Abstract
Background:
The CREST-2 Registry (C2R) was approved by NINDS-NIH in September 2014 with CMS, FDA and industry collaboration to enroll patients undergoing CAS. The Registry credentials interventionists and promotes optimal patient selection, procedural-technique and outcomes.
Objectives:
We report periprocedural outcomes in a cohort of carotid artery stenting (CAS) performed for asymptomatic and symptomatic carotid stenosis.
Methods:
Asymptomatic patients with ≥70% and symptomatic patients with ≥50% carotid stenosis, aged ≤80 years, and at standard or high-risk for carotid endarterectomy are eligible for enrollment. Interventionists are credentialed by a multi-specialty committee that reviews experience, lesion-selection, technique, and outcomes. The primary endpoint was a composite of stroke and death (S/D) in the 30-day periprocedural period. Myocardial infarction and access-site complications were assessed as secondary outcomes.
Results:
As of December 2018, 187 interventionists from 98 sites in the US performed 2219 CAS procedures in 2141 patients with primary atherosclerosis (78 were bilateral). The mean age of the cohort was 68 years, 65% were male, and 92% were white; 1180 (55%) were for asymptomatic disease, and 961 (45%) were for symptomatic disease. All FDA-approved stents and embolic protection devices were represented. The 30-day rate of S/D was 1.4% for asymptomatic, 2.8% for symptomatic, and 2.0% for all patients.
Conclusions:
C2R is the first national registry for CAS co-sponsored by federal and industry partners. CAS was performed by experienced operators using appropriate patient selection and optimal technique. In that setting, a broad group of interventionists achieved very low periprocedural S/D rates for asymptomatic and symptomatic patients.
Keywords: Carotid, stenosis, stenting, symptomatic, asymptomatic, registry
CONDENSED ABSTRACT
The CREST-2 Registry (C2R) was approved on September 17, 2014 and is the first national registry for carotid artery stenting (CAS) co-sponsored by federal and industry partners. Patients with asymptomatic ≥70% and symptomatic ≥50% stenosis, aged ≤80 years, and at standard or high risk for carotid endarterectomy are eligible for enrollment. As of December 31, 2018, 187 interventionists from 98 sites performed 2219 procedures in 2141 patients with primary atherosclerosis (78 bilateral, 1228 asymptomatic, 991 symptomatic). All FDA-approved devices were represented. The 30-day rate of stroke or death was 1.4% for asymptomatic, 2.8% for symptomatic, and 2.0% for all patients.
INTRODUCTION
Carotid artery stenting (CAS) has been shown in two prospective multicenter, randomized trials to have equivalent outcomes compared to carotid endarterectomy (CEA) in terms of the composite endpoint of periprocedural stroke, death, and myocardial infarction (MI) and late ipsilateral stroke prevention.(1–3) The carotid procedures in these and other trials were performed over a decade ago. At that time, higher periprocedural stroke rates after CAS were offset by higher rates of MI after CEA.(1,3–5) Since then, CAS methods have evolved with better patient selection, refinement of technique and improved technology.(6)
The Medicare coverage decision in 2005 allowed reimbursement for CAS performed in a subset of patients with carotid artery stenosis deemed to be at high risk for CEA.(7) However, most carotid occlusive disease exists in an asymptomatic standard surgical risk patient population. The Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Study (CREST-2) is a set of two multicenter randomized trials run in parallel.(8,9) One trial assesses treatment differences between CEA plus intensive medical management (IMM) versus IMM alone. The other assesses treatment differences between CAS plus IMM versus IMM alone. Reimbursement rules over the last decade have caused the number of CAS procedures performed in the US to decline considerably.(10),(11) The CREST-2 Registry (C2R) was therefore initiated as a companion to the CREST-2 trial to provide the means to maintain, enhance, and ensure requisite expertise in CAS in order to conduct a rigorous randomized controlled clinical trial comparing CAS to IMM alone.
There are no large, prospective, comprehensive analyses reporting contemporary CAS outcomes in North America. The C2R provides such a multicenter dataset by ensuring responsible procedural oversight through a national quality assurance program based on a collaborative effort by the National Institutes of Health (NIH), Centers for Medicare and Medicaid Services (CMS), the Society for Vascular Surgery, the American College of Cardiology, Industry partners, and the CREST-2 leadership. It represents outcomes from the full spectrum of specialties and operators performing CAS with acceptable experience. A large number of operators from academic and community medical centers are included. We report the periprocedural outcomes for CAS performed in patients with asymptomatic and symptomatic, high and low surgical risk carotid stenosis from primary atherosclerosis.
METHODS
Design
In September 2014, the National Institute of Neurological Disorders and Stroke (NINDS)-NIH approved the C2R protocol. The CMS subsequently expanded reimbursement coverage for CAS within C2R on September 17, 2014, to include all categories of patients with carotid stenosis. Coverage was predicated on rigorous accreditation mechanisms by a multi-specialty physician group from CREST-2. The C2R oversight model is designed to achieve safety by ensuring that patients are carefully selected and treated by qualified, proficient providers at centers experienced in caring for patients with carotid disease. The goal is to enroll consecutive patients with asymptomatic or symptomatic carotid stenosis undergoing CAS by the selected interventionists at participating sites. A small number of CREST-2 trial-eligible standard-risk asymptomatic patients are unable to join the trial due to patient refusal. These patients are permitted enrollment into C2R to undergo CAS. Information on patient demographics, vascular risk factors, symptomatic status, and degree of stenosis is collected at baseline. Procedural details such as stent and filter type, as well as intra-procedural adverse events, are also recorded. Periprocedural stroke and other adverse events are recorded at the local site 30 days post-procedure. Enrollment in C2R is ongoing, and this report includes patients enrolled from September 17, 2014 through December 31, 2018, inclusive. It also analyzes outcomes of a subset of CREST-2 trial-eligible patients enrolled in C2R. The clinicaltrials.gov identifier for the Registry is . The C2R administrative center performs data quality checks to ensure data accuracy. The C2R protocol was reviewed by the University of Maryland institutional review board and granted a waiver of written informed consent.
Patient eligibility
Patients are ≥18 and ≤80 years of age. They are eligible with asymptomatic ≥70% carotid stenosis, or symptomatic (transient ischemic attack [TIA], stroke, or amaurosis fugax within 180 days before CAS procedure) ≥50% carotid stenosis. They can be standard surgical risk, high anatomic risk or high physiologic risk for CEA. Patients are excluded for any one of the following conditions: New York Heart Association (NYHA) Class IV congestive heart failure, chronic obstructive pulmonary disease on chronic continuous oxygen therapy, severe (Childs Class D) liver failure, cancer with metastatic spread and/or undergoing active chemotherapy, any dementia considered greater than “mild,” and a modified Rankin Scale (mRS) >3.
Site selection
Sites are selected based on a high and consistent caseload of patients with carotid stenosis, a history of successful participation in clinical trials, collaborative and multidisciplinary expertise, availability of staff certified in the NIH stroke scale (NIHSS)(12) and mRS(13), and availability of a facility certified for carotid ultrasound.
Interventionist selection
As part of the operation of C2R, prospective interventionists are reviewed by the multi-specialty Interventional Management Committee (IMC) of CREST-2. The IMC reviews interventionist experience, technique, appropriate lesion-selection, and quality of outcomes based on procedural notes and discharge summaries to identify the group of interventionists who will be invited to participate in C2R. A minimum career case-volume of 50 CAS procedures, and recent-case rate of 8 CAS procedures in the past 12 months are required to be considered for the Registry.
Procedural protocol
Prospective interventionists have to undergo a web-based or slide-based training program for the CREST-2 CAS protocol prior to enrolling patients into C2R. The training program is based on lessons learned from CREST and describes optimal patient and lesion selection, procedural technique and reporting methodology.(1,14,15) Avoidance of adverse aortic arch anatomy (Type 3), tortuous common or internal carotid arterial anatomy (two 90 degree turns), and non-contiguous or long lesions (>3 cm) is strongly encouraged. Prolonged filter dwell times, overuse of intraarterial contrast runs, and aggressive post-stenting angioplasty with large (>5.0 mm) balloons is discouraged. The use of pre-procedural statins, peri-procedural dual antiplatelet therapy, and intra-procedural anticoagulation is reinforced. Interventionists are permitted to use any Food and Drug Administration (FDA)-approved carotid stent (open- or closed-cell and straight or tapered configurations) and embolic protection device (distal filter or proximal flow-reversal) of their choice. Permitted closed-cell stent configurations include Xact (Abbott Vascular, Santa Clara, CA), WallStent (Boston Scientific, Marlborough, MA) or Scaffold (W. L. Gore & Associates, Inc., Flagstaff, AZ), and open-cell stents include Rx Acculink (Abbott Vascular), Precise (Cordis-Cardinal Health, Santa Clara, CA) or Protégé Rx (Medtronic, Minneapolis, MN). Protective filters that are permitted include Rx Accunet and Emboshield Nav6 (Abbott Vascular), Filterwire EZ (Boston Scientific), Angioguard (Cordis-Cardinal Health), Spider Fx (Medtronic), or Gore embolic filter (W. L. Gore & Associates, Inc.) and flow-reversal protection devices include MoMa (Medtronic) or Gore devices (W. L. Gore & Associates); transcarotid flow reversal was excluded. The choice of anesthesia is left to the discretion of the interventionist, though general anesthesia is discouraged.
Assessment of endpoints
The primary endpoint in this analysis is a composite of stroke and death (S/D) during the periprocedural period. Neurologic examination and the NIHSS assessments are performed at baseline and 30 days after stenting at which time patients are also queried for any events that may have occurred after discharge and prior to their follow-up visit. A stroke is defined as a clinically detected neurological deficit consistent with stroke or an increase in the NIHSS of ≥2 points. An evaluation for MI is initiated on clinical suspicion and defined by an elevation of cardiac enzymes based on the thresholds of the local clinical center, in addition to either chest pain or symptoms consistent with ischemia or EKG evidence of ischemia. Access site complications are considered significant if there is a need for blood transfusion or surgical evacuation/repair.
Data management and analysis
The Registry takes advantage of pre-existing infrastructure to create a cost-effective National Quality Assurance Program for carotid stenting. Data is submitted prospectively through the Society for Vascular Surgery Vascular Quality Initiative (VQI) and the American College of Cardiology National Cardiovascular Data Registry (NCDR) data-entry portals, from sites participating in those respective registries. Interventionists submit additional supplementary data on each procedure, directly to the C2R Administrative Center. The Administrative center then collates the information from all three sources to create the C2R database. The supplementary data includes repeated questions on primary outcome measures and procedural details. This allows assessments for internal consistency and data accuracy between two separate sources of information. In addition, data for each procedure are examined for internal consistency between direct information on clinical events (stroke, TIA) and stroke severity measures recorded by sites (NIHSS and mRS). Sites are contacted for corrections when any discordant data are identified. A third layer of quality control includes the subset of C2R cases submitted for review to the IMC by interventionists for consideration to commence enrollment into CREST-2. The number of such cases submitted for IMC review ranges from 3–20 cases per interventionist. The information reviewed includes operative notes, discharge summaries and procedural angiograms. These provide an additional opportunity to cross-check the data submitted to C2R. Over 200 such case reviews were possible within the procedures reported in this analysis, and sites were contacted for corrections when any discordant data were identified. Data aggregation was conducted by the C2R statistical team. The procedural characteristics, patient characteristics and adverse event rates (events per 100 procedures) were tabulated using SAS Version 9.4 (SAS Institute, Cary, North Carolina). The data were analyzed by an independent statistical team with no input from the industry funding agencies which also did not have access to the data.
RESULTS
Patient Demographics and Procedural Details
Over the 52-month enrollment period, 3461 CAS procedures were entered into the Registry by the selected interventionists. Indications for the procedures included asymptomatic or symptomatic carotid stenosis from primary atherosclerosis (2330, 67.3%), ipsilateral restenosis (912, 26.4%), and other less frequent conditions (219, 6.3%) such as carotid trauma, dissection or fibromuscular dysplasia. Of the 2330 cases performed for primary atherosclerosis, follow-up data was not available for 111 cases (4.8%). The remaining 2219 CAS procedures were done in 2141 patients (78 for bilateral disease). All subsequent results pertain to the first procedure for each patient (n=2141) in this group. Among the asymptomatic patients in C2R, 264 (22.4%) were potentially eligible for the CREST-2 trial but could not be enrolled in the trial due to patient refusal; these patients underwent CAS under the Registry, and their data were included in C2R. Demographic and clinical features of the patients, and characteristics of lesions undergoing CAS for primary atherosclerosis are presented in Table 1. A total of 1180 CAS procedures were performed on patients with asymptomatic stenoses (55.1%) and 961 (44.9%) on symptomatic stenoses. The mean age of patients undergoing CAS was 67.8 ± 7.8 years (mean ± SD) and a majority were male (65.3%) and white (91.8%). The prevalence of vascular risk factors in the patients was predictably high. The mean percent diameter stenosis was 84.2 ± 9.8% and mean lesion length 21 ± 11 mm.
Table 1.
Baseline Demographic and Clinical Characteristics of Patients Undergoing Carotid Artery Stenting in C2R for Primary Atherosclerosis
| Characteristic - n (%) | All patients 2141* (100%) |
Asymptomatic 1180 (55.1%) |
Symptomatic 961 (44.9%) |
|---|---|---|---|
| Age -yr., mean (SD†) | 67.8 (7.8) | 68.3 (7.5) | 67.1 (8.1) |
| Male sex | 1399 (65.3) | 744 (63.1) | 655 (68.2) |
| White race‡ | 1965 (91.8) | 1082 (91.7) | 883 (92.0) |
| Risk factors§ | |||
| Hypertension | 1824 (88.7) | 1040 (91.5) | 784 (85.2) |
| Diabetes | 846 (39.9) | 488 (41.6) | 358 (37.9) |
| Prior or current smoker | 1597 (74.7) | 894 (75.9) | 703 (73.2) |
| Previous cardiovascular disease | 955 (46.2) | 594 (51.9) | 361 (39.0) |
| Previous coronary-artery bypass | 467 (21.8) | 291 (24.7) | 176 (18.3) |
| Lesion Characteristics | |||
| Percent stenosis at enrollment -mean (SD) | 84.2 (9.8) | 85.0 (8.6) | 83.2 (11.1) |
| Length of lesion, mm - mean (SD) | 21.0 (11.0) | 21.9 (10.8) | 19.8 (11.0) |
| Left carotid artery treated | 1085 (50.7) | 584 (49.5) | 501 (52.1) |
The first C2R-eligible revascularization for 2141 patients
SD = Standard Deviation
Race was self-reported
Missing data for hypertension (86), diabetes (24), previous cardiovascular disease (75), stenosis (14), lesion length (315)
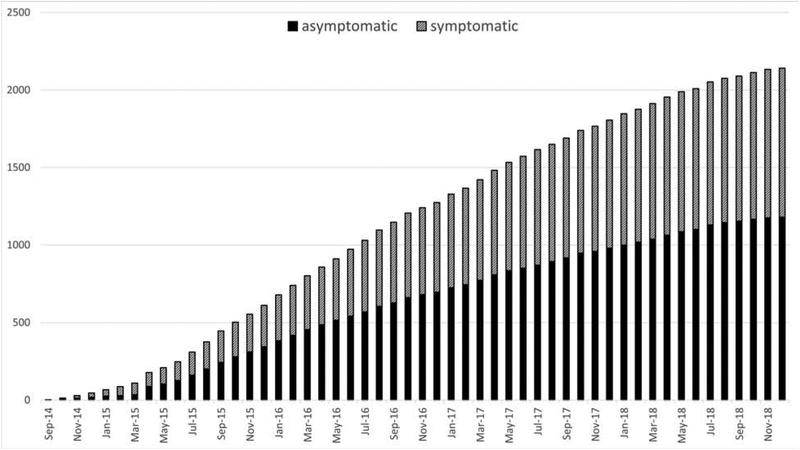
The Registry had 187 selected interventionists from 98 selected sites across 37 states, and included 85 (45.5%) interventional cardiologists, 44 (23.5%) vascular surgeons, 25 (13.4%) interventional radiologists/neuro-radiologists, 23 (12.3%) neurosurgeons and 10 (5.3%) interventional neurologists. Figure 1 shows the cumulative enrollment in C2R. The median number of patients enrolled per interventionist was 8 (IQR 3–18, range 1 to 122). The rate of enrollment has a median of 40.5 patients per month (IQR 25.5–57, range 3–70).
Figure 1. Cumulative Enrollment of Primary Atherosclerosis Cases in the CREST-2 Registry Over Time.
Cumulative enrollment of asymptomatic and symptomatic carotid artery stenting procedures performed from the inception of the CREST-2 Registry through December 31, 2018. The median rate of enrollment was 40.5 (inter-quartile range 25.5 – 57.0) per month and the number of patients enrolled per interventionist was 8 (inter-quartile range 3–18) per month.
Procedural details are presented in Table 2. Most patients were treated with pre-procedural dual antiplatelet therapy (80.9%), pre-procedural statin therapy (76.7%), and post-procedural dual antiplatelet therapy (93.5%). All FDA-approved stents and embolic protection devices were represented in the Registry. A total of 1500 (70.1%) patients were implanted with a closed-cell stent (XACT, Wallstent, and Scaffold), 639 (29.9%) with an open-cell stent (Rx Acculink, Precise Pro, and Protégé), and 1 (0.05%) was treated with balloon angioplasty alone. The procedure was performed with embolic protection in 98.7% of patients. Among these, 1960 (91.5%) used filter-protection and 153 (7.1%) used a flow-reversal device; only 28 (1.3%) were performed without protection. Most of the procedures (94.1%) were performed under local anesthesia.
Table 2.
Details of Carotid Stenting Procedures Performed in C2R for Primary Atherosclerosis
| Characteristic - n (%) | All patients 2141* (100%) |
Asymptomatic 1180 (55.1%) |
Symptomatic 961 (44.9%) |
|---|---|---|---|
| Medications | |||
| Pre-procedure† DAPT‡ | 1733 (80.9) | 964 (81.7) | 769 (80.0) |
| Pre-procedure statin therapy | 1641 (76.7) | 910 (77.2) | 731 (76.1) |
| Post-procedure§ DAPT | 1999 (93.5) | 1096 (93.1) | 903 (94.1) |
| Stents | |||
| Closed cell stents | 1500 (70.1) | 805 (68.3) | 695 (72.5) |
| Open-cell stents | 639 (29.9) | 373 (31.6) | 266 (27.7) |
| No stent used (balloon angioplasty) | 1 (0.05) | 1 (0.08) | 0 (0) |
| Embolic protection | |||
| Distal filter protection | 1960 (91.5) | 1074 (91.0) | 886 (92.2) |
| Proximal flow-reversal | 153 (7.1) | 94 (8.0) | 59 (6.1) |
| No embolic protection | 28 (1.3) | 12 (1.0) | 16 (1.7) |
| Anesthesia** | |||
| Local | 1919 (94.1) | 1066 (95.3) | 853 (92.7) |
| General | 120 (5.9) | 53 (4.7) | 67 (7.3) |
The first C2R-eligible revascularization for 2141 patients
Pre-procedure includes medication taken within 36 hours of the CAS procedure
DAPT (dual anti-platelet therapy) is defined as aspirin + any other antiplatelet
Post-procedure includes medications prescribed to the patient at the time of discharge
“Local” includes minimal sedation (anxiolysis), moderate sedation/analgesia (conscious sedation), local, and regional anesthesia. “General” includes deep sedation/analgesia, local converted to general, and general anesthesia
Clinical Outcomes
Details of outcomes are provided in Table 3. Among all patients with primary atherosclerosis, the periprocedural rate of S/D was 2.0%. Among the 961 patients with symptomatic primary atherosclerosis, the rate of S/D was 2.8%, and there were 4 deaths, 23 strokes, 2 MIs, and 4 major access site complications. Among the 1180 patients with asymptomatic primary atherosclerosis, the rate of S/D was 1.4%, and there were 4 deaths, 12 strokes, 2 MIs, and 9 major access site complications. A portion (n=264, 22.4%) of potentially CREST-2 trial-eligible patients (normal-risk asymptomatic) were enrolled into the Registry and underwent CAS by the selected interventionists. Their results are displayed in Table 4. The rate of S/D among these patients was 0.8% compared to 1.5% for the remaining trial-ineligible patients enrolled in C2R.
Table 3.
Periprocedural Adverse Events among Patients Undergoing Carotid Stenting in C2R for Primary Atherosclerosis
| Adverse event rates - n (%) | All patients 2141* (100%) |
Asymptomatic 1180 (55.1%) |
Symptomatic 961 (44.9%) |
|---|---|---|---|
| Stroke or death (S/D) | 43 (2.0) | 16 (1.4) | 27 (2.8) |
| Any stroke, regardless of laterality | 35 (1.6) | 12 (1.0) | 23 (2.4) |
| Stroke ipsilateral to the index artery | 31 (1.5) | 9 (0.8) | 22 (2.3) |
| Stroke contralateral to the index artery | 10 (0.5) | 5 (0.4) | 5 (0.5) |
| Death | 8 (0.4) | 4 (0.3) | 4 (0.4) |
| Stroke, death, or myocardial infarction | 47 (2.2) | 18 (1.5) | 29 (3.0) |
| Myocardial infarction | 4 (0.2) | 2 (0.2) | 2 (0.2) |
| Stroke, death, MI, or major† access site complications | 59 (2.8) | 27 (2.3) | 32 (3.3) |
| Major access site complications | 13 (0.6) | 9 (0.8) | 4 (0.4) |
The first C2R-eligible revascularization for 2141 patients
Access site complications are considered major if there is a need for blood transfusion or surgical evacuation/repair
Table 4.
Periprocedural Adverse Events among Asymptomatic Patients Undergoing Carotid Stenting in C2R for Primary Atherosclerosis, Stratified by Potential CREST-2 Trial Eligibility
| Adverse event rates - n (%) | All asymptomatic patients 1180* (100%) |
CREST-2 trial ineligible 916 (77.6%) |
CREST-2 trial eligible 264 (22.4%) |
|---|---|---|---|
| Stroke or death | 16 (1.4) | 14 (1.5) | 2 (0.8) |
| Any stroke, regardless of laterality | 12 (1.0) | 11 (12) | 1 (0.4) |
| Stroke ipsilateral to the index artery | 9 (0.8) | 8 (0.9) | 1 (0.4) |
| Stroke contralateral to the index artery | 5 (0.4) | 5 (0.6) | 0 (0) |
| Death | 4 (0.3) | 3 (0.3) | 1 (0.4) |
| Stroke, death, or myocardial infarction | 18 (1.5) | 16 (1.8) | 2 (0.8) |
| Myocardial infarction (MI) | 2 (0.2) | 2 (0.2) | 0 (0) |
| Stroke, death, MI, or major† access site complications | 27 (2.3) | 23 (2.5) | 4 (1.5) |
| Major access site complications | 9 (0.8) | 7 (0.8) | 2 (0.8) |
1180 asymptomatic patients out of the total 2141 C2R patients with primary atherosclerosis
Access site complications are considered major if there is a need for blood transfusion or surgical evacuation/repair
DISCUSSION
This study is the largest, prospective, multicenter registry evaluating contemporary CAS outcomes in the United States. It is the first national registry for CAS co-sponsored by federal and industry partners that creates a cost-effective national quality assurance program for carotid stenting. CAS was performed in C2R by experienced operators using appropriate patient selection and optimal technique. In that setting, a broad group of interventionists achieved very low periprocedural rate of stroke or death for asymptomatic (1.4%) and symptomatic patients (2.8%) undergoing CAS for primary atherosclerosis. C2R represents a protocol-driven disciplined approach to CAS using currently accepted best practices that maintains safe and efficacious CAS outcomes while assisting the credentialing of interventionists for the CREST-2 randomized trial.(11,16–18)
The C2R 30-day rate of S/D of 1.4% for asymptomatic patients compares favorably to the 2.5% rate reported by the Stent Protected Angioplasty versus Carotid Endarterectomy (SPACE-2) trial in 2019.(6) SPACE-2 is a randomized, controlled, multi-center trial comparing CAS to CEA in asymptomatic patients with ≥70% stenosis. SPACE-2 was halted prematurely because of slow enrollment; 513 patients were enrolled, and 197 were randomized to CAS. Thus far patients have been followed for one-year. Similar to CREST-2 utilizing C2R, the SPACE-2 trial required that centers and interventionists enroll in a registry. Interventionists had to have performed ≥40 CAS or ≥20 CAS in the SPACE-1 study with a peri-procedural complication rate ≤6%.
The C2R 30-day rate of S/D is lower than those reported in prior prospective trials and registries where there was comparatively less focus on interventionist competency, patient selection, lesion selection, procedural technique and adjunctive medical therapy.(4,5). Experience and certain elements of technique have previously been documented to be associated with event rates from CAS.(16,19,20) Compared to these earlier studies, interventionists in C2R were more experienced in the performance of CAS. The training for CAS in the low risk asymptomatic patients for C2R and CREST-2 has been further refined. For example, arch and great vessel atherosclerosis, type 3 arches and some type 2 arches, modest circumferential lesion calcification and specific types of vessel and lesion tortuosity are excluded. Adjunctive therapy with dual antiplatelet agents, pre procedure statins, antithrombotic agents and hypertension and hypotension management are better specified. Further, technique has been updated to include specific recommendations for limited procedural angiography, procedure time, balloon size, limited balloon dilatation, short embolic protection filter dwell time and use of closed cell stents if available. The IMC reviewed each interventionist in detail for prior training, total career experience and current procedural volume. Procedural reports and angiographic records were examined. Interventionists had to participate in an interactive webinar on the ideal CAS protocol. They were counselled on cases representing poor patient selection, lesion selection, technique, or medical therapy.
Earlier rigorous, prospective randomized trials have demonstrated good stroke prevention outcomes from both CAS and CEA.(1–3) For example, the 30-day rate of S/D in CREST was 2.5% in asymptomatic patients and 6% in symptomatic patients. In the International Carotid Stenting Study, the 30-day rate of S/D was 7.4%.(21) The results of the present study suggest that if these trials were to be repeated with current CAS experience and practice, the results observed with CAS may be even more favorable.(3,6) The low event rates we observed are also consistent with outcomes in recent but far smaller FDA registries examining emerging device technologies.(22–24) The results are concordant with recently completed registries from Europe.(25) They reported a rate of stroke or death of 2.9% in their large cohort of mostly symptomatic patients, quite similar to the 2.7% seen in symptomatic patients in C2R. The low event rates from C2R for CAS are also comparable to those reported for Trans Carotid Revascularization.(26)
In any randomized trial comparing the efficacy of treatment strategies, it is necessary to optimize the therapies to reflect “state of the art” management practices. CREST-2 is currently examining the efficacy of intensive medical management alone (IMM) against the standard practices of treatment with CEA or CAS plus IMM. For the trial to be credible, it must be performed with minimal periprocedural stroke events in each treatment group. The 1.4% periprocedural rate of S/D observed in the asymptomatic cohort of C2R is reassuring and suggest that the NINDS was sanguine in including CAS as a treatment option in CREST-2. There is no certainty that these outcomes will be reproduced in the trial. However, an analysis of patients in the Registry that met criteria for inclusion in the trial demonstrated nominally more favorable outcomes. The rate of S/D in this subset of CREST-2 eligible patients who could not be enrolled was 0.8%.
Medicare reimbursement for CAS is currently restricted to a small subset of patients designated to be at high risk for CEA. When established, C2R negotiated exemptions that allowed selected practitioners and medical centers to perform CAS for broader indications under controlled conditions and to bill for these services. With this arrangement, the Registry has met its mission of facilitating the credentialing of interventionists to enroll into the randomized trial, while maintaining the safety and efficacy of outcomes. Starting with 30 approved interventionists in 2014, CREST-2 has 187 interventionists entering patients into the trial.
C2R is supported by infrastructure funded by NINDS-NIH with additional support from industry partners and shares some data-collection infrastructure from academic societies. This unique collaboration provides a model for supervision, monitoring and roll-out of new technology requiring specific skill-sets among practitioners across the country. Additional unique aspects of the Registry include careful scrutiny of data submitted by clinical centers to ensure complete and accurate information. Validation of the information is facilitated by a review of multiple streams of data submitted from centers. The model is flexible and scalable, allowing the collection of data (2330 procedures) from a large number of providers (n=187) from multiple centers (n=98) across the country allowing robust analyses that permit reliable conclusions to be drawn.
Limitations
These data from credentialed interventionists may not reflect the results observed in the general community. The results represent estimates of adverse events that can be achieved with experience and appropriate patient selection and technique. However, interventionists from five different specialties succeeded in performing 2141 CAS procedures with low periprocedural events underlining the unique, multidisciplinary character of this endovascular procedure. The registry thus provides valuable guidance on patients suitable for CAS, how the procedure should be performed and what experience is optimal to achieve safe outcomes. It also provides a guide for monitoring performance outcomes for all practitioners. The endpoints were reported by the interventionists rather than by independent evaluators or stroke neurologists, though the protocol incorporated as much cross-checking of information as was possible within a registry framework.
Conclusions
C2R is the first national registry for CAS co-sponsored by federal, industry and academic society partners. Protocol-specified credentialing and training with the goal of optimal patient and lesion selection and optimal technique was accomplished. In that setting, CAS performed by a broad group of experienced interventionists achieved low periprocedural rate of stroke or death in a large cohort of patients with asymptomatic and symptomatic carotid stenosis.
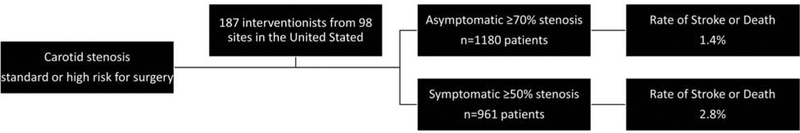
Central Illustration. Outcomes for Stenting of Atherosclerotic Carotid Stenosis in the CREST-2 Registry.
Consecutive patients with standard or high-risk stenosis were enrolled to undergo stenting with all FDA-approved stents and embolic protection devices. Adverse events achieved by interventionists from five different specialties were low for both symptomatic and asymptomatic patients.
CLINICAL PERSPECTIVES.
Competency in Practice-Based Learning and Improvement:
A quality assurance program developed through federal, professional society and industry cooperation that addresses training, credentialing and monitoring of procedural technique and outcomes can assure high quality carotid artery stenting.
Translational Outlook.
Randomized trials will help clarify the role of carotid revascularization as an adjunct to intensive risk factor modification for patients with asymptomatic carotid stenosis.
Acknowledgements:
We acknowledge the contributions of the Earl & Nyda Swanson Neurosciences Research Fund and the Harley N. and Rebecca N. Hotchkiss Endowed Fund in Neuroscience Research, Honoring Ken and Marietta.
Funding: CREST-2 is funded by the National Institute of Neurological Disorders and Stroke through two U01 awards: U01 NS080168 and U01 NS08016, CREST-2 Registry is funded by NINDS-NIH and by Abbott Vascular, Boston Scientific, Cordis-Cardinal, W. L. Gore and Associates, Medtronic and Silk Road Medical.
Abbreviations list:
- CAS
Carotid artery stenting
- CEA
Carotid endarterectomy
- S/D
Stroke or death
- MI
Myocardial infarction
- NINDS
National Institute of Neurological Disorders and Stroke
- TIA
Transient ischemic attack
- mRS
modified Rankin Scale
- NIHSS
NIH stroke scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brott TG, Hobson RW, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus Endarterectomy for Treatment of Carotid-Artery Stenosis. N Engl J Med. 2010. July 30;363(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W, et al. Long-Term Results of Stenting versus Endarterectomy for Carotid-Artery Stenosis. N Engl J Med. 2016. March 17;374(11):1021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC, et al. Randomized Trial of Stent versus Surgery for Asymptomatic Carotid Stenosis. N Engl J Med. 2016. March 17;374(11):1011–20. [DOI] [PubMed] [Google Scholar]
- 4.Brown MM. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): A randomised trial. Lancet. 2001. June 2;357(9270):1729–37. [PubMed] [Google Scholar]
- 5.Gurm HS, Yadav JS, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, et al. Long-Term Results of Carotid Stenting versus Endarterectomy in High-Risk Patients. N Engl J Med. 2008. April 10;358(15):1572–9. [DOI] [PubMed] [Google Scholar]
- 6.Reiff T, Eckstein H, Mansmann U, Jansen O, Fraedrich G, Mudra H, et al. Angioplasty in asymptomatic carotid artery stenosis vs. endarterectomy compared to best medical treatment: One-year interim results of SPACE-2. Int J Stroke. 2019;0(0):174749301983301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CMS. Coverage Decision Memorandum for Carotid Artery Stenting. CMS.gov; 2005. [Google Scholar]
- 8.Howard VJ, Meschia JF, Lal BK, Turan TN, Roubin GS, Brown RD, et al. Carotid revascularization and medical management for asymptomatic carotid stenosis: Protocol of the CREST-2 clinical trials. Int J Stroke. 2017;12(7):770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lal BK, Meschia JF, Brott TG. Clinical need, design, and goals for the Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis trial. Semin Vasc Surg. 2017. March 1;30(1):2–7. [DOI] [PubMed] [Google Scholar]
- 10.Lichtman JH, Jones MR, Leifheit EC, Sheffet AJ, Howard G, Lal BK, et al. Carotid Endarterectomy and Carotid Artery Stenting in the US Medicare Population, 1999–2014. JAMA. 2017. September 19;318(11):1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lal BK, Meschia JF, Roubin GS, Jankowitz B, Heck D, Jovin TG, et al. Factors Influencing Credentialing of Interventionists in the CREST-2 Trial. J Vasc Surg. 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brott T, Adams HP Jr., Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–70. [DOI] [PubMed] [Google Scholar]
- 13.Banks JL, Marotta CA. Outcomes Validity and Reliability of the Modified Rankin Scale: Implications for Stroke Clinical Trials. Stroke. 2007. March 1;38(3):1091–6. [DOI] [PubMed] [Google Scholar]
- 14.Roubin GS, Iyer S, Halkin A, Vitek J, Brennan C. Realizing the Potential of Carotid Artery Stenting. Circulation. 2006. April 25;113(16):2021–30. [DOI] [PubMed] [Google Scholar]
- 15.Lal BK, Brott TG. The Carotid Revascularization Endarterectomy vs. Stenting Trial completes randomization: Lessons learned and anticipated results. Vol. 50, Journal of Vascular Surgery. 2009. p. 1224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore WS, Popma JJ, Roubin GS, Voeks JH, Cutlip DE, Jones M, et al. Carotid angiographic characteristics in the CREST trial were major contributors to periprocedural stroke and death differences between carotid artery stenting and carotid endarterectomy. J Vasc Surg. 2016. April 1;63(4):851–858.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roubin GS. Credentialing operators for carotid artery stenting accepting the occasional bad apple or insisting on airline industry proficiency. JACC Cardiovasc Interv. 2014;7(11):1318–9. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins LN, Roubin GS, Chakhtoura EY, Gray WA, Ferguson RD, Katzen BT, et al. The Carotid Revascularization Endarterectomy versus Stenting Trial: Credentialing of Interventionalists and Final Results of Lead-in Phase. J Stroke Cerebrovasc Dis. 2010. March 1;19(2):153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shishehbor MH, Venkatachalam S, Gray WA, Metzger C, Lal BK, Peng L, et al. Experience and Outcomes With Carotid Artery Stenting: An Analysis of the CHOICE Study (Carotid Stenting for High Surgical-Risk Patients; Evaluating Outcomes Through the Collection of Clinical Evidence). JACC Cardiovasc Interv. 2014. November 1;7(11):1307–17. [DOI] [PubMed] [Google Scholar]
- 20.Roubin GS. Credentialing Operators for Carotid Artery Stenting: Accepting the Occasional “Bad Apple” or Insisting on Airline Industry “Proficiency”. JACC Cardiovasc Interv. 2014. November 1;7(11):1318–9. [DOI] [PubMed] [Google Scholar]
- 21.Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ansel GM, Hopkins LN, Jaff MR, Rubino P, Bacharach JM, Scheinert D, et al. Safety and effectiveness of the INVATEC MO.MA® proximal cerebral protection device during carotid artery stenting: Results from the ARMOUR pivotal trial. Catheter Cardiovasc Interv. 2010. January 25;76(1):1–8. [DOI] [PubMed] [Google Scholar]
- 23.Schneider PA, Levy E, Bacharach JM, Metzger DC, Randall B, Garcia A, et al. A First-in-Human Evaluation of a Novel Mesh-Covered Stent for Treatment of Carotid Stenosis in Patients at High Risk for Endarterectomy. JACC Cardiovasc Interv. 2018. December 10;11(23):2396–404. [DOI] [PubMed] [Google Scholar]
- 24.Langhoff R, Schofer J, Scheinert D, Schmidt A, Sedgewick G, Saylors E, et al. Double Filtration During Carotid Artery Stenting Using a Novel Post-Dilation Balloon With Integrated Embolic Protection. JACC Cardiovasc Interv. 2019. February 25;12(4):395–403. [DOI] [PubMed] [Google Scholar]
- 25.González García A, Moniche F, Escudero-Martínez I, Mancha F, Tomasello A, Ribó M, et al. Clinical Predictors of Hyperperfusion Syndrome Following Carotid Stenting: Results From a National Prospective Multicenter Study. JACC Cardiovasc Interv. 2019. May 13;12(9):873–82. [DOI] [PubMed] [Google Scholar]
- 26.Kwolek CJ, Jaff MR, Leal JI, Hopkins LN, Shah RM, Hanover TM, et al. Results of the ROADSTER multicenter trial of transcarotid stenting with dynamic flow reversal. J Vasc Surg. 2015. November;62(5):1227–1234.e1. [DOI] [PubMed] [Google Scholar]