In Baltimore, increasing gonorrhea screening among population aged 15 to 24 years and in San Francisco increasing screening among all men who have sex with men was estimated to most likely reduce gonorrhea burden.
Supplemental digital content is available in the text.
Abstract
Background
Baltimore and San Francisco represent high burden areas for gonorrhea in the United States. We explored different gonorrhea screening strategies and their comparative impact in the 2 cities.
Methods
We used a compartmental transmission model of gonorrhea stratified by sex, sexual orientation, age, and race/ethnicity, calibrated to city-level surveillance data for 2010 to 2017. We analyzed the benefits of 5-year interventions which improved retention in care cascade or increased screening from current levels. We also examined a 1-year outreach screening intervention of high-activity populations.
Results
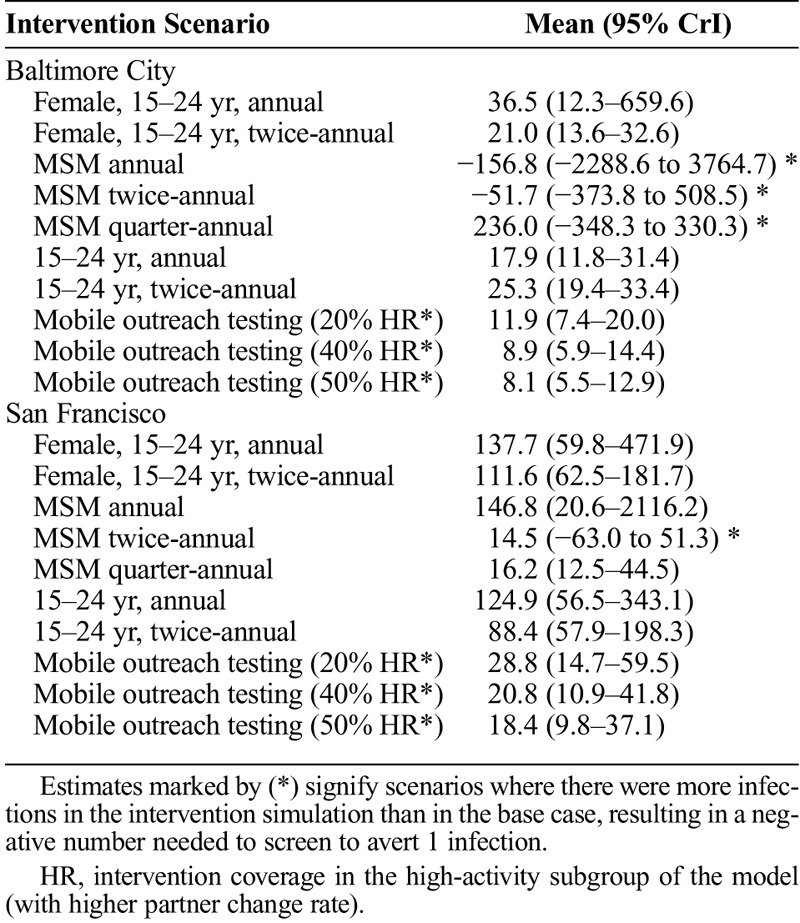
In Baltimore, annual screening of population aged 15 to 24 years was the most efficient of the 5-year interventions with 17.9 additional screening tests (95% credible interval [CrI], 11.8–31.4) needed per infection averted while twice annual screening of the same population averted the most infections (5.4%; 95% CrI, 3.1–8.2%) overall with 25.3 (95% CrI, 19.4–33.4) tests per infection averted. In San Francisco, quarter-annual screening of all men who have sex with men was the most efficient with 16.2 additional (95% CrI, 12.5–44.5) tests needed per infection averted, and it also averted the most infections (10.8%; 95% CrI, 1.2–17.8%). Interventions that reduce loss to follow-up after diagnosis improved outcomes. Depending on the ability of a short-term outreach screening to screen populations at higher acquisition risk, such interventions can offer efficient ways to expand screening coverage.
Conclusions
Data on gonorrhea prevalence distribution and time trends locally would improve the analyses. More focused intervention strategies could increase the impact and efficiency of screening interventions.
Gonorrhea is the second most reported notifiable infection in the United States.1 Gonococcal infection increases risks of adverse outcomes, such as pelvic inflammatory disease and infertility, and there is potential for increased human immunodeficiency virus (HIV) acquisition among people uninfected with HIV.2 Antibiotic resistant gonorrhea strains have amplified the urgency to improve control and surveillance of gonorrhea. Reported gonorrhea diagnosis rates in the United States are increasing.1 This increase is attributable in part to changes in reporting systems (moving from paper-based to computerized reporting), increased uptake of more sensitive testing technologies,3 and increased use of nucleic acid amplification testing for extragenital infections.4 It is also possible that rising gonorrhea diagnosis rates reflect real increases in incidence, due to changes in partner-seeking and risk behaviors, such as finding sexual partners online,5 and increases in condomless sex.6
Gonorrhea burden differs by geographic region and population group.7 San Francisco, California, and Baltimore City, Maryland, represent high-burden areas for gonorrhea. In both cities, gonorrhea diagnosis rates are higher than the national average: in 2016, there were over 1000 reported gonorrhea diagnoses per 100,000 population aged 15 to 39 years, compared with 377 per 100,000 at the national level,8 and diagnosis rates in the cities have risen over the past 6 years. The cities differ in their gonorrhea epidemiology: in San Francisco, gonorrhea diagnoses are concentrated among men who have sex with men (MSM), whereas in Baltimore City, the majority of diagnoses are observed among the heterosexual population.9 Local data describing these differences can be used to better characterize gonorrhea transmission, and explore more focused intervention strategies.
National guidelines recommend annual gonorrhea screening among sexually active women younger than 25 years and older women at increased risk,10 and the Centers for Disease Control and Prevention also recommends screening of MSM.11 Local differences in disease burden and resource availability drive different priorities for sexually transmitted infection (STI) service providers. In this study, we examined how local data can be used to explore more effective gonorrhea prevention efforts and to estimate how differences in epidemic characteristics influence the impact of screening interventions.
MATERIALS AND METHODS
Metapopulation Model
We simulated gonorrhea transmission dynamics using a published deterministic compartmental metapopulation model.12 The heterosexual model population of men and women is stratified to subpopulations of non-Hispanic black, Hispanic, and “white and other.” The model includes MSM, who are not stratified by race/ethnicity due to the scarcity of data for this population. Each subpopulation is further stratified to 15 to 24 years and 25 to 39 years populations. Sexual activity levels are defined as people who are not sexually active and those who are sexually active. In addition to the different partner change rates by sex, sexual orientation, age, and race/ethnicity, the modeled sexually active population is stratified to those with higher or lower partner change rate; 10% of sexually active population are set to belong to the higher partner change rate category. We refer to the 10% of the population as the “high-activity” group in this study. Partner change rates within each subpopulation are varied in calibration. Differential equations and model specification can be found in the supplemental material of the original study.12
Gonorrhea health states are represented by a susceptible-infectious-susceptible model, with the infectious stage stratified into asymptomatic and symptomatic categories. The model simulates the transmission dynamics and natural history of gonorrhea in the metapopulation, capturing the estimated underlying burden of infection (eg, model-estimated incidence and prevalence). The model then simulates the diagnosis of gonorrhea via screening (to identify asymptomatic infections) and care-seeking by patients (for symptomatic infections). We further assume that not all diagnoses are reported, and that reporting may differ between asymptomatic and symptomatic infections due to presumptive treatment of symptomatic individuals. The model was implemented in R and C++. Model code is available at: https://github.com/PPML/gc-regional.
Data
The model population represented the population composition of each city by race/ethnicity and sexual orientation. In San Francisco, 18.5% of the adult male population is estimated to be MSM, whereas in Baltimore City, the proportion is close to 4%.13 Further differences are seen by race/ethnicity: in San Francisco, 16.2% of the population is Hispanic and 4.5% is non-Hispanic black, compared with 6.0% and 57.0%, respectively, in Baltimore City.14,15
We calibrated the model to data from San Francisco and Baltimore City for the years 2010 to 2017 and 2010 to 2016, respectively, based on the data available from each city. The model was calibrated to 5 sources of data:
i) City-level rates of gonorrhea diagnoses by age, sex, and race/ethnicity.
ii) City-level data from the sexually transmitted disease (STD) Surveillance Network (SSuN, described below),16 on the proportion of male diagnoses that are among MSM and the proportion of diagnoses that are symptomatic by sex and sexual orientation.
iii) National-level prevalence estimates for women by age and race/ethnicity from the National Health and Nutrition Examination Survey (NHANES).17 Local estimates of the distribution of prevalent gonococcal infections are not available in NHANES. Prevalence data pertaining to ages 15 to 17 years is part of NHANES restricted-use variables, and these data were accessed through the Research Data Center at National Center for Health Statistics.18 Prevalence for ages 18 to 39 years are available as public-use data. Use of NHANES data assumes that the burden and disparities observed in women at the national level are also represented at the local level.
iv) Prospective cohort studies to estimate a plausible range of gonorrhea incidence for MSM.19,20 Men who have sex with men are a key population in gonorrhea epidemiology, yet we have limited data to inform the model of the subpopulation. Based on available cohort studies we estimated a median incidence of 7.8% (95% range, 2–20%) per year.
v) National Survey of Family Growth (2011–2013 survey) was used to calibrate age-assortative mixing. National Survey of Family Growth provides national-level estimates, and in absence of local-level data, we assumed that the age mixing observed at the national level resemble local-level patterns.21
Using SSuN Data on Treatment Delays and Loss to Follow-up
The SSuN is an enhanced surveillance system, where a network of STD clinics collect data on key behavioral, biological, and clinical aspects related to STIs.16 The SSuN data from Baltimore, 2010 to 2017, were used to examine treatment delays and loss to follow-up among asymptomatic people with gonorrhea diagnosis by sex, race/ethnicity, and sexual orientation. After a diagnosis, there can be a delay in treatment initiation, or the patient can be lost to follow-up (LTFU) and not receive treatment at all.22,23 The outcomes were captured as time from sample collection to treatment record among those treated (delay in treatment initiation) and no record of treatment (assumed to be LTFU).
In Baltimore City, the average treatment delay for the years 2010 to 2017 was relatively short and differed little across subpopulations: among asymptomatic patients, the average delay was below 10 days (range, 0–118 days) for each sex and sexual orientation group. Therefore, we focused on analyzing the impact that could be achieved by reductions in LTFU. Among asymptomatic women and MSM, approximately 10% were missing treatment documentation. This varied between 3% and 14% by race/ethnicity. Among men who have sex with women (MSW), LTFU was approximately 20%, with variation between groups (17–27% between race/ethnicity groups). There was also variation in the total LTFU over time from 1.5% in 2011 to 17.9% in 2014. The Baltimore City Health Department does not routinely follow up on men for treatment verification but does for women, and it is unclear to what degree men's LTFU is overestimated in the data. We varied the proportion of diagnosed asymptomatic gonorrhea infections assumed to be LTFU in the base case model between 10% and 20% among the total modeled population.
Analysis
We calibrated the model using a Bayesian framework (Supplement 1, http://links.lww.com/OLQ/A440). Time-varying parameters were introduced from 2002 onward, and model predictions were calibrated to empirical data for the years 2010 to 2016 for San Francisco and 2010 to 2017 for Baltimore City. Time-varying parameters allowed for changes in screening rates (allowed to vary by sex, race/ethnicity, and sexual orientation), reporting probabilities for a diagnosed infection and transmission probabilities. As the base case models, we used the calibrated models of Baltimore and San Francisco and projected estimates 5 years after the calibration data period. For each setting, we simulated multiple epidemic trajectories using the parameter sets obtained during calibration. For each simulation, time-varying parameter values were held constant at the level estimated in the last calibration year in the simulation.
Interventions were implemented after the last calibration year using the same assumptions for both settings (Table 1). We examined a set of 5-year screening interventions, where a subpopulation is screened at constant coverage. The intervention scenarios assumed that coverage would not be reduced in any subpopulation from base case levels. For example, in the annual screening intervention, a subpopulation's screening frequency was only altered if it was less than once per year in the base case simulation.
TABLE 1.
Description of the Screening Interventions in the Analysis
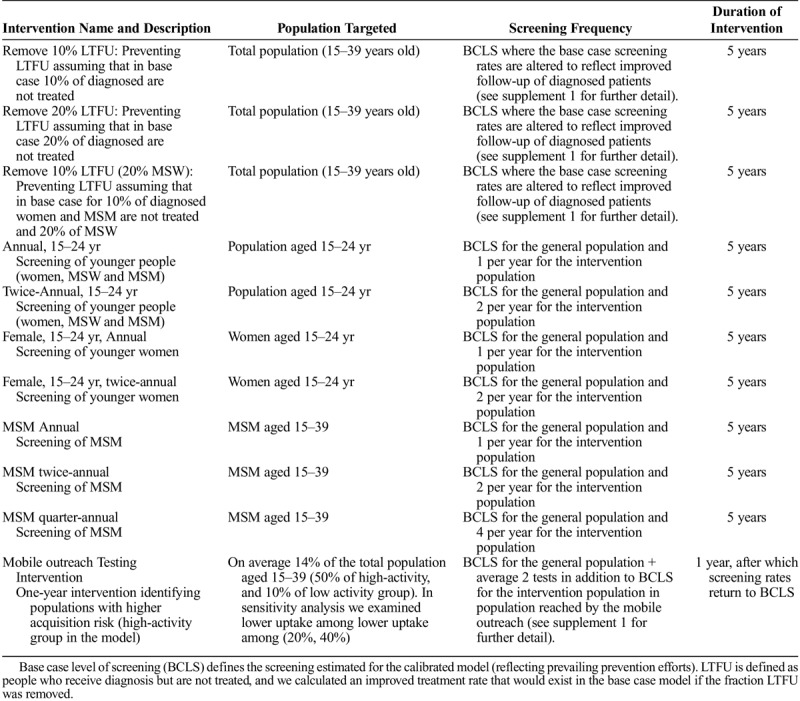
Improvements in patient retention in the care cascade (via reductions in LTFU) were assumed to produce a proportional increase in the number of individuals successfully completing treatment. Details on how this was operationalized are in Supplement 1. Finally, we examined the impact of a short-term screening intervention. In 2016, Baltimore City implemented urogenital and rectal gonorrhea screening as part of their mobile outreach testing program focusing on neighborhoods with high burden of HIV and STI (unpublished data). We modeled the impact of a similar intervention, assuming the program lasted 1 year, and it was able to target the high-activity population in the model. As it is likely that not all high-activity individuals would uptake screening, we assumed that, on average, 50% of all high-activity individuals and 10% of all low-activity individuals would be reached by the mobile outreach intervention (14% of the model population). We assumed the outreach screening is additive to the base case. We modeled an intervention with an average 2 tests increase to base case screening coverage for those reached by the intervention.
We performed 2 sensitivity analyses (Supplement 1, http://links.lww.com/OLQ/A440). To examine the population-level impact of increasing MSM screening, we varied screening frequency 1 to 5 times per year. We had assumed that the mobile outreach intervention would reach 50% of the cities' high-activity population. We had no data of the ability of the outreach screening to reach populations at higher acquisition risk. We analyzed 2 alternative scenarios where the same intervention coverage is maintained with 20% and 40% uptake among high-activity population and the remaining population screened are low-activity individuals.
Outcomes
Outcomes were calculated using 1000 simulations from the parameter posterior distributions. We estimated population-level prevalence trends, infections averted, additional tests needed and additional screening tests required to avert an infection relative to the base case. Fraction of infections averted was calculated as (i0 − ia) / i0, where i0 is the cumulative incidence in base case and ia is the respective estimate in the intervention scenario, and additional screening tests were calculated similarly. We estimated the number of additional screening tests required to avert an additional incident infection relative to base case as (sa − s0) / (i0 − ia), where sa is the number of cumulative screening tests in the intervention scenario, and s0 is the respective measure in the base case. For additional screening tests required to avert an infection, we calculated the point estimate as the ratio of mean additional tests and mean averted infections. The 95% credible intervals (CrI) were calculated as the 2.5th and 97.5th percentiles of the distribution of the ratios calculated for each simulation. For other outcomes, the estimates relative to the base case were calculated within each model simulation. Outcomes are presented as mean and 95% CrI or as Tukey box plots.
RESULTS
Model Fit and Base Case Estimates
Model fits to reported diagnosis rates by sex and age are shown in Figure 1. The model reproduced the main epidemiological features of both cities. Further model fits by race/ethnicity, sex, and age are in Supplement 2, http://links.lww.com/OLQ/A441 for Baltimore City and in Supplement 3, http://links.lww.com/OLQ/A441 for San Francisco. The overall gonorrhea prevalence on the last year calibration data were available was estimate mean 1.3% (95% CrI, 1.0–1.6%) in Baltimore City and 1.0% (95% CrI, 0.8–1.3%) in San Francisco. Model-estimated breakdown of new infections by subpopulation follows the distribution of observed gonorrhea diagnoses for each city reflecting the significance of observed gonorrhea diagnoses in the calibration of the transmission model (Supplement 1, Fig. S1, http://links.lww.com/OLQ/A440). To reproduce the increase in reported diagnosis rates observed for both cities, the model estimated changes in screening rates (Supplement 2, http://links.lww.com/OLQ/A441; and Supplement 3, http://links.lww.com/OLQ/A442, page 5) and modest increases in acquisition risk for both the MSM and heterosexual populations (Supplement 2, http://links.lww.com/OLQ/A441; and Supplement 3, http://links.lww.com/OLQ/A442, page 6).
Figure 1.
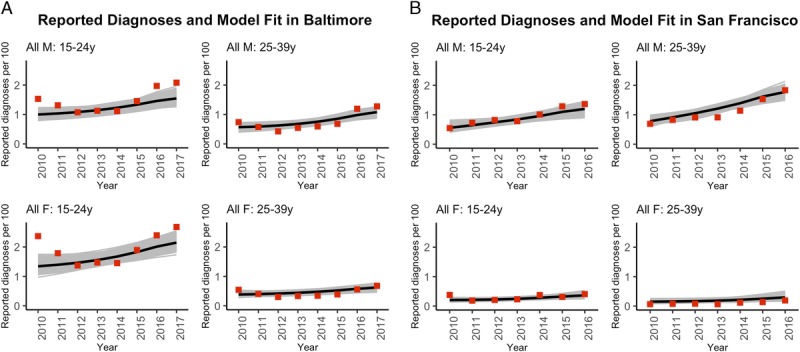
Model fit to overall gonorrhea diagnosis rate for (A) Baltimore City and (B) San Francisco. With men (M) on top row and women (F) on bottom row. Diagnosis rate data are presented as red squares, and model posterior simulations as mean (black line) and 100% range (gray lines). Note the different x-axes between plots. Further calibration results for both cities are in the Supplementary materials 2 and 3.
5-Year Screening Interventions
The intervention most effective in reducing population-level gonorrhea prevalence in Baltimore City was twice-annual screening of the 15- to 24-year-old population, and in San Francisco quarter-annual screening of MSM (Fig. 2). For Baltimore City, increasing testing in the younger age groups resulted in more infections averted than focusing on the MSM (Fig. 3).
Figure 2.
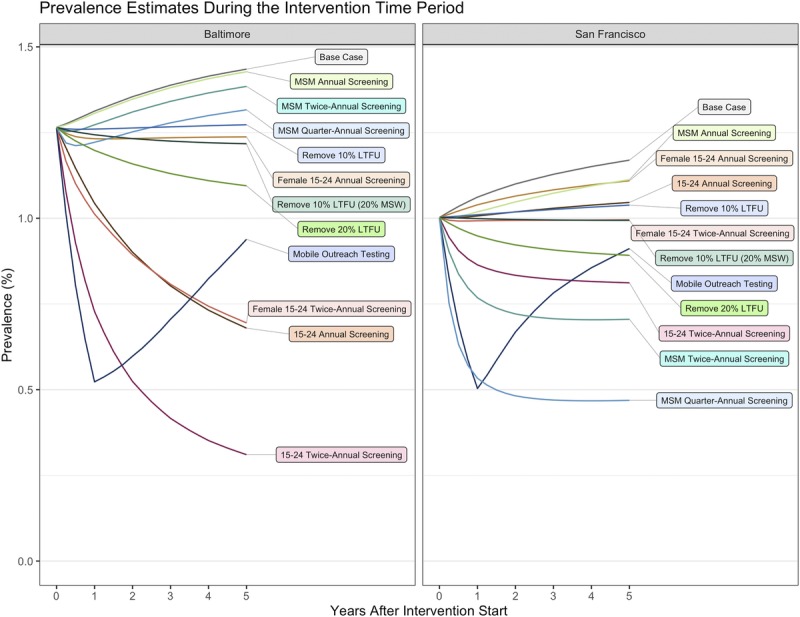
Population prevalence estimates per 100 persons during the intervention period, presented as the mean of the calibrated model (base case) and for the counterfactual interventions. Footnote: Mobile Outreach Testing (50% HR/high-activity, 10% LR/low-activity population scenario presented; see supplement 1, Fig. S4 for the sensitivity analysis results). Remove 10% LTFU: assume 10% LTFU for all asymptomatic, which is removed in the counterfactual; remove 20% LTFU: assume 20% LTFU for all asymptomatic, which is removed in the counterfactual; remove 10% LTFU (20% MSW): assume 10% LTFU for all MSM and women, and 20% for MSW.
Figure 3.
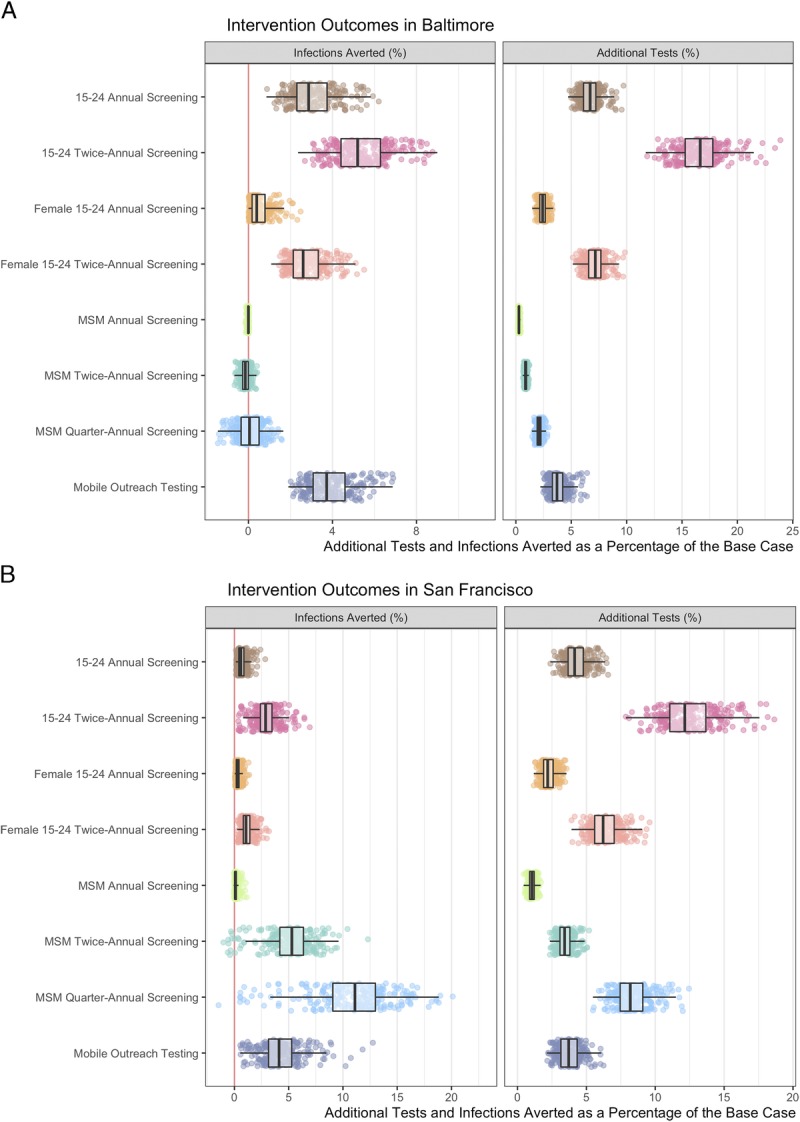
Cumulative infections averted and additional tests relative to the calibrated model (%) for the population in (A) Baltimore City and (B) San Francisco for the 5-year time period. The red vertical line at 0 defines a point at which the same number of incident infections occurred in base case than in the intervention. When less than 0, the 5-year incidence is higher in the intervention scenario than in the base case. Footnote: Mobile Outreach Testing (50% high-activity, 10% low-activity population scenario presented; see supplement 1, Fig. S4 for the sensitivity analysis results). Scatter plots present a sample of 250 model simulations to display the underlying distribution. Boxplots represent summary statistics for all 1000 simulations.
Twice-annual screening of the population aged 15 to 24 years averted the most infections with a mean of 5.4% (95% CrI, 3.1–8.2%) of infections averted requiring 16.6% (12.9–20.9%) additional tests compared with base case (Fig. 3; Supplement 1, Table S3 and S4, http://links.lww.com/OLQ/A440). Twice-annual screening of the population aged 15 to 24 years in San Francisco averted 2.9% (95% CrI, 0.6–5.5%) of incident infections with 12.5% (95% CrI, 9.4–16.8%) additional screening tests. In San Francisco, quarter-annual screening of all MSM resulted in the most infections averted. We estimated that it averted 10.8% (95% CrI, 1.2–17.8%) of infections, and it required 8.3% (95% CrI, 6.4–11.1%) additional tests.
In Baltimore City, annual screening of 15- to 24-year-old population required 17.9 (95% CrI, 11.8–31.4) screening tests per infection averted compared with 25.3 (95% CrI, 19.4–33.4) tests needed in the twice-annual screening (Table 2). In San Francisco, quarter-annual screening of MSM required 16.2 (95% CrI, 12.5–44.5) screening tests per infection averted. The MSM screening interventions had the largest uncertainty associated characterized by the negative results and broad credible intervals. Overall, there was asymmetry in the outcomes associated with additional tests per infection averted. In both cities, MSM were estimated to be screening at near-annual levels in the base case, and the incremental impact of annual screening is limited. We examined the relationship between screening frequency in MSM and its impact on population-level transmission dynamics (Supplement 1, Fig. S2 and Figure S3, http://links.lww.com/OLQ/A440). In San Francisco, with a sizeable MSM population, increasing screening frequency in MSM population increased the population-level benefits. In Baltimore City, with a smaller MSM population, the model estimated that large increases in screening MSM population would be needed to avert infections at population-level, whereas smaller increases in screening could increase incidence via rapid reinfection after treatment.
TABLE 2.
Model-Estimated Number of Additional Screening Tests Required to Avert One Additional Incident Infection Relative to the Base Case During the 5-Year Intervention Time Period
Impact of Removing LTFU
Improving retention of treatment cascade by removing LTFU was estimated to reduce gonorrhea prevalence from the base case (Fig. 2). It was also estimated to avert between 0.5% (95% CrI, 0.3–0.9%) and 1.1% (95% CrI, 0.6–1.8%) of the infections that occurred in the base case in Baltimore City and 1.0% (95% CrI, 0.1–1.7%) and 2.1% (95% CrI, 0.2–3.8%) in San Francisco (Fig. 4).
Figure 4.
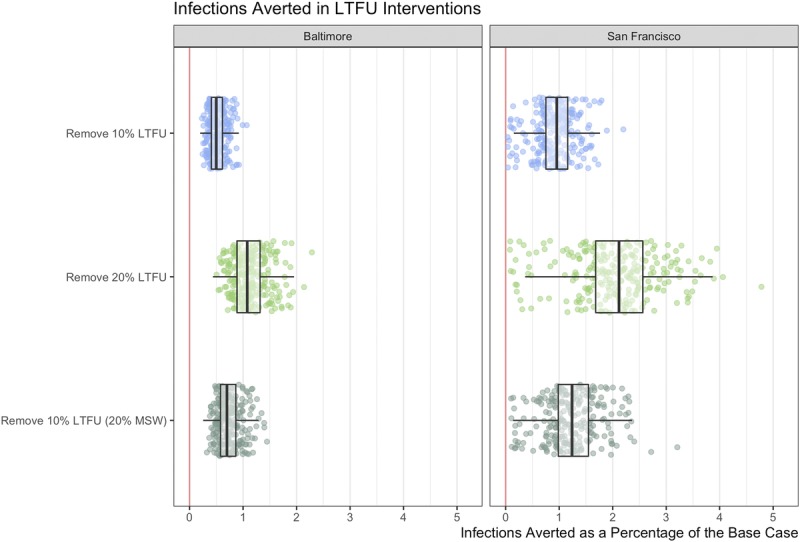
Cumulative infections averted and additional tests relative to the calibrated model (%) for population in Baltimore and in San Francisco for the 5-year period. For the LTFU scenarios, infections are averted through better follow-up of diagnosed patients, by adjusting the treatment rate of the base case model. Footnote: Remove 10% LTFU: assume 10% LTFU for all asymptomatic, which is removed in the counterfactual; Remove 20% LTFU: assume 20% LTFU for all asymptomatic, which is removed in the counterfactual; Remove 10% LTFU (20% MSW) assume 10% LTFU for all MSM and women, and 20% for MSW, which is removed in the counterfactual. Scatter plots present a sample of 250 model simulations to display the underlying distribution. Boxplots represent summary statistics for all 1000 simulations.
Mobile Outreach Testing
The 1-year mobile outreach testing intervention focused on increasing screening in high-activity populations (model subgroup with the highest partner change rate). It was estimated to temporarily reduce population prevalence, which rebounded after the intervention ended. Five years later, the estimated prevalence was still lower than in the base case. The results were sensitive to the assumptions of what proportion of the cities high-activity population would uptake additional screening (Supplement 1, Fig. S4 and Figure S5, http://links.lww.com/OLQ/A440). When assuming that 50% of all the high-activity population in the city were reached, we estimated the outreach screening to avert 3.9% (95% CrI, 2.3–6.3%) and 4.3% (95% CrI, 0.9–8.7%) of infections in Baltimore City and San Francisco, respectively. If only 20% of high-activity population were reached, the outreach screening was estimated to avert 2.6% (95% CrI, 1.4–4.5%) and 2.7% (95% CrI, 0.6–6.0%), respectively. The outreach intervention required 3.8% (95% CrI, 2.6–5.8%) and 3.8% (95% CrI, 2.4–5.8%) additional screening tests in Baltimore City and San Francisco. Although the mobile outreach testing resulted in similar levels of infections averted relative to the cities' base case, fewer screening tests were required to avert an infection in Baltimore. In Baltimore, when 20% or 50% screening uptake among high-activity were assumed, between 8.1 (95% CrI, 5.5–12.9) and 11.9 (95% CrI, 7.4–20.0) tests were required per infection averted compared with San Francisco where between 18.4 (95% CrI, 9.8–37.1) and 28.8 (95% CrI, 14.7–59.5) were required per infection averted (Table 2).
DISCUSSION
We reproduced the observed epidemiological trends in Baltimore City and San Francisco using local surveillance data. In Baltimore, screening the 15- to 24-year-old population annually or twice-annually was associated with the largest gains. Although twice-annual screening was associated with the largest prevalence decline and most infections averted, annual screening required fewer screening tests per infection averted. In San Francisco, increasing screening in MSM population had the largest population-level impact. Quarter-annual screening of all sexually active MSM had the largest gains at the population level. This aligns with the recommendation for pre-exposure prophylaxis (PrEP) users and people with multiple sex partners.24,25
The reference point used were the calibrated base case models, which estimated high levels of screening in the most at-risk populations. The analyses illustrate the challenges in achieving additional gains in the presence of prevention efforts. Preventing LTFU among those diagnosed with gonorrhea will allow for improved gonorrhea control with no increase in screening coverage, but it requires additional efforts from service providers and public health departments. Assessing the demographic characteristics of the patients with highest risk of LTFU and identifying providers where LTFU and long treatment delays are more likely to occur could improve patient treatment cascade. Short-term interventions, such as the modeled mobile outreach testing intervention, could provide meaningful gains, if they are truly focused toward those with the highest gonorrhea acquisition risk and achieve a high uptake. We can identify the high-activity populations in the model, but there is little empirical data on how well different screening strategies identify persons at the highest risk. We implemented the mobile outreach intervention in a simplistic manner, assuming high screening coverage among the high-activity populations in the city, and we are likely overestimating the impact of the intervention.
In San Francisco, MSM continue to be a large at-risk population, whereas in Baltimore non-Hispanic black heterosexuals carry the largest burden of gonorrhea. Reflecting an evidence gap, the model had more stratification of the heterosexual population than of the MSM population, which allowed for a more detailed description of the Baltimore epidemic than of the San Francisco epidemic. Modeling HIV status and race/ethnicity would provide a better representation of the MSM population. PrEP uptake has increased in San Francisco since 2014.25 Screening rates in the MSM population are impacted by PrEP use,25 and they likely remain highly variable among MSM. We did not explicitly model site-specific infections, and our outcome measures, relative to base case model, assume there is an increase in screening coverage, but the number of sites screened remains unchanged. More data on risk behaviors, screening practices, and site-specific infection would allow for further refinement of the model, and individual-based model would facilitate a more detailed examination of screening, particularly when site of infection is of interest.
The absence of data on screening uptake in the MSM population as a whole limited the extent to which we could make an inference about screening within this population. Data are also limited on screening uptake among heterosexual population, and differences by race/ethnicity and age are poorly understood. Although we modeled risk heterogeneity by sex, age, race/ethnicity, sexual orientation, and levels of partner change rate, this represents a simplification of the true risk heterogeneity present in a population. There are limited data on the underlying burden of infection among the population at large and for the subpopulations studied. Having local prevalence estimates would allow us to analyze disparities and levels of infection in greater detail. The estimates could be used directly for service targeting and would provide data inputs for modeling analyses.
We did not consider antimicrobial resistance (AMR) in the model framework.26 Increased detection and treatment will increase exposure to antimicrobials, which could accelerate the emergence of AMR.27 However, continued screening is likely maintaining gonorrhea at lower levels and continues to be the main mode of gonorrhea control. Uncertainty in the future of gonorrhea prevention was a reason to model only short-term interventions. Future research on point-of-care testing of STIs and rapid antimicrobial susceptibility testing could strengthen the preventative arsenal. In the future, it may be possible to combine novel technologies such as point-of-care testing of AMR with screening for more effective surveillance and gonorrhea control.28,29 Implementing novel technologies, or old technologies in novel ways, will benefit from a better understanding of the epidemiology of the infection. Even absent improved diagnostics, these results suggest that an understanding of local epidemiology is necessary to prioritize potential strategies for gonorrhea control.
Supplementary Material
Footnotes
Acknowledgments: The authors thank Trang Nguyen and Robert Kohn for providing SSuN data for San Francisco and for their feedback on the manuscript. The computations in this paper were run on the Odyssey cluster supported by the FAS Division of Science, Research Computing Group at Harvard University.
Conflict of Interest: None declared.
Sources of Funding: This work was supported by the U.S. Centers for Disease Control and Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement (5NU38PS004644).
STD Surveillance Network (SSuN) activities are supported by the U.S. Centers for Disease Control and Prevention, Division of STD Prevention (PS08-865 and PS13-1306).
Minttu M. Rönn and Christian Testa contributed equally.
Previous presentation: Poster at the 2018 National STD Prevention Conference in Washington, DC, August 27-30, 2018.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
REFERENCES
- 1.Centers for Disease Control and Prevention. Sexually transmitted diseases surveillance 2017. 2018. Available from: https://www.cdc.gov/std/stats17/default.htm. Accessed February 25, 2019.
- 2.Grad YH, Goldstein E, Lipsitch M, et al. Improving control of antibiotic-resistant gonorrhea by integrating research agendas across disciplines: Key questions arising from mathematical modeling. J Infect Dis 2016; 213:883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornelisse VJ, Chow EP, Huffam S, et al. Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing. Sex Transm Dis 2017; 44:114–117. [DOI] [PubMed] [Google Scholar]
- 4.Patton ME, Kidd S, Llata E, et al. Extragenital gonorrhea and chlamydia testing and infection among men who have sex with men—STD surveillance network, United States, 2010-2012. Clin Infect Dis 2014; 58:1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabecinha M, Mercer CH, Gravningen K, et al. Finding sexual partners online: Prevalence and associations with sexual behaviour, STI diagnoses and other sexual health outcomes in the British population. Sex Transm Infect 2017; 93:572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holt M, Lea T, Mao L, et al. Community-level changes in condom use and uptake of HIV pre-exposure prophylaxis by gay and bisexual men in Melbourne and Sydney, Australia: Results of repeated behavioural surveillance in 2013-17. Lancet HIV 2018; 5:e448–e456. [DOI] [PubMed] [Google Scholar]
- 7.Chesson HW, Kent CK, Owusu-Edusei K, Jr., et al. Disparities in sexually transmitted disease rates across the ‘eight Americas’. Sex Transm Dis 2012; 39:458–464. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2016. Sexually transmitted diseases surveillance. Available from: https://www.cdc.gov/std/stats16/default.htm. Accessed November 16, 2017.
- 9.Baltimore City Health Department. HIV/STD Data &Resources. Available from: https://health.baltimorecity.gov/hivstd-data-resources. Accessed March 29, 2019.
- 10.U.S.Preventive Services Task Force. Screening for chlamydia and gonorrhea : Recommendation statement. Am Fam Physician 2015; 91:1–4. [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2015. STD treatment guidelines: STD screening recommendations. Available from: https://www.cdc.gov/std/tg2015/screening-recommendations.htm. Accessed October 27, 2019.
- 12.Tuite AR, Rönn MM, Wolf EE, et al. Estimated impact of screening on gonorrhea epidemiology in the United States: Insights from a mathematical model. Sex Transm Dis 2018; 45:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grey JA, Bernstein KT, Sullivan PS, et al. Estimating the population sizes of men who have sex with men in US states and counties using data from the American community survey. JMIR Public Health Surveill 2016; 2:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baltimore City Health Department. Available from: https://health.baltimorecity.gov/. Accessed May 5, 2019.
- 15.San Francisco Department of Public Health. Available from: https://www.sfdph.org/dph/default.asp. Accessed May 5, 2019.
- 16.Centers for Disease Control and Prevention. STD Surveillance Network (SSuN). Available from: https://www.cdc.gov/std/ssun/default.htm Accessed February 25, 2019.
- 17.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and nutrition and examination survey data. Available from: https://wwwn.cdc.gov/nchs/nhanes/default.aspx. Accessed May 5, 2019.
- 18.National Center for Health Statistics. Research data center. Available from: https://www.cdc.gov/rdc/index.htm. Accessed April 5, 2019.
- 19.Kelley CF, Vaughan AS, Luisi N, et al. The effect of high rates of bacterial sexually transmitted infections on HIV incidence in a cohort of black and white men who have sex with men in Atlanta, Georgia. AIDS Res Hum Retroviruses 2015; 31:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris SR, Klausner JD, Buchbinder SP, et al. Prevalence and incidence of pharyngeal gonorrhea in a longitudinal sample of men who have sex with men: The EXPLORE study. Clin Infect Dis 2006; 43:1284–1289. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Survey of family growth.
- 22.Wong D, Berman SM, Furness BW, et al. Time to treatment for women with chlamydial or gonococcal infections: A comparative evaluation of sexually transmitted disease clinics in 3 US cities. Sex Transm Dis 2005; 32:194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwebke JR, Sadler R, Sutton JM, et al. Positive screening tests for gonorrhea and chlamydial infection fail to lead consistently to treatment of patients attending a sexually transmitted disease clinic. Sex Transm Dis 1997; 24:181–184. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Pre-exposure prophylaxis (PrEP). Available from: https://www.cdc.gov/hiv/risk/prep/index.html. Accessed May 26, 2019.
- 25.San Francisco Department of Public Health. The HIV epidemiology annual report 2016. Available from: https://www.sfdph.org/dph/files/reports/RptsHIVAIDS/Annual-Report-2016-20170831.pdf. Accessed March 1, 2019.
- 26.Melendez J, Hardick J, Barnes M, et al. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates in Baltimore, Maryland, 2016: The importance of sentinel surveillance in the era of multi-drug-resistant gonorrhea. Antibiotics 2018; 7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fingerhuth SM, Bonhoeffer S, Low N, et al. Antibiotic-resistant Neisseria gonorrhoeae spread faster with more treatment, not more sexual partners. PLoS Pathog 2016; 12:e1005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuite AR, Gift TL, Chesson HW, et al. Impact of rapid susceptibility testing and antibiotic selection strategy on the emergence and spread of antibiotic resistance in gonorrhea. J Infect Dis 2017; 216:1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rönn MM, Menzies NA, Gift TL, et al. Potential for point-of-care tests to reduce chlamydia-associated burden in the United States: A mathematical modeling analysis. Clin Infect Dis 2019: Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


