Nest Program. She has stakeholder equity for Mayo Clinic as a Clinical Innovator for Mayo Innovator HeraMED, as it relates to the Orion project and HeraBEAT Fetal heart rate Dopplers, all proceeds go to Mayo Clinic. Curtis Lowery received money paid to them by Angel Eye Board of Directors. They received money paid to their institution by Air Toco Board of Directors. The other authors did not report any potential conflicts of interest.
The authors thank Mary Hyde, MSLS, AHIP, Jean Riedlinger, MSLS, AHIP, Beth DeFrancis Sun, MLS, and Yvonnada McNeil, MSLS, for their assistance with the database searches, and Mary Liu, MHSA, PMP, and Nancy O'Reilly, MHS, PMP for facilitating with the management of the systematic review process.
Each author has indicated that he or she has met the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/B690.
REFERENCES
- 1.Center for Connected Health Policy. What is telehealth? Available at: https://www.cchpca.org/about/about-telehealth. Retrieved June 25, 2019.
- 2.Farag S, Chyjek K, Chen KT. Identification of iPhone and iPad applications for obstetrics and gynecology providers. Obstet Gynecol 2014;124:941–5. [DOI] [PubMed] [Google Scholar]
- 3.IMS Institute for Healthcare Informatics. Patient adoption of mHealth: use, evidence and remaining barriers to mainstream acceptance. Available at: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/patient-adoption-of-mhealth.pdf. Retrieved May 31, 2019. [Google Scholar]
- 4.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PRISMA Group. Ann Intern Med 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 5.United Nations Development Programme. Table 1. Human development Index and its components. Human development reports. New York, NY: United Nations; 2018. [Google Scholar]
- 6.Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0. London, United Kingdom: The Cochrane Collaboration 2011. [Google Scholar]
- 7.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abroms LC, Johnson PR, Leavitt LE, Cleary SD, Bushar J, Brandon TH, et al. A randomized trial of text messaging for smoking cessation in pregnant women. Am J Prev Med 2017;53:781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsoh JY, Kohn MA, Gerbert B. Promoting smoking cessation in pregnancy with Video Doctor plus provider cueing: a randomized trial. Acta Obstet Gynecol Scand 2010;89:515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans WD, Wallace Bihm J, Szekely D, Nielsen P, Murray E, Abroms L, et al. Initial outcomes from a 4-week follow-up study of the Text4baby program in the military women's population: randomized controlled trial. J Med Internet Res 2014;16:e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naughton F, Cooper S, Foster K, Emery J, Leonardi-Bee J, Sutton S, et al. Large multi-centre pilot randomized controlled trial testing a low-cost, tailored, self-help smoking cessation text message intervention for pregnant smokers (MiQuit). Addiction 2017;112:1238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yudin MH, Mistry N, De Souza LR, Besel K, Patel V, Blanco Mejia S, et al. Text messages for influenza vaccination among pregnant women: a randomized controlled trial. Vaccine 2017;35:842–8. [DOI] [PubMed] [Google Scholar]
- 13.Stockwell MS, Westhoff C, Kharbanda EO, Vargas CY, Camargo S, Vawdrey DK, et al. Influenza vaccine text message reminders for urban, low-income pregnant women: a randomized controlled trial. Am J Public Health 2014;104(suppl 1):e7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moniz MH, Hasley S, Meyn LA, Beigi RH. Improving influenza vaccination rates in pregnancy through text messaging: a randomized controlled trial. Obstet Gynecol 2013;121:734–40. [DOI] [PubMed] [Google Scholar]
- 15.Dodd JM, Louise J, Cramp C, Grivell RM, Moran LJ, Deussen AR. Evaluation of a smartphone nutrition and physical activity application to provide lifestyle advice to pregnant women: the SNAPP randomised trial. Matern Child Nutr 2018;14:e12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redman LM, Gilmore LA, Breaux J, Thomas DM, Elkind-Hirsch K, Stewart T, et al. Effectiveness of SmartMoms, a novel eHealth intervention for management of gestational weight gain: randomized controlled pilot trial. JMIR Mhealth Uhealth 2017;5:e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham ML, Strawderman MS, Demment M, Olson CM. Does usage of an eHealth intervention reduce the risk of excessive gestational weight gain? Secondary analysis from a randomized controlled trial. J Med Internet Res 2017;19:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herring SJ, Cruice JF, Bennett GG, Rose MZ, Davey A, Foster GD. Preventing excessive gestational weight gain among African American women: a randomized clinical trial. Obesity (Silver Spring) 2016;24:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huberty JL, Buman MP, Leiferman JA, Bushar J, Hekler EB, Adams MA. Dose and timing of text messages for increasing physical activity among pregnant women: a randomized controlled trial. Transl Behav Med 2017;7:212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fjeldsoe BS, Miller YD, Marshall AL. MobileMums: a randomized controlled trial of an SMS-based physical activity intervention. Ann Behav Med 2010;39:101–11. [DOI] [PubMed] [Google Scholar]
- 21.Choi J, Lee JH, Vittinghoff E, Fukuoka Y. mHealth physical activity intervention: a randomized pilot study in physically inactive pregnant women. Matern Child Health J 2016;20:1091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilmore LA, Klempel MC, Martin CK, Myers CA, Burton JH, Sutton EF, et al. Personalized mobile health intervention for health and weight loss in postpartum women receiving women, infants, and children benefit: a randomized controlled pilot study. J Womens Health (Larchmt) 2017;26:719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herring SJ, Cruice JF, Bennett GG, Darden N, Wallen JJ, Rose MZ, et al. Intervening during and after pregnancy to prevent weight retention among African American women. Prev Med Rep 2017;7:119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Pligt P, Ball K, Hesketh KD, Teychenne M, Crawford D, Morgan PJ, et al. A pilot intervention to reduce postpartum weight retention and central adiposity in first-time mothers: results from the mums OnLiNE (Online, Lifestyle, Nutrition & Exercise) study. J Hum Nutr Diet 2018;31:314–28. [DOI] [PubMed] [Google Scholar]
- 25.Phelan S, Hagobian T, Brannen A, Hatley KE, Schaffner A, Munoz-Christian K, et al. Effect of an internet-based program on weight loss for low-income postpartum women: a randomized clinical trial. JAMA 2017;317:2381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang H, Li M, Wen LM, Hu Q, Yang D, He G, et al. Effect of short message service on infant feeding practice: findings from a community-based study in Shanghai, China. JAMA Pediatr 2014;168:471–8. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed AH, Roumani AM, Szucs K, Zhang L, King D. The effect of interactive web-based monitoring on breastfeeding exclusivity, intensity, and duration in healthy, term infants after hospital discharge. J Obstet Gynecol Neonatal Nurs 2016;45:143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallegos D, Russell-Bennett R, Previte J, Parkinson J. Can a text message a week improve breastfeeding? BMC Pregnancy Childbirth 2014;14:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalfra MG, Nicolucci A, Lapolla A. The effect of telemedicine on outcome and quality of life in pregnant women with diabetes. TISG J Telemed Telecare 2009;15:238–42. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Ferre N, Galindo M, Fernandez MD, Velasco V, Runkle I, de la Cruz MJ, et al. The outcomes of gestational diabetes mellitus after a telecare approach are not inferior to traditional outpatient clinic visits. Int J Endocrinol 2010;2010:386941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ladyzynski P, Wojcicki JM. Home telecare during intensive insulin treatment–metabolic control does not improve as much as expected. J Telemed Telecare 2007;13:44–7. [DOI] [PubMed] [Google Scholar]
- 32.Homko CJ, Santamore WP, Whiteman V, Bower M, Berger P, Geifman-Holtzman O, et al. Use of an internet-based telemedicine system to manage underserved women with gestational diabetes mellitus. Diabetes Technol Ther 2007;9:297–306. [DOI] [PubMed] [Google Scholar]
- 33.Homko CJ, Deeb LC, Rohrbacher K, Mulla W, Mastrogiannis D, Gaughan J, et al. Impact of a telemedicine system with automated reminders on outcomes in women with gestational diabetes mellitus. Diabetes Technol Ther 2012;14:624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackillop L, Hirst JE, Bartlett KJ, Birks JS, Clifton L, Farmer AJ, et al. Comparing the efficacy of a mobile phone-based blood glucose management system with standard clinic care in women with gestational diabetes: randomized controlled trial. JMIR Mhealth Uhealth 2018;6:e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.di Biase N, Napoli A, Sabbatini A, Borrello E, Buongiorno AM, Fallucca F. Telemedicine in the treatment of diabetic pregnancy. Ann Ist Super Sanita 1997;33:347–51. [PubMed] [Google Scholar]
- 36.Miremberg H, Ben-Ari T, Betzer T, Raphaeli H, Gasnier R, Barda G, et al. The impact of a daily smartphone-based feedback system among women with gestational diabetes on compliance, glycemic control, satisfaction, and pregnancy outcome: a randomized controlled trial. Am J Obstetrics Gynecol 2018;218:453.e1–7. [DOI] [PubMed] [Google Scholar]
- 37.Caballero-Ruiz E, Garcia-Saez G, Rigla M, Villaplana M, Pons B, Hernando ME. A web-based clinical decision support system for gestational diabetes: automatic diet prescription and detection of insulin needs. Int J Med Inform 2017;102:35–49. [DOI] [PubMed] [Google Scholar]
- 38.Lanssens D, Vandenberk T, Smeets CJ, De Canniere H, Molenberghs G, Van Moerbeke A, et al. Remote monitoring of hypertension diseases in pregnancy: a pilot study. JMIR Mhealth Uhealth 2017;5:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez-Ferre N, Galindo M, Fernandez MD, Velasco V, de la Cruz MJ, Martin P, et al. A telemedicine system based on internet and short message service as a new approach in the follow-up of patients with gestational diabetes. Diabetes Res Clin Pract 2010;87:e15–7. [DOI] [PubMed] [Google Scholar]
- 40.Zairina E, Abramson MJ, McDonald CF, Li J, Dharmasiri T, Stewart K, et al. Telehealth to improve asthma control in pregnancy: a randomized controlled trial. Respirology 2016;21:867–74. [DOI] [PubMed] [Google Scholar]
- 41.Hirshberg A, Downes K, Srinivas S. Comparing standard office-based follow-up with text-based remote monitoring in the management of postpartum hypertension: a randomised clinical trial. BMJ Qual Saf, 2018;27:871–7. [DOI] [PubMed] [Google Scholar]
- 42.Chernick LS, Stockwell MS, Wu M, Castano PM, Schnall R, Westhoff CL, et al. Texting to increase contraceptive initiation among adolescents in the emergency department. J Adolesc Health 2017;61:786–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thiel de Bocanegra H, Bradsberry M, Lewis C, Maguire F. Do Bedsider family planning mobile text message and e-mail reminders increase kept appointments and contraceptive coverage? Womens Health Issues 2017;27:420–5. [DOI] [PubMed] [Google Scholar]
- 44.Bull S, Devine S, Schmiege SJ, Pickard L, Campbell J, Shlay JC. Text messaging, teen outreach program, and sexual health behavior: a cluster randomized trial [published erratum appears in Am J Public Health 2016;106:e14]. Am J Public Health 2016;106:S117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsur L, Kozer E, Berkovitch M. The effect of drug consultation center guidance on contraceptive use among women using isotretinoin: a randomized, controlled study. J Womens Health (Larchmt) 2008;17:579–84. [DOI] [PubMed] [Google Scholar]
- 46.Hou MY, Hurwitz S, Kavanagh E, Fortin J, Goldberg AB. Using daily text-message reminders to improve adherence with oral contraceptives: a randomized controlled trial [published erratum appears in Obstet Gynecol 2010;116:1224]. Obstet Gynecol 2010;116:633–40. [DOI] [PubMed] [Google Scholar]
- 47.Buchanan CRM, Tomaszewski K, Chung SE, Upadhya KK, Ramsey A, Trent ME. Why didn't you text me? Poststudy trends from the DepoText trial. Clin Pediatr (Phila) 2018;57:82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castano PM, Bynum JY, Andres R, Lara M, Westhoff C. Effect of daily text messages on oral contraceptive continuation: a randomized controlled trial. Obstet Gynecol 2012;119:14–20. [DOI] [PubMed] [Google Scholar]
- 49.Trent M, Thompson C, Tomaszewski K. Text messaging support for urban adolescents and young adults using injectable contraception: outcomes of the DepoText pilot trial. J Adolesc Health 2015;57:100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bracken H, Lohr PA, Taylor J, Morroni C, Winikoff B. RU OK? The acceptability and feasibility of remote technologies for follow-up after early medical abortion. Contraception 2014;90:29–35. [DOI] [PubMed] [Google Scholar]
- 51.Grossman D, Grindlay K. Safety of medical abortion provided through telemedicine compared with in person. Obstet Gynecol 2017;130:778–82. [DOI] [PubMed] [Google Scholar]
- 52.Grossman D, Grindlay K, Buchacker T, Lane K, Blanchard K. Effectiveness and acceptability of medical abortion provided through telemedicine. Obstet Gynecol 2011;118:296–303. [DOI] [PubMed] [Google Scholar]
- 53.Grossman DA, Grindlay K, Buchacker T, Potter JE, Schmertmann CP. Changes in service delivery patterns after introduction of telemedicine provision of medical abortion in Iowa. Am J Public Health 2013;103:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reed JL, Huppert JS, Taylor RG, Gillespie GL, Byczkowski TL, Kahn JA, et al. Improving sexually transmitted infection results notification via mobile phone technology. J Adolesc Health 2014;55:690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang AJ, Phillips S, Schembri M, Vittinghoff E, Grady D. Device-guided slow-paced respiration for menopausal hot flushes: a randomized controlled trial. Obstet Gynecol 2015;125:1130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asklund I, Nystrom E, Sjostrom M, Umefjord G, Stenlund H, Samuelsson E. Mobile app for treatment of stress urinary incontinence: a randomized controlled trial. Neurourol Urodyn 2017;36:1369–76. [DOI] [PubMed] [Google Scholar]
- 57.Hoffman V, Soderstrom L, Samuelsson E. Self-management of stress urinary incontinence via a mobile app: two-year follow-up of a randomized controlled trial. Acta Obstet Gynecol Scand 2017;96:1180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haggerty AF, Huepenbecker S, Sarwer DB, Spitzer J, Raggio G, Chu CS, et al. The use of novel technology-based weight loss interventions for obese women with endometrial hyperplasia and cancer. Gynecol Oncol 2016;140:239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joseph RP, Keller C, Adams MA, Ainsworth BE. Print versus a culturally-relevant Facebook and text message delivered intervention to promote physical activity in African American women: a randomized pilot trial. BMC Womens Health 2015;15:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vonk Noordegraaf A, Anema JR, van Mechelen W, Knol DL, van Baal WM, van Kesteren PJ, et al. A personalized eHealth programme reduces the duration until return to work after gynaecological surgery: results of a multicentre randomised trial. BJOG 2014;121:1127–35. [DOI] [PubMed] [Google Scholar]
- 61.Aiken A, Gomperts R, Trussell J. Experiences and characteristics of women seeking and completing at-home medical termination of pregnancy through online telemedicine in Ireland and Northern Ireland: a population-based analysis. BJOG 2017;124:1208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aiken ARA, Digol I, Trussell J, Gomperts R. Self reported outcomes and adverse events after medical abortion through online telemedicine: population based study in the Republic of Ireland and Northern Ireland. BMJ 2017;357:j2011. [DOI] [PMC free article] [PubMed] [Google Scholar]