Abstract
Purpose of review
Most microinvasive glaucoma surgery (MIGS) procedures bypass outflow resistance residing proximally in the trabecular meshwork and inner wall of Schlemm's canal. A novel procedure combining trabeculotomy with viscodilation adds to this by also addressing distal resistance of the canal and collector channel ostia. This review examines the development and evidence for both trabeculotomy and canaloplasty separately and the combination in a single procedure.
Recent findings
Recent aqueous angiography studies have confirmed the segmental nature of outflow through Schlemm's canal highlighting the need to address distal outflow pathway resistance. Combined trabeculotomy and viscodilation ab interno is a novel approach with a new purpose-designed device (OMNI Surgical System) becoming available to surgeons in early 2018. Recent results as both a standalone and combined with cataract procedure demonstrate significant intraocular pressure reductions with an average 41% reduction from baseline in the pseudophakic group.
Summary
Targeting both distal as well as proximal points of outflow resistance in the conventional pathway may prove to be a highly efficacious MIGS modality. Additional large prospective studies are currently ongoing to confirm these preliminary results.
Keywords: microinvasive glaucoma surgery, outflow resistance, trabeculotomy, viscodilation
INTRODUCTION
Elevated intraocular pressure (IOP) is the cardinal risk factor for development and progression of glaucoma [1▪▪]. Landmark studies such as OHTS and AGIS established the importance of IOP reduction [2,3]. Elevated IOP is a consequence of increased outflow resistance in the conventional (trabeculocanalicular) outflow pathway. Surgical interventions to reduce IOP aim to either circumvent the conventional pathway, as with filtration procedures, or remove or bypass resistance facilitating physiological outflow. Outflow resistance in the conventional pathway can be ascribed to three areas: the juxtacanalicular meshwork and inner wall of Schlemm's canal (50–75%); Schlemm's canal; collector channels (latter two up to 50%) [4–6]. Opening Schlemm's canal to the anterior chamber to bypass trabecular resistance was first attempted over 100 years ago by De Vincentiis [7]. Dilation of Schlemm's canal by various means to address downstream resistance is a more recent approach having its roots in ab externo nonpenetrating procedures like sinusotomy and viscocanalostomy [8,9]. Neither of these approaches address all three areas of outflow resistance while combining trabeculotomy/goniotomy with viscodilation of Schlemm's canal may.
For more than 100 years glaucoma surgeons have been trying to alter anterior chamber angle anatomy to enhance outflow and treat glaucoma. Angle surgery ab interno is currently undergoing a renaissance including trabecular and supraciliary implants, new surgical instruments for partial trabeculotomy (≤120°), ab-interno procedures and systems allowing circumferential trabeculotomy, circumferential viscodilation, and most recently combined circumferential viscodilation with trabeculotomy (Fig. 1). This article reviews the clinical evidence for ab-interno trabeculotomy, ab-interno viscodilation, and the combination of both together.
FIGURE 1.
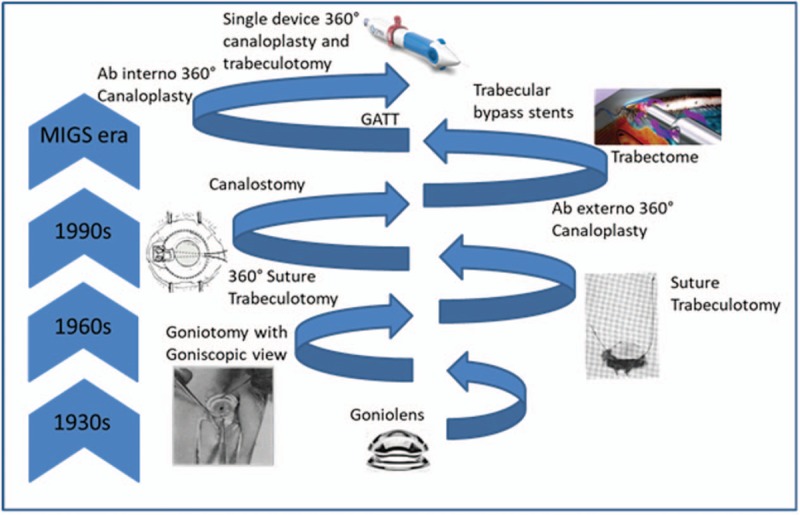
Innovation and evolution in canal-based glaucoma surgery.
Box 1.
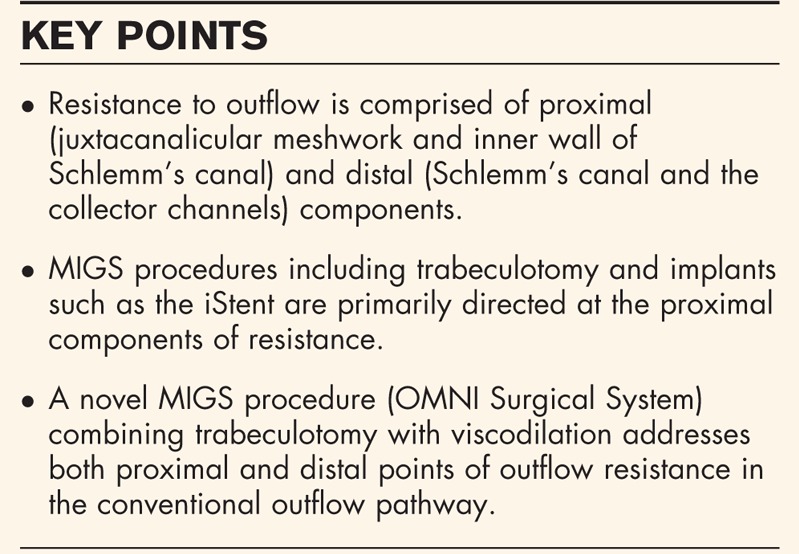
no caption available
A BRIEF HISTORY
Goniotomy, arguably the first micro-invasive glaucoma surgery (MIGS), became practical with the arrival the operating microscope and surgical goniolens. Barkan initially considered this operation ideal for ‘chronic simple glaucoma’ (i.e., open-angle glaucoma or OAG). While detailed outcomes are not available, where the incision was at least 90° pressure was ‘normalized’ through 24 months. Where the incision was less than 90°, normalized pressure was achieved only with adjunctive medication (miotics) although miotics alone (preoperation) were insufficient to achieve normal pressure [10]. Subsequently, goniotomy for adult OAG was largely abandoned due to inconsistent efficacy while becoming the treatment of choice for congenital glaucoma [11,12]. More recently, there has been a resurgence in ab interno goniotomy including the Trabectome, a device that electro-ablates the trabecular meshwork and inner wall of Schlemm's canal, and the Kahook Dual Blade, a single-use knife that makes parallel incisions in the trabecular meshwork creating a free strip of tissue. Like traditional goniotomy both are limited to treatment of the nasal arc from a temporal incision [13▪,14▪].
Belief that the trabecular meshwork was responsible for obstruction to outflow and disappointment with goniotomy as a surgical solution for OAG led to development of ab externo trabeculotomy. In addition the procedure could be difficult for many surgeons and there was a need for a procedure in children that could be done in the presence of corneal edema which prevented gonioscopy. This technique employed a nylon suture threaded and pulled through Schlemm's canal via three limbal incisions about 2 clock hours apart. When held at the third incision and pulled from the first, the suture ‘appeared like a bowstring’ in the anterior chamber having burst through the inner wall of Schlemm's canal and the trabecular meshwork [15]. This technique foreshadowed single incision approaches although provided only an approximately 120° trabeculotomy rather than a full 360°.
The 360° suture trabeculotomy was first developed as a pediatric procedure. A single cut-down is used for both entry and exit from Schlemm's canal. The suture's blue color allows visualization under transillumination with a goniolens ensuring accurate placement and the ability to follow progress as it is fed through the canal [16]. Better outcomes were obtained using the 360° technique in comparison with the partial 120° method in a head-to-head comparison [17,18]. A subsequent enhancement was the iTrack illuminated microcatheter (Ellex, Adelaide, South Australia, Australia) for improved visualization [19,20]. In adult eyes with either primary open-angle glaucoma OAG (POAG) (n = 25) or secondary OAG (n = 18) success (IOP reduced 30% and <18 mmHg on the same or fewer medications) was achieved by 84% (POAG) and 89% (secondary OAG) [21]. Circumferential ab-externo trabeculotomy came with several advantages over other trabeculotomy procedures. Fully opening Schlemm's canal to the anterior chamber results in a higher proportion of surgical successes [18]. Enhanced visualization allows more accurate placement in Schlemm's canal and consequently decreased likelihood of creating false passages into the anterior chamber or suprachoroidal space. Moreover, since the entirety of the angle is treated fewer repeated procedures with consequent increase in conjunctival scarring are required [16]. However, the required cutdown and manipulation of conjunctiva and the creation of a scleral flap, diminish the likelihood of a successful outcome for future filtration surgery, if required.
A minimally invasive ab interno approach, ‘gonioscopy-assisted transluminal trabeculotomy’ (GATT), was developed to avoid the tissue trauma of the ab externo procedure while maintaining the effectiveness [22]. In brief, a nasal paracentesis is created for suture entry. A second, temporal, paracentesis allows for entry of the blade used to create a small (1–2 mm) nasal goniotomy for suture insertion into Schlemm's canal. Micro forceps entering through the temporal paracentesis grasp the suture and feed it circumferentially around the canal. Grasping the tip of the suture and bringing it out through the temporal opening creates a 180° trabeculotomy; Applying tension to the proximal suture end completes the 360° trabeculotomy [22]. In a retrospective review of 72 POAG cases at 24 months including GATT with cataract surgery (n = 27), GATT in pseudophakic patients (n = 19), and GATT in phakic patients (n = 26), IOP reductions were 8.4 mmHg (baseline of 22.5 mmHg), 8.9 mmHg (baseline of 24.7 mmHg), and 10.4 mmHg (baseline of 26.0 mmHg) [23▪▪]. Medication use was reduced from 2.9, 2.6, and 3.2 to 1.0, 1.6, and 1.8, respectively [23▪▪]. Success defined as IOP reduction of at least 20%, IOP of 21 mmHg or less and no reoperation for IOP was between approximately 60% (pseudophakic) and 80% (with cataract surgery) [23▪▪]. IOP reduction in a mixed cohort of secondary glaucomas was even greater (Table 1). As with most trabeculotomy procedures the most common adverse event was transient hyphema resolving within the first postoperative week.
Table 1.
Intraocular pressure reductions (mmHg ± SD) in circumferential trabeculotomy studies
| Reference | Procedure | Diagnosis | N | Baseline IOP | IOP at last follow-up (length of follow-up) | Percentage change |
| Chin et al. [21] | Ab externo 360° | POAG/SOAG | 43 | 27.8 ± 12.2 | 12.9 ± 2.5 (18 months) | −53.6 |
| Grover et al. [23▪▪] | Ab interno 360° | POAG | ||||
| Phakic | 46 | 26.0 ± 6.9 | 15.6 ± 5.7 | −40.0 | ||
| w/Phaco | 36 | 22.5 ± 5.4 | 14.1 ± 3.2 | −37.3 | ||
| Pseudo | 37 | 24.7 ± 6.2 | 15.8 ± 7.4 | −36.0 | ||
| Othera | ||||||
| Phakic | 30 | 30.9 ± 10.0 | 13.8 ± 4.5 | −55.3 | ||
| w/Phaco | 25 | 25.7 ± 6.3 | 14.5 ± 4.4 | −43.6 | ||
| Pseudo | 24 | 26.8 ± 7.9 | 13.4 ± 4.7 (24 month) | −50.0 | ||
| Sarkisian et al. [25▪▪] | Ab interno 360° | POAG (83%) | 81 | 23.7 ± 6.3b | 15.7 ± 5.5b (12 month) | −33.8 |
IOP, intraocular pressure; POAG, primary open-angle glaucoma; SOAG, secondary open-angle glaucoma.
aOther included chronic angle closure, pseudoexfoliation, pigment dispersion, uveitic, mixed mechanism. Other OAG, trauma, steroid.
bSD estimated from error bars in published figure.
A similar operation can be accomplished with a purpose-designed device, the TRAB360 (Sight Sciences, Menlo Park, California, USA). The TRAB360 consists of a handpiece and cannula with a retractable microcatheter. Entering the eye through a single temporal clear corneal incision, the anterior chamber is crossed and a small goniotomy is made with the tip of the cannula. The microcatheter is advanced into Schlemm's canal through 180° with a control wheel on the handpiece. Withdrawing the cannula tears the meshwork and inner wall. Repeating the procedure in the opposite direction creates a full circumferential trabeculotomy [24▪]. A series of 81 eyes with refractory glaucoma was treated 360° with the TRAB360 as a standalone procedure (not with cataract surgery). Two-thirds of eyes were pseudophakic, most (91%) were OAG, all had a preoperative IOP at least 18 mmHg on medication, half had failed prior laser trabeculoplasty, and 19% had failed filtration surgery. Mean IOP was reduced from 23.7 to 15.7 mmHg at 12 months, a 34% reduction, while medications were reduced from 1.7 to 1.1. IOP reduction was even greater in the subset of eyes with baseline IOP at least 25 mmHg; from an average of 30.2 to 16.1 mmHg at 12 months. Mild hyphema occurred in about half of the eyes and, not surprisingly in this refractory group, 20 eyes required additional surgical intervention [25▪▪]. Outcomes for GATT and TRAB360 (Table 1) are similar. The main difference being the complexity of the procedure, number of incisions, and cost. The TRAB360 procedure is simpler and requires just one incision rather than two, however GATT can be performed with an inexpensive suture.
In contrast to trabeculotomy, canaloplasty addresses distal resistance in addition to its effects on the inner wall and trabecular meshwork. Canaloplasty originated with the realization that the IOP-lowering effect of canalostomy was due more to the viscodilation of Schlemm's canal and consequent disruption of lateral walls, inner wall endothelium, and bridging structures than it was to aqueous flow through a Descemet's ‘window’ and into the cut ends of Schlemm's canal [26]. As for circumferential trabeculotomy ab externo, access to Schlemm's canal is under a scleral flap. In contrast to trabeculotomy, in canaloplasty the microcatheter is not pulled through the inner wall of Schlemm's canal and the trabecular meshwork. Instead small amounts of viscoelastic are injected through the microcatheter around the full circumference of Schlemm's canal. In one variant a prolene suture is attached to the distal end of the microcatheter and looped through the canal as the microcatheter is withdrawn. The suture is tightened and tied off to stretch the inner wall and the trabecular meshwork [27].
Cameron et al. followed a series of 56 OAG patients that underwent ab externo 360° canaloplasty for 6 months [28]. The series included five eyes that had failed prior glaucoma surgery. The mean IOP at 6 months was decreased 34% from baseline (Table 2) and medications decreased from 2.1 to 0.2 [28]. In addition, ultrasound imaging showed that Schlemm's canal, often not visible prior to the surgery, could be visualized at 6 months [28]. Mean IOP reduction was not related to the extent of dilation (no dilation, 1 or 2, 3, or 4 dilation sites, 360° dilation) however the proportion of patients meeting IOP targets (<15, <18 or <21 mmHg) was directly related to degree of dilation [28]. Evaluation of canaloplasty in 90 African patients with POAG and using a tensioning suture resulted in substantial IOP reductions at 15 months (Table 2) [27]. In a large (n = 127 eyes), prospective study including adult OAG patients undergoing either canaloplasty alone (n = 97) or canaloplasty with cataract surgery (n = 30), IOP (all patients) was decreased 32% at 24 months and 36% at 36 months from preoperative baseline of 23.6 mmHg [29,30]. While only 5% of eyes were medication-free at baseline, 61 and 49% were medication-free at 24 and 36 months [29,30]. Interestingly, there was a significant relationship between distension of Schlemm's canal and IOP and medication reduction at 24 months although a comparison of the patients with and without a tensioning suture revealed no difference in terms of IOP or medication reduction at 36 months [29,30]. In a small series of 20 eyes with OAG (95%), Voykov et al.[31] reported sustained IOP reductions through 60 months of follow-up (Table 2) while decreasing medication use from 3.4 to 1.7 medications.
Table 2.
Intraocular pressure reductions (mmHg ± SD) in published canaloplasty studies
| Reference | Procedure | Diagnosis | N | Baseline IOP | IOP at last follow-up (length of follow-up) | Percentage change | |
| Cameron et al. [28] | Ab externo 360° | POAG | 56 | 25.1 ± 8.7 | 16.7 ± 4.4 (6 month) | −33.5 | |
| Grieshaber et al. [27] | Ab externo 360° w/suture | POAG in Black patients | 90 | 42.7 ± 12.5 (6–0 suture) | 19.2 ± 6.4 (15 month) | −55.0 | |
| 45.0 ± 12.1 (10–0 suture) | 16.4 ± 4.9 (15 month) | −63.6 | |||||
| Voykov et al. [31] | Ab externo 360° w/suture | OAG | 18 | 25.7 ± 6.6 | 14.2 ± 3.4 (60 month) | −44.8 | |
| Lewis et al. [29] | Ab externo 360° w/suture | OAG | 127 | 23.6 ± 4.8 | 16.0 ± 4.2 (24 month) | −32.2 | |
| Körber [33▪] | Ab interno 360° | POAG | 20a | 18.5 ± 3.4 | 15.5 ± 2.4 (9 month) | −16.2 | |
| Vastardis et al. [32▪▪] | Ab externo 360° w/suture | POAG | Standalone | (12 month) | |||
| Advanced | 172 | 19.2 ± 6.4 | 13.3 ± 4.5 | −27.9 | |||
| Moderate | 51 | 20.7 ± 5.0 | 15.2 ± 4.0 | b | |||
| Early | 39 | 21.3 ± 5.7 | 18.1 ± 3.8 | b | |||
| With phaco | |||||||
| Advanced | 212 | 19.4 ± 7.5 | 14.5 ± 4.7 | −16.5 | |||
| Moderate | 51 | 19.5 ± 5.9 | ∼14.5b | b | |||
| Early | 39 | 19.4 ± 7.3 | ∼14.5b | b | |||
| Ondrejka and Körber [34▪▪] | Ab interno 360° | POAG | IOP ≥ 18 mmHg | (12 month) | |||
| 72 | 24.6 ± 7.1 | 14.6 ± 2.8 | −41% | ||||
| IOP < 18 mmHg | |||||||
| 34 | 14.9 ± 1.8 | 13.6 ± 2.3 | b | ||||
| Brusini et al. [35▪] | Ab externo 360° w/suture | Steroid OHT | 9 | 30.4 ± 6.8 | 13.7 ± 1.9 (12 month) | −54.9 | |
IOP, intraocular pressure; OAG, open-angle glaucoma; POAG, open-angle glaucoma open-angle glaucoma.
an = 6 at 9 months.
bData not published, IOP estimated from published figure.
Vastardis et al. followed a cohort of over 500 POAG patients treated with ab externo canaloplasty for 1 year. Patients were stratified by standalone procedure or combined with phaco, and by glaucoma severity (early, moderate, advanced). Meaningful IOP reductions were achieved in all groups (Table 2) of between 3 mmHg (early, standalone) and 6 mmHg (advanced, standalone) while reducing medications from approximately 2.5 to less than 1 for all subgroups [32▪▪].
The ab interno approach (ABiC for AB Interno Canaloplasty) differs from the ab externo in that Schlemm's canal is accessed through a small goniotomy internally requiring only a small clear corneal incision rather than conjunctival dissection and a scleral flap. There is no tensioning suture. A small case series showed modest IOP reductions, however average baseline IOP was relatively low (18.5 mmHg). While patients were on one to three medications at baseline, 16 patients (80%) were medication free at last follow-up (maximum follow-up was 12 months; nine patients with ≥6 months follow-up) [33▪]. In a series of 106 eyes treated with the VISCO360 (Sight Sciences Inc.) stratified by baseline IOP, patients with baseline IOP at least 18 mmHg had an average 41% reduction in IOP (24.6 to 14.6 mmHg) at 12 months (Table 2). In patients with baseline IOP less than 18 mmHg, IOP was maintained while medications were reduced from 1.8 to 0.2 [34▪▪]. Promising results with canaloplasty have also been achieved in steroid-induced IOP elevation (Table 2) [35▪].
Both ab interno and ab externo 360° canaloplasty provide similar IOP-lowering efficacy (Table 2). This was confirmed in a paired-eye study where one eye was treated ab externo and the other ab interno. Results were essentially identical. At 12 months ab externo eyes had mean IOP of 13.5 mmHg on 0.9 medications (18.1 mmHg preoperative on 2.4 medications) while ab interno eyes had mean IOP of 13.8 mmHg on 0.8 medications (18.5 mmHg preoperative on 2.4 medications) [36▪▪].
TRABECULOTOMY COMBINED WITH VISCODILATION
In patients previously treated (1–54 months) with ab externo canaloplasty but whom were inadequately controlled, Voykov et al.[37] demonstrated additional IOP reduction by using the tensioning suture to perform an ab interno 360° trabeculotomy. In a larger, 88 patient cohort, Seuthe et al.[38] showed at 12 months a 41.2% reduction in IOP and reduction in medications from 2.7 to 1.6. These studies added trabeculotomy to canaloplasty, but only after many months, and only in patients not achieving sufficient IOP reduction. Combining the two different modalities and simultaneously addressing multiple points of outflow resistance could therefore prove more effective than either alone.
The OMNI Surgical System (Sight Sciences), provides the ability to perform a circumferential viscodilation and trabeculotomy using the same device. Given the short time this device has been marketed limited data are currently available. Brown et al. reported promising preliminary results for a series of 54 eyes (41 where the OMNI was used with cataract surgery and 13 in pseudophakic patients). In combined cases and the majority of standalone (8/13) the treatment consisted of 180° viscodilation and trabeculotomy. Five eyes in the standalone group with more advanced disease were treated 360°. Mean IOP reductions were 5.8 mmHg in the combined cases and 10.0 mmHg for the standalone group [39▪▪,40▪]. In a European study of 24 eyes with early, moderate or advanced glaucoma and 18 months of follow-up, and including both combined with cataract surgery (n = 10) and standalone cases (N = 14), IOP was reduced from preoperative baseline of 18.7 to 11.4 mmHg for combined cases while medications were reduced 50% from an average of 2.8 to 1.4. In standalone cases IOP went from 23.4 to 14.0 (−40%) and medications from 3.0 to 2.0 over the same time period [41▪]. A large prospective study of this device used with cataract surgery is in progress (clinicaltrials.gov NCT03861169).
Elevated IOP is the leading risk factor for glaucoma and currently the only one treatable [42]. Outflow resistance is the result of contributions from distinct portions of the physiologic outflow pathway. In primates up to 75% of the resistance comes from the trabecular meshwork and in particular the juxtacanalicular tissue [4,43]. The inner wall accounts for 10% or less of resistance in normal eyes [44]. Morphometric and perfusion studies show that the cross-sectional area of Schlemm's canal averages 54% less and mean outflow facility 55% less in POAG eyes compared with normal, and that these measures are correlated, implicating canal atrophy as a source of resistance [45]. Moreover, the entire canal is not active, showing a ‘segmental’ pattern in tracer and OCT imaging studies [46,47▪▪]. A third point of resistance is the collector channels ostia (Fig. 2). In bovine eyes, light microscopy showed that increasing IOP resulted in herniation of the inner wall and juxtacanalicular tissue into the ostia of the collector channels [6]. Addressing all three of these points of resistance by combining viscodilation with trabeculotomy is appealing theoretically and practically. A single surgery with a minimally invasive approach provides maximum safety and minimum tissue disruption. No implant is left behind. The conjunctiva is not injured. The promise of this multimodal, ab interno approach targeting all three sources of outflow resistance is supported today by historical data on the two individual procedures. Ongoing clinical studies and, importantly, clinical experience in the hands of surgeons will allow fair evaluation of safety, effectiveness, and the most appropriate patients. Laboratory studies including imaging/visualization in perfused cadaver eyes and aqueous dynamics studies can confirm the hypothesized mechanism of action.
FIGURE 2.
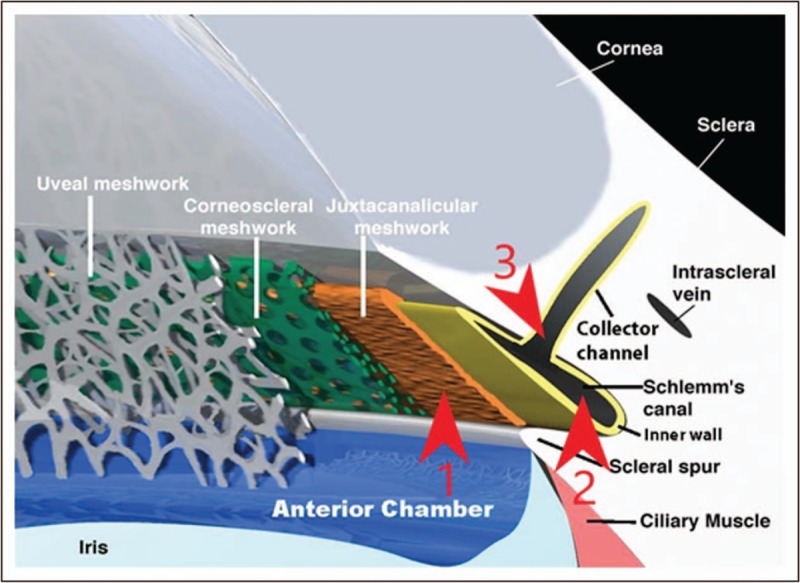
Conventional outflow pathway. Red arrowheads indicate three major components of outflow resistance. 1. Juxtacanalicular trabecular meshwork. 2. Schlemm's canal. 3. Ostia of collector channels.
CONCLUSION
The objective of glaucoma surgery has always been to facilitate egress of aqueous humor and restore IOP to levels consistent with optic nerve health, ideally restoring fluid dynamics to a normal equilibrium [48]. MIGS has revolutionized treatment and is changing the norms of clinical practice. The quest for glaucoma surgeries that provide efficacy while minimizing risk is over a century old and has culminated in the MIGS techniques of today providing the surgeon and the patient with an unprecedented diversity of options [7,49].
Acknowledgements
None.
Financial support and sponsorship
Publication costs were paid for by Sight Sciences, Inc.
Conflicts of interest
J.D. and R.B. are employees of Sight Sciences.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪▪.Kim JH, Rabiolo A, Morales E, et al. Risk factors for fast visual field progression in glaucoma. Am J Ophthalmol 2019; 207:268–278. [DOI] [PubMed] [Google Scholar]; The retrospective cohort study followed 1317 eyes for a mean follow-up of over 11 years with at least six visual fields each. The study confirmed age, and peak intraocular pressure (IOP) as predictors of glaucomatous visual field progression underscoring the importance of IOP control.
- 2.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002; 120:701–713. [DOI] [PubMed] [Google Scholar]
- 3.AGIS Investigators. The Advanced Glaucoma Intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 2000; 130:429–440. [DOI] [PubMed] [Google Scholar]
- 4.Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol 1963; 69:783–801. [DOI] [PubMed] [Google Scholar]
- 5.Rosenquist R, Epstein D, Melamed S, et al. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr Eye Res 1989; 8:1233–1240. [DOI] [PubMed] [Google Scholar]
- 6.Battista SA, Lu Z, Hofmann S, et al. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci 2008; 49:5346–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkan O. A new operation for chronic glaucoma. Am J Ophthalmol 1936; 19:951–966. [PubMed] [Google Scholar]
- 8.Krasnov MM. Sinusotomy. Trans Am Acad Ophthalmol Otolaryngol 1972; 76:368–374. [PubMed] [Google Scholar]
- 9.Stegmann R, Pienaar A, Miller D. Viscocanalostomy for open-angle glaucoma in Black African patients. J Cataract Refract Surg 1999; 25:316–322. [DOI] [PubMed] [Google Scholar]
- 10.Barkan O. Technique of goniotomy. Arch Ophthalmol 1938; 19:217–223. [Google Scholar]
- 11.Barkan O. Operation for congenital glaucoma. Am J Ophthalmol 1942; 25:552–568. [Google Scholar]
- 12.Barkan O. Goniotomy for the relief of congenital glaucoma. Br J Ophthalmol 1948; 32:701–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13▪.Kaplowitz K, Bussel II, Loewen NA. Francis BA, Sarkisian SR, Jr, Tan JC. Trabeculotomy by internal approach: trabectome. Minimally invasive glaucoma surgery: a practical guide. New York: Thieme; 2017. 82–91. [Google Scholar]; The article provides a concise description of the Trabectome instrument and technique as well as a comprehensive review of clinical results current through 2015.
- 14▪.Akil H, Francis B A. Francis BA, Sarkisian SR, Jr, Tan JC. Trabeculotomy by internal approach: dual blade. Minimally invasive glaucoma surgery: a practical guide. New York: Thieme; 2017. 92–93. [Google Scholar]; A short account of the dual blade device along with a summation of published evidence through 2016.
- 15.Smith R. A new technique for opening the canal of Schlemm: preliminary report. Br J Ophthalmol 1960; 44:370–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck AD, Lynch MG. 360° trabeculotomy for primary congenital glaucoma. Arch Ophthalmol 1995; 113:1200–1202. [DOI] [PubMed] [Google Scholar]
- 17.Girkin CA, Rhodes L, McGwin G, et al. Goniotomy versus circumferential trabeculotomy with an illuminated microcatheter in congenital glaucoma. J AAPOS 2012; 16:424–427. [DOI] [PubMed] [Google Scholar]
- 18.Lim ME, Neely DE, Wang J, et al. Comparison of 360-degree versus traditional trabeculectomy in pediatric glaucoma. J AAPOS 2015; 19:145–149. [DOI] [PubMed] [Google Scholar]
- 19.Sarkisian SR., Jr An illuminated microcatheter for 360-degree trabeculotomy [corrected] in congenital glaucoma: a retrospective case series. J AAPOS 2010; 14:412–416. [DOI] [PubMed] [Google Scholar]
- 20.Girkin CA, Marchase N, Cogen MS. Circumferential trabeculotomy with an illuminated microcatheter in congenital glaucomas. J Glaucoma 2012; 21:160–163. [DOI] [PubMed] [Google Scholar]
- 21.Chin S, Nitta T, Shinmei Y, et al. Reduction of intraocular pressure using a modified 360-degree suture trabeculectomy technique in primary and secondary open-angle glaucoma: a pilot study. J Glaucoma 2012; 21:401–407. [DOI] [PubMed] [Google Scholar]
- 22.Grover DS, Godfrey DG, Smith O, et al. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy. Technique report and preliminary results. Ophthalmology 2014; 121:855–861. [DOI] [PubMed] [Google Scholar]
- 23▪▪.Grover DS, Smith O, Fellman RL, et al. Gonioscopy-assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy: 24 months follow-up. J Glaucoma 2018; 27:393–401. [DOI] [PubMed] [Google Scholar]; This was a retrospective study of 198 patients treated with gonioscopy-assisted transluminal trabeculotomy (GATT). Results are given for GATT as a standalone procedure in phakic or pseudophakic eyes and combined with phacoemulsification. Patients had primary open-angle glaucoma open-angle glaucoma (POAG) and other glaucomas including CACG. GATT was generally successful across all groups with few side effects but with notably higher failure rates in those with more advanced glaucoma.
- 24▪.Sarkisian SR, Jr, Allan E. Francis BA, Sarkisian SR, Jr, Tan JC. 360-degree ab interno trabeculotomy. Minimally invasive glaucoma surgery: a practical guide. New York: Thieme; 2017. 104–108. [Google Scholar]; The article provides a good overview of the TRAB360 (a precursor to the OMNI) and clinical results with this device.
- 25▪▪.Sarkisian SR, Mathews B, Ding K, et al. 360° ab-interno trabeculotomy in refractory primary open-angle glaucoma. Clin Ophthalmol 2019; 13:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]; The TRAB360 device was evaluated in 81 eyes with refractory OAG in this retrospective case series. Safety and IOP-lowering efficacy (IOP < 18 mmHg and at least 20% reduction from baseline) was demonstrated at 1-year postsurgery with the majority of patients also reducing medications.
- 26.Smit BA, Johnstone MA. Effects of viscoelastic injection into Schlemm's canal in primate and human eyes: potential relevance to viscocanalostomy. Ophthalmology 2002; 109:786–792. [DOI] [PubMed] [Google Scholar]
- 27.Grieshaber MC, Pienaar A, Olivier J, Stegmann R. Comparing two tensioning suture sizes for 360° viscocanalostomy (canaloplasty): a randomized controlled trial. Eye 2010; 24:1220–1226. [DOI] [PubMed] [Google Scholar]
- 28.Cameron B, Field M, Ball S, Kearney J. Circumferential viscodilation of Schlemm's canal with a flexible microcannula during non-penetrating glaucoma surgery. Digit J Ophthalmol [Internet] 2006; 12:1.http://www.djo.harvard.edu/site.php?urlZ/physicians/oa/929Available at: http://www.djo.harvard.edu/site.php?urlZ/physicians/oa/929.[. [Google Scholar]
- 29.Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: two-year interim clinical study results. J Cataract Refract Surg 2009; 35:814–824. [DOI] [PubMed] [Google Scholar]
- 30.Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: three year results of circumferential viscodilation and tensioning of Schlemm canal using a microcatheter to treat open-angle glaucoma. J Cataract Refract Surg 2011; 37:682–690. [DOI] [PubMed] [Google Scholar]
- 31.Voykov B, Blumenstock G, Leitritz MA, et al. Treatment efficacy and safety of canaloplasty for open-angle glaucoma after 5 years. Clin Exp Ophthalmol 2015; 43:768–771. [DOI] [PubMed] [Google Scholar]
- 32▪▪.Vastardis I, Fili S, Gatzioufas Z, Kohlhaas M. Ab externo canaloplasty results and efficacy: a retrospective cohort study with a 12-month follow-up. Eye Vis 2019; 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This was a very large retrospective cohort study including 602 eyes with POAG followed for 12 months. Results were stratified by canaloplasty alone or with phacoemulsification and also by glaucoma severity. Overall success rates were between 69 and 75% for canaloplasty alone and between 77 and 83% when combined with cataract surgery. The percentage of eyes requiring reoperation was low (<4%).
- 33▪.Körber N. Ab interno canaloplasty for the treatment of glaucoma: a case series study. Spektrum Augenheilkd 2018; 32:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]; A small case series of 20 patients with POAG undergoing ab-interno canaloplasty.
- 34▪▪.Ondrejka S, Körber N. 360° ab-interno Schlemm's canal viscodilation in primary open-angle glaucoma. Clin Ophthalmol 2019; 13:1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]; The retrospective analysis of 106 eyes with POAG treated with 360 degree ab interno canaloplasty stratified results by baseline IOP; IOP controlled at baseline (<18 mmHg) or IOP at least 18 mmHg at baseline. For the eyes controlled at baseline, IOP was maintained at 12 months while a mean 41% reduction was achieved for those with IOP at least 18 mmHg. Across both groups at 12 months 86% of eyes were medication free.
- 35▪.Brusini P, Tosoni C, Zeppieri M. Canaloplasty in corticosteroid-induced glaucoma. Preliminary results. J Clin Med 2018; 7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]; The report of a small series of eyes shows canaloplasty to be efficacious in lowering IOP in eyes with steroid-induced ocular hypertension.
- 36▪▪.Gallardo MJ, Supnet RA, Ahmed IIK. Circumferential viscodilation of Schlemm's canal for open-angle glaucoma: ab-interno vs ab-externo canaloplasty with a tensioning suture. Clin Ophthalmol 2018; 12:2493–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]; A paired eye study that provides a head-to-head comparison of canaloplasty ab externo with tensioning suture and canaloplasty ab interno. IOP reductions and reduction in medication burden were essentially identical but the adverse event profile was superior in the ab interno eyes.
- 37.Voykov B, Szurman P, Dimopoulos S, et al. Micro-invasive suture trabeculotomy after canaloplasty: preliminary results. Clin Exp Ophthalmol 2015; 43:409–414. [DOI] [PubMed] [Google Scholar]
- 38.Seuthe AM, Januschowski K, Szurman P. Micro-invasive 360-degree suture trabeculotomy after successful canaloplasty – one year results. Graefes Arch Clin Exp Ophthalmol 2016; 254:155–159. [DOI] [PubMed] [Google Scholar]
- 39▪▪.Brown RH, Dhamdhere K, Tsegaw S, Lynch MG. Viscodilation of Schlemm's canal and trabeculotomy combined with cataract surgery for reducing intraocular pressure in open angle glaucoma. J Cataract Refract Surg 2020; 46: in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first published results for the OMNI device. This article describes outcomes for 41 eyes with OAG treated with combined trabeculotomy and viscodilation combined with cataract surgery. All eyes had a postsurgical IOP reduction while the mean reduction for those with a preop IOP more than 22 mmHg was 10 mmHg. No eyes required additional glaucoma surgery.
- 40▪.Brown R, Dhamdhere K. Ab-interno trabeculectomy combined with viscodilation of Schlemm's canal for reducing intraocular pressure in patients with mild to severe open-angle glaucoma. ASCRS 2019, Paper 56726. [Google Scholar]; In addition to data included in Ref. [39▪▪], data for use in standalone cases is also provided.
- 41▪.Grabska-Liberek I, Majszyk-Ionescu J, Duda P, et al. OMNI in open-angle glaucoma treatment: an 18-month follow-up. ESCRS 2019, Presented Poster Session: Glaucoma II (https://www.escrs.org/paris2019/programme/poster-village-details.asp?id=33863&day=0). [Google Scholar]; Longest follow-up currently available for circumferential trabeculotomy combined with viscodilation performed with the OMNI device. in press.
- 42.Van Buskirk EM, Cioffi GA. Glaucomatous optic neuropathy. Am J Ophthalmol 1992; 113:447–452. [DOI] [PubMed] [Google Scholar]
- 43.Mäepea O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm's canal in monkeys. Exp Eye Res 1992; 54:879–883. [DOI] [PubMed] [Google Scholar]
- 44.Bill A, Svedbergh B. Scanning electron microscopic studies of the trabecular meshwork and the canal of Schlemm – an attempt to localize the main resistance to outflow of aqueous humor in man. Acta Ophthalmol 1972; 50:295–320. [DOI] [PubMed] [Google Scholar]
- 45.Allingham RR, De Kater AW, Ethier CR. Schlemm's canal and primary open angle glaucoma: correlation between Schlemm's canal dimensions and outflow facility. Exp Eye Res 1996; 62:101–109. [DOI] [PubMed] [Google Scholar]
- 46.Hann CR, Bahler CK, Johnson DH. Cationic ferritin and segmental flow through the trabecular meshwork. Invest Ophthalmol Vis Sci 2005; 46:1–7. [DOI] [PubMed] [Google Scholar]
- 47▪▪.Huang AS, Peneteado RC, Saha SK, et al. Fluorescein aqueous angiography in live normal human eyes. J Glaucoma 2018; 27:957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]; An elegant study employing fluorescein aqueous angiography and OCT imaging in live human eyes in contrast to earlier work in bovine, nonhuman primates or cadaver eyes. This study demonstrates the segmental nature of aqueous outflow and shows larger intrascleral episcleral vein lumens in angiographically positive regions compared with negative areas.
- 48.Duke-Elder S, Jay B. Duke-Elder S. Chapter VII: simple glaucoma. Part VIIIc. Surgical treatment. System of ophthalmology vol XI: diseases of the lens and vitreous; glaucoma and hypotony. London: Henry Kimpton; 1969. 527–549. [Google Scholar]
- 49.Saheb H, Ahmed IIK. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol 2012; 23:96–104. [DOI] [PubMed] [Google Scholar]


