Abstract
Background:
Recent studies have reported the prevalence of cardiovascular diseases (CVDs) among cancer patients following the use of the vascular endothelial growth factor (VEGF) signaling inhibitors. However, data for patients with a history of cancer before active cancer treatment are lacking. This study aims to investigate the distribution of CVD-related comorbidities before cancer treatment in potential VEGF antagonists candidates.
Methods:
A total of 22 500 newly diagnosed cancer patients registered from 1 January 2011 to 31 December 2017 were included. Cancer patients with colorectal cancer (CRC), renal cell carcinoma (RCC), thyroid cancer, hepatocellular carcinoma (HCC), and lung cancer were selected.
Results:
Hypertension (HTN), coronary heart diseases, atrial fibrillation, and heart failure were top CVD comorbidities among studied cancers. HTN was the most prevalent CVD (26.0%). The prevalence of HTN in RCC, CRC (33.5 and 29.4% respectively) was significantly higher than that in HCC, lung cancer, and thyroid cancer patients (25.1, 24.5, and 23.1%, respectively). Among cancer patients with HTN, the majority of cancer patients fall in grade III (75.7%) and very high cardiovascular risk level (85.4%). Out of the 5847 HTN patients, 26% were not in antihypertensive use, and 34.2% failed to achieve the target blood pressure.
Conclusion:
Cancer patients carry a high burden of CVD-related comorbidities before the application of VEGF antagonists. HTN is the most prevalent comorbid condition, and cancer patients with HTN constitute substantial cardiovascular risks and a higher co-prevalence of other CVDs.
Keywords: cancer, cardiovascular diseases, hypertension, vascular endothelial growth factor inhibitors
INTRODUCTION
In recent years, an increasing number of cancer survivors with cardiovascular comorbidities are emerging, mainly attributed to the advances in treatment [1]. For instance, drugs that block the vascular endothelial growth factor (VEGF) signaling pathway (VSP) have expanded the therapeutic options for several solid tumor cancers, such as metastatic colorectal cancer, nonsmall cell lung cancer, renal cell carcinoma, thyroid cancer, and hepatocellular carcinoma [2–7]. However, among cancer survivors treated with VSP inhibitors, increasing evidence demonstrates that cardiovascular (CV) diseases (CVDs) have become a growing concern leading to premature morbidity and mortality. Evidence showed that 25–66% of VSP-treated fatal events in cancer patients occur with vascular diseases, especially including hypertension (HTN), arterial thromboembolism, and myocardial infarction [8]. As such, a careful assessment of risk factors for cardiovascular events is necessary for patients before receiving antiangiogenic therapies.
Currently, there are two major classes of VEGF inhibitors in clinical practice (monoclonal VEGF antibodies and small molecule VEGF receptor tyrosine kinase inhibitors). Both classes carry a different list of indications for different solid tumors and their application is associated with an increased incidence of HTN during administration [9]. Previous studies reported the prevalence or incidence rate of CVD following VEGF antagonists or any anticancer therapy. However, no study reported the prevalence of the common comorbid cardiovascular conditions in cancer patients who are either ready for the VEGF antagonist use or potentially eligible for VEGF use because of their clinical conditions. Therefore, this study aims to investigate the distribution of CVD-related comorbidities before the VEGF antagonists application in the cancer-affected population.
MATERIALS AND METHODS
Study design and participants
This cross-sectional study was based on data from the Electronic Medical Record Research Database (EMRRD) of the first affiliated hospital of Dalian Medical University (FAHDM). The EMRRD is developed to establish a computerized clinical database, and the clinical records are updated continuously. A total of 24 487 histologically confirmed cancer patients, who were hospitalized at FAHDM between 1 January 2011 and 31 December 2017 were retrieved for this study. Inclusion criteria include age above 18 years, free of any previous use of anticancer therapy, and potentially be treated with VEGF antagonist. After excluding patients who were not candidates for VEGF antagonist use (whether during their first visit or potentially in the near future), had a history of any anticancer therapy, and/or had data errors, 22 500 patients were included in the analysis (Fig. 1). FAHDM approved this study, and all patients were informed about their participation and provided their consent to participate in the present study.
FIGURE 1.
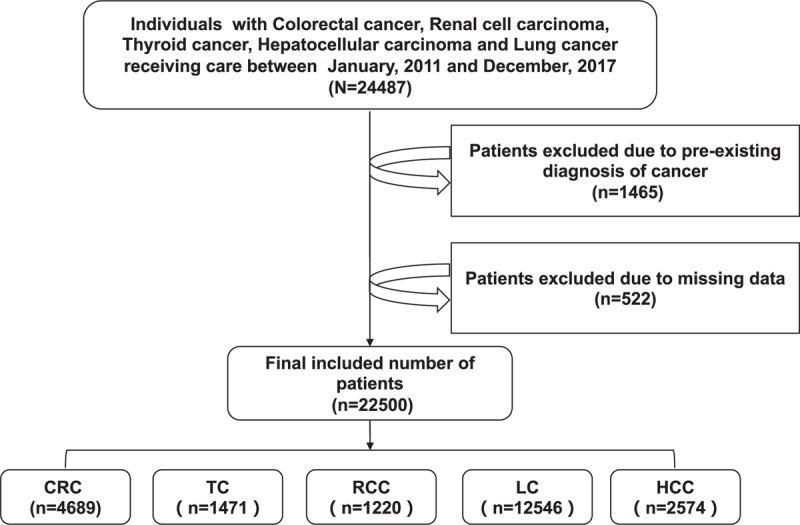
Flow chart of study population. CRC, colorectal cancer; HCC, hepatocellular carcinoma; LC, lung cancer; RCC, renal cell carcinoma; TC, thyroid cancer.
Tumor site selection
Choice of tumor sites was based on the criteria for VEGF antagonists use or potential eligibility for the VEGF antagonists use in the future. The VEGF antagonists are prescribed for advanced cancer and known to be useful for different solid tumors including, colorectal cancer, advanced renal cell carcinoma, symptomatic, progressive or unresectable medullary thyroid cancer, unresectable hepatocellular carcinoma, nonsmall cell lung cancer [2–7]. Therefore, histologically confirmed colorectal cancer (CRC), renal cell carcinoma (RCC), thyroid cancer, hepatocellular carcinoma (HCC) and lung cancer were included for this study.
Data collection and covariates
Presence of comorbid CVD conditions was assessed using all available data at the cancer diagnosis. The researcher focused primarily on medical records. Trained health professionals examined the hospital medical records, and they retrieved information related to demographic characteristics of patients, health problems (past and current medical conditions), comorbid conditions, and lifestyle-related data, such as alcohol use, cigarette smoking. A patient was considered to have HTN if a SBP at least 140 mmHg, mean DBP at least 90 mmHg, and/or current use of an antihypertensive was present in their medical history [10]. Whereas the grade and cardiovascular risk stratification of HTN were defined based on 2018 Chinese Guidelines for Prevention and Treatment of Hypertension [11]. The three grades of HTN were defined as follows: grade 1 (SBP 140–159 mmHg; DBP 90–99 mmHg), grade 2 (SBP 160–179 mmHg; DBP 100–109 mmHg) and grade 3 (SBP ≥180 mmHg; DBP ≥110 mmHg). The cardiovascular risk stratification of HTN was categorized into four levels including low risk, moderate risk, high risk, and very high risk. Coronary heart disease (CHD) was defined based on the presence of either angina or history of heart attack evidenced by medical records. Dyslipidemia was defined as total cholesterol at least 240 mg/dl, low-density lipoprotein at least 160 mg/dl, high-density lipoprotein less than 40 mg/dl, or use of lipid-lowering drugs [12]. Diabetes mellitus was defined as fasting plasma glucose at least 126 mg/dl or treatment with insulin or oral hypoglycemic medication [13]. Smoking was defined as current smoking status or a lifetime consumption of more than 100 cigarettes.
Statistical method
Continuous variables were expressed using the mean ± SD, and categorical data were presented using frequency and percentage. Statistical significance of differences for categorical variables was tested using chi-square. Comparison between continuous data for two independent groups between the HTN and without HTN groups was conducted using the independent sample t-test. One-way ANOVA was used to compare the difference between three or more groups. Age and sex-adjusted binary logistic analysis was employed to examine the associations between HTN and the risk factors of CVD among different studied cancers. A two-sided P-value of less than 0.05 was considered statistically significant. All analyses were performed using SPSS software version 24.0 (SPSS, Chicago, Illinois, USA).
RESULTS
Prevalence and distribution of comorbid cardiovascular conditions for different tumor types
Table 1 summarizes the baseline demographic characteristics of the study. Of the 22 500 patients included, women (43%) were slightly lower than men, and the mean age of the participants was 67 ± 12 years. Cancer patients bear a high burden of CVD-related comorbidities. When examining all cancer patients, HTN was the most prevalent cardiovascular comorbid condition (26.0%). Other comorbid conditions include CHD (7.8%), HF (3.3%), and atrial fibrillation (4.2%). The rates of the above comorbid conditions vary among the studied cancers. As shown in Fig. 2, the prevalence of HTN in RCC and CRC was significantly higher(33.5 and 29.4%, respectively) than the prevalence of HTN in thyroid cancer, lung cancer, and HCC (23.1, 24.5, and 25.1%, respectively).
TABLE 1.
Baseline characteristics of the participants
| Variables | Total (22 500) | CRC (4689) | TC (1471) | RCC (1220) | LC (12 546) | HCC (2574) | P value |
| Age (years) | 66.9 ± 12.2 | 70.3 ± 12.1 | 55.6 ± 13.9 | 65.3 ± 12.7 | 67.6 ± 11.4 | 64.9 ± 11.0 | <0.001 |
| Female [(n) (%)] | 9724 (43.2) | 1882 (40.1) | 1125 (76.5) | 380 (31.1) | 5768 (46.0) | 569 (22.1) | <0.001 |
| HTN [n (%)] | 5847 (26.0) | 1380 (29.4) | 340 (23.1) | 409 (33.5) | 3072 (24.5) | 646 (25.1) | <0.001 |
| SBP (mmHg) | 127.2 ± 12.5 | 127.3 ± 11.9 | 124.3 ± 12.3 | 130.4 ± 11.9 | 127.6 ± 12.7 | 125.5 ± 12.6 | <0.001 |
| DBP (mmHg) | 77.7 ± 7.5 | 77.2 ± 6.4 | 77.3 ± 7.1 | 79.4 ± 6.8 | 77.9 ± 7.9 | 77.2 ± 7.6 | <0.001 |
| CHD [n (%)] | 1762 (7.8) | 411 (8.8) | 71 (4.8) | 115 (9.4) | 1043 (8.3) | 112 (4.7) | <0.001 |
| HF [n (%)] | 732 (3.3) | 175 (3.7) | 20 (1.4) | 37 (3.0) | 452 (3.6) | 48 (2.0) | <0.001 |
| AF [n (%)] | 950 (4.2) | 265 (5.7) | 28 (1.9) | 50 (4.1) | 530 (4.2) | 77 (3.0) | <0.001 |
| TC (mg/dl) | 191.0 ± 49.0 | 192.0 ± 48.7 | 201.1 ± 46.1 | 190.0 ± 48.6 | 193.9 ± 47.5 | 169.6 ± 53.5 | <0.001 |
| TG (mg/dl) | 138.9 ± 102.4 | 142.9 ± 108.7 | 155.8 ± 118.0 | 162.5 ± 138.0 | 138.9 ± 96.7 | 110.7 ± 79.6 | <0.001 |
| LDL-C (mg/dl) | 112.6 ± 33.9 | 112.9 ± 34.2 | 116.9 ± 31.0 | 111.9 ± 31.7 | 114.3 ± 32.8 | 100.9 ± 38.5 | <0.001 |
| HDL-C (mg/dl) | 43.3. ± 12.6 | 42.9. ± 12.2 | 46.6 ± 12.6 | 41.2 ± 11.3 | 44.4 ± 12.1 | 37.6 ± 14.4 | <0.001 |
| CV risk factors [n (%)] | |||||||
| Dyslipidemia | 5856 (52.6) | 1375 (53.9) | 314 (48.2) | 310 (56.1) | 3072 (49.7) | 785 (65.8) | <0.001 |
| TCh ≥240 mg/dl | 1574 (14.1) | 378 (14.8) | 122 (18.7) | 73 (13.2) | 893 (14.5) | 108 (9.0) | <0.001 |
| LDL-C ≥160 mg/dl | 876 (7.9) | 195 (7.6) | 59 (9.1) | 41 (7.4) | 499 (8.1) | 82 (6.9) | 0.458 |
| HDL-C ≤40 mg/dl | 4585 (41.2) | 1086 (42.5) | 211 (32.4) | 260 (46.9) | 2319 (37.5) | 709 (59.4) | <0.001 |
| Current smoking | 5687 (25.6) | 853 (18.6) | 99 (6.9) | 252 (21.0) | 3580 (28.8) | 903 (35.9) | <0.001 |
| Alcohol consumption | 3243 (15.0) | 587 (13.0) | 64 (4.5) | 157 (13.4) | 1731 (14.4) | 704 (28.6) | <0.001 |
| DM | 3247 (14.4) | 827 (17.6) | 151 (10.3) | 199 (16.3) | 1584 (12.6) | 486 (18.9) | <0.001 |
Continuous variables were expressed using the mean ± SD, and categorical data were presented using frequency and percentage. P values are derived from one-way ANOVA for continuous variables and χ2 for categorical variables among different cancer sites. AF, atrial fibrillation; CHD, coronary heart disease; CRC, colorectal cancer; CV, cardiovascular; DM, diabetes mellitus; HCC, hepatocellular carcinoma; HDL-C, high-density lipoprotein cholesterol; HF, heart failure; HTN, hypertension; LC, lung cancer; LDL-C, low-density lipoprotein cholesterol; RCC, renal cell carcinoma; TC, thyroid cancer; TCh, total cholesterol; TG, triglycerides.
FIGURE 2.
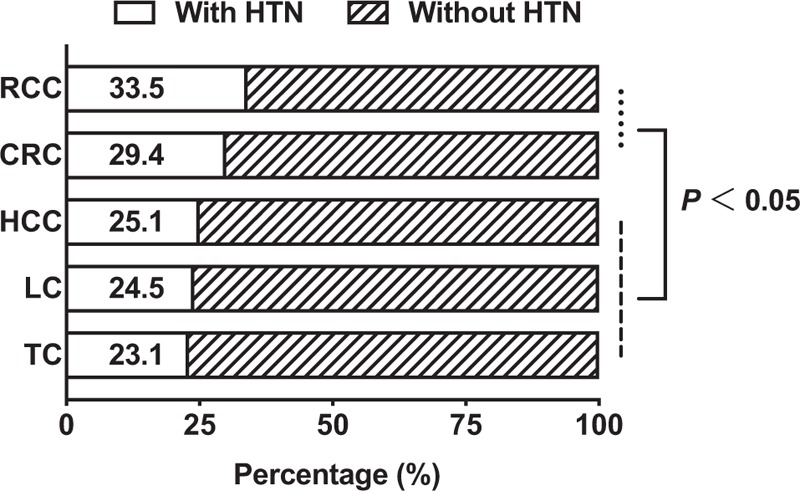
Distribution of hypertension among different cancer patients. CRC, colorectal cancer; HCC, hepatocellular carcinoma; HTN, hypertension; LC, lung cancer; RCC, renal cell carcinoma; TC, thyroid cancer.
The prevalence of hypertension according to different grades and cardiovascular risk stratifications
When examining HTN prevalence among the cancer patients based on the grades and stratifications of cardiovascular risk, we found that the majority of cancer with HTN cases fall at the higher grades, and higher cardiovascular risks as well. In this study, cancer patients with grade III hypertension account 75.7% out of the total hypertension cases. Whereas, cancer patients diagnosed with grade I and II HTN accounts for 3.9 and 20.4%, respectively (Table 2, Fig. 3). Similar findings were observed following the evaluation of cancer patients based on the levels of cardiovascular risk stratification of HTN. Out of the total cancer patients with HTN, the prevalence of very high-risk level was found to be 85.4%. However, high risk, moderate risk, and low risk present a smaller percentage of hypertension prevalence (10.2, 3.6, and 0.8, respectively). This trend was similar for all the studied cancers. The proportion of very high cardiovascular risk level in RCC was the highest than other cancers (88.6%).
TABLE 2.
The prevalence of hypertension based on grades and cardiovascular risk stratifications among cancer patients
| Total | CRC | TC | RCC | LC | HCC | |
| HTN (n) | 5847 | 1380 | 340 | 409 | 3072 | 646 |
| Grade classification [n (%)] | ||||||
| Grade I | 184 (3.9) | 38 (3.6) | 21 (7.7) | 7 (2.4) | 99 (3.8) | 19 (3.9) |
| Grade II | 955 (20.4) | 228 (21.8) | 46 (16.9) | 65 (22.6) | 521 (20.2) | 95 (19.4) |
| Grade III | 3541 (75.7) | 779 (74.5) | 205 (75.4) | 216 (75) | 1965 (76) | 376 (76.7) |
| Cardiovascular risk stratification [n (%)] | ||||||
| Low risk | 35 (0.8) | 9 (0.9) | 5 (2.0) | 1 (0.4) | 18 (0.7) | 2 (0.4) |
| Moderate risk | 157 (3.6) | 35 (3.6) | 11 (4.5) | 5 (1.9) | 90 (3.7) | 16 (3.6) |
| High risk | 445 (10.2) | 105 (10.9) | 27 (11) | 25 (9.3) | 247 (10.2) | 41 (8.9) |
| Very high risk | 3729 (85.4) | 818 (84.6) | 203 (82.5) | 239 (88.6) | 2066 (85.3) | 403 (87.2) |
Data were presented using frequency and percentage. CRC, colorectal cancer; HCC, hepatocellular carcinoma; HTN, hypertension; LC, lung cancer; RCC, renal cell carcinoma; TC, thyroid cancer.
FIGURE 3.
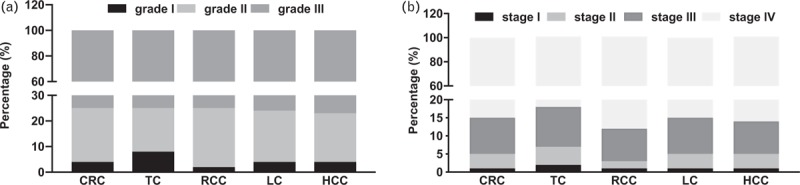
The prevalence of hypertension according to different grades (a) and cardiovascular risk stratifications (b). CRC, colorectal cancer; HCC, hepatocellular carcinoma; LC, lung cancer; RCC, renal cell carcinoma; TC, thyroid cancer.
Co-prevalence of hypertension with other cardiovascular diseases or risk factors
As shown in Table 3, the burden of HTN tend to increase in advanced age groups (72.3 ± 10.6 vs. 65.1 ± 12.2, P < 0.001) and was higher in women (45.7 vs. 42.4%, P < 0.001). Also, cancer patients with HTN had higher proportion of cardiovascular comorbidities, such as CHD (21.3 vs. 3.1%, P < 0.001), HF (8 vs 1.6%, P < 0.001), and atrial fibrillation (9.7 vs. 2.3%, P < 0.001). When examining co-prevalence by CVD risk factors, the cancer patients with HTN had a higher proportion of dyslipidemia (56.6 vs. 50.5%, P < 0.001), diabetes mellitus (32.8 vs. 8%, P < 0.001), and hyperuricemia (51.2 vs. 36.6%, P < 0.001). Also the mean values of creatinine (103.7 ± 126.4 vs. 78 ± 61.3, P < 0.001) was higher in cancer patients with HTN. However, there was no statistically significant difference in the proportion of smokers between the hypertensive and nonhypertensive cancer patients (25.4 vs. 25.7%, P = 0.596).
TABLE 3.
Comparison of cardiovascular disease and cardiovascular risk factors between hypertension and without hypertension groups
| Total (22 500) | CRC (4689) | TC (1471) | RCC (1220) | LC (12 546) | HCC (2574) | |||||||
| Variables | HTN (5847) | Non-HTN (16 653) | HTN (1380) | Non-HTN (3309) | HTN (340) | Non-HTN (1131) | HTN (409) | Non-HTN (811) | HTN (3072) | Non-HTN (9474) | HTN (646) | Non-HTN (1928) |
| Age (years) | 72.3 ± 10.6 | 65.1 ± 12.2* | 75.6 ± 9.7 | 68.1 ± 12.4§ | 65.0 ± 10.8 | 52.8 ± 13.5‡ | 70.2 ± 11.2 | 62.9 ± 12.6& | 72.4 ± 10.2 | 66.0 ± 11.3£ | 69.7 ± 10.4 | 63.3 ± 10.7† |
| Female [n (%)] | 2670 (45.7) | 7054 (42.4)* | 623 (45.1) | 1259 (38.0) § | 253 (74.4) | 872 (77.1) | 149 (36.4) | 231 (28.5) & | 1477 (48.1) | 4291 (45.3) £ | 168 (26.0) | 401 (20.8) † |
| CHD [n (%)] | 1243 (21.3) | 519 (3.1)* | 300 (21.7) | 111 (3.4) § | 52 (15.3) | 19 (1.7) ‡ | 88 (21.5) | 27 (3.3) & | 707 (23.0) | 336 (3.5) £ | 96 (14.9) | 26 (1.3) † |
| HF [n (%)] | 465 (8.0) | 267 (1.6)* | 121 (8.8) | 54 (1.6) § | 14 (4.1) | 6 (0.5) ‡ | 32 (7.8) | 5 (0.6) & | 269 (8.8) | 183 (1.9) £ | 29 (4.5) | 19 (1.0) † |
| AF [n (%)] | 565 (9.7) | 385 (2.3)* | 171 (12.4) | 94 (2.8) § | 22 (6.5) | 6 (0.5) ‡ | 35 (8.6) | 15 (1.8) & | 290 (9.4) | 240 (2.5) £ | 47 (7.3) | 30 (1.6) † |
| CV risk factors [i (%)] | ||||||||||||
| Dyslipidemia [n (%)] | 2224 (56.6) | 3632 (50.5)* | 524 (57.1) | 851 (52.1) § | 138 (58.5) | 176 (42.4) ‡ | 167 (63.7) | 143 (49.1) & | 1134 (53.5) | 1938 (47.8) £ | 261 (66.2) | 524 (65.6) |
| LDL (mg/dl) | 113.3 ± 33.9 | 112.2 ± 33.9 | 111.3 ± 33.5 | 113.9 ± 34.7 | 117.4 ± 30.8 | 116.5 ± 31.2 | 114.9 ± 32.9 | 109.1 ± 30.3& | 115.3 ± 33.6 | 113.8 ± 32.3 | 104.0 ± 36.9 | 99.3 ± 39.3† |
| HDL (mg/dl) | 42.0 ± 12.2 | 44.0 ± 12.8* | 41.2 ± 11.9 | 43.8 ± 12.3§ | 44.1 ± 12.2 | 48.0 ± 12.6‡ | 40.1 ± 11.5 | 42.2 ± 11.1& | 43.2 ± 12.0 | 45.0 ± 12.2£ | 37.4 ± 13.0 | 37.6 ± 15.0 |
| TG (mg/dl) | 150.8 ± 111.8 | 132.5 ± 96.2* | 147.7 ± 102.9 | 140.2 ± 111.8 | 171.3 ± 131.2 | 146.9 ± 109.0‡ | 184.4 ± 172.9 | 143.0 ± 92.6& | 150.3 ± 105.2 | 132.9 ± 91.4£ | 125.9 ± 93.7 | 103.2 ± 70.4† |
| TCh (mg/dl) | 191.6 ± 49.2 | 190.8 ± 48.9 | 188.3 ± 48.3 | 194.0 ± 48.8§ | 199.7 ± 45.9 | 201.9 ± 46.2 | 194.2 ± 51.4 | 186.3 ± 45.6 | 194.8 ± 48.8 | 193.4 ± 46.8 | 175.1 ± 50.3 | 166.8 ± 54.8† |
| DM | 1920 (32.8) | 1327 (8.0)* | 488 (35.4) | 339 (10.2) § | 94 (27.6) | 57 (5.0) ‡ | 144 (35.2) | 55 (6.8) & | 949 (30.9) | 635 (6.7) £ | (245)37.9% | 241 (12.5) † |
| Smoking | 1467 (25.4) | 4220 (25.7) | 232 (17.1) | 621 (19.2) | 38 (11.4) | 61 (5.6) ‡ | 87 (21.6) | 165 (20.7) | 884 (28.9) | 2696 (28.7) | 226 (35.5) | 677 (36.1) |
| Alcohol consumption | 892 (15.8) | 2351 (14.8) | 163 (12.2) | 424 (13.4) | 23 (7) | 41 (3.8) ‡ | 157 (15.9) | 94 (12.1) | 1731 (15.4) | 1273 (14.4) | 185 (28.6) | 519 (28.3) |
| UA≥360 μmol/l | 2916 (51.2) | 5884 (36.6)* | 668 (50.4) | 1142 (36.3) § | 127 (38.1) | 263 (24.0) ‡ | 300 (75.6) | 477 (60.4) & | 1506 (50.0) | 3272 (35.5) £ | 315 (50.2) | 730 (39.4) † |
| Creatinine (μmol/l) | 103.7 ± 126.4 | 78.0 ± 61.3* | 107.5 ± 135.3 | 83.0 ± 72.4§ | 77.7 ± 92.0 | 59.4 ± 24.3‡ | 168.3 ± 199.3 | 115.6 ± 109.6& | 95.0 ± 103.1 | 74.7 ± 53.3£ | 110.4 ± 149.1 | 80.5 ± 56.5† |
Continuous variables were expressed using the mean ± SD, and categorical data were presented using frequency and percentage. P values are derived from t-test for continuous variables and χ2 for categorical variables; *,§, ‡, &, £ and † means P < 0.05 among total and different cancers when compared the group with HNT and without HTN. AF, atrial fibrillation; CHD, coronary heart disease; CRC, colorectal cancer; CV, cardiovascular; DM, diabetes mellitus; HCC, hepatocellular carcinoma; HDL-C, high-density lipoprotein cholesterol; HF, heart failure; HTN, hypertension; LC, lung cancer; LDL-C, low-density lipoprotein cholesterol; RCC, renal cell carcinoma; TC, thyroid cancer; TCh, total cholesterol; TG, triglycerides; UA, uric acid.
Among the different tumor sites, the proportion of cancer patients who were diagnosed with heart failure, CHD, and atrial fibrillation was significantly higher in the HTN group compared with those without HTN. Also, the mean age and proportion of diabetes mellitus were significantly higher in the HTN group compared with non-HTN (P < 0.05). Moreover, women were significantly higher in HTN group compared with their counters in all tumor sites, except in thyroid cancer (74.4 vs. 77.1%, P = 0.305). Similarly, dyslipidemia was higher in the hypertension group compared with those without HTN, except for HCC (66.2 vs. 65.6%, P > 0.05). There was not a statistically significant difference in smoking between the two groups in all cancer sites but not in thyroid cancer patients (11.4 vs. 5.6%, P < 0.001). Similarly, alcohol consumption was significantly higher in the HTN group compared with their counterparts in thyroid cancer patients, but there were no significant differences in the other types of cancers. Regardless of cancer sites, patients with higher uric acid and creatinine values had a higher likelihood of HTN.
Associated factors of hypertension among cancer patients
Table 4 shows the associations between the presence of HTN and conventional risk factors of CVD among different studied cancers. Age and sex-adjusted binary logistic regression showed that CRC, RCC, lung cancer, and HCC patients with CHD, heart failure or atrial fibrillation had an increased likelihood of HTN. Similarly, thyroid cancer patients with CHD [odds ratio (OR) = 6.064, 95% CI: 3.433–10.712] and atrial fibrillation (OR = 5.335, 95% CI: 1.983–14.355) had a higher risk of having HTN. The patients diagnosed with HCC and CHD [OR: 9.401; 95% confidence interval (CI) 5.966–14.814] had increased risk of HTN. In contrast, there was no statistically significant association between heart failure in thyroid cancer patients and HTN.
TABLE 4.
Associated factors of hypertension among cancer patients
| CRC | TC | RCC | LC | HCC | |||||||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| CHD | 5.655 | 4.461–7.168 | <0.001 | 6.064 | 3.433–10.712 | <0.001 | 5.915 | 3.710–9.429 | <0.001 | 6.014 | 5.217–6.934 | <0.001 | 9.401 | 5.966–14.814 | <0.001 |
| HF | 3.428 | 2.438–4.82 | <0.001 | 2.798 | 0.970–8.073 | 0.057 | 7.804 | 2.958–20.588 | <0.001 | 3.002 | 2.455–3.671 | <0.001 | 3.270 | 1.774–6.027 | <0.001 |
| AF | 3.167 | 2.414–4.154 | <0.001 | 5.335 | 1.983–14.355 | 0.001 | 3.278 | 1.723–6.237 | <0.001 | 2.786 | 2.317–3.350 | <0.001 | 3.802 | 2.342–6.172 | <0.001 |
| Dyslipidemia | 1.150 | 0.97–1.37 | 0.117 | 1.840 | 1.30–2.63 | 0.001 | 1.800 | 1.26–2.57 | 0.001 | 1.230 | 1.10–1.37 | <0.001 | 0.970 | 0.74–1.26 | 0.969 |
| TCh | 0.999 | 0.997–1.001 | 0.341 | 1.000 | 0.996–1.004 | 0.936 | 1.005 | 1.001–1.008 | 0.015 | 1.002 | 1.000–1.003 | 0.010 | 1.003 | 1.001–1.006 | 0.008 |
| LDL | 0.999 | 0.997–1.002 | 0.497 | 1.000 | 0.994–1.005 | 0.941 | 1.007 | 1.001–1.013 | 0.015 | 1.002 | 1.001–1.004 | 0.006 | 1.003 | 1.000–1.007 | 0.042 |
| HDL | 0.987 | 0.979–0.994 | <0.001 | 0.976 | 0.961–0.991 | 0.002 | 0.989 | 0.973–1.005 | 0.179 | 0.989 | 0.984–0.994 | <0.001 | 1.000 | 0.992–1.009 | 0.941 |
| TG | 1.002 | 1.001–1.003 | <0.001 | 1.003 | 1.001–1.004 | <0.001 | 1.005 | 1.003–1.007 | <0.001 | 1.003 | 1.002–1.003 | <0.001 | 1.004 | 1.002–1.006 | <0.001 |
| DM | 4.232 | 3.592–4.986 | <0.001 | 4.907 | 3.350–7.188 | <0.001 | 7.350 | 5.153–10.484 | <0.001 | 5.417 | 4.830–6.076 | <0.001 | 3.890 | 3.137–4.823 | <0.001 |
| Smoking | 1.106 | 0.918–1.333 | 0.290 | 2.605 | 1.496–4.539 | 0.001 | 1.320 | 0.954–1.826 | 0.093 | 1.097 | 0.984–1.223 | 0.096 | 1.225 | 0.989–1.517 | 0.063 |
| Alcohol consumption | 1.235 | 0.997–1.529 | 0.053 | 1.876 | 1.006–3.497 | 0.048 | 1.980 | 1.356–2.890 | <0.001 | 1.279 | 1.123–1.458 | <0.001 | 1.392 | 1.112–1.741 | 0.004 |
| UA≥360 μmol/l | 2.040 | 1.77–2.35 | <0.001 | 2.050 | 1.51–2.78 | <0.001 | 2.150 | 1.62–2.85 | <0.001 | 1.960 | 1.79–2.14 | <0.001 | 1.480 | 1.22–1.78 | <0.001 |
| Creatine | 1.002 | 1.001–1.003 | <0.001 | 1.009 | 1.003–1.015 | 0.002 | 1.002 | 1.001–1.003 | <0.001 | 1.003 | 1.002–1.004 | <0.001 | 1.003 | 1.002–1.004 | <0.001 |
Data were expressed as odds ratio (OR) and 95% confidence interval (CI) adjusted by age and sex and calculated with logistic regression models. AF, atrial fibrillation; CHD, coronary heart disease; CRC, colorectal cancer; CV, cardiovascular; DM, diabetes mellitus; HCC, hepatocellular carcinoma; HDL-C, high-density lipoprotein cholesterol; HF, heart failure; HTN, hypertension; LC, lung cancer; LDL-C, low-density lipoprotein cholesterol; RCC, renal cell carcinoma; TC, thyroid cancer; TCh, total cholesterol; TG, triglycerides; UA, uric acid.
In the present study, cancer patients diagnosed with cardiovascular disease were found to have an increased likelihood of HTN among patients diagnosed with RCC, with the highest OR observed for CHD (OR: 7.804; 95% CI: 2.985–20.588) and heart failure (OR: 5.915; 95% CI: 3.710–9.429). Moreover, increased triglycerides, uric acid, and creatinine levels and a prior diagnosis of diabetes mellitus were significantly associated with the risk of having HTN in all the studied cancers. Also, patients diagnosed with thyroid cancer, RCC, lung cancer, and HCC who were recorded as alcohol drinkers had a higher risk of HTN. The ORs and 95% CI for HTN among alcohol drinkers for thyroid cancer, RCC, lung cancer, and HCC were (OR: 1.876; 95% CI: 1.006–3.497), (OR: 1.980; 95% CI: 1.356–2.890), (OR: 1.279; 95% CI: 1.123–1.458), and (OR: 1.392; 95% CI: 1.112–1.741), respectively. Also, dyslipidemia was significantly associated with the risk of having HTN in thyroid cancer, RCC, lung cancer, and HCC. However, it was not significantly associated with increased risk of HTN in CRC and HCC patients.
Blood pressure control among the hypertension patients
Only 74.1% of cancer patients with HTN were in antihypertensive use. Out of the total HTN patients, only 65.8% achieved the normal blood pressure readings (SBP ≤140 mmHg and DBP ≤90 mmHg). Among those patients that did not achieve the normal range of blood pressure readings, 33% were failed to achieve normal SBP, whereas 7.6% failed to meet the normal DBP readings. Regardless of tumor sites, most of the patients with the higher HTN grade or cardiovascular risk level tend to have poor blood pressure control (Tables 5 and 6 and Fig. 4).
TABLE 5.
Blood pressure control in different grades
| Total | CRC | TC | RCC | LC | HCC | |||||||||||
| (5685) | I (38) | II (223) | III (758) | I (21) | II (45) | III (202) | I (6) | II (64) | III (215) | I (95) | II (504) | III (1899) | I (19) | II (94) | III (367) | |
| Target BP [n (%)] | 3741 (65.8) | 33 (86.8) | 174 (78.0) | 492 (64.9) | 19 (90.5) | 37 (82.2) | 137 (67.8) | 6 (100.0) | 45 (70.3) | 119 (55.3) | 71 (74.7) | 364 (72.2) | 1181 (62.2) | 18 (94.7) | 71 (75.5) | 226 (61.6) |
| SBP≥140 mmHg [n (%)] | 1877 (33) | 5 (13.2) | 46 (20.6) | 261 (34.4) | 2 (9.5) | 8 (17.8) | 61 (30.2) | 0 (0.0) | 19 (29.7) | 94 (43.7) | 22 (23.2) | 135 (26.8) | 699 (36.8) | 1 (5.3) | 21 (22.3) | 138 (37.6) |
| DBPe≥90 mmHg [n (%)] | 433 (7.6) | 0 (0.0) | 9 (4.0) | 41 (5.4) | 0 (0.0) | 1 (2.2) | 22 (10.9) | 0 (0.0) | 3 (4.7) | 22 (10.2) | 5 (5.3) | 30 (6.0) | 141 (7.4) | 0 (0.0) | 4 (4.3) | 39 (10.6) |
BP, blood pressure; CRC, colorectal cancer; HCC, hepatocellular carcinoma; HTN, hypertension; LC, lung cancer; RCC, renal cell carcinoma; TC, thyroid cancer.
TABLE 6.
Blood pressure control in different cardiovascular risk stratifications
| Total | CRC | TC | RCC | LC | HCC | ||||||
| (5685) | I+II (43) | III+IV (904) | I+II (16) | III+IV (227) | I+II (6) | III+IV (262) | I+II (98) | III+IV (2241) | I+II (18) | III+IV (435) | |
| Target BP [n (%)] | 3741 (65.8) | 35 (81.4) | 611 (67.6) | 15 (93.8) | 161 (70.9) | 4 (66.7) | 156 (59.5) | 74 (75.5) | 1443 (64.4) | 13 (72.2) | 282 (64.8) |
| SBP ≥140 mmHg, [n (%)] | 1877 (33) | 8 (18.6) | 287 (31.7) | 1 (6.3) | 62 (27.3) | 2 (33.3) | 104 (39.7) | 24 (24.5) | 774 (34.5) | 5 (27.8) | 148 (34.0) |
| DBP ≥90 mmHg, [n (%)] | 433 (7.6) | 1 (2.3) | 46 (5.1) | 0 (0.0) | 21 (9.3) | 0 (0.0) | 23 (8.8) | 6 (6.1) | 158 (7.1) | 0 (0.0) | 41 (9.4) |
The cardiovascular risk stratifications of HTN were categorized into four levels including: I (low risk), II (moderate risk), III (high risk), and IV (very high risk). BP, blood pressure; CRC, colorectal cancer; HCC, hepatocellular carcinoma; HTN, hypertension; LC, lung cancer; RCC, renal cell carcinoma; TC, thyroid cancer.
FIGURE 4.
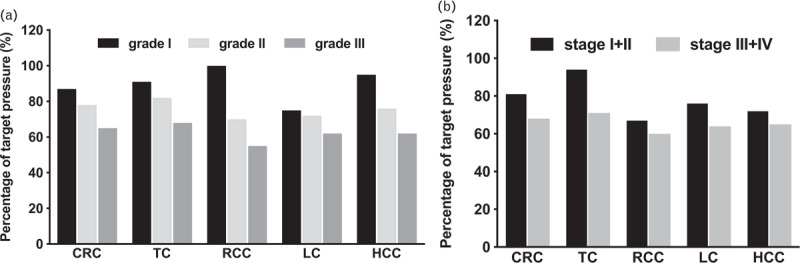
Condition of blood pressure among different grades (a) and cardiovascular risk stratifications among different cancers (b). The cardiovascular risk stratification of hypertension was categorized into four levels including: I (low risk), II (moderate risk), III (high risk), and IV (very high risk). CRC, colorectal cancer; HCC, hepatocellular carcinoma; LC, lung cancer; RCC, renal cell carcinoma; TC, thyroid cancer.
DISCUSSION
The main findings of our study reported that cancer patients, with the potential indication for the treatment of VSP inhibitors, carry a significant burden of CVD-related comorbidities, with HTN prevalence tops other comorbid conditions. Patients with higher HTN grades and cardiovascular risk level account for the higher proportion of hypertensive patients in all the studied cancers. Patients with HTN tend to carry more co-existent CVDs and cardiovascular risk factors compared with those without HTN. Also, this study reported that the majority of HTN patients were in antihypertensive use, but only 65.8% achieved the target BP, suggesting that HTN management of blood pressure was lacking.
Emerging evidence suggests an intimate relationship between CVDs and cancer, which may result from several shared risk factors such as inflammation, reactive oxygen species, and so on [14]. In this study, CVD conditions, including HTN, CHD, heart failure, and atrial fibrillation were common CVD comorbidities among the studied cancer cases. According to the present study, HTN was the most prevalent CVD (26.0%), which was similar to that in the general population according to the latest China Hypertension Survey [15]. The increase in blood pressure is indeed associated with a higher risk of cardiovascular events, arterial thromboembolism, and proteinuria [16], and may also limit the therapeutic benefits of VEGF inhibitors, bringing to dose reduction or therapy withdrawal. For instance, a previous study reported that 25–66% of cancer patients die of fatal events because of VSP-associated cardiotoxicity that eventually develops to HTN, arterial thromboembolism, myocardial infarction, and so on [8].
The present study showed that the distribution of HTN was found to differ from various types of cancer. According to our results, the higher prevalence was observed in RCC (33.5%) and CRC (29.4%). Although many studies and meta-analyses showed that HTN could be considered as the main risk factor for the development and progression of certain types of cancer, the association between these clinical entities is still not clear [17]. However, increasing evidence showed that HTN affects the possibility for the development of RCC. It was also suggested that chronic renal hypoxia during HTN could induce up-regulation of hypoxia-inducible factors, which in turn plays a significant role in oncogenesis [18,19]. A shred of evidence established that oxidative stress [19] and lipid peroxidation [20,21] are associated with HTN, which are also known to play a critical role in the pathogenesis of RCC. However, the biological mechanism is still controversial and remained unclear. In addition, it is reported that HTN increase the risk of colorectal cancer [22,23]. The current study reported that HTN patients account for 25.1, 24.5, and 23.1% in HCC, lung cancer and thyroid cancer patients, respectively. However, whether there is a causal relationship between HTN and these cancers remains uncertain. Therefore, follow-up studies are needed to investigate the possible pathways that connect HTN with these cancers [24].
In this study, patients with higher HTN grades and very high risk level of cardiovascular risk account for the higher proportion of hypertensive patients in all the studied cancers. Within the cancer patients with HTN, the proportion of high cardiovascular risk, moderate cardiovascular risk, and the low risk was 10.2, 3.6, and 0.8%, respectively, and the proportion of grade I and II HTN was 3.9 and 20.4%, respectively. Meanwhile, patients who were in very high cardiovascular risk level and grade III HTN account for 85.4% and 75.7%, respectively, implying that the cancer patients had a high degree of HTN severity. This could be attributed to the presence of multiple risk factors in HTN patients, such as glucose intolerance and dyslipidemia [25,26], which were also previously implicated in increasing the risk of cancer [14]. Another reason could be because of the higher proportion of CVD comorbidities, including diabetes mellitus, heart failure, and CHD among cancer patients with HTN.
The Cardiovascular Toxicities Panel, Convened by the Angiogenesis Task Force of the National Cancer Institute Investigational Drug Steering Committee, recommended less than 140/90 mmHg as the goal for blood pressure control in patients on VEGF inhibitor therapy in general and less than 130/80 mmHg for patients with diabetes and/or chronic kidney disease [27]. However, there is no guideline for the management of pre-exciting HTN in cancer patients. Our study found that, before the active cancer treatment, almost 26% of the hospitalized cancer patients with HTN did not undergo any hypertensive therapy. Moreover, over 34% patients’ blood pressure did not meet the recommended target SBP of less than 140 mmHg and target DBP pressure of less than 90 mmHg, which may be because of the less awareness of the importance of cardiovascular risk assessment among the oncologists. This finding shows that there is still a need for HTN management optimization in hospital settings. In the present study, cancer patients had a higher burden of CVD comorbidities during hospital admission, which calls a strict need for BP control and HTN management among cancer patients. Also, the present study showed that cancer patients with HTN had a higher proportion of cardiovascular risk factors, including diabetes mellitus, dyslipidemia, hyperuricemia, and elevated creatinine. Previously, elevated uric acid and creatinine have also been reported to be independent risk factors for cardiovascular risks in hypertensive patients [10]. In addition, it should be noted that an uncontrolled BP may further complicate the care of cancer patients significantly [16]. For this reason, blood pressure and other CVD comorbidities should be assessed prior to chemotherapy, and careful attention should be given during chemotherapy.
Limitation
There are several limitations to this study. First, we do not have detailed information about tumor size and stages information, which otherwise could help to further confirm the indication for the treatment with VEGF inhibitors. Second, although trained health professionals carefully examined the medical records, we may underestimate the prevalence of comorbidities. Third, the data comes from a single center with relatively small sample size.
In conclusion, cancer patients, with the potential indication for the treatment of VSP inhibitors, generally carry a high burden of CVD-related comorbidity. HTN, CHD, atrial fibrillation, and heart failure were top CVD comorbidities among candidates for VEGF antagonist use. Especially, pre-existing HTN was the most prevalent comorbid condition. Among HTN patients, those with grade III and very high cardiovascular risk level constitute the largest proportion. Also, HTN patients had a significant burden of CVD comorbidity among the candidates for VEGF antagonist use. In addition to this, a significant proportion of patients did not undergo any antihypertensive therapy, and management of blood pressure during hospitalization was not optimized, which may limit the therapeutic benefits of VEGF inhibitors. Therefore, the complex issue of CVD and cancer treatment urgently needs outcome evidence allowing to optimize prophylactic and therapeutic approaches.
ACKNOWLEDGEMENTS
Previous presentations: This work has not been presented in any conference or journal as a whole or in part.
This study was supported by grants from Liaoning Clinical Capacity Construction Funding (LNCCC-D20-2015).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Fei Liu and Tesfaldet H. Hidru contributed equally to this article and are considered co-first authors.
Abbreviations: BP, blood pressure; CHD, coronary heart disease; CRC, colorectal cancer; CVDs, cardiovascular diseases; DM, diabetes mellitus; HCC, hepatocellular carcinoma; HDL-C, high-density lipoprotein cholesterol; HTN, hypertension; LDL-C, low-density lipoprotein cholesterol; RCC, renal cell carcinoma; TC, total cholesterol; VEGF, vascular endothelial growth factor
REFERENCES
- 1.Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin Proc 2014; 89:1287–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol 2006; 3:24–40. [DOI] [PubMed] [Google Scholar]
- 3.Manzo A, Montanino A, Carillio G, Costanzo R, Sandomenico C, Normanno N, et al. Angiogenesis Inhibitors in NSCLC. Int J Mol Sci 2017; 18: pii: E2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barata PC, Rini BI. Treatment of renal cell carcinoma: current status and future directions. CA Cancer J Clin 2017; 67:507–524. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10:25–34. [DOI] [PubMed] [Google Scholar]
- 7.Wells SA, Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 2012; 30:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidinger M. Understanding and managing toxicities of vascular endothelial growth factor (VEGF) inhibitors. EJC Suppl 2013; 11:172–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wulkersdorfer B, Zeitlinger M, Schmid M. Pharmacokinetic aspects of vascular endothelial growth factor tyrosine kinase inhibitors. Clin Pharmacokinet 2016; 55:47–77. [DOI] [PubMed] [Google Scholar]
- 10.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018; 39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 11.Joint Committee for Guideline R. 2018 Chinese Guidelines for Prevention and Treatment of Hypertension-a report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J Geriatr Cardiol 2019; 16:182–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 13.Pearson TA, Palaniappan LP, Artinian NT, Carnethon MR, Criqui MH, Daniels SR, et al. American Heart Association Council on Epidemiology and Prevention. American Heart Association Guide for Improving Cardiovascular Health at the Community Level, 2013 update: a scientific statement for public health practitioners, healthcare providers, and health policy makers. Circulation 2013; 127:1730–1753. [DOI] [PubMed] [Google Scholar]
- 14.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation 2016; 133:1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Z, et al. Status of Hypertension in China: Results From the China Hypertension Survey, 2012-2015. Circulation 2018; 137:2344–2356. [DOI] [PubMed] [Google Scholar]
- 16.Faruque LI, Lin M, Battistella M, Wiebe N, Reiman T, Hemmelgarn B, et al. Systematic review of the risk of adverse outcomes associated with vascular endothelial growth factor inhibitors for the treatment of cancer. PloS One 2014; 9:e101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radisauskas R, Kuzmickiene I, Milinaviciene E, Everatt R. Hypertension, serum lipids and cancer risk: a review of epidemiological evidence. Medicina (Kaunas) 2016; 52:89–98. [DOI] [PubMed] [Google Scholar]
- 18.Kaelin WG., Jr The von Hippel-Lindau gene, kidney cancer, and oxygen sensing. J Am Soc Nephrol 2003; 14:2703–2711. [DOI] [PubMed] [Google Scholar]
- 19.Weikert S, Boeing H, Pischon T, Weikert C, Olsen A, Tjonneland A, et al. Blood pressure and risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition. Am J Epidemiol 2008; 167:438–446. [DOI] [PubMed] [Google Scholar]
- 20.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18. [DOI] [PubMed] [Google Scholar]
- 21.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004; 4:579–591. [DOI] [PubMed] [Google Scholar]
- 22.Pelucchi C, Negri E, Talamini R, Levi F, Giacosa A, Crispo A, et al. Metabolic syndrome is associated with colorectal cancer in men. Eur J Cancer 2010; 46:1866–1872. [DOI] [PubMed] [Google Scholar]
- 23.Stocks T, Lukanova A, Bjorge T, Ulmer H, Manjer J, Almquist M, et al. Metabolic Syndrome Cancer Project Me-Can Group. Metabolic factors and the risk of colorectal cancer in 580,000 men and women in the metabolic syndrome and cancer project (Me-Can). Cancer 2011; 117:2398–2407. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Kim YA, Hwangbo B, Kim MJ, Cho H, Hwangbo Y, Lee ES. Effect of antihypertensive medications on sepsis-related outcomes: a population-based cohort study. Crit Care Med 2019; 47:e386–e393. [DOI] [PubMed] [Google Scholar]
- 25.Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, et al. REACH Registry Investigators. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006; 295:180–189. [DOI] [PubMed] [Google Scholar]
- 26.Mancia G, Facchetti R, Bombelli M, Polo Friz H, Grassi G, Giannattasio C, et al. Relationship of office, home, and ambulatory blood pressure to blood glucose and lipid variables in the PAMELA population. Hypertension 2005; 45:1072–1077. [DOI] [PubMed] [Google Scholar]
- 27.Maitland ML, Bakris GL, Black HR, Chen HX, Durand JB, Elliott WJ, et al. Cardiovascular Toxicities Panel, Convened by the Angiogenesis Task Force of the National Cancer Institute Investigational Drug Steering Committee. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst 2010; 102:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]


