Over the past decades, immunomodulatory drugs, pro-teasome inhibitors, and targeted antibodies have significantly improved the overall survival of multiple myeloma (MM) patients. Nonetheless, the disease remains incurable as patients ultimately develop resistance to all available modalities. One of the well-known mechanisms of therapy resistance in MM is evasion of apoptosis, characterized by an up-regulation of anti-apoptotic proteins of the Bcl-2 family and/or inhibitor of apoptosis (IAP) proteins including Survivin, whose expression is associated with a worse clinical outcome.1,2 Apoptosis resistance can be MM cell intrinsic, but can also be induced by their interactions with accessory cells and the extracellular matrix in the bone marrow (BM). Inside-out signaling via integrins, signaling via NOTCH, or soluble factors like IL-6, can significantly contribute to this microenvironment-mediated drug resistance (EM-DR).3 To overcome apoptosis resistance, Li et al. have recently screened for compounds that could alter the expression of Survivin in tumor cells and described a new small molecule, FL118.4 Although FL118 was identified as a camptothecin analog, it appeared to inhibit the promoter activity of Survivin and its gene expression with significantly greater efficien cy than it could inhibit DNA topoisomerase-I.5 In addition, FL118 was shown to selectively and independently inhibit additional anti-apoptotic genes including Mcl-1, XIAP and cIAP2. In preclinical settings, FL118 showed a favorable toxicity profile in experimental animals,6 and an effective antitumor activity against human colon and head-and-neck tumors in a topoisomerase I- and P53 status-independent manner.5,7 The latter is of importance for MM, since mutations or the loss of the P53 encoding gene, through the deletion of chromosome 17p, are characteristics of high-risk MM with poor prognosis.8,9 Prompted by these encouraging results, we have addressed here for the first time the anti-MM activity of FL118 in vitro and in vivo.
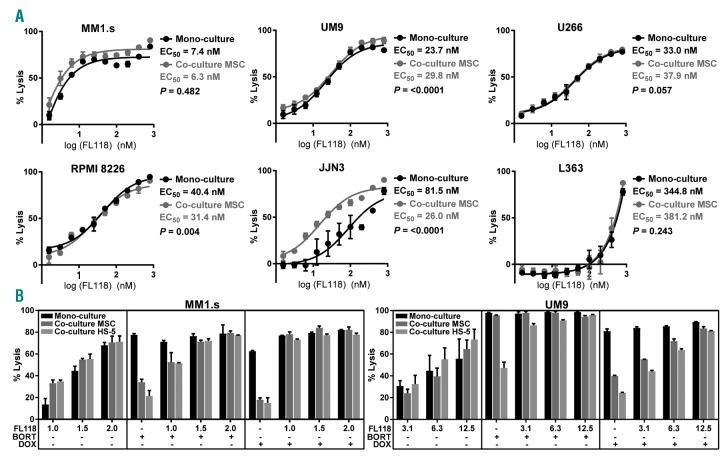
We first evaluated FL118 against a panel of six MM cell lines with a different P53 status (Online Supplementary Table S1). Since stromal cells can induce EM-DR, the assays were executed in the presence or absence of MM patient-derived BM mesenchymal stromal cells (BMMSC). While FL118 showed only minimal toxicity against BMMSC (Online Supplementary Figure S1), it exhibited a clear dose-dependent anti-MM activity in the MM cell lines, independent of the P53 status, with the half maximal effective concentration (EC50) values ranging from 7.4 nmol/L to 344.8 nmol/L (Figure 1A). Importantly, lysis of MM cell lines by FL118 was not reduced, or even increased, in the presence of BMMSC for five of six MM cell lines, except for a minimal reduction for the cell line UM9. Further analyses indicated that the efficacy of FL118 to induce lysis in MM cell lines was not related to the baseline protein expression levels of its known direct or indirect targets, including Mcl-1, Survivin, XIAP, Bcl-2, PUMA, or NOXA (Online Supplementary Figure S2). Rather, the activity of FL118 seemed to correlate with its capacity to modulate these genes; in three MM cell lines with high, intermediate, and low FL118 susceptibility, respectively, FL118 promoted pro-apoptotic signaling and cleavage of caspase-3 and PARP only in the high- and intermediate-susceptible cell lines but not in the low-susceptible cell line (Online Supplementary Figure S3).
Figure 1.
FL118 is effective against MM cell lines regardless of the presence of bone marrow (BM) mesenchymal stromal cells (MSC) and abrogates stromal cell-induced drug resistance against bortezomib and doxorubicin. (A) Luciferin (LUC)-transduced MM cell lines were treated with serial concentrations of FL118 in the presence or absence of BM mesenchymal stromal cells (BMMSC) derived from 12 MM patients at the time of diagnosis. MM cell viability was determined by bioluminescence imaging (BLI), after 24 hours of treatment with FL118. The half maximal effective concentration (EC50) values were determined for both culture conditions from the log dose-response using Graphpad Prism version 7. The differences in the dose response curves were analyzed by nonlinear regression (*P<0.05; ****P<0.0001). Error bars represent the standard deviation (SD) of four independent experiments executed in duplicate. (B) The MM cell lines MM1.s and UM9 were treated with serial concentrations of FL118 (1.0-2.0 and 3.1-12.5 nmol/L for MM1.s and UM9 respectively) and with predetermined concentrations of bortezomib (BORT; 2 and 4 nmol/L for MM1.s and UM9 respectively) or doxorubicin (DOX; 14 and 108 nmol/L for MM1.s and UM9 respectively) in the presence or absence of BMMSC or HS-5 for 48 hours. Results are representative of three independent assays. Error bars represent the standard deviation (SD) of duplicate cultures.
As previously mentioned, one of the well-described contributors to therapy resistance in MM is EM-DR. Crosstalk of MM cells with BMMSC via physical contact or soluble factors can result in the upregulation of several anti-apoptotic proteins.2,10,11 Therefore, we questioned whether FL118 could modulate the stromal cell-mediated resistance against other anti-MM drugs. Co-culture of two MM cell lines with BMMSC or, to a larger extent, with the stromal cell line HS-5 significantly inhibited the lysis of MM cell lines by two anti-MM drugs, bortezomib and doxorubicin (Figure 1B). This stroma-cell induced drug resistance was effectively abrogated by FL118 in a dose-dependent manner. These observations, in particular the reversal of stroma-induced bortezomib resistance by FL118, may be relevant because in combination with other possible resistance mechanisms, such as proteasome subunit mutations or increased expression of pro-teasome subunits, EM-DR also contributes to clinical bortezomib resistance.12
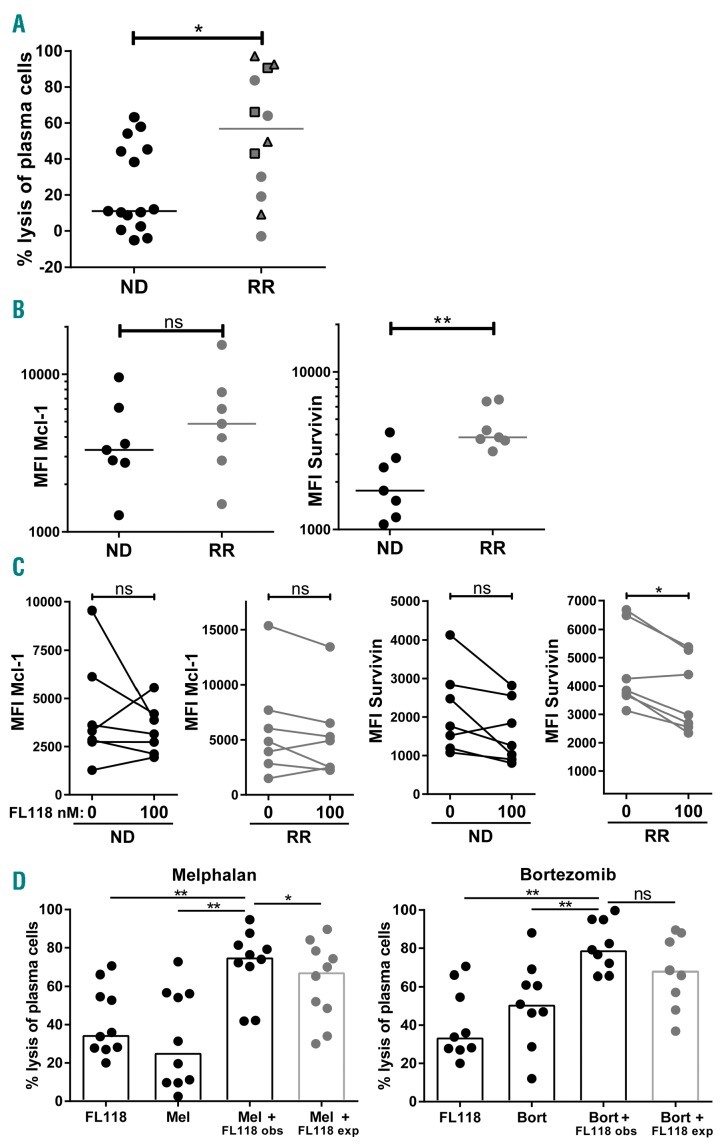
To evaluate the activity of FL118 in a preclinical setting, we assessed its efficacy against primary MM cells present in BM mononuclear cell (BMMNC) samples derived from 15 newly diagnosed (ND) and 12 relapsed and/or refractory (RR) MM patients. In these samples we measured the FL118-induced MM cell lysis by flow cytometry via enumeration of the surviving CD138+ CD38+ MM cells as reported earlier,13 after incubation with FL118 for 24 hours. In 15 of 27 samples, FL118 induced MM cell lysis equal or above 20% (Figure 2A). Interestingly, FL118 was significantly more effective in the samples of RR patients compared to ND patients. Furthermore, similar to the results obtained with MM cell lines, anti-MM activity of FL118 was independent of the P53 status, since among all well-responsive FL118 patients, there were also three patients who showed a deletion of chromosome 17p (Figure 2A). In an attempt to clarify the superior activity of FL118 in RR as compared to ND patients, we measured two FL118 target molecules, Survivin and Mcl-1, in primary MM cells by flow cytometry. Even though RR patients showed enhanced Survivin expression as compared to ND patients, the anti-MM efficacy of FL118 was not associated with the baseline expression of either Survivin or Mcl-1 (Figure 2B). However, again in agreement with the results from cell lines, the anti-MM efficacy of FL118 seemed related to its ability to modulate these anti-apoptotic proteins, with Survivin modulation being more pronounced in RR patients compared to ND patients (Figure 2C). Interestingly, in many FL118-susceptible RR patients, the levels of Survivin expression, although significantly reduced by FL118, were still relatively higher compared to ND patients. This observation suggests that RR patients become dependent on elevated levels of anti-apoptotic proteins for their survival and may explain why FL118 is more efficient in RR patients than in ND patients. Alternatively, differential expression of efflux pumps could explain the differential efficacy of FL118, but this scenario seems unlikely since recent reports indicate that FL118 is not a substrate for ABCG/CRP and MDR1/P-glycoprotein (P-gp) efflux pumps.7,14
Figure 2.
FL118 is more effective in relapsed and/or refractory (RR) MM as compared to newly diagnosed (ND) MM patients and it enhances melphalan and bortezomib-induced MM cell lysis. (A) BM mononuclear cell (BMMNC) samples from 15 ND and 12 RR MM patients were treated with 100 nmol/L FL118 for 24 hours. Viable CD138+ CD38+ MM cells were enumerated via flow cytometry. The percentage lysis of MM cells was calculated relative to untreated samples. Within the RR MM group, patients without known cytogenetic anomalies (n=5), with a deletion of chromosome 17p (n=3), and with intact chromosome 17p (n=4) are depicted with circles, squares, and triangles, respectively. The bars indicate the median values. The differences between groups were tested using the Mann-Whitney test (*P<0.05). (B) Mcl-1 and Survivin expression levels (Median fluorescence intensity (MFI)) in CD138+ CD38+ MM cells of untreated BMMNC from seven ND and seven RR patients were determined by flow cytometry. The bars indicate the median values. The differences between groups were tested using the Mann-Whitney test (**P<0.01; ns: not significant). (C) Mcl-1 and Survivin expression levels (MFI) in CD138+ CD38+ MM cells that were untreated or treated with FL118 for 16 hours. The differences between groups were tested using Wilcoxon matched-pairs rank test (*P<0.05; ns: not significant). Note that Survivin modulation was more pronounced in RR MM patients P=0.031) compared to ND MM patients (P=0.078). (D) BMMNC from MM patients were treated with predetermined suboptimal concentrations of FL118 (12.5-100 nmol/L) and/or with predetermined suboptimal concentrations of melphalan (5-10 mmol/L) (n=10) or bortezomib (2-3 nmol/L) (n=9) for 48 hours. The observed lysis levels (obs) upon co-treatment were compared to the expected lysis levels (exp), which were calculated with the assumption that the combinatorial effect is achieved by additive effects (Bliss-model) thus using the following formula: % expected lysis = (% lysis with FL118 + % lysis with the second drug) - % lysis with FL118 × % lysis with the second drug. The null hypothesis of “additive effects” was rejected if the observed values were significantly different than the expected values. Bars represent the median values of the groups. The statistical differences between the indicated groups were calculated using the Wilcoxon matched-pairs rank test (*P<0.05; **P<0.01; ns: not significant).
Since efficacious treatment of MM only has been achieved through combination therapy, we investigated whether FL118 could enhance MM cell lysis when combined with currently used anti-MM drugs, including melphalan, bortezomib, lenalidomide, pomalidomide, or dexamethasone. Assays with MM cell lines suggested at least an additive activity of FL118 combination with melphalan or bortezomib (Online Supplementary Figure S4). In primary BMMNC samples, FL118 showed additive effects with bortezomib and synergistic effects with melphalan (Figure 2D). Noteworthy here is the recently reported inhibitory activity of FL118 on ERCC6, a critical regulator of DNA repair.6 This could explain the favorable combination of FL118 with melphalan and bortezomib, as both drugs can induce DNA damage. FL118 did not improve anti-MM activity of immunomodulatory drugs pomalidomide or lenalidomide and even had antagonistic effects with dexamethasone (Online Supplementary Figure S5). Nonetheless, when we used lenalidomide, borte-zomib and dexamethasone simultaneously, similar to the clinically applied RVD treatment, addition of FL118 increased MM cell lysis in an additive manner. Possibly, bortezomib mainly contributed to the favorable combination of RVD treatment with FL118 and, thereby, neutralized the adverse interactions between dexamethasone and FL118. Thus, this multidrug combination could still be beneficial even though this outcome was not predicted by individual combination assays.
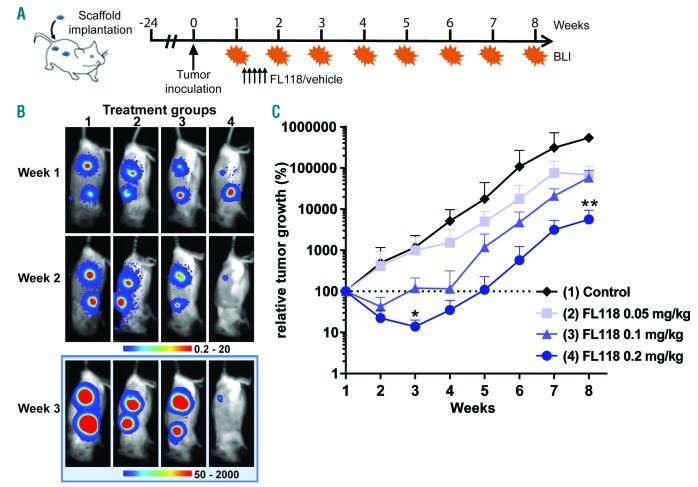
Finally, to determine the potential in vivo anti-MM activity of FL118 we used a unique xenotransplant mouse model, in which MM tumors were grown in a humanized BM niche generated by subcutaneous inoculation of human MSC-coated scaffolds.15 In this model, treatment of UM9 cell line-derived MM tumors with FL118 for five days induced a clear, dose-dependent anti-MM activity (Figure 3). At the highest dose of 0.2 mg/kg, FL118 reduced the initial tumor volume to 14% and delayed tumor growth up to five weeks (Figure 3C). At week 8, this dose resulted in a twenty-fold tumor reduction compared to the control group.
Figure 3.
In vivo anti-tumor activity of FL118. (A) Schematic overview of the experimental design: Hybrid scaffolds, in vitro coated with MSC, were implanted subcutaneously at the back of RAG2−/−γc−/− mice (four scaffolds per mice) and inoculated with tumors (LUC-transduced MM cell line UM9). After one week, mice were treated with FL118 or vehicle via intravenous administration, daily for five times (arrowed). (B) Bioluminescense images (BLI) of representative mice per treatment group at week 1 (before the start of the treatment), week 3 (two weeks after the start of the treatment), and week 8 (end of the experiment). Four treatment groups included: (1) vehicle control (n=3); (2) 0.05 mg/kg FL118 (n=4); (3) 0.1 mg/kg FL118 (n=4); (4) 0.2 mg/kg FL118 (n=4). (C) Analysis of tumor loads per treatment group. BLI results are expressed as relative tumor growth with the BLI signal at week 1 set to 100% (indicated by the dashed line). Each tumor growth curve represents the mean with SD. The statistical differences between mice treated with the vehicle and mice treated with FL118 were calculated using Kruskal-Wallis ANOVA (*P<0.05; **P<0.01).
Taken together, our results extend earlier findings obtained in colon and head-and-neck cancer cells5,7 and indicate that FL118, with its effects on multiple anti-apoptotic molecules such as Survivin, Mcl-1, XIAP, and Bcl-2, could be an effective anti-MM agent. Moreover, the potency of FL118 to overcome stromal cell-induced drug resistance could be of importance since EM-DR is considered a relevant mechanism of clinical resistance to several drugs including bortezomib. The most interesting observation may be the high anti-MM efficacy of FL118 especially in RR patients, including patients with P53 dysfunction, a patient group that urgently needs new therapeutic options. Furthermore, our data suggest that FL118 could also be beneficial when combined with standard anti-MM agents such as melphalan, bortezomib, and the currently applied RVD treatment. The potent preclinical efficacy of FL118 described here therefore warrants further evaluation of this novel drug as a therapy option for RR MM patients in clinical trials as a single agent or in combination with currently available anti-MM agents.
Supplementary Material
Acknowledgments
The authors would like to thank the company of Canget BioTekpharma LLC (www.canget-biotek.com), Buffalo, NY, USA, for providing the FL118 drug.
Footnotes
Funding: this study was supported by research funding from the Dutch Cancer Society (VU2014-6567) and Worldwide Cancer Research (14-1223).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Nakagawa Y, Abe S, Kurata M, et al. IAP family protein expression correlates with poor outcome of multiple myeloma patients in association with chemotherapy-induced overexpression of multidrug resistance genes. Am J Hematol. 2006;81(11):824–831. [DOI] [PubMed] [Google Scholar]
- 2.Gupta VA, Matulis SM, Conage-Pough JE, et al. Bone marrow microenvironment-derived signals induce Mcl-1 dependence in multiple myeloma. Blood. 2017;129(14):1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Marzo L, Desantis V, Solimando AG, et al. Microenvironment drug resistance in multiple myeloma: emerging new players. Oncotarget. 2016;7(37):60698–60711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li F. Discovery of survivin inhibitors and beyond: FL118 as a proof of concept. Int Rev Cell Mol Biol. 2013;305:217–252. [DOI] [PubMed] [Google Scholar]
- 5.Ling X, Cao S, Cheng Q, Keefe JT, Rustum YM, Li F. A novel small molecule FL118 that selectively inhibits survivin, Mcl-1, XIAP and cIAP2 in a p53-independent manner, shows superior antitumor activity. PLoS One. 2012;7(9):e45571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling X, Wu W, Fan C, et al. An ABCG2 non-substrate anticancer agent FL118 targets drug-resistant cancer stem-like cells and overcomes treatment resistance of human pancreatic cancer. J Exp Clin Cancer Res. 2018;37(1):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling X, Liu X, Zhong K, Smith N, Prey J, Li F. FL118, a novel camptothecin analogue, overcomes irinotecan and topotecan resistance in human tumor xenograft models. Am J Transl Res. 2015;7(10):1765–1781. [PMC free article] [PubMed] [Google Scholar]
- 8.Chin M, Sive JI, Allen C, et al. Prevalence and timing of TP53 mutations in del(17p) myeloma and effect on survival. Blood Cancer J. 2017;7(9):e610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tessoulin B, Eveillard M, Lok A, et al. p53 dysregulation in B-cell malignancies: More than a single gene in the pathway to hell. Blood Rev. 2017;31(4):251–259. [DOI] [PubMed] [Google Scholar]
- 10.McMillin DW, Delmore J, Weisberg E, et al. Tumor cell-specific bio-luminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med. 2010;16(4):483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Haart SJ, van de Donk NW, Minnema MC, et al. Accessory cells of the microenvironment protect multiple myeloma from T-cell cyto-toxicity through cell adhesion-mediated immune resistance. Clin Cancer Res. 2013;19(20):5591–5601. [DOI] [PubMed] [Google Scholar]
- 12.Markovina S, Callander NS, O’Connor SL, et al. Bortezomib-resistant nuclear factor-kappaB activity in multiple myeloma cells. Mol Cancer Res. 2008;6(8):1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Veer MS, de Weers M, van Kessel B, et al. Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Haematologica. 2011;96(2):284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westover D, Ling X, Lam H, et al. FL118, a novel camptothecin derivative, is insensitive to ABCG2 expression and shows improved efficacy in comparison with irinotecan in colon and lung cancer models with ABCG2-induced resistance. Mol Cancer. 2015;14:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groen RW, Noort WA, Raymakers RA, et al. Reconstructing the human hematopoietic niche in immunodeficient mice: opportunities for studying primary multiple myeloma. Blood. 2012;120(3):e9–e16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.