Abstract
BACKGROUND:
Insufficient fluid administration intra- and postoperatively may lead to delayed renal graft function (DGF), while fluid overload increases the risk of heart failure, infection, and obstipation. Several different fluid protocols have been suggested to ensure optimal fluid state. However, there is a lack of evidence of the clinical impact of these regimens. This study aimed to determine whether individualized goal-directed fluid therapy (IGDT) positively affects the initial renal function compared to a high-volume fluid therapy (HVFT) and to examine the effects on renal endothelial glycocalyx, inflammatory and oxidative stress markers, and medullary tissue oxygenation. The hypothesis was that IGDT improves early glomerular filtration rate (GFR) in pigs subjected to renal transplantation.
METHODS:
This was an experimental randomized study. Using a porcine renal transplantation model, animals were randomly assigned to receive IGDT or HVFT during and until 1 hour after transplantation from brain-dead donors. The kidneys were exposed to 18 hours of cold ischemia. The recipients were observed until 10 hours after reperfusion, which included GFR measured as clearance of chrom-51-ethylendiamintetraacetat (51Cr-EDTA), animal weight, and renal tissue oxygenation by fiber optic probes. The renal expression of inflammatory and oxidative stress markers as well as glomerular endothelial glycocalyx were analyzed in the graft using polymerase chain reaction (PCR) technique and immunofluorescence.
RESULTS:
Twenty-eight recipient pigs were included for analysis. We found no evidence that IGDT improved early GFR compared to HVFT (P = .45), while animal weight increased more in the HVFT group (a mean difference of 3.4 kg [1.96–4.90]; P < .0001). A better, however nonsignificant, preservation of glomerular glycocalyx (P = .098) and significantly lower levels of the inflammatory marker cyclooxygenase 2 (COX-2) was observed in the IGDT group when compared to HVFT. COX-2 was 1.94 (1.50–2.39; P = .012) times greater in the HVFT group when compared to the IGDT group. No differences were observed in outer medullary tissue oxygenation or oxidative stress markers.
CONCLUSIONS:
IGDT did not improve early GFR; however, it may reduce tissue inflammation and could possibly lead to preservation of the glycocalyx compared to HVFT.
See Editorial, p 596
KEY POINTS.
Question: Does individualized goal-directed fluid therapy (IGDT) improve early renal function in pigs subjected to renal transplantation?
Findings: IGDT did not improve early renal function but may reduce tissue inflammation and lead to better preservation of the glycocalyx.
Meaning: IGDT takes resources, and its introduction in the clinic should be preceded by further studies on long-term renal graft function because our primary end point early graft function was not positively affected.
Fluid management during renal transplantation is important for optimal organ perfusion. It reduces the risk of renal graft thrombosis, delayed graft function (DGF), and postoperative complications such as pneumonia and heart failure.1 Sufficient intravascular fluid is required for maintenance of an adequate cardiac output, blood pressure, and thus initial graft perfusion during anesthesia. Conversely, fluid overload may not only cause heart failure but also affect the microcirculation due to damage of the vascular endothelium leading to increased permeability and transport of fluids from the vascular bed to the interstitium.2 The vascular endothelium is lined with the glycocalyx, which plays a fundamental role in the regulation of vascular endothelial permeability, coagulation, and leukocyte adhesion and inflammation.3–5
In renal transplantation, the glycocalyx is disrupted through various mechanisms, including ischemia–reperfusion injury and the presence of proinflammatory cytokines. Inflammatory changes in the glycocalyx disrupt the microcirculation causing edema and loss of antioxidative properties.3 In addition, disturbance in the microcirculation can affect the tissue oxygenation.6 The complex interactions of these mechanisms, however, are poorly understood, and it is not clear whether more restrictive fluid therapies affect these parameters.
Fluid infusion during kidney transplantations has previously been done using a continuous high-volume fluid infusion therapy (HVFT) 10–15 mL/kg/h. Recently, individualized goal-directed fluid therapy (IGDT) is suggested to be the preferred method for optimizing the fluid infusion during various surgical procedures such as pancreaticoduodenectomies7 and colorectal cancer surgery.8 Interestingly, IGDT has recently been suggested to reduce the incidence of DGF and the level of serum creatinine after 7 and 30 days when compared to HVFT.9
IGDT is defined as an individually targeted fluid therapy, where fluid is administered depending on hemodynamic parameters, such as stroke volume (SV), SV variation (SVV), and cardiac output. Administration of norepinephrine (NE) has also been investigated as a strategy to optimize renal blood flow; however, there is no clear evidence of any beneficial effects on graft function.10–14
Using a porcine renal transplantation model, we are able to study both interventions in a vascular system that is comparable to humans. Our hypothesis is that IGDT improves early glomerular filtration rate (GFR) in pigs subjected to renal transplantation. The aim of this study was to evaluate the potentially beneficial effect of IGDT versus a HVFT used intra- and postoperatively on early GFR posttransplantation. We also evaluated kidney tissue oxygenation as well as vascular glycocalyx integrity and markers of oxidative stress and inflammation.
METHODS
Design
This is a randomized controlled experimental study which is investigating pigs receiving a kidney from a brain-dead donor and comparing 2 different fluid regimens for intra- and posttransplantation use: IGDT and HVFT with or without NE infusion. Randomization was achieved by drawing a fluid regimen and NE regimen randomly. Randomization was always for the right kidney. The left kidney would receive the opposite fluid regimen but the same NE regimen.
An overview of the experiment is presented on the time line in Supplemental Digital Content, Figure 2, http://links.lww.com/AA/C941.
Animals and Experimental Procedures
Ethical conditions and all animal procedures for this study were approved by the Danish Animal Experiments Inspectorate (protocol no. 2014-15-0201-00378). This manuscript adheres to the applicable Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines.16 The study was performed using female Danish landrace pigs weighing approximately 60 kg. They were kept at a standard diet with free access to water before transport to the facility. On day 1, both kidneys were explanted from a brain-dead donor, and on day 2, the kidneys were transplanted into 2 recipients.
Induction of Brain Death in the Donors
Brain death was achieved as described previously.15 After 4 hours of brain death, the kidneys were removed. Before removal, heparin (15,000 IU) was administered and kidneys were flushed in situ with 4°C Custodiol (bridge to life) and stored in a fridge at 4°C for 18 hours.
Anesthesia
On arrival at the operation facilities, initial sedation was achieved using intravenous ketamine (5 mg/kg) and midazolam (0.05 mg/kg). The pigs were weighed, intubated, and ventilated using 40% oxygen, a respiratory tidal volume of 600 mL, and kept at expiratory carbon dioxide (CO2) levels between 4.5 and 5.5 kPa. Anesthesia was maintained with continuous infusion of propofol (3 mg/kg/h) and fentanyl (15 µg/kg/h) throughout the study.
All animals received 1 L of Ringer acetate (Baxter International, Deerfield, IL) during the first hour to compensate for fluid losses during fasting. Cefuroxime (750 mg) was administered intramuscularly after sedation, and vascular sheaths (Edwards FloTrac; Edwards Lifesciences, Irvine, CA) were inserted in the carotid artery and in both jugular veins to monitor hemodynamic parameters.
Transplantation
The native kidneys were removed from the recipients by a retroperitoneal access. The donor kidneys were anastomosed end-to-end to the left native renal vessels of the recipients. Transplantations were performed simultaneously by 2 experienced transplant surgeons. A feeding tube Chariére 8 (Ch8) was inserted in the ureter for collection of urine and assessment of GFR. The pigs were observed for 10 hours after reperfusion and the kidneys were removed, weighed, and tissue samples were stored in formalin or at −80°C.
Fluid Therapy and Interventions
Ringer acetate was used for fluid infusion at all times. The fluid regimens were started immediately after sedation and continued until 1-hour posttransplantation. Regimens of the IGDT and HVFT groups are shown in Supplemental Digital Content, Figure 1, http://links.lww.com/AA/C941. The HVFT regime was 10 mL/kg/h with addition of 500-mL bolus if mean arterial pressure (MAP) decreased below 60 mm Hg. The IGDT regime was 2 mL/kg/h with SV continuously measured. At the beginning of the nephrectomy, a 250-mL fluid challenge was administered, and if SV increased by ≥12%, 250-mL bolus was administered until the increase was <12%. Whenever the new SV decreased >2 mL, another fluid challenge was administered. If MAP decreased below 60 mm Hg, a 500-mL bolus was administered.
The groups receiving NE were continuously infused with a low dose (0.5 ng/kg/min) throughout the experiment. This dose was unfortunately extremely low due to a miscalculation resulting in a dose 1/60 of the intended dose.
Management of B-Glucose
Arterial blood samples were taken regularly, and blood glucose levels were kept above 3.5 mmol/L by infusion of 20-mL 50% glucose if levels were <3.5 mmol/L.
Measurement of GFR
GFR was measured for 10 hours after graft reperfusion as urinary clearance of chrom-51-ethylendiamintetraacetat (51Cr-EDTA). A bolus injection of 0.06 MBq/kg was administered 3 hours before reperfusion of the graft followed by continuous infusion at 0.03 MBq/kg/h. Blood and urine were collected for isotope measurements as stated below.
Samples
Blood was sampled, and urine output was measured every 30 minutes during the first 2 hours, and every 1 hour for the remaining 8 hours. Blood samples were centrifuged, and plasma and urine were stored at −80°C.
Samples of renal cortex were collected from the transplanted kidneys. Control renal tissue was obtained from healthy Danish Landrace pigs weighing approximately 60 kg (n = 5). All samples were processed for pathology, immunofluorescent microscopy, and quantitative polymerase chain reaction (QPCR) analysis.
Glomerular Glycocalyx Assessment
Paraffin-embedded tissue sections were labeled for glycocalyx glycoproteins using Dolichos biflorus agglutinin (DBA) lectin. DBA lectin binds α-linked N-acetylgalactosamine (GalNAc). GalNAc is present in all tissue but to a much greater density in areas with glycosylated proteins such as the glycocalyx. Sections were deparaffinized and incubated with DBA lectin (VEC-B-1035; VectorLabs, Burlingame, CA) and further incubation with fluorescein isothiocyanate (FITC)-conjugated streptavidin. The labeled sections were photographed using a Leica TCS SL Spectral Confocal Microscope (TM Leica Microsystems, Wetzlar, Germany). Three glomeruli from each tissue sample were randomly selected and photographed. The fluorescent labeling was quantitated using ImageJ (National Institutes of Health, Bethesda, MD). The operator was blinded to the origin of the sections throughout both microscopy and image analysis.
Quantitative Polymerase Chain Reaction
Ribonucleic acid (RNA) was isolated from renal cortex with a Nucleospin RNA II mini kit (Macherey Nagel, Düren, Germany). Complementary deoxyribonucleic acid (cDNA) synthesis and QPCR analysis were performed as previously described.17 β-actin was used as a control gene. Primer sequences used are given in Supplemental Digital Content, Table 1, http://links.lww.com/AA/C941.
Hematoxylin and Eosin and Periodic Acid–Schiff Staining
Sections from paraffin-embedded kidney cortex were deparaffinized by immersion into graded alcohol solutions. The sections were stained using Mayer hematoxylin and eosin (HE) or periodic acid–Schiff (PAS) to assess the following parameters: tubular and glomerular injury, casts, inflammation, glomerular dilation, and edema. Assessment of the histology was done by an experienced pathologist, who was blinded to treatment, using a semiquantitative scoring system. For each section, the pathologist would score for each parameter on a scale 0–4 based on how much of the section was affected: 0 if <1% was affected, 1 (1%–5%), 2 (6%–25%), 3 (25%–50%), and 4 (51%–100%).
Neutrophil Gelatinase–Associated Lipoprotein Excretion in Urine
Urinary neutrophil gelatinase–associated lipocalin (NGAL) concentration was measured by enzyme-linked immunosorbent assay (ELISA; BioPorto Diagnostics, Hellerup, Denmark) and the excretion expressed as NGAL excretion rate.
Glutathione Assay
Glutathione (GSH) levels in renal cortical tissue were measured using a total GSH (glutathione disulfide [GSSG]/GSH) assay kit (OxiSelect assay kit; Nordic Biosite, Copenhagen, Denmark). Cortical samples were assayed in triplicates and analyzed at 405 nm. The results are expressed as the total GSH levels (µM)/µg of tissue.
Renal Oxygen Tension
Tissue oxygenation was measured using NX-BF/OFT/E fiber optic probes (Oxford Optronix, Oxford, UK). The probes were used in conjunction with the OxyLite monitor (Oxford Optronix, Abingdon, UK) to enable continuous renal tissue oxygenation in the outer medulla (tPo2) measurements. Data were collected using the WinDaq software (DATAQ Instruments, Akron, OH). The probes were inserted in renal outer medulla through a venflon guided by micro-ultrasound imaging. Measurements were conducted for 10 minutes at each hour. The mean value of the period was used to cancel momentary fluctuations.
Termination of the Experiment
At the end of the 10-hour observation period, the transplanted kidney was removed for weighing and sample collection. The animals were then euthanized by administration of an overdose of pentobarbital.
Statistics
Baseline data are shown as mean ± standard deviation (SD). Treatment effect is shown as mean ± 95% confidence interval (CI). All continuous data and data on glycocalyx were analyzed using a linear mixed regression effects model. The linear mixed regression effects model was used to allow for repeated measures, taking the study design into account and to allow for analysis of parameters containing missing data. The model was used to compare outcome between intervention groups with intervention group and time as fixed effects and donor as random effect. Adjustment for multiple comparisons was done using Scheffe method. P value < .05 was considered statistically significant. Data on inflammatory markers were analyzed using 1-way analysis of variance (ANOVA) with a Scheffe post hoc test. Data on oxidative stress were analyzed by paired t test. Data were analyzed in Stata/IC 14.2 (StataCorp, College Station, TX).
Power calculation was with GFR as primary end point. The power calculation was conducted for a 2-sided ANOVA for repeated measurements with a power of 80% using an α of 1.25% to reduce the risk of an overall type 1 error. Number of repeated measures set at 12 and a correlation between measurements on 0.4. From prior experiments conducted at our facilities, we expect a variance explained by the GFR to be approximately 0.44 and the variance of the error to be approximately 1. This gives a sample size of 6 in each of the 4 groups.
RESULTS
A total of 16 donor pigs and 32 recipient pigs were included in the experiment. Two recipients (both with no-NE infusion, 1 receiving IGDT and 1 receiving HVFT) were excluded due to surgical complications. One donor was excluded because of incidental hydroureter. This leaves a total of 28 full transplantations with completed follow-up (n = 7 per group). Some parameters include missing data due to technical malfunctions during sampling. There were no significant differences in baseline parameters neither between the recipient groups nor the donor animals (Supplemental Digital Content, Table 2, http://links.lww.com/AA/C941).
Data from arterial blood gas tests during the observation period are presented in Supplemental Digital Content, Table 3, http://links.lww.com/AA/C941.
Effects of NE
Administration of NE did not affect the primary end point of GFR (Figure 1A) nor did it affect any of the other analyzed parameters when compared to no NE (data not shown). Hence, and because the dose of NE was likely too low to be with any effects, the NE and no-NE groups were pooled together leaving 2 groups for the final analyses: the IGDT group and the HVFT group.
Figure 1.
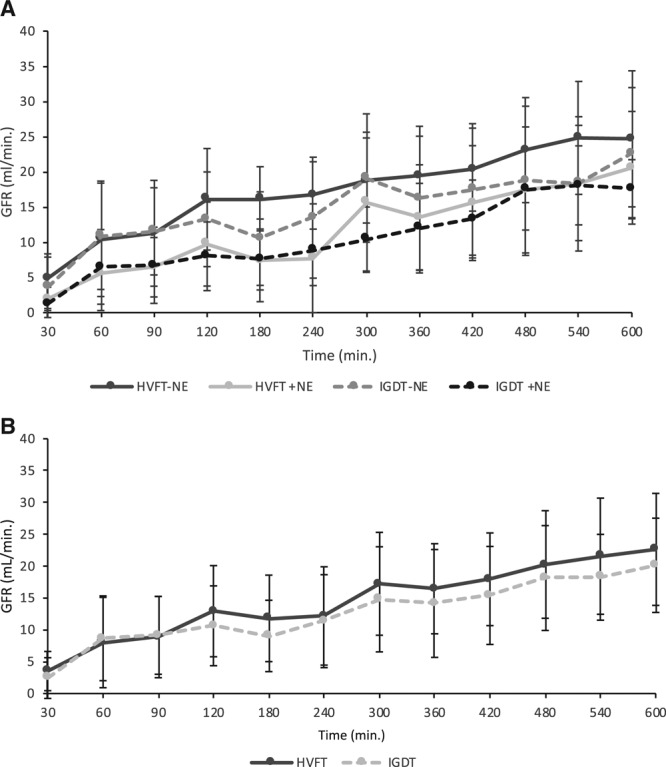
Effect of fluid therapy on GFR. The recovery of renal function during follow-up of 10 h. GFR measured by 51Cr-EDTA. A, GFR in all 4 groups; HVFT with and without NE and IGDT with and without NE. No difference was observed after low-dose NE; hence, the groups were pooled. B, GFR in the HVFT and IGDT groups. Error bars represent SD. 51Cr-EDTA indicates chrom-51-ethylendiamintetraacetat; GFR, glomerular filtration rate; HVFT, high-volume fluid infusion therapy; IGDT, individual goal-directed fluid therapy; NE, norepinephrine; SD, standard deviation.
Effects of Fluid Therapy on GFR
Over time we observed a significant rise in GFR in both groups (mean increase in GFR of 18.6 mL/min [14.1–23.0]; P < .0001) but with no difference between IGDT and HVFT (mean difference, −1.72 mL/min [−5.11 to 1.67]; P = .29; Figure 1B).
Effect of Fluid Therapy on Animal and Kidney Weight
Pigs in the HVFT group received more fluid throughout the experiment compared to the pigs in the IGDT group (10.0 ± 1.93 L vs 6.06 ± 1.70 L; mean difference, −3.94 L; P = .0002; Figure 2A). The weight of the HVFT-treated animals increased significantly more than in the IGDT-treated animals (7.6 ± 2.3 kg vs 4.2 ± 1.3 kg; mean weight increase of 3.43 kg [1.96–4.90]; P < .0001). Mean weight ± SD of the kidneys just after ischemia was for the IGDT 112.9 ± 16.7 g and for the HVFT 114.6 ± 13.1 g. The mean increase in kidney weight from transplantation to 10 hours posttransplantion in the IGDT was by a factor of 1.92 CI (1.79–2.05) and in the HVFT group by a factor of 1.86 CI (1.81–1.91). Between the groups, no significant differences were observed in neither initial kidney weight, kidney weight increase nor final kidney weight.
Figure 2.
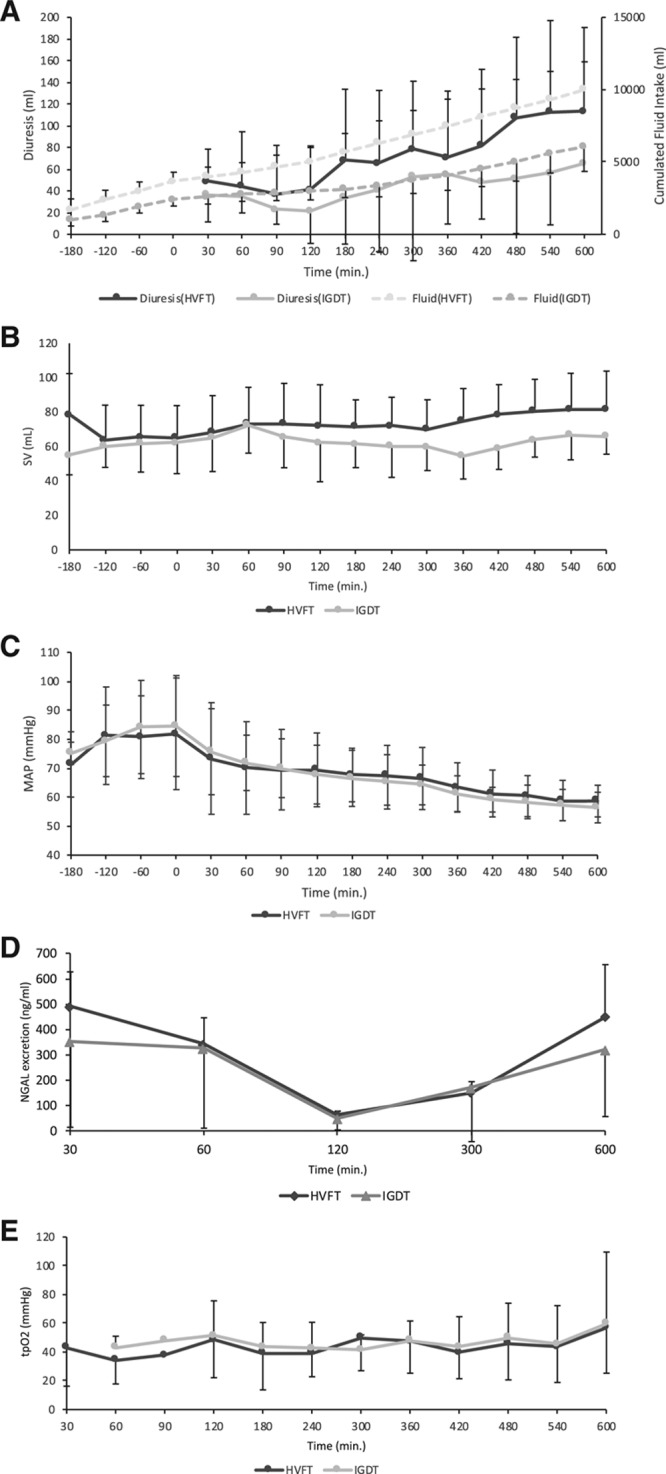
Effect of fluid therapy on hemodynamic parameters, urine output, urinary NGAL, and tissue oxygenation. A, Diuresis and accumulated fluid intake in the HVFT and IGDT groups. B, Stroke volume (B) and MAP (C) in the HVFT and IGDT groups. D, Urinary NGAL excretion from the HVFT and IGDT groups. E, tPo2 from the HVFT and IGDT groups (HVFT n = 9 and IGDT n = 10). HVFT indicates high-volume fluid infusion therapy; IGDT, individual goal-directed fluid therapy; MAP, mean arterial pressure; NGAL, neutrophil gelatinase–associated lipocalin; tPo2, renal tissue oxygenation in the outer medulla.
Effect of Fluid Therapy on Blood Pressure, Urine Output, Urinary NGAL, and Tissue Oxygenation
Both the accumulated fluid infusion and urine output were increased to a greater extent in the HVFT-treated animals compared to IGDT (Figure 2A). MAP and SV did not differ between the 2 groups and showed no change in either of the groups during the experimental follow-up (Figure 2B–C). No difference was observed in the urinary NGAL excretion concentration between the 2 groups (Figure 2D). tPo2 did not change during follow-up and showed no significant difference between the groups (Figure 2E).
Indexed parameters of SV, GFR, and diuresis can be found in Supplemental Digital Content, Figure 3, http://links.lww.com/AA/C941.
Effect of Fluid Therapy on Renal Endothelial Glycocalyx
Fluorescent staining for GalNAc revealed a strong staining of the glomerulus (Figure 3A). Quantitation of the fluorescent label revealed reduced labeling in the HVFT group compared to IGDT-treated animals although it did not reach significance (mean difference in fluorescence, 9.2 mean fluorescent intensity [−1.98 to 20.5]; P = .098; Figure 3B). There was no difference in the staining of glomeruli within the same kidney (data not shown).
Figure 3.
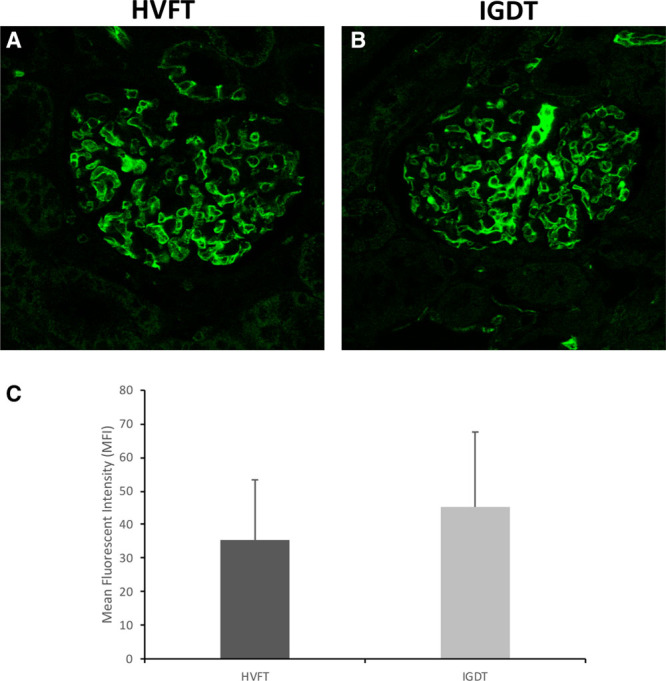
Effect of fluid therapy on glycocalyx preservation. Representative immunofluorescent staining of the glomeruli using DBA lectin in the HVFT group (A) and the IGDT group (B). C, Mean fluorescent intensity of the glomeruli after staining with DBA lectin in the HVFT (n = 14) and IGDT groups (n = 14). Error bars represent SD. DBA indicates Dolichos biflorus agglutinin; HVFT, high-volume fluid infusion therapy; IGDT, individual goal-directed fluid therapy; MFI, mean fluorescent intensity; SD, standard deviation.
Effects of Fluid Therapy on Renal Inflammation and Oxidative Stress
Cyclooxygenase 2 (COX-2) expression was significantly increased in the HVFT group compared to the IGDT group (mean expression of COX-2 increased with a factor of 1.94 [1.50–2.39]; P = .012; Figure 4A). An increased expression of tumor necrosis factor α (TNF-α), monocyte chemoattractant protein 1 (MCP1), and interleukin 6 (IL-6) group was also identified in the HVFT group when compared to the IGDT group although this was not significant (Figure 4B–D). Both the HVFT and IGDT groups revealed a higher expression of all inflammatory markers compared to the control pig kidneys. No difference was observed between groups in the expression of heme oxygenase 1 (HO-1) and GSH (Figure 4E–F), suggesting no difference in oxidative stress levels.
Figure 4.
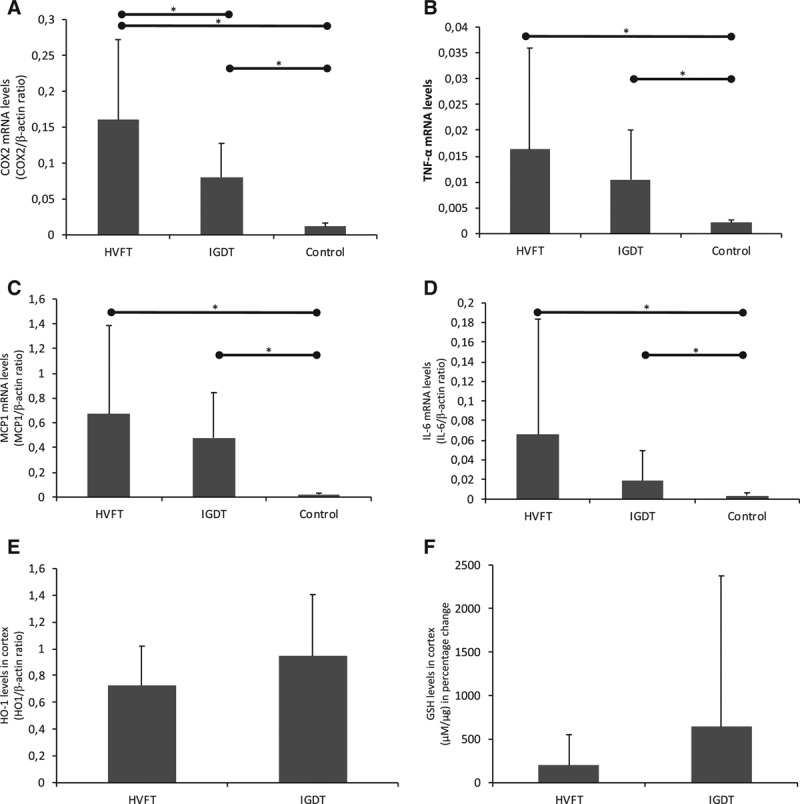
Effects of fluid therapy on inflammatory markers. A, COX-2 mRNA levels in HVFT (n = 14), IGDT (n = 14), and control group (n = 4). B, TNFα mRNA levels in HVFT (n = 14), IGDT (n = 14), and control group (n = 5). C, MCP1 mRNA levels in HVFT (n = 14), IGDT (n = 14), and control group (n = 5). D, IL-6 mRNA levels in HVFT (n = 14), IGDT (n = 14), and control group (n = 5). E, HO-1 levels in HVFT (n = 14) an IGDT groups (n = 14). F, GSH levels in cortex as percentage change in HVFT (n = 11) and IGDT groups (n = 11). mRNA levels were normalized to β-actin. Data are presented as means, and error bars represent SD, *P < .05. COX-2 indicates cyclooxygenase 2; GSH, glutathione; HO-1, heme oxygenase 1; HVFT, high-volume fluid infusion therapy; IGDT, individualized goal-directed fluid therapy; IL-6, interleukin 6; MCP1, monocyte chemoattractant protein 1; mRNA, messenger ribonucleic acid; SD, standard deviation; TNFα, tumor necrosis factor α.
Effects of Fluid Therapy on Renal Histology
Table.
Semiquantitative Histological Evaluation of Renal Specimens
| HVFT | IGDT | |
|---|---|---|
| Tubular damage | 0.64 (0–3) | 0.57 (0–2) |
| Casts | 0.50 (0–2) | 0.57 (0–2) |
| Inflammation | 0.57 (0–2) | 0.42 (0–1) |
| Glomerular damage | 0.29 (0–3) | 0.21 (0–3) |
| Edema | 0 (0–0) | 0 (0–0) |
Abbreviations: HVFT, high-volume fluid infusion therapy; IGDT, individualized goal-directed fluid therapy.
Histological assessment of tubular damage, casts, inflammation, glomerular damage, and edema showed no difference between the HVFT and IGDT groups (Figure 5; Table).
Figure 5.
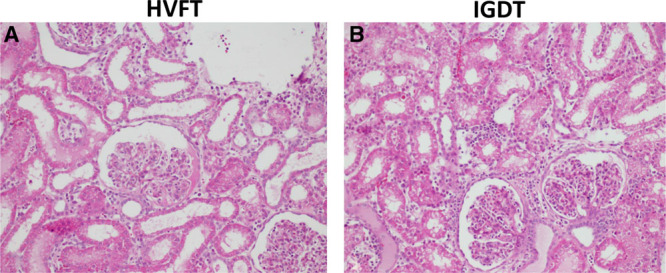
Histological evaluation of induced kidney damage after fluid therapy. Representative PAS-stained kidney sections demonstrating histological changes of fluid therapy A, Cortex of the kidney in HVFT group. B, Cortex of the kidney in IGDT group. Original magnification: ×40 C: Semiquantitative histological evaluation of renal specimens. Values are presented as means; ranges within each group are shown in parentheses. Each sampled was scored as follows: 0, no change (<1%); 1, mild affection (1%–5%); 2, incipient affection (6%–25%); 3, profound affection (25%–50%); and 4, severe affection (50%–100%). HVFT indicates high-volume fluid infusion therapy; IGDT, individualized goal-directed fluid therapy; PAS, periodic acid–Schiff.
DISCUSSION
With our established porcine model of renal transplantation after brain death, this study did not show any effect of IGDT on the immediate renal graft function assessed by GFR, histology, and urinary NGAL. GFR and MAP did not differ between IGDT and HVFT, as shown previously by Byrne et al18 and Lima et al,19 providing further support of the challenge that is IGDT. However, while not reaching significance, reduced glycocalyx labeling in the kidney was observed in the HVFT compared to IGDT together with increased expression of the inflammatory marker COX-2.
Our model combining brain death with 18-hour cold ischemia time15 is suitable to study interventions against DGF. IGDT protocols have been shown to be beneficial in abdominal surgical procedures7 and are widely used to reduce complications like wound infection, pneumonia, sepsis, respiratory failure, and prolonged mechanical ventilation.20,21 In renal transplantation, a recent study by Cavaleri et al9 showed a lower incidence of DGF within the first postoperative week when using IGDT. Consistent with the reduction in DGF incidences, Cavaleri et al9 also observe a better 7-day and 30-day serum creatinine in the IGDT patients. However, a significant higher serum creatinine was observed after IGDT within the first 24 hours indicating no effect of IGDT at early time points. Our data from a more detailed observation showed no difference in early GFR between IGDT and HVFT. However, we only observed the pigs for 10 hours. Alongside a retrospective study including 404 kidney recipients has concluded that restricted management of fluid balance did not decrease graft survival.22
Reduced glycocalyx, indicating endothelial damage, was observed in the HVFT group. Suggesting that excessive fluid administration negatively affects the kidney over time. Increased fluid administration leads to a rise in atrial natriuretic peptide (ANP). ANP injures the glycocalyx by cleavage of various glycocalyx membrane-bound proteoglycans and glycoproteins including syndecan-1 and hyaluronic acid, which increases the extravasation of fluid from the intravascular space23 and negatively impact the glycocalyx.24,25 The shown trend toward difference in glycocalyx preservation suggests that glycocalyx might play a role in the optimization of fluid therapy. This is further supported by a study using an experimental porcine model of severe acute pancreatitis where IGDT reduced serum levels of heparan sulfate components of the endothelial glycocalyx, indicated lesser glycocalyx damage,26 an observation further supported by Byrne et al18 showing a significant loss of glycocalyx after high-volume fluid resuscitation in septic pigs.18 Interstitial edema and even “compartment syndrome” can lead to early graft loss.27
A potential advantage of IGDT is indicated by the significantly lower COX-2 expression in this group. Other inflammatory biomarkers TNF-α, MCP1, and IL-6 showed likewise lower values in IGDT pigs, although this did not reach statistical significance.26,28 COX-2 may be more sensitive to fluid therapy in the short term. Several studies have demonstrated an important role for the regulation of COX-2 in acute renal allograph rejection,29–31 indicating that COX-2 might play a role in the early inflammatory response during renal transplantation. We analyzed a broad panel of inflammatory markers that are induced in response to renal transplantation,29,30 which we also observed in our study. Although not all the inflammatory markers showed significant changes, our data overall suggest that inflammation is less after IGDT compared to HVFT during renal transplantation. It is well recognized that an inflammatory environment due to parenchymal injury during transplantation makes the graft more prone to acute and chronic rejection.32,33 Ischemia–reperfusion injury and the response to brain death in the donor will initiate an inflammatory response,34 as also evident from our results. It is important that all therapy, including the initial fluid therapy, minimizes this because it may not only cause immediate tissue damage but also initiate further immunological and fibrotic processes that will potentially shorten graft survival. In our study, the observation period after reperfusion was limited to 10 hours. In animals without any preimmunization, we expect any signs of rejection of the transplanted kidney to be unlikely within this time frame. Using our advanced catheter technique, we found no indication that microcirculation or oxygenation differed between IGDT and HVFT.
Because human kidney transplant recipients have various comorbidities and receive several interfering medications/drugs including immunosuppressants, we believe that studies such as ours using selected interventions in a controlled pig model are important to reveal effects and complications of new therapies. However, the model has limitations: (1) the animals were young, unlike the typical kidney transplant recipient. Young subjects can compensate for physiological imbalances to a higher degree. However, the pig is an excellent model for renal transplantation due to the anatomical and physiological similarities to human kidneys. (2) Periods of anesthesia lasting 10 hours are seldomly used in the clinic, but this allowed the measurements in the pig and could still imitate the complicated patients. There was a decrease in MAP from the time of reperfusion and throughout the follow-up for both the IGDT and the HVFT groups, and MAP reached 60 mm Hg after 7–8 hours, a situation that is also a challenge in the clinical setting, where bolus injection of Ringer acetate is used like we did. (3) Our follow-up period was too short to get a steady-state GFR. However, graft onset is a reliable indicator of the level of acute kidney injury which in renal transplantation after brain death is known to predict later graft function.35 (4) It is a statistical limitation that the 4 study groups had to be pooled into 2 due to the miscalculation regarding NE administration. (5) In addition, the kidneys were instrumented for measurements of tissue oxygen, which may have influenced the renal function slightly.
In conclusion, no significant difference in early GFR was demonstrated comparing IGDT and HVFT, indicating that reduced and individualized fluid administration does not affect graft onset. Our data indicated a better preservation of glycocalyx as well as lower levels of inflammatory markers in the IGDT group. Taken together this indicate that an IGDT might be applicable in renal transplantation; however, more studies are needed to clarify any benefits associated with this.
ACKNOWLEDGMENTS
The authors thank technicians Birgitte Sahl (Department of Renal Medicine, Aarhus University Hospital, Aarhus, Denmark) as well as Gitte Skou, Gitte Kall, and Lene Elsebeth Nielsen (Department of Clinical Medicine, Aarhus University, Aarhus, Denmark) for expert technical assistance. We also thank Gertrude Nieuwenhuijs-Moeke, MD, DMSci, University Medical Center Groningen, the Netherlands, for valuable advice and support.
DISCLOSURES
Name: Jonathan Kunisch Eriksen, MD.
Contribution: This author helped conceive the study, design the experiments, collect and analyze the data, and write the article.
Name: Lise H. Nielsen, MD.
Contribution: This author helped conceive the study, design the experiments, collect and analyze the data, and write the article.
Name: Niels Moeslund, MD.
Contribution: This author helped conceive the study, design the experiments, collect and analyze the data, and write the article.
Name: Anna K. Keller, MD, PhD.
Contribution: This author helped conceive the study, design the experiments, and write the article.
Name: Søren Krag, MD, PhD.
Contribution: This author helped analyze the data.
Name: Michael Pedersen, PhD.
Contribution: This author helped design the experiments, analyze the data, and write the article.
Name: Jens Aage K. Pedersen, MD, PhD.
Contribution: This author helped design the experiments and write the article.
Name: Henrik Birn, MD, PhD.
Contribution: This author helped design the experiments, analyze the data, and write the article.
Name: Bente Jespersen, MD, PhD.
Contribution: This author helped design the experiments, analyze the data, and write the article.
Name: Rikke Norregaard, PhD.
Contribution: This author helped design the experiments, analyze the data, and write the article.
This manuscript was handled by: Alexander Zarbock, MD.
Supplementary Material
FOOTNOTES
GLOSSARY
- 51Cr-EDTA =
- chrom-51-ethylendiamintetraacetat
- ANOVA
- analysis of variance
- ANP =
- atrial natriuretic peptide
- ARRIVE =
- Animal Research: Reporting In Vivo Experiments
- cDNA =
- complementary deoxyribonucleic acid
- Ch8 =
- Chariére 8
- CI =
- confidence interval
- CO2 =
- carbon dioxide
- COX-2 =
- cyclooxygenase 2
- DBA =
- Dolichos biflorus agglutinin
- DGF =
- delayed graft function
- ELISA =
- enzyme-linked immunosorbent assay
- FITC =
- fluorescein isothiocyanate
- GalNAc =
- N-acetylgalactosamine
- GFR =
- glomerular filtration rate
- GSH =
- glutathione
- GSSG =
- glutathione disulfide
- HE =
- hematoxylin and eosin
- HO-1 =
- heme oxygenase 1
- HVFT =
- high-volume fluid infusion therapy
- IGDT =
- individualized goal-directed fluid therapy
- IL-6 =
- interleukin 6
- MAP =
- mean arterial pressure
- MCP1 =
- monocyte chemoattractant protein 1
- NE =
- norepinephrine
- NGAL =
- neutrophil gelatinase–associated lipocalin
- PAS =
- periodic acid–Schiff
- QPCR =
- quantitative polymerase chain reaction
- RNA =
- ribonucleic acid
- SD
- standard deviation
- SV =
- stroke volume
- SVV =
- stroke volume variation
- TNFα =
- tumor necrosis factor α
- tPo2 =
- renal tissue oxygenation in the outer medulla
Published ahead of print 10 September, 2019.
Funding: This study was supported by the Danish Council for Independent Research | Medical Sciences, Aarhus University Foundation, and Novo Nordisk Foundation.
The authors declare no conflicts of interest.
J. K. Eriksen, L. H. Nielsen, and N. Moeslund contributed equally and share first authorship.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
Reprints will not be available from the authors.
REFERENCES
- 1.Barone CP, Martin-Watson AL, Barone GW. The postoperative care of the adult renal transplant recipient. Medsurg Nurs. 2004;13:296–302. [PubMed] [Google Scholar]
- 2.Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M. A rational approach to perioperative fluid management. Anesthesiology. 2008;109:723–740. [DOI] [PubMed] [Google Scholar]
- 3.Chelazzi C, Villa G, Mancinelli P, De Gaudio AR, Adembri C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit Care. 2015;19:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebong EE, Lopez-Quintero SV, Rizzo V, Spray DC, Tarbell JM. Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1. Integr Biol (Camb). 2014;6:338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alphonsus CS, Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia. 2014;69:777–784. [DOI] [PubMed] [Google Scholar]
- 6.Jacob M, Chappell D, Becker BF. Regulation of blood flow and volume exchange across the microcirculation. Crit Care. 2016;20:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinberg L, Banting J, Churilov L, et al. The effect of a surgery-specific cardiac output-guided haemodynamic algorithm on outcomes in patients undergoing pancreaticoduodenectomy in a high-volume centre: a retrospective comparative study. Anaesth Intensive Care. 2017;45:569–580. [DOI] [PubMed] [Google Scholar]
- 8.Asklid D, Segelman J, Gedda C, Hjern F, Pekkari K, Gustafsson UO. The impact of perioperative fluid therapy on short-term outcomes and 5-year survival among patients undergoing colorectal cancer surgery - a prospective cohort study within an ERAS protocol. Eur J Surg Oncol. 2017;43:1433–1439. [DOI] [PubMed] [Google Scholar]
- 9.Cavaleri M, Veroux M, Palermo F, et al. Perioperative goal-directed therapy during kidney transplantation: an impact evaluation on the major postoperative complications. J Clin Med. 2019;8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaer GL, Fink MP, Parrillo JE. Norepinephrine alone versus norepinephrine plus low-dose dopamine: enhanced renal blood flow with combination pressor therapy. Crit Care Med. 1985;13:492–496. [DOI] [PubMed] [Google Scholar]
- 11.Anderson WP, Korner PI, Selig SE. Mechanisms involved in the renal responses to intravenous and renal artery infusions of noradrenaline in conscious dogs. J Physiol. 1981;321:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richer M, Robert S, Lebel M. Renal hemodynamics during norepinephrine and low-dose dopamine infusions in man. Crit Care Med. 1996;24:1150–1156. [DOI] [PubMed] [Google Scholar]
- 13.Bellomo R, Wan L, May C. Vasoactive drugs and acute kidney injury. Crit Care Med. 2008;36:S179–S186. [DOI] [PubMed] [Google Scholar]
- 14.Bellomo R, Giantomasso DD. Noradrenaline and the kidney: friends or foes? Crit Care. 2001;5:294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Rijt WG, Secher N, Keller AK, et al. α-Melanocyte stimulating hormone treatment in pigs does not improve early graft function in kidney transplants from brain dead donors. PLoS One. 2014;9:e94609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson L, Madsen K, Krag S, Frøkiær J, Jensen BL, Nørregaard R. Disruption of cyclooxygenase type 2 exacerbates apoptosis and renal damage during obstructive nephropathy. Am J Physiol Renal Physiol. 2015;309:F1035–F1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrne L, Obonyo NG, Diab SD, et al. Unintended consequences: fluid resuscitation worsens shock in an ovine model of endotoxemia. Am J Respir Crit Care Med. 2018;198:1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima A, van Rooij T, Ergin B, et al. Dynamic contrast-enhanced ultrasound identifies microcirculatory alterations in sepsis-induced acute kidney injury. Crit Care Med. 2018;46:1284–1292. [DOI] [PubMed] [Google Scholar]
- 20.Navarro LH, Bloomstone JA, Auler JO, Jr, et al. Perioperative fluid therapy: a statement from the international fluid optimization group. Perioper Med (Lond). 2015;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong MA, Wang Y, Berbenetz NM, McConachie I. Does goal-directed haemodynamic and fluid therapy improve peri-operative outcomes? Eur J Anaesthesiol. 2018;35:1. [DOI] [PubMed] [Google Scholar]
- 22.Miñambres E, Rodrigo E, Ballesteros MA, et al. Impact of restrictive fluid balance focused to increase lung procurement on renal function after kidney transplantation. Nephrol Dial Transplant. 2010;25:2352–2356. [DOI] [PubMed] [Google Scholar]
- 23.Jespersen B, Eiskjaer H, Pedersen EB. Effect of atrial natriuretic peptide on blood pressure, guanosine 3’:5’-cyclic monophosphate release and blood volume in uraemic patients. Clin Sci (Lond). 1990;78:67–73. [DOI] [PubMed] [Google Scholar]
- 24.Jacob M, Saller T, Chappell D, Rehm M, Welsch U, Becker BF. Physiological levels of A-, B- and C-type natriuretic peptide shed the endothelial glycocalyx and enhance vascular permeability. Basic Res Cardiol. 2013;108:347. [DOI] [PubMed] [Google Scholar]
- 25.Chappell D, Bruegger D, Potzel J, et al. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care. 2014;18:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wodack KH, Poppe AM, Tomkötter L, et al. Individualized early goal-directed therapy in systemic inflammation: is full utilization of preload reserve the optimal strategy? Crit Care Med. 2014;42:e741–e751. [DOI] [PubMed] [Google Scholar]
- 27.Horrow MM, Parsikia A, Zaki R, Ortiz J. Immediate postoperative sonography of renal transplants: vascular findings and outcomes. AJR Am J Roentgenol. 2013;201:W479–W486. [DOI] [PubMed] [Google Scholar]
- 28.Funk DJ, HayGlass KT, Koulack J, Harding G, Boyd A, Brinkman R. A randomized controlled trial on the effects of goal-directed therapy on the inflammatory response open abdominal aortic aneurysm repair. Crit Care. 2015;19:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rangel EB, Moura LA, Franco MF, Pacheco-Silva A. Up-regulation of cyclooxygenase-2 in different grades of acute human renal allograft rejection. Prostaglandins Leukot Essent Fatty Acids. 2007;76:235–243. [DOI] [PubMed] [Google Scholar]
- 30.Rangel EB, Moura LA, Franco MF, Pacheco-Silva A. Up-regulation of cyclooxygenase-2 during acute human renal allograft rejection. Clin Transplant. 2005;19:543–550. [DOI] [PubMed] [Google Scholar]
- 31.Hausknecht B, Voelkl S, Riess R, Gauer S, Goppelt-Struebe M. Expression of cyclooxygenase-2 in biopsies obtained from human transplanted kidneys undergoing rejection. Transplantation. 2003;76:109–114. [DOI] [PubMed] [Google Scholar]
- 32.Halloran PF, Melk A, Barth C. Rethinking chronic allograft nephropathy: the concept of accelerated senescence. J Am Soc Nephrol. 1999;10:167–181. [DOI] [PubMed] [Google Scholar]
- 33.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. [DOI] [PubMed] [Google Scholar]
- 34.Saat TC, Susa D, Roest HP, et al. A comparison of inflammatory, cytoprotective and injury gene expression profiles in kidneys from brain death and cardiac death donors. Transplantation. 2014;98:15–21. [DOI] [PubMed] [Google Scholar]
- 35.Yarlagadda SG, Coca SG, Formica RN, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2008;24:1039–1047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


