Abstract
A facultative parasite of cereals, Fusarium culmorum is a soil-, air- and seed-borne fungus causing foot and root rot, fusarium seedling blight, and especially Fusarium head blight, a spike disease leading to decreased yield and mycotoxin contamination of grain. In the present study, we tested changes in expression of wheat genes (B2H2, ICS, PAL, and PR2) involved in defence against diseases. We first compared expression of the analysed genes in seedlings of non-inoculated and artificially inoculated wheat (variety Bohemia). The second part of the experiment compared expression of these genes in seedlings grown under various treatment conditions. These treatments were chosen to determine the effects of prochloraz, sodium bicarbonate, ergosterol, aescin and potassium iodide on expression of the analysed defence genes. In addition to the inoculated and non-inoculated cultivar Bohemia, we additionally examined two other varieties of wheat with contrasting resistance to Fusarium sp. infection. These were the blue aleurone layer variety Scorpion that is susceptible to Fusarium sp. infection and variety V2-49-17 with yellow endosperm and partial resistance to Fusarium sp. infection. In this manner, we were able to compare potential effects of inductors upon defence gene expression among three varieties with different susceptibility to infection but also between inoculated and non-inoculated seedlings of a single variety. The lowest infection levels were detected in the sodium bicarbonate treatment. Sodium bicarbonate had not only negative influence on Fusarium growth but also positively affected expression of plant defence genes. Expression of the four marker genes shown to be important in plant defence was significantly affected by the treatments. The greatest upregulation in comparison to the water control was identified under all treatments for the B2H2 gene. Only expression of PAL under the ergosterol and prochloraz treatments were not statistically significant.
Introduction
Fusarium culmorum is a ubiquitous soil-borne fungus with a highly competitive saprophytic capability. As a facultative parasite, it can cause foot and root rot [1]. Fusarium culmorum is also seed-borne and causes fusarium seedling blight when infected seed is used in sowing. Seedling blight can cause extensive damage to growing seedlings [2] that can lead to reduced plant establishment, number of heads per square meter, and also grain yield [3]. Fusarium head blight (FHB) is one of the most severe diseases responsible for decrease in grain yield and quality. Furthermore, presence of mycotoxins produced by this fungus (deoxynivalenol, nivalenol, zearalenone, and many others) can harm human and animal health. FHB in wheat is mainly caused by Fusarium graminearum, F. culmorum, and F. poae. Fusarium culmorum infection is dominant in colder regions, such as north, east, and central Europe [1]. The major reservoirs of Fusarium sp. inoculum are crop residues on the soil surface. The fungus can survive on a wide range of living plant species (wheat, corn, barley, soybean, and rice) (see Bai and Shaner for a review [4]).
There are several means to fight this disease: use of fungicides, cultural practices, resistant cultivars, and biological agents [5]. Although seed treatment is used to control soil-borne infection caused by Fusarium spp. [6], there is no definite way to defeat this complex of Fusarium diseases. Efficacy of fungicide treatments against FHB is only 15–30% [7]. Fully resistant cultivars are not available to date, but some cultivars have useable levels of partial resistance that limit yield loss and mycotoxins accumulation [8]. FHB resistance has a quantitative nature and identification of responsible genes is difficult. Even though numerous quantitative trait loci have been described to date (see Duba et al. for a review [9]), just a few such genes have been definitively identified, sequenced, and their causal mutations determined. Kage et al. [10] identified an FHB resistance gene on chromosome 2DL as the TaACT gene encoding agmatine coumaroyl transferase. They suggest that several single nucleotide polymorphisms (SNPs) and two inversions may be important for gene function. The second identified gene, Fhb1, confers resistance in variety Sumai 3. It is pore-forming toxin-like (PFT) gene [11]. Further, a number of pathogenicity and virulence factors have been characterized [11, 12, 13].
The expression of defence-related genes also can be important in the plant’s reaction against pathogens. PR-1, PR-2 (glucanases), PR-3 (chitinase), PR-4 (thaumatin-like proteins), PR-5, and peroxidase have been shown to be induced in both resistant and susceptible cultivars after point inoculation [14]. These proteins were detected as early as 6 to 12 h after inoculation and peaked after 36 to 48 h. Earlier and greater expression of PR-4 and PR-5 transcripts were observed, however, in resistant cultivar Sumai 3 than in susceptible cultivar Wheaton [14]. Larger amounts of β-1,3-glucanase and chitinase enzymes also have been detected in resistant cultivar Sumai [15]. The overexpression of defence response genes in wheat could enhance FHB resistance in both greenhouse and field conditions.
A large number of organic and inorganic compounds have previously been described as affecting plant defence mechanisms [16, 17]. For example, such plant or fungal-derived compounds as monoterpenes or ergosterol can induce plant defence [18, 19].
Our study is focused upon comparing expression of different genes in non-inoculated and inoculated varieties and under various treatment conditions. The first part of the experiment compares expression of the genes β-1,3-glucanase (PR2), chitinase (B2H2), phenylalanine ammonia-lyase (PAL), and isochorismate synthase (ICS) (the last two being genes from the salicylate pathway) in healthy seedlings versus seedlings of Triticum aestivum var. Bohemia artificially inoculated with F. culmorum. The second part of the experiment focused on how expression of these genes is influenced by different treatment solutions (based upon prochloraz, aescin, ergosterol, sodium bicarbonate and potassium iodide) within which seedlings were grown. Prochloraz, potassium iodide and sodium bicarbonate were chosen for their antifungal effects in preliminary in vitro experiments (S1 Fig) and aescin and ergosterol because of their expected effect on plant triggered immunity. How these treatments influence expression of the aforementioned genes is compared with a water control in inoculated and healthy seedlings of Bohemia as well as in the moderately Fusarium-resistant yellow endosperm variety V2-49-17 and the susceptible blue aleurone layer variety Scorpion. The originality of this research consists in using artificially infected seeds during anthesis of mother plants and continuous exposure of seedlings to potential inductors.
Materials and methods
Plant material
Inoculated and non-inoculated (healthy) groups of wheat seeds (var. Bohemia) were used for the first part of the experiment. Inoculated and healthy seeds were acquired from plants grown under field conditions during the 2017 season. Inoculated seeds were collected from plants that had been sprayed during mid-anthesis phase by F. culmorum (tribe KM16902) macroconidia at concentration 5 × 105 conidia ml−1. A previous study had shown a high level of virulence and strong production of deoxynivalenol (DON) by this tribe [20]. The seeds from inoculated and healthy variants were collected and stored at room temperature and low humidity. The plant material further consisted of two bread wheat cultivars differing in grain colour and in susceptibility to F. culmorum infection (V2-49-17 –yellow kernels, medium resistant to infection; Scorpion–blue aleurone layer, highly susceptible to infection).
Growth chamber test under controlled conditions
Growth chamber test of 50 kernels of all three cultivars (Bohemia–inoculated and healthy; Scorpion, and V2-49-17) were laid with 1 cm separation distance into two layers of filtrate paper and rolled up. The rolls were immersed into the treatment solutions. Four replications (200 kernels in total) were made for each combination of cultivar and treatment solution. The treatment solutions consisted of 25 μg ml−1 solution of ergosterol, 25 μg ml−1 solution of aescin, 1 μg ml−1 solution of prochloraz, 1% solution of potassium iodide and 0.1 M solution of sodium bicarbonate. The sodium bicarbonate solution was boiled at 120°C for half an hour. During boiling, the sodium bicarbonate gradually decomposed to sodium carbonate, water and carbon dioxide. This reaction led to alkalization of pH. Distilled water was used as a control solution.
Seedlings were cultivated under controlled conditions (20°C/18°C, 12/12 h of light/dark) until the two-leaves growth stage. At this stage, the whole leaves were collected from three plants representing three biological replicates. The leaves showed no signs of F. culmorum infection. Symptoms of F. culmorum infection were visible only on the lower parts of plants and around the seeds (Fig 1). The leaves were immediately frozen in liquid nitrogen and preserved at −80°C until RNA isolation. The numbers of infected seeds and seeds with no sign of infection were counted and the results were statistically analysed by ANOVA (Statistica 12 software).
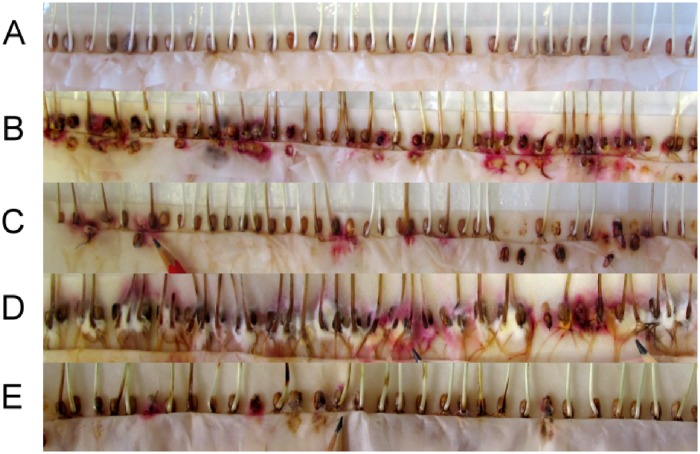
Fig 1. Inoculated and non-inoculated seedlings of cv. Bohemia under different treatment conditions.
(A) non-inoculated seedlings in water, (B) inoculated seedlings in water, (C) inoculated seedlings in prochloraz, (D) inoculated seedlings in potassium iodide, (E) inoculated seedlings in sodium bicarbonate. Pink–white coloration around kernels and dark discoloration of coleoptiles and stem bases are symptoms of F. culmorum infection.
RNA isolation and qPCR
Leaves were homogenized in a TissueLyser II (Qiagen) for 2 minutes at 27 Hz. Caution was taken during homogenization to avoid sample melting. The homogenized samples were immediately placed into liquid nitrogen. The RNA was isolated using the RNeasy Plant Mini Kit (Qiagen) while following the manufacturer’s instructions. DNA was removed during the RNA purification using the RNase-Free DNase Set (Qiagen). The isolated RNA was stored at −80°C. cDNA was synthesized using the Transcriptior High Fidelity cDNA Synthesis Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions with 1 μg of total RNA and anchored-oligo (dT) primers. The concentration of cDNA was measured with Qubit (Thermo Fisher Scientific) and cDNA was diluted to concentration 10 ng μl−1. The expression analysis of the chosen plant defence genes (chitinase (B2H2), β-1,3-glucanase (PR2), isochorismate synthase (ICS), and phenylalanine ammonia-lyase (PAL)) was performed using the CFX96TM Real-Time PCR Detection System (Bio-Rad). The qPCR mix consisted of 1× SYBR Green (Top Bio), 0.2 μM forward and reverse primers (Table 1), 15 ng cDNA, and water to final volume 15 μl. The reference gene was glyceraldehyde-3-phosphate dehydrogenase (GAPDH) according to Travella et al. (2006) [21] and Sun et al. (2014) [22]. The control sample consisted of equal amounts of cDNA from all three replicates of healthy, untreated Bohemia seedlings diluted to 10 ng μl−1. The primers specificity and presence of primer dimers were verified by melting analysis. The data were analysed using the 2–ΔΔCq method with CFX Manager 3.0 software (Bio-Rad, USA). Three biological as well as three technical replicates were run.
Table 1. Primer pairs used in the study.
Names, sequences of forward and reverse primers, publication sources of primer pairs, and gene functions are listed.
| Gene name | Forward primer | Reverse primer | Publication | Function |
|---|---|---|---|---|
| B2H2 | TCTATCGAAACGCCATTGTTACA | AGAGGCCGTTCGCATAGTCA | [42] | chitinase |
| PR2 | CCGCACAAGACACCTCAAGATA | CGATGCCCTTGGTTTGGTAGA | [43] | β-1,3-glucanase |
| PAL | TTGATGAAGCCGAAGCAGGACC | ATGGGGGTGCCTTGGAAGTTGC | [44] | salicylate pathway |
| ICS | AGAAATGAGGACGACGAGTTTGAC | CCAAGTAGTGCTGATCTAATCCCAA | [44] | salicylate pathway |
| GAPDH | TTAGACTTGCGAAGCCAGCA | AAATGCCCTTGAGGTTTCCC | [22] | reference gene |
Results
Determination of Fusarium infection level in growth chamber test
Inoculated and healthy seeds of wheat cv. Bohemia were treated with different solutions: water, prochloraz, aescin, ergosterol, sodium bicarbonate and potassium iodide. Inoculated seeds contained high levels of F. culmorum DNA (analysed by qPCR, S1 File), the mean of three replications being 5,048 μg kg−1 of DON (analysed by ELISA, S1 File). No F. culmorum DNA was detected in the non-inoculated seeds, and their DON content (if any) was under the detection limit. The plants were visually inspected at the two-leaves stage for presence of Fusarium infection. The number of infected seeds under every treatment was compared to that for the control (treated only with water). The presence of Fusarium infection was detectable by pink–white mycelia growing around kernels and dark discoloration of the coleoptile and stem (Fig 1).
In the variant without inoculation there were no infected seeds. On the contrary, the inoculated seeds that had been submerged in water showed high level of infection (Fig 1). This level of infection was decreased under every treatment except for that of potassium iodide. The level of infection in the potassium iodide treatment group was even increased in comparison to the water-treated inoculated seeds. In evaluating the 200 seeds from each combination, significant differences were detected between individual groups. The results suggest that the lowest level of infection in inoculated seeds was detected in the sodium bicarbonate treatment, followed (in order from lowest to highest) by prochloraz, ergosterol, aescin, water, and potassium iodide (Fig 2). Thus, the treatment with 0.1M sodium bicarbonate was more potent in suppressing fungal growth than was the treatment with 1 μg ml−1 prochloraz.
Fig 2. Percentage of F. culmorum-infected plants in different treatments with cultivar Bohemia.
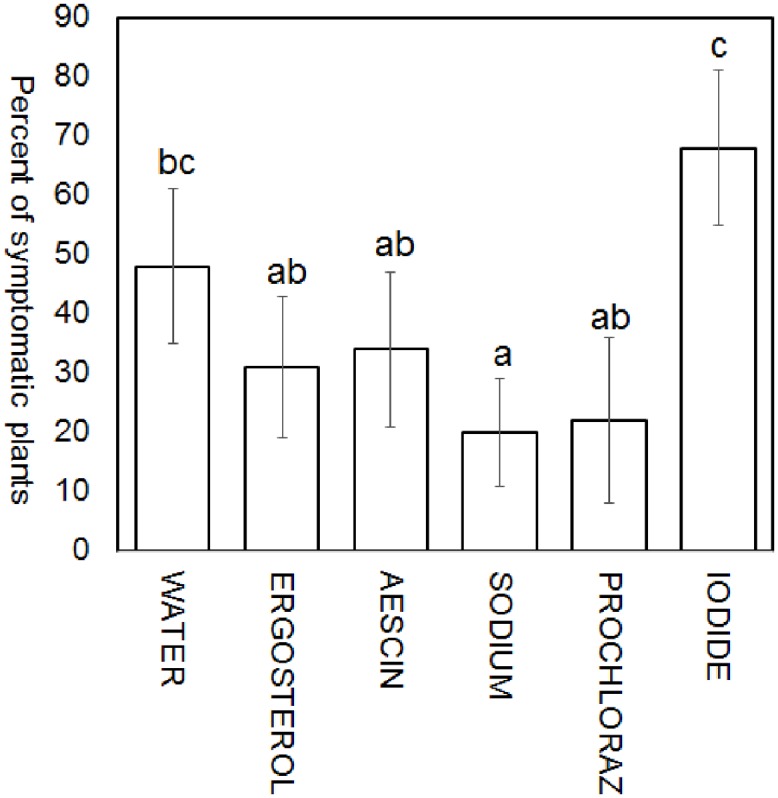
Two hundred inoculated seeds were evaluated for each treatment solution (water [control], ergosterol, aescin, sodium bicarbonate, prochloraz and potassium iodide). Error bars indicate 95% confidence intervals around the mean.
Expression levels of four genes involved in plant–pathogen interaction in wheat seedlings
The expression of four important plant defence genes (B2H2, ICS, PAL, and PR2) against Fusarium infection was detected. These genes were analysed in three technical and biological replications of each studied variety under each treatment. The fold differences (FDs) in their expression levels were first compared among the reference (water control) and other experimental conditions (ergosterol, aescin, sodium bicarbonate, prochloraz, and potassium iodide) of all studied varieties (Table 2). In this manner, the efficiency of different treatments in enhancing expression of plant defence genes with and without pathogen infection was examined. The treatments did not influence expression of the genes in an even manner, as expression was stronger in some genes and weaker in others. Only insignificant changes were detected in inoculated Bohemia under the ergosterol treatment (ICS, PAL) as well as in Scorpion under the aescin treatment (PAL).
Table 2. Expression levels of four chosen genes in leaves of healthy and inoculated plants of wheat variety Bohemia and healthy V2-49-17 and Scorpion varieties.
The expression of four genes (B2H2, ICS, PAL, and PR2) were detected in healthy and inoculated Bohemia, V2-49-17 variety with yellow endosperm, and cv. Scorpion with blue aleurone layer in different treatment conditions (ergosterol, aescin, sodium bicarbonate, prochloraz and potassium iodide) and indicated as a fold difference (FD) to control (treatment water).
| Variant | Gene | Ergosterol | Aescin | Sodium bicarbonate | Prochloraz | Potassium iodide | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy Bohemia | B2H2 | -0,99 | *** | 10,16 | **** | 838,05 | **** | 72,96 | **** | 7370,13 | **** |
| ICS | -0,76 | *** | 3,76 | **** | 2,71 | *** | 4,53 | **** | -0,39 | *** | |
| PAL | -0,83 | ** | 0,94 | ** | 5,99 | **** | 5,06 | **** | 4,98 | **** | |
| PR2 | -0,84 | ** | 3,97 | **** | 1,07 | ** | 0,42 | ** | 20,75 | **** | |
| Inoculated Bohemia | B2H2 | 2,29 | ** | 2,80 | ** | 0,95 | ** | 5,20 | **** | 7,45 | **** |
| ICS | -0,27 | 1,33 | *** | 0,41 | ** | -0,85 | *** | -0,57 | ** | ||
| PAL | 2,10 | ** | 1,98 | ** | 4,99 | **** | 7,12 | **** | 3,86 | **** | |
| PR2 | 1,30 | *** | 3,11 | **** | 1,96 | *** | 1,31 | *** | 30,80 | **** | |
| V2-49-17 variety with yellow endosperm | B2H2 | 42,73 | **** | 46,67 | **** | 1060,53 | **** | 2040,09 | **** | 1362,64 | **** |
| ICS | 1,21 | **** | 8,27 | **** | 6,26 | **** | 12,29 | **** | -0,33 | *** | |
| PAL | 8,57 | *** | 4,39 | *** | 57,79 | **** | 82,05 | **** | -1,00 | * | |
| PR2 | 23,42 | **** | 58,49 | **** | 25,16 | **** | 20,09 | **** | 48,52 | **** | |
| Scorpion with blue aleurone layer | B2H2 | -0,98 | **** | -0,98 | **** | 122,92 | **** | -0,42 | *** | 44,48 | **** |
| ICS | 1,22 | **** | -0,69 | *** | -0,87 | *** | 1,59 | **** | -0,09 | * | |
| PAL | 5,85 | *** | -0,02 | 14,02 | **** | 43,83 | **** | -1,00 | *** | ||
| PR2 | 4,08 | **** | 2,73 | **** | 20,16 | **** | 21,71 | **** | 49,85 | **** | |
Significant fold differences between water control and experimental treatments are indicated by asterisks (P < 0.05 (*); P < 0.01 (**); P < 0.001 (***); P < 0.0001 (****). The statistical analyses were carried out separately within each gene and treatment.
B2H2 expression was upregulated by almost all treatments in all studied varieties (Table 2). The highest expression level of this gene was achieved under the sodium bicarbonate (V2-49-17) treatment followed by the prochloraz (V2-49-17) and potassium iodide (healthy Bohemia) treatments. It was downregulated just under the ergosterol (healthy Bohemia and Scorpion), aescin (Scorpion), and prochloraz (Scorpion) treatments. This gene achieved the largest FD (up to 7,370; potassium iodide) in comparison to the other genes. The FD was lowest in inoculated Bohemia. The FD of B2H2 was in some cases near to 1,000 in comparison to the water control. This high FD was not achieved solely in the inoculated Bohemia and the Scorpion variety. In inoculated Bohemia, for example, the FD ranged from 0.95 under the sodium to 7.45 under the iodide treatment. In healthy Bohemia, the expression ranged from −0.99 to 7,370 FD in comparison to the water control. A similarly large increase of expression in comparison to the water control as in healthy Bohemia was identified also in V2-49-17. The V2-49-17 variety showed significant increase of B2H2 expression in comparison to healthy Bohemia while the expression of all other genes was lower.
The ICS gene manifested the smallest fold increase under almost all analysed treatments. Its expression was often downregulated under some experimental treatments in some analysed varieties (Table 2). The largest fold difference was detected in the V2-49-17 variety under the prochloraz treatment, the smallest in inoculated Bohemia under the prochloraz treatment. Under the iodide treatment, all FDs were in negative values for all analysed varieties.
FDs for PAL expression were increased under almost every treatment. These increases were not to such large extent as seen for B2H2. The largest FDs were achieved under prochloraz (82.05 FD) and sodium bicarbonate (57.79 FD) treatments in the V2-49-17 variety. Downregulation of PAL expression in comparison to the water control was identified under the ergosterol treatment in healthy Bohemia, under potassium iodide treatment in V2-49-17, and under potassium iodide and aescin treatments in the Scorpion variety (Table 2). The lowest FD was detected under iodide in the yellow variety.
The FDs for PR2 expression in comparison to the water control were elevated under almost all treatments and in all analysed varieties except for the ergosterol treatment in healthy Bohemia. The largest FD was seen in the V2-49-17 variety under all treatments except for prochloraz and iodide, in which cases the FDs were greater in the Scorpion variety. The largest FD for PR2 was observed under the iodide treatment (from 20.75 to 49.85 FD). The lowest FD in almost all cases was found in healthy Bohemia (Table 2).
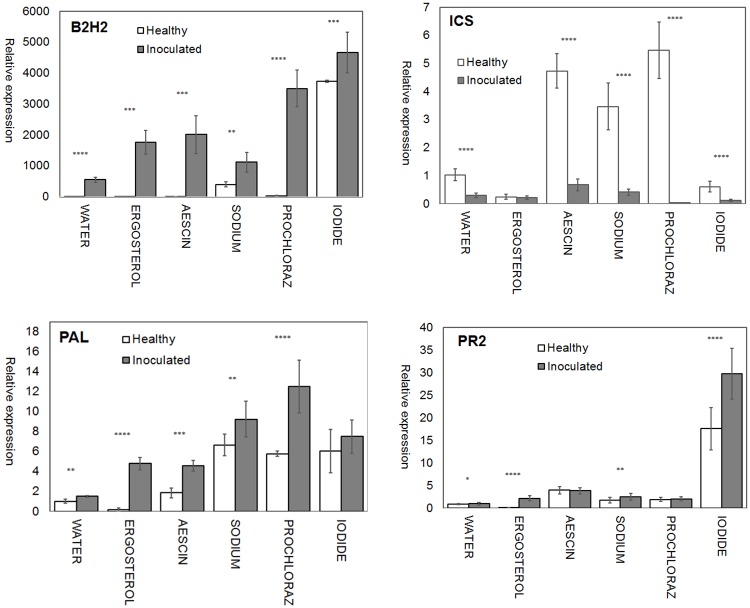
We further compared the expression of all four genes between the inoculated and healthy Bohemia under all treatments. The strongest B2H2 expression in inoculated Bohemia was identified under potassium iodide treatment and the weakest under the control. The strongest B2H2 expression in healthy Bohemia was identified under potassium iodide treatment followed by that for sodium bicarbonate (Fig 3). The expression of B2H2 was increased in inoculated Bohemia under all treatments.
Fig 3. Expression profiles of B2H2, ICS, PAL, and PR2 in leaves of inoculated and healthy plants of wheat variety Bohemia under different treatment conditions (A, B, C, D).
Expression levels were relative to healthy cv. Bohemia seeds and were normalized with the wheat reference gene GAPDH. Expression levels shown are mean values and standard deviation for three replications. Statistically significant differences between healthy and inoculated cv. Bohemia plants are indicated by asterisks above every treatment (P < 0.05 (*); P < 0.01 (**); P < 0.001 (***); P < 0.0001 (****).
Expression of ICS was significantly downregulated in inoculated Bohemia under almost all treatments, the exception being the ergosterol treatment, in which case the difference was not statistically significant (Fig 3). The strongest ICS expression was detected under the prochloraz treatment in healthy Bohemia. The weakest was under the iodide and ergosterol treatments.
Comparison of healthy versus inoculated Bohemia showed increase of PAL expression in inoculated Bohemia under all treatments. The strongest expression of PAL in healthy Bohemia was identified under the sodium bicarbonate treatment. The greatest expression in inoculated Bohemia was identified under the prochloraz and sodium bicarbonate treatments (Fig 3).
PR2 expression was elevated in all treatments other than the aescin treatment in inoculated Bohemia. The difference between healthy and inoculated Bohemia under the aescin treatment was not statistically significant. The expression of PR2 in inoculated Bohemia was elevated under the iodide (in comparison to healthy Bohemia), and ergosterol treatments with high significance. Small increases were detected under the water and sodium bicarbonate treatments (Fig 3).
Discussion
In current study was tested the expression of chosen marker genes of wheat seedlings after various treatments by potential plant defence inductors. The effect of plant defence inductors was previously widely studied [18, 19] and their effect on defence genes expression was taken to the account. Effect of chitinase genes in increasing plant resistance to fungal diseases has been observed in previous studies (see Fahmy et al. for a review [23]). Transgenic wheat with barley chitinase II was shown to be resistant against powdery mildew, leaf rust pathogens, and F. graminearum [24, 25, 26]. Chitinase from wheat, barley, and maize kernels has been shown to inhibit hyphal elongation of the fungi [27]. In the present study, expression of the chitinase gene in the V2-49-17 variety was greater than was its expression in healthy Bohemia under the control variant (water) as well as under the experimental treatments. This higher value of chitinase expression can be connected to partial resistance of the yellow variety to Fusarium sp. infection. Higher chitinase levels in resistant cultivars already have been detected in previous studies [28, 29]. The largest fold increase for B2H2 expression in healthy Bohemia was detected under the potassium iodide treatment. This 7,000 FD could be explained by the negative effect of this treatment on seedlings growth. This retardation of growth was connected to increase of F. culmorum infection in potassium iodide-treated varieties regardless of variety or presence of Fusarium infection. However, more than 1,000 FD [1,363] in comparison to the water control was detected just in V2-49-17. The growth retardation of seedlings under iodide treatment in our experiment is substantiated by the previous study of Brenchley [30], who reported a negative effect of high concentration of potassium iodide on germination of barley seeds and even of low concentration on the survival of barley seedlings. Iodine at concentration 10 ppm was observed to be toxic to barley. Nevertheless, a concentration 0.5–1 ppm had a positive effect on barley plants [31]. The iodine is most toxic in its iodide form [32]. This is the form we used in our experiments at 10,000 ppm concentration. Used concentration inhibited F. culmorum growth in preliminary in vitro test (S1 Fig). The observed toxicity of potassium iodide for wheat seedlings is thus understandable and such a high concentration suppress natural plant immunity, thus allowing us to see the effect of immunity on the intensity of the pathogen development (Fig 1). The strong expression of B2H2 is just a result of this treatment and does not reflect an effect of potassium iodide’s increasing resistance in the plant. This inhibitory effect was more potent than the effects of sodium bicarbonate and prochloraz. Significant increase of B2H2 expression could be seen also in the sodium bicarbonate treatment. This increase exceeded those under the prochloraz treatments. It can be seen across all analysed varieties with the exception of the inoculated Bohemia. Our results imply that sodium bicarbonate is significantly effective in enhancing expression of defence genes. Contrary to potassium iodide, sodium bicarbonate has no negative effect on wheat seedlings’ growth. Comparison of all treatments in inoculated wheat showed that the lowest numbers of seedlings with detectable Fusarium infection at the three-leaves stage were under the prochloraz and sodium bicarbonate treatments. This suggests that sodium bicarbonate has potential for increasing plant resistance. The addition of sodium bicarbonate to experimental treatments was conditioned by its alkaline pH. This was in accordance with studies showing inhibition of TRI genes expression in Fusarium by alkaline pH [33, 34, 35]. TRI genes expression is important for synthesis of DON, which is known to be a virulent factor aiding in the establishment and propagation of Fusarium infection within the spikes [36, 37]. In preliminary experiments, the sodium bicarbonate showed potential for inhibiting Fusarium growth in vitro (S1 Fig). Indeed, the sodium bicarbonate showed great potential also in planta (Fig 1). Sodium bicarbonate had not only a negative influence on Fusarium growth but also a positive effect on expression of plant defence genes. The sodium bicarbonate has been shown to be potent in inhibiting growth also of other Fusarium species [38, 39].
We analysed the SA pathway’s function in plant defence by examining expression of the two genes ICS and PAL, both of whose biosynthetic pathways are known to be involved in SA production within Arabidopsis [40]. Hao et al. [40] had previously detected that suppression of the ICS gene compromised plant resistance to F. graminearum but that similar suppression of PAL genes had no significant effect. Those authors also found that F. graminearum-inoculated plants with stronger expression of ICS were comparable to wild-type control plants [40] and that plants with ICS suppressed did not accumulate SA during pathogen infection and were more susceptible to Fusarium. In the present study, the suppression of growth and higher rate of Fusarium infection connected to lower ICS expression were detectable predominantly under the potassium iodide treatment, where ICS had negative FD in comparison to the water treatment in every analysed variety. Similarly, ICS was downregulated under all treatments in the inoculated wheat cv. Bohemia. On the other hand, ICS expression was upregulated in all other treatments except for a few exceptions in Scorpion and inoculated Bohemia. It was upregulated in V2-49-17 under all treatments other than that of potassium iodide, which can be connected to this variety’s partial Fusarium resistance. Upregulation of ICS in inoculated Bohemia in comparison to the water control was detected only under sodium bicarbonate and aescin treatments, which can indicate effects of these treatments on expression of defence genes. Hao et al. [40] have suggested that ICS plays a unique role in SA biosynthesis in barley, which, in turn, confers a basal resistance to F. graminearum by modulating the accumulation of H2O2, O2, and reactive oxygen-associated enzymatic activities. In the present study, the greatest increase in PAL expression was detected in the V2-49-17 and Scorpion varieties. We found no correlation between higher PAL expression and the level of resistance to F. culmorum, because V2-49-17 is partially resistant but Scorpion is susceptible. In healthy versus inoculated Bohemia, PAL expression was generally increased under most treatments and especially under the potassium iodide treatments. Wildermuth et al. had detected increased PAL and decreased ICS expression after F. graminearum infection [41]. They also found a difference in timing whereby earlier increase of PAL expression was detected in a partially resistant variety (Wangshuibai). They found no difference, however, between resistant and susceptible varieties in the timing of decrease in ICS expression.
Conclusion
We conclude that prochloraz and sodium bicarbonate has the greatest potential for suppression of fungal development without having a negative effect on plant growth. According to our findings, the sodium bicarbonate had not only a negative influence on Fusarium growth but also a positive effect on upregulating the expression of plant defence genes.
Supporting information
(DOCX)
HCl pH4 –PDA balanced to pH4 by HCl, NaOH pH10 –PDA balanced to pH10 by NaOH, IODIDE–PDA with potassium iodide (1% concentration), SODIUM–PDA with sodium bicarbonate (0.1 M), ACETATE–PDA with ammonium acetate (0.1 M), SDHI–PDA with fluxapyroxad (1 μg ml−1), QoI–PDA with picoxystrobin (1 μg ml−1), DMI–PDA with prochloraz (1 μg ml−1).
(EPS)
Acknowledgments
We thank Peter Antal for his advising us with the establishment of in vitro experiments.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work and ZA, DB, PeM and PaM have been supported by the Ministry of Agriculture of the Czech Republic (project no. MZE RO1118). PaM have been supported by the Ministry of Agriculture of the Czech Republic (project no. QK1910197) and Internal Grant of Palacky University (project no. IGA_PrF_2020_003). PeM have been supported by the Ministry of Agriculture of the Czech Republic (project no. QK1910343). The funder Agrotest Fyto, Ltd provided support in the form of salaries for all authors, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Parry DW, Jenkinson P, McLeod L. Fusarium ear blight in small grain cereals–a review. Plant Pathol. 1995; 44:207–238. 10.1111/j.1365-3059.1995.tb02773.x [DOI] [Google Scholar]
- 2.Khan MR, Fischer S, Egan D, Doohan FM. Biological control of Fusarium seedling blight disease of wheat and barley. Phytopathology. 2006; 96:386–394. 10.1094/PHYTO-96-0386 [DOI] [PubMed] [Google Scholar]
- 3.Wong LSL, Tekauz A, Leslie D, Abramson D, McKenzie RIH. Prevalence, distribution and importance of Fusarium head blight in winter in Manitoba. Can J Plant Pathol. 1992; 14:233–238. 10.1080/07060669209500882 [DOI] [Google Scholar]
- 4.Bai G, Shaner G. Management and resistance in wheat and barley to fusarium head blight. Annu Rev Phytopathol. 2004; 42:135–161. 10.1146/annurev.phyto.42.040803.140340 [DOI] [PubMed] [Google Scholar]
- 5.Wagacha JM, Muthomi JW. Fusarium culmorum: Infection process, mechanisms of mycotoxin production and their role in pathogenesis in wheat. Crop Protection. 2007; 26(7):877–885. 10.1016/j.cropro.2006.09.003 [DOI] [Google Scholar]
- 6.Jørgensen LN, Nielsen LK, Nielsen BJ. Control of seedling blight in winter wheat by seed treatments–impact on emergence, crop stand, yield and deoxynivalenol. Acta Agr Scand B-Sp. 2012; 62:431–440. 10.1080/09064710.2011.641028 [DOI] [Google Scholar]
- 7.D’Angelo DL, Bradley CA, Ames KA, Willyerd KT, Madden LV, Paul PA. Efficacy of fungicide applications during and after anthesis against Fusarium head blight and deoxynivalenol in soft red winter wheat. Plant Dis. 2014; 98:1387–1397. 10.1094/PDIS-01-14-0091-RE [DOI] [PubMed] [Google Scholar]
- 8.Pereyra SA, Dill-Macky R, Sims AL. Survival and inoculum production of Gibberella zeae in wheat residue. Plant Dis. 2004; 88:724–730. 10.1094/PDIS.2004.88.7.724 [DOI] [PubMed] [Google Scholar]
- 9.Duba A, Goriewa-Duba K, Wachowska U. A review of the interactions between wheat and wheat pathogens: Zymoseptoria tritici, Fusarium spp. and Parastagonospora nodorum. Int J Mol Sci. 2018; 19(4). 10.3390/ijms19041138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kage U, Karre S, Kushalappa AC, McCartney C. Identification and characterization of a fusarium head blight resistance gene TaACT in wheat QTL-2DL. Plant Biotechnol J. 2017; 15(4):447–457. 10.1111/pbi.12641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawat N, Pumphrey MO, Liu S, Zhang X, Tiwari VK, Ando K, et al. Wheat Fhb1 encodes a chimeric lectin with agglutinin domains and a pore-forming toxin-like domain conferring resistance to Fusarium head blight. Nat Genet. 2016; 48:1576 10.1038/ng.3706 [DOI] [PubMed] [Google Scholar]
- 12.Cuthbert PA, Somers DJ, Thomas J, Cloutier S, Brulé-Babel A. Fine mapping Fhb1, a major gene controlling fusarium head blight resistance in bread wheat (Triticum aestivum L.). Theor Appl Genet. 2006; 112(8):1465 10.1007/s00122-006-0249-7 [DOI] [PubMed] [Google Scholar]
- 13.Buerstmayr H, Ban T, Anderson A. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: a review. Plant Breeding. 2009; 128:1–26. 10.1111/j.1439-0523.2008.01550.x [DOI] [Google Scholar]
- 14.Pritsch C, Muehlbauer GJ, Bushnell WR, Somers DA, Vance CP. Fungal development and induction of defense response genes during early infection of wheat spikes by Fusarium graminearum. Mol Plant Microbe Interact. 2000; 13(2):159–69. 10.1094/MPMI.2000.13.2.159 [DOI] [PubMed] [Google Scholar]
- 15.Kang Z, Buchenauer H, Huang L, Han Q, Zhang H. Cytological and immunocytochemical studies on responses of wheat spikes of the resistant Chinese cv. Sumai 3 and the susceptible cv. Xiaoyan 22 to infection by Fusarium graminearum. Eur J Plant Pathol. 2007; 120(4):383–396. 10.1007/s10658-007-9230-9 [DOI] [Google Scholar]
- 16.Wiesel L, Newton AC, Elliott I, Booty D, Gilroy EM, Birch PR, et al. Molecular effects of resistance elicitors from biological origin and their potential for crop protection. Front Plant Sci. 2014; 5:655 10.3389/fpls.2014.00655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakraborty N, Chandra S, Acharya K. Biochemical basis of improvement of defense in tomato plant against Fusarium wilt by CaCl2. Physiol Mol Biol Plants. 2017; 23(3):581–596. 10.1007/s12298-017-0450-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardoza RE, McCormick SP, Malmierca MG, Olivera ER, Alexander NJ, Monte E, et al. Effects of trichothecene production on the plant defense response and fungal physiology: overexpression of the trichoderma arundinaceum tri4 gene in T. harzianum. Appl Environ Microbiol. 2015; 81(18): 6355–6366. 10.1128/AEM.01626-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tugizimana F, Steenkamp PA, Piater LA, Dubery IA. Ergosterol-induced sesquiterpenoid synthesis in tobacco cells. Molecules. 2012; 17(2):1698–1715. 10.3390/molecules17021698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matušinsky P, Polišenská I, Kadlíková M, Tvarůžek L, Spitzerová D, Spitzer T. Dynamics of T-2 toxin synthesis on barley ears. J Food Agr Environ. 2013; 11(3–4):1114–1122. [Google Scholar]
- 21.Travella S, Klimm TE, Keller B. RNA interference-based gene silencing as an efficient tool for functional genomics in hexaploid bread wheat. Plant Physiol. 2006; 142:6–20. 10.1104/pp.106.084517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H, Guo Z, Gao L, Zhao G, Zhang W, Zhou R, et al. DNA methylation pattern of Photoperiod-B1 is associated with photoperiod insensitivity in wheat (Triticum aestivum). New Phytol. 2014; 204(3): 682–692. 10.1111/nph.12948 [DOI] [PubMed] [Google Scholar]
- 23.Fahmy AH, Hassanein RA, Hashem HA, Ibrahim AS, El Shihy OM, Qaid EA. Developing of transgenic wheat cultivars for improved disease resistance. J App Biol Biotech. 2018; 6(2):31–40. 10.7324/JABB.2018.60206 [DOI] [Google Scholar]
- 24.Shin S, Mackintosh CA, Lewis J, Heinen SJ, Radmer L, Dill-Macky R, et al. Transgenic wheat expressing a barley class II chitinase gene has enhanced resistance against Fusarium graminearum. J Exp Bot. 2008; 59(9):2371–2378. 10.1093/jxb/ern103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bliffeld M, Mundy J, Potrykus I, Futterer J. Genetic engineering of wheat for increased resistance to powdery mildew disease. Theor Appl Genet. 1999; 98:1079–1086. 10.1007/s001220051170 [DOI] [Google Scholar]
- 26.Oldach KH, Becker D, Lörz H. Heterologous expression of genes mediating enhanced fungal resistance in transgenic wheat. Mol Plant Microbe Interact. 14(7):832–838. 10.1094/MPMI.2001.14.7.832 [DOI] [PubMed] [Google Scholar]
- 27.Selitrennikoff CP. Antifungal proteins. Appl Environ Microb. 2001; 67(7):2883–2894. 10.1128/AEM.67.7.2883-2894.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li WL, Faris JD, Muthukrishnan S, Liu DJ, Chen PD, Gill BS. Isolation and characterization of novel cDNA clones of acidic chitinases and β−1,3-glucanases from wheat spikes infected by Fusarium graminearum. Theor Appl Genet. 2000; 102:353–362. 10.1007/s001220051653 [DOI] [Google Scholar]
- 29.Pritsch C, Muehlbauer GJ, Bushnell WR, Somer DA, Vance CP. Fungal development and induction of defense response genes during early infection of wheat spikes by Fusarium graminearum. Mol Plant Microbe Interact. 2000; 13:159–169. 10.1094/MPMI.2000.13.2.159 [DOI] [PubMed] [Google Scholar]
- 30.Brenchley WE. The effect of iodine on soils and plants. Ann Appl Biol. 1924; 11:86–112. 10.1111/j.1744-7348.1924.tb05695.x [DOI] [Google Scholar]
- 31.Umaly RC, Poel LW. Effects of iodine in various formulations on the growth of barley and pea plants in nutrient solution culture. Ann Bot-London. 1971; 35:127–131. 10.1093/oxfordjournals.aob.a084451 [DOI] [Google Scholar]
- 32.Duborská E, Urík M, Kubová J. Interaction with soil enhances the toxic effect of iodide and iodate on barley (Hordeum vulgare L.) compared to artificial culture media during initial growth stage. Arch Agron Soil Sci. 2018; 64(1):46–57. 10.1080/03650340.2017.1328104 [DOI] [Google Scholar]
- 33.Merhej J, Boutigny AL, Pinson-Gadais L, Richard-Forget F, Barreau C. Acidic pH as a determinant of TRI gene expression and trichothecene B biosynthesis in Fusarium graminearum. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010; 27(5):710–717. 10.1080/19440040903514531 [DOI] [PubMed] [Google Scholar]
- 34.Merhej J., Richard-Forget F., Barreau Ch. The pH regulatory factor Pad1 regulates Tri gene expression and trichothecene production in Fusarium graminearum. Fungal Genet Biol. 2011; 48(3): 275–284. 10.1016/j.fgb.2010.11.008 [DOI] [PubMed] [Google Scholar]
- 35.Merhej J., Urban M., Dufresne M., Hammond-Kosack KE, Richard-Forget F., Barreau Ch. The velvet gene, FgVe1, affects fungal development and positively regulates trichothecene biosynthesis and pathogenicity in Fusarium graminearum. Mol Plant Pathol. 2012; 13(4):363–374. 10.1111/j.1364-3703.2011.00755.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jansen C, von Wettstein D, Schäfer W, Kogel KH, Felk A, Maier FJ. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. P Natl Acad Sci USA. 2005; 102(46):16892–16897. 10.1073/pnas.0508467102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proctor RH, Hohn TM, McCormick SP. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol Plant Microbe Interact. 1995; 8:593–601. 10.1094/mpmi-8-0593 [DOI] [PubMed] [Google Scholar]
- 38.Hang YD, Woodams EE. Control of Fusarium oxysporum by baking soda. LWT-Food Sci Technol. 2003; 36(8):803–805. 10.1016/S0023-6438(03)00095-1 [DOI] [Google Scholar]
- 39.Zaker M. Antifungal evaluation of some inorganic salts against three phytopathogenic fungi. Int J Agr Crop Sci. 2014; 7(14):1352–1358. [Google Scholar]
- 40.Hao J, Pétriacq P, de Bont L, Hodges M, Gakière B. Characterization of L-aspartate oxidase from Arabidopsis thaliana. Plant Sci. 2018; 271:133–142. 10.1016/j.plantsci.2018.03.016 [DOI] [PubMed] [Google Scholar]
- 41.Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001; 414(6863):562–565. 10.1038/35107108 [DOI] [PubMed] [Google Scholar]
- 42.Kong L, Anderson JM, Ohm HW. Induction of wheat defense and stress-related genes in response to Fusarium graminearum. Genome. 2005; 48(1): 29–40. 10.1139/g04-097 [DOI] [PubMed] [Google Scholar]
- 43.Gao CS, Kou XJ, Li HP, Zhang JB, Saad ASI, Liao YC. Inverse effects of Arabidopsis NPR1 gene on fusarium seedling blight and fusarium head blight in transgenic wheat. Plant Pathology. 2013; 62(2): 383–392. 10.1111/j.1365-3059.2012.02656.x [DOI] [Google Scholar]
- 44.Ding L, Xu H, Yi H, Yang L, Kong Z, Zhang L. et al. Resistance to hemi-biotrophic F. graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PLoS One. 2011; 6(4), e19008 10.1371/journal.pone.0019008 [DOI] [PMC free article] [PubMed] [Google Scholar]