Abstract
This review summarizes the current evidence on the potential role of phytol, a microbial metabolite of chlorophyl A, and its metabolites, phytanic and pristanic acids, in carcinogenesis. Primary food sources in Western diets are the nut skin for phytol and lipids in dairy, beef and fish for its metabolites. Phytol and its metabolites gained interest as dietary compounds for cancer prevention because, as natural ligands of peroxisome proliferator-activated receptor-α and -γ and retinoid X receptor, phytol and its metabolites have provided some evidence in cell culture studies and limited evidence in animal models of anti-carcinogenic, anti-inflammatory and anti-metabolic-syndrome properties at physiological concentrations. However, there may be a narrow range of efficacy, because phytol and its metabolites at supra-physiological concentrations can cause in vitro cytotoxicity in non-cancer cells and can cause morbidity and mortality in animal models. In human studies, evidence for a role of phytol and its metabolites in cancer prevention is currently limited and inconclusive. In short, phytol and its metabolites are potential dietary compounds for cancer prevention, assuming the challenges in preventing cytotoxicity in non-cancer cells and animal models and understanding phytol metabolism can be mitigated.
Keywords: cancer, phytanic acid, phytanic acid, phytol
Introduction
Cancer is the second leading cause of deaths with an estimated 609 604 cancer deaths in 2018 in the US (Siegel et al. 2018). Dietary phytol, a diterpene alcohol and microbial metabolite of chlorophyl A, and its poly-methylated branched-chain fatty acid metabolites, phytanic acid (PA; 3,7,11,15-tetramethylhexadecanoic acid) and pristanic acid (PRA; 2,6,10,14-tetramethylpentadecanoic acid), have garnered the interest of cancer prevention researchers due to their ability to serve as natural ligands for peroxisome proliferator-activated receptor (PPAR)-α, PPAR-γ and retinoid X receptor (RXR) (Roca-Saavedra et al., 2017). These receptors are transcription factors leading to downstream effects on metabolism, proliferation and apoptosis (Roca-Saavedra et al., 2017). Furthermore, they upregulate the expression of α-methylacyl-CoA racemase (AMACR), especially in prostate cancer tissue (Verhoeven and Jakobs, 2001). Besides being involved in PA catabolism, AMACR is also critical in the degradation of bile acids, ibuprofen and other methylated fatty acids (Lloyd et al., 2008). Hence, phytol, PA and PRA may be linked to carcinogenesis through involvement in peroxisomal and mitochondrial functions, oxidative stress, inflammatory pathways, cell signal transduction, glucose/energy metabolism and microbial effects.
The Western diet contains about 10 mg/day of phytol (Steinberg, 1989; Brown et al., 1993; Vetter and Schroder, 2010; Roca-Saavedra et al., 2017), which mostly comes from the skin of nuts (Steinberg et al., 1967), 50–100 mg/day of PA and 10–30 mg/day of PRA, which are primarily from lipids in dairy, beef and fish (Steinberg, 1989; Brown et al., 1993; Vetter and Schroder, 2010; Roca-Saavedra et al., 2017). The average circulating PA concentration is below 10 µM, and according to diagnostic criteria for PA-related diseases, the PA concentration below 30 µM is considered within the normal physiological range (Baldwin et al., 2010; Lloyd et al., 2013). Tissue PA concentrations are below 0.3% of total fatty acids (Kataria et al., 2015). Both concentrations are sufficient to elicit chemo-preventive properties in cell culture studies, which are summarized in this review.
Current epidemiological evidence about dietary PA intake or circulating PA concentrations is limited to prostate cancer and non-Hodgkin’s Lymphoma (NHL) with varied results. Out of five studies (Xu et al., 2005; Price et al., 2010; Wright et al., 2012; Ollberding et al., 2013; Wright et al., 2014), dietary intake or circulating concentration of PA or PRA have been linked to increased cancer risk overall or in subgroups in four studies and no association was observed in one study. Major food sources of phytol and its metabolites have been implicated in the etiology of cancer. The World Cancer Research Fund/American Institute for Cancer Research expert panel review (World Cancer Research Fund and American Institute for Cancer Research, 2018) reported strong evidence for a positive association of consuming red meats (including beef) and processed meats with colorectal cancer risk and an inverse association between dairy products and colorectal cancer risk. Further, they reported strong evidence for a positive association between salted fish intake and nasopharyngeal cancer risk. For the other major food sources of phytol and its metabolites, current evidence is insufficient or inconsistent to draw conclusions.
This article reviews the current evidence from cell culture studies, animal feeding studies and human intervention and observational studies, regarding phytol and its metabolites as potential dietary compounds for cancer prevention. Furthermore, this review recommends potential future research directions, which, if proven successful, will allow us to move forward with formulating prevention strategies involving phytol and its metabolites in order to decrease the public health burden of cancer. The goal of this review is to raise awareness of phytol and its metabolites and to foster future research, as outlined in this review.
Metabolism and physiological concentrations of phytol and its metabolites
The metabolism of phytol and its metabolites is shown in Fig. 1. Free phytol occurs at low concentrations in plant and animal tissues except for the skin of nuts and plant leaves, where it accumulates and acts as an antimicrobial agent (Steinberg et al., 1967; Islam et al., 2015), suggesting a potential role of phytol in altering the microbiome population. Phytol is derived from plant and phytoplankton chlorophyl. Nearly all phytol in plants is in the bound form as side-chain of chlorophyl or pheophytin and requires microbial enzymes to be released as free phytol (Hansen, 1980). Chlorophyl or pheophytin cannot be absorbed by humans, whereas PA has an absorption rate of 80% (Baxter, 1968). The free phytol is readily converted to PA in the gastrointestinal tract and lymph (Baxter, 1968). As phytol is not produced endogenously (Steinberg et al., 1967), circulating phytol concentrations are probably found at trace amounts (Hansen, 1980). PRA in circulation is derived primarily from endogenous conversion of PA in the liver and a smaller portion from the diet. To our knowledge, the role of phytol and its metabolites on the microbiome is currently unknown and warrants research.
Fig. 1.
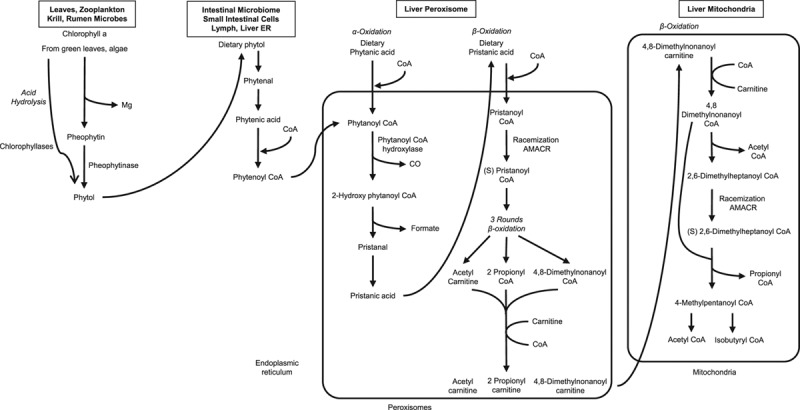
Metabolism of phytol, phytanic acid and pristanic acid. Phytol is derived from plant and phytoplankton chlorophyll. Rumen microbiota, marine zooplankton and human intestinal bacteria are able to cleave the chlorophyll porphyrin ring and release phytol. After intestinal absorption, dietary phytol is metabolized in the endoplasmic reticulum (ER) via phytenal and phytenic acid to phytanoyl CoA. Dietary PA and PRA are activated on the cytosolic side of the peroxisomes to phytanoyl CoA and pristanoyl CoA. In the peroxisomes, phytanoyl CoA is α-oxidized to PRA, the racemic mixture of PRA is converted by α-methylacyl-CoA racemase (AMACR) to (2S) PRA. After three rounds of β-oxidation, 4,8-dimethylnonanoyl CoA is shuttled by carnitine into the mitochondria for complete β-oxidation, which include four rounds of β-oxidation and one racemization step using AMACR (Verhoeven and Jakobs, 2001). PA, phytanic acid; PRA, pristanic acid.
However, at supra-physiological levels, phytol can accumulate in the liver after conversion to PA (Hansen, 1980; Gloerich et al., 2007). Circulating PA is derived primarily from the diet and a small portion (<20%) from endogenous conversion of phytol into PA (Hansen, 1980). As shown in Fig. 1, in the liver, PA is activated to phytanoyl CoA and then transported in the peroxisomes, where phytanoyl CoA is α-oxidized to PRA using various enzymes (Verhoeven and Jakobs, 2001). Mutations in enzymes involved in this step, such as phytanoyl CoA hydroxylase, can result in accumulation of PA as in Refsum disease, but PRA does not accumulate due to such mutations (Hansen, 1980; Verhoeven and Jakobs, 2001). As poly-methylated branched-chain fatty acids, PA and PRA replace primarily n-3 or n-6 polyunsaturated fatty acids in tissues (Hansen, 1965; Skrbic and Cumings, 1969; Malmendier et al., 1974). The only reported human tissue concentration of PA is 0.30 wt% of total fatty acids in the prostate tissue (Kataria et al., 2015), but no other tissues. In human populations, serum and plasma PA concentrations were on average under 10 µM [2.6 µM in the EPIC study (Price et al., 2010), 2.9 µM in the North Carolina study (Xu et al., 2005) and 9.8 µM in the Alpha-Tocopherol Beta-Carotene (ATBC) study (Wright et al., 2014)] and differed among meat consumers, vegetarians and vegans (5.77, 3.93 and 0.86 μM, respectively) (Allen et al., 2008), indicating that intake of animal products can increase circulating PA concentrations.
In short, our knowledge about the physiological processes involved in the control of PA and PRA concentrations in absorption, circulation and tissue distribution as well as the effect of the intestinal microbiome on circulating PA and PRA concentrations is limited and requires further research.
Human consumption patterns of phytol and its metabolites
The current knowledge on phytol and PRA contents in foods is limited (Steinberg et al., 1967; Coppack et al., 1988; Steinberg, 1989; Brown et al., 1993). Major food sources of phytol are the skin of nuts (about 1 g/100 g of food), whereas concentrations in other foods were below 15 mg/100 g of food (Steinberg et al., 1967; Brown et al., 1993). Major food sources of PA are lipids from dairy, beef and fish. Foods with the highest PA content are butter, followed by salmon, halibut and fatty cheeses (Roca-Saavedra et al., 2017). Besides foods, 1 g of fish-oil supplements contain about 10% of the total dietary PA intake. In two previous epidemiological studies, the primary dietary sources of PA were cheese, butter and milk, which in total accounted for up to 53% in the Nebraska Lymphoma Study (NLS) (Ollberding et al., 2013) and 90% in ATBC cohort (Wright et al., 2012). The correlation between circulating PA concentration and fat intake from butter, a major food source of PA, is modest (r = 0.35–0.44) (Price et al., 2010; Wright et al., 2014; Kataria et al., 2015). Hence, circulating PA concentrations may serve as dietary biomarker of ruminant fat intake in populations with low fatty fish consumption.
An average Western diet with dairy products contains between 50 and 100 mg/day of PA (Roca-Saavedra et al., 2017) and is estimated to contain about 10–30 mg/day and 10 mg/day for PRA and phytol, respectively, based on the ratio of PA to PRA in dairy products (Steinberg, 1989; Brown et al., 1993; Vetter and Schroder, 2010). This level is similar to the largest feeding study (tested 156 vs. 78 mg/day of PA) (Werner et al., 2013). A few observational studies also reported an estimated intake of PA. In the NLS, the middle tertile of PA ranged from 43.0 to 63.8 mg/day (Ollberding et al., 2013). In the ATBC Cancer Prevention cohort of Finnish male smokers, the average intake in the lowest and highest quartiles of PA were 64 and 199 mg/day, respectively (Wright et al., 2012). Hence, a relatively wide range of intake was reported in healthy populations, although the direct comparison of estimated intake amounts from different studies needs a caution as the studies used different approaches (e.g. the number of food items included in their food frequency questionnaires and statistical methods used to estimate the intake).
Role of phytol and its metabolites in carcinogenesis
Cell culture studies
Phytol and its metabolites at concentrations in the physiological range (≤10 μM) can alter pathways involved in carcinogenesis, such as increasing apoptosis and decreasing proliferation (Komiya et al., 1999; Kim et al., 2015; Thakor et al., 2017). Proposed mechanisms by which phytol and its metabolites exert their chemo-preventive effects include inducing mitochondrial dysfunction, oxidative damage and intracellular Ca2+ deregulation, as well as epigenetic changes such as histone deacetylation (Idel et al., 2002; Schönfeld et al., 2004; Schönfeld et al., 2006; Schönfeld and Wojtczak, 2007; Leipnitz et al., 2010; Kruska and Reiser, 2011; Grings et al., 2012; Borges et al., 2015; Dhaunsi et al., 2016; Dhaunsi et al., 2017) (Table 1). The concentrations that resulted in these changes varied from 1 µM of PA for inducing oxidative damage in rat heart cells (Schönfeld et al., 2004) to 100 µM of PA for causing nitric oxide-dependent apoptosis in vascular cells (Schluter et al., 2002). In human embryonic kidney cells (HEK293), 50 μM of PA increased intracellular release of Ca2+ reserves, which was mediated via activation of free fatty acid receptor, GPR40 (Kruska and Reiser, 2011). Thus, PA may alter various pathways in different cell types in a dose-dependent manner. Studies of brain tissues showed effects on membrane depolarization, oxidative damage, mitochondrial reactive oxygen species (ROS) generation and histone deacetylation (epigenetic transcription regulation) at concentrations as low as 80 nM of PA (Schönfeld et al., 2004; Reiser et al., 2006; Schönfeld et al., 2006; Schönfeld and Reiser, 2006; Rönicke et al., 2009; Leipnitz et al., 2010; Borges et al., 2015; Nagai, 2015). In HepG2 cells, 40 µM of phytol can suppress the epithelial-mesenchymal transition signaling, which is important for tumor invasion (Kim et al., 2015). Conversely, phytol and its metabolites may also have tumor-promoting properties, as ROS generation can induce DNA damage that either causes apoptosis or transforms normal cells into cancerous cells (Gu et al., 2018; Samimi et al., 2018). Moreover, 0.3 µM of PA or PRA in normal prostate cells was sufficient to increase the protein content of AMACR (Mobley et al., 2003), an enzyme known to be elevated in prostate cancer tissue (Thornburg et al., 2006). Thus, PA may be both friend and foe for cancer prevention and treatment, which warrant further investigation.
Table 1.
Effect of phytanic acid on cancer pathways in cell culture studies
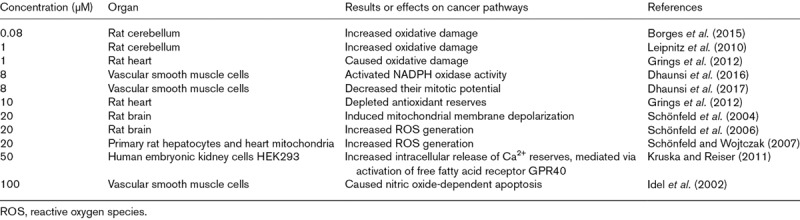
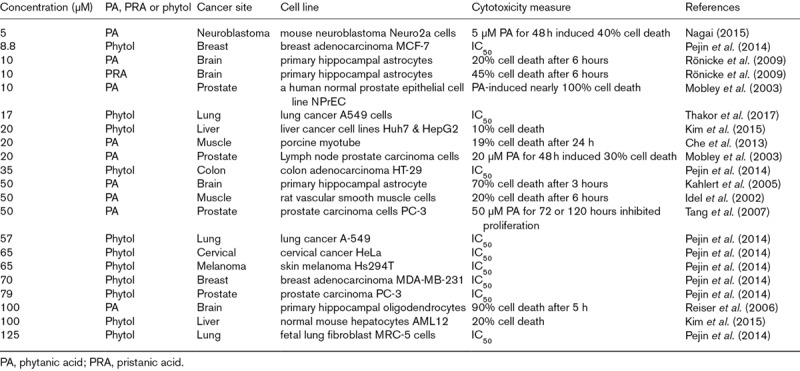
In the physiological range (≤10 μM), phytol and its metabolites can be cytotoxic to various cancer cell lines (Idel et al., 2002; Mobley et al., 2003; Kahlert et al., 2005; Reiser et al., 2006; Tang et al., 2007; Rönicke et al., 2009; Che et al., 2013; Pejin et al., 2014; Kim et al., 2015; Nagai, 2015; Thakor et al., 2017), as shown in Table 2. Although the induction period and measures of cytotoxicity slightly vary among studies, the lowest concentrations at which cytotoxicity was observed were 5 µM of PA in neuroblastoma Neuro2a cells (Nagai, 2015) and 8.8 µM of phytol in breast adenocarcinoma MCF-7 (Pejin et al., 2014). The highest concentration at which cytotoxicity was observed was 79 µM of phytol in prostate carcinoma PC-3 (Pejin et al., 2014), suggesting that cancer cell lines may differ in their sensitivity to phytol and its metabolites. Of note is that, in a previous study of lung cancer A549 cells, the IC50 for phytol (17 μM) was lower than for the cancer drug, methotrexate (20 µM) (Thakor et al., 2017). Of concern is that 10 µM of PA was already cytotoxic to a human normal prostate epithelial cell line (NPrEC), but not to lymph node prostate carcinoma cells (Mobley et al., 2003), suggesting a narrow and sometimes overlapping range between beneficial and detrimental effects. Additional studies are needed to investigate cytotoxicity of phytol and its metabolites in non-cancer and cancer cells and to elucidate why cell lines from different organs vary in their sensitivity to phytol and its metabolites.
Table 2.
Effect of phytol, phytanic acid or pristanic acid on cytotoxicity in cell culture studies
Another desirable aspect of phytol and its metabolites as potential pharmacological agents is that they may improve markers of metabolic syndrome in the physiological range (≤10 μM). Organs may differ in their sensitivity to phytol and its metabolites, as minimal concentrations required for beneficial effects varied from 0.1 and 100 μM of PA. At concentration of as low as 0.1 μM, PA increased brown adipocyte differentiation (Schluter et al., 2002), whereas 40 or 100 μM was needed for white adipocyte differentiation (Heim et al., 2002; Schluter et al., 2002). In muscle cells, 1 μM of PA was sufficient to stimulate glucose uptake (Che et al., 2013) and 5 μM of phytol was sufficient to induce genes involved in muscle differentiation in C2C12 cells (Yang et al., 2017). In contrast, 100 μM of PA was needed to increase transcription of genes that increase glucose uptake in HepG2 cells (Heim et al., 2002).
Proposed pathways by which phytol and its metabolites exert their chemopreventive effects are summarized in Fig. 2. The primary proposed mode of action is that phytol and its metabolites act as natural ligands of various nuclear receptors, especially PPAR-α and -γ and RXR, and induce transcription of nuclear receptor-responsive genes. Such induction can occur at concentrations similar to synthetic ligands that can be used for insulin-sensitizing effects and chemoprevention and treatment (Peters et al., 2012). Phytol concentrations as low as 1, 50 and 10 μM induced PPAR-α, -β and -γ activity, respectively, in monkey kidney CV-1 cells and HepG2 hepatocytes (Heim et al., 2002; Goto et al., 2005). Concentrations of 1 μM of PRA and 3 μM of PA induced RXR activity, potentially through retinol esters of PA (Tang et al., 2007), in monkey kidney COS-1 cells and human HepG2 cells (Zomer et al., 2000). Other proposed pathways are the PI3kinase/AKT pathway (Wang et al., 2017) and as ligands of TRAIL, FAS, and TNF receptors, glucose-6-phosphate dehydrogenase receptor (Thakor et al., 2017), and the free fatty acid receptor, GPR40 (Kruska and Reiser, 2011).
Fig. 2.
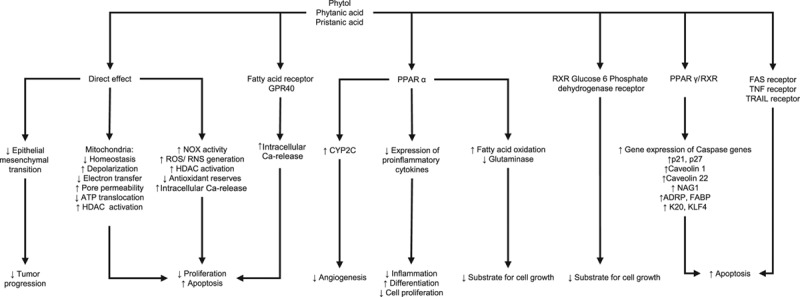
Proposed mechanisms by which phytol and its metabolites exert their chemo-preventive properties. Phytol, phytanic acid and pristanic acid are proposed to affect pathways involved in carcinogenesis such as cell proliferation, differentiation and apoptosis, angiogenesis and inflammation. These effects are direct or indirect by inducing activation of transcription factors such as PPAR and receptors related to fatty acid and energy metabolism or inflammation. PPAR, peroxisome proliferator-activated receptor.
In summary, it is encouraging that phytol and its metabolites can alter pathways involved in carcinogenesis at concentrations within the physiological range (≤10 μM). It is, however, concerning that limited evidence also suggests that phytol or PA at the same concentrations can be cytotoxic to breast and brain non-cancer cell lines and that the chemo-preventive effect of phytol and its metabolites may differ by organs and cancer cell lines.
Animal model studies
Current evidence from animal models on a role of phytol and its metabolites in carcinogenesis is limited to one study (Liska et al., 2012); hence, this review includes animal models of metabolic syndrome and nonalcoholic fatty liver disease (NAFLD) and animal toxicity studies (Ellinghaus et al., 1999; Elmazar and Nau, 2004; Mönnig et al., 2004; Hashimoto et al., 2006; Mackie et al., 2009; Elmazar et al., 2013; Silva et al., 2014; Selkälä et al., 2015; Wang et al., 2017; Yang et al., 2017) (Table 3). Dietary concentrations of phytol and its metabolites are expressed in wt%, if possible, to facilitate comparison of dietary concentrations among species. In female Sprague–Dawley rats, the combination of phytol at 0.05 wt% and a vitamin D analogue seocalcitol at 7 ppb, starting 7 weeks after mammary tumor induction, slowed mammary tumor growth, but not tumor malignancy or invasiveness; (Liska et al., 2012). Four animal feeding studies evaluated the effect of oral phytol on indicators of metabolic syndrome and NAFLD and showed beneficial effects (van den Brink et al., 2005; Hashimoto et al., 2006; Elmazar et al., 2013; Wang et al., 2017). Dietary concentrations ranged from 0.025 to 0.5 wt% of phytol for 2–5 weeks and resulted in decrease in body fat percentage and TNF-α concentrations, and improvement in blood lipid and glucose profiles (Hashimoto et al., 2006; Elmazar et al., 2013). Interestingly, in one animal study, the 0.05 wt% phytol increased adipogenesis, but decreased total body fat mass, as the average size of adipocytes was smaller (Wang et al., 2017). In addition to indicators of metabolic syndrome and NAFLD, phytol also showed anti-inflammatory properties in two studies, specifically decreased concentrations of TNF-α in one study (Elmazar et al., 2013) and decreased cytokine concentrations and inhibiting leukocyte migration in the other study (Silva et al., 2014).
Table 3.
Effects of phytol, or phytanic acid on cancer or biomarkers in animal feeding studies
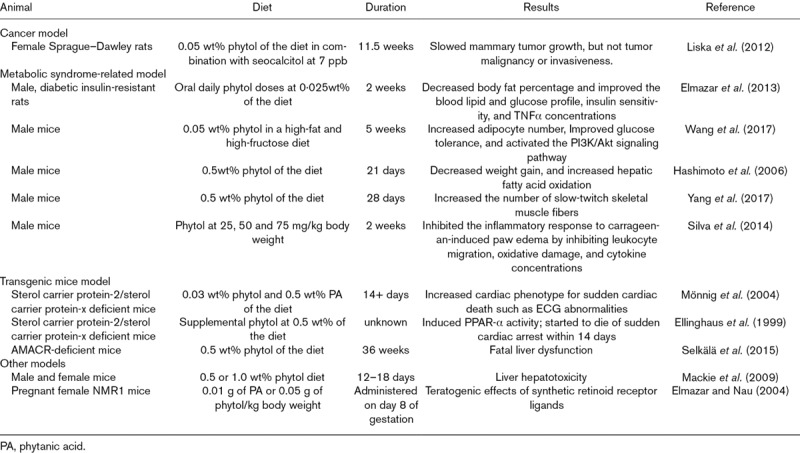
Detrimental effects of oral phytol and its metabolites were reported in four animal studies, three of which used transgenic animal models that cannot catabolize PA, which accumulates in tissues and subsequently leads to death. In two studies of sterol carrier protein-2/sterol carrier protein-x deficient mice, phytol at 0.5 wt% of the diet-induced PPAR-α activity (Ellinghaus et al., 1999), and mice started to die of sudden cardiac arrest by two weeks (Mönnig et al., 2004). In AMACR-deficient mice, phytol at 0.5 wt% of the diet resulted in morbidity by 2 weeks, killed most mice by 6 weeks, and all by 36 weeks due to liver dysfunction (Selkälä et al., 2015). Hepatotoxicity, consistent with chronic PPAR-α upregulation, was also observed in male and female mice after being on a 0.5 or 1.0 wt% phytol diet for 12 days (Mackie et al., 2009). Not based on transgenic animal model, but in pregnant NMR1 mice, teratogenic effects of synthetic retinoid receptor ligands were potentiated with oral administration of 0.01 g of PA or 0.05 g of phytol/kg of body weight (Elmazar and Nau, 2004).
In summary, results of oral phytol at concentrations of 0.05 wt% of the diet for prevention or treatment of metabolic syndrome and inflammation are encouraging; however, 10-fold higher concentrations may be harmful, indicating the need for dose-response studies. Given that a Western diet (assuming 500 g/day of macronutrient consumption) contains phytol at 10% of the PA intake, an average person would consume 5 mg/day (equal to 0.001 wt% of the diet) and 20 mg/day of phytol (0.004 wt% of the diet), which is 10-fold lower than the lowest concentrations shown effective in animal models (Elmazar et al., 2013; Wang et al., 2017). Furthermore, future animal studies should use dietary PA and PRA rather than phytol because humans have limited ability to metabolize phytol to PA or PRA and biologically effective PA and PRA contents can be obtained from human diet, assuming that phytol and its metabolites have the same efficacy (Steinberg, 1989; Brown et al., 1993).
Human studies
Current evidence from intervention studies on a role of phytol and its metabolites is extremely limited. As to PA, initially, Werner et al. (2011) examined the effect of PA on biomarkers of metabolic syndrome among 14 healthy participants in a double-blind, randomized, 4-week parallel intervention study in Denmark. They were given 45 g of milk fat/day from test butter and cheese with 0.13 wt% of PA for the control group or 0.24 wt% of PA for the intervention group. Plasma PA concentrations increased in both groups significantly (P < 0.05) with a tendency of larger changes in the control group than the intervention group and no group differences in biomarkers of metabolic syndrome. In the follow-up study, Werner et al. (2013) increased both the PA amount and the length of intervention and compared the effect of replacing 15% of the participants’ caloric intake from fat in the form of butter (39 g/day of butter that included 156 vs. 78 mg/day of PA) for 12 weeks on blood lipid, glucose, and inflammatory marker profiles among 38 healthy participants. To standardize the PA content and minimize residual confounding, the milk fat was obtained either from pasture-grazing or conventionally-fed cows and the former had twice the PA concentrations. Overall, there were no significant group differences in biomarker profiles related to metabolic syndrome (Werner et al., 2013). This null result can be explained by no meaningful difference in plasma PA concentrations (3.6 vs. 3.3 µM; P = 0.15), as only 15% of caloric intake from fat was replaced. Hence, future dietary intervention studies need to increase the difference in the PA content between two groups to achieve at least 2-fold differences in circulating PA concentration, potentially by providing the entire meals, instead of partially replacing their food source of fat. In short, there is currently lack of evidence from human intervention studies that would suggest a beneficial effect of phytol and its metabolites on chronic disease prevention.
Current evidence from epidemiological studies on a role of phytol and its metabolites in chronic diseases is limited to five cancer studies. Two studies (Wright et al., 2012; Ollberding et al., 2013) have examined the association between dietary PA intake and cancer risk. A retrospective case-control study (336 cases with 185 women and 460 controls with 236 women) in Nebraska, NLS (Ollberding et al., 2013), reported no association between total dietary PA intake and NHL risk. When stratified by NHL subtype, total dietary PA was positively associated with follicular lymphoma and small lymphocytic lymphoma/chronic lymphocytic leukemia. Interestingly, the association between PA and NHL subtype differed by dietary PA source (Ollberding et al., 2013). PA from beef, but not dairy products, was positively associated with diffuse large B-cell lymphoma. PA from milk, but not other dairy products, was associated with follicular lymphoma. This observation makes it unlikely that PA was driving the association with NHL subtype risk, leaving the possibility of residual confounding by other compounds in these food sources of PA. In the prospective ATBC cohort (27,111 participants, 1,929 incident cases, 438 advanced cases), PA intake was not associated with overall prostate cancer risk, but was positively associated with advanced prostate risk (Wright et al., 2012). Moreover, this association was linked to high-fat dairy products and there was no association with PA from beef or fish. Thus, the association is subject to residual confounding as in NLS and could be due to compounds unique to dairy fat, such as short- and medium-chain fatty acids, which warrant further investigations. However, both studies did not further elucidate this possibility by mutually adjusting for the intake of these compounds, which needs to be explored in future studies and would help to clarify which bioactive compounds in dairy and beef products (e.g., mutagens) are driving the association between PA intake and cancer risk.
Three epidemiological studies measured serum or plasma PA concentrations and assessed the association with prostate cancer risk in men (Xu et al., 2005; Price et al., 2010; Wright et al., 2014). A small, population-based, retrospective case-control study (49 cases and 55 control) conducted in North Carolina reported significantly higher serum PA levels among prostate cancer cases than controls (Xu et al., 2005). Two prospective case-control studies nested within the European Prospective Investigation into Cancer and Nutrition (EPIC) and ATBC cohorts followed up on the finding. In the EPIC cohort (566 cases and 566 controls), plasma PA concentrations were not associated with overall prostate cancer risk; however, there was a positive association among participants who fasted at least three hours before blood draw (Price et al., 2010). In the ATBC cohort (300 cases and 300 controls), in which dietary PA intake was associated with aggressive, but not overall prostate cancer risk (Wright et al., 2012), neither serum PA or PRA concentrations were associated with overall or aggressive prostate cancer risk (Wright et al., 2014).
As a note of caution, circulating PA concentrations do not necessarily reflect tissue PA concentrations. The correlation between serum and benign prostate tissue PA concentrations in 26 participants from Chicago was low (r = 0.28; P = 0.15) (Kataria et al., 2015). More research is needed to determine factors that alter tissue PA concentrations and their relation with cancer risk and circulating PA concentrations. Besides prostate cancer, future epidemiologic studies are warranted to investigate cancer sites that have a lower IC50, such as breast or brain, or that accumulate more PA and have a higher lipid content, specifically liver and kidney (Hansen, 1965; Skrbic and Cumings, 1969; Malmendier et al., 1974), which are more likely to show an association.
In summary, current evidence in human studies for a role of phytol and its metabolites in cancer prevention is insufficient, inconsistent, and could be explained by residual confounding by other food components. Another concern is that primary food sources of phytol and its metabolites have other food components that are known to have cancer-promoting properties (e.g. mutagens and iron in meats) (World Cancer Research Fund and American Institute for Cancer Research, 2018). The question arises whether dietary intervention studies for cancer prevention can be formulated to achieve large differences in circulating concentrations of phytol, its metabolites, or both and their chemo-preventive effects without residual confounding by other food components. Thus, a better strategy for future human intervention studies would be to use a supplement rich in phytol and its metabolites. As to cancer site, we recommend that future epidemiological studies should also examine cancers other than prostate cancer and NHL, especially cancer sites whose cells are known to be more sensitive to phytol and its metabolites such as brain, liver, kidney and blood. Furthermore, future studies should examine the interrelation between dietary, circulating and tissue levels of phytol and its metabolites in normal and cancer tissue.
Role of phytol and its metabolites on α-methylacyl-CoA racemase expression and cancer risk
The enzyme AMACR is critical for catabolism of PA and PRA, as it converts the (R)-form of PRA to the (S)-form, which can be further oxidized in humans. The expression of AMACR is upregulated in vitro by PA or PRA (Mobley et al., 2003) via PPAR-α to prevent accumulation of PA or PRA (Gloerich et al., 2007). Thus, elevated PA levels in circulation may be linked to decreased AMACR expression and may differ by AMACR polymorphisms. There is continued interest in the association of AMACR expression and polymorphisms with cancer, as elevated AMACR protein or gene overexpression has been reported in various cancer tissues such as prostate, colon, rectum, ovaries, breast, bladder, lung, kidney, skin and lymphoma (Zhou et al., 2002). Currently, AMACR gene expression is used in prostate cancer diagnosis in combination with other biomarkers such as 34E12, CK5/6 or p63 (Amin et al., 2014) or with prostate cancer antigen 3 (Ouyang et al., 2009), as AMACR is most consistently upregulated in prostate cancer tissue (Thornburg et al., 2006). A meta-analysis combining five case-control studies found that D175G and M9V polymorphisms of the AMACR gene were associated with prostate cancer risk (Chen et al., 2015).
A study conducted in Chicago using benign prostate tissue did not find an association between AMACR gene expression and dietary PA intake or serum PA concentration (Kataria et al., 2015). Furthermore, AMACR polymorphism did not modify the associations between dietary red meat or dairy intake on prostate cancer in the population-based case-control study in the state of Washington (Wright et al., 2011). AMACR is generally required to convert (R)-forms of fatty acids with chiral centers (usually methylated fatty acids of microbial origin) to (S)-forms of fatty acids and, thus, are essential for the degradation of bile acids, ibuprofen and other methylated fatty acids (Wanders, 2014). Therefore, elevated AMACR expression may be due to PPAR-α stimulation by compounds other than phytol or its metabolites, such as increased bile acid turnover, increased microbial fatty acid degradation, and low activity of AMACR or enzymes downstream required for methylated fatty acid degradation. Future research is needed to determine factors that elevate AMACR expression in some cancer tissues, but not in others. In summary, while the association of AMACR gene and protein expression with cancer risk and prognosis is well-established for prostate cancer, there is limited evidence that this association is modified by PA.
Conclusion
There is a gap in research and knowledge related to phytol and its metabolites, PA and PRA and carcinogenesis. Phytol and its metabolites are potential dietary compounds for cancer prevention because, based on some evidence in cell culture studies and limited evidence in animal models, they can alter pathways involved in carcinogenesis at physiological concentrations that can be achieved by dietary modifications. However, there are concerns regarding the cytotoxicity in non-cancer cells and various cell types. More research is needed to determine if and how normal and cancer tissues react similarly and differently to phytol and its metabolites. Animal feeding studies at phytol concentrations that can be achieved with dietary modification alone show promise; however, the number of studies is insufficient to draw conclusions. Moreover, studies in transgenic animal models report higher morbidity and mortality if PA accumulates in tissues. Previous studies on circulating and tissue PA and PRA concentrations suggest that little is known about the physiological processes involved in the control of PA and PRA concentrations in absorption, circulation and tissue distribution as well as the effect of the intestinal microbiome on circulating PA and PRA concentrations. Population-based studies that examine the association between phytol and its metabolites and cancer risk are few and largely limited to prostate cancer. Based on the cytotoxicity and tissue accumulation data, other cancer sites such as brain, liver, kidney and blood are more sensitive to PA and, thus, have a greater chance to show associations in epidemiological studies than other cancer sites. In conclusion, phytol and its metabolites are potential natural agents for cancer prevention if current challenges including cytotoxicity in non-cancer cells and morbidity and mortality associated with PA accumulation in tissue can be mitigated.
Acknowledgements
This study was supported by the Knight Cancer Institute OHSU/OSU Cancer Prevention and Control Initiative (PHR030-PV07 to Y.T.). The funders had no role in the submission of the manuscript. We thank Ms. Alexandra Heisler and Dr. Jessica Keune for their assistance in editing the manuscript.
G.B. and Y.T. was responsible for the conceptualization of this review. G.B., Z.Z. and Y.T. conducted literature search and G.B. drafted the initial manuscript. R.K., M.G., J.S. and Y.T. provided critical comments to the manuscript. All authors reviewed, revised the manuscript and approved the final version.
Conflicts of Interest
There are no conflicts of interest.
References
- Allen NE, Grace PB, Ginn A, Travis RC, Roddam AW, Appleby PN, Key T. Phytanic acid: measurement of plasma concentrations by gas-liquid chromatography-mass spectrometry analysis and associations with diet and other plasma fatty acids. Br J Nutr. 2008; 99:653–659 [DOI] [PubMed] [Google Scholar]
- Amin MB, Epstein JI, Ulbright TM, Humphrey PA, Egevad L, Montironi R, et al. Best practices recommendations in the application of immunohistochemistry in urologic pathology: report from the International Society of Urological Pathology consensus conference. Am J Surg Pathol. 2014; 38:1017–1022 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68:7–30 [DOI] [PubMed] [Google Scholar]
- Baldwin EJ, Gibberd FB, Harley C, Sidey MC, Feher MD, Wierzbicki AS. The effectiveness of long-term dietary therapy in the treatment of adult Refsum disease. J Neurol Neurosurg Psychiatry. 2010; 81:954–957 [DOI] [PubMed] [Google Scholar]
- Baxter JH. Absorption of chlorophyll phytol in normal man and in patients with Refsum’s disease. J Lipid Res. 1968; 9:636–641 [PubMed] [Google Scholar]
- Borges CG, Canani CR, Fernandes CG, Zanatta Â, Seminotti B, Ribeiro CA, et al. Reactive nitrogen species mediate oxidative stress and astrogliosis provoked by in vivo administration of phytanic acid in cerebellum of adolescent rats: a potential contributing pathomechanism of cerebellar injury in peroxisomal disorders. Neuroscience. 2015; 304:122–132 [DOI] [PubMed] [Google Scholar]
- Brown PJ, Mei G, Gibberd FB, Burston D, Mayne PD, Mcclinchy JE, et al. Diet and Refsums disease - the determination of phytanic acid and phytol in certain foods and the application of this knowledge to the choice of suitable convenience foods for patients with Refsums disease. J Hum Nutr Diet. 1993; 6:295–305 [Google Scholar]
- Che BN, Oksbjerg N, Hellgren LI, Nielsen JH, Young JF. Phytanic acid stimulates glucose uptake in a model of skeletal muscles, the primary porcine myotubes. Lipids Health Dis. 2013; 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Wang JR, Huang L, Yang Y, Jiang YM, Guo XJ, et al. Significant association of alpha-methylacyl-coa racemase gene polymorphisms with susceptibility to prostate cancer: a meta-analysis. Asian Pac J Cancer Prev. 2015; 16:1857–1863 [DOI] [PubMed] [Google Scholar]
- Coppack SW, Evans R, Gibberd FB, Clemens ME, Billimoria JD. Can patients with Refsum’s disease safely eat green vegetables?. Br Med J (Clin Res Ed). 1988; 296:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaunsi GS, Alsaeid M, Akhtar S. Phytanic acid activates NADPH oxidase through transactivation of epidermal growth factor receptor in vascular smooth muscle cells. Lipids Health Dis. 2016; 15:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaunsi GS, Alsaeid M, Akhtar S. Phytanic acid attenuates insulin-like growth factor-1 activity via nitric oxide-mediated γ-secretase activation in rat aortic smooth muscle cells: possible implications for pathogenesis of infantile Refsum disease. Pediatr Res. 2017; 81:531–536 [DOI] [PubMed] [Google Scholar]
- Ellinghaus P, Wolfrum C, Assmann G, Spener F, Seedorf U. Phytanic acid activates the peroxisome proliferator-activated receptor alpha (pparalpha) in sterol carrier protein 2-/ sterol carrier protein x-deficient mice. J Biol Chem. 1999; 274:2766–2772 [DOI] [PubMed] [Google Scholar]
- Elmazar MM, El-Abhar HS, Schaalan MF, Farag NA. Phytol/phytanic acid and insulin resistance: potential role of phytanic acid proven by docking simulation and modulation of biochemical alterations. PLoS One. 2013; 8:e45638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmazar MM, Nau H. Potentiation of the teratogenic effects induced by coadministration of retinoic acid or phytanic acid/phytol with synthetic retinoid receptor ligands. Arch Toxicol. 2004; 78:660–668 [DOI] [PubMed] [Google Scholar]
- Gloerich J, van den Brink DM, Ruiter JP, van Vlies N, Vaz FM, Wanders RJ, Ferdinandusse S. Metabolism of phytol to phytanic acid in the mouse, and the role of pparalpha in its regulation. J Lipid Res. 2007; 48:77–85 [DOI] [PubMed] [Google Scholar]
- Goto T, Takahashi N, Kato S, Egawa K, Ebisu S, Moriyama T, et al. Phytol directly activates peroxisome proliferator-activated receptor alpha (pparalpha) and regulates gene expression involved in lipid metabolism in pparalpha-expressing hepg2 hepatocytes. Biochem Biophys Res Commun. 2005; 337:440–445 [DOI] [PubMed] [Google Scholar]
- Grings M, Tonin AM, Knebel LA, Zanatta A, Moura AP, Filho CS, et al. Phytanic acid disturbs mitochondrial homeostasis in heart of young rats: a possible pathomechanism of cardiomyopathy in Refsum disease. Mol Cell Biochem. 2012; 366:335–343 [DOI] [PubMed] [Google Scholar]
- Gu H, Huang T, Shen Y, Liu Y, Zhou F, Jin Y, et al. Reactive oxygen species-mediated tumor microenvironment transformation: the mechanism of radioresistant gastric cancer. Oxid Med Cell Longev. 2018; 2018:5801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen RP. 3,7,11,15-tetramethylhexadecanoic acid: its occurrence in the tissues of humans afflicted with Refsum’s syndrome. Biochim Biophys Acta. 1965; 106:304–310 [DOI] [PubMed] [Google Scholar]
- Hansen RP. Phytol - its metabolic products and their distribution - a review. New Zeal J Sci. 1980; 23:259–275 [Google Scholar]
- Hashimoto T, Shimizu N, Kimura T, Takahashi Y, Ide T. Polyunsaturated fats attenuate the dietary phytol-induced increase in hepatic fatty acid oxidation in mice. J Nutr. 2006; 136:882–886 [DOI] [PubMed] [Google Scholar]
- Heim M, Johnson J, Boess F, Bendik I, Weber P, Hunziker W, Fluhmann B. Phytanic acid, a natural peroxisome proliferator-activated receptor (PPAR) agonist, regulates glucose metabolism in rat primary hepatocytes. FASEB J. 2002; 16:718–720 [DOI] [PubMed] [Google Scholar]
- Idel S, Ellinghaus P, Wolfrum C, Nofer JR, Gloerich J, Assmann G, et al. Branched chain fatty acids induce nitric oxide-dependent apoptosis in vascular smooth muscle cells. J Biol Chem. 2002; 277:49319–49325 [DOI] [PubMed] [Google Scholar]
- Islam MT, de Alencar MV, da Conceição Machado K, da Conceição Machado K, de Carvalho Melo-Cavalcante AA, de Sousa DP, de Freitas RM. Phytol in a pharma-medico-stance. Chem Biol Interact. 2015; 240:60–73 [DOI] [PubMed] [Google Scholar]
- Kahlert S, Schönfeld P, Reiser G. The Refsum disease marker phytanic acid, a branched chain fatty acid, affects ca2+ homeostasis and mitochondria, and reduces cell viability in rat hippocampal astrocytes. Neurobiol Dis. 2005; 18:110–118 [DOI] [PubMed] [Google Scholar]
- Kataria Y, Wright M, Deaton RJ, Rueter EE, Rybicki BA, Moser AB, et al. Dietary influences on tissue concentrations of phytanic acid and AMACR expression in the benign human prostate. Prostate. 2015; 75:200–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CW, Lee HJ, Jung JH, Kim YH, Jung DB, Sohn EJ, et al. Activation of caspase-9/3 and inhibition of epithelial mesenchymal transition are critically involved in antitumor effect of phytol in hepatocellular carcinoma cells. Phytother Res. 2015; 29:1026–1031 [DOI] [PubMed] [Google Scholar]
- Komiya T, Kyohkon M, Ohwaki S, Eto J, Katsuzaki H, Imai K, et al. Phytol induces programmed cell death in human lymphoid leukemia molt 4B cells. Int J Mol Med. 1999; 4:377–380 [DOI] [PubMed] [Google Scholar]
- Kruska N, Reiser G. Phytanic acid and pristanic acid, branched-chain fatty acids associated with Refsum disease and other inherited peroxisomal disorders, mediate intracellular ca2+ signaling through activation of free fatty acid receptor GPR40. Neurobiol Dis. 2011; 43:465–472 [DOI] [PubMed] [Google Scholar]
- Leipnitz G, Amaral AU, Zanatta A, Seminotti B, Fernandes CG, Knebel LA, et al. Neurochemical evidence that phytanic acid induces oxidative damage and reduces the antioxidant defenses in cerebellum and cerebral cortex of rats. Life Sci. 2010; 87:275–280 [DOI] [PubMed] [Google Scholar]
- Liska J, Macejova D, Ondkova S, Brtko J. Morphology of 1-methyl-1-nitrosourea induced rat mammary tumours after treatment with precursor of phytanic acid or its combination with vitamin D analogue. Endocr Regul. 2012; 46:21–26 [DOI] [PubMed] [Google Scholar]
- Lloyd MD, Darley DJ, Wierzbicki AS, Threadgill MD. Alpha-methylacyl-coa racemase–an ‘obscure’ metabolic enzyme takes centre stage. FEBS J. 2008; 275:1089–1102 [DOI] [PubMed] [Google Scholar]
- Lloyd MD, Yevglevskis M, Lee GL, Wood PJ, Threadgill MD, Woodman TJ. Α-methylacyl-coa racemase (AMACR): metabolic enzyme, drug metabolizer and cancer marker P504S. Prog Lipid Res. 2013; 52:220–230 [DOI] [PubMed] [Google Scholar]
- Mackie JT, Atshaves BP, Payne HR, McIntosh AL, Schroeder F, Kier AB. Phytol-induced hepatotoxicity in mice. Toxicol Pathol. 2009; 37:201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmendier CL, Jonniaux G, Voet W, Van Den Bergen CJ. Fatty acid composition of tissues in Refsum’s disease (herodopathia atactica polyneuritiformis). Estimation of total phytanic acid accumulation. Biomedicine. 1974; 20:398–407 [PubMed] [Google Scholar]
- Mobley JA, Leav I, Zielie P, Wotkowitz C, Evans J, Lam YW, et al. Branched fatty acids in dairy and beef products markedly enhance alpha-methylacyl-coa racemase expression in prostate cancer cells in vitro. Cancer Epidemiol Biomarkers Prev. 2003; 12:775–783 [PubMed] [Google Scholar]
- Mönnig G, Wiekowski J, Kirchhof P, Stypmann J, Plenz G, Fabritz L, et al. Phytanic acid accumulation is associated with conduction delay and sudden cardiac death in sterol carrier protein-2/sterol carrier protein-x deficient mice. J Cardiovasc Electrophysiol. 2004; 15:1310–1316 [DOI] [PubMed] [Google Scholar]
- Nagai K. Phytanic acid induces neuro2a cell death via histone deacetylase activation and mitochondrial dysfunction. Neurotoxicol Teratol. 2015; 48:33–39 [DOI] [PubMed] [Google Scholar]
- Ollberding NJ, Aschebrook-Kilfoy B, Caces DB, Wright ME, Weisenburger DD, Smith SM, Chiu BC. Phytanic acid and the risk of non-hodgkin lymphoma. Carcinogenesis. 2013; 34:170–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang B, Bracken B, Burke B, Chung E, Liang J, Ho SM. A duplex quantitative polymerase chain reaction assay based on quantification of alpha-methylacyl-coa racemase transcripts and prostate cancer antigen 3 in urine sediments improved diagnostic accuracy for prostate cancer. J Urol. 2009; 181:2508–2513; discussion 2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejin B, Kojic V, Bogdanovic G. An insight into the cytotoxic activity of phytol at in vitro conditions. Nat Prod Res. 2014; 28:2053–2056 [DOI] [PubMed] [Google Scholar]
- Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer. 2012; 12:181–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AJ, Allen NE, Appleby PN, Crowe FL, Jenab M, Rinaldi S, et al. Plasma phytanic acid concentration and risk of prostate cancer: results from the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2010; 91:1769–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser G, Schönfeld P, Kahlert S. Mechanism of toxicity of the branched-chain fatty acid phytanic acid, a marker of Refsum disease, in astrocytes involves mitochondrial impairment. Int J Dev Neurosci. 2006; 24:113–122 [DOI] [PubMed] [Google Scholar]
- Roca-Saavedra P, Mariño-Lorenzo P, Miranda JM, Porto-Arias JJ, Lamas A, Vazquez BI, et al. Phytanic acid consumption and human health, risks, benefits and future trends: a review. Food Chem. 2017; 221:237–247 [DOI] [PubMed] [Google Scholar]
- Rönicke S, Kruska N, Kahlert S, Reiser G. The influence of the branched-chain fatty acids pristanic acid and Refsum disease-associated phytanic acid on mitochondrial functions and calcium regulation of hippocampal neurons, astrocytes, and oligodendrocytes. Neurobiol Dis. 2009; 36:401–410 [DOI] [PubMed] [Google Scholar]
- Samimi A, Kalantari H, Lorestani MZ, Shirzad R, Saki N. Oxidative stress in normal hematopoietic stem cells and leukemia. Apmis. 2018; 126:284–294 [DOI] [PubMed] [Google Scholar]
- Schluter A, Giralt M, Iglesias R, Villarroya F. Phytanic acid, but not pristanic acid, mediates the positive effects of phytol derivatives on brown adipocyte differentiation. FEBS Lett. 2002; 517:83–86 [DOI] [PubMed] [Google Scholar]
- Schönfeld P, Kahlert S, Reiser G. In brain mitochondria the branched-chain fatty acid phytanic acid impairs energy transduction and sensitizes for permeability transition. Biochem J. 2004; 383:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfeld P, Kahlert S, Reiser G. A study of the cytotoxicity of branched-chain phytanic acid with mitochondria and rat brain astrocytes. Exp Gerontol. 2006; 41:688–696 [DOI] [PubMed] [Google Scholar]
- Schönfeld P, Reiser G. Rotenone-like action of the branched-chain phytanic acid induces oxidative stress in mitochondria. J Biol Chem. 2006; 281:7136–7142 [DOI] [PubMed] [Google Scholar]
- Schönfeld P, Wojtczak L. Fatty acids decrease mitochondrial generation of reactive oxygen species at the reverse electron transport but increase it at the forward transport. Biochim Biophys Acta. 2007; 1767:1032–1040 [DOI] [PubMed] [Google Scholar]
- Selkälä EM, Nair RR, Schmitz W, Kvist AP, Baes M, Hiltunen JK, Autio KJ. Phytol is lethal for AMACR-deficient mice. Biochim Biophys Acta. 2015; 1851:1394–1405 [DOI] [PubMed] [Google Scholar]
- Silva RO, Sousa FB, Damasceno SR, Carvalho NS, Silva VG, Oliveira FR, et al. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam Clin Pharmacol. 2014; 28:455–464 [DOI] [PubMed] [Google Scholar]
- Skrbic TR, Cumings JN. Phytanic acid in tissue lipids in Refsum’s disease. Clin Chim Acta. 1969; 23:17–21 [DOI] [PubMed] [Google Scholar]
- Steinberg D. Scriver CR, Beaudet AA, Sly WS, Valle D. Refsum disease. Metabolic Basis of Inherited Disease. 1989, New York, New York: McGraw Hill; 1533–1550 [Google Scholar]
- Steinberg D, Vroom FQ, Engel WK, Cammermeyer J, Mize CE, Avigan J. Refsum’s disease--a recently characterized lipidosis involving the nervous system. Combined clinical staff conference at the National Institutes of Health. Ann Intern Med. 1967; 66:365–395 [DOI] [PubMed] [Google Scholar]
- Tang XH, Suh MJ, Li R, Gudas LJ. Cell proliferation inhibition and alterations in retinol esterification induced by phytanic acid and docosahexaenoic acid. J Lipid Res. 2007; 48:165–176 [DOI] [PubMed] [Google Scholar]
- Thakor P, Subramanian RB, Thakkar SS, Ray A, Thakkar VR. Phytol induces ROS mediated apoptosis by induction of caspase 9 and 3 through activation of TRAIL, FAS and TNF receptors and inhibits tumor progression factor glucose 6 phosphate dehydrogenase in lung carcinoma cell line (A549). Biomed Pharmacother. 2017; 92:491–500 [DOI] [PubMed] [Google Scholar]
- Thornburg T, Turner AR, Chen YQ, Vitolins M, Chang B, Xu J. Phytanic acid, AMACR and prostate cancer risk. Future Oncol. 2006; 2:213–223 [DOI] [PubMed] [Google Scholar]
- van den Brink DM, van Miert JN, Dacremont G, Rontani JF, Wanders RJ. Characterization of the final step in the conversion of phytol into phytanic acid. J Biol Chem. 2005; 280:26838–26844 [DOI] [PubMed] [Google Scholar]
- Verhoeven NM, Jakobs C. Human metabolism of phytanic acid and pristanic acid. Prog Lipid Res. 2001; 40:453–466 [DOI] [PubMed] [Google Scholar]
- Vetter W, Schroder M. Concentrations of phytanic acid and pristanic acid are higher in organic than in conventional dairy products from the German market. Food Chem. 2010; 119:746–752 [Google Scholar]
- Wanders RJ. Metabolic functions of peroxisomes in health and disease. Biochimie. 2014; 98:36–44 [DOI] [PubMed] [Google Scholar]
- Wang J, Hu X, Ai W, Zhang F, Yang K, Wang L, et al. Phytol increases adipocyte number and glucose tolerance through activation of PI3K/akt signaling pathway in mice fed high-fat and high-fructose diet. Biochem Biophys Res Commun. 2017; 489:432–438 [DOI] [PubMed] [Google Scholar]
- Werner LB, Hellgren LI, Raff M, Jensen SK, Petersen RA, Drachmann T, Tholstrup T. Effect of dairy fat on plasma phytanic acid in healthy volunteers–a randomized controlled study. Lipids Health Dis. 2011; 10:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner LB, Hellgren LI, Raff M, Jensen SK, Petersen R A, Drachmann T, Tholstrup T. Effects of butter from mountain-pasture grazing cows on risk markers of the metabolic syndrome compared with conventional danish butter: a randomized controlled study. Lipids Health Dis. 2013; 12:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Cancer Research Fund and American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. 2018, Washington, DC.: American Association for Cancer Research [Google Scholar]
- Wright ME, Albanes D, Moser AB, Weinstein SJ, Snyder K, Männistö S, Gann PH. Serum phytanic and pristanic acid levels and prostate cancer risk in Finnish smokers. Cancer Med. 2014; 3:1562–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright ME, Bowen P, Virtamo J, Albanes D, Gann PH. Estimated phytanic acid intake and prostate cancer risk: a prospective cohort study. Int J Cancer. 2012; 131:1396–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JL, Neuhouser ML, Lin DW, Kwon EM, Feng Z, Ostrander EA, et al. AMACR polymorphisms, dietary intake of red meat and dairy and prostate cancer risk. Prostate. 2011; 71:498–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Thornburg T, Turner AR, Vitolins M, Case D, Shadle J, et al. Serum levels of phytanic acid are associated with prostate cancer risk. Prostate. 2005; 63:209–214 [DOI] [PubMed] [Google Scholar]
- Yang K, Wang L, Zhou G, Lin X, Peng J, Wang L, et al. Phytol promotes the formation of slow-twitch muscle fibers through PGC-1α/mirna but not mitochondria oxidation. J Agric Food Chem. 2017; 65:5916–5925 [DOI] [PubMed] [Google Scholar]
- Zhou M, Chinnaiyan AM, Kleer C G, Lucas PC, Rubin MA. Alpha-methylacyl-coa racemase: a novel tumor marker over-expressed in several human cancers and their precursor lesions. Am J Surg Pathol. 2002; 26:926–931 [DOI] [PubMed] [Google Scholar]
- Zomer AW, Jansen GA, Van Der Burg B, Verhoeven NM, Jakobs C, Van Der Saag PT, et al. Phytanoyl-coa hydroxylase activity is induced by phytanic acid. Eur J Biochem. 2000; 267:4063–4067 [DOI] [PubMed] [Google Scholar]


