Summary:
The mammalian sex chromosomes harbor an abundance of newly acquired ampliconic genes, although their functions require elucidation[1–9]. Here we demonstrate that the X-linked Slx and Slxl1 ampliconic gene families represent mouse-specific neofunctionalized copies of a meiotic synaptonemal complex protein, Sycp3. In contrast to the meiotic role of Sycp3, CRISPR-loxP-mediated multi-megabase deletions of the Slx (5Mb) and Slxl1 (2.3Mb) ampliconic regions result in post-meiotic defects, abnormal sperm and male infertility. Males carrying Slxl1 deletions sire more male offspring whereas males carrying Slx and Slxl1 duplications sire more female offspring, which directly correlates with Slxl1 gene dosage and gene expression levels. SLX and SLXL1 proteins interact with spindlin protein family members (SPIN1 and SSTY1/2) and males carrying Slxl1 deletions downregulate a sex chromatin modifier, Scml2, leading us to speculate that Slx and Slxl1 function in chromatin regulation. Our study demonstrates how newly-acquired X-linked genes can rapidly evolve new and essential functions and how gene amplification can increase sex chromosome transmission.
eTOC blurb:
A common assumption is that genes essential to a species survival must be deeply conserved. Kruger et al. report how two newly-acquired X-linked gene families, Slx and Slxl1, evolved from an autosomal single-copy gene, massively duplicated, became essential for male fertility, and mediate the sex ratio of offspring by gene copy number changes.
Results and Discussion:
The megabase size and complexity of ampliconic (large (>10kb) and nearly-identical (>99% nucleotide identity) segmental duplications) regions have made it difficult to assess their evolutionary history and associated genes functions. Here, we examined the evolution and function of the Slx and Slxl1 (Sycp3-like X-linked and Slx-like 1) ampliconic gene families in mice. The Slx and Slxl1 gene families share sequence similarity with autosomal Synaptonemal complex protein-3 (Sycp3), but the evolutionary history of Slx and Slxl1 remains unclear[10, 11]. Double knockdown studies of Slx/Slxl1 exhibit defects in post-meiotic spermatogenesis, male fertility and meiotic drive[12, 13]. However, the lack of complete deletion mutants has hindered functional studies, because Slx/Slxl1 knockdowns are incomplete, may have off-target effects, are on a mixed genetic background, and do not allow one to assess the independent functions of Slx versus Slxl1. To more thoroughly characterize the functions of Slx and Slxl1, we generated precise, complete, and independent deletions of the megabase-sized Slx and Slxl1 ampliconic regions on an inbred genetic background.
We first investigated the evolutionary history of Slx and Slxl1, by retracing Slx and Slxl1 gene families origination from Sycp3. Our sequence analyses indicate that in the mouse-rat ancestor, a single copy of Sycp3 transposed via a copy and paste mechanism to an X-linked region syntenic to mouse Slxl1. Slxl1 is thus the first family member to evolve, as no additional copies of Slxl1, Slx or Sly are detectable on rat autosomal or Y chromosomal sequences (Figure 1A, Figure S1A). In rats, Slxl1 remains single-copy and appears non-functional, based upon low to no gene expression across rat tissues (Figure S1B, C). In mice, Slxl1 maintains the intron-exon structure of Sycp3 (Figure S2A) and a Slxl1-related gene family member, Slx, was acquired on an additional region of the mouse X chromosome (Figure 1A). Slx and Slxl1 underwent repeated segmental duplications resulting in ~5Mb (Slx) and ~2.3Mb (Slxl1) ampliconic regions, comprising 4% of the mouse X chromosome (Figure 1A, Figure S3A)[1]. Mouse SLX and SLXL1 protein sequence is highly diverged from SYCP3 (<40% amino acid identity) (Figure S2B) and from rat SLXL1 (<39% amino acid identity) (Figure S1D), as compared to the median of all mouse-rat orthologs (95% amino acid identity)[14]. The high level of divergence suggests SLX/SLXL1 evolved new functions. Our findings establish the evolutionary acquisition and trajectory of the Slx, Slxl1 and Sly gene family, which was previously unclear.
Figure 1. Slx and Slxl1 are mouse-lineage specific neofunctionalized copies of Sycp3.
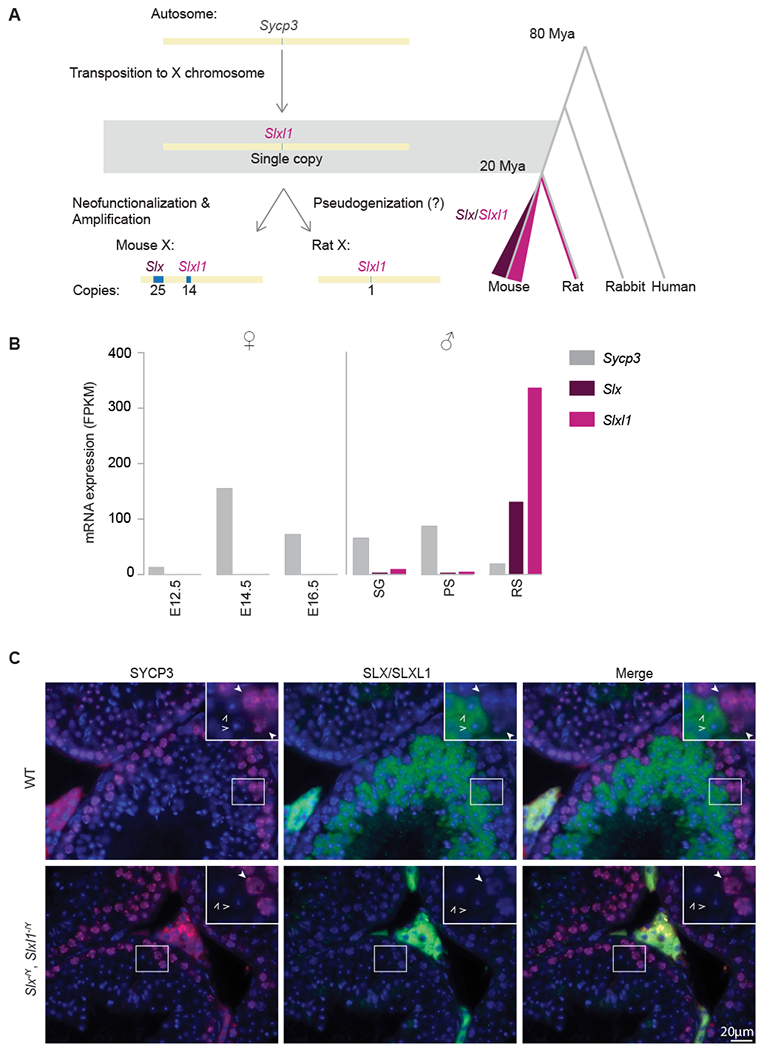
(A) Sycp3 transposed to the X chromosome in the mouse-rat ancestor. Following mouse and rat speciation, Slx was acquired and both Slx/Slxl1 were segmentally duplicated within the mouse-lineage. Sycp3 (light grey), Slx (purple), Slxl1 (pink). (B) mRNA-seq analysis on publically available data sets [31, 32]. In female mice, Sycp3 expression is detected embryonically at E14.5 and in male mice, Sycp3 expression is detected in spermatogonia (SG) and pachytene spermatocytes (PS). Slx and Slxl1 are expressed exclusively in the male germline, post-meiotically, in round spermatids (RS). (C) Immunofluorescence of SYCP3 (red) and SLX/SLXL1 (green) on DAPI stained testis histological sections from wildtype mice and mice lacking SLX/SLXL1 (Slx−/Y, Slxl1−/Y). RS (open arrow) and PS (closed arrow). Staining between tubules (Leydig cells) is non-specific. See also Figure S1, S2 and S3.
The rapid sequence divergence of Slx and Slxl1 from Sycp3 appears to reflect neofunctionalization, based on their gene expression patterns and protein localization. SYCP3, a component of the meiotic synaptonemal complex, is expressed in male and female meiotic germ cells and localizes to the nucleus (Figure 1B, C)[15]. In contrast, SLX and SLXL1 are expressed exclusively post-meiosis in testicular germ cells (Figure 1B, Figure S3B) and are present within the cytoplasm (Figure 1C), supporting previous studies[16]. The cytoplasmic localization of SLX and SLXL1 is consistent with their loss of the putative nuclear localization signal and DNA-binding domain of SYCP3 (Figure S2B)[16–18]. Our findings suggest Slx and Slxl1 are neofunctionalized copies of Sycp3.
To explore the independent functions of Slx and Slxl1, we used CRISPR and Cre/loxP (Figure 2B) to generate precise and complete multi-megabase deletions of Slx (Slx−/Y) and Slxl1 (Slxl1−/Y). We generated individual deletions as they may have independent functions with 61% protein identity and having rapidly diverged from each other (dN/dS=0.947) (Figure 2A–C, Figure S1D, S4A, B). Slx−/Y and Slxl1−/Y mice exhibit wildtype fertility, fecundity, testis weights, sperm counts, and no observable spermatogenic defects as observed via histology, which is in contrast to previous studies observing male fertility phenotypes in Slx knockdown mice (Figure 2D, Figure S4C, D)[12].
Figure 2. Slx and Slxl1 are essential for male fertility and exhibit post-meiotic spermatogenesis specific defects.
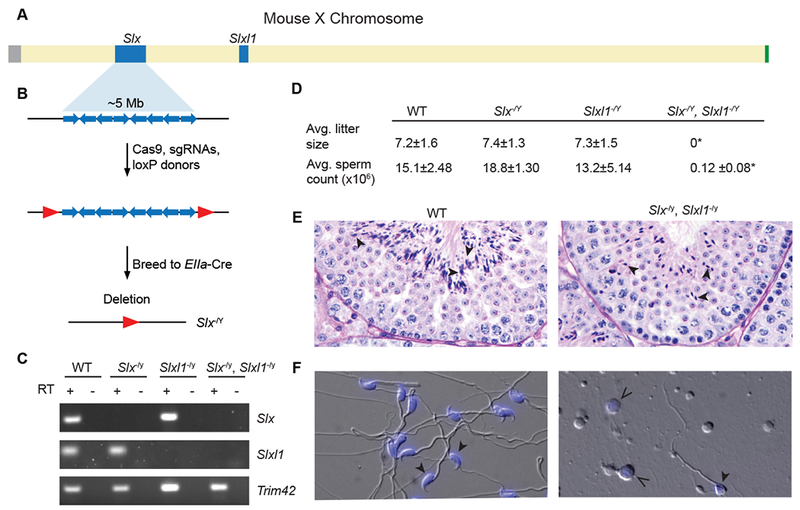
(A) Schematic representation of the mouse X chromosome. Ampliconic regions are shown in blue, centromere in grey and pseudoautosomal region (PAR) in green. (B) Schematic representation of Slx ampliconic region consisting of segmental duplications (blue arrows), red arrows denote loxP sites. Mice carrying loxP sites flanking the ampliconic region were mated to Ella-Cre carrying mice. Slxl1−/Y mice were generated in a complementary manner. (C) RT-PCR (+) and no RT controls (−) performed on RNA isolated from adult testis. Trim42 is a testis specific gene that was used as a control. All primers are intron-spanning. (D) Average litter size and cauda epididymal sperm count including standard deviation. Average litter size was determined by mating three males from each genotype against five females each for a total of 15 litters. Sperm were counted from at least three males. Two tailed t-tests were used to compare to WT, * p-value <0.0001 (E) Periodic-acid Schiff stained testis histology from WT and Slx−/Y, Slxl1−/Y males. Stage 7 tubules, elongated spermatids (see black arrowheads) exhibit abnormal morphology in Slx−/Y, Slxl1−/Y testis. (F) Cauda epididymal sperm from a WT and Slx−/Y, Slxl1−/Y male, DIC and DAPI (closed arrows). Open arrows indicate cells that resemble round spermatids based upon DAPI stain present in Slx−/Y, Slxl1−/Y. See also Figure S4.
To test for genetic redundancy between Slx and Slxl1, we generated double mutants (Slx−/Y, Slxl1−/Y), via recombination of the independent deletions that are complete null mutants at the RNA and protein levels (Figure 1C, 2C, Figure S4A, B). In contrast to the independent deletions, double deletion mice (Slx−/Y, Slxl1−/Y) are infertile and produce <1% the wildtype number of cauda epididymal sperm, which when present are morphologically severely abnormal (Figure 2D, F). Our double deletion mice resemble previous knockdown studies, but the complete loss of Slx and Slxl1 in Slx−/Y, Slxl1−/Y mice now allow us to precisely identify that defects in post-meiotic germ cells occur during the transition from round spermatids to elongated spermatids at stage 7/8 (Figure 2E, Figure S4F)[12]. The timing of elongation defects is consistent with our stage specific determination of SLX/SLXL1 protein presence in wildtype males (Figure 1C, Figure S4F). To assess whether Slx−/Y, Slxl1−/Y mice produce competent meiotic products (round spermatids), we performed round spermatid injections (ROSI). Oocytes injected with round spermatids from WT or Slx−/Y, Slxl1−/Y males develop to blastocysts at the same frequency (Figure S4G, B), indicating that Slx−/Y, Slxl1−/Y round spermatids successfully progress through meiosis, supporting Slx and Slxl1 being functionally distinct from meiotic Sycp3 [19, 20]. To help explain why Slx−/Y, Slxl1−/Y exhibit defects in spermatid elongation, we performed mRNA-seq on round spermatids from Slx−/Y, Slxl1−/Y and wildtype males and identified 990 misregulated genes (adjusted P<0.01), which in combination likely perturb round spermatid elongation (Data S1). These results demonstrate that Slx and Slxl1 acquired new functions in post-meiotic spermatids and function redundantly with each other[20].
While our results confirm that Slx and Slxl1 are essential for male fertility, they do not explain their massive gene amplification (Figure S1B, S3A). Slx and Slxl1’s massive gene amplification has been proposed to be a result of their involvement in meiotic drive (the non-Mendelian inheritance of chromosomes), however the importance of gene dosage has never been formally tested[3, 13]. If increases in gene dosage, via gene amplification, serve to increase chromosome transmission (meiotic drive), then complete deletions (Slxl1−/Y or Slx−/Y) or duplications (Slxdup/Y or Slxl1dup/Y or Slxdup/Y, Slxl1dup/Y) (Figure 3A, B) of these regions should decrease or increase X chromosome transmission, respectively. Slxl1−/Y, but not Slx−/Y, sire more male offspring (58.5% male, 141/241 pups) (two tailed Fisher’s Exact test, P <0.05) as compared to wildtype males (48.5% male, 94/194 pups; Figure 3C). Conversely, Slxdup/Y, Slxl1dup/Y sire more female offspring (58.2% female, 181/311 pups; Figure 3C) as compared to WT (two tailed Fisher’s Exact test, P =0.1672) and is statistically significant when compared to Slxl1−/Y (two tailed Fisher’s Exact test, P <0.0001). We suspect differences between observing sex ratio distortion in SlxDup/Y, Slxl1Dup/Y mice, but not Slxl1Dup/Y mice, is due to unequal crossing over decreasing Slxl1 copy number in the Slxl1Dup/Y line. Previous knockdown studies were unable to decipher Slxl1’s specific role and the impact of gene dosage in meiotic drive [12]. We suspect the distinct functions of Slx and Slxl1 in meiotic drive are due to diverged residues (they share 61% amino acid identity) or gene expression levels, and the redundant functions in fertility are due to their conserved residues (Figure S2B). Our findings support Slxl1s involvement in meiotic drive and via a gene dosage-dependent mechanism.
Figure 3. Changes in Slxl1 gene dosage results in sex ratio distortion of offspring and Slxl1 gene expression levels.
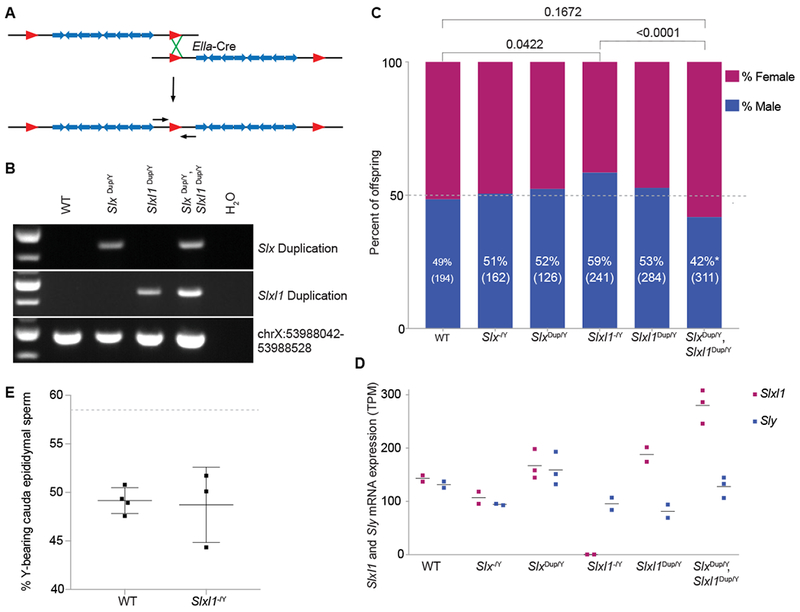
(A) Schematic representation of a floxed (red triangles) ampliconic region with segmental duplications (blue arrows). Duplications were generated by expression of Ella-Cre and pups were genotyped using PCR primers spanning the duplication junction (small black arrows). (B) Representative genotyping of wildtype (WT), Slx duplication (Slx dup/Y), Slxl1 duplication (Slxl1 dup/Y) and Slx/Slxl1 (Slx dup/Y, Slxl1 dup/Y) duplication mice. ChrX:53988042-53988528 is unique sequence, which controls for retaining the intervening sequence present between the Slx and Slxl1 ampliconic regions. (C) Males of each genotype were mated against wildtype females and progeny were sex genotyped. The percent of male offspring is shown as a percentage along with the number of pups screened in parentheses. P-values were calculated using a two-tailed Fisher’s Exact test, compared to experimental WT data (bars on top) and theoretical 50:50 data with an equivalent population size (p<0.05, asterisk near male percentage). (D) mRNA-seq was performed on isolated round spermatids. The horizontal line represents average expression across biological replicates (bullets) for Slxl1 and Sly. TPM, Transcripts per million. (E) X and Y chromosome paint was performed on sperm collected from the cauda epididymis of wildtype and Slxl1−/Y males. Each bullet represents the percentage of Y-bearing sperm from one male, with overall mean and standard deviation. Dotted line denotes expected percentage of Y-bearing sperm if ratio of X- to Y-bearing sperm reflected observed Slxl1−/Y sired offspring sex ratio. Differences between wildtype and Slxl1−/Y are not significant, Fisher’s exact test two-tailed. See also Data S1.
Slxl1 has been proposed to be in meiotic conflict, resulting in drive with a related Y-linked gene family Sly (Sycp3-like Y-linked), where Sly represses Slx and Slxl1 gene expression[13, 21]. Our finding that Slxl1 gene dosage contributes to meiotic drive (Figure 3C) led us to test if mice with sex ratio distortion exhibit corresponding gene expression changes in Sly, Slxl1, or both. We performed mRNA-seq on round spermatids and found that increases in Slxl1 gene dosage have corresponding increases in Slxl1 expression, while Sly expression remains relatively unchanged (Figure 3D). Our findings suggest the ratio of Slxl1 to Sly gene expression levels mediates meiotic drive (Figure 3D); however, the ratio does not affect the number of X versus Y-bearing sperm from Slxl1−/Y mice (Figure 3E). Meiotic drive likely impacts the relative fitness of X- versus Y-bearing sperm via changes in SLXL1 to SLY protein abundance and interactions with protein partners.
We next identified SLX- and SLXL1-interacting proteins that may contribute to meiotic drive. We performed endogenous SLX/SLXL1 co-immunoprecipitation and mass spectrometry on testis lysates from wildtype, Slx−/Y, Slxl1−/Y and Slx−/Y, Slxl1−/Y mice. Candidates were prioritized by their reproducibility across multiple wildtype samples and absence in Slx−/Y, Slxl1−/Y samples. We identified spindlin family members SPIN1, SSTY1, SSTY2 (SSTY1/2) and 26S proteasome components PSMC1 and PSMD4 (Figure 4A, Table S1). We did not immunoprecipitate SLY, suggesting SLXL1 and SLY conflict does not occur through direct protein interactions. Instead, SLX, SLXL1 and SLY may compete for spindlin family members, as SSTY1/2 have been shown to interact with SLX, SLXL1 and SLY[22]. SLX and SLXL1 are present in all haploid spermatids (Figure 1C) and therefore could compete with SLY for SPIN1 and SSTY1/2 by diffusing across the cytoplasmic bridges connecting haploid spermatids (Figure 4C). SPIN1 is a chromatin reader and post-transcriptional regulator of mRNA stability [23, 24]. SPIN1 binds H3K4me3-R8me2a via its Spin/Ssty domains, which are shared with SSTY1/2 [24]. We hypothesize that changes in SLXL1 to SLY abundance could alter SPIN1 distribution and may have transcriptomic consequences that impact the downstream fitness of X- versus Y-bearing sperm. PSMC1 and PSMD4 may be non-specific interactions, as our antibody has non-specific localization to the acrosome (present in Slx−/Y, Slxl1−/Y) where proteases are known to reside (Figure S4E).
Figure 4. SLX/SLXL1 interact with spindlin family members and regulate chromatin.
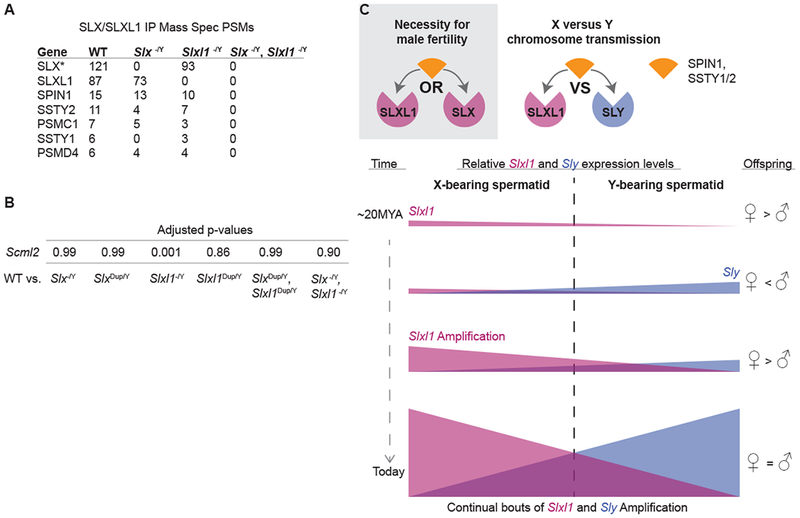
(A) Mass spectrometry results from SLX/SLXL1 immunoprecipitations on wildtype, Slx−/Y, Slxl1−/Y, and Slx−/Y, Slxl1−/Y whole testis lysates. PSMs (peptide-to-spectrum matches) were quantified to determine relative protein abundance. Candidates were filtered by high confidence (<1% FDR) and reproducibility. (*, additional SLX accessions were removed for simplicity, see Table S1 for biological replicates) (B) Expression level adjusted p-values for Scml2 isoform NM_001290652 when comparing wildtype versus deletion or duplication genotype round spermatid mRNA-seq samples. (C) Our model in which SLX or SLXL1 interactions with spindlin family members, SPIN1 and SSTY1/2, is essential for fertility and how SLXL1 and SLY competition for spindlin family members, SPIN1 and SSTY1/2, influences chromosome transmission through the sharing of proteins and transcripts across cytoplasmic bridges. Changes in the relative expression of Slxl1 to Sly result in corresponding changes in the fitness of X and Y sperm that influence offspring sex ratio.
Based on SLXL1 and SLY protein interaction with spindlin family members SPIN1 and SSTY1/2 we speculated that misregulated X- or Y-linked genes may cause X- versus Y-sperm fitness differences. Comparison of round spermatid mRNA-seq data from wildtype versus Slxl1−/Y males identified five X-linked (not including Slxl1 gene family members), two Y-linked and 50 autosomal genes that are significantly misregulated (adjusted P<0.01) (Data S1). One of the seven is an alternative splice form of Scml2 that is significantly downregulated only in Slxl1−/Y males (Figure 4B, Data S1). SCML2 is a germline-specific polycomb group protein that regulates sex chromosome-wide silencing in meiotic and post-meiotic cells via chromatin modifications[25]. Together, the interactions of SLXL1 with SPIN1 and SSTY1/2 and the downregulation of Scml2, lead us to speculate that changes in round spermatid chromatin may mediate meiotic drive.
The neofunctionalization and amplification of Slx and Slxl1 evolved in the past ~20 MY of mouse-lineage evolution, creating a gene family that functions in a post-meiotic manner that is essential for male fertility (Figure 4C). Our findings build upon previous studies, providing new insights into the evolution, neofunctionalization and the independent contributions of Slx versus Slxl1[12, 13]. Additionally, we demonstrate the impact gene dosage has on gene expression and conflict between Slxl1 and Sly. We propose that the acquisition of Slxl1 in the rat-mouse ancestor conferred a selective advantage to increase X chromosome transmission (Figure 4C). In response, Sly evolved on the Y chromosome specifically in the mouse-lineage to equalize the sex ratio. The rapid changes in gene dosage, gene expression levels and protein sequence chronicle the continued bouts of Slx/Slxl1 versus Sly genetic conflict within the mouse-lineage (Figure 4C). In the present day C57BL/6J mouse strain, Slxl1 is likely a primary mediator of meiotic drive, because the frequency of female offspring correlates with increases (Slxdup/Y, Slxl1dup/Y) and decreases (Slxl1−/Y) in Slxl1 gene dosage (Figure 3C, D). These evolutionary signatures inform our proposed model in which SLXL1 and SLY compete for interactions with spindlin family members SPIN1 and SSTY1/2 in a dose-dependent manner (Figure 4C). We speculate the interaction of SLX/SLXL1 with the conserved and ubiquitously expressed SPIN1 mediates the male fertility and SLX/SLXL1 interaction with the newly-acquired and ampliconic Y-linked gene family SSTY1/2 mediates meiotic drive (Figure 4C)[3, 26]. Consistent with this idea, the chromatin reader SSTY1/2 localizes to X and Y chromatin, where it can have impacts on the localization and regulation of transcription[22].
Mammalian sex chromosomes harbor a plethora of newly acquired ampliconic genes expressed predominantly in the testis[1–6]. Is X-Y co-amplification of newly acquired genes a universal phenomenon continually reshaping sex chromosome evolution? In mice, Ssty1/2 and Sstx are X-Y co-amplified gene families that pre-date Slx, Slxl1 and Sly[3, 10]. Ssty1/2 and Sstx may represent a former or current system of genetic conflict. Given SLX, SLXL1 and SLY proteins interact with SSTY1/2, it is tempting to speculate that conflict between gene families on the X and Y chromosome can continuously re-emerge and converge upon pre-existing pathways. The sex chromosomes are a known battleground for genes in conflict and competition between co-amplified X- and Y-linked genes could provide the genetic basis for speciation and hybrid male sterility; both processes known to be associated with the X chromosome[27–29]. Indeed, in other mammals there are hints of entirely new co-amplified X-Y gene families[30]. More complete assemblies of X and Y chromosomes will determine the extent of X-Y gene co-amplification across mammals. We conclude that prime candidates of X-Y genetic conflict can be readily identified in future studies by the presence of three defining signatures: new acquisition, X-Y co-amplification, and rapid sequence evolution.
STAR Methods
Lead Contact and Materials Availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jacob L. Mueller (jacobmu@umich.edu). The SLX/SLXL1, Rabbit polyclonal antibody we generated is available from our lab. Mice will be available from our lab until they become available from the Mutant Mouse Resource and Research Center Repository.
Experimental model and subject details
Transgenic mice used in this study were generated from injected zygotes (see Generation of transgenic lines) derived from the cross of F1 (DBA2xC57B/6N) and C57B/6N mice, ordered from Envigo. Heterozygous transgenic female mice were mated to C57BL/6J male mice ordered from Jackson Laboratory (Bar Harbor, ME) to backcross. Breeding was conducted with one male and one or two females, all mice were older than 6 weeks of age. Resulting pups were weaned at 21dpp, separated by sex and no more than 5 pups per cage (Allentown, 11.5in x 7.5in x 5in). All experiments were conducted with adult males (2-6 months of age) transgenic males were compared to wildtype littermates or age matched wildtype controls. Cages were kept on ventilated racks (Allentown) at 72°F, 30-70% humidity, on 12hr: 12hr light:dark cycle. Cages are monitored daily and changed at least every two weeks in a room that is specific-pathogen free, monitored by sentinel mice. Mice were provided water and fed Lab Diet 5008 food ad libitum. Adult mice were sacrificed by CO2 asphyxiation followed by cervical dislocation and pups were euthanized using decapitation in compliance with the ULAM standard procedures of euthanasia. The Institutional Animal Care and Use Committee at the University of Michigan Medical School (PRO00007498) approved all procedures involving mice.
Method Details
Gene expression of mouse and rat X/Y co-amplified genes
RNA-seq analyses were conducted by analyzing previously published datasets. Specifically, mouse tissue panel data were analyzed from SRP016501[33], fetal ovary data from SRP058992[32], ovary data from SRP017959[34], and sorted testicular germ cell populations from SRP113417[31]. Rat tissue panel data was used from SRP016501[33] and rat sorted testicular germ cell populations from SRP026340[35]. Alignments were performed using Tophat with the mm10 mouse reference genome, a refFlat file with RefSeq gene annotations and --max-multihits set to 240; otherwise standard default parameters were used. We used Cufflinks, using the refFlat RefSeq gene annotation file, to estimate expression levels as fragments per kilobase per millions of mapped fragments (FPKM).
Round spermatid FACS isolation and library generation
We largely followed a previously published protocol to isolate round spermatids (1n)[36]. Briefly, we disassociated cells from a pair of testes by enzymatic treatment with Collagenase type I (Worthington Biochemical Corporation), DNase I (Worthington Biochemical Corporation), and Trypsin (Life Technologies). The cell suspension was passed through cell strainers (100μm and 40μm) and incubated with Hoechst 33342 (Setareh Biotech) to determine DNA content and propidium iodide (Acros Organics) to evaluate cell viability. Cell sorting was performed on a Synergy Head Cell Sorter cell sorter (Sony). The purity of each sort was determined via fluorescence microscopy visual inspection of 100 cells morphology and nuclear staining with DAPI for round spermatids. The purity of round spermatids was typically >85%, which is consistent with previous studies[36].
TRIzol LS (Thermo Fisher Scientific) was used to extract total RNA. RNA was DNAse treated to remove any genomic DNA contamination using RNeasy Mini Kit and RNase-Free DNase Set (QIAGEN). Libraries were generated using samples with >7 RIN scores and >5ng of total RNA. Stranded mRNAseq libraries were generated using the NEBNext Ultra II Directional RNA Library Prep Kit (NEB) and Agencourt AMPure XP beads (Beckman Coulter) following the manufacturers protocol. We generated at least two biological replicate round spermatid mRNA-seq libraries per genotype. All libraries were sequenced on a single Illumina NovaSeq lane (~50 million sequenced fragments per library) and in NCBI SRA: PRJNA545829. Alignments were performed with Kallisto and DESeq2 using the RefSeq index with bootstrapping set to 100[37, 38].
Generation of transgenic lines
To generate mice with multi-megabase deletions of the Slx and Slxl1 ampliconic region, loxP sites were sequentially integrated upstream and downstream of either the Slx or Slxl1 ampliconic region via CRISPR. sgRNA targeting unique sequence flanking the ampliconic region was selected based on scores from either the CRISPR Design Tool (http://www.genome-engineering.org/) or CRISPOR (http://crispor.tefor.net)[39]. sgRNA and Cas9 mRNA were synthesized in vitro, using the MEGAshorscript T7 kit (ThermoFisher) and mMESSAGE mMACHINE T7 ULTRA kit (ThermoFisher), respectively, followed by MEGAclear Kit (ThermoFisher) for clean-up. sgRNA, Cas9 mRNA, and single stranded oligo donor carrying the loxP sequence (IDT; Table S2) were mixed for microinjection at concentration of 50, 100, and 100 ng/ul, respectively. Cytoplasmic microinjection was performed on zygotes derived from the cross of F1 (DBA2xC57BL/6N) and C57BL/6N mice, using a piezo-driven microinjection technique[40]. Floxed mice were mated against C57B/6J Ella-Cre mice resulting in mice carrying either a Slx or Slxl1 deletion or duplication (Figure 2B, C, Figure 3A, B). Two independent deletion and duplication lines were generated for each Slx and Slxl1 ampliconic region. No differences were observed between independent lines and data was therefore compiled. All deletion or duplication carrying males were derived from heterozygous female mice that had been backcrossed to C57BL/6J males for at least four generations. Mice were genotyped by extracting DNA out of a tail biopsy using Viagen DirectPCR lysis reagent using primers that flank loxP sites (Table S2).
Testis Histology
Testes were collected from 2-6 months old mice. The tunica albuginea was nicked and the testes were fixed with Bouins Fixative overnight at 4°C. Testes were then washed through a series of ethanol washes (25%, 50%, 75% EtOH) before being stored in 75% EtOH at 25°C. Testes were paraffin embedded and 5μm sections were made that were stained with Periodic acid Schiff (PAS) and hematoxylin. Sections were studied using a light microscope and staged[41]. Specific types of germ cells were identified based upon their location, nuclear size and nuclear staining pattern[41].
Immunohistochemistry
Immunohistochemistry was performed on 5μm paraffin embedded sections. Sections were deparaffinized and rehydrated prior to antigen presentation. Antigen presentation was performed by boiling slides in 0.01M Citrate Buffer pH 6 for 10 minutes. Sections were blocked for 1hr at room temperature using 3% BSA and 0.05% Triton X-100 in PBS and subsequently probed with primary and secondary antibodies.
Our SLX/SLXL1 antibody was generated against a CVSFSEEWQRFARS-KLH conjugated peptide. Genemed Synthesis Inc. synthesized and injected the peptide into rabbits and affinity purified the antibody. Antibody specificity was assessed by probing whole testis lysates from wildtype (C57BL/6J), Slx−/Y and Slxl1−/Y mice with both pre-immune serum and purified antibodies on western blots. The following commercially available antibodies were used: SCP3 (Abcam, ab97672), Peroxidase AffiniPure (Jackson ImmunoResearch, 711-035-152), FITC AffiniPure (Jackson ImmunoResearch, 711-095-152), Rhodamine RedX AffiniPure (Jackson ImmunoResearch, 715-295-151).
RT-PCR
Total RNA was extracted using Trizol (Life Technologies) according to manufacturer’s instructions. Ten μg of total RNA was DNase treated using Turbo DNAse (Life Technologies) and reverse transcribed using Superscript II (Invitrogen) using oligo(dT) primers following manufactures instructions. Intron-spanning primers were used to perform RT-PCR on adult testis cDNA preparations for Slx, Slxl1 and a round spermatid specific gene Trim42 (Table S2).
Assaying Fertility
The fecundity of males was assessed by mating at least three males 2-6 months of age of a given genotype to 2-6 month old C57B/6J females and monitoring females for copulatory plugs. The fertility of all lines was compared to that of C57B/6J males (wildtype). Offspring sex ratios data was compiled by sex genotyping offspring from the aforementioned crosses as well as pups resulting from males bred with CD1 females.
Sperm counts were conducted on sperm isolated from the cauda epididymis. Briefly, the cauda epididymis was isolated and nicked three times to allow sperm to swim out. The nicked epididymis was then rotated for 1hr at 37°C in Toyoda Yokoyama Hosi media (TYH). Sperm were fixed in 4%PFA and counted using a hemocytometer. For each genotype, at least three male mice were counted with three technical replicates performed for each mouse and averaged. Testes were collected from 2-6 month old males for all experiments and weighed, along with total body, in order to determine relative testis weight.
X-Y Chromosome Paint
Cauda epididymal sperm were collected and fixed onto slides using 3:1 methanol: acetic acid. Slides were stored at −20°C. Slides were immersed twice in 2x SSC for three minutes followed by a series of ethanol washes (70%, 90%, 100% ethanol) for two minutes each. Slides were incubated at room temperature for 30 minutes in 5mM DTT, 1% Triton X-100, 50mM Tris. Slides were again washed with 2x SSC and the ethanol series. Slides were incubated in 70% formamide, 2x SSC at 78°C for five minutes and then washed in ethanol series for one minute each. Mouse X and Y paint probes from MetaSystems Probes were mixed and denatured at 80°C for ten minutes before placing on slide. Slides were incubated overnight at 37°C in a humid chamber. Slides were washed in 50% formamide, 2x SSC three times at 45°C for five minutes, 2x SSC once at 45°C for five minutes and rinsed in Photo-Flo 200 (Kodak). Slides were mounted with VECTASHIELD with DAPI (Vector Laboratories).
Co-Immunoprecipitation and Mass Spectrometry
SLX/SLXL1 co-immunoprecipitation was performed on whole testis lysate using our SLX/SLXL1 antibody conjugated to protein A Dynabeads (Life Technologies). Briefly, testes from wildtype, Slx−/Y, Slxl1−/Y and Slx−/Y, Slxl1−/Y males were lysed (150 mM KCl, 0.05% NP-40, 0.5 mM DTT, and protease inhibitors (EDTA-free Complete PI) and centrifuged at full speed in a tabletop centrifuge at 4C. The top lipid layer was discarded and the remaining supernatant was transferred to a new tube and incubated with SLX/SLXL1 antibody. Protein A dynabeads were rinsed 3 times with lysis buffer before adding the lysate/antibody solution and incubated at 4°C for at least 1hr. Beads were subsequently washed 2X with lysis buffer and 1X with lysis buffer containing no detergent and 1X with PBS containing protease inhibitors. A small aliquot of beads was removed for western blot analysis. The remaining beads were stored at −20°C after removing all liquid. Prior to trypsin digestion, beads were resuspended in 50ul of 0.1M ammonium bicarbonate buffer (pH~8). Cysteines were reduced by adding 50ul of 10 mM DTT and incubated at 45°C for 30 min. To alkylate cysteines, the samples incubated at room temperature, protected from light, with 65 mM 2-Chloroacetamide. Proteins were digested overnight with 1ug sequencing grade modified trypsin at 37°C with constant shaking in a Thermomixer. Digestion was stopped by acidification and peptides were desalted using SepPak C18 cartridges using manufacturer’s protocol (Waters). Samples were dried using a vacufuge. Samples were dissolved in 8ul of 0.1% formic acid, 2% acetonitrile solution and then 2ul of the peptide solution was resolved on a nano-capillary reverse phase column (Acclaim PepMap C18, 2 micron, 50 cm, ThermoScientific) using a 0.1% formic acid, 2% acetonitrile (Buffer A) and 0.1% formic acid, 95% acetonitrile (Buffer B) gradient at 300 nl/min over a period of 180 min (2-22% buffer B in 110 min, 22-40% in 25 min, 40-90% in 5 min followed by holding at 90% buffer B for 5 min and requilibration with Buffer A for 25 min). Eluent was directly introduced into Orbitrap Fusion tribrid mass spectrometer (Thermo Scientific, San Jose CA) using an EasySpray source. MS1 scans were acquired at 120K resolution (AGC target=1x106; max IT=50 ms). Data-dependent collision induced dissociation MS/MS spectra were acquired using Top speed method (3 seconds) following each MS1 scan (NCE ~32%; AGC target 1x105; max IT 45 ms).
Proteins were identified by searching the MS/MS data against Mus Musculus (UniProt; 60627 entries) using Proteome Discoverer (v2.1, Thermo Scientific). Search parameters included MS1 mass tolerance of 10 ppm and fragment tolerance of 0.2 Da; two missed cleavages were allowed; carbamidimethylation of cysteine was considered fixed modification and oxidation of methionine, deamidation of aspergine and glutamine were considered as potential modifications. False discovery rate (FDR) was determined using Percolator and proteins/peptides with a FDR of ≤1% were retained for further analysis.
Candidate interacting proteins were filtered based upon high confidence (<1% FDR), reproducible presence across multiple wildtype samples (PSM >5) and reproducible absence across all Slx−/Y, Slxl1−/Y samples. Three biological replicates-wildtype and Slx−/Y, Slxl1−/Y, two biological replicates Slx−/Y and Slxl1−/Y were performed. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE[42] partner repository with the dataset identifier PXD014276.
Estimation of amplicon copy number
For estimating the number of Slxl1 copies in the rat genome, read depth analysis was performed using the mrsFAST[43] aligner and the fastCN pipeline[44]. Reads were mapped against the rn6 reference genome in which sequences identified by RepeatMasker, Tandom Repeat Finder, and 50-mers with an occurrence greater than 50 were masked. Read depth was normalized to account for GC content, averaged in windows containing 3,000 unmasked positions, and converted to copy-number based on the observed depth in autosomal regions not previously identified as copy-number variable[45]. Analysis was performed using male and female genomic data obtained from SRP029216[46].
Comparisons of exon-intron structure
Complete RefSeq mRNA sequences were obtained for mouse Sycp3 (NM_011517), Slx (NM_001136476), Slxl1 (NM_029181), and Sly (NM_201530). Pairwise comparisons for each of the four genes were performed via Exalign[47] to determine conservation on exon-intron structure.
Dot-plots
Self-symmetry triangular dot plots that show repeats within a sequenced region were generated from a custom Perl script that can be found at http://pagelab.wi.mit.edu/materials-request.
Round spermatid Injections (ROSI)
Round spermatids were collected and enriched from the testes of 2-6 month old WT and Slx−/Y,Slxl1−/Y males, as described previously[48]. Briefly, testes were placed in red blood cell lysis solution (Miltenyi Biotec) to remove Tunica albuginea. Seminiferous tubules were transferred to GL-PBS (0.01% PVP in Dulbecco’s PBS containing glucose and pyruvate from GIBCO), cut into small pieces and pipetted gently to release spermatogenic cells. The cell suspension was filtered through a 50-um nylon mesh, centrifuged at 200xg for 5 min at 4°C, and resuspended in GL-PBS containing 0.4 mg/ml pronase E (SERVA). The cell suspension was centrifuged again at 400xg for 5 min at 4°C to precipitate most elongating spermatids and spermatozoa. The remaining suspension containing most round spermatids was washed twice at 200xg for 5 min at 4°C and stored in 7.5% glycerol, 7.5% FBS, 0.01% PVP in GL-PBS at −80C.
Unfertilized eggs were harvested from 5-weeks-old superovulated C57BL6/DBA2 F1 females. Round spermatids were thawed and washed twice with 7.5% FBS in GL-PBS at 400xg for 5 min at 4°C. More than 90% cells were alive after thawing, as determined by the trypan blue extrusion test. On the injection dish, eggs and round spermatids were placed in a droplet of the M2 medium (Sigma) and a droplet of GL-PBS, respectively. Using a needle with 6um inner diameter and 15 um outer diameter for piezo-driven microinjection, individual round spermatids were drawn into the needle to break the plasma membrane, and the nucleus was injected into the cytoplasm of the egg. Following round spermatid injection, eggs were activated in the M16 medium (Sigma) containing 5 mM SrCl2 and 5 mM EGTA for 20 min at 37 °C[49]. Eggs were then washed and cultured in KSOM media for development.
Quantification and Statistical Analysis
Two-tailed Fisher’s Exact tests were used to calculate statistical significance between wildtype and transgenic groups in offspring sex ratio (Figure 3) and X versus Y sperm counts (Figure 3) as the outcomes are binary. The number of pups or sperm screened are noted in the figure and figure legend, respectively. To determine statistical significance for average litter size, sperm counts and testis weights between wildtype and transgenic groups, a two-tailed T-test was performed. The number of mice used is denoted in the figure legends. All error bars denote standard deviation.
Data and Code Availability
Sequencing data is available on NCBI SRA: PRJNA545829. Mass spectrometry data is available on the PRIDE database: PXD014276.
Supplementary Material
Data S1. Excel file of Round Spermatid mRNAseq. Related to Figure 3.
Highlights:
Slx and Slxl1 are mouse lineage-specific copies of their autosomal progenitor Sycp3
Slx and Slxl1 gene families are essential for male fertility
Changes in Slxl1 gene copy number distorts the ratio of male to female offspring
Slxl1 versus Sly competition for spindlin proteins may mediate sex ratio distortion
Acknowledgments:
We acknowledge Cincinnati Children’s Hospital Medical Center Transgenic Animal and Genome Editing Core for mutant mouse production, Celvie Yuan and Yang Yu for their assistance with ROSI experiments, the University of Michigan Flow Cytometry Shared Resource Laboratory for FACS, DNA Sequencing Core for Sanger and Next Generation Sequencing, Venkatesha Basrur at the Proteomics Resource facility for mass spectrometry and Cancer Center Tissue Core for generating testis histological sections. We thank D. Ginsburg for sharing EIIa-Cre mice. We thank M. Arlt, S. Kalantry, J. Moran, C. Swanepoel and Y. Yamashita for their comments.
Funding: This work was supported by National Institutes of Health grants HD064753 and HD094736 to JLM, T32GM007544 to ANK and a National Science Foundation Graduate Research Fellowship DGE 1256260 to ANK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests:
The authors declare no competing interests.
References:
- 1.Mueller JL, Mahadevaiah SK, Park PJ, Warburton PE, Page DC, and Turner JM (2008). The mouse X chromosome is enriched for multicopy testis genes showing postmeiotic expression. Nat Genet 40, 794–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller JL, Skaletsky H, Brown LG, Zaghlul S, Rock S, Graves T, Auger K, Warren WC, Wilson RK, and Page DC (2013). Independent specialization of the human and mouse X chromosomes for the male germ line. Nat Genet 45, 1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soh YQ, Alfoldi J, Pyntikova T, Brown LG, Graves T, Minx PJ, Fulton RS, Kremitzki C, Koutseva N, Mueller JL, et al. (2014). Sequencing the mouse Y chromosome reveals convergent gene acquisition and amplification on both sex chromosomes. Cell 159, 800–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, Repping S, Pyntikova T, Ali J, Bieri T, et al. (2003). The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 423, 825–837. [DOI] [PubMed] [Google Scholar]
- 5.Hughes JF, Skaletsky H, Pyntikova T, Graves TA, van Daalen SK, Minx PJ, Fulton RS, McGrath SD, Locke DP, Friedman C, et al. (2010). Chimpanzee and human Y chromosomes are remarkably divergent in structure and gene content. Nature 463, 536–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy WJ, Pearks Wilkerson AJ, Raudsepp T, Agarwala R, Schaffer AA, Stanyon R, and Chowdhary BP (2006). Novel gene acquisition on carnivore Y chromosomes. PLoS Genet 2, e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janecka JE, Davis BW, Ghosh S, Paria N, Das PJ, Orlando L, Schubert M, Nielsen MK, Stout TAE, Brashear W, et al. (2018). Horse Y chromosome assembly displays unique evolutionary features and putative stallion fertility genes. Nature communications 9, 2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skinner BM, Sargent CA, Churcher C, Hunt T, Herrero J, Loveland JE, Dunn M, Louzada S, Fu B, Chow W, et al. (2016). The pig X and Y Chromosomes: structure, sequence, and evolution. Genome Res 26, 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuroda-Kawaguchi T, Skaletsky H, Brown LG, Minx PJ, Cordum HS, Waterston RH, Wilson RK, Silber S, Oates R, Rozen S, et al. (2001). The AZFc region of the Y chromosome features massive palindromes and uniform recurrent deletions in infertile men. Nature genetics 29, 279–286. [DOI] [PubMed] [Google Scholar]
- 10.Ellis PJ, Bacon J, and Affara NA (2011). Association of Sly with sex-linked gene amplification during mouse evolution: a side effect of genomic conflict in spermatids? Hum Mol Genet 20, 3010–3021. [DOI] [PubMed] [Google Scholar]
- 11.Toure A, Clemente EJ, Ellis P, Mahadevaiah SK, Ojarikre OA, Ball PA, Reynard L, Loveland KL, Burgoyne PS, and Affara NA (2005). Identification of novel Y chromosome encoded transcripts by testis transcriptome analysis of mice with deletions of the Y chromosome long arm. Genome Biol 6, R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cocquet J, Ellis PJ, Yamauchi Y, Riel JM, Karacs TP, Rattigan A, Ojarikre OA, Affara NA, Ward MA, and Burgoyne PS (2010). Deficiency in the multicopy Sycp3-like X-linked genes Slx and Slxl1 causes major defects in spermatid differentiation. Mol Biol Cell 21, 3497–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cocquet J, Ellis PJ, Mahadevaiah SK, Affara NA, Vaiman D, and Burgoyne PS (2012). A genetic basis for a postmeiotic X versus Y chromosome intragenomic conflict in the mouse. PLoS Genet 8, e1002900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbs RA, Weinstock GM, Metzker ML, Muzny DM, Sodergren EJ, Scherer S, Scott G, Steffen D, Worley KC, Burch PE, et al. (2004). Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature 428, 493–521. [DOI] [PubMed] [Google Scholar]
- 15.Page SL, and Hawley RS (2004). The genetics and molecular biology of the synaptonemal complex. Annu Rev Cell Dev Biol 20, 525–558. [DOI] [PubMed] [Google Scholar]
- 16.Reynard LN, Turner JM, Cocquet J, Mahadevaiah SK, Toure A, Hoog C, and Burgoyne PS (2007). Expression analysis of the mouse multi-copy X-linked gene Xlr-related, meiosis-regulated (Xmr), reveals that Xmr encodes a spermatid-expressed cytoplasmic protein, SLX/XMR. Biol Reprod 77, 329–335. [DOI] [PubMed] [Google Scholar]
- 17.Baier A, Alsheimer M, Volff JN, and Benavente R (2007). Synaptonemal complex protein SYCP3 of the rat: evolutionarily conserved domains and the assembly of higher order structures. Sex Dev 1, 161–168. [DOI] [PubMed] [Google Scholar]
- 18.Syrjanen JL, Pellegrini L, and Davies OR (2014). A molecular model for the role of SYCP3 in meiotic chromosome organisation. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato T, Katagiri K, Yokonishi T, Kubota Y, Inoue K, Ogonuki N, Matoba S, Ogura A, and Ogawa T (2011). In vitro production of fertile sperm from murine spermatogonial stem cell lines. Nature communications 2, 472. [DOI] [PubMed] [Google Scholar]
- 20.Yuan L, Liu JG, Zhao J, Brundell E, Daneholt B, and Hoog C (2000). The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol Cell 5, 73–83. [DOI] [PubMed] [Google Scholar]
- 21.Cocquet J, Ellis PJ, Yamauchi Y, Mahadevaiah SK, Affara NA, Ward MA, and Burgoyne PS (2009). The multicopy gene Sly represses the sex chromosomes in the male mouse germline after meiosis. PLoS Biol 7, e1000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comptour A, Moretti C, Serrentino ME, Auer J, Ialy-Radio C, Ward MA, Toure A, Vaiman D, and Cocquet J (2014). SSTY proteins co-localize with the post-meiotic sex chromatin and interact with regulators of its expression. FEBS J 281, 1571–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chew TG, Peaston A, Lim AK, Lorthongpanich C, Knowles BB, and Solter D (2013). A tudor domain protein SPINDLIN1 interacts with the mRNA-binding protein SERBP1 and is involved in mouse oocyte meiotic resumption. PLoS One 8, e69764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su X, Zhu G, Ding X, Lee SY, Dou Y, Zhu B, Wu W, and Li H (2014). Molecular basis underlying histone H3 lysine-arginine methylation pattern readout by Spin/Ssty repeats of Spindlin1. Genes Dev 28, 622–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams SR, Maezawa S, Alavattam KG, Abe H, Sakashita A, Shroder M, Broering TJ, Sroga Rios J, Thomas MA, Lin X, et al. (2018). RNF8 and SCML2 cooperate to regulate ubiquitination and H3K27 acetylation for escape gene activation on the sex chromosomes. PLoS Genet 14, e1007233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staub E, Mennerich D, and Rosenthal A (2002). The Spin/Ssty repeat: a new motif identified in proteins involved in vertebrate development from gamete to embryo. Genome Biol 3, RESEARCH0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Presgraves DC (2008). Sex chromosomes and speciation in Drosophila. Trends Genet 24, 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forejt J (1996). Hybrid sterility in the mouse. Trends Genet 12, 412–417. [DOI] [PubMed] [Google Scholar]
- 29.Zanders SE, and Unckless RL (2019). Fertility Costs of Meiotic Drivers. Curr Biol 29, R512–R520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larson EL, Kopania EEK, and Good JM (2018). Spermatogenesis and the Evolution of Mammalian Sex Chromosomes. Trends Genet 34, 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammoud SS, Low DH, Yi C, Carrell DT, Guccione E, and Cairns BR (2014). Chromatin and transcription transitions of mammalian adult germline stem cells and spermatogenesis. Cell Stem Cell 15, 239–253. [DOI] [PubMed] [Google Scholar]
- 32.Soh YQ, Junker JP, Gill ME, Mueller JL, van Oudenaarden A, and Page DC (2015). A Gene Regulatory Program for Meiotic Prophase in the Fetal Ovary. PLoS Genet 11, e1005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merkin J, Russell C, Chen P, and Burge CB (2012). Evolutionary dynamics of gene and isoform regulation in Mammalian tissues. Science 338, 1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Necsulea A, and Kaessmann H (2014). Evolutionary dynamics of coding and non-coding transcriptomes. Nat Rev Genet 15, 734–748. [DOI] [PubMed] [Google Scholar]
- 35.Chalmel F, Lardenois A, Evrard B, Rolland AD, Sallou O, Dumargne MC, Coiffec I, Collin O, Primig M, and Jegou B (2014). High-resolution profiling of novel transcribed regions during rat spermatogenesis. Biol Reprod 91, 5. [DOI] [PubMed] [Google Scholar]
- 36.Gaysinskaya V, Soh IY, van der Heijden GW, and Bortvin A (2014). Optimized flow cytometry isolation of murine spermatocytes. Cytometry A 85, 556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bray NL, Pimentel H, Melsted P, and Pachter L (2016). Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34, 525–527. [DOI] [PubMed] [Google Scholar]
- 38.Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haeussler M, Schonig K, Eckert H, Eschstruth A, Mianne J, Renaud JB, Schneider-Maunoury S, Shkumatava A, Teboul L, Kent J, et al. (2016). Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol 17, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott MA, and Hu YC (2019). Generation of CRISPR-Edited Rodents Using a Piezo-Driven Zygote Injection Technique. Methods Mol Biol 1874, 169–178. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed EA, and de Rooij DG (2009). Staging of mouse seminiferous tubule cross-sections. Methods Mol Biol 558, 263–277. [DOI] [PubMed] [Google Scholar]
- 42.Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, Hewapathirana S, Kundu DJ, Inuganti A, Griss J, Mayer G, Eisenacher M, et al. (2019). The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res 47, D442–D450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hach F, Hormozdiari F, Alkan C, Hormozdiari F, Birol I, Eichler EE, and Sahinalp SC (2010). mrsFAST: a cache-oblivious algorithm for short-read mapping. Nat Methods 7, 576–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pendleton AL, Shen F, Taravella AM, Emery S, Veeramah KR, Boyko AR, and Kidd JM (2018). Comparison of village dog and wolf genomes highlights the role of the neural crest in dog domestication. BMC Biol 16, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guryev V, Saar K, Adamovic T, Verheul M, van Heesch SA, Cook S, Pravenec M, Aitman T, Jacob H, Shull JD, et al. (2008). Distribution and functional impact of DNA copy number variation in the rat. Nat Genet 40, 538–545. [DOI] [PubMed] [Google Scholar]
- 46.Cortez D, Marin R, Toledo-Flores D, Froidevaux L, Liechti A, Waters PD, Grutzner F, and Kaessmann H (2014). Origins and functional evolution of Y chromosomes across mammals. Nature 508, 488–493. [DOI] [PubMed] [Google Scholar]
- 47.Pavesi G, Zambelli F, Caggese C, and Pesole G (2008). Exalign: a new method for comparative analysis of exon-intron gene structures. Nucleic Acids Res 36, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogura A, Matsuda J, Asano T, Suzuki O, and Yanagimachi R (1996). Mouse oocytes injected with cryopreserved round spermatids can develop into normal offspring. J Assist Reprod Genet 13, 431–434. [DOI] [PubMed] [Google Scholar]
- 49.Kishigami S, and Wakayama T (2007). Efficient strontium-induced activation of mouse oocytes in standard culture media by chelating calcium. J Reprod Dev 53, 1207–1215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Excel file of Round Spermatid mRNAseq. Related to Figure 3.
Data Availability Statement
Sequencing data is available on NCBI SRA: PRJNA545829. Mass spectrometry data is available on the PRIDE database: PXD014276.


