Supplemental Digital Content is available in the text
Keywords: nicotine lozenge, nicotine replacement therapy, quit attempts, smoking cessation
Abstract
Objective:
To evaluate the efficacy in smoking cessation and safety of 2 and 4 mg nicotine mint lozenges in Chinese adult smokers.
Methods:
This was a multicenter, randomized, stratified, double-blind, placebo-controlled, parallel-group study. The low-dependence stratum included 483 smokers (241 randomized to active 2 mg nicotine lozenge and 242 to placebo lozenge). The high-dependence stratum included 240 smokers (120 randomized to active 4 mg nicotine lozenge and 120 to placebo lozenge). The primary endpoint was successful smoking cessation at 6 weeks postquit, defined as continuous abstinence from smoking for the 28-day period up to and including the 6-week visit (verified by CO measurement). Cochran–Mantel–Haenszel tests were performed to compare quit rates between active nicotine and placebo separately for the high-dependence and low-dependence strata.
Results:
The primary analysis showed that in the low-dependence (2 mg) stratum, 59 subjects (24.5%) of 241 in the active nicotine group and 52 subjects (21.5%) of 242 in the placebo group were successful quitters (P = .3851). In the high-dependence (4 mg) stratum, 37 subjects (30.8%) of 120 in the active nicotine group and 24 subjects (20.2%) of 119 in the placebo group were successful quitters (P = .0565).
Conclusions:
The 4 mg nicotine lozenge provided a directionally significant improvement in smoking cessation rates compared with placebo in Chinese adult smokers with high nicotine dependence for the primary endpoint. The 2 mg nicotine lozenge provided higher, but nonsignificant, smoking cessation rates than placebo. Both nicotine lozenges were generally well tolerated in Chinese adult smokers.
Smoking is a major global public health challenge with serious health consequences and is the leading cause of preventable disease and death in both developed and developing countries (U.S. Department of Health and Human Services, 2014). Of >7000 components of tobacco smoke, nicotine is most associated with tobacco addiction (Benowitz, 1996; U.S. Department of Health and Human Services, 2010). In 2013, 21% of adults globally were current smokers and the total number of global tobacco users was 1.1 billion (World Health Organization, 2015). China has become the world's largest center for tobacco consumption, accounting for one-third of the world's tobacco users. In the 2015 China Adult Tobacco Survey (CATS), smoking prevalence among Chinese people aged 15 years and over was reported at 27.7%, representing 316 million smokers (Liang, 2015). In 2005, an estimated 0.67 million deaths in Chinese adults aged over 40 years were attributable to smoking (Gu et al., 2009). It is predicted that smoking-related mortality for China will rise to more than 2 million people per year by 2030 (Asma et al., 2015).
Male smokers predominate in China (Zheng et al., 2014). In the 2015 GATS, reported smoking prevalence among Chinese men was 52.1% versus 2.7% among women (Liang, 2015). Smoking prevalence is also higher in men in Western countries, but the gender gap is far less compared with China (Giovino et al., 2012). According to the 2010 GATS report, only 16.9% of ever smokers in China had quit smoking and most quit attempts occurred without use of cessation aids (Asma et al., 2015).
Current first-line pharmacological alternatives for smoking cessation include nicotine replacement therapy (NRT), bupropion (antidepressant, not targeting nicotine), and varenicline (selective partial nicotine receptor agonist) (Wu et al., 2006; Jain et al., 2013). NRT was developed the earliest (1970s), has established efficacy and safety (Silagy et al., 2004; Jain et al., 2013), and remains one of the most popular and recommended therapies for smoking cessation (Silagy et al., 2004; Gonzales et al., 2006; Hughes et al., 2007). NRT is available in a number of different formulations, including chewing gum, transdermal patch, inhalers, nasal or oral spray, and lozenge to accommodate individual preference or medical circumstance (eg, temporomandibular joint pain, odontopathy). NRTs contain about 50% of the nicotine dose obtained from smoking, providing some relief of withdrawal symptoms to help the smoker quit completely (Benowitz, 1996).
The pharmacologic treatment of nicotine addiction is a relatively new concept in China, from both the patient and the healthcare provider perspective (Hou and Cai, 2014; Xiao et al., 2014). Although NRT has been investigated in Chinese smokers from Hong Kong (Lam et al., 2005) and Taiwan (Hsueh et al., 2010), data on NRT effectiveness in mainland Chinese smokers are few (Xiao et al., 2014). Given the pervasiveness of tobacco use in China and its associated harms, more research is needed to assess the effectiveness of pharmacologic options that facilitate smoking cessation in this population.
Toward that end, this article details a phase 3 clinical study evaluating the effectiveness of NRT in adult Chinese smokers who are motivated to quit. The primary objective was to demonstrate the efficacy of 2 and 4 mg nicotine mint lozenges in smoking cessation, defined as continuous abstinence for the 28-day period up to and including the visit at week 6. Secondary objectives included assessment of continuous cessation rates at week 12 and week 24; long-term smoking cessation rate at week 24; 7-day point-prevalence cessation rates at weeks 1, 2, 4, 6, 12, and 24; relief of nicotine craving and withdrawal symptoms associated with cessation; body weight change from baseline to weeks 6, 12, and 24; electrocardiogram (ECG), vital signs, laboratory, and adverse events (AEs).
METHODS
Study Design
This was a multicenter, randomized, stratified (low- and high-dependence smoking status), double-blind, placebo-controlled, parallel-group study (ClinicalTrials.gov registration: NCT00985985) to evaluate the efficacy and safety of 2 and 4 mg nicotine mint lozenges in Chinese adult smokers during a 12-month period of smoking cessation.
Study Sites and Ethics Approval
The study was conducted at 10 academic or hospital-associated smoking cessation clinics in 6 cities (Beijing, Shenyang, Tianjin, and Shanghai [2 sites each]; Hangzhou and Guangzhou [1 site each]) in China between May 8, 2009, and February 9, 2011. Study participants were recruited from smoking cessation clinics and via advertisements published in the hospitals. The study was conducted in compliance with the ethical principles of the Declaration of Helsinki and its amendments, International Conference on Harmonisation Guideline for Good Clinical Practice, and other applicable regulations. The study protocol, protocol amendments, and subject-informed consent form were reviewed and approved by the Ethics Committees of all 10 investigational sites and by the China Food and Drug Administration. All subjects provided written informed consent prior to participation in this study.
Study Procedure
Subject demographics, past medical history, vital signs, physical examinations, laboratory (including pregnancy) tests, ECGs, concomitant medications, baseline CO levels, smoking history questionnaires, and diary cards were completed and recorded during screening.
This 12-month study had a maximum of 13 visits, which included both outpatient visits and telephone visits. A complete schedule of study procedures by visit can be found in Supplementary Table 1. Visit 1 was for screening and recording of subjects’ baseline characteristics and medical history. At visit 2 (the subject's designated target smoking quit date), subjects were stratified by nicotine dependence level (see below) and randomized to NRT or placebo; a User's Guide for smoking cessation using nicotine lozenges was provided. Body weight, concomitant medications, and vital signs were recorded. Subjects received low-intensity behavioral support (10 minutes) beginning at visit 2 through visit 5 (ie, Weeks 0, 1, 2, and 4 of treatment). Subjects were considered treatment failures if they had either an expiratory CO level greater than 10 ppm or reported smoking between visit 5 (4 weeks postquit ± 3 days) and visit 13 (12 months postquit ± 5 days). Investigators followed up with subjects who reported no more than 6 days of smoking at week 24 (visit 10) by telephone during visit 11 (8 months postquit ± 5 days), visit 12 (10 months postquit ± 5 days), and visit 13 (12 months postquit ± 5 days).
All study treatments were provided by Sino-American Tianjin Smith Kline & French Laboratories Ltd, Tianjin, China. The study treatments were administered buccally for absorption through the oral mucosa. Lozenges were moved from side to side in the mouth until completely dissolved and were not to be chewed or swallowed. The dose schedule was split into stages (Supplementary Table 2). Diary cards were provided to record cravings, withdrawal symptoms, and daily lozenge usage.
Randomization, Sample Size, and Blinding
Subjects were stratified into high-dependence and low-dependence strata based on “time to first cigarette” given that previous investigations of individual items from the Fagerström Test for Nicotine Dependence (FTND) have shown that this item provided the strongest predictor of early (1 week) and late (6 month) quitting success (Baker et al., 2007). The high-dependence stratum, consisting of subjects who smoked their first cigarette within 30 minutes of awakening, were randomized to receive either the 4 mg nicotine lozenge or matching 0 mg nicotine placebo lozenge. The low-dependence stratum, consisting of subjects who smoked their first cigarette >30 minutes after awakening, were randomized to receive either the 2 mg nicotine lozenge or matching 0 mg nicotine placebo lozenge. The randomization schedule was computer-generated and was provided to the site by the GSK Biostatistics Department.
The sample size for the 2 mg stratum was calculated by assuming 6-week cessation rates of 46% for active nicotine versus 30% for placebo lozenge. To obtain 90% power for detection of a significant difference at the 2-sided 5% significance level, 200 subjects per group were required, so an enrollment target of 480 participants was used. The sample size for the 4 mg stratum was calculated by assuming 6-week cessation rates of 49% for active nicotine versus 30% for placebo lozenge. To obtain 90% power for detection of a significant difference at the 2-sided 5% significance level, 65 subjects per group were required. However, to meet the recommendation of the regulatory body, China Food and Drug Administration, 120 subjects per group were recruited.
This was a double-blind study. Sponsors, sponsor's representatives, the investigator, staff, and subjects were blinded to treatment regimen.
Inclusion and Exclusion Criteria
The study included Chinese adult smokers (≥18 years old who smoked daily for at least 1 year before study enrollment) who had a strong motivation to quit smoking as assessed by an affirmative response to the question “Are you motivated to quit smoking within the next 30 days using NRT or placebo lozenges?” and the investigator's clinical opinion. The subjects’ smoking histories were recorded, including FTND scores, time to first cigarette, difficulty refraining from smoking, number of cigarettes smoked per day, smoking during illness, and other variables. Subjects had to be capable of understanding instructions and providing written informed consent.
Subjects were excluded if they used tobacco other than cigarettes (eg, pipes, cigars, snuff) or nicotine delivery systems other than lozenge (eg, nicotine gum, nicotine patch, nicotine inhaler, or nasal spray); smoked illicit substances (eg, cannabis, cocaine, heroin, ice drug); used smoking cessation aids within 30 days before study entry; had a history of alcohol or drug use; were currently involved in any other clinical trial or previous participation in this study; were pregnant, breastfeeding, or of childbearing potential but refused use of medical contraception; had unstable or uncontrolled systemic medical conditions, hyperthyroidism, or used insulin for diabetes mellitus; recent myocardial infarction or cerebral vascular accidents; allergy to aspartame or phenylpyruvic acid; diagnosis of phenylketonuria; or medical history endangering subject safety or study result validity.
Study Parameter Measurements
The study subjects made 8 in-person outpatient visits to the study site through 24 weeks. Outpatient visits occurred at screening and weeks 0, 1, 2, 4, 6, 12, and 24 after initiation of treatment. In addition, subjects underwent 5 telephone visits with study investigators at 16 and 20 weeks, and at 8, 10, and 12 months. The measurements and procedures conducted at each visit are summarized in Supplementary Table 1.
The primary efficacy endpoint was 28-day continuous smoking abstinence at 6 weeks. This endpoint was selected because previous studies indicate that early quitting success may be a predictor of long-term quitting success (Hurt et al., 1994; Kenford et al., 1994; Ashare et al., 2013). Abstinence was confirmed by expiratory CO levels threshold of ≤10 ppm at each in-person visit to the study site (Supplementary Table 1). This cutoff was chosen because this threshold was used in a previous trial of the 2 and 4 mg lozenges and has been recommended for use in clinical trials (Shiffman et al., 2002; SRNT Subcommittee on Biochemical Verification, 2002). Subjects who failed to meet the success criteria and those with unknown smoking status were counted as failures.
Secondary efficacy parameters were point prevalence of abstinence at time points through week 24, defined as complete abstinence from smoking for the 7 days up to and including the evaluation day; long-term successful cessation at week 24 (defined as meeting the 6-week success criteria and subsequently smoking on ≤6 days between weeks 6 and 24); relief of craving/total withdrawal symptoms; and changes in body weight. The safety parameters were PE, vital signs, laboratory tests, ECG, and AEs.
Statistical Analysis
Efficacy and safety statistical analyses were performed separately for the 2 and 4 mg strata. Three analysis populations were defined: full analysis set (FAS; all randomized subjects except those known not to have taken any study medication) for the primary analysis, per-protocol set (PPS; subjects in good compliance with study protocol), and safety set (SS; all randomized subjects having taken at least 1 dose of study medication).
All smoking cessation rates were compared using a Cochran-Mantel-Haenszel test with adjustment for center effect. The relief of craving/withdrawal symptoms was analyzed by a Wilcoxon rank sum test to compare nicotine with placebo (separately within each stratum). Change in body weight among subjects who quit was analyzed with an analysis of covariance model that included effects of treatment, dosing amount, baseline body weight, response, and center (separate analysis within each stratum). All statistical tests were 2-sided at the 5% significance level. Safety analysis consisted of tabulation of AEs.
A post hoc efficacy analysis was conducted on the continuous abstinence endpoints at 6, 12, and 24 weeks in subpopulations of the FAS based on various baseline characteristics including total FTND score (<6 or ≥6), number of prior quit attempts (<2 or ≥2), number of cigarettes per day (≥10, <20, and ≥20), and average lozenge use over the first 6 weeks (<9/day or ≥9 per day) and were compared using a Cochran-Mantel-Haenszel test.
RESULTS
Disposition of Subjects
A total of 781 subjects were screened; 58 failed to meet the inclusion/exclusion criteria and therefore 723 subjects were randomized and comprised the FAS, including 240 high-dependence and 483 low-dependence smokers. The disposition of subjects in the FAS analysis is provided in Supplementary Figure 1. Discontinuation rates were similar between treatment groups in each stratum. The most common reasons for discontinuation were “other” (n = 6) and lost to follow-up (n = 5) in the high-dependence stratum and lost to follow-up (n = 15) and lack of efficacy (n = 11) in the low-dependence stratum. The PPS included a total of 688 subjects. There were 459 subjects (227 nicotine and 232 placebo) in the 2 mg stratum and 229 subjects (115 nicotine and 114 placebo) in the 4 mg stratum. The SS included 720 subjects and included all 483 subjects (241 nicotine and 242 placebo) randomized to the 2 mg stratum and 237 subjects (119 nicotine and 118 placebo) randomized to the 4 mg stratum. Three subjects were excluded from the SS because of nonuse of study drug.
Demographics and Other Baseline Characteristics
The 2 mg stratum enrolled 465 (96.27%) male and 18 (3.73%) female subjects. Baseline age was 39.1 ± 11.78 years; height, 171.6 ± 5.72 cm; body weight, 72.1 ± 11.23 kg. In the 4 mg stratum, subjects were 234 (97.91%) male and 5 (2.09%) female; age, 42.2 ± 10.45 years; height, 172.6 ± 5.73 cm; body weight, 75.1 ± 11.9 kg. All subjects showed tobacco dependence. Both strata had more than 50% of subjects smoking 11–20 cigarettes daily, and greater than 50% of subjects had previously tried to quit smoking without success (Supplementary Table 3).
Primary Efficacy Analysis
In the FAS, the primary analysis showed higher week 6 smoking cessation rates for the nicotine mint lozenge compared with placebo in both strata. However, only the 4 mg stratum approached statistical significance (P = 0.0565). In the low-dependence (2 mg) stratum, 59 subjects (24.48%) of 241 in the active nicotine group and 52 subjects (21.49%) of 242 in the placebo group were successful quitters (P = .3851). In the high-dependence (4 mg) stratum, 37/120 subjects (30.83%) in the active nicotine group and 24/119 subjects (20.17%) in the placebo group were successful quitters (P = .0565) (Table 1).
TABLE 1.
Analysis of Successful Smoking Cessation Rate at Week 6 (Primary Efficacy; Full Analysis Set)
| Low-dependence Stratum (2 mg) | High-dependence Stratum (4 mg) | |||||
| NRT | Placebo | Total | NRT | Placebo | Total | |
| N | 241 | 242 | 483 | 120 | 119 | 239 |
| Failed, n (%) | 182 (75.52) | 190 (78.51) | 372 (77.02) | 83 (69.17) | 95 (79.83) | 178 (74.48) |
| Succeeded, n (%) | 59 (24.48) | 52 (21.49) | 111 (22.98) | 37 (30.83) | 24 (20.17) | 61 (25.52) |
| P value* | 0.3851 | 0.0565 | ||||
| OR* | 1.22 | 1.82 | ||||
| 95% CI | 0.78–1.92 | 0.99–3.32 | ||||
*P value based on Cochran-Mantel-Haenszel test (adjusted by center); OR based on logistic model (adjusted by center).
CI, confidence interval; NRT, nicotine replacement therapy; OR, odds ratio.
Secondary Efficacy Analysis
The week 6 quit rates in the PPS was a secondary efficacy measure (Fig. 1) that was analyzed using the same method as the primary analysis. Results were consistent with the primary FAS results.
FIGURE 1.
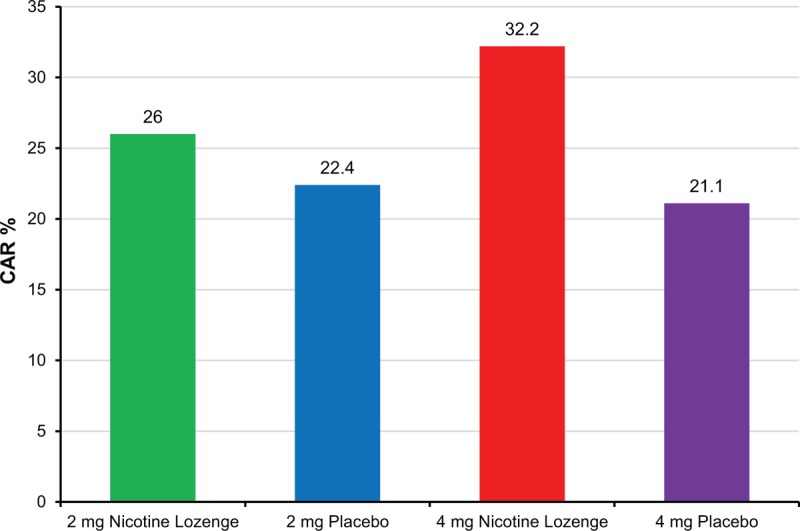
Continuous abstinence rate at week 6 in per-protocol set (secondary efficacy parameter). CAR, continuous abstinence rate.
There were no significant between-treatment differences (nicotine vs placebo) in the 2 mg stratum for the rate of continuous successful smoking cessation in subjects from the FAS at week 12, week 24, and month 12 (all P values >0.05; Table 2 and Fig. 2). However, in the 4 mg stratum, significantly more subjects in the nicotine lozenge group quit smoking continuously and successfully versus placebo at week 12 (22.5% vs 10.9%; OR: 2.46; 95% CI: 1.18–5.10; P = 0.0160) and week 24 (19.2% vs 10.1%; OR: 2.18; 95% CI: 1.02–4.67; P = 0.0464), although there was no significant difference between treatments at month 12 (15.8% vs 9.2%; OR: 1.85; 95% CI: 0.84, 4.07; P = 0.1226). Results of the PPS analysis were consistent with those of the FAS.
TABLE 2.
Secondary Efficacy Endpoints (Full Analysis Set)
| Low-dependence Stratum (2 mg) | High-dependence Stratum (4 mg) | |||
| Parameter | NRT (n = 241) | Placebo (n = 242) | NRT (n = 120) | Placebo (n = 119) |
| Continuous successful smoking cessation* | ||||
| Week 12 | ||||
| Failed, n (%) | 198 (82.2) | 201 (83.1) | 93 (77.5) | 106 (89.1) |
| Succeeded, n (%) | 43 (17.8) | 41 (16.9) | 27 (22.5) | 13 (10.9) |
| P value† | 0.740 | 0.016 | ||
| OR† | 1.09 | 2.46 | ||
| 95% CI | 0.66–1.78 | 1.18–5.10 | ||
| Week 24 | ||||
| Failed, n (%) | 210 (87.1) | 210 (86.8) | 97 (80.8) | 107 (89.9) |
| Succeeded, n (%) | 31 (12.9) | 32 (13.2) | 23 (19.2) | 12 (10.1) |
| P value† | 0.951 | 0.046 | ||
| OR† | 0.98 | 2.18 | ||
| 95% CI | 0.56–1.72 | 1.02–4.67 | ||
| Month 12 | ||||
| Failed, n (%) | 215 (89.2) | 214 (88.4) | 101 (84.2) | 108 (90.8) |
| Succeeded, n (%) | 26 (10.8) | 28 (11.6) | 19 (15.8) | 11 (9.2) |
| P value† | 0.822 | 0.123 | ||
| OR† | 0.93 | 1.85 | ||
| 95% CI | 0.51–1.69 | 0.84–4.07 | ||
| Long-term successful smoking cessation rates at week 24† | ||||
| Failed, n (%) | 195 (80.9) | 199 (82.2) | 91 (75.8) | 100 (84.0) |
| Succeeded, n (%) | 46 (19.1) | 43 (17.8) | 29 (24.2) | 19 (16.0) |
| P value† | 0.648 | 0.111 | ||
| OR† | 1.12 | 1.72 | ||
| 95% CI | 0.69–1.82 | 0.89–3.32 | ||
| 7-Day point prevalence abstinence (%) | ||||
| Week 1 | 12.5 | 12.0 | 20.0§ | 10.1 |
| Week 2 | 21.2 | 23.1 | 33.3 | 26.1 |
| Week 4 | 32.0 | 26.3 | 36.7 | 30.3 |
| Week 6 | 35.7 | 37.2 | 41.7 | 32.8 |
| Week 12 | 41.5 | 39.3 | 40.8§ | 26.1 |
| Week 24 | 41.5 | 43.1 | 50.0 | 43.7 |
| Relief of craving/withdrawal symptoms, total scores (successful quitters) | ||||
| Week 1 | 7.42 | 6.14 | 5.89 | 9.20 |
| Week 2 | 6.42 | 4.67 | 5.07 | 8.02 |
| Week 4 | 4.52 | 4.18 | 3.01 | 7.33 |
| Week 6 | 4.09 | 3.71 | 3.13 | 6.56 |
| Change from baseline in body weight (successful quitters), kg (SD) | ||||
| Week 6 | 1.5 (1.76) | 1.3 (2.45) | 1.7 (2.93) | 1.5 (1.76) |
| Week 12 | 1.9 (2.24) | 1.8 (2.43) | 1.8 (2.94) | 1.8 (2.22) |
| Week 24 | 2.0 (2.75)§ | 1.2 (2.56) | 2.4 (4.05) | 2.8 (2.80) |
*Proportion of subjects who achieved the primary endpoint at week 6 and quit smoking completely by week 12 and week 24.
†P value based on Cochran-Mantel-Haenszel test (adjusted by center); OR based on logistic model (adjusted by center).
‡Proportion of subjects who achieved the primary endpoint with no more than 6 cumulative days of smoking from week 6 to week 24.
§P < 0.05.
CI, confidence interval; NRT, nicotine replacement therapy; OR, odds ratio.
FIGURE 2.
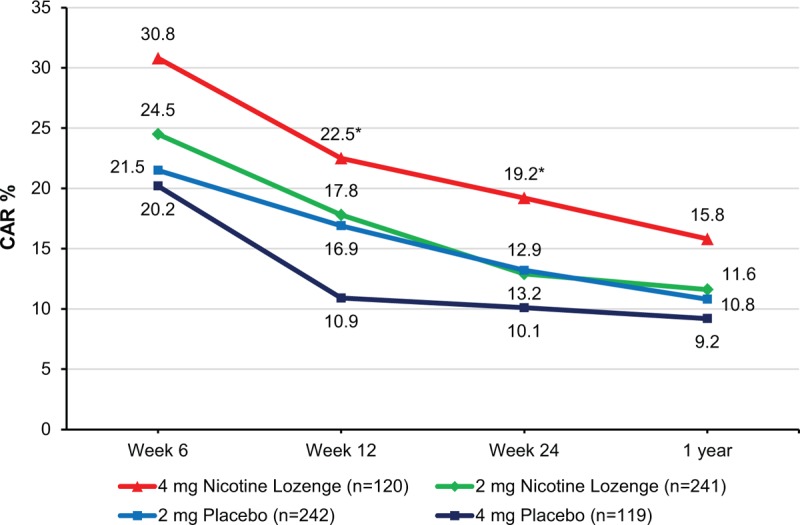
Continuous abstinence rate in full analysis set over time. ∗P < 0.05 versus corresponding placebo based on Cochran–Mantel–Haenszel test (adjusted by center). CAR, continuous abstinence rate.
Nicotine mint lozenge provided higher rates of long-term successful smoking cessation versus placebo (Table 2) at week 24 in both the 4 and 2 mg strata of the FAS (4 mg, 24.17% vs placebo, 15.97%; 2 mg, 19.09% vs placebo, 17.77%), but without statistical significance (4 mg, OR: 1.72; 95% CI: 0.89–3.32; P = 0.1105; 2 mg, OR: 1.12; 95% CI: 0.69–1.82; P = 0.6484). Results of the PPS analysis were consistent with those of FAS.
In the FAS, the 7-day point-prevalence success rates (Table 2) increased over time from week 1 to week 24 in both the 4 and 2 mg strata and in both the nicotine and placebo treatment groups. Week 24 success rates were 1.5–3.0 times those of week 1. In the 2 mg stratum, treatment differences between nicotine and placebo were not statistically significant. In the 4 mg stratum, nicotine lozenge provided significantly higher success rates than placebo at week 1 (20% vs 10.1%; P = 0.0233) and week 12 (40.83% vs 26.05%; P = 0.0159). PPS analysis results were consistent with those of FAS.
Nicotine Cravings and Withdrawal Symptoms
Nicotine craving and withdrawal symptoms were evaluated daily with the Minnesota Nicotine Withdrawal Scale (MNWS). MNWS scores for craving/withdrawal symptoms in both strata declined gradually during the first 6 weeks (Table 2). There were no significant differences in MNWS scores between the 2 mg nicotine lozenge group and the 2 mg placebo groups at any time point. The 4 mg nicotine lozenge provided significantly higher rates of continuous abstinence versus 4 mg placebo at weeks 1 (20% vs 10.1%; P = 0.023) and 12 (40.8% vs 26.1%; P = 0.016). In the subpopulation of successful quitters only, however, the total MNWS scores in the nicotine group were significantly lower than those in the placebo group at weeks 3, 4, 5, and 6 for the 4 mg stratum (Table 2).
Weight Gain
Weight gain may accompany smoking cessation; therefore, the most meaningful comparison of weight gain between nicotine and placebo lozenge in both strata is in the subpopulation of successful smoking quitters. In that population, there was no significant difference in weight gain at any time point except in the 2 mg stratum at 24 weeks (nicotine, 2.0 kg vs placebo, 1.2 kg, P = 0.0497) (Table 2).
Safety Analysis
Adverse Events
Table 3 summarizes AE rates by preferred terms according to the Medical Dictionary for Regulatory Activities. AEs were more frequent with active nicotine lozenges than with placebo; most AEs were mild or moderate in severity. In the 2 mg nicotine group, a total of 219 AEs occurred in 111 subjects (46.1%); 1 event (0.4%) was definitely related to the study drug, 19 (7.9%) were probably related to study drug, and 28 (11.6%) were possibly related to study drug. In the 4 mg nicotine group, 129 AEs occurred in 61 (51.3%) subjects; 4 events (3.4%) were definitely related to study drug, 11 (9.2%) were probably related to study drug, and 16 (13.5%) were possibly related to study drug. The most common AEs encountered were upper respiratory tract infections, nasopharyngitis, and nausea; all are known side effects of NRT. A total of 8 subjects discontinued treatment because of AEs: 3 in the 2 mg nicotine group (1.2%), 2 in the 2 mg placebo group (0.8%), 1 in the 4 mg nicotine group (0.8%), and 2 in the 4 mg placebo group (1.7%).
TABLE 3.
Adverse Events by Preferred Term
| Low-dependence Stratum (2 mg) | High-dependence Stratum (4 mg) | |||
| AE, n % | Nicotine (n = 241) | 2 mg Placebo (n = 242) | Nicotine (n = 119) | 4 mg Placebo (n = 118) |
| Any AE | 111 (46.1) | 91 (37.6) | 61 (51.3) | 58 (49.2) |
| URTI | 27 (11.2) | 19 (7.9) | 17 (14.3) | 11 (9.3) |
| Nasopharyngitis | 16 (6.6) | 14 (5.8) | 13 (10.9) | 5 (4.2) |
| Nausea | 15 (6.2) | 10 (4.1) | 7 (5.9) | 8 (6.8) |
| Diarrhea | 9 (3.7) | 9 (3.7) | 2 (1.7) | 2 (1.7) |
| Abdominal distension | 6 (2.5) | 2 (0.8) | 1 (0.8) | 3 (2.5) |
| Oropharyngeal discomfort | 7 (2.9) | 0 | 0 | 0 |
| Vomiting | 5 (2.1) | 0 | 0 | 1 (0.9) |
| Dizziness | 10 (4.2) | 0 | 3 (2.5) | 4 (3.4) |
| Palpitations | 5 (2.1) | 1 (0.4) | 3 (2.5) | 2 (1.7) |
| Hypertension | 5 (2.1) | 0 | 0 | 2 (1.7) |
| Somnolence | 0 | 0 | 3 (2.5) | 0 |
| Insomnia | 1 (0.4) | 0 | 3 (2.5) | 2 (1.7) |
| Dyspnea | 1 (0.4) | 2 (0.8) | 6 (5.0) | 2 (1.7) |
AE, adverse event; URTI, upper respiratory tract infection.
Three subjects experienced 5 serious AEs (SAEs). One subject in the 4 mg placebo arm had 3 SAEs (bile duct stone, biliary tract infection, and cholelithiasis) that were considered by the investigator as irrelevant to the study drug. One subject in the 2 mg nicotine group had 1 SAE (acute onset of cough and expectoration in a patient with underlying chronic obstructive pulmonary disease) and 1 subject in the 4 mg active nicotine arm had 1 SAE (skin laceration); the 2 SAEs were assessed by the investigator as possibly irrelevant and irrelevant, respectively, to the study drug. No deaths or disabilities were reported. All 3 subjects with SAEs fully recovered. In the 2 mg nicotine group, 6 subjects (2.5%) had increased blood pressure and 2 subjects (0.8%) had increased blood glucose. Also, the blood glucose of 2 subjects (1.7%) increased in the 4 mg nicotine group. During the study, no other clinically relevant AEs were noted in terms of changes in vital signs, physical exam status, or laboratory tests.
Post Hoc Efficacy Analysis
A post hoc efficacy analysis was conducted on the continuous abstinence endpoints at 6, 12, and 24 weeks in subpopulations of the FAS, according to 3 baseline characteristics: nicotine dependency (FTND <6, FTND ≥6), number of previous smoking quit attempts (<2, ≥2), and number of cigarettes smoked per day at baseline (>10, ≤20, >20); the 2 and 4 mg strata were analyzed separately. Results were similar to those of the entire FAS except for the subsets with prior quit attempts. In those with fewer than 2 prior quit attempts (approximately two-thirds of FAS subjects), the 2 mg stratum had higher quit rates with placebo. In the subset with 2 or more prior quit attempts (approximately one-third of FAS subjects), the 2 mg stratum had a significantly higher quit rate with nicotine than placebo at all 3 time points (P = 0.0009 at week 6, P = 0.0133 at week 12, P = 0.0039 at week 24).
DISCUSSION
The primary efficacy parameter in this study was 28-day continuous successful smoking quit rate at the end of week 6. In the 2 mg stratum, the success rates were 24.48% for nicotine versus 21.49% for placebo, which was similar between groups and did not reach statistical significance (OR: 1.22; 95% CI: 0.78–1.92; P = 0.3851). In the 4 mg stratum, the success rate of 30.8% for nicotine versus 20.2% for placebo approached statistical significance (OR: 1.82; 95% CI: 0.99–3.32; P = 0.0565).
The low-intensity behavioral support employed in this study may help explain why the efficacy of NRT appeared lower than expected in this population. Behavioral support is an important component of smoking cessation efforts with NRT in the real world, and such support has been shown to have a significant positive effect on the efficacy of NRT and other cessation therapies (Kotz et al., 2014; Lancaster and Stead, 2017). Only minimal behavioral support was offered as part of this study in order to avoid confounding the effects of the treatment, but this lack of additional support may have reduced the observed effects of NRT.
The results of the current study, and the efficacy of smoking cessation therapies in general, have significant implications for efforts to reduce rates of smoking in China. The push to reduce smoking in China is not as well established as cessation campaigns in the US and Europe. The availability of NRT is low and medication use for smoking cessation has been reported to be less than 6% among smokers (Yang et al., 2011; Katanoda et al., 2014), so the need for effective pharmacotherapeutic options for nicotine dependence is great in this population.
Few smoking cessation studies in mainland Chinese subjects can be used for comparison. An open-label, multicenter clinical study tested the effectiveness of 12 weeks of NRT treatment (including a 12-week off-treatment follow-up) provided as either nicotine gum (2 or 4 mg) or nicotine patches in 300 Chinese physicians from 6 general hospitals in Beijing, Shanghai, and Guangzhou who were motivated to quit smoking (Xiao et al., 2014). Enrolled patients smoked at least 10 cigarettes per day for at least 3 years; only 2% had previously used any type of stop-smoking medication. Continuous abstinence from weeks 2 to 24 occurred in 17% of subjects overall and was the highest in subjects treated with 2 mg nicotine gum (20.9%). The point-prevalence abstinence rate at week 24 was 35%; 38% had reduced daily cigarette consumption by 50% (Xiao et al., 2014). Although the 2 mg nicotine lozenge provided no significant benefit versus placebo in rate of successful smoking cessation at 12 or 24 weeks in our study, this was not the case with the 4 mg nicotine lozenge, where the odds of successful abstinence at 12 and 24 weeks was more than 2-fold that found with placebo treatment.
Nicotine sublingual tablets were investigated in a randomized, double-blind, placebo-controlled, 3-month smoking cessation trial in adult smokers who smoked at least 10 cigarettes per day for at least 1 year from communities in Beijing, China (Guo Song et al., 2006). The primary study outcome was self-reported abstinence rates at 2 and 3 months. At the end of 3 months, 52.2% of subjects taking nicotine sublingual tablets reported abstinence at 3 months versus 19.1% taking placebo. A 50% reduction in smoking was found in 42.6% on NRT versus 14.8% on placebo (Guo Song et al., 2006).
Smokers’ demographic and baseline characteristics, as well as NRT option and use, have been shown in previous clinical studies to influence smoking cessation (Shiffman et al., 2005; Uhl et al., 2007; Fu et al., 2008; Cox et al., 2011). It is speculated that a good statistical design and analysis model (eg, the adjusted multivariate logistic regression model) adjusted and controlled for as many key demographic and baseline characteristics as possible might produce a clinical outcome more tailored to Chinese smokers.
LIMITATIONS
The use of CO measurements to confirm smoking status is an important limitation to this study. The 10 ppm threshold used to identify smoking relapse has been recommended and utilized in past studies of NRT, but more recently, concerns that this threshold misclassifies too many smokers as abstainers has led to lower thresholds (3–4 ppm) being recommended and used (Perkins et al., 2013; Cropsey et al., 2014). Additionally, exhaled CO has a half-life of 2–8 hours (SRNT Subcommittee on Biochemical Verification, 2002) and is an indicator of acute smoking only. Consequently, this study may not have captured smoking relapse in participants who did not smoke shortly before in-person visits, which took place weekly initially, and monthly later in the study. Other outcomes widely used in smoking cessation trials, including point-prevalence abstinence (a secondary outcome in this study) and prolonged abstinence, may better detect true rates of cessation than continuous abstinence (Cheung et al., 2017). Finally, the calculated sample sizes needed for the study were based on efficacy estimates (46% for nicotine treatment and 30% for placebo) that were higher than the success rate actually observed in the study population. The 2 mg stratum was, therefore, limited by being underpowered, and the significance of the results may have been affected by enrollment of too few study subjects.
CONCLUSIONS
In both the FAS and PPS populations, we found no statistical differences between the active and placebo groups in the 2 mg stratum, whereas in the 4 mg stratum, we observed a directionally significant improvement in smoking cessation rates compared with placebo in Chinese adult smokers with high nicotine dependence on the primary endpoint at 6 weeks. This finding was supported by statistically significant longer term smoking cessation rates at 12 and 24 weeks with the 4 mg nicotine mint lozenge compared with the placebo lozenge. Both 2 and 4 mg nicotine mint lozenges were generally well tolerated in this study based on the study safety data.
Unlike results from the FAS analysis, the post hoc analysis revealed significant between-treatment differences in smoking cessation rates at all time points in subjects in the 2 mg stratum with ≥2 previous quit attempts and suggests that the 2 mg nicotine mint lozenge may help Chinese adult smokers to quit and maintain the cessation for at least 6 months. This is potentially due to greater motivation to quit in subjects with more previous quit attempts. Further studies are needed to confirm these outcomes in Chinese adult smokers.
Supplementary Material
Supplementary Material
Acknowledgments
Herein we give our sincere thanks to all the subjects, investigators, and healthcare providers from the 10 clinical sites who were involved in this study. Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Footnotes
Current affiliation: Accenture, Berwyn, PA, USA.
On behalf of the following Principal Investigators: Chunxue Bai (Zhongshan Hospital Fudan University, Shanghai); Kejing Ying (Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou); Ping Chen (General Hospital of Shenyang Military District PLA, Shenyang); Qiang Li (Changhai Hospital, Second Medical Military University, Shanghai); Nanshan Zhong (The First Affiliated Hospital of Guangzhou Medical University, Guangzhou); Gengzhi Ge (The 2nd Hospital of Tainjin Medical University, Tianjin); Jiangtao Lin (China-Japan Friendship Hospital, Beijing); Baoyuan Chen (Tianjin Medical University General Hospital, Tianjin).
This study was sponsored by Tianjin Sino-American SmithKline & French Laboratory Ltd. Medical writing assistance was provided by Peloton Advantage, LLC, an OPEN Health company, and was funded by GlaxoSmithKline Consumer Healthcare. GlaxoSmithKline Consumer Healthcare provided a full review of the article.
The authors report no conflicts of interest.
REFERENCES
- Ashare RL, Wileyto EP, Perkins KA, et al. The first 7 days of a quit attempt predicts relapse: validation of a measure for screening medications for nicotine dependence. J Addict Med 2013; 7:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asma S, Mackay J, Song SY, et al. The Global Adult Tobacco Survey Atlas. Atlanta, GA: CDC Foundation; 2015. [Google Scholar]
- Baker TB, Piper ME, et al. Transdisciplinary Tobacco Use Research Center (TTURC) Tobacco Dependence. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tobacco Res 2007; 9: Suppl 4: S555–S570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol 1996; 36:597–613. [DOI] [PubMed] [Google Scholar]
- Cheung KL, de Ruijter D, Hiligsmann M, et al. Exploring consensus on how to measure smoking cessation. A Delphi study. BMC Public Health 2017; 17:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Okuyemi K, Choi WS, et al. A review of tobacco use treatments in U.S. ethnic minority populations. Am J Health Promot 2011; 25:S11–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey KL, Trent LR, Clark CB, et al. How low should you go? Determining the optimal cutoff for exhaled carbon monoxide to confirm smoking abstinence when using cotinine as reference. Nicotine Tob Res 2014; 16:1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SS, Kodl MM, Joseph AM, et al. Racial/Ethnic disparities in the use of nicotine replacement therapy and quit ratios in lifetime smokers ages 25 to 44 years. Cancer Epidemiol Biomarkers Prev 2008; 17:1640–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovino GA, Mirza SA, Samet JM, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet 2012; 380:668–679. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006; 296:47–55. [DOI] [PubMed] [Google Scholar]
- Gu D, Kelly TN, Wu X, et al. Mortality attributable to smoking in China. N Engl J Med 2009; 360:150–159. [DOI] [PubMed] [Google Scholar]
- Guo Song JZN, Sun HQ, Niu GS, et al. The smoking cessation efficacy of nicotine sublingual tablet: a double-blind, placebo-controlled trial. Chin J Psychiatry 2006; 39:102–105. [Google Scholar]
- Hou XM, Cai BQ. Smoking cessation among rural populations in Beijing. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2014; 36:501–505. [DOI] [PubMed] [Google Scholar]
- Hsueh KC, Chen CY, Yang YH, et al. Smoking cessation program in outpatient clinics of Family Medicine Department in Taiwan: a longitudinal evaluation. Eval Health Prof 2010; 33:12–25. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2007. Cd000031. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Dale LC, Fredrickson PA, et al. Nicotine patch therapy for smoking cessation combined with physician advice and nurse follow-up. One-year outcome and percentage of nicotine replacement. JAMA 1994; 271:595–600. [PubMed] [Google Scholar]
- Jain R, Majumder P, Gupta T. Pharmacological intervention of nicotine dependence. BioMed Res Int 2013; 2013:278392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katanoda K, Jiang Y, Park S, et al. Tobacco control challenges in East Asia: proposals for change in the world's largest epidemic region. Tob Control 2014; 23:359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, et al. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA 1994; 271:589–594. [DOI] [PubMed] [Google Scholar]
- Kotz D, Brown J, West R. ‘Real-world’ effectiveness of smoking cessation treatments: a population study. Addiction 2014; 109:491–499. [DOI] [PubMed] [Google Scholar]
- Lam TH, Abdullah AS, Chan SS, et al. Adherence to nicotine replacement therapy versus quitting smoking among Chinese smokers: A preliminary investigation. Psychopharmacology (Berl) 2005; 177:400–408. [DOI] [PubMed] [Google Scholar]
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2017; 3:Cd001292. [DOI] [PubMed] [Google Scholar]
- Liang X. Report of China City Adult Tobacco Survey 2013-14. Atlanta, GA: CDC Foundation; 2015. [Google Scholar]
- Perkins KA, Karelitz JL, Jao NC. Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence. Nicotine Tobacco Res 2013; 15:978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Di Marino ME, Sweeney CT. Characteristics of selectors of nicotine replacement therapy. Tob Control 2005; 14:346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dresler CM, Hajek P, et al. Efficacy of a nicotine lozenge for smoking cessation. Arch Intern Med 2002; 162:1267–1276. [DOI] [PubMed] [Google Scholar]
- Silagy C, Lancaster T, Stead L, et al. Nicotine replacement therapy for smoking cessation. The Cochrane Database of Systematic Reviews 2004. Cd000146. [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tobacco Res 2002; 4:149–159. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking–50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- Uhl GR, Liu QR, Drgon T, et al. Molecular genetics of nicotine dependence and abstinence: whole genome association using 520,000 SNPs. BMC Genet 2007; 8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO Report on the Global Tobacco Epidemic. 2015: Raising taxes on tobacco. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- Wu P, Wilson K, Dimoulas P, et al. Effectiveness of smoking cessation therapies: A systematic review and meta-analysis. BMC Public Health 2006; 6:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Zhong N, Bai C, et al. Nicotine gum or patch treatment for smoking cessation and smoking reduction: a multi-centre study in Chinese physicians. Front Med 2014; 8:84–90. [DOI] [PubMed] [Google Scholar]
- Yang J, Hammond D, Driezen P, et al. The use of cessation assistance among smokers from China: Findings from the ITC China Survey. BMC Public Health 2011; 11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, McLerran DF, Rolland BA, et al. Burden of total and cause-specific mortality related to tobacco smoking among adults aged ≥ 45 years in Asia: a pooled analysis of 21 cohorts. PLoS Med 2014; 11:e1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


