Abstract
The Bruton tyrosine kinase inhibitor ibrutinib has become a leading therapy against chronic lymphoid leukemia. Recently, ibrutinib has been associated with the occurrence of invasive fungal infections, in particular invasive aspergillosis. The mechanisms underlying the increased susceptibility to fungal infections associated with exposure to ibrutinib are currently unknown. Innate immunity, in particular polymer-phonuclear neutrophils, represents the cornerstone of anti-Aspergillus immunity; however, the potential impact of ibrutinib on neutrophils has been little studied. Our study investigated the response to Aspergillus fumigatus and neutrophil function in patients with chronic lymphoid leukemia or lymphoma, who were undergoing ibrutinib therapy. We studied the consequences of ibrutinib exposure on the functions and anti-Aspergillus responses of neutrophils obtained from healthy donors and 63 blood samples collected at different time points from 32 patients receiving ibrutinib for lymphoid malignancies. We used both flow cytometry and video-microscopy approaches to analyze neutrophils’ cell surface molecule expression, cytokine production, oxidative burst, chemotaxis and killing activity against Aspergillus. Ibrutinib is associated, both in vitro and in patients under treatment, with multiple functional defects in neutrophils, including decreased production of reactive oxygen species, impairment of their capacity to engulf Aspergillus and inability to efficiently kill germinating conidia. Our results demonstrate that ibrutinib-exposed neutrophils develop significant functional defects that impair their response against Aspergillus fumigatus, providing a plausible explanation for the emergence of invasive aspergillosis in ibrutinib-treated patients.
Introduction
Bruton tyrosine kinase (BTK), a member of the TEC kinase family, plays a key role in the signaling pathway of the B-cell receptor, which controls B-cell development, activation and proliferation. In humans, inactivating BTK mutations are responsible for a maturation block in early B-cell development at the pro-B-cell stage which results in a nearly complete absence of B cells and agammaglobulinemia, a disease known as X-linked agammaglobulinemia (XLA).1 Ibrutinib, a small covalent BTK inhibitor, has ushered a new era in the therapeutic strategy for chronic lymphocytic leukemia and displays interesting clinical efficacy in other types of lymphoid malignancies.2 Recently, ibrutinib has been linked to the occur rence of invasive fungal infections, in particular invasive aspergillosis with an unusually high number of cases with central nervous system involvement.3–5 This association was somewhat unexpected since invasive fungal infections do not belong to the spectrum of infections observed in patients with XLA and patients with chronic lymphocytic leukemia are considered to be at low-risk of invasive aspergillosis.6
It has been known for decades that neutrophils represent the major effectors in the host’s defense mechanisms against Aspergillus.7,8 Quantitative and qualitative (e.g. NADPH oxidase component deficiency in chronic granulomatous disease) defects affecting neutrophils are among the most important risk factors for developing invasive aspergillosis.7,9 BTK has been extensively studied in B cells but data on its role in neutrophils are limited. Although previous work indicates that BTK is expressed by neutrophils and plays a role in neutrophil development and function,10 very little is known about the potential effect of BTK or its inhibition on antifungal immunity.11
This prompted us to test the hypothesis that ibrutinib administration might impair neutrophil responses against Aspergillus. We performed in vitro experiments on neutrophils from healthy donors and analyzed neutrophil functions in patients receiving ibrutinib at different time points during the course of treatment. Here we show that ibrutinib treatment is associated with multiple defects in neutrophil functions, in particular decreased CD11b surface expression, reduction of reactive oxygen species (ROS) production and impairment of the cells’ capacity to kill Aspergillus fumigatus.
Methods
Patients
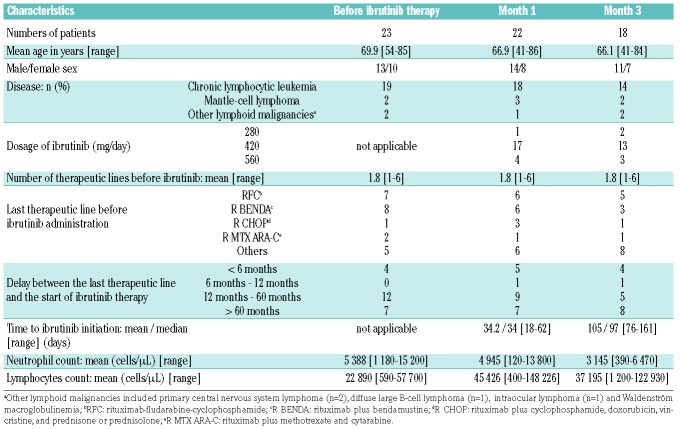
Thirty-three patients from three centers treated with ibrutinib for a lymphoid malignancy were enrolled in the study (Table 1). The main indications for ibrutinib treatment were chronic lymphocytic leukemia (n=23; 72% of patients) and mantle-cell lymphoma (n=4; 12.5% of patients). Patients receiving corticoid therapy or granulocyte colony-stimulating factors were not considered. Neutrophils were collected from patients just before ibrutinib treatment was initiated (M0; n=23) then approximately 1 month (M1; n=22) and 3 months later (M3; n=18). No patient was neutropenic. Because a complete longitudinal (M0, M1 and M3) follow up could not be obtained in all patients, the sample size varies between experiments and time points. No available data have been subtracted. No cases of invasive aspergillosis were diagnosed throughout the study.
Table 1.
Characteristics of patients and blood samples included in the study.
Neutrophil isolation
Neutrophils were isolated using the dextran-Ficoll method, as detailed in the Online Supplement.
Stimulation conditions
Whole-blood samples (500 μL) were stimulated for 2 h at 37°C with either 106 germinating conidia from an Aspergillus fumigatus sensu stricto clinical isolate or 5 ng/mL bacterial lipopolysaccharide (LPS) (Sigma Aldrich) or phosphate-buffered saline (PBS) as a control. Details are provided in the Online Supplement.
Surface molecule expression of neutrophils
For cytometry analysis anti-human antibodies directed against the following human antigens were used: CD11b, CD14, CD15, CD16, CD62L, Dectin 1, TLR2 and TLR4 (BD Biosciences) and CD66b (Biolegend).
Measurement of neutrophil oxidative burst
Neutrophils contained in 500 μL heparinized whole-blood samples were incubated with 123-dihydro-rhodamine (Life Technologies) for 5 min at 37°C, then stimulated with the aforementioned stimuli for 2 h at 37°C.
Intracellular cytokine analysis
Whole-blood samples (500 μL) were stimulated with the above-mentioned stimuli for 4 h at 37°C. Brefeldin A (Sigma-Aldrich) was added after 30 min of stimulation. Cells were stained with membrane antibodies, permeabilized using the Intracellular Fixation & Permeabilisation Buffer Set (eBioscience) and stained with the following anti-human antibodies: interleukin (IL)-8, IL-6, tumor necrosis factor alpha (TNFα) (BD Biosciences) and IL-1β (R&D Systems). The concentration of IL-8 was determined using a Duo-Set enyme-linked immunosorbent assay kit from R&D Systems (Minneapolis, MN, USA).
Video-microscopy experiments
Three thousand Aspergillus fumigatus conidia were seeded in black, 96-well clear-bottom plates (Greiner) and allowed to germinate. After two washes, 48,000 isolated neutrophils in RPMI medium and Sytox green (final concentration 2 μM) were added. Interactions were visualized during 16 h of co-culture at 37°C using a Zeiss Axio Z1 fluorescent microscope (Carl Zeiss, Germany). Images were processed and analyzed using Imaris® software. The chemotaxis assay was performed using IncuCyte® ClearView 96-Well Chemotaxis Plates. Purified neutrophils stained by Hoechst (final concentration 10 μM) were placed in the membrane insert and formyl-methionine-leucyl-phenylalanine (fMLP), as a chemo-attractant (final concentration 10 μM), was put in the reservoir plate. More details are given in the Online Supplement.
Statistical analysis
GraphPad Prism 6 was used for statistical analyses (GraphPad software, La Jolla, CA, USA).
Ethics
This non-interventional study was performed in accordance with national regulations regarding the use of human material from healthy volunteers and patients. The local medical ethics committee (CPP Ile de France IV) approved the study, which was conducted in accordance with the principles of the Declaration of Helsinki. Signed informed consent to participation in the study was obtained from all patients.
Results
Effect of ibrutinib on CD11b, CD62L and Dectin-1 expression on neutrophils
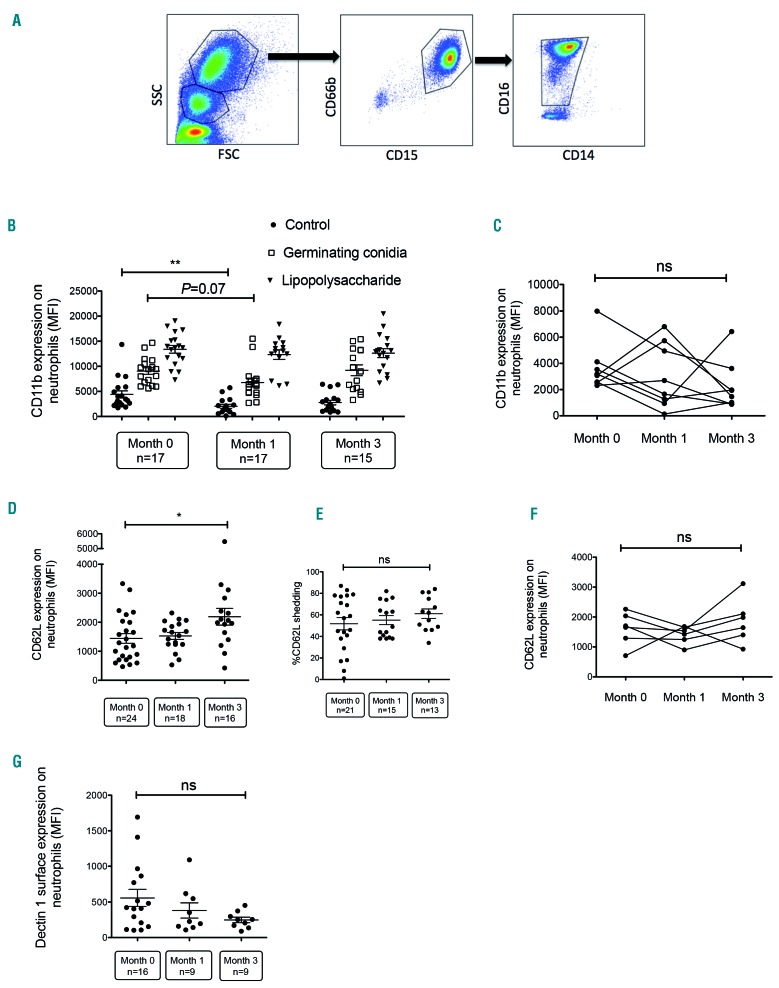
Neutrophils were gated according to size and granularity by forward versus side scatter (FSC vs. SSC) and identified as CD66b+, CD15+, CD16+/low, CD14− cells (Figure 1A). Surface expression of CD11b and CD62L (L-selectin) was evaluated at baseline then following stimulation by germinating conidia or LPS. In association with CD18 (β2 integrin), CD11b forms the heterodimeric integrin complement receptor 3 (CR3), also called macrophage-1 antigen, which is not only involved in the adhesion and migration of leukocytes but has also been recognized as a major receptor for β-glucan.12 CD11b is overexpressed at the surface of neutrophils after degranulation as it is contained in secondary and tertiary neutrophil granules.13 CD62L is involved in transient tethering of the neutrophils to the endothelial surface. Shedding of CD62L marks activation of neutrophils. As previously reported,14 germinating conidia and LPS induced significant activation of neutrophils, as evidenced by an increase in CD11b and a decrease in CD62L expression (Figure 1B and data not shown). Expressed as mean fluorescence intensity (MFI), mean CD11b surface expression of the M0 group increased 2-fold after stimulation with germinating conidia (9,053 vs. 4,414) and by 3-fold after LPS stimulation (13,392 vs. 4,414). CD11b surface expression on unstimulated neutrophils decreased after starting ibrutinib treatment (n=17 patients) in comparison with the expression prior to treatment initiation (n=17 patients) (Figure 1B). However, no statistically significant difference was observed when a paired analysis of eight patients sampled at M0, M1 and M3 was done (Figure 1C). After stimulating whole blood with germinating conidia, the increase of CD11b surface expression in M1 patients was statistically not different from that of the M0 group. Expressed as MFI, mean CD11b surface expression of the M1 group increased 3.3-fold after germinating conidia stimulation (6,857 vs. 2,070) and by 6-fold after LPS stimulation (12,394 vs. 2,070). Basal CD62L expression tended to increase over time with a statistically significant difference between M0 and M3 (Figure 1D) but CD62L shedding following stimulation by germinating conidia did not change over time (Figure 1E). Paired analysis of six patients sampled at M0, M1 and then M3 indicated no difference over time (Figure 1F). We also analyzed basal expression of Dectin-1, TLR2 and TLR4, which play important roles in antifungal defenses.15,16 Dectin-1 expression decreased over time but the difference was not statistically significant. No difference was observed for TLR2 and TLR4 (Figure 1G and data not shown). Collectively, these results suggest that neutrophils from patients receiving ibrutinib slightly altered CD11b surface expression but no other important immune receptors. In addition, ibrutinib-exposed neutrophils seemed to maintain their ability to trigger a marked response to Aspergillus and LPS stimuli.
Figure 1.
Effect of ibrutinib on CD11b, CD62L and Dectin-1 expression on neutrophils. (A) Representative fluorescence-activated cell-sorted plots: neutrophils were gated according to size and granularity by forward (FSC) vs. side scatter (SSC) properties and as CD66b+, CD15+, CD16+/low, CD14- cells. (B) Neutrophil surface expression of CD11b in samples of whole blood taken from patients before starting ibrutinib therapy, and 1 month and 3 months after initiation of ibrutinib therapy, as assessed by flow cytometry. Blood samples (500 μL) were incubated for 2 h without stimulation (phosphate-buffered saline) or after stimulation with 106 germinating Aspergillus conidia or bacterial lipopolysaccharide plus formyl-methionine-leucyl-phenylalanine. (C) Paired analysis of CD11b surface expression in neutrophils collected from eight patients at different time points during ibrutinib therapy. (D, E) Neutrophil surface expression of CD62L in whole blood samples from patients before ibrutinib therapy, 1 month after starting ibrutinib treatment, and 3 months after initiation of ibrutinib therapy, as assessed by flow cytometry. The percentage of CD62L shedding was determined after stimulation with germinating conidia compared with non-stimulated cells. (F) Paired analysis of CD62L surface expression in neutrophils collected from six patients at different time points during ibrutinib therapy. (G) Neutrophil surface expression of Dectin-1 in whole blood taken from patients before ibrutinib therapy, 1 month after treatment initiation and 3 months after initiation of ibrutinib therapy, as assessed by flow cytometry. Long bars represent the mean fluorescence intensity (MFI), and short bars represent the standard error of the mean. Multiple groups were analyzed using the Kruskal-Wallis test with the Dunn multiple comparison post-test. Paired data were analyzed using the Friedman test. *P<0.05; ** P<0.01; ns: non-significant.
Reactive oxygen species production after Aspergillus challenge is decreased in patients receiving ibrutinib
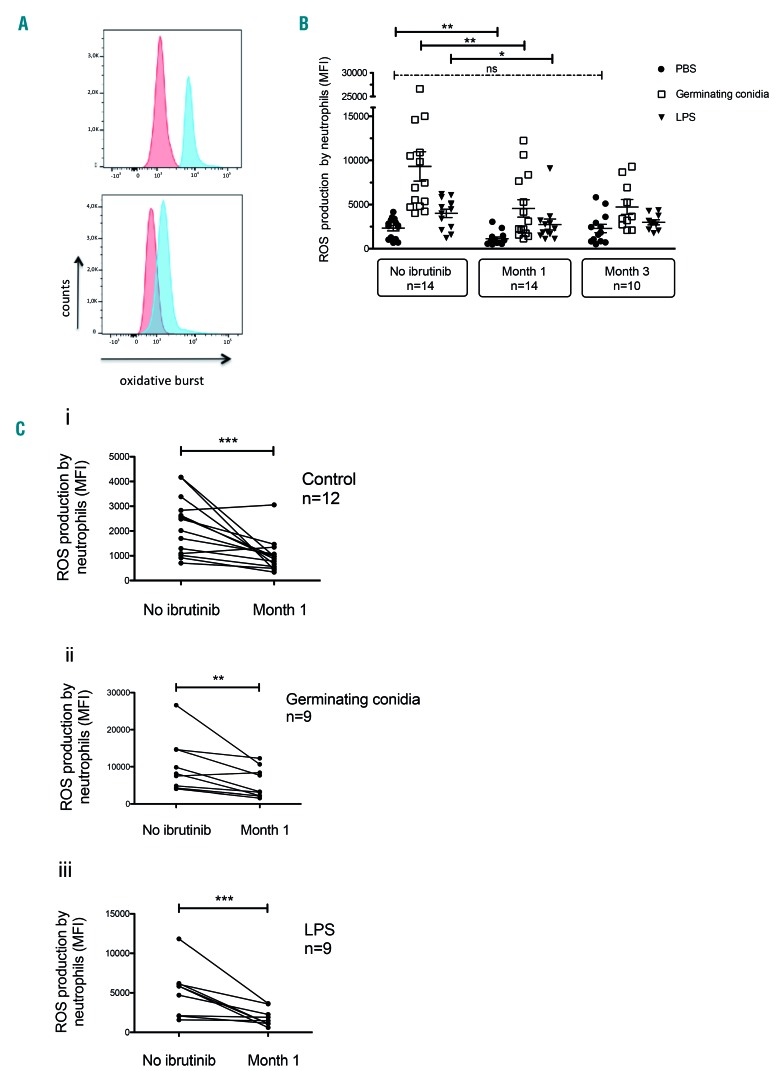
As previously reported,14 ROS production increased after 2 h of stimulation by germinating conidia or LPS plus fMLP (Figure 2A). ROS production by neutrophils sampled at M1 was statistically lower for all conditions, i.e. PBS control, Aspergillus stimulation and LPS stimulation (Figure 2A, B). Expressed as MFI, mean ROS production decreased at M1 by 51.5% for the basal PBS condition (2,333 vs. 1,131; P<0.05), 51% after stimulation with germinating conidia (9,330 vs. 4,569; P<0.05) and 31.6% after LPS stimulation (4,008 vs. 2,742; P<0.05) in comparison with M0. The defect in ROS production persisted in neutrophils at M3. Paired analysis of samples collected at M0 and M1 after stimulation with germinating conidia or LPS was achievable in 12 and 9 patients, respectively. As shown in Figure 2C, ROS production decreased markedly after 1 month of ibrutinib treatment both before and after stimulation by Aspergillus conidia or LPS.
Figure 2.
Evolution of reactive oxygen species production by neutrophils during ibrutinib therapy. (A) Whole-blood neutrophils from a 61-year old man with chronic lymphoid leukemia were analyzed by flow cytometry for production of reactive oxygen species (ROS) in the basal condition (red) and after stimulation with 106 Aspergillus fumigatus germinating conidia (blue) before (upper panel) and after 62 days of ibrutinib therapy (lower panel). (B) ROS production by neutrophils in whole blood collected from patients before ibrutinib therapy, 1 month after starting ibrutinib treatment and 3 months after initiation of ibrutinib therapy and stimulated with 106 Aspergillus germinating conidia or bacterial lipopolysaccharide plus formyl-methionine-leucyl-phenylalanine (LPS). Long bars represent the mean fluorescence intensity (MFI), and short bars represent the 10-90th percentile. The Kruskal-Wallis test with the Dunn multiple comparison post-test was applied *P<0.05; **P<0.01; ns: non-significant. (C) Paired analysis of ROS production by neutrophils in 500 μL whole blood from patients before and after 1 month of ibrutinib therapy under various conditions: (i) no stimulation (basal level); (ii) after stimulation with 106 Aspergillus fumigatus germinating conidia; or (iii) challenge with bacterial lipopolysaccharide plus formyl-methionine-leucyl-phenylalanine (LPS). Wilcoxon matched pairs test was applied; **P<0.01; ***P<0.005
Neutrophils from ibrutinib-treated patients fail to produce interleukin-8 upon Aspergillus stimulation
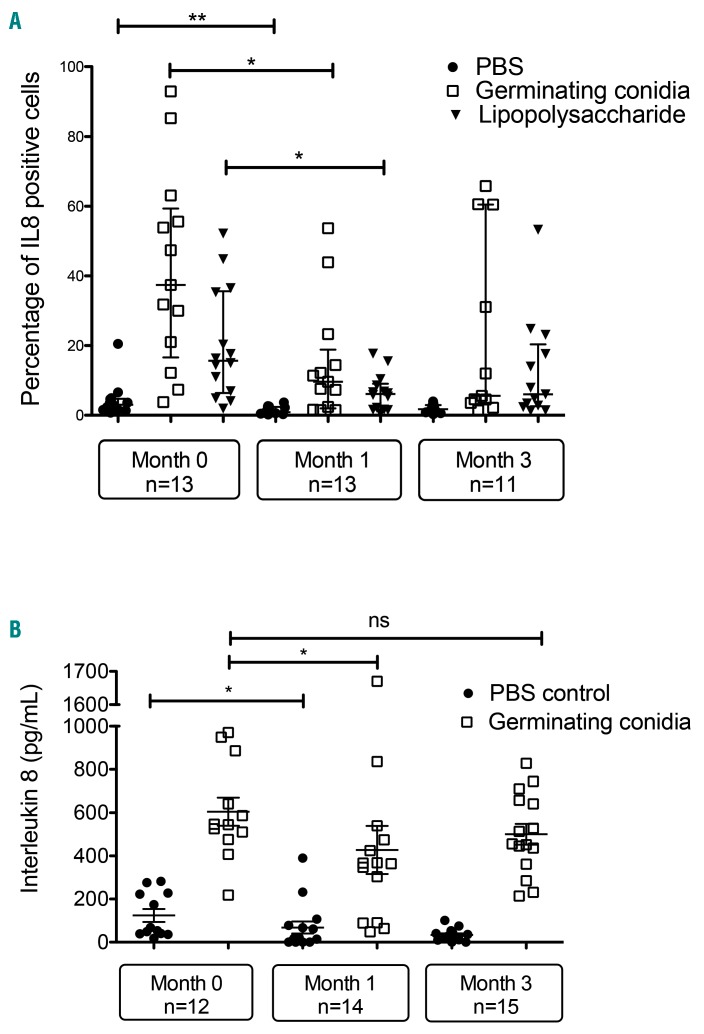
We evaluated intracellular production of IL-1β, IL-6, TNFα and IL-8 by neutrophils in whole blood by flow cytometry. In our experimental conditions, we did not detect changes in IL-1β, IL-6 or TNFα either before or after Aspergillus or LPS challenge over time (data not shown). We next focused on IL-8, a major chemoattractant for neutrophils. As shown in Figure 3A, the mean percentage of IL-8-positive cells increased 4.7-fold (20.1% vs. 4.3%) and 9.7-fold (41.7% vs. 4.3%) after stimulation for 4 h with LPS or germinating conidia, respectively (Figure 3A). The percentage of IL-8-positive cells decreased in the M1 group in comparison with the M0 group for all conditions (control, germinating conidia and LPS). and was not modified when comparing the M1 to M3 group. We next measured the concentration of IL-8 in whole blood collected from at M0, M1 and M3 and infected for 2 h by A. fumigatus germinating conidia. These results confirmed that ibrutinib therapy was related to a decrease of IL-8 production after Aspergillus challenge in the M1 group (Figure 3B).
Figure 3.
Production of interleukin 8 by neutrophils is impaired during ibrutinib therapy. (A) Neutrophils from whole blood taken from patients before ibrutinib treatment, and 1 and 3 months after starting ibrutinib therapy were untreated (exposed to phosphate-buffered saline, PBS), or stimulated with Aspergillus germinating conidia or lipopolysaccharide for 4 h at 37°C. Brefeldin A was added after 30 min of stimulation. Cells were stained with membrane antibodies, permeabilized, stained with interleukin 8 (IL-8) antibody and then analyzed by flow cytometry. The results are expressed as the percentage of positive cells. (B) Whole blood samples were stimulated with Aspergillus germinating conidia for 2 h. The supernatants were collected and tested for IL8 concentration.
Ibrutinib has no effect on neutrophil mobility and chemotaxis
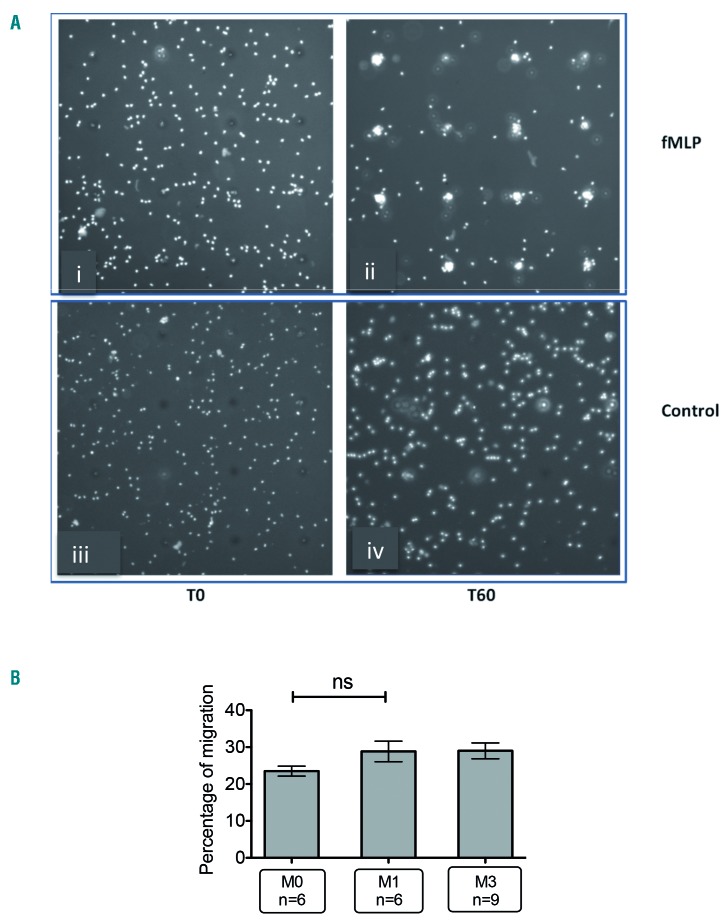
Because migration to infected sites is a prerequisite to mounting an efficient anti-fungal response, we tested the ability of neutrophils to react to the chemoattractant fMLP (Figure 4A). Percentage of chemotaxis was defined as the number of neutrophils that reached the pore against the total number of neutrophils in the surrounding area. As shown in Figure 4B, neutrophils derived from treated and untreated patients displayed similar capacity to reach the reservoir containing fMLP, demonstrating that ibrutinib treatment did not alter the ability of neutrophils to migrate and respond to the fMLP signal.
Figure 4.
Ibrutinib therapy does not alter neu-trophil chemotaxis. Neutrophil migration assays were performed using IncuCyte® ClearView 96-Well Chemotaxis Plates (BioScience). Ten thousand purified neutrophils were aliquoted into the membrane insert, while formyl-methionine-leucyl-phenylalanine (fMLP) was added to the reservoir plate. Migration of neutrophils towards the reservoir from the insert was observed in real time over the course of 60 min. The migration index was defined as the proportion of neutrophils migrating into the pore compared with all neutrophils crossing a square zone of 260 x 260 μm centered by the pore of interest. (A) At the beginning of the experiment (T0), the presence of fMLP (i) led to migration of neutrophils towards the pore after 60 minutes (T60) (ii), whereas the vehicle control has no effect (iii. iv). (B) Neutrophils collected from patients before (M0) or after 1 month (M1) and 3 months (M3) of ibrutinib therapy were submitted to the chemotaxis assay. Statistical analysis was performed by applying the Kruskall Wallis test with the Dunn multiple comparison post-test. ns: non-significant.
Engulfment and killing of Aspergillus fumigatus by neutrophils are impaired in patients receiving ibrutinib
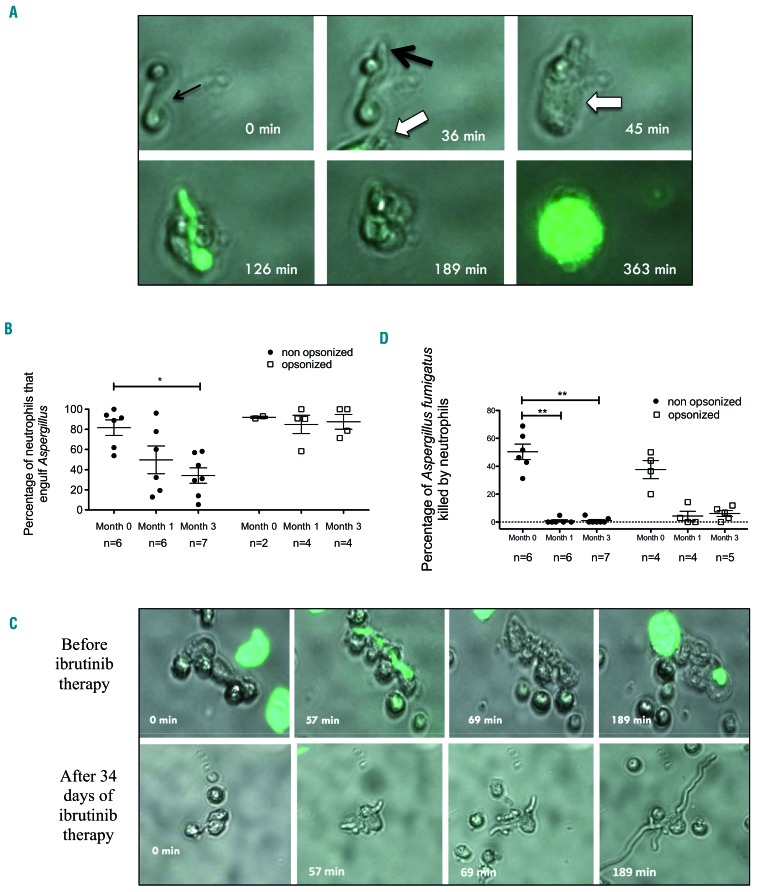
Video-microscopy was used to investigate the behavior of neutrophils and fungi (at the germinating conidia stage) as well as their interactions. Preliminary experiments using Aspergillus exposed to voriconazole, an antifungal drug with fungicidal activity against Aspergillus, indicated that dying Aspergillus stained transiently with Sytox green dye. As opposed to human cells, the fluorescence of the dead fungi was lost after approximately 15-20 min (data not shown). We therefore determined Aspergillus killing as the Sytox staining of the fungus and its inability to grow further. Because killing germinating conidia requires close contact, we focused on germinating conidia engulfed by neutrophils and calculated the percentage of killing as the number of dying Aspergillus as a proportion of the whole number of germinating conidia engulfed by neutrophils.
Aspergillus killing proceeds in two steps. First, neutrophils engulf i.e. deform and bind firmly to Aspergillus, next they prevent its growth and ultimately they kill the fungus 60 to 120 min later (Figure 5A). First, we found that the proportion of neutrophils that could engulf Aspergillus decreased after 1 and 3 months of treatment with ibrutinib (Figure 5B). This defect was restored when Aspergillus conidia were opsonized by addition of human autologous serum (Figure 5B). Strikingly, exposure to ibrutinib was associated with a major impairment of the ability of neutrophils to kill Aspergillus. The percentage of germinating conidia killed by neutrophils fell from 50% at M0 to 1% at M1 and 0.8% at M3 (Figure 5C, D). Opsonization by autologous serum had no effect on the killing defect (Figure 5D).
Figure 5.
Engulfment and killing of Aspergillus fumigatus by neutrophils is impaired in patients receiving ibrutinib. Neutrophil-Aspergillus fumigatus interactions were tracked in real-time by video-microscopy during co-culture for 16 h. Three thousand Aspergillus fumigatus conidia were first allowed to germinate in a 96-well plate before 48,000 purified neutrophils were added. For opsonized conditions, autologous serum was added for 15 min and then removed before neutrophils were added. (A) The time sequence shows elongation of one germinating conidium (black arrows; time: 0 to 36 min after the start of experiment) before engulfment by a neutrophil from a patient with lymphoid malignancy prior to ibrutinib therapy (white arrow; time: 45 min). The neutrophil kills the Aspergillus conidium (as indicated by the fungus stained with Sytox green) after approximately 80 min of close non-discontinuous interactions. Approximately 4 h after killing the fungus, the neutrophil dies (time: 363 min). (B) Percentage of Aspergillus fumigatus engulfed by neutrophils in patient samples before ibrutinib therapy and 1 or 3 months after the start of ibrutinib therapy, with or without previous opsonization with autologous serum. The Kruskal-Wallis test with the Dunn multiple comparison post-test were applied; *P<0.05. (C) Comparison of two different time sequences of a sample from a single patient with chronic lymphoid leukemia before ibrutinib therapy (upper) and after 34 days of ibrutinib therapy (lower). In the sample taken after ibrutinib treatment, the inability of the neutrophil to kill the germinating conidia led to uncontrolled fungal growth. (D) Rate of killing of Aspergillus fumigatus germinating conidia by neutrophils from patients before ibrutinib therapy and 1 or 3 months after the start of ibrutinib therapy, with or without previous opsonization with autologous serum. The Kruskal-Wallis test with the Dunn multiple comparison post-test were applied; **P<0.005.
Neutrophils exposed to ibrutinib in vitro have altered responses to Aspergillus
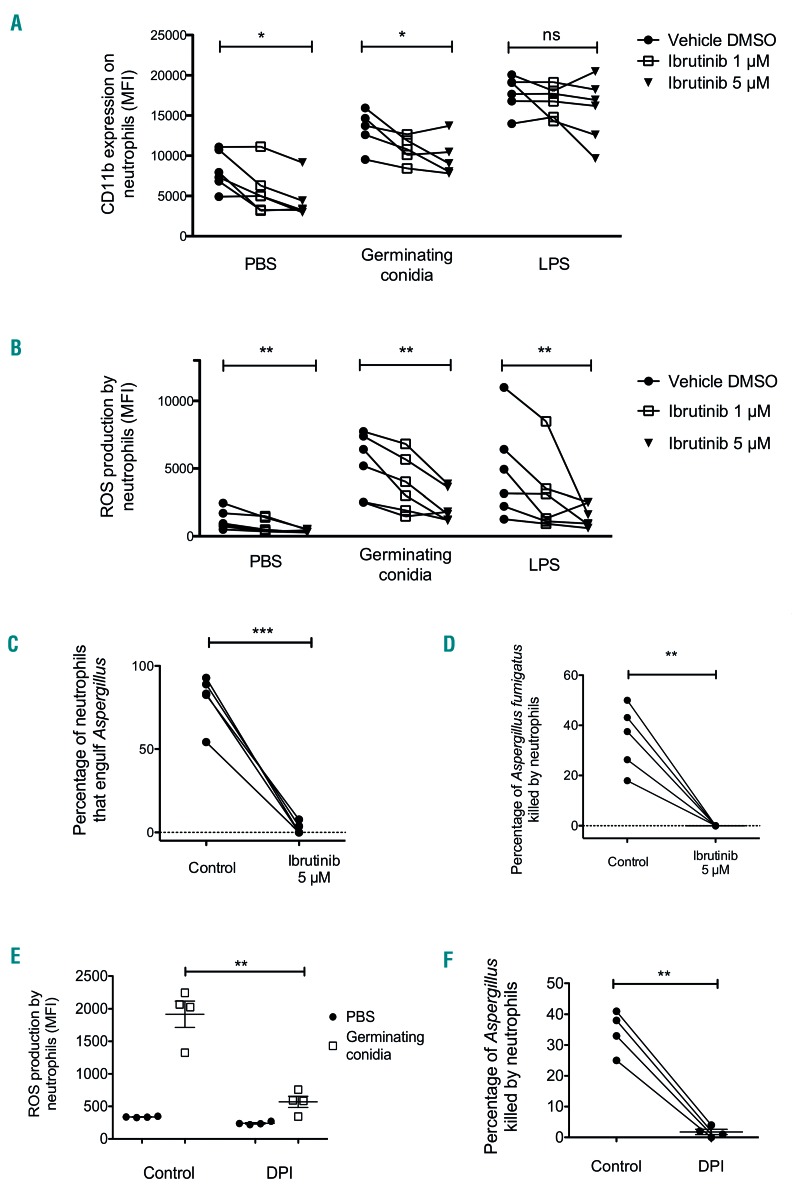
Similar experiments were conducted on neutrophils obtained from healthy donors. Addition of ibrutinib was associated with decreased cell surface expression of CD11b both at baseline (8,143 vs. 4,396; 46% decrease; P<0.05) and after challenge with germinating conidia (13,298 vs. 9,811; 26.2% decrease; P<0.05) (Figure 6A). Interestingly, CD11b expression was unmodified after LPS stimulation (17,789 vs. 15,674; decrease: 11.9%; P=NS), suggesting that the effect of ibrutinib was specific to the antifungal response pathway. Ibrutinib also impaired ROS production in all conditions, as evidenced by decreases of 68.2% from basal level (1,205 vs. 383; P<0.005), 58.3% after challenge with germinating conidia (5,298 vs. 2,209; P<0.005) and 69% after LPS stimulation (4,834 vs. 1,500; P<0.005) (Figure 6B).
Figure 6.
In vitro effect of ibrutinib on neutrophil reactive oxygen species production, CD11b surface expression and killing of Aspergillus fumigatus. Blood collected from six healthy donors was incubated for 10 min at 37°C with ibrutinib (final concentration of 1 μM or 5 μM) or dimethylsulfoxide (DMSO) vehicle as a control and then stimulated for 2 h before flow cytometry analysis. The long horizontal bars indicate the mean, and the short bars indicate the standard error of the mean. (A) Expression of surface CD11b on neutrophils without stimulation (phosphate-buffered saline, PBS) or after stimulation with germinating Aspergillus conidia or bacterial lipopolysaccharide (LPS) plus formyl-methionine-leucyl-phenylalanine (fMLP). (B) Reactive oxygen species (ROS) production by neutrophils without stimulation (PBS) or stimulated by germinating Aspergillus conidia or LPS plus fMLP. Neutrophils were purified from blood collected from five healthy donors and then incubated for 10 min with ibrutinib (final concentration: 5 μM) or DMSO. (C) Engulfment of Aspergillus germinating conidia by neutrophils was observed in real-time by video-microscopy during co-culture for 16 h. (D) The rate of killing of Aspergillus germinating conidia by neutrophils was determined in real-time by video-microscopy during co-culture for 16 h. Blood collected from four healthy donors was incubated for 10 min with the NADPH oxidase inhibitor diphenyleneiodonium (DPI; final concentration 5 μM) or DMSO before stimulation or neutrophil purification. (E) ROS production by neutrophils in blood with or without DPI and stimulated for 2 h with germinating conidia, assessed by flow cytometry analysis. (F) Rate of killing of Aspergillus germinating conidia by neutrophils isolated from blood incubated with or without DPI determined in real-time by video-microscopy during a 16 h co-culture. Multiple group analysis was assessed by the Friedman test for paired data with the Dunn multiple comparison post-test; two-group analysis was assessed by a paired t-test; *P<0.05; **P<0.005; ***P<0.0005; ns: non-significant.
Finally, we confirmed the video-microscopy observations, using blood from healthy donors exposed to 5 μM ibrutinib for 10 min. Ibrutinib led to a decrease of engulfment ability and a dramatic impairment of Aspergillus killing by neutrophils (Figure 6C, D). Because we found that ibrutinib markedly impaired ROS production, we examined whether the inhibition of ROS production by diphenyleneiodonium (DPI), a NADPH oxidase inhibitor, could lead to similar results. DPI caused not only a pre dictable decrease in ROS in response to germinating conidia, but also decreased killing activity against growing Aspergillus, in a manner similar to that observed with ibrutinib (Figure 6E, F and data not shown). Interestingly, DPI had no effect on the engulfment process, which suggests that the inhibitory effect of ibrutinib is not caused by the sole inhibition of ROS production.
Discussion
Ibrutinib, an irreversible BTK inhibitor, displays significant clinical efficacy in some lymphoid malignancies.2 Ibrutinib has, however, recently been associated with the occurrence of invasive fungal infections, in particular invasive aspergillosis.3–5 Intriguingly, patients with XLA do not experience aspergillosis and patients with lymphoid malignancies are usually considered to be at low risk of invasive aspergillosis. Consequently, the mechanism behind the emergence of invasive aspergillosis among patients receiving ibrutinib has remained unclear. The association of ibrutinib and fungal infections, in particular invasive aspergillosis,3–5 suggested that the drug could have a detrimental effect on antifungal immune responses.17
Because neutrophils are the cornerstone in the fight against fungi, the impact of ibrutinib on neutrophil functions had to be evaluated. Neutrophils act against Aspergillus through different mechanisms, depending on the stage of life of the fungus. Inhibition of the growth of resting conidia involves non-oxidative pathways and CD11b/CD18 recognition while hyphae are destroyed by oxidative derivatives after recognition by Fc-gamma receptor or CLR.18,19
Analysis of CD11b, which like Dectin-1 is a very important receptor for β-glucan,20 showed conflicting results. An unpaired comparison between 17 samples obtained before treatment and 17 samples after approximately 1 month of ibrutinib indicated that basal levels were reduced after initiating ibrutinib therapy. This result was further confirmed in vitro. However, paired analysis on eight longitudinally followed patients was inconclusive. The reason for this discrepancy is unclear. It might be due to an inadequate cohort size or to the patients’ heterogeneity in terms of disease stage and treatment response. Interestingly, both in vivo and in vitro analyses showed that ibrutinib had no effect on CD11b surface expression in response to LPS, which suggests that the defect may be specific to the antifungal response. The C-type lectin receptor of β-glucan Dectin-1 is considered to be an essential component of the antifungal immune response. In human neutrophils Dectin-1 neutralization by monoclonal antibodies leads to an impairment of binding and phagocytosis of zymosan and diminished ROS production21 although contradictory results have been published.20 We found a not statistically significant defect in Dectin-1 surface expression during ibrutinib therapy. Collectively, our results suggest that ibrutinib may alter CD11b surface expression on neutrophils from treated patients but has no effect on other important immune receptors and has little effect on their upregulation after Aspergillus and LPS stimuli.
ROS production is a major mechanism to kill fungi7,8 as exemplified by the phenotype of patients with chronic granulomatous disease. Neutrophils of these patients display a profound defect in ROS production caused by mutations in components of the NADPH oxidase22 and therefore are unable to kill Aspergillus hyphae.19 Mangla et al. showed that neutrophils from X-linked immunodeficient mice lacking functional Btk had defective production of both ROS and nitric oxide after LPS challenge.23 In agreement with these observations, a recent study also found that superoxide production in neutrophils stimulated with fMLP was reduced in Btk−/− mice.24 After 1 month of ibrutinib treatment, we found that neutrophil ROS decreased by approximately 50% in the presence of Aspergillus germinating conidia. A similar alteration in ROS production was also observed when ibrutinib was added to blood from healthy donors, which indicates that the impact of ibrutinib is prompt and seems to rule out an effect occurring during granulopoiesis. In contradiction with our results, Stadler et al. found no difference in ROS production by neutrophils challenged with zymosan or LPS in patients treated with ibrutinib.11 It should however be noted that these results were obtained from a cohort of only six patients, for whom the duration and posology of ibrutinib therapy were not reported. Moreover, zymosan is a yeast extract containing β-glucan, the structure of which differs from that of Aspergillus. Since CD11b is important for neutrophil ROS production,25 one may hypothesize that reduced CD11b expression after ibrutinib exposure may affect ROS, although this requires further investigation.
In human neutrophils, it has been shown that fMLP-induced IL-8 release is dependent on ROS production.26 IL-8 is a major neutrophil chemoattractant and its levels have been found to be elevated in the serum and bronchoalveolar lavage of patients with invasive aspergillosis.27 IL-8 production by neutrophils was reduced in samples from ibrutinib-treated patients upon Aspergillus stimulation. By contrast, the production of other cytokines did not change upon stimulation or during the course of ibrutinib therapy. Whether or not defective IL-8 production may result in insufficient neutrophil recruitment to the sites of Aspergillus infection deserves further investigation, including the use of animal models.
While ibrutinib did not seem to impair neutrophil chemotaxis, video-microscopy revealed that it reduced the cells’ ability to interact closely with and engulf Aspergillus. The molecular mechanism by which ibrutinib hinders these interactions remains to be determined. More striking was the dramatic decrease in the ability of neutrophils to kill Aspergillus when exposed to ibrutinib. Altogether, given that the oxidative burst is the major mechanism of killing hyphae,18 it is very likely that ibrutinib impairs neutrophil killing at least in part through inhibition of ROS. We observed that in vitro exposure of neutrophils to the NADPH oxidase inhibitor DPI recapitulated the effect of ibrutinib except it did not impair Aspergillus engulfment by neutrophils, which suggests that the effect of ibrutinib on neutrophils extends beyond that of ROS inhibition and involves different pathways.
Collectively, our results show that ibrutinib induces multiple functional defects in neutrophils, which result in their inability to kill Aspergillus, and provide a first clue to explain clinical cases. One caveat of our study is that it was not designed to determine whether the effect of ibrutinib is caused by the inhibition of BTK itself. Essentially known for its key role in B cells, experimental evidence suggests that BTK is functional in neutrophils. In a Btk-deficient mouse model, Fiedler et al. showed that Btk was important during granulopoiesis and that neutrophils from Btk−/− mice lacked granule components important for their antimicrobial activity, such as elastase, lactoferrin and myeloperoxidase.10 Btk is involved in the activation of the PLCγ2, AKT and p38MAPK pathways in neutrophils after stimulation.24 Btk is also involved in phosphorylation of Myd88 and NFκB activation after TLR2 or TLR4 engagement.28,29 Lionnakis et al. reported that Btk−/−mice died after Aspergillus challenge whereas wildtype animals did not.4 However, not all experimental evidence agrees with such a role for Btk. Indeed, Btk was found by Honda et al. to be a critical gatekeeper of neutrophil responses, preventing excessive inflammation through inhibition of ROS.30 More recently Cavaliere et al. concluded that monocyte and neutrophil maturation and function were unaffected in XLA patients.31 Another limitation of our in vitro experiments is that we, as others,17 used higher concentrations of ibrutinib than those observed in patients32 to compensate for the very short time of exposure. However, several neutrophil functions, such as an increase in CD11b expression after LPS challenge, were unchanged, suggesting that the concentrations used did not lead to a global impairment of the neutrophils’ cellular processes.
XLA patients are not particularly susceptible to fungal infections, unless one considers that alternative mechanisms may develop over time to compensate for BTK deficiency in humans. At present, whether neutrophil defects are caused by the sole inhibition of BTK by ibrutinib thus remains an open question. Like most kinase inhibitors, ibrutinib is not highly specific, which raises the hypothesis that impairment of antifungal activity may be caused by inhibition of other targets. Further work is required to unravel the precise molecular mechanisms responsible for the observed neutrophil defects. The development of a murine model would provide inter esting information on the antifungal response dynamics in vivo, e.g., whether the impact on CD11b alters the neutrophils’ ability to reach infected tissues. A comparison with other BTK inhibitors, in particular acalabrutinib, which is more selective than ibrutinib, might help to explore the hypothesis of an off-target effect. Finally, it should be highlighted that despite these experimental results, invasive aspergillosis remains a relatively rare complication. This suggests that ibrutinib exposure is probably not sufficient by itself in most cases and that additional environmental and host factors, e.g., TLR or Dectin polymorphisms, the amount of conidia exposure, acquired immune defects related to the underlying disease or previous therapies, are required to enable the development of a clinical infection.
In conclusion, our study, which demonstrates that exposure to ibrutinib impairs the anti-Aspergillus responses of neutrophils both in vitro and in vivo, provides the first step in the road to understanding the relationship between invasive fungal infections and treatment with ibrutinib. The emergence of invasive aspergillosis in this population may be due to neutrophil defects in ROS production in response to fungi, inability of neutrophils to attach firmly to hyphae, and marked impairment in the neutrophils’ capacity to kill fungi. Further work is required to unravel the exact mechanisms underlying this effect, in particular whether it is caused by the inhibition of BTK itself in neutrophils or by an off-target inhibition of other kinases in neutrophils and/or other cell types in the microenvironment.
Supplementary Material
Acknowledgments
We thank all patients and express our gratitude to healthcare providers for their assistance in the inclusion of patients.
This work was supported by a grant from The Janssen Company and grants from “Fondation pour la Recherche Médicale - Equipe Labelisée” and from “Agence Nationale de la Recherche”, project CMOS 2015 (ANR-EMMA-050).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/2/478
References
- 1.Block H, Zarbock A. The role of the tec kinase Bruton’s tyrosine kinase (Btk) in leukocyte recruitment. Int Rev Immunol. 2012;31(2):104–118. [DOI] [PubMed] [Google Scholar]
- 2.Itchaki G, Brown JR. Experience with ibrutinib for first-line use in patients with chronic lymphocytic leukemia. Ther Adv Hematol. 2018;9(1):3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghez D, Calleja A, Protin C, et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood. 2018;131(17):1955–1959. [DOI] [PubMed] [Google Scholar]
- 4.Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31(6):833–843.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grommes C, Younes A. Ibrutinib in PCNSL: The curious cases of clinical responses and aspergillosis. Cancer Cell. 2017;31(6): 731–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tisi MC, Hohaus S, Cuccaro A, et al. Invasive fungal infections in chronic lymphoproliferative disorders: a monocentric retrospective study. Haematologica. 2017;102(3):e108–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond RD, Clark RA. Damage to Aspergillus fumigatus and Rhizopus oryzae hyphae by oxidative and nonoxidative microbicidal products of human neutrophils in vitro. Infect Immun. 1982;38(2):487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaffner A, Douglas H, Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982;69(3):617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker RD. Leukopenia and therapy in leukemia as factors predisposing to fatal mycoses. Mucormycosis, aspergillosis, and cryptococcosis. Am J Clin Pathol. 1962;37: 358–373. [DOI] [PubMed] [Google Scholar]
- 10.Fiedler K, Sindrilaru A, Terszowski G, et al. Neutrophil development and function critically depend on Bruton tyrosine kinase in a mouse model of X-linked agammaglobulinemia. Blood. 2011;117(4):1329–1339. [DOI] [PubMed] [Google Scholar]
- 11.Stadler N, Hasibeder A, Lopez PA, et al. The Bruton tyrosine kinase inhibitor ibrutinib abrogates triggering receptor on myeloid cells 1-mediated neutrophil activation. Haematologica. 2017;102(5):e191–e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thornton BP, Vetvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J Immunol. 1996;156(3):1235–1246. [PubMed] [Google Scholar]
- 13.Cowland JB, Borregaard N. Granulopoiesis and granules of human neutrophils. Immunol Rev. 2016;273(1):11–28. [DOI] [PubMed] [Google Scholar]
- 14.Imbert S, Bresler P, Boissonnas A, et al. Calcineurin inhibitors impair neutrophil activity against Aspergillus fumigatus in allogeneic hematopoietic stem cell transplant recipients. J Allergy Clin Immunol. 2016;138(3):860–868. [DOI] [PubMed] [Google Scholar]
- 15.Hohl TM, Van Epps HL, Rivera A, et al. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 2005;1(3):e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubourdeau M, Athman R, Balloy V, et al. Aspergillus fumigatus induces innate immune responses in alveolar macrophages through the MAPK pathway independently of TLR2 and TLR4. J Immunol. 2006;177(6): 3994–4001. [DOI] [PubMed] [Google Scholar]
- 17.Bercusson A, Colley T, Shah A, Warris A, Armstrong-James D. Ibrutinib blocks Btk-dependent NF-kB and NFAT responses in human macrophages during Aspergillus fumigatus phagocytosis. Blood. 2018;132(18):1985–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gazendam RP, van Hamme JL, Tool AT, et al. Human neutrophils use different mechanisms to kill Aspergillus fumigatus conidia and hyphae: evidence from phagocyte defects. J Immunol. 2016;196(3): 1272–1283. [DOI] [PubMed] [Google Scholar]
- 19.Zarember KA, Sugui JA, Chang YC, Kwon-Chung KJ, Gallin JI. Human polymorphonu-clear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. J Immunol. 2007;178(10): 6367–6373. [DOI] [PubMed] [Google Scholar]
- 20.van Bruggen R, Drewniak A, Jansen M, et al. Complement receptor 3, not Dectin-1, is the major receptor on human neutrophils for beta-glucan-bearing particles. Mol Immunol. 2009;47(2-3):575–581. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy AD, Willment JA, Dorward DW, Williams DL, Brown GD, DeLeo FR. Dectin-1 promotes fungicidal activity of human neutrophils. Eur J Immunol. 2007;37(2):467–478. [DOI] [PubMed] [Google Scholar]
- 22.Lekstrom-Himes JA, Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. N Engl J Med. 2000;343(23):1703–1714. [DOI] [PubMed] [Google Scholar]
- 23.Mangla A, Khare A, Vineeth V, et al. Pleiotropic consequences of Bruton tyrosine kinase deficiency in myeloid lineages lead to poor inflammatory responses. Blood. 2004;104(4):1191–1197. [DOI] [PubMed] [Google Scholar]
- 24.Volmering S, Block H, Boras M, Lowell CA, Zarbock A. The neutrophil Btk signalosome regulates integrin activation during sterile inflammation. Immunity. 2016;44(1):73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark HL, Abbondante S, Minns MS, Greenberg EN, Sun Y, Pearlman E. Protein deiminase 4 and CR3 regulate aspergillus fumigatus and beta-glucan-induced neu-trophil extracellular trap formation, but hyphal killing is dependent only on CR3. Front Immunol. 2018;9:1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hidalgo MA, Carretta MD, Teuber SE, et al. fMLP-induced IL-8 release is dependent on NADPH oxidase in human neutrophils. J Immunol Res. 2015;2015:120348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heldt S, Eigl S, Prattes J, et al. Levels of interleukin (IL)-6 and IL-8 are elevated in serum and bronchoalveolar lavage fluid of haematological patients with invasive pulmonary aspergillosis. Mycoses. 2017;60(12):818–825. [DOI] [PubMed] [Google Scholar]
- 28.Gray P, Dunne A, Brikos C, Jefferies CA, Doyle SL, O’Neill LA. MyD88 adapter-like (Mal) is phosphorylated by Bruton’s tyrosine kinase during TLR2 and TLR4 signal transduction. J Biol Chem. 2006;281(15): 10489–10495. [DOI] [PubMed] [Google Scholar]
- 29.Doyle SL, Jefferies CA, O’Neill LA. Bruton’s tyrosine kinase is involved in p65-mediated transactivation and phosphorylation of p65 on serine 536 during NFkappaB activation by lipopolysaccharide. J Biol Chem. 2005;280(25):23496–23501. [DOI] [PubMed] [Google Scholar]
- 30.Honda F, Kano H, Kanegane H, et al. The kinase Btk negatively regulates the production of reactive oxygen species and stimulation-induced apoptosis in human neutrophils. Nat Immunol. 2012;13(4):369–378. [DOI] [PubMed] [Google Scholar]
- 31.Cavaliere FM, Prezzo A, Bilotta C, Iacobini M, Quinti I. The lack of BTK does not impair monocytes and polymorphonuclear cells functions in X-linked agammaglobulinemia under treatment with intravenous immunoglobulin replacement. PLoS One. 2017;12(4):e0175961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.