Abstract
Background:
Pulmonary hypertension (PH) is a characterized by increased pulmonary vascular resistance leading to right heart failure. Elevated right atrial (RA) pressure reflects right ventricular (RV) pressure overload and is an established risk factor for mortality in PH. We hypothesized that PH patients with an increased ratio of RA to LA volume index, RAVI/LAVI, would have increased mortality.
Methods:
We evaluated the association of RAVI/LAVI with mortality in 124 patients seen at a single academic center’s PH clinic after adjusting for the REVEAL risk score, an established risk score in PH. LA and RA volume indices were measured in the four and two chamber views by two independent researchers. Multivariable logistic regression was used to model the independent association of RAVI/LAVI with survival.
Results:
Among 124 patients (mean age 62 ± 12.7 years, 68.6% female), each unit increase in RAVI/LAVI was associated with a nearly two-fold increase in mortality (OR 1.91, 95% Cl 1.20-3.04). In a multivariable logistic regression, each unit increase in RAVI/LAVI was associated with a nearly two-fold increase in mortality (OR 1.734, 95% CI 1.003-2.998). Furthermore, RAVI/LAVI in the highest quartile (>1.42) was significantly associated with elevated right atrial pressure (RAP) to pulmonary artery wedge pressure ratio (RAP/PAWP) (0.76±0.41, p=0.02) compared with the lowest quartile (<0.77), suggesting an interaction between invasive hemodynamic data, atrial structural changes, and mortality in PH.
Conclusions:
Increased RAVI/LAVI in PH is associated with decreased survival and accounts for atrial structural remodeling related to invasive hemodynamics. These findings support further study of this
Keywords: Pulmonary Hypertension, Left Atrial Volume Index, Right Atrial Volume Index, REVEAL risk score
Introduction
Pulmonary hypertension (PH) is hemodynamically defined as elevated mean pulmonary arterial pressure greater than 25 mmHg at rest with a right heart catheterization [1]. The disease is inexorably progressive, and is characterized by pulmonary vascular remodeling leading to increased vascular impedance This excessive afterload on the right ventricle (RV) leads to dilatation, failure and ultimately death [2–4]. In contrast to the left ventricle (LV), the RV is thin-walled and extremely vulnerable to increased pulmonary pressure; however, it is important to note the remarkable ability of the RV to adapt to significant afterload quickly when compared to the LV because the RV wall stress is greater than LV wall stress for comparable pressures and the fact that there is less fibrosis in patients with RV pressure overload when compared to LV pressure overload [5–6]. As a result, the status of the RV chamber in the face of an increased afterload primarily determines the overall prognosis. The hemodynamic profile of the failing RV is characterized by increased right atrial pressure (RAP) and a low cardiac output. Increased RAP leads to RA structural remodeling that manifests as chamber enlargement. Furthermore, RA enlargement may often occur before RV enlargement [7]. Prior studies have shown that RV enlargement results in leftward septal shift which increases left end diastolic pressure and as a result pulmonary arterial wedge pressure. This suggests that volume changes may precede significant pressure changes in the ventricles and atria. Consequently, it is possible that volume changes may be more sensitive than pressure changes in detecting early right heart failure [8,28]. With this in mind, we hypothesized that patients that the RA to LA volume index (RAVI/LAVI), which can be easily and noninvasively measured by two-dimensional Doppler echocardiography (2D Echo), would be independently associated with survival in PH.
Methods:
Study Design and Patient Cohort
We retrospectively evaluated consecutive patients referred to the PH clinic at a single academic center with from March 2012 to December 2017. Patients were included in the study if they met the hemodynamic definition of PH (mean PAP > 25 mmHg) and had sufficient echocardiographic images for the calculation of RAVI/LAVI. Of note, because both the numerator and denominator of this ratio are indexed by body surface area (BSA), RAV/LAV would give equivalent results; however, considering that the analysis involves analysis of RAVI and LAVI separately, and RAVI/LAVI has been reported in the literature for other conditions, we use RAVI/LAVI throughout this analysis.
Clinical variables, functional status and pertinent hemodynamic variables were also obtained. The following baseline variables were used for the calculation of the REVEAL risk score: PH subgroup, age, gender, modified WHO functional class, heart rate and systolic blood pressure, six-minute walk distance (6MWD), brain natriuretic peptide (BNP) levels, creatinine, height, weight, and presence or absence of pericardial effusion by echocardiogram, percent predicted carbon dioxide diffusion capacity (DLCO), and right heart catheterization (RHC) data, including right atrial (RA) pressure and pulmonary vascular resistance (PVR). Patients were only included in the study if both an echocardiogram and RHC were performed within one year of each other. In particular, the median difference in months between RHC and TTE was 2.33 (IQR 0.53-6.17).
Baseline characteristics and hospitalization data were obtained from the Clinical Data Repository (CDR). Patients were excluded if they had PH associated with congenital heart disease. Survival was ascertained from the CDR, which routinely queries the national death index registry at regular intervals. The entire cohort was then stratified into RAVI/LAVI quartiles which were then analyzed for the association of clinical outcomes. The study was approved by the institutional review board of the University of Virginia.
Echocardiography:
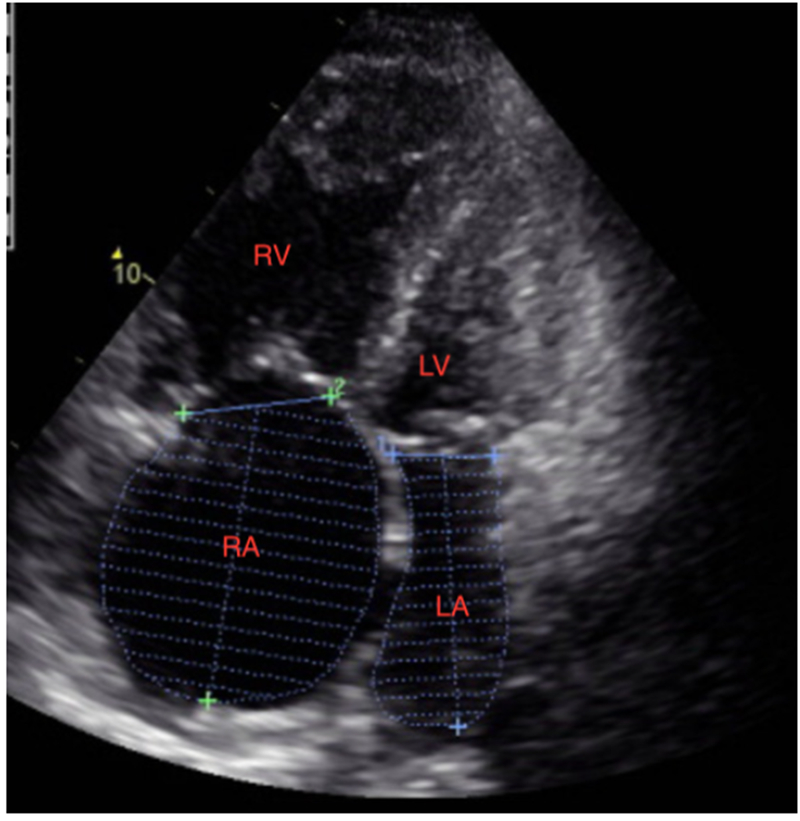
All patients underwent a clinically indicated 2D Echo according to the published guidelines [9]. The echocardiographic images were obtained in the standard parasternal, apical, and subcostal views by experienced echo technologists. Images were obtained with patients lying in the left lateral decubitus position. LAVI was measured using the biplane disc summation method in the four- and two-chamber views, while RAVI was measured using the single plane disc summation method in the four-chamber view (Figure 1). All studies were performed using the Philips IE33 or Epiq 7CV (Philips Medical Systems, Andover, MA) or GE Vivid E9 Ultrasound (GE, Milwaukee, WI) systems. All studies were analyzed using Enterprise Imaging (Agfa Healthcare N.V., Mortsel, Belgium). Offline echocardiographic analysis was performed by two investigators that were blinded to hemodynamic and clinical outcomes. Echocardiographic images were processed using the Enterprise Imaging software.
Figure1:
Apical 4 Chamber View Measuring Right Atrial Volume and Left Atrial Volume. Left atrial (LA) and Right atrial (RA) endocardium were traced at end diastole. Volumes were calculated using the biplane disc summation method
Invasive Hemodynamics
All patients underwent standard clinical hemodynamic assessment by RHC. Hemodynamic tracings were individually reviewed and recorded at end-expiration [10]. Cardiac output was estimated using the indirect Fick method (with an assumed O2 consumption of 125 mL/min/m2 ×BSA).
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as frequency and percentages. Analyses of differences in categorical variables by RAVI/LAVI groups were performed using chi square tests, while differences in continuous variables were assessed using t-tests or the Wilcoxon test depending on normality. Bivariable and multivariable logistic regression was used to evaluate the associations of RAVI, LAVI, RAVI/LAVI, and the REVEAL risk score with survival during a five-year period after adjustment for other covariates. Receiver operating characteristic (ROC) curves were developed based on the logistic regression analysis in order to assess the area under the curve for RAVI, LAVI, RAVI/LAVI, and the REVEAL risk score with survival. Variables chosen for the multivariable model were those with P < 0.05 in the bivariable models. ROC curves were also constructed for survival using the multivariable logistic regression model with covariates of RAVI/LAVI and the REVEAL score. Mann-Whitney 95% confidence limits were determined for the ROC area values. Bland-Altman plots were used to assess the inter-observer agreeability for the RAVI/LAVI measurements. An alpha value of less than 0.05 was used for statistical significance. Statistical analysis was performed using SAS 9.4 (SAS Institute, Cary, NC).
Results:
Baseline Characteristics
There were 124 consecutive patients with complete echocardiographic and hemodynamic data available. The baseline characteristics of these 124 patients stratified by quartiles of RAVI/LAVI are shown in Table 1. Median age was 62.3 ± 12.7 years, and 62% of patients were female. As shown in Table 1, the distribution of etiology of PH in the cohort was characterized by: Group I – idiopathic, porto-pulmonary, and connective tissue disease (CREST, Sjogren’s), 47 patients (37.9%); Group II – left heart disease, 29 patients (23.4%); Group III – intrinsic lung disease (COPD, OSA, ILD), 13 (10.5%); Group IV - chronic thromboembolism, 4 (3.2%); Group V – sarcoid and ESRD, 14 patients (11.3%); and Mixed 17, (13.7%). There were no statistically significant differences in the burden of co-morbid conditions with respect to atrial fibrillation, hypertension, diabetes or chronic kidney disease between the RAV/LAVI quartiles.
Table 1–
Baseline Demographic Characteristics by RAVI/LAVI Quartile
| RAVI/LAVI Q1 (<0.773) | RAVI/LAVI Q2 (0.773-1.101) | RAVI/LAVI Q3 (1.102-1.421) | RAVI/LAVI Q4 (>1.421) | P value | |
|---|---|---|---|---|---|
| Age | 63.6±12.8 | 60.1±12.3 | 60.9±13.3 | 63.4±12.5 | 0.60 |
| Female gender, n (%) | 25 (80.7) | 21 (67.7) | 21 (67.7) | 18 (58.1) | 0.30 |
| BMI | 35.2±7.72 | 34.9±13.1 | 31.8±11.6 | 31.0±9.22 | 0.14 |
| Diabetes Mellitus | 13 (41.9) | 7 (22.6) | 10 (32.3) | 8 (25.8) | 0.28 |
| CKD | 8 (25.8) | 8 (25.8) | 15 (48.3) | 8 (25.8) | 0.18 |
| ESRD | 2 (6.5) | 2 (6.5) | 5 (16.1) | 0 (0) | 0.12 |
| AF | 6 (19.3) | 6 (19.3) | 8 (25.8) | 7 (22.6) | 0.95 |
Values are presented as mean ± SD or n(%).A fib=Atrial fibrillation, BMI, body mass index. RAVI-LAVI, right atrial- left atrial volume ratio
RAVI/LAVI and Mortality
Hemodynamic and clinical profiles by quartiles of RAVI/LAVI are shown in Table 2. There were significantly more deaths in the highest quartile of RAVI/LAVI compared with the remaining quartiles. Overall, increasing RAVI/LAVI was associated with clinical and hemodynamic markers of severe disease status as demonstrated by low cardiac output, increasing RV chamber dimensions, and pulmonary artery pressures (p < 0.05).
Table 2–
Hemodynamic and Clinical Parameters by RAVI/LAVI Quartile
| RAVI/LAVI Q1 (<0.77) | RAVI/LAVI Q2 (0.77-1.10) | RAVI/LAVI Q3 (1.02-1.42) | RAVI/LAVI Q4 (>1.42) | p value | |
|---|---|---|---|---|---|
| RAVI (ml/m2) | 20.9±9.7 | 27.4±13.1 | 36.0±13.9 | 51.94±35.0 | 0.0004 |
| LAVI (ml/m2) | 33.2±15.3 | 30.9±14.3 | 31.0±11.8 | 22.1±9.91 | <0.0001 |
| PASP (mmHg) | 58.2±19.9 | 60.5±17.8 | 64.4±21.3 | 78.7±24.8 | 0.004 |
| PADP (mmHg) | 21.2±9.04 | 23.0±8.85 | 23.6±10.0 | 26.8±10.5 | 0.18 |
| MPAP (mmHg) | 35.6±14.2 | 38.2±11.1 | 40.0±13.3 | 46.0±14.8 | 0.02 |
| PAWP (mmHg) | 17.7±8.09 | 16.7±8.43 | 15.8±8.81 | 13.3±7.72 | 0.16 |
| Fick CI (L/min/m2) | 3.20±1.07 |
3.08±0.81 | 2.93±0.83 | 2.36±0.52 | 0.004 |
| RAP/PAWP | 0.63±0.17 | 0.57±0.19 | 0.69±0.24 | 0.76±0.41 | 0.02 |
| REVEAL | 8.07±2.60 | 8.38±2.32 | 8.40±2.76 | 9.90±1.84 | 0.01 |
| LVEF (%) | 57.1±8.04 | 56.8±8.90 | 58.1±4.77 | 59.5±3.73 | 0.68 |
| RVIDD (mm) | 4.18±0.86 | 4.49±1.00 | 4.44±0.93 | 4.84±0.85 | 0.02 |
| 6MW (mm) | 237.7±87.6 | 236.6±86.0 | 242.2±102.8 | 206.7±102.1 | 0.42 |
| Deaths (%) | 2(6.45) | 3(9.68) | 4(12.90) | 9(29.0) | 0.097 |
Values are presented as mean ± SD. RA-LAVI, right atrial – left atrial volume index; BMI, body mass index; RAVI, right atrial volume index; LAVI, left atrial volume index; PASP, pulmonary artery systolic pressure; PADP, pulmonary artery diastolic pressure; MPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; CI – cardiac index; RAP/PAWP, right atrial pressure – pulmonary capillary wedge pressure; EF, ejection fraction; RVIDD, right ventricular internal diameter index; 6MW, six minute walk.
Interobserver Variability Analysis
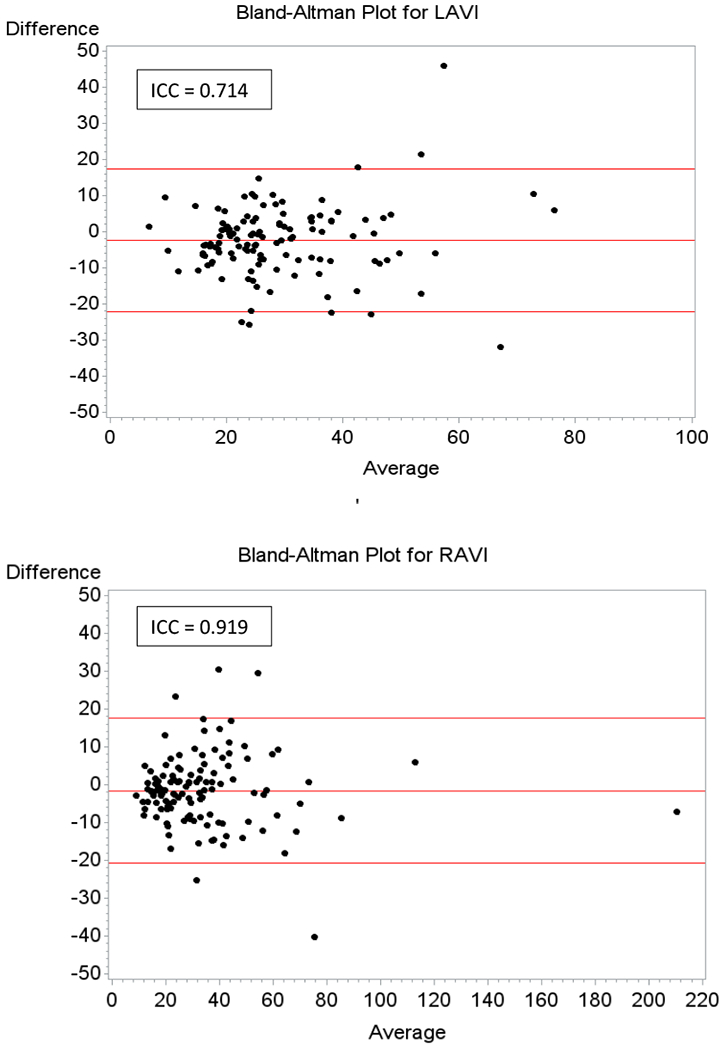
The inter-observer variability was shown using a Bland Altmann plot. The intra-class correlation cofficient for the RAVI was 0.92, p < 0.0001 while for LAVI was 0.729, p<0.0001 as shown in Figure 2, illustrating remarkable similarities between observers for RAVI and LAVI respectively.
Figure2:
Bland-Altman Plot for Right Atrial Volume and Left Atrial Volume. Inter-observer variability was shown using a Bland-Altman Plot for both RAVI and LAVI. The intra-class correlation coefficient for RAVI was 0.92 (P < 0.0001) while the intra-class correlation for LAVI was 0.71 (P < 0.0001).
Regression Analysis
Results of the bivariable logistic regression analysis are shown in Table 3 for variables with significant associations with increased mortality. As shown in Table 3, each unit increase in RAVI/LAVI was associated with an approximately two-fold increase in mortality during follow-up (OR 1.91, 95% CI 1.20-3.04, P=0.0063). As only RAVI/LAVI and the REVEAL risk score were found to be significant in the bivariable models, these two variables were included in the multivariable model. As shown in Table 3, this association of RAVI/LAVI with mortality remained significant in this multivariable Cox model with an adjusted OR of 1.73 (95% CI 1.003-2.998, P=0.048). Of note, there was also a correlation between RAVI/LAVI and an elevated RAP to pulmonary artery wedge pressure (PAWP) ratio (RAP/PAWP) (r=0.76, p=0.02)
Table 3–
Bivariable and Multivariable Logistic Regression Analysis for Mortality.
| OR (95% CI) | P value | |
|---|---|---|
| Bivariable Regression (Unadjusted) | ||
| RAVI/LAVI (per 1 unit ) alone | 1.91 (1.20- 3.04) | 0.006 |
| REVEAL- RISK (per 0.01) alone | 1.70 (1.28- 2.26) | 0.0003 |
| RAVI (per 1 unit) | 1.02 (0.997-1.03) | 0.10 |
| LAVI (per 1 unit) | 1.00 (0.97-1.04) | 0.94 |
| Multivariable Model | ||
| RAVI/LAVI (per 1 unit) | 1.73 (1.003- 3.00) | 0.049 |
| REVEAL-RISK (per 0.01) | 1.62 (1.21-2.17) | 0.001 |
RA-LAVI, right atrial to left atrial volume index
ROC Analysis
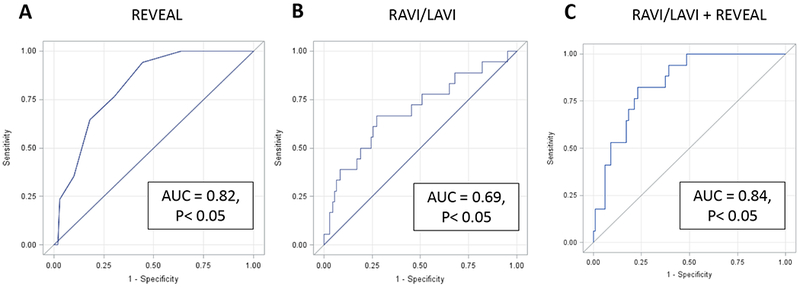
We also found that the AUC for RAVI/LAVI and the REVEAL risk score in the same model was greater than that for the REVEAL risk score alone. As shown in Figure 3, the AUC was 0.82 with the REVEAL risk score alone and 0.84 with the model including both RAVI/LAVI and the REVEAL risk score.
Figure3:
Receiver Operating Characteristic for REVEAL Risk Model, RAVI/LAVI, and RAVI/LAVI adjusted for REVEAL Risk Model. A, Area Under the Curve for the REVEAL Risk Model = 0.82 (P < 0.05) B, Area Under the Curve for the RAVI/LAVI Model = 0.69 (P < 0.05), and C, Area Under the Curve for the RAVI/LAVI Model Adjusted for REVEAL Risk = 0.84 (P < 0.05)
Discussion
Among the main findings of the present study were that there was a significant association between increased RAVI/LAVI and mortality in PH patients. There was nearly a two-fold increase in mortality in the highest quartile of RAVI/LAVI. In addition, RAVI/LAVI by 2D Echo was significantly associated with the RAP/PAWP obtained from invasive hemodynamics demonstrating a strong correlation between structural and hemodynamic parameters in PH.
Despite increase in the general awareness and detection of PH [11], survival and outcomes remain poor across the spectrum of PH groups [12–15]. Furthermore, risk assessment remains a challenge [11]. Robust risk assessment models allow clinicians to determine prognosis, monitor disease progression and treatment response to therapy [16]. RAVI/LAVI is a novel index that reflects important inter-atrial interactions. Previous studies have reported that RA enlargement independently predicted adverse outcomes and advanced disease status in PH [17]. The RA is a relatively thin walled structure with a limited capacity to compensate for increased pressure load leading to chamber dilatation rather than hypertrophy as one would anticipate from the law of Laplace. In advanced stages of PH, the characteristic hemodynamic profile includes severely elevated RAP, elevated pulmonary vascular resistance and a low cardiac output [18–19]. RA enlargement as already noted can lead to reduced LA volume and eventually reduced stroke volume as observed by a prior study looking at left atrial size and mortality in patients with PE. These mechanisms highlight important hemodynamic-structural interactions of the heart especially in a setting of chronic hemodynamic stress on the RV [20]. Thus, RAVI/LAVI is an index that constitutively integrates multiple hemodynamic variables between the left and right sides of the heart, and more specifically the pressure-volume interactions with inter-atrial structural remodeling. PH is primarily a disease of the right heart system, but the close interactions between the left and right systems are a common feature of PH even as the disease progresses [21]. For example, it has been shown that leftward deviation of the interventricular septum as well as increase in RV/LV wall ratio has significant diagnostic specificity in PH [22]. Another metric, septal angle, the angle between the interventricular septum and a line connecting the sternal midpoint and thoracic vertebral spinous process, signals right ventricular overload [23]. As previously described, increased RV overload in the setting of PH, can cause interventricular septal bowing which leads to impaired diastolic filling and decreased LV stroke volume [24]. Chronic pressure overload in PH not only affects RV directly but also significantly impinges on the LV chamber, impairing LV diastolic filling and overall cardiac output. The RV initially compensates for the increased afterload by augmenting systolic contractility; however, in the face of sustained pressure, the compensatory mechanisms are overwhelmed leading to ventricular-arterial uncoupling. When this occurs, the RV undergoes maladaptive changes leading to RV chamber enlargement, leftward septal bowing with compression of the LV cavity, low cardiac output, hypotension and myocardial ischemia. These interactions between the left and right ventricular systems are facilitated by a shared limited pericardial space, a common septum, and interlacing apical muscle fibers [8,25–27].
Studies have also shown that RAP/PAWP has an important relationship to the pulmonary vasculature and by extension a proxy indicator of RV function [28].However, RAP/PAWP does not always emerge as an independent predictor of outcomes in severe PH [29]. In this regard RAVI/LAVI may conceivably be more attractive in predicting outcomes in PH as we have demonstrated. These results not only highlight the important interactions between left and right heart systems but also underscore the importance of the close interplay between structural and invasive hemodynamic data in PH patients.
Limitations
Our sample size was limited by the number of patients with complete hemodynamic data and with good quality echocardiographic images for the measurement of RA/LA volumes. Although 2D Echo is widely available and considered a useful tool for the measurement of the atrial dimensions and function, it is however limited by the absence of an orthogonal plane and reliance on geometric assumptions of atrial chambers [30]. For these reasons, 3D Echo is considered to be superior to 2D Echo [31]. This study was not powered to demonstrate the changes in RAVI/LAVI with pulmonary vasodilators as well as neurohormonal therapies that can attenuate cardiac remodeling process in PH patients [32]. Further, the hemodynamic measurements were not obtained simultaneously with 2D Echo measurements. Even so, the fact that structural changes of the heart develop over time, does offset this limitation Lastly, our PH cohort was heterogeneous but represents a real world PH patient population likely seen in most clinical practices. Although the REVEAL risk score was initially derived and validated in group 1 PH patients [33], it has recently been validated to have comparable survival prediction when applied to a broad population of PH patients [34].
Conclusions
RAVI/LAVI is an easily assessed novel echocardiographic parameter. The correlation with RAP/PAWP suggests that this noninvasive parameter may be used as a surrogate marker for the effects of chronically elevated pressures on the RA. In summary, RAVI/LAVI has important independent prognostic implications beyond current risk models in patients with PH.
Acknowledgments
Disclosures
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose. Dr. Bilchick is supported by National Institutes of Health grants R56 HL135556 and R03 HL135463, and American Heart Association grant 17GRNT33671086. Dr. Breathett received support from National Institute of Health (NIH) L60 MD010857, and the University of Arizona Health Sciences, Strategic Priorities Faculty Initiative Grant.
References
- 1.Gaile N, Vacheiry JL, Gibbs S, et al. : 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur. Heart J 2016: 37(1):67–119. [DOI] [PubMed] [Google Scholar]
- 2.Bossone E, D’Andrea A, D’Alto M, et al. : Echocardiography in pulmonary arterial hypertension from diagnosis to prognosis. J. Am. Soc. Echocardiogr 2013: 26(1):1–14. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z and Chesler NC: Pulmonary vascular wall stiffness: An important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulmonary Circulation 2011:1(2):212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimoda L and Laurie S: Vascular remodeling in pulmonary hypertension. J Mol Med 2014:91(3):297–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naeije R, and Manes A: The right ventricle in pulmonary arterial hypertension. European Respiratory Review 2014:23:476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. : Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 2013:62(25):D22–D33. [DOI] [PubMed] [Google Scholar]
- 7.Deng Y, Guo SL, Wu WF, et al. : Right Atrial Evaluation in Patients with Pulmonary Hypertension: A Real Time 3-Dimensional Transthoracic Echocardiogram Study. J Ultrasound Med 2016:35(1):49–61. [DOI] [PubMed] [Google Scholar]
- 8.Naeije R, Badagliacca R: The overloaded right heart and ventricular interdependence. Cardiovasc Res 2017:113: 1474–1485. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Badano LP, Mor-Avi V, et al. : Recommendation for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr 2015: 28(1):1–39. [DOI] [PubMed] [Google Scholar]
- 10.Hoeper MM, Bogaard HJ, Condliffe R, et al. : Definitions and Diagnosis of Pulmonary Hypertension. JACC 2013:62(25):D42–D50. [DOI] [PubMed] [Google Scholar]
- 11.Deano RC, Glassner-Kolmin C, Rubenfire M, et al. : Referral of Patients with Pulmonary Hypertension Diagnoses to Tertiary Pulmonary Hypertension Centers: The Multicenter rePHerral Study. JAMA Intern Med . 2013:173(10):887–893. [DOI] [PubMed] [Google Scholar]
- 12.Benza RL, Farber HW, Selej M, et al. : Assessing Risk in Pulmonary Arterial Hypertension: What We Know, What We Don’t. Eur Respir J . 2017:50(2):1701353. [DOI] [PubMed] [Google Scholar]
- 13.Gomberg-Maitland M, Glassner-Kolmin C, Watson S, et al. : Survival in pulmonary arterial hypertension patients awaiting lung transplantation. J Heart Lung Transplant 2013:32:1179–1186. [DOI] [PubMed] [Google Scholar]
- 14.Wei-Ting C, Shih-Feng W, Chih-Hsin H, et al. : Prognostic Factors in Patients with Pulmonary Hypertension – A Nationwide Cohort Study. Am Heart J . 2016:5(9):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abramson SV, Burke JF, Kelly JJ, et al. : Pulmonary Hypertension Predicts Mortality and Morbidity in Patients with Dilated Cardiomyopathy. Ann Intern Med . 1992:116:888–895. [DOI] [PubMed] [Google Scholar]
- 16.Raina A, Humbert M. Risk Assessment in Pulmonary Arterial Hypertension. Eur Respir Rev . 2016:142:390–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cioffi G, de Simone G, Mureddu G, and Tarantini L: Right atrial size and function in patients with pulmonary hypertension associated with disorders of respiratory system or hypoxemia. Eur J Echocardiography 2007:8:322–331. [DOI] [PubMed] [Google Scholar]
- 18.Humphrey J, Strumpf R, Halperin H, et al. : Toward a Stress Analysis in the Heart In Glass L, Hunter P, and McCulloch A (eds): Theory of Heart. New York, NY: Springer; 1991, p 59–76 [Google Scholar]
- 19.Regen DM and Maurer CR Jr: The Dependence of Chamber Dynamics on Chamber Dimensions. J. Theor. Biol 1983:105(4):679.-. [DOI] [PubMed] [Google Scholar]
- 20.Aviram G, Soikher E, Bendet A, et al. : Prediction of Mortality in Pulmonary Embolism Based on Left Atrial Volume Measured on CT Pulmonary Angiography. Chest 2016: 149(3):667–675. [DOI] [PubMed] [Google Scholar]
- 21.Ha B, Lucas CL, Henry GW, et al. : Effects of Chronically Elevated Pulmonary Arterial Pressure and Flow on Right Ventricular Afterload. Am J Physiol 1994:267(1 Pt 2):H155–65. [DOI] [PubMed] [Google Scholar]
- 22.Gan C, Lankhaar JW, Marcus JT, et al. : Impaired left ventricular filling due to right-to-left ventricular interaction in patients with pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 2006:290(4):H1528–33. [DOI] [PubMed] [Google Scholar]
- 23.Sitbon O, Humbert M, Nunes H, et al. : Long-Term Intravenous Epoprostenol Infusion in Primary Pulmonary Hypertension: Prognostic Factors and Survival. Am Coll Cardiol 2002:40:780–788 [DOI] [PubMed] [Google Scholar]
- 24.Raymond RJ, Hinderliter AL, Willis PW, et al. : Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. JACC 2002:39:1214–1219. [DOI] [PubMed] [Google Scholar]
- 25.Belenkie I, Smith ER, and Tybert JV: Ventricular Interaction: From Bench to Bedside. Ann Med 2001:33(4): 236–241. [DOI] [PubMed] [Google Scholar]
- 26.Dell’Italia LJ: Anatomy and Physiology of the Right Ventricle. Cardiol Clin . 2012:30(2): 167–187. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda Y, Tanaka H, Motoji Y, et al. : Utility of Combining Assessment of Right Ventricular Function and Right Atrial Remodeling as a Prognostic Factor for Patients with Pulmonary Hypertension. Int J Cardiovasc Imaging 2014:30:1269–1277. [DOI] [PubMed] [Google Scholar]
- 28.Fares W, Bellumkonda L, Tonelli A, et al. : Right atrial pressure/pulmonary artery wedge pressure ratio: A more specific predictor of survival in pulmonary hypertension. J. Heart Lung Transplant 2016:35(6): 760–767 [DOI] [PubMed] [Google Scholar]
- 29.D’Alto M, Motoji Y, Romeo E, et al. : Fluid challenge predicts clinical worsening in pulmonary arterial hypertension. Int J Cardiol 2018:261:167–171. [DOI] [PubMed] [Google Scholar]
- 30.Marsan NA, Tops LF, Holman ER, et al. : Comparison of Left Atrial Volumes and Function by Real-Time Three-Dimensional Echocardiography in Patients Having Cathete Ablation for Atrial Fibrillation with Persistence of Sinus Rhythm Versus Recurrent Atrial Fibrillation Three Months Later. Am J Cardiol 2008:102: 847–853. [DOI] [PubMed] [Google Scholar]
- 31.Lang RM, Badano LP, Tsang W, et al. : EAE/ASE Recommendations for Image Acquisition and Display Using Three-Dimensional Echocardiography. Eur Heart J Cardiovasc Imaging 2012:13(1):1–46. [DOI] [PubMed] [Google Scholar]
- 32.de Man FS, Handoko ML, Guignbert C, et al. : Neurohormonal Axis in Patients with Pulmonary Arterial Hypertension: Friend or Foe. Am J Respir Crit Care Med 2013: 187(1):14–19. [DOI] [PubMed] [Google Scholar]
- 33.Benza RL, Gomberg-Maitland M, Miller DP, et al. : The REVEAL Registry Risk Score Calculator in Patients Newly Diagnosed with Pulmonary Arterial Hypertension. Chest 2012:141(2):354–362. [DOI] [PubMed] [Google Scholar]
- 34.Cogswell R, McGlothlin D, Kobashigawa E, et al. : Performance of the REVEAL model in WHO Group 2 to 5 Pulmonary Hypertension: Application Beyond Pulmonary Arterial Hypertension. J. Heart Lung Transplant 2013:32:293–298. [DOI] [PubMed] [Google Scholar]


