Abstract
Ionizing radiation is a risk factor for myeloid neoplasms including myelodysplastic syndromes (MDS), and atomic bomb survivors have been shown to have a significantly higher risk of MDS. Our previous analyses demonstrated that MDS among these survivors had a significantly higher frequency of complex karyotypes and structural alterations of chromosomes 3, 8, and 11. However, there was no difference in the median survival time between MDS among survivors compared with those of de novo origin. This suggested that a different pathophysiology may underlie the causative genetic aberrations for those among survivors. In this study, we performed genome analyses of MDS among survivors and found that proximally exposed patients had significantly fewer mutations in genes such as TET2 along the DNA methylation pathways, and they had a significantly higher rate of 11q deletions. Among the genes located in the deleted portion of chromosome 11, alterations of ATM were significantly more frequent in proximally exposed group with mutations identified on the remaining allele in 2 out of 5 cases. TP53, which is frequently mutated in therapy-related myeloid neoplasms, was equally affected between proximally and distally exposed patients. These results suggested that the genetic aberration profiles in MDS among atomic bomb survivors differed from those in therapy-related and de novo origin. Considering the role of ATM in DNA damage response after radiation exposure, further studies are warranted to elucidate how 11q deletion and aberrations of ATM contribute to the pathogenesis of MDS after radiation exposure.
Introduction
Myelodysplastic syndromes (MDS) are clonal myeloid disorders characterized by cytopenias related to ineffective hematopoiesis, dysplasia, and progression to acute myeloid leukemia.1 The pathogenesis of MDS is not yet fully understood; however, recently developed DNA sequencing technologies have clearly demonstrated the important roles of genome alterations. The most frequent mutations observed in de novo MDS are in the genes coding splicing factors (e.g. SF3B1 and SRSF2), followed by mutations in the genes for DNA methylation (e.g. TET2 and DNMT3A) and histone modification (e.g. ASXL1 and EZH2).2–4 Typically, these somatic mutations are sequentially acquired de novo with age, and lead to the development of MDS through the aging-related hematopoietic condition called clonal hematopoiesis of indeterminate potential (CHIP).5–8
Chemotherapy and radiotherapy are well-known risk factors for the development of myeloid neoplasms (therapy-related myeloid neoplasms, t-MN) including MDS (t-MDS), and the clinical and genetic features of t-MDS are different from those of de novo MDS with some overlap.9 For example, response to treatment and survival rates are very poor for t-MDS, and the karyotypes of t-MDS frequently show deletions of the long arms or the whole of chromosomes 5 and 7, often associated with complex karyotypes.10 The most frequently mutated gene in t-MDS is TP53 followed by RUNX1. However, TET2 mutations are less frequent in t-MDS than in de novo cases. Several reports have demonstrated that chemotherapy/radiotherapy provides an opportunity for the proliferation of pre-existing hematopoietic stem cells carrying mutations in genes such as TP53 and RUNX1, which eventually progresses to overt t-MN.11
Atomic bomb (A-bomb) radiation has also been reported to be a risk factor for developing MDS, with the degree of risk being associated with radiation dose exposure and distance from the hypocenter.12 Our previous reports showed an increase in chromosomal aberrations and complex karyotypes in MDS among the proximally exposed survivors. However, the median survival time and time to progression to leukemia did not differ between the proximally and distantly exposed groups.13 Detailed comparisons of chromosomal aberrations between A-bomb survivors and unexposed patients with MDS demonstrated that structural alterations in chromosomes 3, 8, and 11 were significantly increased in MDS among survivors, while alterations in chromosomes 5 and 7 were equally frequent in both groups.14
These observations suggest that MDS among A-bomb survivors may have a different pathogenesis compared with de novo and t-MDS cases, which may be reflected by their different patterns of genome alterations. To address these issues, we analyzed MDS among A-bomb survivors using next generation sequencing technologies. We found different profiles of driver mutations among proximally exposed patients, as well as frequent deletion of the long arm of chromosome 11 associated with aberrations of ATM.
Methods
We analyzed 32 patients diagnosed as having MDS, and three patients as idiopathic cytopenia of undetermined significance among A-bomb survivors (Online Supplementary Table S1), and we divided them into two groups: patients exposed within 2.7 km of the hypocenter were categorized as the proximally exposed (PE) group, and the others as the distally exposed (DE) group according to the approach adopted by the Radiation Effect Research Foundation15 (Online Supplementary Methods). In this study, we compared clinical/genome data between PE and DE groups because these two groups would have lived in similar circumstances (stayed in Nagasaki after A-bomb under similar environmental circumstances including medical access) except for the dose of A-bomb radiation, more than 5mGy (at 2.7 km) or less, which suggested DE as an appropriate control for PE. Based on our previous epidemiological analysis, excess relative risk (ERR) of MDS among survivors that had been exposed to 5mGy would be 0.02 (ERR, 4.3 / Gy).12 Several different sequencing methods were applied in this study depending on the amount and the quality of DNA samples (Online Supplementary Tables S1 and S2).
First, we performed whole exome sequencing (WES) with matched germline controls for five patients in the PE group, coded as unbiased-WES (U-WES), then WES without matched germline controls (B-WES-T) for three and eight patients in the PE and DE groups, respectively. Limited number of genes (356 genes) were validated for B-WES-T, which were putative drivers of hematologic malignancies3,4,16,17 or candidate genes identified through U-WES (Online Supplementary Table S3). Targeted capture sequencing (T-S) of 154 genes was performed for another ten and nine patients in the PE and DE groups, respectively, without matched controls. Target genes were selected based on published data3,4,16,17 and results of the U-WES cases (Online Supplementary Table S4).
We also investigated three cases (U-WES-3, 4, and 7) using whole genome sequencing (WGS) with matched germline controls (Online Supplementary Methods and Online Supplementary Table S5).
The DNA copy number alterations (CNA) were analyzed with a SNP array (CytoScan HD Array, Affymetrix, Santa Clara, CA, USA), and CNA in T-S cases were identified in the sequencing data using the CNACS pipeline,18 because of the insufficient quality and quantity of DNA for SNP array. Although copy number states of whole chromosomes could not be evaluated, frequently affected regions in MDS, such as the long arms of chromosome 5 (5q), 7q, and 20q, were included among the targets. We also targeted the genes affected by 11q deletion to evaluate the whole arm of 11q because this region was of interest in this study. This study was approved by the ethics committee of Nagasaki University.
Further details of the methods used for this study are available in the Online Supplementary Methods.
Results
Clinical features of patients in this study
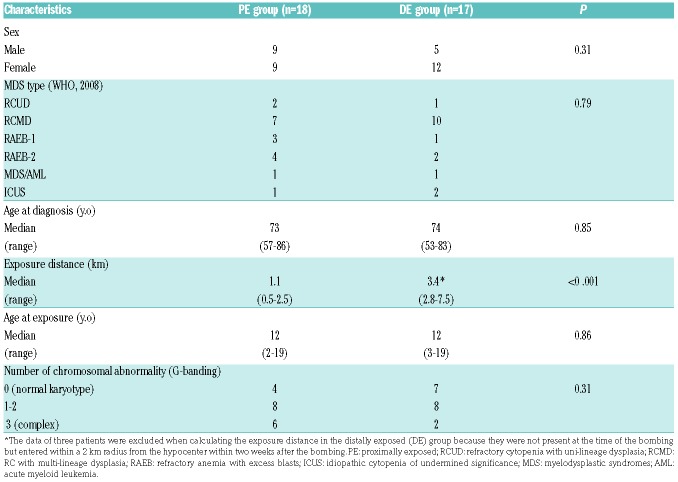
The major clinical characteristics of the 35 patients who took part in this study are listed in Table 1 with further details provided in Online Supplementary Table S1. The median exposure distance from the hypocenter was 1.1 km in the PE group and 3.4 km in the DE group (P<0.001). There were no significant differences in the sex, subtype of MDS, age at diagnosis, or age at the time of the bombing between the two groups. The frequencies of abnormal karyotype and complex karyotype were higher in PE but without statistical significance (Table 1). There was no difference in survival time after diagnosis between two groups (P=0.652) (Online Supplementary Figure S7).
Table 1.
Patients’ characteristics.
Somatic mutations, mutational spectrum, and clonal architecture of myelodysplastic syndromes in the proximally exposed group
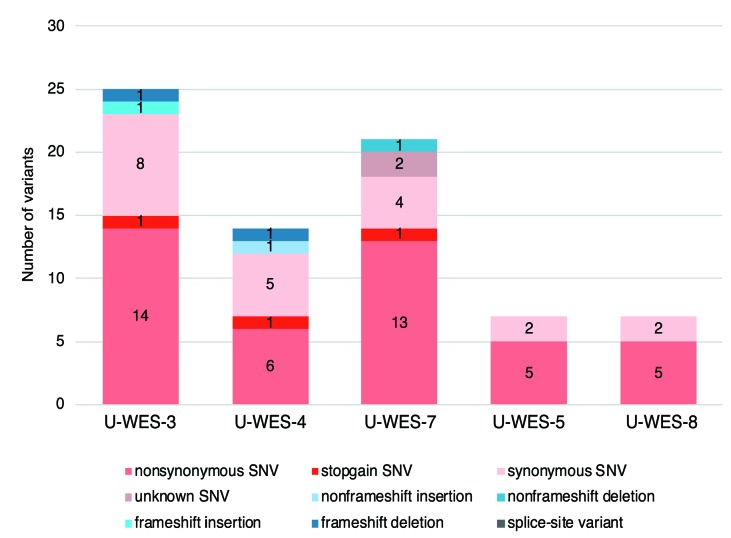
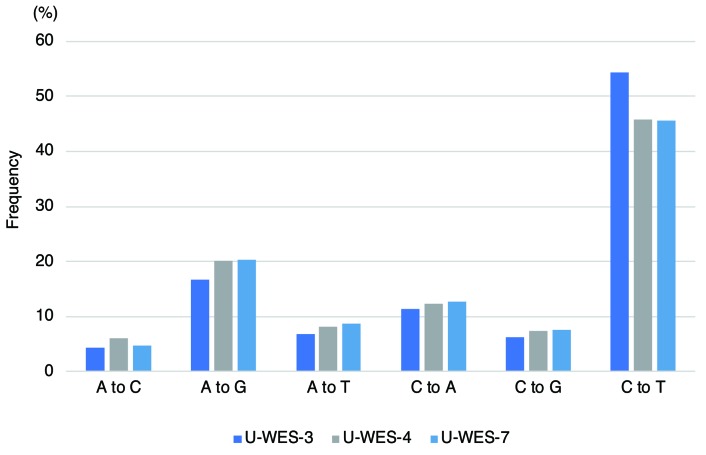
Among the five patients (U-WES-3, 4, 5, 7, 8) in the PE group who were analyzed using WGS and/or WES, we identified 5-15 somatic missense and nonsense SNV (mean 9.2 per sample), and 0-2 somatic INDEL (insertions or deletions; mean 1 per sample) on coding exons (Figure 1 and Online Supplementary Table S6) per patient. The number of somatic SNV identified using WGS of three patients (U-WES-3, 4 and 7) in the PE group was 1,695, 573, and 756, respectively (Online Supplementary Tables S7-1, -2, and -3). The most frequent pattern of nucleotide substitution was cytosine-to-thymine (C to T) (Figure 2 and Online Supplementary Figure S3). Clonal heterogeneities of MDS in these patients were inferred from the analysis of variant allele frequencies of the identified somatic SNV (Online Supplementary Figure S4).
Figure 1.
Somatic mutations identified in coding exons of five patients in the proximally exposed group. Each numerical number on the bar charts represents the number of variants of each mutation type identified using whole exome sequencing. No splice site variants were identified among these five patients. U-WES: unbiased-whole exome sequencing.
Figure 2.
Pattern of nucleotide substitutions in the whole genomes of three patients in the proximally exposed group. The pattern of nucleotide substitution was examined in three patients in the proximally exposed group who were analyzed using whole genome sequencing. Frequencies of each pattern of substitution are represented on the y-axis.
Comparison of mutated genes between the proximally exposed and distally exposed groups
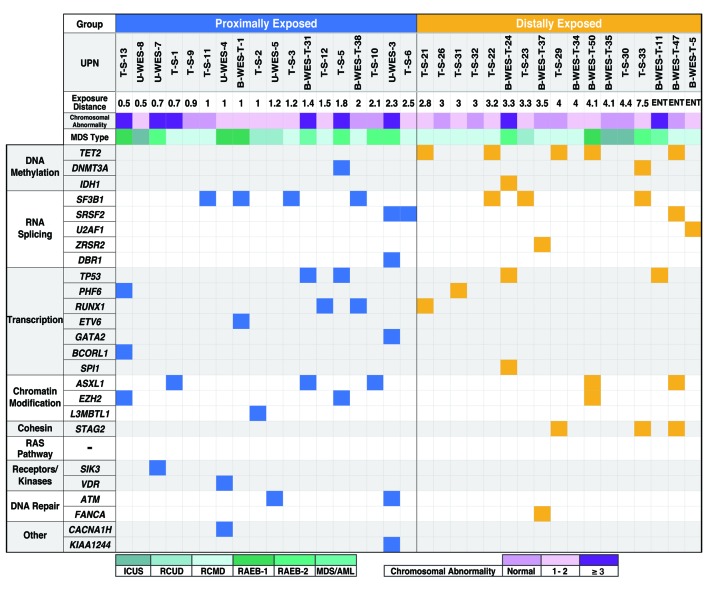
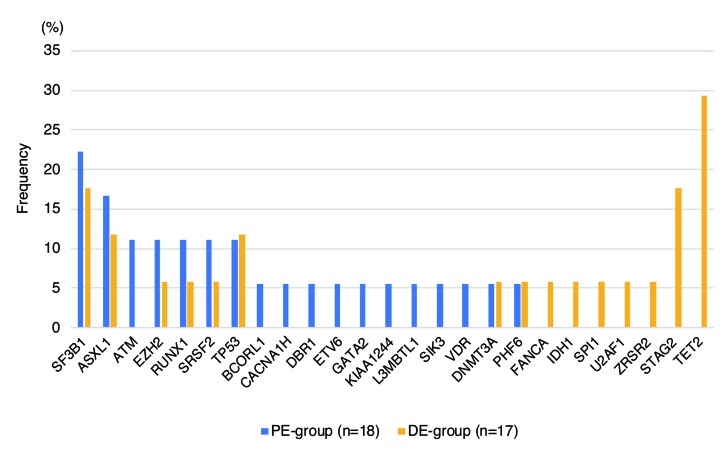
Using the U-WES, B-WES-T, and T-S methods, somatic and oncogenic mutations were identified in 16 out of 18 patients (89%) in PE, and 12 out of 17 patients (71%) in DE groups (Figure 3 and Online Supplementary Tables S6, S8 and S9). Among these mutations, in DE group, TET2 was most frequently affected (5 out of 17 patients, 29%), followed by SF3B1 (3 out of 17, 18%) and STAG2 (18%) (Figure 4). However, none of the PE patients had TET2 or STAG2 mutations, and the most frequently mutated gene was SF3B1 (4 out of 18 patients, 22%). There was a statistically significant difference in the frequency of TET2 mutations between the two groups (P=0.019), but not for STAG2 (P=0.104). Mutations in TP53 were identified at very similar frequencies in the two groups: PE: 2 out of 18 patients (11%); DE: 2 out of 17 (12%) (P=1.00). There was also no significant difference in the frequency of RUNX1 mutations between the two groups (11% and 6% in PE and DE, respectively; P=1.00).
Figure 3.
Somatic mutations in myelodysplastic syndromes (MDS) among A-bomb survivors. Each row and column represents a mutated gene and patient, respectively. Identified gene mutations are shown as blue (proximally exposed group) or yellow (distally exposed group) squares. Assumed functional pathways are shown on the far left. UPN: unique patient number; ENT: entered within a 2 km radius from the hypocenter within two weeks after the atomic bombing. RCUD: refractory cytopenia with unilineage dysplasia; RCMD: RC with multi-lineage dysplasia; RAEB: refractory anemia with excess blasts; ICUS: idiopathic cytopenia of undetermined significance; AML: acute myeloid leukemia. DE: distally exposed group; PE: proximally exposed group.
Figure 4.
Frequencies of somatic gene mutations in the proximally and distally exposed groups. Frequencies were calculated as the percentage of patients in each group carrying the different mutated genes.
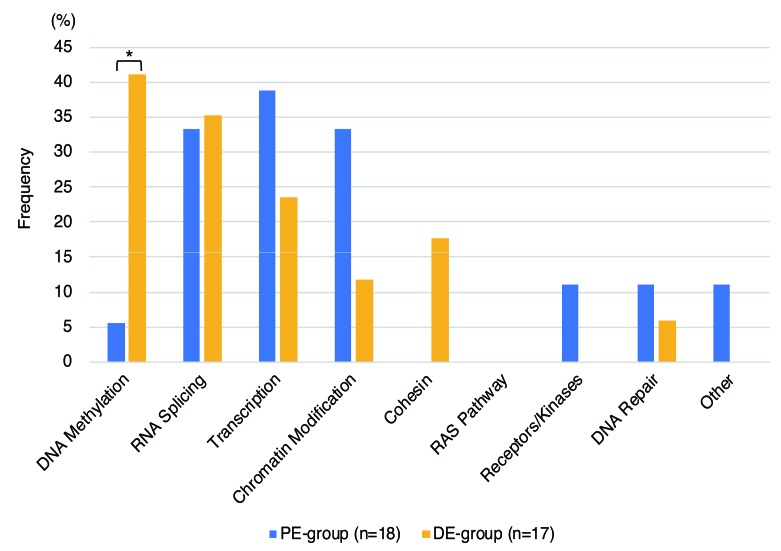
Mutated genes were categorized on the basis of their assumed roles in functional pathways (Figure 5 and Online Supplementary Table S10). We found that gene mutations along the DNA methylation pathways were significantly less frequent in the PE group (1 out of 18 patients, 5.6%) than in the DE group (7 out of 17, 41%; P=0.018). Genes coding RNA splicing factors were mutated with equal frequency in both groups. Mutations in genes for transcription factors and the chromatin modification pathway were more frequent in PE than in DE without statistical significance (transcription factors, 39% and 24%, respectively, P=0.47; chromatin modification pathway, 33% and 12%, respectively, P=0.23).
Figure 5.
Frequencies of mutated genes categorized by assumed functional pathway. Frequencies were calculated as the percentage of patients in each group carrying mutated genes within the different functional pathways. *P=0.018 using Fisher’s exact test. DE: distally exposed group; PE: proximally exposed group.
Copy number alterations and affected genes on 11q
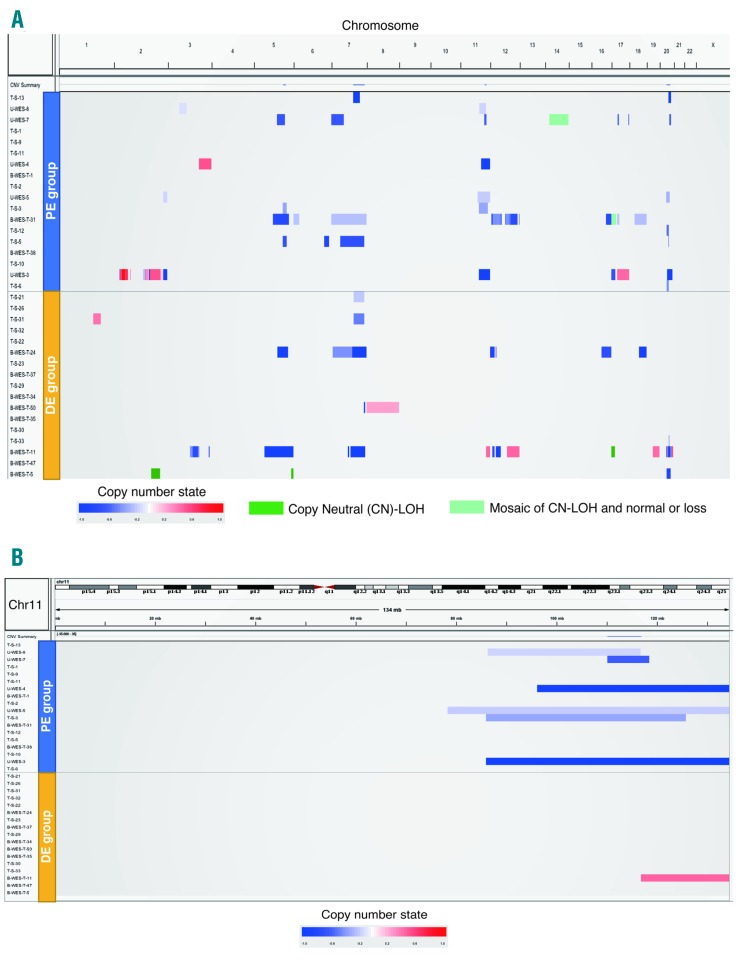
Copy number alterations were evaluated by SNP array or T-S as described in the Methods and the Online Supplementary Methods, although the T-S data did not cover whole chromosomes. Using these methods, we identified CNA in 11 out of 18 (61%), and 7 out of 17 patients (41%) in the PE and DE groups, respectively (Figure 6A and Online Supplementary Table S11). CNA in chromosomes 11 and 20 were more frequent in PE (Figure 6B and Online Supplementary Figure S5A). Among the CNA, 11q deletion was identified only in PE with statistically significant difference (33% and 0% in PE and DE, respectively; P=0.019). Copy number loss of chromosome 5q and chromosome 7 were identified with almost equal frequency in both groups [chromosome 5q: PE: 4 out of 18 patients (22%); DE: 2 out of 17 (12%), P=0.66; chromosome 7: PE: 22%; DE: 5 out of 17 (29%), P=0.71] (Online Supplementary Figure S5B and C).
Figure 6.
Copy number alterations in myelodysplastic syndromes (MDS) among A-bomb survivors. Each row represents the copy number alterations (CNA) in each patient. The order of patients was in accordance with the exposure distance. (A) CNA on whole chromosomes except for chromosome Y, (B) CNA on chromosome 11. DE: distally exposed group; PE: proximally exposed group.
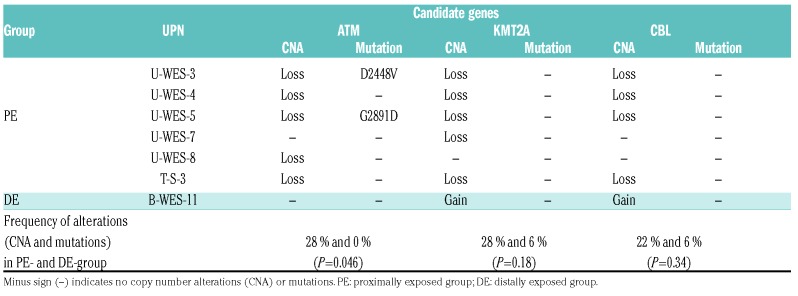
There are several genes observed to be recurrently affected in MDS and acute myeloid leukemia on 11q, such as ATM, KMT2A, and CBL.3,11,16,17 Copy number loss of ATM, KMT2A, and CBL were identified in five, five, and four patients in the PE group, respectively (Table 2), while copy number gain of KTM2A and CBL were found in one patient in the DE group (Online Supplementary Figure S6). We also identified mutations in ATM on the remaining allele in two patients (U-WES-3 and -5) in the PE group; thus, both ATM alleles were affected in these patients. Alterations of ATM were significantly more frequent in PE than in DE patients (28% and 0%, respectively; P=0.046).
Table 2.
Copy number alterations and mutations of candidate genes in the frequently affected region on chromosome 11q.
Discussion
To better understand how A-bomb radiation contributed to the pathogenesis of MDS among survivors, we analyzed DNA samples from these MDS patients using next generation sequencing and SNP arrays for the first time. We found no apparent increase in the number of SNV among MDS patients proximally exposed to A-bomb radiation compared with those reported for patients with de novo or secondary MDS/AML.5,11 The pattern of nucleotide substitutions was also similar to that observed in de novo cases with C-to-T substitution being the most frequent, although this analysis was performed for only three patients in our cohort. We previously reported that the number of chromosomal aberrations was significantly increased in MDS among A-bomb survivors, especially in proximally exposed patients.13 This finding led us to predict increased nucleotide alterations in MDS among patients in the PE group but this was not the case. In spite of the genotoxic effects of ionizing radiation, it did not apparently contribute to increase the mutational burden in MDS. This may be related to the specific nature of A-bomb radiation: one-off, whole-body exposure that was mostly external. This could explain, at least in part, the reason why there was no difference in survival of MDS patients between the PE and DE groups (Online Supplementary Figure S7), and between exposed and unexposed de novo MDS, or by the distance from the hypocenter, as we previously reported.13,14 Considering that our previous study showed cytogenetic risk categories (by revised-International Prognostic Scoring System) significantly divided survival for both MDS among survivors and those unexposed, the same cytogenetic hits seemed to have had a similar influence on their survival.13,14
However, we found significant differences in the profile of mutated genes between proximally and distally exposed patients. TET2 mutations, which are one of the most frequent alterations in myeloid neoplasms including MDS,3,4 were not detected in the PE group but were observed in the DE group, as frequently as reported for de novo MDS (approx. 29%). This was related, at least in part, to the significantly less mutations along with DNA methylation pathways in the PE group, as TET2 is one of the major genes in this pathway. Mutations in TP53 and genes coding splicing factors, such as SF3B1, were comparable to those in de novo cases,3,4 and were equally frequent in the PE and DE groups. TP53 is the most frequently mutated gene in t-MN including t-MDS, and it is highly correlated with poor outcome.11 Our previous work demonstrated no significant difference in survival time between A-bomb survivors with MDS and unexposed MDS patients,13,14 which could be partly explained by their similar frequency of TP53 mutations. Taken together, these findings suggest that the profile of gene mutations in MDS among proximally exposed survivors is different to that of de novo MDS patients (reduction or lack of TET2 mutations in PE cases) and t-MDS patients (fewer TP53 mutations in PE cases).
A study on mutations of RUNX1 in MDS among A-bomb survivors (both proximally and distally exposed cases) in Hiroshima noted an increased alteration rate of 46% (6 out of 13 cases) and a missense/frameshift mutation rate of 31% (4 out of 13 cases).19 In the present study, however, 3 out of 35 cases (8.6%) had RUNX1 mutations, which was as frequent as reported for de novo MDS (approx. 10%). There is no clear explanation for this difference in the frequency of RUNX1 mutations but the small number of cases examined in each study (13 cases in the Hiroshima study, and 35 cases in this study) might have played a role. It is also possible that the differences in MDS subtypes influenced the results between two studies, as RUNX1 mutations are enriched in high-risk MDS.3 In the Hiroshima study, among 13 patients analyzed, there were one patient with refractory anemia with excess blasts (RAEB), eight with RAEB in transformation (RAEB-t), and one AML, sharing 76.9% (10 out of 13) by MDS with increased blasts. Our patient cohort contained 12 patients with RAEB or AML, which was 34.3% (12 out of 35) of all participants.
Copy number alterations are frequently found in MDS.20,21 In this study, we found a significantly higher frequency of copy number loss for 11q in the PE group than in the DE group (P=0.019). Loss of chromosomes 5 or 7, which occurs more frequently in t-MDS (40-50%), and is usually accompanied by a complex karyotype, was observed at an almost equal frequency in the PE and DE groups but less frequently than in t-MDS. Although we did not analyze the entire genes within the commonly deleted region of 11q in the PE group, we detected mutations on the residual ATM allele in 2 out of 5 cases (U-WES-3 and U-WES-5) but not in KMT2A or CBL. The ATM mutations, p.D2448V and p.G2891D, were located in the FAT and PI3K domains, respectively. Because of the pathogenic nature of these mutations, U-WES-3 and U-WES-5 appeared to lack expression of functional ATM protein. Deletions or mutations of ATM have been reported in de novo MDS3,4,7,8,22 and it does not seem to be specific to MDS among A-bomb survivors. However, the significantly higher frequency of 11q deletion and the presence of biallelic alterations of ATM strongly suggested its importance in the pathogenesis of MDS among survivors.
Since ionizing radiation induces DNA double-strand breaks (DSB),23,24 deletions and translocations are frequently observed as a consequence of exposure. Accordingly, our previous study demonstrated that chromosomal translocations were significantly increased in MDS among A-bomb survivors; however, the translocations involving 11q23 where KMT2A locates were rare.14 We observed a significantly higher frequency of 11q aberrations but not translocations among survivors, compared with MDS of unexposed patients.14 Taken together, these results indicated that aberrations of 11q, especially hemizygous deletion of 11q, could be caused by A-bomb radiation.
ATM protein is a key molecule in DNA damage response, in particular, for DSB caused by ionizing radiation,25,26,27 and it is possible that the loss of one allele of ATM was the initial event for clonal selection towards the development of MDS among A-bomb survivors. It is assumed that immature hematopoietic cells that lost ATM following A-bomb radiation either responded poorly or incorrectly to other DNA damage generated at the same time. This might also explain why TET2 mutations, which are common in de novo MDS, and are usually thought to be an initiating mutation for de novo MDS, were observed at a low frequency in the PE group in this study. Considering the gain-of-function alterations of KMT2A and CBL in MDS,28,29,30 the defect in ATM function generated by 11q deletion that has also been found in de novo MDS3,22 would have a greater impact on the initiation of MDS among A-bomb survivors. It is necessary to investigate whether alterations in ATM, rather than TET2, are frequently present in A-bomb survivors who have clonal hematopoiesis of indeterminate potential (CHIP).
In conclusion, we reported a profile of genetic alterations in MDS among survivors exposed to A-bomb radiation, such as fewer mutations in genes along DNA methylation pathways, and frequent 11q deletions and aberrations in ATM. Further investigations are warranted to elucidate the role of these genetic alterations in the pathogenesis of MDS after radiation exposure.
Supplementary Material
Acknowledgments
We thank Mariko Yozaki, Naoko Ito, Hiroe Urakami, Azumi Yukawa, and Chihiro Yoshikawa for their technical assistance, and Natasha Beeton-Kempen (Edanz Group, www.edanzediting.com/ac) for editing a draft of this manuscript.
For original sequence data, please contact Masataka Taguchi (mtaguchi-ngs@umin.org), and Yasushi Miyazaki (y-miyaza@nagasaki-u.ac.jp).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/2/358
Funding
This work was partly supported by JSPS KAKENHI (Grant Number 26461426) (Y. Miyazaki), MEXT KAKENHI (Grant number 17H04209) (K-IY, Y. Miyazaki), the Program of the Network-type Joint Usage/Research Center for Radiation Disaster Medical Science (MT, MH, K-IY, Y. Miyazaki), the Takeda Science Foundation (K-IY, Y. Miyazaki), Practical Research for Innovative Cancer Control (Grant number 16ck0106073h0003) (SO), the Project for Cancer Research and Therapeutic Evolution (Grant number P-CREATE, 16cm0106501h0001) from Japan AMED (SO), and JSPS KAKENHI (Grant number 15H05909) (SO).
References
- 1.Adès L, Itzykson R, Fenaux P. Myelodysplastic syndromes. Lancet. 2014; 383(9936):2239–2252. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida K, Sanada M, Shiraishi Y, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478(7367):64–69. [DOI] [PubMed] [Google Scholar]
- 3.Haferlach T, Nagata Y, Grossmann V, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28(2):241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papaemmanuil E, Gerstung M, Malcovati L, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122(22):3616–3627; quiz 3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makishima H, Yoshizato T, Yoshida K, et al. Dynamics of clonal evolution in myelodysplastic syndromes. Nat Genet. 2017;49(2):204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015; 126(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genovese G, Kähler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganser A, Heuser M. Therapy-related myeloid neoplasms. Curr Opin Hematol. 2017;24(2):152–158. [DOI] [PubMed] [Google Scholar]
- 10.Smith SM, Le Beau MM, Huo D, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102(1):43–52. [DOI] [PubMed] [Google Scholar]
- 11.Wong TN, Ramsingh G, Young AL, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2015;518(7540):552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwanaga M, Hsu W-L, Soda M, et al. Risk of myelodysplastic syndromes in people exposed to ionizing radiation: a retrospective cohort study of Nagasaki atomic bomb survivors. J Clin Oncol. 2011;29(4):428–434. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo M, Iwanaga M, Kondo H, et al. Clinical features and prognosis of patients with myelodysplastic syndromes who were exposed to atomic bomb radiation in Nagasaki. Cancer Sci. 2016;107(10):1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horai M, Satoh S, Matsuo M, et al. Chromosomal analysis of myelodysplastic syndromes among atomic bomb survivors in Nagasaki. Br J Haematol. 2018; 180(3):381–390. [DOI] [PubMed] [Google Scholar]
- 15.Young R, Kerr G, eds. Reassessment of the Atomic Bomb Radiation Dosimetry for Hiroshima and Nagasaki, Dosimetry System 2002, Report of the Joint US-Japan Working Group Hiroshima: Radiation Effects Research Foundation; 2005. [Google Scholar]
- 16.Kihara R, Nagata Y, Kiyoi H, et al. Comprehensive analysis of genetic alterations and their prognostic impacts in adult acute myeloid leukemia patients. Leukemia. 2014;28(8):1586–1595. [DOI] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Research Network. Ley TJ, Miller C, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013; 368(22):2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshizato T, Nannya Y, Atsuta Y, et al. Genetic abnormalities in myelodysplasia and secondary acute myeloid leukemia: impact on outcome of stem cell transplantation. Blood. 2017;129(17):2347–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harada H, Harada Y, Tanaka H, Kimura A, Inaba T. Implications of somatic mutations in the AML1 gene in radiation-associated and therapy-related myelodysplastic syndrome/acute myeloid leukemia. Blood. 2003;101(2):673–680. [DOI] [PubMed] [Google Scholar]
- 20.Tiu RV, Gondek LP, O’Keefe CL, et al. Prognostic impact of SNP array karyotyping in myelodysplastic syndromes and related myeloid malignancies. Blood. 2011; 117(17):4552–4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens-Kroef MJ, Olde Weghuis D, ElIdrissi-Zaynoun N, et al. Genomic array as compared to karyotyping in myelodys-plastic syndromes in a prospective clinical trial. Genes Chromosom. Cancer. 2017; 56(7):524–534. [DOI] [PubMed] [Google Scholar]
- 22.Wang SA, Abruzzo LV, Hasserjian RP, et al. Myelodysplastic syndromes with deletions of chromosome 11q lack cryptic MLL rearrangement and exhibit characteristic clinicopathologic features. Leuk Res. 2011; 35(3):351–357. [DOI] [PubMed] [Google Scholar]
- 23.Cannan WJ, Pederson DS. Mechanisms and Consequences of Double-Strand DNA Break Formation in Chromatin. J Cell Physiol. 2016;231(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price BD, D’Andrea AD. Chromatin remodeling at DNA double-strand breaks. Cell. 2013;152(6):1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banin S, Moyal L, Shieh S, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281(5383):1674–1677. [DOI] [PubMed] [Google Scholar]
- 26.Canman CE, Lim DS, Cimprich KA, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281(5383):1677–1679. [DOI] [PubMed] [Google Scholar]
- 27.Guleria A, Chandna S. ATM kinase: Much more than a DNA damage responsive protein. DNA Repair. 2016;39:1–20. [DOI] [PubMed] [Google Scholar]
- 28.Dicker F, Haferlach C, Sundermann J, et al. Mutation analysis for RUNX1, MLL-PTD, FLT3-ITD, NPM1 and NRAS in 269 patients with MDS or secondary AML. Leukemia. 2010;24(8):1528–1532. [DOI] [PubMed] [Google Scholar]
- 29.Dorrance AM, Liu S, Chong A, et al. The Mll partial tandem duplication: differential, tissue-specific activity in the presence or absence of the wild-type allele. Blood. 2008;112(6):2508–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanada M, Suzuki T, Shih L-Y, et al. Gain-of-function of mutated C-CBL tumour suppressor in myeloid neoplasms. Nature. 2009;460(7257):904–908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.